Introduction
Hepatocellular carcinoma (HCC) was the sixth most
common cancer type and fourth most common cause of
cancer-associated mortalities worldwide in 2018 (1). Although the various therapeutic
modalities for HCC have improved significantly in recent years,
patients with HCC exhibit a poor survival rate, with a five-year
survival rate of ~30% (2). Two
leading causes of the unfavorable prognosis are delays in the
diagnosis of HCC and a lack of appropriate treatment for advanced
HCC (3). Although researchers have
evaluated the treatment of advanced HCC with immunotherapy or
molecular-targeted therapy, the survival rate of patients with HCC
has not significantly improved. The occurrence of HCC is thought to
be associated with disturbances in the relationships between
various genes (4). Therefore,
identifying the genes and proteins that regulate HCC development is
important for developing novel therapeutic targets (4).
The Hippo signaling pathway antagonizes the
oncogenic transcriptional co-activators yes-associated protein
(YAP) and Tafazzin (TAZ) and serves an important role
in limiting organ size during developmental processes (5,6).
Without the activation of Hippo signaling, non-phosphorylated
YAP and TAZ enter the nucleus and activate the
expression of antiapoptotic and proliferative genes with TEA domain
transcription factors (TEADs) (5,6).
Mammals harbor four TEAD genes (TEAD1-4). VGLL
is a hippo-independent coactivator that regulates gene expression
via interaction with TEAD, and there are four VGLL
genes (VGLL1-4). Notably, VGLL4 inhibits tumor growth
by binding to TEAD and VGLL1 inhibits YAP/TAZ
target genes by competitively binding to TEAD (7–10).
Several previous studies revealed that TEADs are involved in
human cancer (11–16). However, these studies were primarily
focused on TEAD1 and TEAD4, while no clinical studies
have examined the association between TEAD2 expression and
prognosis in HCC. The purpose of the present study was to
investigate the mRNA expression status of TEAD2 in HCC
tumors, as well as the prognostic clinical significance of
TEAD2 in HCC. Therefore, the role of TEAD2 as a
possible prognostic marker and therapeutic target for treating HCC
was evaluated.
Materials and methods
Gene expression profiling
Level 3 mRNA expression aggregated data and clinical
data from 50 normal and 377 HCC samples were acquired from The
Cancer Genome Atlas Liver Hepatocellular Carcinoma (TCGA-LIHC) data
portal (https://portal.gdc.cancer.gov/) and
RNAseqV2-RSEM_genes were obtained from Firebrowse v.1.1.39.
(http://firebrowse.org/) for gene expression
analysis. All 377 dataset samples were included for profiling of
mRNA expression.
Analysis of mRNA expression
R software (v.3.6.1; http://www.r-project.org) was used to analyze the raw
data. mRNA expression levels were analyzed with Firebrowse
(http://firebrowse.org) and Gene Expression
Profiling Interactive Analysis software (GEPIA; http://gepia.cancer-pku.cn/). GeneNeighbors and
ClassNeighbors, which are modules in GenePattern (http://broadinstitute.org/cancer/software/genepattern),
were used to calculate the nearest gene neighbors and identify
genes most significantly correlated with a class template for a
specific Hippo pathway gene, respectively. The dataset, which is
Illuminahiseq_maseqv2-RSEM_genes_normalized RNA-Seq data obtained
from Firebrowse (http://firebrowse.org/?cohort=LIHC&download_dialog=true),
was processed using R software (v.3.6.1; http://www.r-project.org) in GeneNeighbors and
ClassNeighbors analyses. The 100 genes most strongly associated
with TEAD2 and VGLL4 were selected for classification
according to Gene Ontology Enrichment Analysis (GO terms) using
Database for Annotation, Visualization and Integrated Discovery
(DAVID; http://david.abcc.ncifcrf.gov).
Differentially expressed genes (DEGs) were classified by GO terms
on the basis of their molecular function, biological process, or
cellular component. DAVID provided not only an overview of
extensive pathways (www.biocarta.com) with various gene interactions but
also the number of DEGs per pathway with a gene enrichment P-value.
Gene enrichment score with P<0.05 considered to indicate a
strong association rather than random probability (17).
Functional enrichment analysis
Gene set enrichment analysis (GSEA) was performed to
identify enriched genes predicted to be associated with pathways in
the hallmark and curated gene sets derived from Kyoto Encyclopedia
of Genes and Genomes (KEGG; http://www.genome.jp/kegg) information. A P-value
<0.05 and false discovery rate <0.1 were considered to
indicate a statistically significant gene.
Survival analysis
Cutoff Finder (http://molpath.charite.de/cutoff) was used to
determine the cut-off values for LIHC mRNA expression.
Illuminahiseq_maseqv2-RSEM_genes_normalised RNA-seq data of Hippo
pathway genes were acquired from a tab-separated files, and the
columns represented variables and the rows represented patients
(http://molpath.charite.de/cutoff/load.jsp). The
determination of optimal cut-off point in Cutoff Finder was based
on overall survival rate for significance on the basis of the
log-rank test for outcome of patient (http://molpath.charite.de/cutoff/assign.jsp). The
Kaplan-Meier method was performed to estimate the cumulative event
(death) rate from the date of operation until death used as the
outcome variable. Survival curves stratified by high and low
expression groups on each Hippo pathway genes were compared using
the log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Human liver samples
In total, 79 patients with HCC were retrospectively
screened who were treated by surgical resection between 1999 and
2016 at Chungnam National University Hospital (Daejeon, South
Korea). The median age of patients was 59 years (range, 18–78
years). All tissue samples were obtained from specimens removed
during lobectomy or segmentectomy. Clinical data was obtained by
reviewing the medical records of all patients. Clinicopathological
review of all cases performed was by JSJ and HSE. The histologic
grade of HCC was determined according to the Edmondson and Steiner
grading system and the Tumor-Node-Metastasis (TNM) staging system
for HCC was determined according to the 8th edition of the American
Joint Committee on Cancer TNM staging system at the time of surgery
(18,19).
Of the 79 HCC cases, fresh-frozen 79 primary HCC and
paired 79 non-tumor liver tissues were acquired for quantitative
(q)PCR from the National Biobank of Korea, Chungnam National
University Hospital, a member of the Korea Biobank Network. In
total, one vial (100 mg) of tumor sample and one vial (100 mg) of
non-tumor sample was obtained from the Biobank of Chungnam National
University Hospital. The present research was approved by the
Institutional Review Board of Chungnam National University Hospital
(approval no. CNUH 2017-05-013).
Reverse transcription (RT)-qPCR
Total RNA was extracted from liver tissues with the
RNeasy Mini kit (Qiagen, Inc.; cat. no. 74106) or TRIzol reagent
(Thermo Fisher Scientific, Inc.) in accordance with the
manufacturers' instructions. We extracted RNA from all liver
tissues used in this experiment using RNeasy Mini kit. Reverse
transcription was performed using the same quantity of total RNA to
generate cDNA using amfiRivert cDNA synthesis master mix
(GenDEPOT). The following temperature protocol were used for RT:
Annealing, 25°C for 5 min and extension at 50°C for 60 min. After
that, Reverse Transcriptase inactivation was performed at 70°C for
15 min. Finally, the samples were held at 4°C. qPCR was performed
using SYBR Green Real-time PCR Master mix (Toyobo Life Science).
The qPCR thermocycling condition were as follows: First,
pre-denaturation step is performed at 95°C for 10 min. This is
followed by 40 cycles of denaturation at 95°C for 45 sec, annealing
at 60°C for 45 sec and extension for 60 sec at 72°C. After that,
the final denaturation reaction is performed for 15 sec at 95°C
followed by a final extension for 5 sec at 65°C. To quantify
transcription, the mRNA expression levels of the target genes were
normalized to those of β-actin. Table
SI shows the primers used in this study. All samples were
evaluated in duplicate, and the relative fold-changes in gene
expression levels were calculated as 2−Δ∆Cq (20).
Experimental and recurrence, survival
analyses
SPSS 13.0 (SPSS, Inc.) and Prism v.5.0 (GraphPad
Software, Inc.) were used for data analysis. In our experimental
analysis, complement DNA (cDNA) was synthesized from paired liver
tissues (tumor and paired non-tumor tissue) and gene expression was
analyzed by amplifying TEAD2 and β-actin on the cDNA. Τhe
mRNA expression data of TEAD2 was analyzed in tumors and
normalized to β-actin. Arbitrary values were then obtained by
defining (Tumor TEAD2/Tumor β-actin)/(Non-tumor
TEAD2/Non-tumor β-actin). Groups stratified into high and
low expression according to the median values and these groups were
converted to categorical variables based on this. Survival analysis
was performed for recurrence and overall survival related with
these two groups. Overall survival (OS) was defined as the duration
between the date of diagnosis and the date of death/final follow
up, from any cause. Recurrence-free survival (RFS) was defined as
the interval between the date of surgery and the date of first
recurrence or the date of the last follow-up. In the experiment,
the gene expression value obtained from duplicated results for each
gene and set the expression value of the patient using the mean
value of the duplicated results. The experiment was performed once
using the paired tissue of the patients. Comparison of the
distributions between two groups were analyzed using the
χ2 test (or Fisher's exact test when the expected
frequency in any group was <5) for categorical variables and by
paired Student's t-test (or by Kolmogorov-Smirnov test when the
expected frequency in any group was <5) for continuous
variables. One-way ANOVA followed by Student Newman-Keuls post-hoc
comparisons were used to compare ≥3 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
mRNA expression of Hippo pathway genes
in HCC
Table I exhibits the
clinicopathological characteristics of patients included in the
present study. The expression of Hippo pathway genes was examined
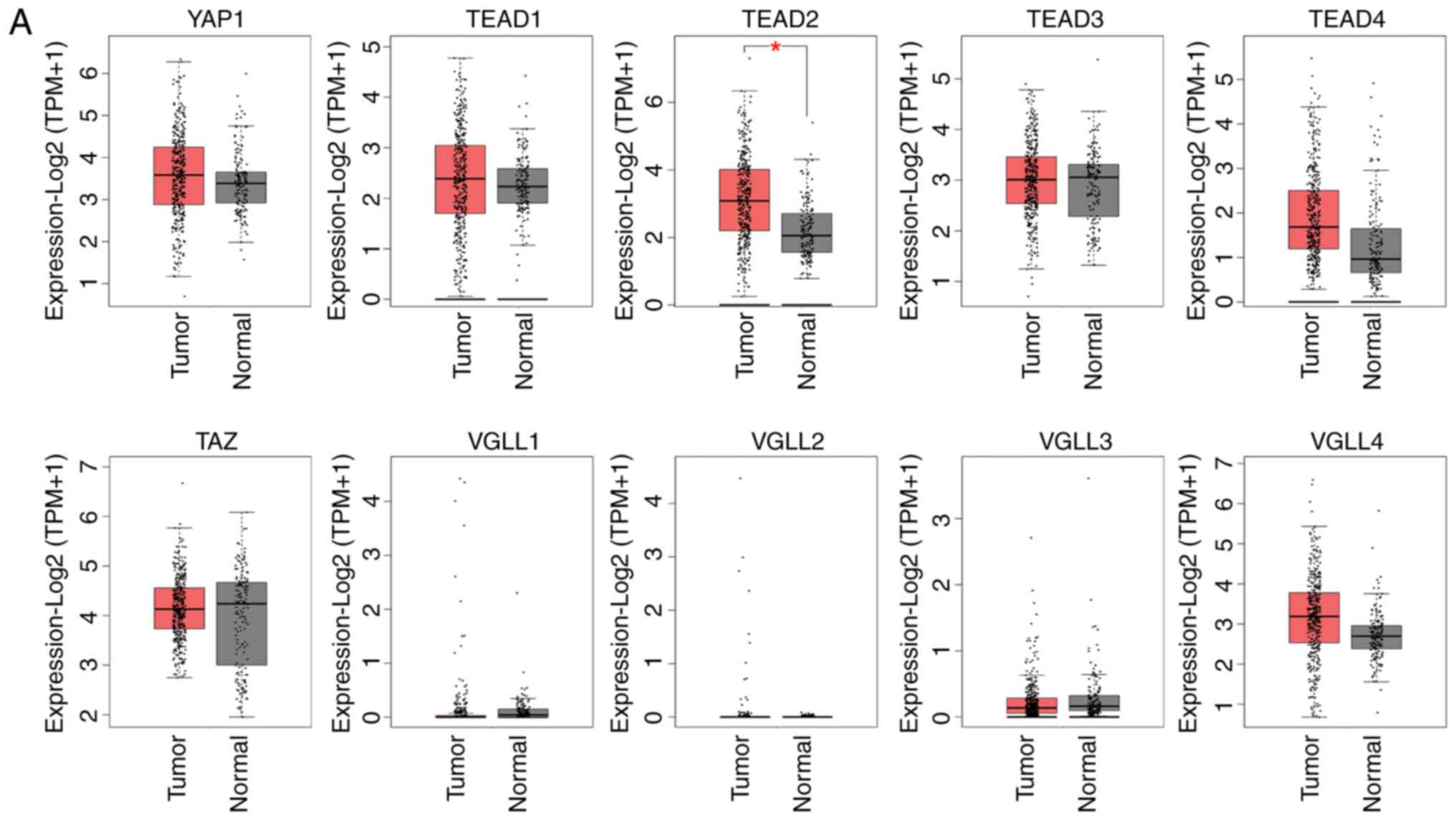
in patients with HCC using the GEPIA database (Fig. 1A and Table II). Comparison of mRNA expression
levels between HCC and normal control samples revealed that
TEAD2 expression was significantly higher in HCC tumor
tissue compared with normal control samples. The mRNA expression
levels of YAP, TEAD4 and VGLL4 were higher in HCC
tissue compared with normal control tissue, although the
differences were not significant (Fig.
1A and Table II).
Specifically, the mRNA expression of TEAD2 was significantly
increased in histologic grades 3–4 samples compared with histologic
grades 1–2 samples and the mRNA expression of TEAD4 was
significantly increased in histologic grades 3–4 samples compared
with histologic grades 1 samples (Fig.
1B). The mRNA expression of TEAD2 was significantly
increased in patients with TNM stage III and IV compared with TNM
stage I and II, and the mRNA expression of VGLL4 was also
significantly increased in TNM stage III and IV compared with in
TNM stage I (Fig. 1C). Among the
ten genes evaluated, TEAD2 exhibited higher expression in
HCC tumor tissue compared with normal control tissue, and this was
significantly associated with histological grade or stage of HCC
(Fig. 1B and C).
 | Table I.Clinicopathologic information of
patients with hepatocellular carcinoma derived from TCGA data
portal. |
Table I.
Clinicopathologic information of
patients with hepatocellular carcinoma derived from TCGA data
portal.
|
Characteristics | Total, n (%) |
|---|
| Patients | 377 (100.0) |
| Sex |
|
|
Female | 122 (32.3) |
|
Male | 255 (67.7) |
| Age, years |
|
|
≤60 | 180 (47.7) |
|
>60 | 196 (52.0) |
| NA | 1 (0.3) |
| TNM stage (8th AJCC
staging system) |
|
| Stage
I | 175 (46.4) |
| Stage
II | 87 (23.1) |
| Stage
III | 86 (22.8) |
| Stage
IV | 5 (1.3) |
| NA | 24 (6.4) |
| Histological grade
(Edmondson-Steiner grade) |
|
| Grade
1 | 55 (14.6) |
| Grade
2 | 180 (47.7) |
| Grade
3 | 124 (33.0) |
| Grade
4 | 13 (3.4) |
| NA | 5 (1.3) |
| Vital status |
|
|
Alive | 245 (65.0) |
|
Dead | 132 (35.0) |
| Child-Pugh
classification |
|
| A | 223 (59.1) |
| B | 21 (5.6) |
| C | 1 (0.3) |
| NA | 132 (35.0) |
| Histological
type |
|
|
Hepatocholangiocarcinoma | 7 (1.9) |
|
Hepatocellular carcinoma | 367 (97.3) |
|
Fibrolamellar carcinoma | 3 (0.9) |
| Adjacent hepatic
tissue inflammation extent type |
|
|
Mild | 101 (26.8) |
|
Severe | 19 (5.0) |
|
None | 119 (31.6) |
| NA | 138 (36.6) |
| Ishak fibrosis
score |
|
| 0-no
fibrosis | 76 (20.1) |
|
1,2-portal fibrosis | 31 (8.2) |
|
3,4-fibrous septa | 30 (8.0) |
|
5-nodular formation and
incomplete cirrhosis | 9 (2.4) |
|
6-established cirrhosis | 72 (19.1) |
| NA | 159 (42.2) |
| Thrombocytopenia
(<150×109/l) |
|
|
Yes | 76 (20.1) |
| No | 234 (62.1) |
| NA | 67 (17.8) |
| Albumin level,
g/dl |
|
|
>3.5 | 217 (57.6) |
|
≤3.5 | 86 (22.8) |
| NA | 74 (19.6) |
| AFP, ng/ml |
|
|
≤20 | 152 (40.3) |
|
>20 | 132 (35.0) |
| NA | 93 (24.7) |
| History of
hepatocellular carcinoma risk |
|
|
Hepatitis B | 105 (27.9) |
|
Hepatitis C | 51 (13.5) |
|
Hepatitis B + C | 7 (1.9) |
| Alcohol
consumption | 118 (31.3) |
| Non-alcoholic fatty
liver disease | 18 (4.8) |
| NA | 78 (20.6) |
 | Table II.Genes regulating the Hippo pathway
hepatocellular carcinoma. The gene alteration contains gene
amplification, deep deletion, missense mutation (unknown
significance), mRNA upregulation and truncating mutation (unknown
significance) of each analysed gene on HCC tissues. |
Table II.
Genes regulating the Hippo pathway
hepatocellular carcinoma. The gene alteration contains gene
amplification, deep deletion, missense mutation (unknown
significance), mRNA upregulation and truncating mutation (unknown
significance) of each analysed gene on HCC tissues.
| Symbol | Gene name | Chromosome
location | Gene alteration
(%) |
|---|
| YAP1 | Yes-Associated
Protein 1 | 11q22.1 | 7 |
| TAZ | Tafazzin | Xq28 | 9 |
| TEAD1 | TEA Domain
Transcription Factor 1 | 11p15.3 | 4 |
| TEAD2 | TEA Domain
Transcription Factor 2 | 19q13.33 | 6 |
| TEAD3 | TEA Domain
Transcription Factor 3 | 6p21.31 | 13 |
| TEAD4 | TEA Domain
Transcription Factor 4 | 12p13.33 | 8 |
| VGLL1 | Vestigial Like
Family Member 1 | Xq26.3 | 3 |
| VGLL2 | Vestigial Like
Family Member 2 | 6q22.1 | 3 |
| VGLL3 | Vestigial Like
Family Member 3 | 3p12.1 | 4 |
| VGLL4 | Vestigial Like
Family Member 4 | 3p25.2 | 6 |
Poor prognosis of patients with HCC
with high expression of TEAD2 and VGLL4
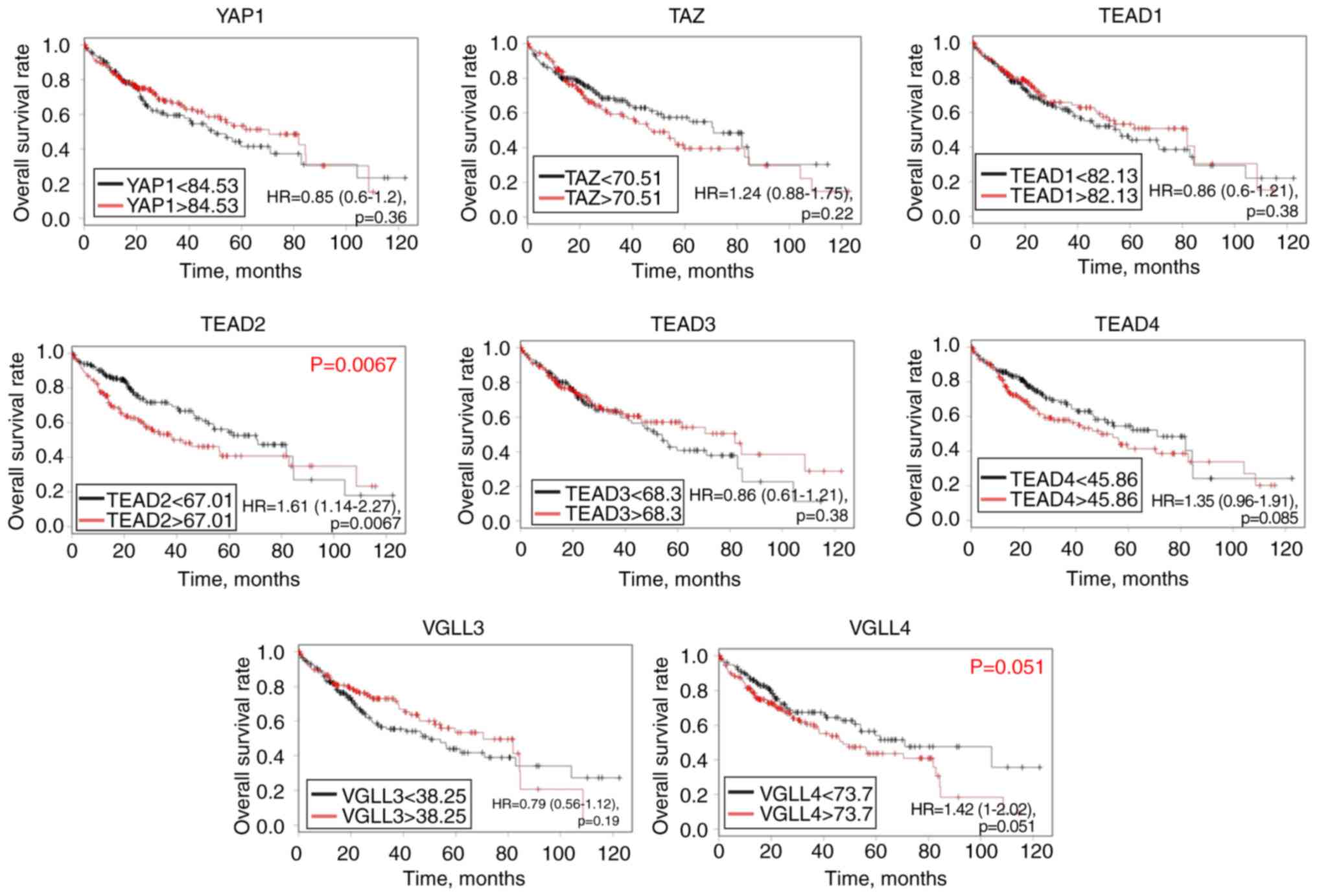
Based on the log-rank test and TCGA database,
abundant mRNA expression of TEAD2 [hazard ratio (HR), 1.61;
95% confidence interval (CI), 1.14–2.27; P=0.0067] was
significantly associated with a poor OS rate in patients with HCC,
and there was a tendency towards significance between TEAD4
(HR, 1.35; 95% CI, 0.95–1.91; P=0.085) and VGLL4 mRNA
expression (HR, 1.42; 95% CI, 1–2.02; P=0.051), and a poor
prognosis (Fig. 2). However,
YAP1, TAZ, TEAD1, TEAD3 and VGLL3 mRNA levels were
not significantly associated with the prognosis of patients with
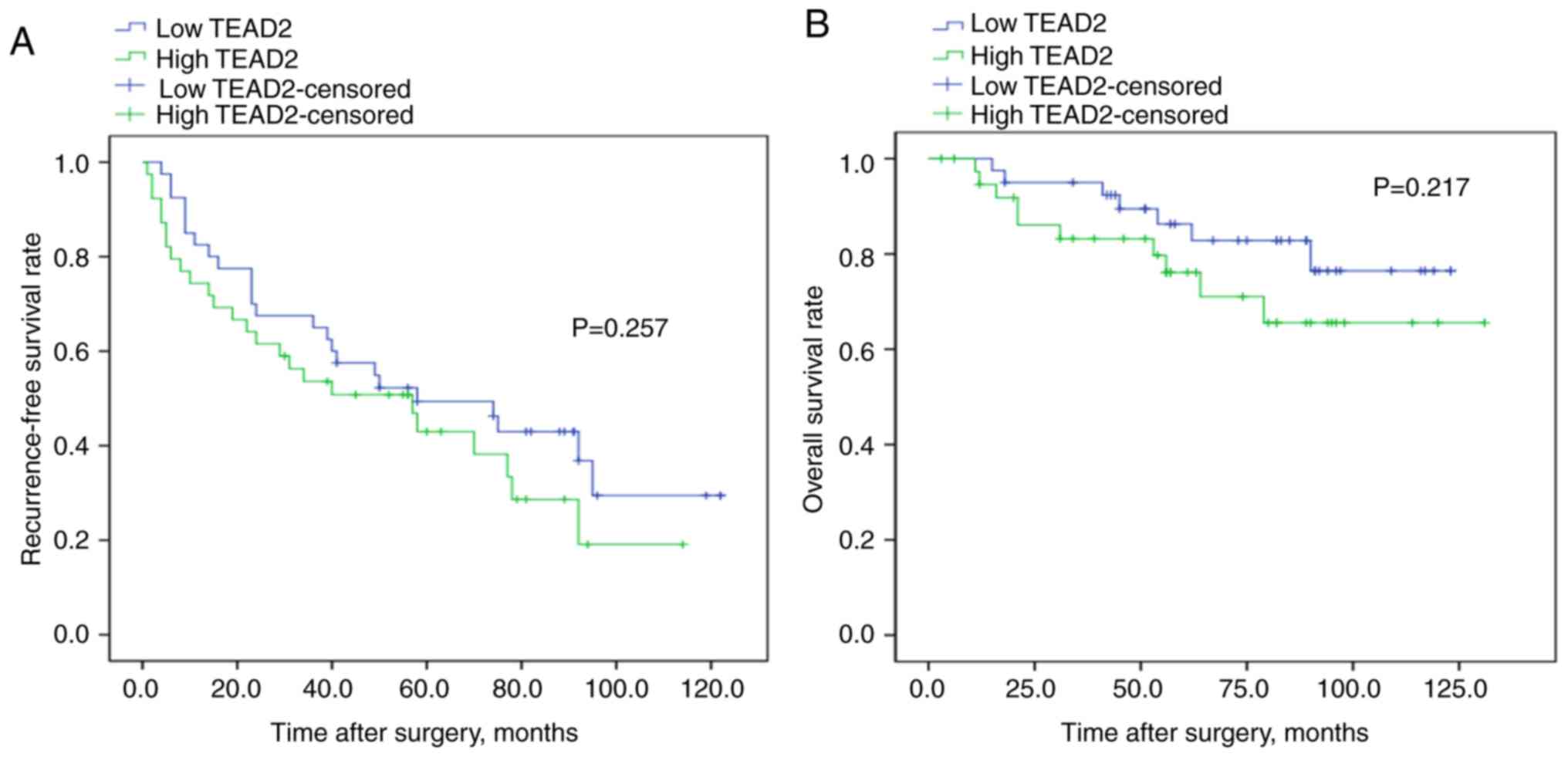
HCC. To validate the association between the mRNA expression of
TEAD2 and prognosis, RT-qPCR was performed and a log-rank
test was conducted on 79 clinical HCC tissues samples. The
clinicopathological characteristics of the patients with HCC are
summarized in Table SII. Although
the results were not significant, there was a tendency for a less
favorable prognosis in patients with increased TEAD2 mRNA
expression compared with those with decreased TEAD2 mRNA
expression, affecting both in recurrence-free and overall survival
rate (P=0.257, P=0.217, respectively; Fig. 3).
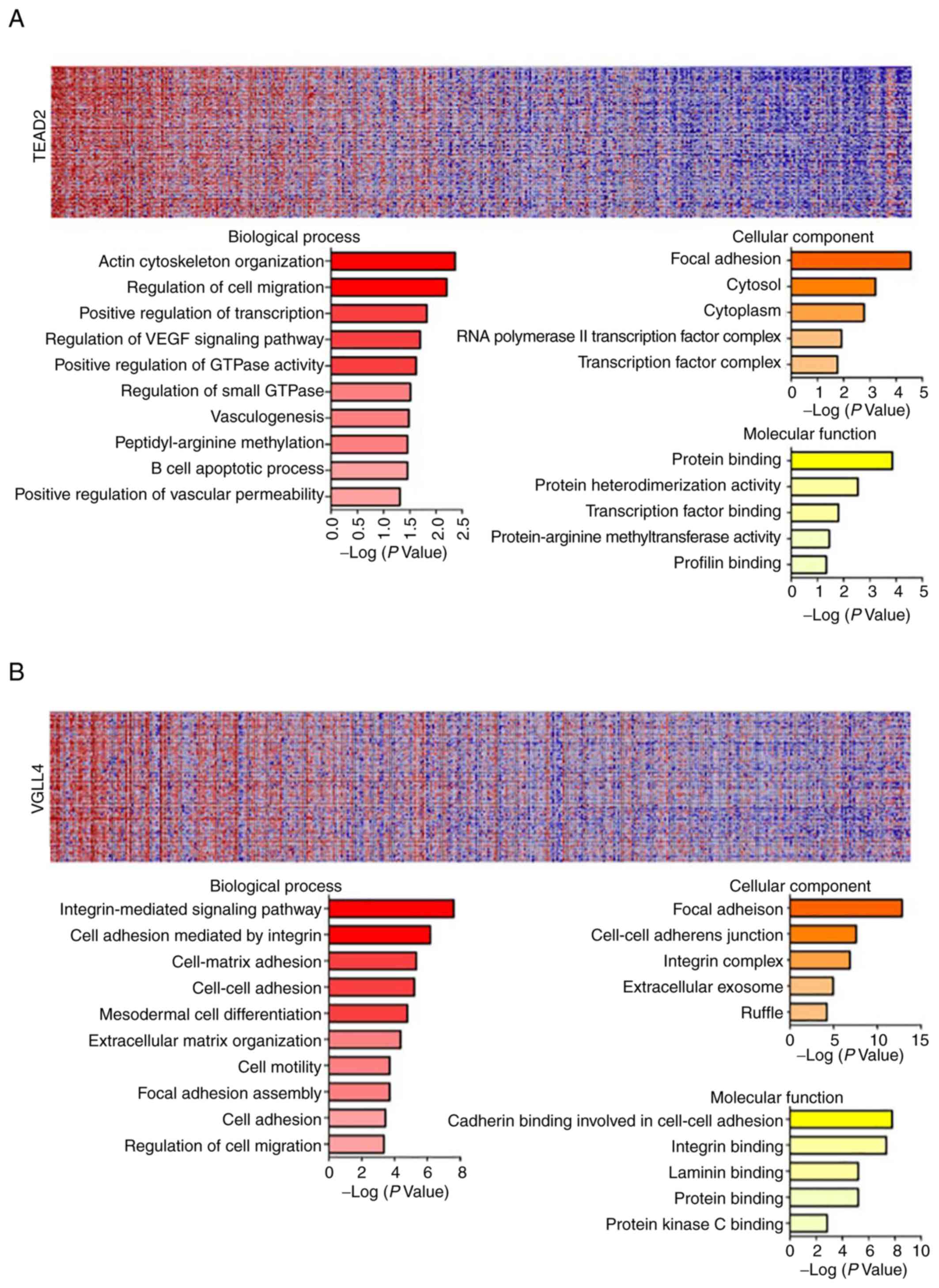
GeneNeighbors analysis of TEAD2 and
VGLL4
The present data revealed that TEAD2 and
VGLL4 are closely associated with a poor prognosis in
overall survival rate analysis using TCGA database, and that
TEAD2 is associated with a poor recurrence-free survival
rate according to the current clinical sample-based analyses
(Figs. 2 and 3). Therefore, GeneNeighbors analysis was
performed to determine the effect of TEAD2 and VGLL4
on the survival of patients with HCC. The 100 genes most strongly
associated with TEAD2 and VGLL4 were identified via
GeneNeighbors analysis (Fig. 4A and
B) and classified using the DAVID (https://david.ncifcrf.gov/). The selected genes were
divided into three categories of biological processes, molecular
functions and cellular components according to gene ontology (GO)
terms. In GeneNeighbors analysis of TEAD2, highly expressed
genes in HCC were primarily associated with ‘positive regulation of
small GTPase’, ‘vasculogenesis’, ‘positive regulation of
transcript’, ‘regulation of cell migration’, ‘regulation of VEGF
signaling’, ‘actin cytoskeleton organization’, ‘peptidyl-arginine
methylation’, ‘B cell apoptotic process’ and ‘positive regulation
of vascular permeability’ in biological processes (Fig. 4A). For cellular components, highly
expressed genes in HCC were strongly associated with ‘focal
adhesion’, ‘cytoplasm’, ‘cytosol’ and ‘RNA polymerase II
transcription factor’. For molecular functions, highly expressed
genes in HCC were mainly associated with ‘transcription factor
binding’, ‘protein heterodimerization activity’, ‘protein-arginine
methyltransferase activity’, ‘profilin binding’ and ‘protein
binding’ (Fig. 4A).
In GeneNeighbors of VGLL4, highly expressed
genes in HCC were primarily associated with ‘integrin-mediated
signaling’, ‘cell adhesion mediated by integrin’, ‘extracellular
matrix (ECM) organization’, ‘cell-matrix adhesion’, ‘cell-cell
adhesion’, ‘mesodermal cell differentiation’, ‘cell motility’,
‘focal adhesion assembly’, ‘cell adhesion’ and ‘regulation of cell
migration in biological processes’ (Fig. 4B).
For cellular components, highly expressed genes in
HCC were associated with ‘focal adhesion’, ‘cell-cell adherent
junction’, ‘integrin complex’, ‘extracellular exosome’ and
‘ruffle’. For molecular functions, highly expressed genes in HCC
were mainly associated with ‘cadherin binding involved in cell-cell
adhesion’, ‘integrin binding’, ‘laminin binding’, ‘protein binding’
and ‘protein kinase C binding’ (Fig.
4B).
ClassNeighbors analysis of TEAD2 and
VGLL4
Analysis by ClassNeighbors yielded two classes of
HCC samples: Class A included the highest 10% expressed
TEAD2 and VGLL4-upregulated HCC samples and class B
included the lowest 10% expressed TEAD2 and
VGLL4-downregulated HCC samples (Fig. 4C and D). The 150 most highly
expressed genes in classes A and B were identified from 20,502
probe sets. These genes were divided into three categories;
biological processes, molecular functions and cellular components
based on gene ontology (GO) terms (Fig.
4C and D). In ClassNeighbors of TEAD2, highly expressed
genes in class A were primarily associated with ‘drug transmembrane
transport’, ‘regulation of cell morphogenesis’, ‘signal
transduction’, ‘cell migration’, ‘regulation of transforming growth
factor beta receptor’, ‘cell growth’, ‘ECM disassembly’, ‘cellular
component organization’, ‘vasculature development’ and
‘L-alpha-amino acid transmembrane transport’ in biological
processes; ‘integral component of plasma membrane’, ‘neuron part’
and ‘ruffle’ in cellular components; and ‘drug transmembrane
transporter activity’ and ‘amino acid transmembrane transporter
activity’ in molecular functions. Highly expressed genes in class B
were closely related to ‘oxidation-reduction’, ‘alpha-amino acid
catabolic process’, ‘ammonium ion metabolic process’, ‘alcohol
metabolic process’, ‘glutamate metabolic process’, ‘amino-acid
betaine catabolic process’, ‘acyl-CoA metabolic process’,
‘alpha-amino acid biosynthetic process’, ‘lipid transport’ and
‘organic hydroxyl compound transport’ in biological process;
oxidoreductase activity and coenzyme binding in molecular function;
mitochondrial matrix and apical part of cell in cellular components
(Fig. 4C).
In ClassNeighbors of VGLL4, highly expressed
genes in class A were closely associated with ‘cell adhesion
mediated by integrin’, ‘cell migration’, ‘epithelial cell
migration’, ‘tyrosine kinase’, ‘cell adhesion’, ‘cellular
compartment organization’, ‘cell-matrix adhesion’, ‘cellular
response to endogenous stimulus’, ‘integrin-mediated signaling
pathway’ and ‘angiogenesis’ in biological processes;
‘collagen-containing ECM’, ‘cell surface’ and ‘integrin complex’ in
cellular components; and ‘integrin binding’ and ‘signaling receptor
binding’ in molecular functions. Highly expressed genes in class B
were closely associated with ‘oxidation-reduction’, ‘xenobiotic
metabolic process’, ‘ammonium ion metabolic process’, ‘glutamate
metabolic process’, ‘carboxylic acid biosynthetic process’,
‘protein targeting to peroxisome’, ‘chemokine-mediated signaling’,
‘monocarboxylic acid catabolic process’, ‘exogenous drug catabolic
process’ and ‘alpha-amino acid catabolic process’ in biological
process; ‘arachidonic acid epoxygenase activity’, ‘iron ion
binding’ and ‘aromatase activity’ in molecular function; and
‘peroxisomal matrix’ and ‘intracellular non-membrane organelle’ in
cellular components (Fig. 4D).
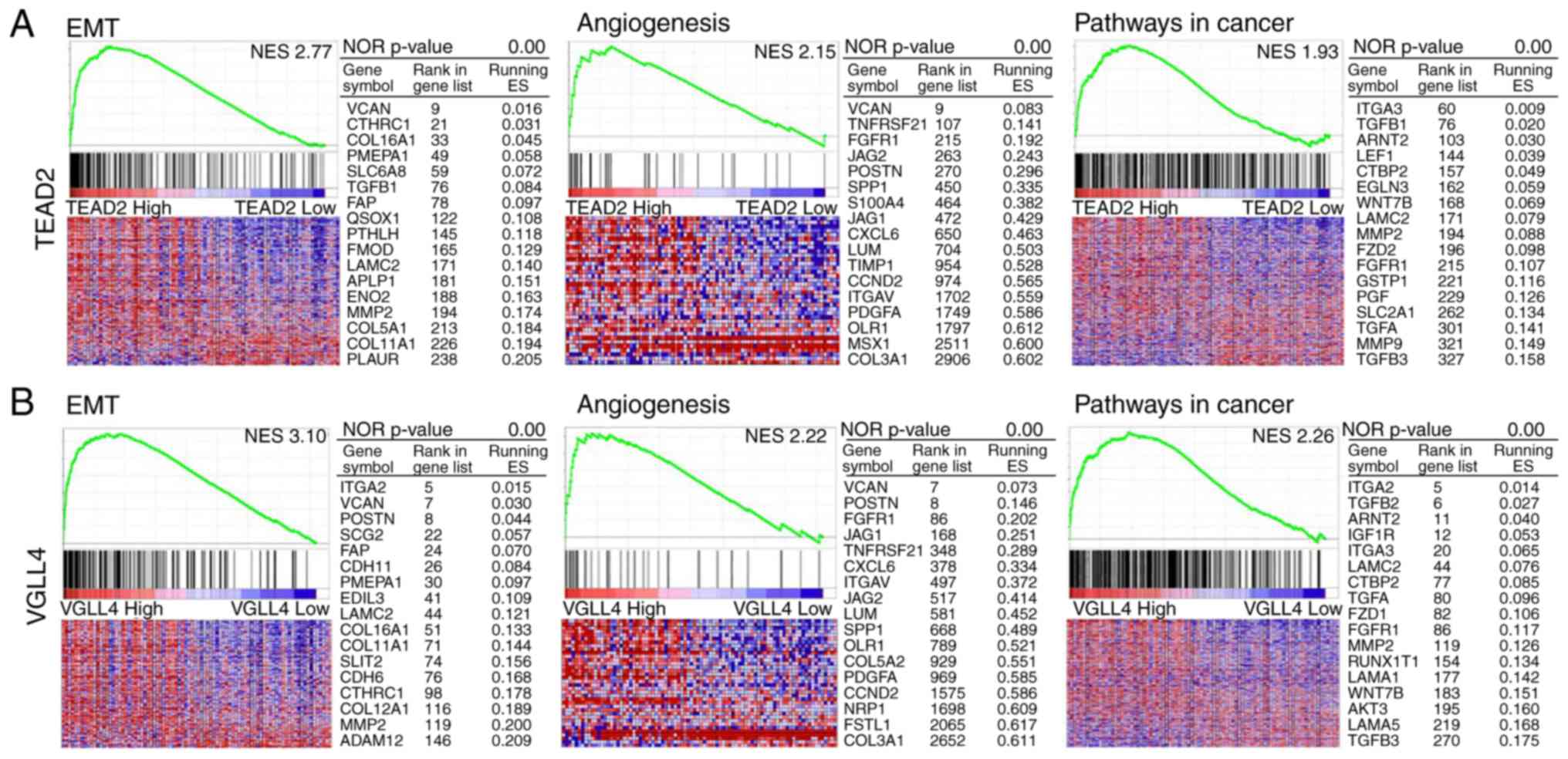
GSEA for TEAD2 and VGLL4
Using GSEA of mRNAs associated with hallmark
pathways and KEGG pathways (Fig.
5), the top 10% of HCC samples with upregulated TEAD2
and VGLL4 and bottom 10% of samples with downregulated
TEAD2 and VGLL4 were investigated. In the hallmarks
pathways, high mRNA expression of TEAD2 and VGLL4 was
strongly associated with genes related to ‘epithelial-mesenchymal
transition (EMT)’ and ‘angiogenesis’. In KEGG pathway analysis,
increased mRNA expression of TEAD2 and VGLL4 was
closely associated with genes involved in cancer pathways (Fig. 5A and B).
Discussion
TEAD protein serves an essential role in the
early stage of the developmental process and cellular ageing
(21–23). It has been demonstrated that
specific TEAD proteins influence cancer as activators of
transcription of pro-growth genes (24). In previous studies, TEAD was
revealed to be upregulated in gastric cancer (16), breast cancer (11,25)
and renal cell carcinoma (15). In
prostate cancer, an increased TEAD1 level was correlated
with unfavorable clinical outcomes (12), and in colorectal cancer and gastric
cancer an increased TEAD4 expression level was associated
with poor outcomes (13,14,26).
Regarding HCC, expression of YAP-TEAD was reported to be
upregulated, with most studies examining the expression of
TEAD1 and TEAD4 (26–28).
According to previous cellular-based assays, inhibition of
LATS2, an upstream regulator of YAP/TAZ in the
Hippo signaling pathway, induces nuclear localization of
YAP1 and YAP1/TEAD2 interactions, which in
turn promotes HCC progression (26). However, this study only explained
the molecular mechanism underlying the action of
YAP1/TEAD2 via LATS2 but did not reveal the
clinical significance of TEAD2 in HCC. In the present study,
based on analyses of TCGA database and clinical samples, as the HCC
stage and histologic grade of tumor increased, TEAD2
expression adversely affected overall survival rate and
recurrence-free survival rate.
In TCGA data analysis, increased mRNA expression of
TEAD2 was significantly associated with a less favorable
prognosis in patients with HCC in overall survival rate, and the
potential of TEAD2 as a prognostic marker was predicted.
Moreover, clinical data analysis revealed a similar tendency as the
results of TCGA data analysis. However, additional studies are
needed to confirm the association between mRNA expression of
TEAD2 and prognosis.
To investigate the function and mechanism of
TEAD2 in HCC, bioinformatics analysis was performed.
GeneNeighbors analyses demonstrated that ‘angiogenesis-associated
genes’ (VEGF, vasculogenesis and vascular permeability),
‘regulation of cell migration’ and ‘actin cytoskeleton
organization’ were highly correlated with TEAD2 in HCC.
ClassNeighbors analysis classified TEAD2-expressing HCC into
Class A, which expresses genes associated with ‘signal
transduction’, ‘cell migration’, ‘ECM disassembly’, ‘cell
morphogenesis’, ‘transforming growth factor β signaling’ and
‘vasculature development’. It was also classified into Class B,
which expresses genes associated with metabolic pathways. Class A
genes enhance EMT and angiogenesis. GSEA was performed on
TEAD2, which was significantly associated with the prognosis
of patients with HCC. EMT, angiogenesis and cancer pathway-related
genes were strongly associated with high mRNA expression of
TEAD2. During EMT, cancer cells lose their apical basal
polarity and cell-cell adhesions and develop a more invasive
phenotype (29). Diepenbruck et
al (30) reported that
increased TEAD2 transcriptional activity promotes the
induction of EMT in breast cancer cells and mammary gland
epithelial cells. Moreover, angiogenesis also serves an important
role in tumor growth and metastasis (31,32).
For a tumor to grow above a certain size the development of new
blood vessels is required, and tumors induce angiogenesis by
secreting various growth factors including VEGF (33,34).
In the current study, the mRNA expression of
VGLL4 was higher in HCC compared with normal control samples
although the difference was not significant, and there was a
tendency towards significance between VGLL4 expression and a
poor prognosis (P=0.051). GeneNeighbors analyses demonstrated that
‘ECM organization’, ‘integrin-mediated signaling pathway’ and ‘cell
adhesion’ associated genes were highly correlated with VGLL4
expression in HCC. ClassNeighbors analysis classified
VGLL4-expressing HCC into Class A, which includes genes
associated with ‘integrin’, ‘cell adhesion’ and ‘angiogenesis’, and
Class B, which includes genes associated with ‘metabolic pathways’.
Class A genes enhance EMT and angiogenesis. Remodeling of the ECM
and changes in cell-ECM interactions are necessary for the
induction and progression of EMT (35). Integrin complexes allow cells to
receive signals from ECM proteins via interactions with signal
transduction mediators. Some integrins play essential roles in EMT
progression (35). GSEA revealed
that EMT-, angiogenesis- and cancer pathway-associated genes were
strongly associated with high mRNA expression of VGLL4.
Previous studies have reported that VGLL4 suppresses
multiple cancer types, such as gastric, lung, colon and breast
carcinoma by suppressing the WNT and Hippo signaling pathways
(36–40). Xie et al (41) demonstrated that VGLL4 causes
G2/M phase arrest in HCC cells, and Shu et al
(42) reported that the
YAP/VGLL4 ratio is higher in patients with HCC, which is
associate with tumor progression and a poor prognosis (41,42).
By contrast to previous studies, the present study reported
tendency towards significance between increased VGLL4 mRNA
expression and the poor prognosis of patients with HCC. Unlike
other cancer types, unknown mechanisms specific to HCC may have
contributed to this discrepancy, and further studies are
needed.
According to previous studies, when Hippo signaling
is activated, YAP/TAZ are phosphorylated and sequestered in
the cytoplasm, whereas TEAD and VGLL bind in the
nucleus. When Hippo signaling is deactivated, YAP/TAZ are
dephosphorylated and translocate to the nucleus to bind
TEAD, activating the downstream transcription of target
genes (Fig. 6) (43,44).
In the present study, mRNA expression and poor prognosis on overall
survival rate for both TEAD2 and VGLL4 exhibited
similar tendencies in patients with HCC. Generally, when Hippo
signaling pathway is ‘On’, TEAD and VGLL maintain
their binding status; therefore, the similar tendency between
TEAD2 and VGLL4 is likely related to our findings.
However, further mechanistic studies are needed to validate this
prediction. The present analysis did not reveal that TEAD2
and VGLL4 significantly influence each other. However,
TEAD2 and VGLL4 expression were significantly
correlated in TCGA HCC cohorts (P<0.001) (data not shown).
Therefore, further experimental studies are required to determine
which factors are the most significant.
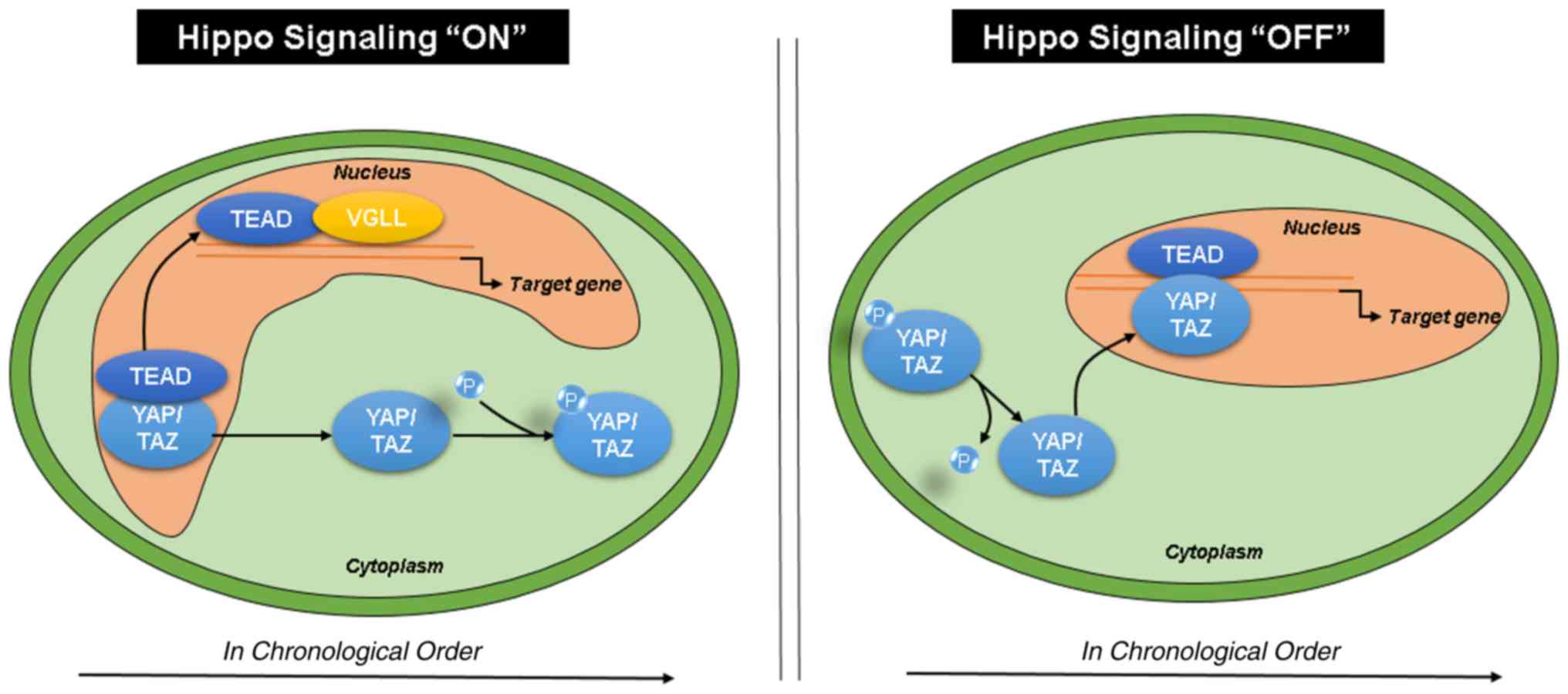 | Figure 6.Hippo signaling pathway. Upon
activation of Hippo signaling, YAP/TAZ are phosphorylated
and sequestered in the cytoplasm, whereas TEAD and
VGLL bind in the nucleus. When Hippo signaling is
deactivated, YAP/TAZ are dephosphorylated and translocate to
the nucleus to bind TEAD, activating the downstream transcription
of target genes. TEAD, transcriptional enhancer associated
domain; VGLL, vestigial like family; HCC,
hepatocellular carcinoma; TEAD2, transcriptional enhancer
associated domain 2; VGLL4, vestigial like family member 4;
YAP, yes-associated protein. |
The present study demonstrated that an increase in
expression of TEAD4 was associated with an increase in
histological grade; however, survival tended to decrease with
increasing TEAD4 expression. Thus, in addition to
histological grades, other factors may influence patient
survival.
In conclusion, the present study was the first to
evaluate the association between TEAD2 and the survival of
patients with HCC. The current data revealed that a high expression
level of TEAD2 in resected tumor tissue from patients with
HCC was positively associated with a poor prognosis. Therefore,
TEAD2 may represent a useful prognostic marker and
therapeutic target for HCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Datasets were both generated and presented in the
present study (patient tissue analysis) and retrieved from online
databases (TCGA). TCGA-LIHC dataset is available in GDC data portal
(https://portal.gdc.cancer.gov/) and
Firebrowse (http://firebrowse.org/?cohort=LIHC&download_dialog=true).
Authors' contributions
BSL and HSE designed the study and conducted
critical revision of the manuscript. JSJ, SYC, WSR, HSE, JSK,
SHKang, ESL, HSM, SHKim and JKS conducted acquisition, analysis and
interpretation of the data. BSL carried out supervision of the
analysis. JSJ and SYC wrote the manuscript. ISK verified and
contributed statistical analysis. All authors read and approved the
final manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This research was approved by the Institutional
Review Board of Chungnam National University Hospital (approval no.
CNUH 2017-05-013).
Patient consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ and
Wu F: Solitary large hepatocellular carcinoma: A specific subtype
of hepatocellular carcinoma with good outcome after hepatic
resection. Ann Surg. 249:118–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aravalli RN, Cressman EN and Steer CJ:
Cellular and molecular mechanisms of hepatocellular carcinoma: An
update. Arch Toxicol. 87:227–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong W and Guan KL: The YAP and TAZ
transcription co-activators: Key downstream effectors of the
mammalian Hippo pathway. Semin Cell Dev Biol. 23:785–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q
and Guan KL: Angiomotin is a novel Hippo pathway component that
inhibits YAP oncoprotein. Genes Dev. 25:51–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HH, Maeda T, Mullett SJ and Stewart
AF: Transcription cofactor Vgl-2 is required for skeletal muscle
differentiation. Genesis. 39:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen HH, Mullett SJ and Stewart AF: Vgl-4,
a novel member of the vestigial-like family of transcription
cofactors, regulates alpha1-adrenergic activation of gene
expression in cardiac myocytes. J Biol Chem. 279:30800–30806. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Günther S, Mielcarek M, Kruger M and Braun
T: VITO-1 is an essential cofactor of TEF1-dependent
muscle-specific gene regulation. Nucleic Acids Res. 32:791–802.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koontz LM, Liu-Chittenden Y, Yin F, Zheng
Y, Yu J, Huang B, Chen Q, Wu S and Pan D: The Hippo effector Yorkie
controls normal tissue growth by antagonizing scalloped-mediated
default repression. Dev Cell. 25:388–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–E2450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knight JF, Shepherd CJ, Rizzo S, Brewer D,
Jhavar S, Dodson AR, Cooper CS, Eeles R, Falconer A, Kovacs G, et
al: TEAD1 and c-Cbl are novel prostate basal cell markers that
correlate with poor clinical outcome in prostate cancer. Br J
Cancer. 99:1849–1858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim B, Park JL, Kim HJ, Park YK, Kim JH,
Sohn HA, Noh SM, Song KS, Kim WH, Kim YS, et al: Integrative
genomics analysis reveals the multilevel dysregulation and
oncogenic characteristics of TEAD4 in gastric cancer.
Carcinogenesis. 35:1020–1027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Wang G, Yang Y, Mei Z, Liang Z, Cui
A, Wu T, Liu CY and Cui L: Increased TEAD4 expression and nuclear
localization in colorectal cancer promote epithelial-mesenchymal
transition and metastasis in a YAP-independent manner. Oncogene.
35:2789–2800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schütte U, Bisht S, Heukamp LC, Kebschull
M, Florin A, Haarmann J, Hoffmann P, Bendas G, Buettner R, Brossart
P and Feldmann G: Hippo signaling mediates proliferation,
invasiveness, and metastatic potential of clear cell renal cell
carcinoma. Transl Oncol. 7:309–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou GX, Li XY, Zhang Q, Zhao K, Zhang CP,
Xue CH, Yang K and Tian ZB: Effects of the hippo signaling pathway
in human gastric cancer. Asian Pac J Cancer Prev. 14:5199–5205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID Gene Functional Classification Tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghassan K A-A and Timothy M: Pawlik,
Junichi Shindoh and Jean-Nicolas Vauthey editors: AJCC Cancer
Staging Manual. (Eighth ed). Chicago, IL: Springer; pp. 287–293.
2017
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Friedrich GA and Soriano P:
Transcriptional enhancer factor 1 disruption by a retroviral gene
trap leads to heart defects and embryonic lethality in mice. Genes
Dev. 8:2293–2301. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawada A, Kiyonari H, Ukita K, Nishioka N,
Imuta Y and Sasaki H: Redundant roles of Tead1 and Tead2 in
notochord development and the regulation of cell proliferation and
survival. Mol Cell Biol. 28:3177–3189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaneko KJ, Kohn MJ, Liu C and DePamphilis
ML: Transcription factor TEAD2 is involved in neural tube closure.
Genesis. 45:577–587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holden JK and Cunningham CN: Targeting the
Hippo pathway and cancer through the TEAD family of transcription
factors. Cancers (Basel). 10(pii): E812018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Nie Z, Zhou Z, Zhang H, Liu R, Wu
J, Qin J, Ma Y, Chen L, Li S, et al: The interplay between TEAD4
and KLF5 promotes breast cancer partially through inhibiting the
transcription of p27Kip1. Oncotarget. 6:17685–17697.
2015.PubMed/NCBI
|
|
26
|
Guo C, Wang X and Liang L: LATS2-mediated
YAP1 phosphorylation is involved in HCC tumorigenesis. Int J Clin
Exp Pathol. 8:1690–1697. 2015.PubMed/NCBI
|
|
27
|
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan
F, Meng S, Wang Y, Yuan Z and Bi W: SIRT1 regulates YAP2-mediated
cell proliferation and chemoresistance in hepatocellular carcinoma.
Oncogene. 33:1468–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai N, Zhang C, Liang N, Zhang Z, Chang A,
Yin J, Li Z, Luo N, Tan X, Luo N, et al: Yes-Associated Protein
(YAP) increases chemosensitivity of hepatocellular carcinoma cells
by modulation of p53. Cancer Biol Ther. 14:511–520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sánchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diepenbruck M, Waldmeier L, Ivanek R,
Berninger P, Arnold P, van Nimwegen E and Christofori G: Tead2
expression levels control the subcellular distribution of Yap and
Taz, zyxin expression and epithelial-mesenchymal transition. J Cell
Sci. 127:1523–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McDougall SR, Anderson AR and Chaplain MA:
Mathematical modelling of dynamic adaptive tumour-induced
angiogenesis: Clinical implications and therapeutic targeting
strategies. J Theor Biol. 241:564–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F,
Han X, Feng Y, Zheng C, Wang Z, et al: VGLL4 functions as a new
tumor suppressor in lung cancer by negatively regulating the
YAP-TEAD transcriptional complex. Cell Res. 24:331–343. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L
and Zhou Z: VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt
and Hippo signalling in colorectal cancer. Nat Commun. 8:140582017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin HS, Park HS, Shin JH, Kim DH, Jun SH,
Lee CJ and Lee TH: A novel inhibitor of apoptosis protein
(IAP)-interacting protein, Vestigial-like (Vgl)-4, counteracts
apoptosis-inhibitory function of IAPs by nuclear sequestration.
Biochem Biophys Res Commun. 412:454–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiao S, Wang H, Shi Z, Dong A, Zhang W,
Song X, He F, Wang Y, Zhang Z, Wang W, et al: A peptide mimicking
VGLL4 function acts as a YAP antagonist therapy against gastric
cancer. Cancer Cell. 25:166–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Shen H, Withers HG, Yang N,
Denson KE, Mussell AL, Truskinovsky A, Fan Q, Gelman IH, Frangou C
and Zhang J: VGLL4 Selectively represses YAP-dependent gene
induction and tumorigenic phenotypes in breast cancer. Sci Rep.
7:61902017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shu B, Zhai M, Miao X, He C, Deng C, Fang
Y, Luo M, Liu L and Liu S: Serotonin and YAP/VGLL4 balance
correlated with progression and poor prognosis of hepatocellular
carcinoma. Sci Rep. 8:97392018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie W, Hao J, Zhang K, Fang X and Liu X:
Adenovirus armed with VGLL4 selectively kills hepatocellular
carcinoma with G2/M phase arrest and apoptosis promotion. Biochem
Biophys Res Commun. 503:2758–2763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou Y, Huang T, Cheng AS, Yu J, Kang W
and To KF: The TEAD family and its oncogenic role in promoting
tumorigenesis. Int J Mol Sci. 17(pii): E1382016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin KC, Park HW and Guan KL: Regulation of
the Hippo pathway transcription factor TEAD. Trends Biochem Sci.
42:862–872. 2017. View Article : Google Scholar : PubMed/NCBI
|