Introduction
Gastric cancer is one of the most commonly diagnosed
malignant tumors (1). The incidence
of gastric cancer is highest in East Asia, followed by Eastern
Europe, South America, and the northeastern region of Japan
(1). Despite the decline in
incidence after the discovery of Helicobacter pylori, the
absolute number of annual cases continues to increase worldwide
(2), which is attributed to an
aging population (2). Furthermore,
the trend toward declining incidence was recently interrupted and
replaced by an upward trend in younger patients for unknown reasons
(2). Although the incidence of
distal gastric cancer has significantly declined, the incidence of
adenocarcinoma at the esophagogastric junction and proximal stomach
has exceeded the incidences of other cancers (3). Aside from surgery, therapy for gastric
cancer has evolved from chemotherapy to targeted therapy and
immunotherapy, which improve prognosis to some extent, although the
survival of patients with late-stage gastric cancer remains poor
(4). Therefore, investigators must
consider different approaches for treating gastric cancer.
Genetic abnormalities occur in gastric cancer that
are associated with altered expression of the cell-cycle regulator
cyclin and cyclin-dependent kinases (CDKs) (5,6).
Overexpression of cyclin E is a particularly frequent event in
gastric carcinomas (5,6) and may therefore serve as an indicator
of the malignant transformation of dysplastic cells (7) and tumor aggressiveness, once an
invasive cancer develops (6,8).
Moreover, overexpression of cyclin D1, cyclin D2, and the catalytic
subunit of CDK4 are associated with tumor progression (9).
Angiotensin II receptor type 1 (AT1) receptor
blockers (ARBs) are used to treat hypertension, chronic heart
failure, and chronic kidney disease. Evidence indicates that
angiotensin II affects cancer growth and that ARBs antagonize AT1
receptors to suppress tumor growth. (10–12).
Telmisartan is an antihypertensive drug that induces apoptosis of
urological and gynecologic cancer cell lines (13,14).
In contrast, telmisartan induces G1 arrest in cancer cell lines
such as those derived from adult T cell leukemias (15), esophageal adenocarcinomas (16), and hepatobiliary malignancies
(17,18). Moreover, ARBs inhibit cell
proliferation and angiogenesis (19). However, we lack sufficient knowledge
of the direct mechanism through which telmisartan suppresses the
growth of gastric cancer cells.
Here we report the antitumor effects of telmisartan
on human gastric cancer cell lines and in a mouse xenograft model
of gastric cancer. We show that telmisartan induced cell cycle
arrest in gastric cancer cell lines but did not induce apoptosis.
Telmisartan caused G0/G1-phase arrest by decreasing the levels of
cyclin D1 and CDK complexes. We investigated the effect of
telmisartan on gastric cancer cell growth in a xenograft model.
Moreover, we analyzed the association of receptor tyrosine kinases
(RTKs), angiogenesis, and microRNAs with tumor suppression.
Materials and methods
Cell culture
The human gastric cancer cell lines MKN74, MKN1 and
MKN45, which were obtained from the Japanese Cancer Research
Resources Bank (Osaka, Japan), were maintained at 37°C in an
atmosphere containing 5% CO2 in Dulbecco's modified
Eagle's medium (Gibco Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS), 20 U/ml penicillin,
and 100 µg/ml streptomycin.
Cell proliferation assay
The cell proliferation assay was performed according
to a previously published procedure (16).
Cell cycle and apoptosis analyses
Cell cycle and apoptosis analyses were performed
according to previously published procedures (16). Detailed information about these
analyses is provided in Data S1.
Gel electrophoresis and western
blotting
Gel electrophoresis and western blotting were
performed according to previously published procedures (16). Briefly, cells were treated with
telmisartan or DMSO and lysed in the presence of a protease
inhibitor cocktail. A NanoDrop 2000 fluorescence spectrometer
(Thermo Fisher Scientific, Inc.) was used for measuring of protein
concentrations. Aliquots (1–10 µg) were added to sample buffer and
heated at 100°C for 5 min. Proteins were separated using 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and
electrophoretically transferred to a nitrocellulose membrane (GE
Healthcare). After blocking in 5% skim milk in 0.05% Tween-20/TBS
buffer, the membranes were incubated with primary antibodies and
then with peroxidase-conjugated secondary antibodies in 5% skim
milk in 0.05% Tween-20/TBS buffer. Immunoreactive proteins were
visualized using an ImageQuant LAS4010 (GE Healthcare, Tokyo,
Japan). Band intensities were semi-quantified using ImageJ software
v1.52q (National Institutes of Health) and normalized to β-actin.
Information concerning the antibodies is provided in Data S1.
Apoptosis assay
Caspase-cleaved cytokeratin 18 (cCK18) expression
was evaluated using an M30 Apoptosense ELISA kit (Funakoshi Co.).
The ELISA assay was performed according to a previously published
procedure (18). Details of the
assay are provided in Data S1.
Antibody array analyses of
phosphorylated receptor tyrosine kinases (p-RTKs) and
angiogenesis-related protein profiles
Arrays were used to determine the levels of p-RTKs
and the expression of angiogenesis-related proteins according to
previously published procedures (16). Detailed information is provided in
Data S1.
Analysis of miRNA arrays
The analysis of miRNA expression is described in
Data S1.
Xenograft model analysis
All mice were treated in accordance with the
guidelines of the Kagawa University Committee on Experimental
Animals. The Kagawa University Animal Care Committee approved the
protocol for animal experiments (Registration no. A155). Male
BALB/c-nu/ nu mice, 6 weeks of age and average weight of 18.6 g
(n=13) were obtained from Japan SLC (Shizuoka, Japan). The mice had
continuous free access to sterilized (γ-irradiated) food (CL-2;
CLEA Japan, Inc.) and autoclaved water. In addition, we placed the
mice in an environment that reduced stress as much as possible,
such as a temperature of 24°C a humidity of approximately 36%, and
turning off the lights at night. Each mouse was subcutaneously
injected in the left flank with 3×106 MKN74 cells. After
approximately 2 weeks, or when tumors reached a maximum diameter
>3 mm, 13 mice were randomly assigned to the groups as follows:
DMSO only (control) (n=7) and intraperitoneally (i.p.) injected
with telmisartan (50 mg/day, n=6). We also used inhalational
anesthesia with 1% sevoflurane prior to drug administration. The
main measurements were body weight and tumor volume, which were
recorded every 3 days. We used a total of 13 mice for this
experiment and all were euthanized at the end of the experiment. We
used carbon dioxide inhalation as a method of euthanasia. The
experiment period was 12 days. No mice died during the experiment.
We decided to euthanize mice if the tumor volume exceeded 2000
(mm3). In addition, it was also applied when a low body
weight of more than 20% was observed as compared with the control
group. Moreover, when we observed behavioral disorders such as
feeding, difficulty in water intake, and continuous lying down in
mice, and when we noticed a poor physical condition visually, we
decided to perform euthanasia as a humane endpoint. Tumor volumes
were calculated as follows: Volume=length × width2/2 as
previously reported (16,19).
Statistical analyses
Results are expressed at the mean ± SD (standard
deviation). Statistical analyses were performed using GraphPad
Prism 6 software (GraphPad Software, Inc.). Comparisons among the
different groups (>2 groups) were analyzed by one-way ANOVA and
Tukey's post hoc test, while Student's t-test was used for
comparisons between 2 groups. P<0.05 was considered to indicate
a significant difference.
Results
Telmisartan suppresses the
proliferation of human gastric cancer cells
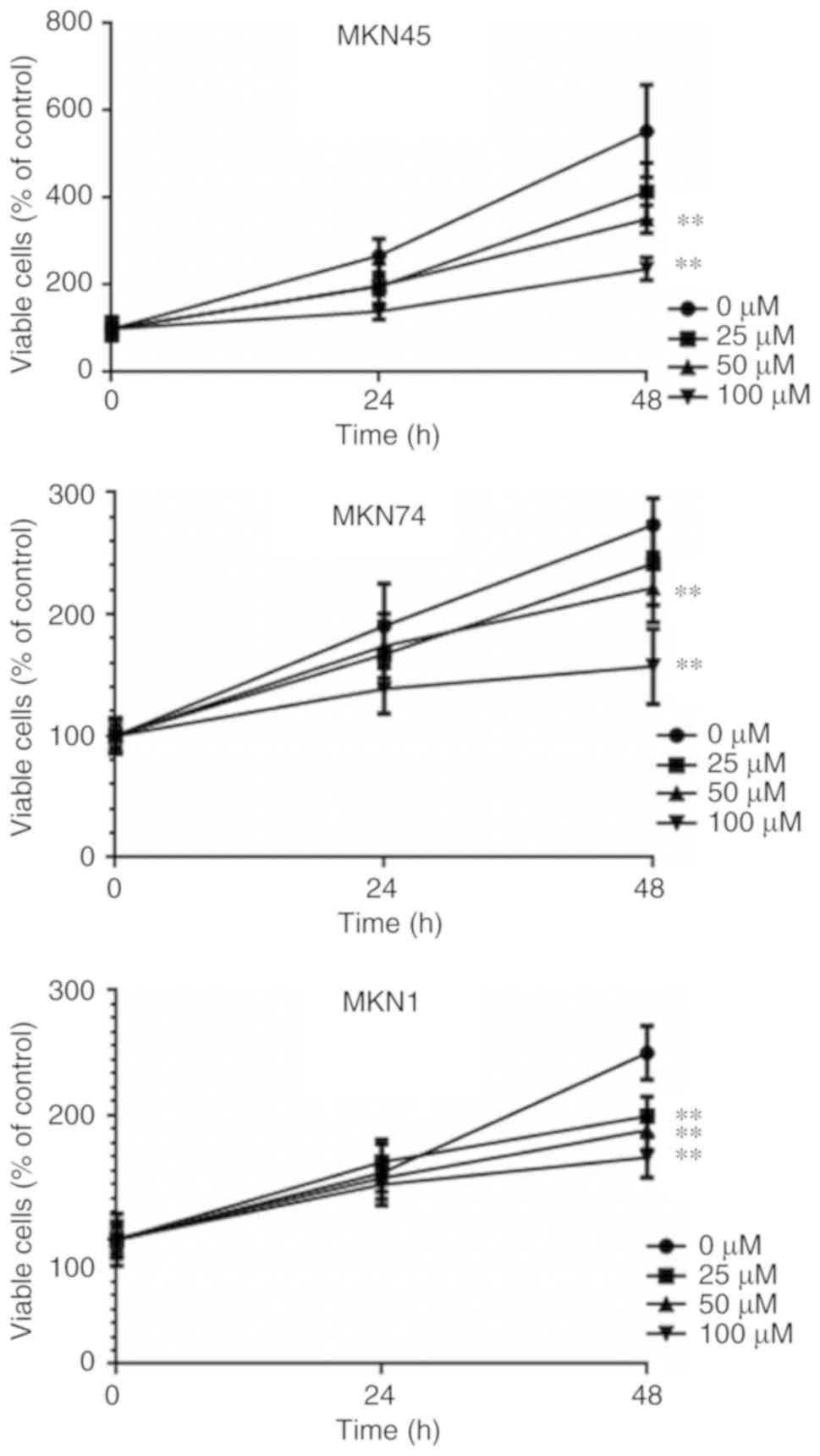
Human gastric cancer cell lines were treated with
different concentrations (0, 25, 50 and 100 µM) of telmisartan for
24 or 48 h. Telmisartan suppressed the proliferation of the gastric
cancer cells, which was time- and concentration-dependent (Fig. 1).
Telmisartan suppresses the
proliferation of human gastric cancer cells by inducing cell cycle
arrest at the G0/G1 phase
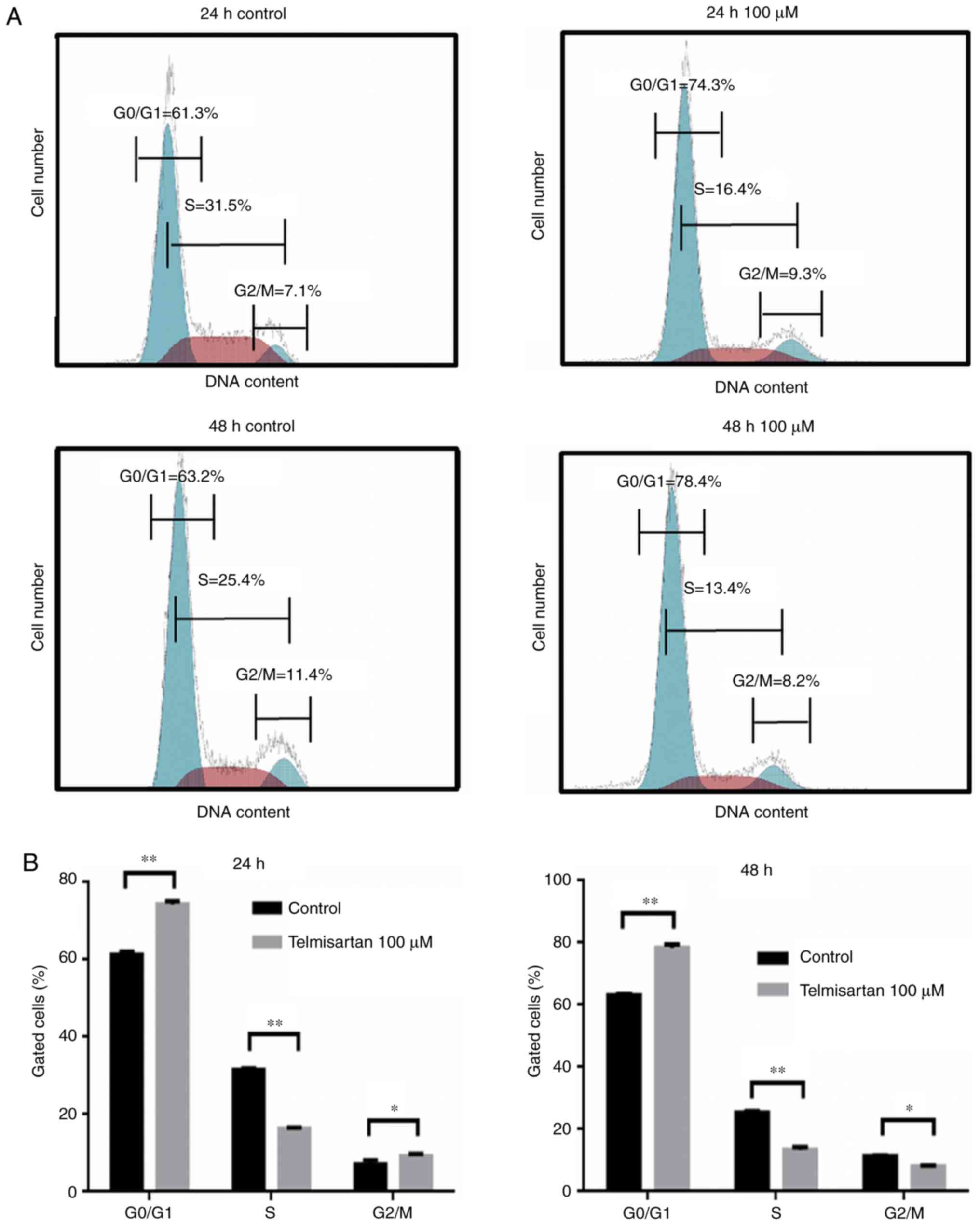
We used flow cytometry to further investigate the
effects of telmisartan on the cell cycle of gastric cancer cells.
When MKN74 cells were incubated with 100 µM telmisartan for 24 and
48 h, the numbers of cells in the S and G0/G1 phases were
significantly decreased and increased, respectively, compared with
the control cells (Fig. 2A and B).
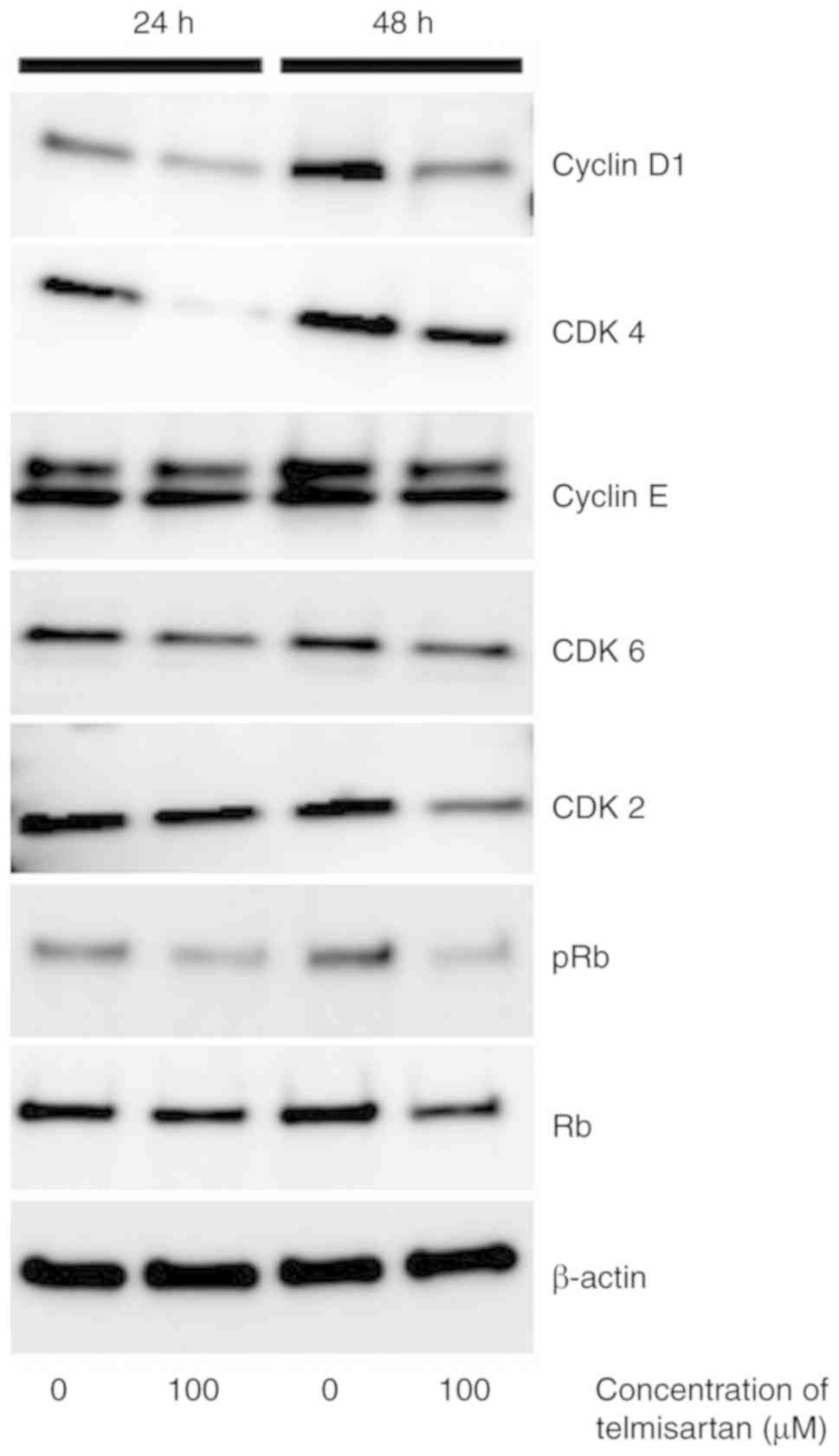
Furthermore, the levels of cyclin D1, CDK4, and phosphorylated
retinoblastoma protein (pRb) were decreased (Fig. 3). These results indicated that
telmisartan suppressed the growth of MKN74 cells by impairing the
progression of the cell cycle.
Telmisartan does not induce MKN74
cells to undergo apoptosis
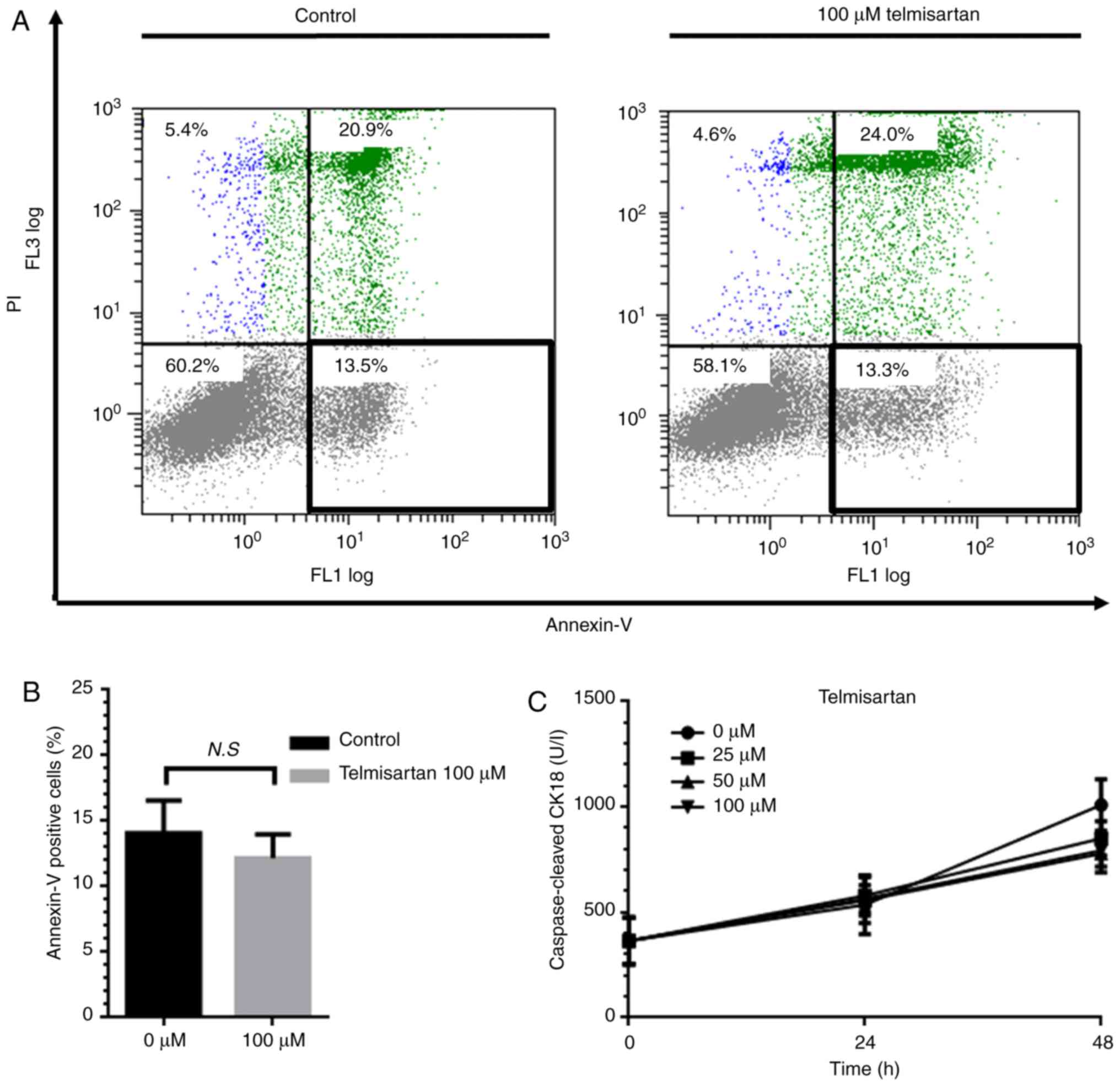
We used flow cytometry to investigate how
telmisartan influences the growth of MKN74 cells (Fig. 4A). Telmisartan did not significantly
alter the proportion of apoptotic cells 24 h after treatment of
MKN74 cells (Fig. 4B). Furthermore,
ELISA analysis revealed that the levels of cCK-18 did not
significantly increase compared with those of the controls
(Fig. 4C). These results indicate
that telmisartan suppressed the proliferation of MKN74 cells
without inducing apoptosis.
Telmisartan increases the levels of
angiogenesis-associated proteins expressed by MKN74 cells
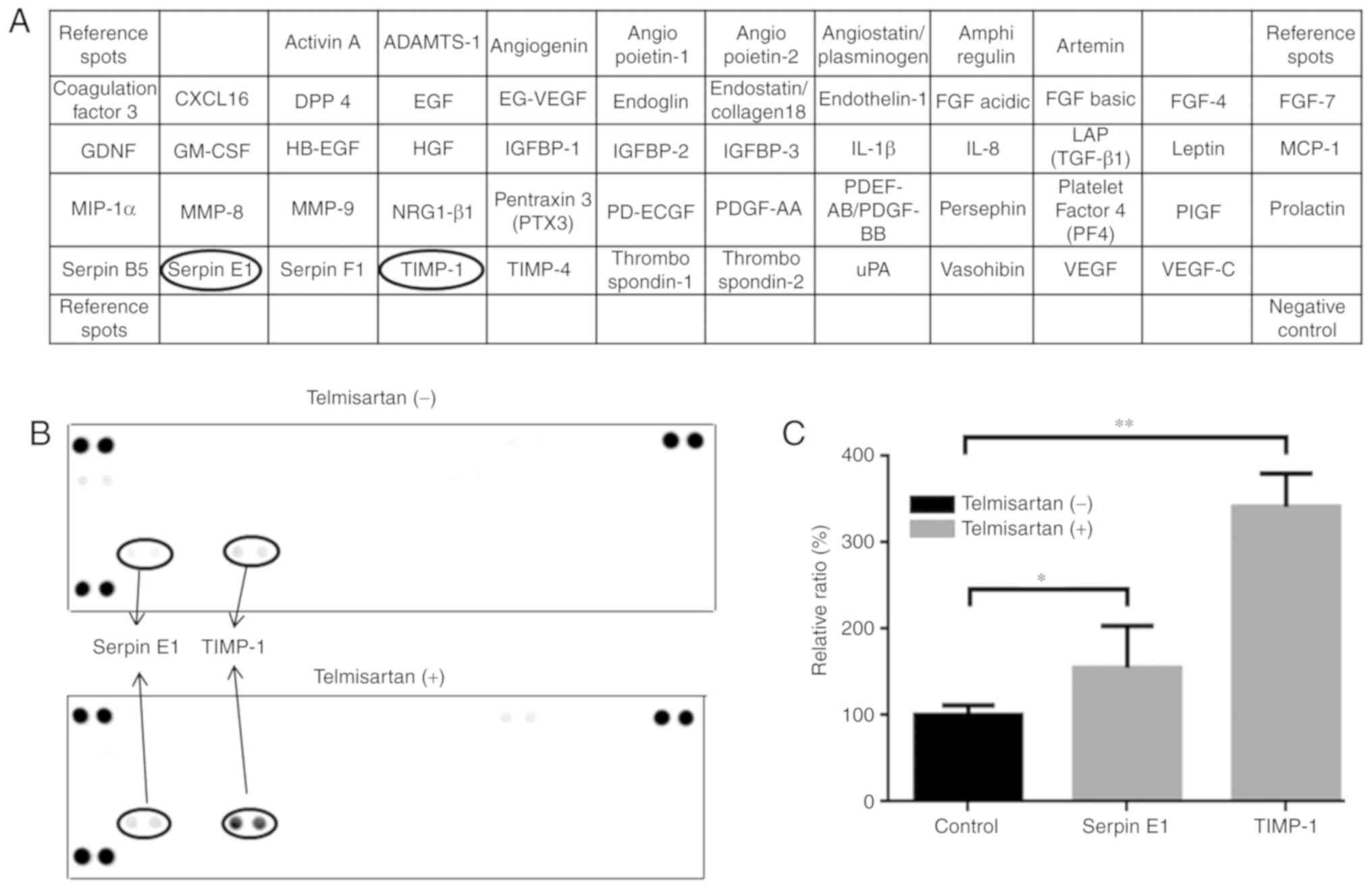
We used an antibody array to detect proteins
associated with angiogenesis expressed by MKN74 cells treated with
telmisartan (Fig. 5A). The levels
of TIMP-1 and Serpin E1 were significantly increased compared with
those of the controls (Fig. 5B and
C).
Phosphorylation of p-RTKs in MKN74
cells treated with telmisartan
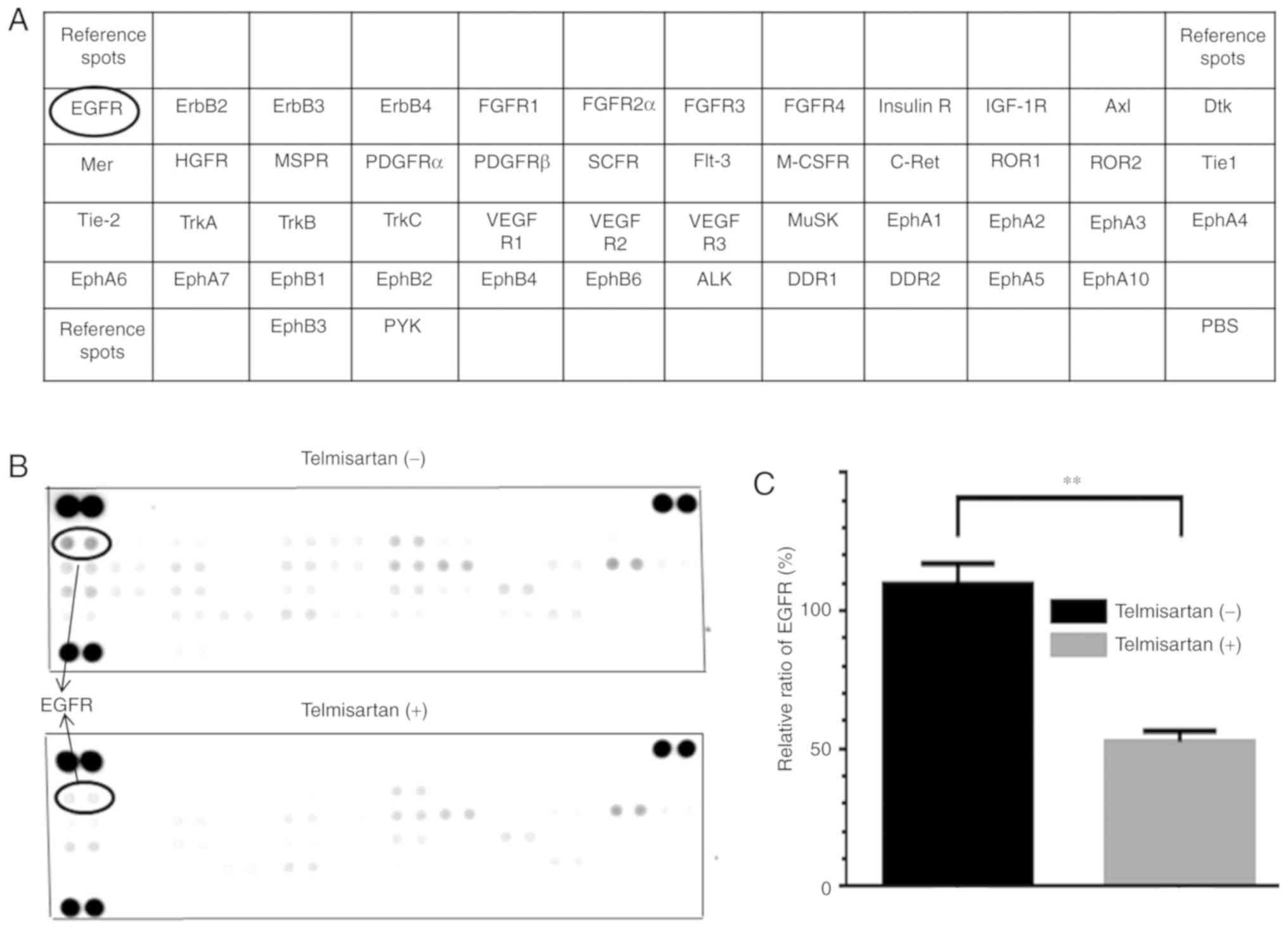
We analyzed an array comprising 49 p-RTKs to
identify RTKs involved in the antiproliferative effect of treating
MKN74 cells for 48 h with 100 µM telmisartan (Fig. 6A). Among the p-RTKs analyzed, the
level of phosphorylated epidermal growth factor receptor (p-EGFR)
was significantly decreased to 44.1% of that of the untreated cells
(Fig. 6B and C).
Telmisartan suppresses tumor growth in
vivo
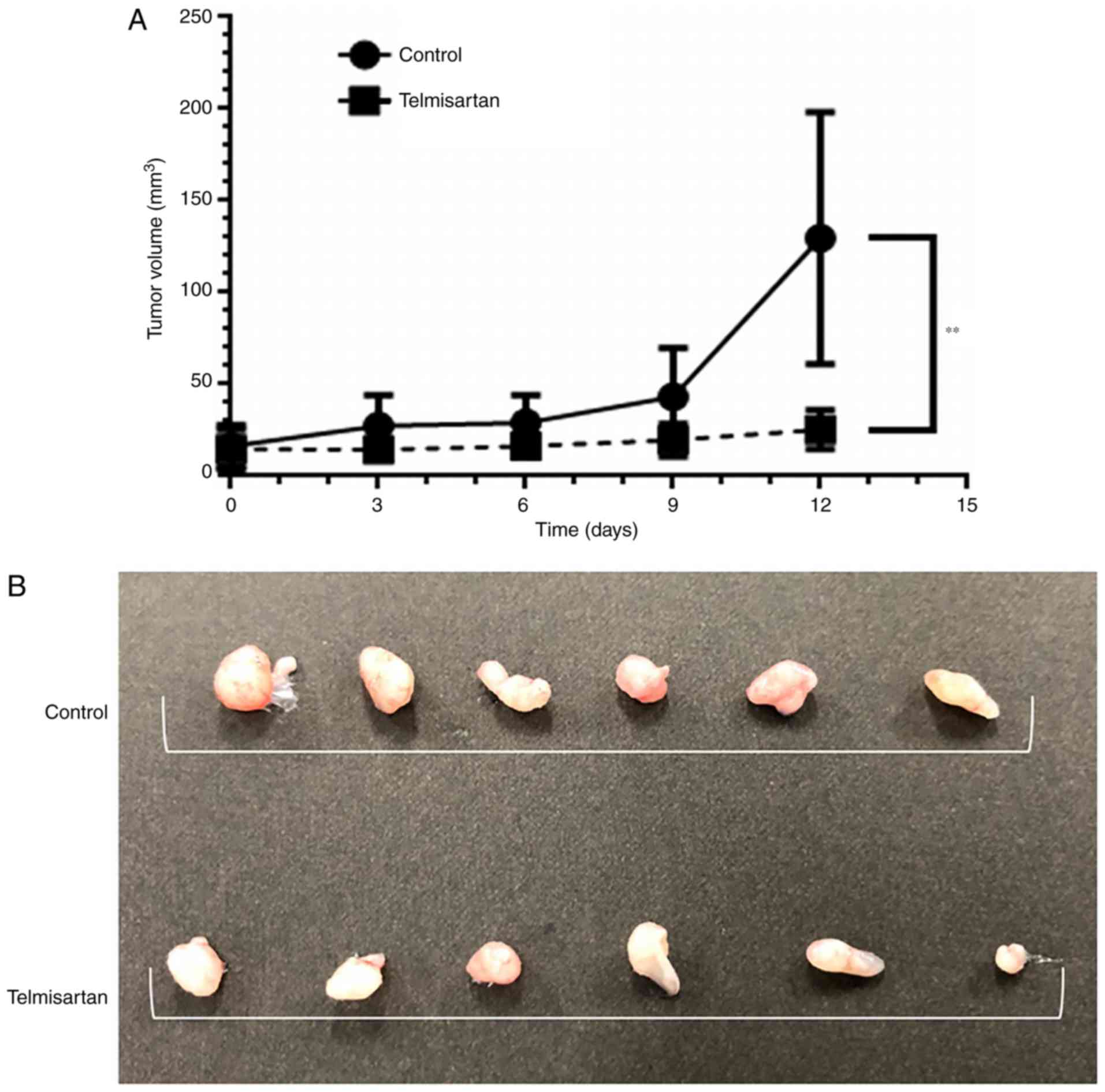
We employed a mouse xenograft model of gastric
cancer to test the potential anticancer effects of telmisartan. The
volumes of tumors induced by MKN74 cells were 19.6% lower compared
with those of the controls without affecting the weight of the mice
(Fig. 7A and B), and there was no
difference in pain-associated behaviors of the telmisartan-treated
and control mice.
Effect of telmisartan on miRNA
expression
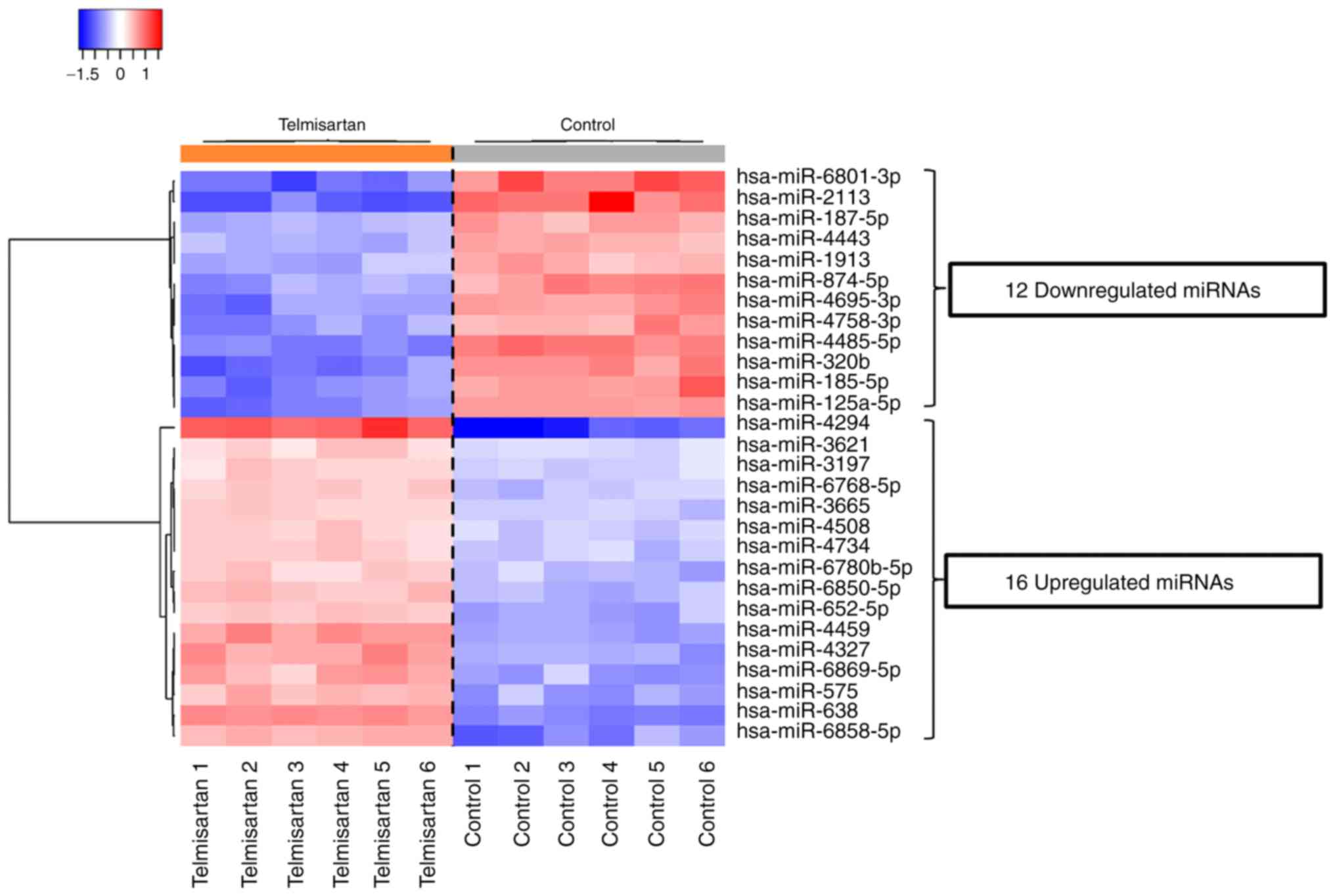
We examined the expression levels of 2,555 miRNAs in
MKN74 cells cultured with or without 100 µM telmisartan. After
normalization and deletion of miRNAs with missing values, the
hierarchical clustering of 369 miRNAs identified 28 miRNAs that
were differentially expressed (Fig.
8). Among these miRNAs, the levels of 16 and 12 were increased
and decreased, respectively (Table
I).
 | Table I.Differential expression of miRNAs in
MKN74 cells cultured with or without telmisartan. |
Table I.
Differential expression of miRNAs in
MKN74 cells cultured with or without telmisartan.
| miRNA | P-value | FC | Chromosomal
localization |
|---|
| Increased |
|
|
|
|
hsa-miR-4294 | 2.18E-08 | 5.01896 | 10q11.23 |
|
hsa-miR-638 | 7.58E-13 | 2.970468 | 19p13.2 |
|
hsa-miR-6858-5p | 7.78E-08 | 2.595125 | Xq28 |
|
hsa-miR-4459 | 1.01E-10 | 2.507542 | 5q11.2 |
|
hsa-miR-4327 | 4.65E-09 | 2.410159 | 21q22.11 |
|
hsa-miR-6869-5p | 8.69E-08 | 2.373717 | 20p13 |
|
hsa-miR-575 | 1.29E-08 | 2.240823 | 4 |
|
hsa-miR-652-5p | 1.12E-08 | 2.040954 | X |
|
hsa-miR-6850-5p | 1.85E-09 | 1.964441 | 8q24.3 |
|
hsa-miR-6780b-5p | 7.1E-08 | 1.842044 | 6p21.1 |
|
hsa-miR-6768-5p | 1.41E-08 | 1.756206 | 16p13.3 |
|
hsa-miR-4734 | 3.67E-08 | 1.713983 | 17q12 |
|
hsa-miR-3665 | 4.44E-10 | 1.69717 | 13q22.3 |
|
hsa-miR-4508 | 1.47E-08 | 1.682616 | 15q11.2 |
|
hsa-miR-3197 | 8.06E-09 | 1.637298 | 21q22.2 |
|
hsa-miR-3621 | 8.39E-08 | 1.576307 | 9q34.3 |
| Decreased |
|
|
|
hsa-miR-2113 | 6.97E-08 | 0.234941 | 6q16.1 |
|
hsa-miR-6801-3p | 1.03E-08 | 0.264563 | 19q13.41 |
|
hsa-miR-4485-5p | 2.32E-12 | 0.309017 | 11p15.4 |
|
hsa-miR-320b | 3.23E-09 | 0.314524 | 1 |
|
hsa-miR-185-5p | 2.3E-08 | 0.345032 | 22q11.21 |
|
hsa-miR-125a-5p | 3.6E-10 | 0.346496 | 19q13.41 |
|
hsa-miR-4695-3p | 2.34E-08 | 0.376426 | 1p36.13 |
|
hsa-miR-874-5p | 1.92E-08 | 0.381128 | 5q31.2 |
|
hsa-miR-4758-3p | 6.51E-08 | 0.402079 | 20q13.33 |
|
hsa-miR-187-5p | 1.46E-09 | 0.442281 | 18q12.2 |
|
hsa-miR-1913 | 4.44E-08 | 0.459243 | 6q27 |
|
hsa-miR-4443 | 6.92E-10 | 0.469228 | 3p21.31 |
Discussion
Environmental factors are strongly related to the
cause of gastric cancer (20).
Nonsteroidal anti-inflammatory drugs and reproductive hormones
decrease the risk of gastric cancer (21–23).
Telmisartan and other angiotensin receptor blockers (ARBs) are
effective for treating hypertension, chronic heart failure, and
chronic kidney disease. ARBs including telmisartan are widely
prescribed to treat these pathologies. Moreover, angiotensin II is
associated with cancer progression, and ARBs suppress tumor growth
by antagonizing the AT1 receptor (10–12,24).
In contrast, telmisartan inhibits different types of cancers; adult
T-cell leukemia cells, esophageal adenocarcinoma, hepatocellular
carcinoma and cholangiocarcinoma (15–18).
Here we evaluated the effect of telmisartan on human gastric cancer
cell lines and found that telmisartan suppressed the proliferation
of MKN74, MKN1 and MKN45 cells, indicating the potential of
telmisartan to serve as an anticancer drug. Moreover, the
antiproliferative effects of telmisartan were associated with cell
cycle arrest. In particular, of the three gastric cancer cell
lines, MKN74 cells were the least sensitive to telmisartan-mediated
cell death. Therefore, we chose MKN74 cells as a model for further
studies.
Dysregulation of the cell cycle and consequent
robust proliferation are hallmarks of cancer cells (25). Cyclins regulate the cell cycle, and
cell proliferation is controlled by complexes formed by cyclin and
cyclin-dependent kinases (CDKs) (26,27).
For example, the cyclin D1-CDK4/6 complex is required for
progression through G1 phase. Here we showed that telmisartan
induced G0/G1-phase arrest of a gastric cancer cell line by
decreasing the levels of cyclin D1, CDK4, as well as the
phosphorylation of the tumor suppressor retinoblastoma (pRb)
protein in the western blotting. Our investigations using a
xenograft model of gastric cancer further verify that telmisartan
inhibited tumor growth through inhibition of the expression of
cyclin D1. Other studies have demonstrated that telmisartan induces
cell cycle arrest in the G0/G1 phase by inhibiting the expression
of cyclin D1 (15–18). In contrast, telmisartan was found to
induce cell cycle arrest at the S phase by inhibiting the
expression of cyclin A2 and CDK2 in esophageal squamous cell
carcinoma (28). This discrepancy
may reflect differences in tumor differentiation, cell phenotypes,
and the characteristics of in vitro models.
Telmisartan was previously found to suppress cell
proliferation by inducing apoptosis of cancer cell lines, including
those derived from cancers of the prostate (29), kidney (30), endometrium (14), and colon (31). Here we were unable to detect
apoptosis of MKN74 cells treated with telmisartan. We conclude
therefore that the induction of apoptosis is not associated with
the suppression of proliferation. These findings suggest that
telmisartan mainly suppresses the proliferation of gastric cancer
cells by arresting the cell cycle and not by inducing
apoptosis.
The ARB candesartan was previously found to
significantly inhibit the expression of transforming growth factor
b1 (TGF-b1) to suppress tumor growth as well as stromal fibrosis
(19). Furthermore, candesartan
significantly suppressed the growth of tumor xenografts and
angiogenesis in mice (19). Here we
showed that MKN74 cells treated with telmisartan expressed elevated
levels of the anti-angiogenesis factor TIMP-1. Overexpression of
tissue inhibitor of metalloproteinase-1 (TIMP-1) shortens
disease-free and overall survival of patients with gastric cancer
(32,33). Therefore, overexpression of TIMP-1
by gastric cancer cells may be associated with poor prognosis,
indicating the transition to a more aggressive malignant
phenotype.
Our results are not consistent with the
antiproliferative effects of telmisartan on gastric cancer cells
published by others (32,33). Our present results are consistent
with the presence of a subpopulation of telmisartan-resistant cells
in cultures of the MKN74 cell line. These results suggest that
telmisartan may be used in combination with conventional anticancer
drugs or other molecularly-targeted therapeutics, particularly
those that inhibit angiogenesis. Evidence indicates that
overexpression of serpin E1, which is similar to TIMP-1, is a
marker of poor prognosis (34) as
is TIMP-1.
Members of the epidermal growth factor receptor
(EGFR) family activate intracellular signaling pathways in response
to extracellular signals (35).
Further, EGFR activation contributes to cell cycle progression, as
it is associated with the expression of cyclin D1 (25). Thus, telmisartan may regulate the
growth of MKN74 cells by inhibiting the activation of EGFR, which
is reflected by its phosphorylation on specific tyrosine residues.
Furthermore, we previously found that telmisartan inhibits the
growth of cancer cells through the regulation of EGFR (16,18).
For example, EGFR is preferentially activated in gastric cancer vs.
normal tissues (36). Here we found
that telmisartan inhibited the phosphorylation of EGFR in MKN74
cells. We further showed in the present study that the
phosphorylation of EGFR was suppressed after 24 h but not at 48 h
after cells were treated with telmisartan (data not shown).
Therefore, these data suggest that early after its addition to
cultures of gastric cancer cells, telmisartan inhibited the
phosphorylation of EGFR.
Telmisartan affects the miRNA expression profile of
cancer cells (16–18). Here we identified 28 differentially
expressed miRNAs in MKN74 cells treated with telmisartan when
compared to controls. miR-185-5p, which was found to be expressed
at lower levels, is a reliable diagnostic biomarker of gastric
cancer (37). In contrast, miR-187,
which was expressed at lower levels in MKN74 cells and is
overexpressed in gastric cancer tissues, is associated with factors
that influence the malignant phenotype (38).
Overexpression of miR-187 was previously found to
promote the proliferation, migration, and invasion of gastric
cancer cells by targeting tumor suppressor CRMP1 (38). Thus, telmisartan may exert its
antitumor effects by inhibiting the expression of miRNAs. The
relationship between cell cycle arrest and these two miRNAs is
unknown and requires further study.
In conclusion, in the present study, it was
demonstrated that telmisartan inhibited the proliferation of a
human gastric cancer cell line in vivo and in vitro
through cell cycle arrest. Moreover, we provide compelling evidence
that the inactivation of the EGFR and the changes in the levels of
an angiogenesis-associated protein and those of two miRNAs
contributed to the antitumor effects of telmisartan.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Kayo Hirose, Miwako Watanabe,
Keiko Fujikawa, Megumi Okamura, Mari Yamada and Fuyuko Kokado from
the Department of Gastroenterology and Neurology, Kagawa University
for their assistance with the experiments.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request. Supplementary information is included in Data S1.
Author's contributions
NF and TM designed the experiments. KF, HI, HKo, TC,
DN, HY, TK, KT, MH, KKo, KKa, HKa, AM, KT, TH, KO and YS conducted
the experiments, analyzed data, and wrote the manuscript. SF and TM
were involved in research design and contributed to writing the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All mice were treated in accordance with the
guidelines of the Kagawa University Committee on Experimental
Animals. The Kagawa University Animal Care Committee approved the
protocol for animal experiments (Registration no. A155).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Correa P: Gastric cancer: Two epidemics?
Dig Dis Sci. 56:1585–1586, author reply 1586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salvon-Harman JC, Cady B, Nikulasson S,
Khettry U, Stone MD and Lavin P: Shifting proportions of gastric
adenocarcinomas. Arch Surg. 129:381–388, discussion 388–389. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilici A: Treatment options in patients
with metastatic gastric cancer: Current status and future
perspectives. World J Gastroenterol. 20:3905–3915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akama Y, Yasui W, Yokozaki H, Kuniyasu H,
Kitahara K, Ishikawa T and Tahara E: Frequent amplification of the
cyclin E gene in human gastric carcinomas. Jpn J Cancer Res.
86:617–621. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bani-Hani KE, Almasri NM, Khader YS,
Sheyab FM and Karam HN: Combined evaluation of expressions of
cyclin E and p53 proteins as prognostic factors for patients with
gastric cancer. Clin Cancer Res. 11:1447–1453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Li JY, He JS, Zhou LX and Chen K:
Tissue microarray analysis of multiple gene expression in
intestinal metaplasia, dysplasia and carcinoma of the stomach.
Histopathology. 46:505–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takano Y, Kato Y, van Diest PJ, Masuda M,
Mitomi H and Okayasu I: Cyclin D2 overexpression and lack of p27
correlate positively and cyclin E inversely with a poor prognosis
in gastric cancer cases. Am J Pathol. 156:585–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shan YS, Hsu HP, Lai MD, Hung YH, Wang CY,
Yen MC and Chen YL: Cyclin D1 overexpression correlates with poor
tumor differentiation and prognosis in gastric cancer. Oncol Lett.
14:4517–4526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto K, Tajima H, Ohta T, Nakanuma S,
Hayashi H, Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa
H, et al: Angiotensin II induces tumor progression and fibrosis in
intrahepatic cholangiocarcinoma through an interaction with hepatic
stellate cells. Int J Oncol. 37:1251–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du N, Feng J, Hu LJ, Sun X, Sun HB, Zhao
Y, Yang YP and Ren H: Angiotensin II receptor type 1 blockers
suppress the cell proliferation effects of angiotensin II in breast
cancer cells by inhibiting AT1R signaling. Oncol Rep. 27:1893–1903.
2012.PubMed/NCBI
|
|
12
|
Kinoshita J, Fushida S, Harada S, Yagi Y,
Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M,
et al: Local angiotensin II-generation in human gastric cancer:
Correlation with tumor progression through the activation of
ERK1/2, NF-kappaB and survivin. Int J Oncol. 34:1573–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuyama M, Funao K, Kuratsukuri K,
Tanaka T, Kawahito Y, Sano H, Chargui J, Touraine JL, Yoshimura N
and Yoshimura R: Telmisartan inhibits human urological cancer cell
growth through early apoptosis. Exp Ther Med. 1:301–306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koyama N, Nishida Y, Ishii T, Yoshida T,
Furukawa Y and Narahara H: Telmisartan induces growth inhibition,
DNA double-strand breaks and apoptosis in human endometrial cancer
cells. PLoS One. 9:e930502014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozako T and Soeda S, Yoshimitsu M, Arima
N, Kuroki A, Hirata S, Tanaka H, Imakyure O, Tone N, Honda S and
Soeda S: Angiotensin II type 1 receptor blocker telmisartan induces
apoptosis and autophagy in adult T-cell leukemia cells. FEBS Open
Bio. 6:442–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujihara S, Morishita A, Ogawa K, Tadokoro
T, Chiyo T, Kato K, Kobara H, Mori H, Iwama H and Masaki T: The
angiotensin II type 1 receptor antagonist telmisartan inhibits cell
proliferation and tumor growth of esophageal adenocarcinoma via the
AMPKα/mTOR pathway in vitro and in vivo. Oncotarget. 8:8536–8549.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oura K, Tadokoro T, Fujihara S, Morishita
A, Chiyo T, Samukawa E, Yamana Y, Fujita K, Sakamoto T, Nomura T,
et al: Telmisartan inhibits hepatocellular carcinoma cell
proliferation in vitro by inducing cell cycle arrest. Oncol
Rep. 38:2825–2835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samukawa E, Fujihara S, Oura K, Iwama H,
Yamana Y, Tadokoro T, Chiyo T, Kobayashi K, Morishita A, Nakahara
M, et al: Angiotensin receptor blocker telmisartan inhibits cell
proliferation and tumor growth of cholangiocarcinoma through cell
cycle arrest. Int J Oncol. 51:1674–1684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okazaki M, Fushida S, Harada S, Tsukada T,
Kinoshita J, Oyama K, Tajima H, Ninomiya I, Fujimura T and Ohta T:
The angiotensin II type 1 receptor blocker candesartan suppresses
proliferation and fibrosis in gastric cancer. Cancer Lett.
355:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamanqar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 5:700–713. 2014. View Article : Google Scholar
|
|
21
|
Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ and
Lin JT: Effective reduction of gastric cancer risk with regular use
of nonsteroidal anti-inflammatory drugs in Helicobacter
pylori-infected patients. J Clin Oncol. 28:2952–2957. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Epplein M, Nomura AM, Wilkens LR,
Henderson BE and Kolonel LN: Nonsteroidal antiinflammatory drugs
and risk of gastric adenocarcinoma: The multiethnic cohort study.
Am J Epidemiol. 170:507–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freedman ND, Chow WH, Gao YT, Shu XO, Ji
BT, Yang G, Lubin JH, Li HL, Rothman N, Zheng W and Abnet CC:
Menstrual and reproductive factors and gastric cancer risk in a
large prospective study of women. Gut. 56:1671–1677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Incalci M, Colombo T, Ubezio P,
Nicoletti I, Giavazzi R, Erba E, Ferrarese L, Meco D, Riccardi R,
Sessa C, et al: The combination of yondelis and cisplatin is
synergistic against human tumor xenografts. Eur J Cancer.
39:1920–1926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The antidiabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cordon-Cardo C: Mutations of cell cycle
regulators. Biological and clinical implications for human
neoplasia. Am J Pathol. 147:545–560. 1995.PubMed/NCBI
|
|
27
|
Hunter T and Pines J: Cyclins and cancer.
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui T, Chiyo T, Kobara H, Fujihara S,
Fujita K, Namima D, Nakahara M, Kobayashi N, Nishiyama N, Yachida
T, et al: Telmisartan inhibits cell proliferation and tumor growth
of esophageal squamous cell carcinoma by inducing s-phase arrest in
vitro and in vivo. Int J Mol Sci. 20:e31972019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan is
a potent target for prevention and treatment in human prostate
cancer. Oncol Rep. 20:295–300. 2008.PubMed/NCBI
|
|
30
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan as
a peroxisome proliferator-activated receptor-γ ligand is a new
target in the treatment of human renal cell carcinoma. Mol Med Rep.
2:193–198. 2009.PubMed/NCBI
|
|
31
|
Lee LD, Mafura B, Lauscher JC, Seeliger H,
Kreis ME and Gröne J: Antiproliferative and apoptotic effects of
telmisartan in human colon cancer cells. Oncol Lett. 8:2681–2686.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mimori K, Mori M, Shiraishi T, Fujie T,
Baba K, Haraguchi M, Abe R, Ueo H and Akiyoshi T: Clinical
significance of tissue inhibitor of metalloproteinase expression in
gastric carcinoma. Br J Cancer. 76:531–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshikawa T, Tsuburaya A, Kobayashi O,
Sairenji M and Miyagi Y: Protein levels of tissue inhibitor of
metalloproteinase-1 in tumor extracts as a marker for prognosis and
recurrence in patients with gastric cancer. Gastric Cancer.
9:106–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao P, Li W, Liu R, Teer JK, Xu B, Zhang
W, Li X, Mcleod HL and He Y: Genome-scale analysis identifies
SERPINE1 and SPARC as diagnostic and prognostic biomarkers in
gastric cancer. OncoTargets Ther. 11:6969–6980. 2018. View Article : Google Scholar
|
|
35
|
Hsieh AC and Moasser MM: Targeting HER
proteins in cancer therapy and the role of the non-target HER3. Br
J Cancer. 97:453–457, 20017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masaki T, Hatanaka Y, Nishioka M, Tokuda
M, Shiratori Y, Reginfo W and Omata M: Activation of epidermal
growth factor receptor kinase in gastric carcinoma: A preliminary
study. Am J Gastroenterol. 95:2135–2136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang Z, Zhu D, Wu L, He M, Zhou X, Zhang
L, Zhang H, Wang W, Zhu J, Cheng W, et al: Six serum-based miRNAs
as potential diagnostic biomarkers for gastric cancer. Cancer
Epidemiol Biomarkers Prev. 26:188–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren L, Li F, Di M, Fu Y, Hui Y, Xiao G,
Sun Q, Liu Y, Ren D and Du X: MicroRNA-187 regulates gastric cancer
progression by targeting the tumor suppressor CRMP1. Biochem
Biophys Res Commun. 482:597–603. 2017. View Article : Google Scholar : PubMed/NCBI
|