Introduction
Cancer is a major public health problem worldwide
and colorectal cancer is one of the leading causes of
cancer-associated mortality (1). In
spite of the considerable progress in cancer therapeutics, tumor
relapse and metastasis remain a major clinical challenge (2). Numerous studies have suggested that
there is a rare subpopulation of poorly-differentiated cancer
cells, cancer stem cells (CSCs), that are responsible for
tumorigenesis, progression, recurrence and metastasis (3,4). It
has been demonstrated that CSCs possess self-renewal properties,
tumor initiation capability, a high proliferation rate and
differentiation potential (5). CSC
populations are associated with chemoradiotherapy resistance and
relapse potential after successful treatment, leading to the
unfavorable outcomes for patients (6).
It is well known that there are small populations of
adult stem cells existing in various tissues, such as the lung,
ovaries, intestines, skin and mammary gland (3,7,8), which
have high proliferative capacity. Adult stem cells possess
self-renewal abilities and multilineage differentiation potential
to maintain tissue homeostasis and repair injured tissues (9,10).
However, dysregulation in the mechanisms underlying proliferation
and differentiation initiates the malignant transformation of adult
stem cells leading to tumorigenesis (11). Previous studies have demonstrated
that intestinal stem cells (ISCs) are the origin of intestinal
neoplasia (8,12,13).
Thus, it was hypothesized that ISCs are the origin of intestinal
CSCs, sustaining tumorigenesis and progression. Resolving the
molecular mechanisms underpinning the malignant transformation of
ISCs to intestinal CSCs may help identify novel targets for
colorectal cancer treatment.
Accumulating evidence suggests that inflammation,
particularly chronic inflammation, serves an important role in
intestinal neoplasia (14–17). Chronic inflammation has been
revealed to promote the hyperactivation of signaling pathways
involved in the regulation of ISCs, including Wnt/β-catenin,
PI3K/AKT/mTOR, NF-κB, STAT3 and Notch (14,15,18).
Then, ISCs undergo genomic alteration and aggressive proliferation,
which eventually transforms normal stem cells into intestinal CSCs
to initiate intestinal tumorigenesis (15). Recent studies have demonstrated that
the crosstalk between the NF-κB and Wnt/β-catenin signaling
pathways may regulate the progression of colorectal cancer in
inflammatory responses (19,20).
Therefore, it is necessary to elucidate the molecular mechanisms
underlying the malignant transformation of ISCs in inflammatory
responses.
The present study focused on the impact of
proinflammatory cytokine tumor necrosis factor (TNF)-α on human
ISCs. The results indicated that TNF-α induced the activation of
the NF-κB and Wnt/β-catenin pathways to promote the malignant
transformation of ISCs, such as increasing proliferation, colony
formation, chemotherapeutic resistance, migration and invasion. It
was further demonstrated that these two signaling pathways
cross-regulated each other in TNF-α-induced malignant
transformation of ISCs.
Materials and methods
Cell culture
The human normal colon epithelial cell line NCM460
was purchased from Ginio Biotechnology Corporation. Cells were
cultured in DMEM-High Glucose medium (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin at 37°C and 5% CO2 in a
humidified atmosphere. For the sphere culture, single NCM460 cells
were transferred in serum-free DMEM/F12 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10 ng/ml bFGF
(PeproTech, Inc.), 20 ng/ml EGF (PeproTech, Inc.), 5 mg/ml insulin
(MCE), 0.4% BSA (Beijing Solarbio Science & Technology Co.,
Ltd.) and 2% B27 Supplements (Gibco; Thermo Fisher Scientific,
Inc.).
NCM460 spheroid (NCM460s) cells were collected using
natural sedimentation at 37°C for 5 min. NCM460s cells were
enzymatically dissociated, resuspended, and then seeded at
2×104 cells per well in serum-free medium (SFM) in
6-well ultra-low attachment culture dishes (Corning, Inc.). Cells
were treated with 0–100 ng/ml TNF-α (PeproTech, Inc.) for various
durations (12, 24, 48 and 72 h) at 37°C to induce an inflammatory
response.
Differentiation assay
To evaluate the differentiation ability of NCM460s
cells from SFM, the 50 NCM460 spheres with >100 µm diameter were
collected and re-cultured for 48 h on a collagen-coated 35-mm dish
in DMEM-High Glucose medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin at
37°C and 5% CO2 in a humidified atmosphere.
Antibodies and reagents
Specific primary antibodies to IKKβ (product no.
8943), phospho-IKKα/β (Ser176/180; cat. no. 2697), IκBα (cat. no.
9242), phospho-IκBα (Ser32/36; cat. no. 9246), NF-κB p65 (cat. no.
8242), phospho-NF-κB p65 (Ser536; cat. no. 3033), TNF-α (cat. no.
8184), APC (cat. no. 2504), GSK-3β (cat. no. 12456), phospho-GSK-3β
(Ser9; cat. no. 9323), β-catenin (cat. no. 8480), phospho-β-catenin
(Ser33/37/Thr41; cat. no. 9561), c-Myc (cat. no. 13987), cyclin D1
(cat. no. 2978), E-cadherin (cat. no. 3195), N-cadherin (cat. no.
13116), vimentin (cat. no. 5741), Snail (cat. no. 3879) and Nanog
(cat. no. 3580) were obtained from Cell Signaling Technology, Inc.
Antibodies to Lgr5 (cat. no. ab75732), Oct4 (cat. no. ab137427) and
Sox2 (cat. no. ab97959) were obtained from Abcam. PE Mouse
Anti-Human CD133 antibody (cat. no. 566593), PE Mouse IgG1, κ
Isotype Control (cat. no. 554680) and 7-AAD (cat. no. 559925) were
obtained from BD Biosciences. The NF-κB inhibitor pyrrolidine
dithiocarbamate (PDTC; cat. no. M4005) and Wnt inhibitor IWP-2
(cat. no. M2237) were purchased from Abmole (Abmole Bioscience,
Inc.). Lipofectamine 3000 transfection reagent was obtained from
Invitrogen; Thermo Fisher Scientific, Inc.
Flow cytometric analysis
For the detection of surface markers in colon cancer
stem cells, cells (1×106 cells) were collected,
incubated with human anti-CD133-PE antibodies (1:20 dilution; BD
Biosciences) at room temperature in the dark for 30 min. Dead cells
were excluded using 7-AAD staining (1:20 dilution; BD Biosciences)
at room temperature for 10 min. Cells were analyzed using a flow
cytometer (BD Biosciences), and the isotype IgG2b (1:20 dilution;
BD Biosciences) was used as the control.
For the cell cycle analysis, cells were pipetted,
washed twice with PBS and fixed in 70% ethanol at 4°C overnight.
Cells were centrifuged at 4,000 rpm for 10 min, re-suspended and
stained with PI (BD Biosciences) at 4°C for 30 min. Then, samples
were also analyzed using a flow cytometer (BD Biosciences).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was analyzed using a CCK-8 assay
according to the manufacturer's instructions (Boshide). Cells were
seeded into a 96-well plate at 5×103 cells/well with 100
µl culture medium which contained the various TNF-α concentrations
(0, 0.01, 0.1, 1, 10, 50 and 100 ng/ml). Cell viability was
quantified by the addition of 10 µl CCK-8 solution. After 2 h of
incubation at 37°C, the absorption was analyzed at 450 nm using a
Multiskan plate reader (Thermo Fisher Scientific, Inc.).
The chemotherapy sensitivity assay to 5-FU
(Sigma-Aldrich; Merck KGaA) was also evaluated using CCK-8 assay
kit as aforementioned.
Soft-agar sphere-formation assay
The 6-well plates were loaded with 2 ml 0.6% soft
agarose. Then, 2 ml 0.375% soft agarose containing 1×104
cells were added on the lower agarose. After 2 weeks of incubation,
the cell spheres were stained with 0.005% crystal violet at room
temperature for 1 h. The number of spheres with >100 µm diameter
in each well were counted using an inverted fluorescence microscope
(magnification, ×100; Olympus Corp.) to analyze the sphere
formation capacity.
Colony formation assay
Cells (1×103 cells) were plated in 35-mm
culture dishes and cultured for 2 weeks. After staining with 0.1%
crystal violet at room temperature for 30 min. The number of
colonies with >1 mm diameter was counted using an inverted
fluorescence microscope (magnification, ×40; Olympus Corp.) to
analyze the colony formation capacity.
Cell migration and invasion assay
Cell migration and invasion assays were performed as
previously described (21). In
brief, cell suspensions containing 2×104 cells in 200 µl
SFM were added into 24-well plates with 8.0-µm upper Transwell
chambers (BD Biosciences) pre-coated without or with Matrigel
(Sigma-Aldrich; Merck KGaA) for migration or invasion assays,
respectively, and lower chambers were filled with 800 µl medium
with 15% FBS. After a 24 h incubation, the migrating and invading
cells were stained with 0.1% crystal violet solution at room
temperature for 30 min and then counted in five random microscopic
fields using an inverted fluorescence microscope (magnification,
×200; Olympus Corp.).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was purified using TRIzol®
reagent (Beyotime Institute of Biotechnology) according to a
standard protocol (22). The
concentration and quality of all the RNA samples were valuated
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.). Then, 1 µg of total RNA was used to synthesize cDNA for the
subsequent reverse transcription with the Reverse Transcription kit
(Takara Biotechnology, Co., Ltd.) according to the manufacturer's
instructions. Quantitative PCR was performed using the SYBR Green
PCR Master Mix kit (Takara Biotechnology, Co., Ltd.) on a qPCR
detection system (Bio-Rad Laboratories, Inc.). The qPCR
thermocycling conditions were as follows: Initial denaturation for
3 min at 95°C followed by 40 cycles of denaturation for 15 sec at
95°C, annealing for 30 sec at 60°C and extension for 30 sec at
72°C. The data were analyzed using the 2−ΔΔCq method
(23) and reported as the
fold-change in gene expression normalized to the expression of the
endogenous control gene GAPDH. The sequences of primers used are
listed in Table SI.
Western blot analysis
After appropriate treatments, cells were harvested
and total cellular proteins were extracted for western blot
analysis. In addition, the nuclear and cytoplasmic proteins were
extracted using a Nuclear and Cytoplasmic Protein Extraction kit
(Sangon Biotech Co., Ltd.) according to the manufacturer's
instructions. Protein concentrations were measured using a BCA
Protein Assay kit (Nanjing KeyGen Biotech Co., Ltd.). Proteins (30
µg/lane) were separated by gel electrophoresis on 8–10% SDS-PAGE
and then transferred onto PVDF membranes (EMD Millipore). After
blocking with 5% non-fat milk at room temperature for 2 h, the PVDF
membranes were incubated with primary antibodies at 4°C overnight.
Membranes were incubated with horseradish peroxidase-labelled
secondary goat anti-rabbit antibody (1:10,000; ProteinTech Group,
Inc.) for 2 h. Proteins were examined by enhanced chemiluminescence
(ECL) detection kit (Nanjing KeyGen Biotech Co., Ltd.), and then
the signals were visualized and analyzed using the ImageJ v5.2.1
Software (Bio-Rad Laboratories, Inc.).
Immunofluorescence staining
Expression levels of p65 and β-catenin were detected
using immunofluorescence staining. Spheres were fixed in 4%
paraformaldehyde at room temperature for 20 min, blocked in
blocking buffer (PBS+2% BSA+0.3% Triton-100) at room temperature
for 1 h, and incubated with primary antibodies against p65 and
β-catenin (1:200, rabbit) at 4°C overnight. Cells were then treated
at room temperature with FITC-conjugated secondary antibody (Boster
Biological Technology) for 1 h and DAPI (Beyotime Institute of
Biotechnology) for 30 min. Fluorescent signals were visualized
using a Leica fluorescence microscope (magnification, ×200; Leica
Microsystems GmbH).
Dual-luciferase reporter assay
The cells were co-transfected with a pGL4.32 vector
(Promega Corporation) containing the NF-κB reporter construct or a
pGL4.49 vector (Promega Corporation) containing the TCF/lymphoid
enhancer-binding factor 1 (LEF) reporter construct linked to a
firefly luciferase reporter plasmid, and with a pRL-TK
Renilla luciferase reporter gene (Promega Corporation). The
luciferase activities were measured using a Dual-Luciferase
Reporter Assay system (Promega Corporation) and normalized as a
relative ratio of luciferase to Renilla luciferase
activities according to the manufacturer's protocol.
Lentiviral transfection of spheroid
cells
Lentiviral vectors bearing p65 short hairpin (sh)RNA
and control non-targeting shRNA were synthesized by Shanghai
GeneChem Co., Ltd. Cells were seeded in 6-well plates at a density
of 3×105 cells/well and cultured at 37°C and 5%
CO2 in a humidified atmosphere overnight. NCM460s cells
were transfected with 100 nM p65 shRNA or control shRNA using
Lipofectamine™ 3000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
12 h of incubation, the medium was replaced with normal SFM under
suspension conditions. After 5 days of transfection, the effect of
p65 shRNA was examined using RT-qPCR and western blotting. Then,
the transfected cells were passaged for further experiments.
Statistical analysis
All data were analyzed using SPSS v20.0 (IMB Corp.).
The results were presented as the mean ± standard deviation from at
least three independent experiments. Differences between two groups
were analyzed using unpaired Student's t-tests. One-way ANOVA
followed by Tukey's post hoc test was used to analyze differences
in multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Intestinal stem cells are enriched
upon spheroidal culture in SFM
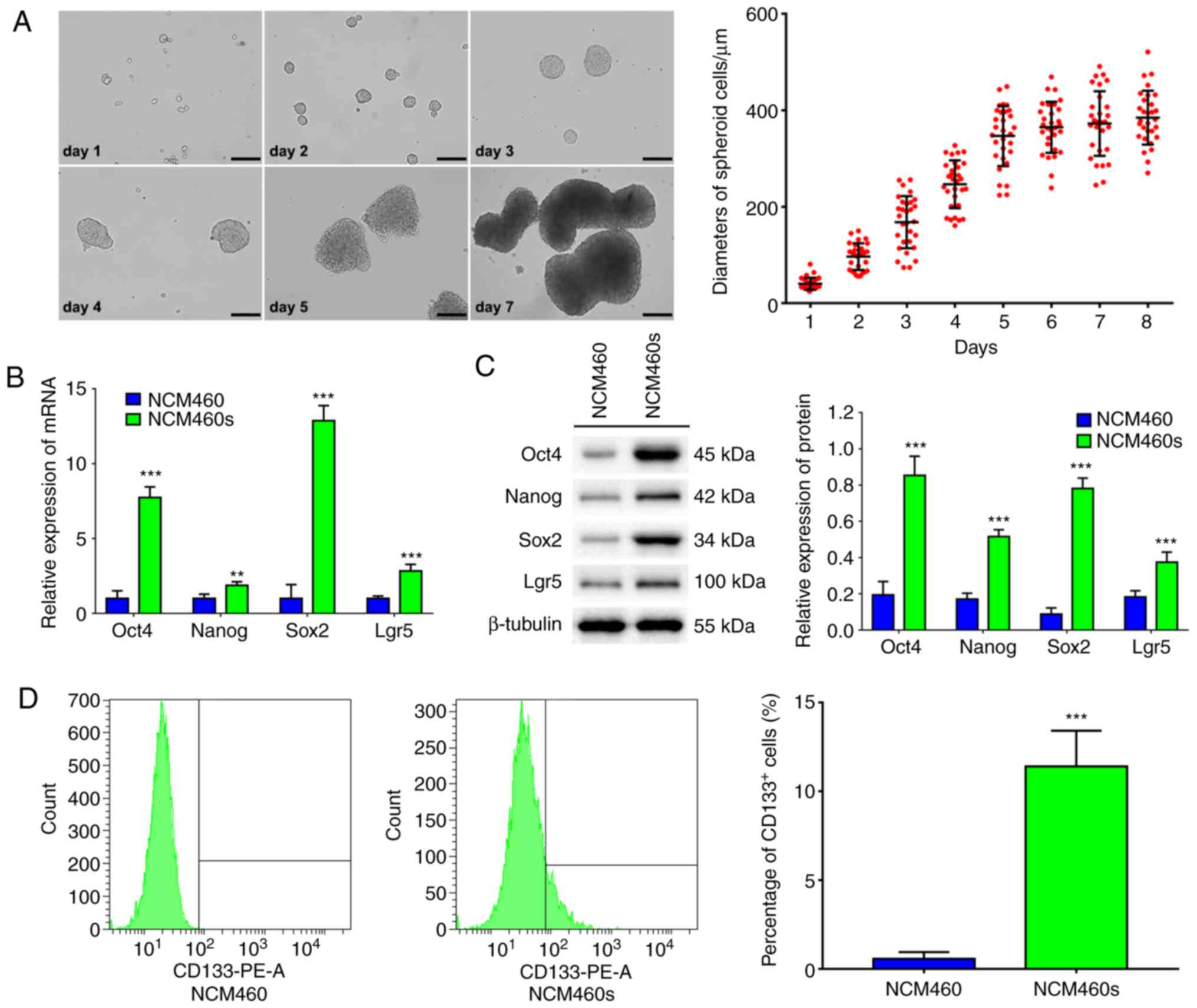
Human normal intestinal epithelial cells, NCM460
cells, formed spheres and the diameters increased gradually when
cultured in SFM under suspension conditions (Fig. 1A). NCM460s cells could be passaged
>30 times in SFM under suspension conditions, indicating that
they possessed the self-renewal ability. Moreover, NCM460s cells
could be induced to differentiate into epithelial-like cells when
cultured in DMEM medium supplemented with 10% FBS (Fig. S1).
In order to validate the stemness of NCM460s cells,
the stem cell genes were analyzed using qPCR, western blotting and
flow cytometric analysis. Results indicated that NCM460s cells
exhibited higher mRNA and protein expression of stem cell genes
such as Oct4, Nanog and Sox2 (24)
and Lgr5, a marker gene of intestinal stem cells (13), compared with those in NCM460
adherent cells (Fig. 1B and C).
Furthermore, flow cytometric analysis revealed that the ratio of
CD133+ cells (one of the specific surface markers in
colorectal CSCs) was <1% in NCM460 adherent cells, but reached
11.40% in NCM460s cells (Fig. 1D).
These results indicated that serum-free suspension culture is an
effective strategy to enrich NCM460s cells with stem cell
characteristics from normal intestinal epithelial NCM460 cells.
TNF-α promotes the malignant
transformation of NCM460s cells
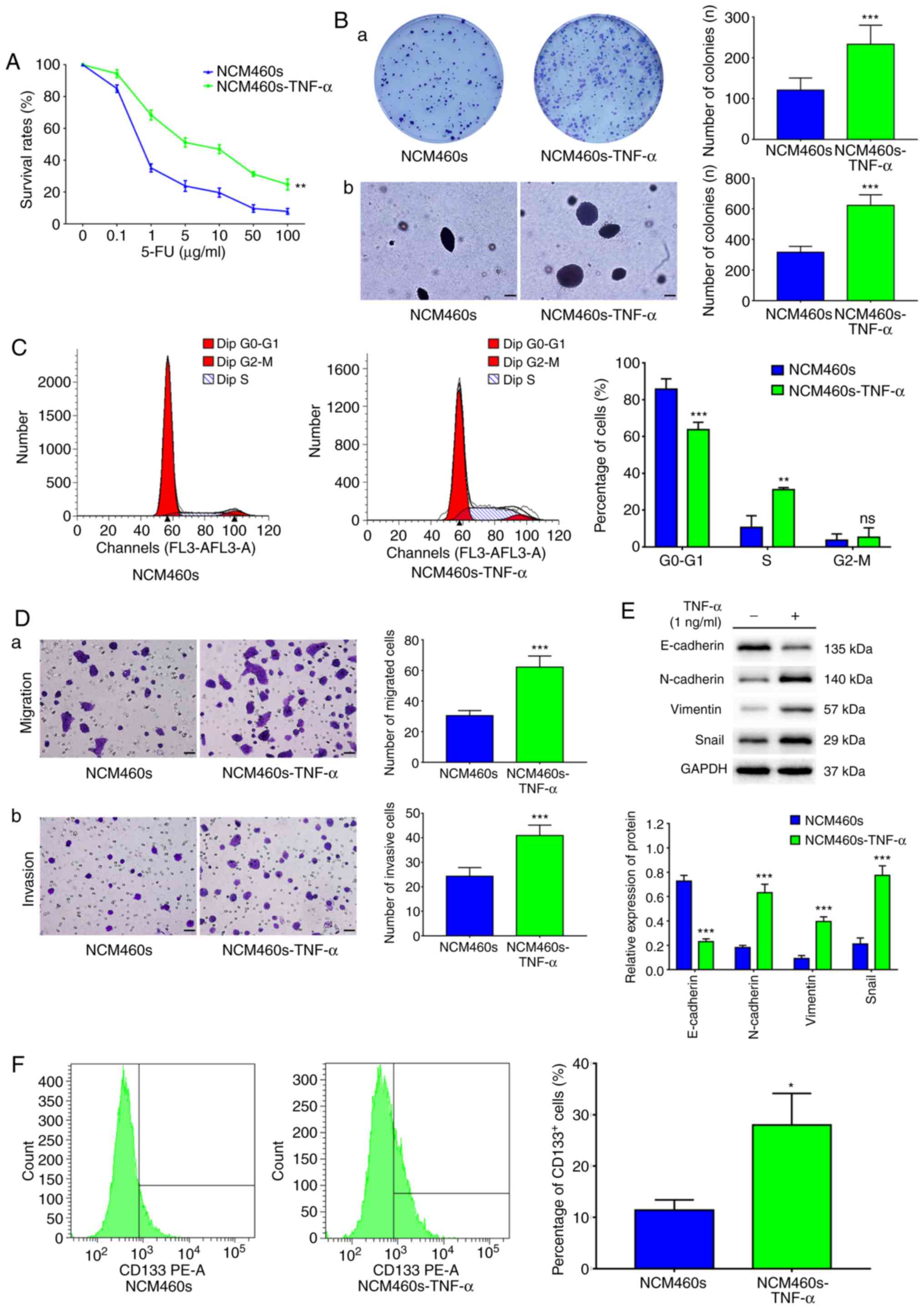
When NCM460s cells were treated with 0–100 ng/ml
TNF-α for various time-points (12, 24, 48 and 72 h), the viability
of NCM460s cells was affected in a dose-dependent and
time-dependent manner. Treatment with 1 ng/ml TNF-α for 24 h
resulted in the maximum increase of the viability of NCM460s cells
(Fig. S2). Therefore, all further
experiments investigating malignant transformation were conducted
for this duration.
To evaluate the impact of TNF-α on chemotherapy
sensitivity, cells were exposed to 5-FU at various concentrations
(0, 0.1, 1, 5, 10, 50 and 100 µg/ml) for 48 h. Then, the
viabilities were analyzed at the indicated concentration of 5-FU
using a CCK-8 assay. The results revealed that the survival rates
of NCM460s cells were significantly increased at each concentration
of 5-FU upon TNF-α treatment, suggesting that TNF-α increased
chemotherapy resistance (Fig.
2A).
The effect of TNF-α on the proliferation ability of
NCM460s cells was assessed using a colony formation and soft agar
assay. The results revealed that TNF-α significantly increased the
colony formation capacity of NCM460s cells (Fig. 2B). In order to explore the
underlying mechanism associated with these phenotypic changes, the
impact of TNF-α on NCM460s cell distribution in the cell cycle was
investigated using flow cytometry. It was reported that TNF-α
significantly reduced the proportion of cells in the
G0-G1 phase and increased the proportion of
cells in the S phase (Fig. 2C).
These data revealed that TNF-α increased the proliferative capacity
of NCM460s cells.
Since the epithelial-mesenchymal transition (EMT) is
a key process in cancer metastasis, the impact of TNF-α on the
migratory and invasive abilities of NCM460s cells was examined
using Transwell assays. It was demonstrated that TNF-α
significantly increased the capacities of migration and invasion of
NCM460s cells (Fig. 2D). In
addition, western blotting revealed that TNF-α decreased the
expression of the epithelial marker E-cadherin, and concurrently
increased the expression of mesenchymal markers, including
N-cadherin, vimentin and transcription factor Snail, in NCM460s
cells (Fig. 2E). These data
revealed that TNF-α induced EMT to promote the migration and
invasion capacities of NCM460s cells.
To further characterize the malignant transformation
of NCM460s cells, the effect of TNF-α on the proportion of
CD133+ cells was investigated using flow cytometry. A
significantly increased proportion of CD133+ cells was
revealed, from 11.40% in NCM460s cells to 28.00% in the
TNF-α-treated NCM460s cells, indicating that TNF-α induced the
phenotypic change from NCM460s cells to CSCs (Fig. 2F). Collectively, these results
indicated that TNF-α induced the malignant transformation of
NCM460s cells.
TNF-α induces inflammatory responses
and initiates activation of the NF-κB and Wnt/β-catenin signaling
pathways
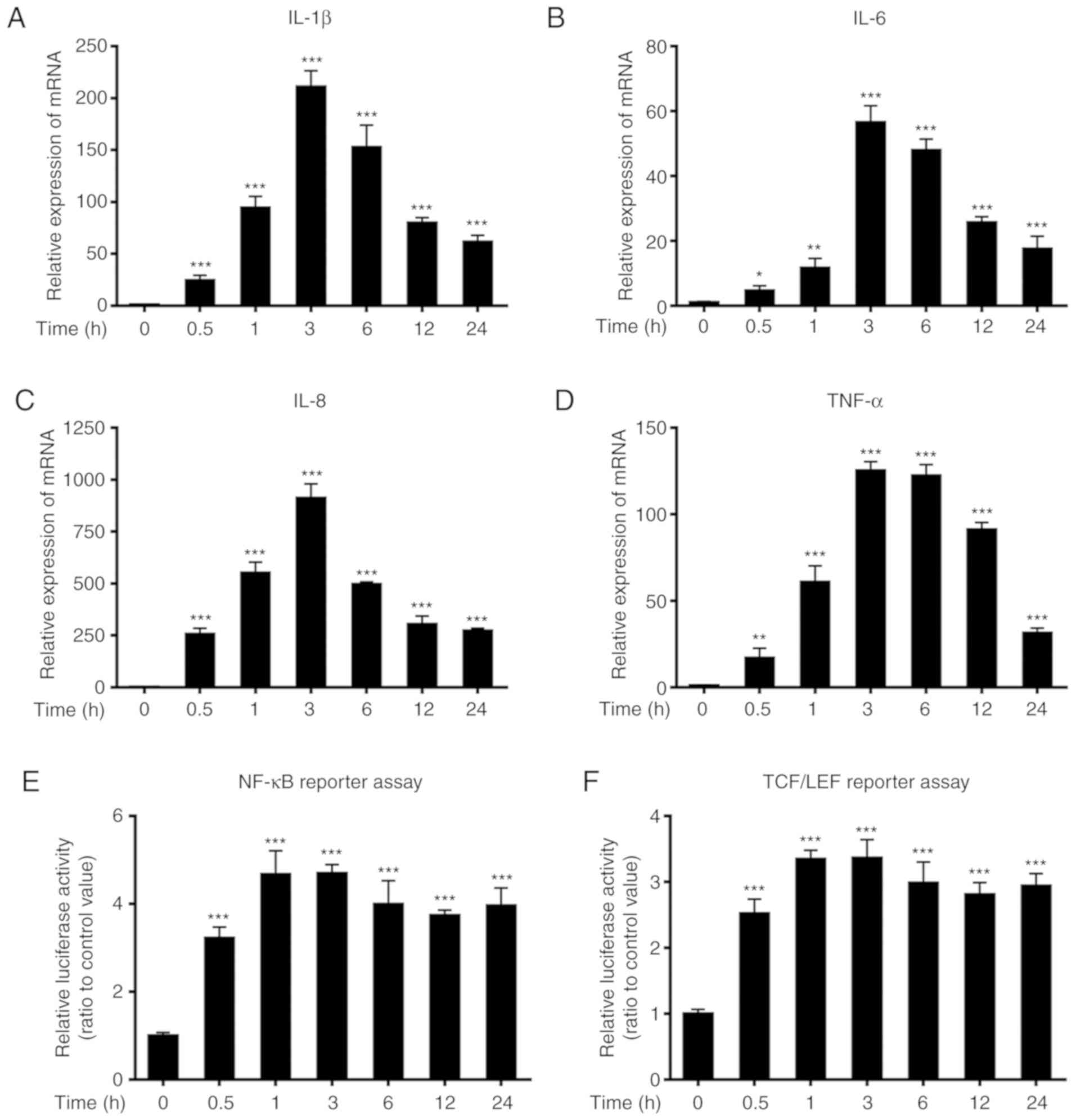
When NCM460s cells were exposed to 1 ng/ml TNF-α for
various time-points, the relative mRNA expression levels of the
proinflammatory cytokines interleukin (IL)-1β, IL-6, IL-8 and TNF-α
were significantly upregulated at 0.5 h after TNF-α stimulation
compared with those of the untreated control, and reached peak
levels ~3 h after stimulation (Fig.
3A-D). The promoter activities of the NF-κB and TCF/LEF
transcription factors were also significantly increased at 0.5 h
after TNF-α stimulation relative to those of the untreated control,
and reached peak levels 1–3 h after stimulation (Fig. 3E-F).
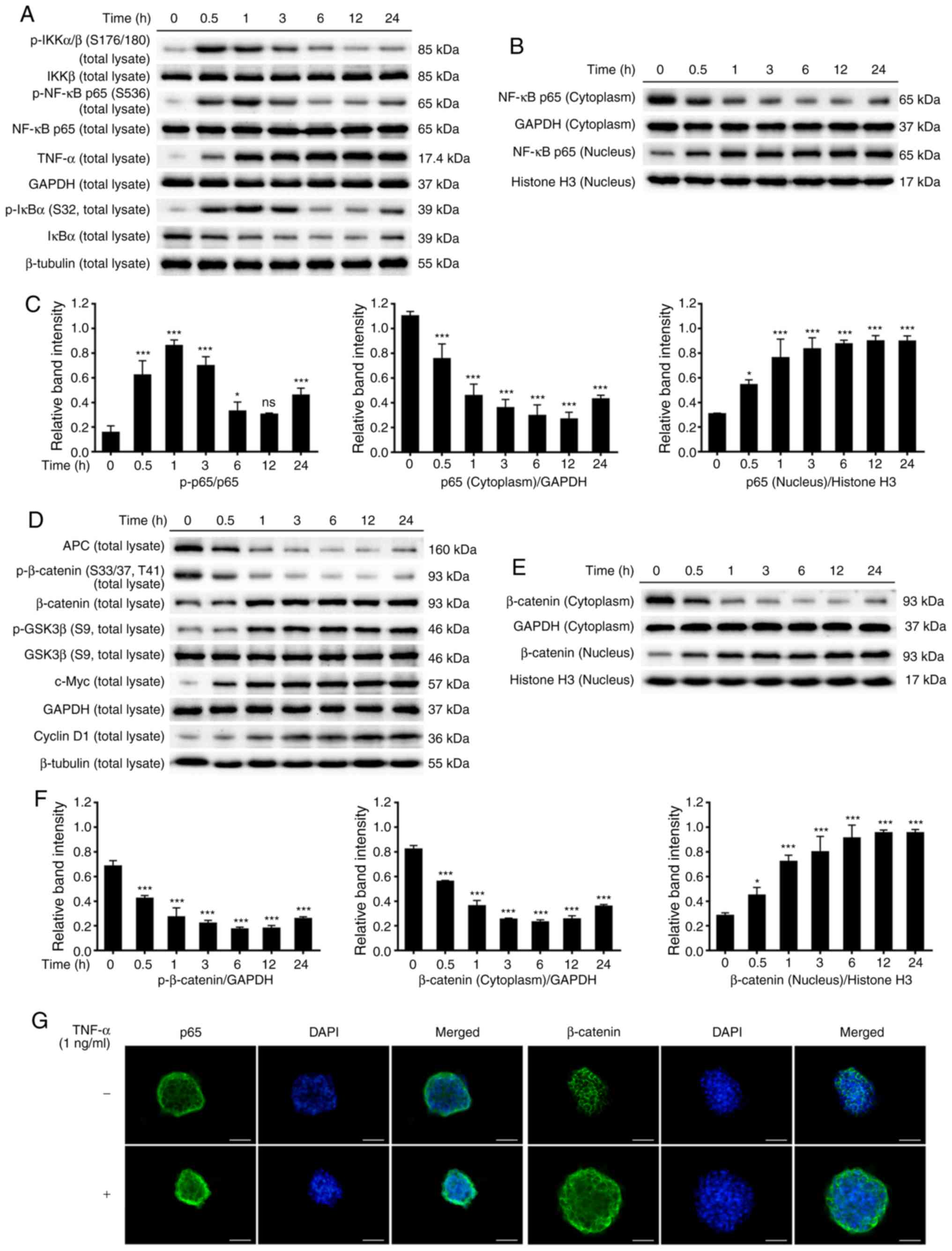
Phosphorylation of IκB kinase complex α/β (IKKα/β)
was increased at 0.5 h after TNF-α stimulation relative to the
untreated control. Moreover, the phosphorylation of IκBα was
upregulated however, the protein levels of IκBα were downregulated
at 0.5 h after stimulation relative to the untreated control, while
TNF-α levels were increased. p65 is an essential subunit of the
NF-κB complex and is key for the regulation of inflammatory
responses. TNF-α increased the phosphorylation of p65 and induced
the nuclear translocation of p65 (Fig.
4A-C).
Conversely, TNF-α decreased the protein levels of
APC, while increasing glycogen synthase kinase (GSK)3β
phosphorylation at 0.5–1 h after stimulation. Phosphorylation of
β-catenin was downregulated and the protein levels of total
β-catenin were upregulated at 0.5 h after stimulation. The
expression of β-catenin was markedly reduced in the cytoplasm but
gradually increased in the nucleus. In addition, the expression of
downstream Wnt target genes c-Myc and cyclin D1 were increased
after TNF-α stimulation (Fig.
4D-F).
To further elucidate the molecular mechanisms by
which TNF-α induced the activation of the NF-κB and Wnt/β-catenin
pathways, immunofluorescence staining was used to observe the
distribution of p65 and β-catenin in NCM460s cells. Exposure of
NCM460s cells to TNF-α for 24 h reduced the expression of p65 and
β-catenin in the cytoplasm but increased their expression in the
nucleus, indicating that TNF-α induced the translocation of p65 and
β-catenin from the cytoplasm to the nucleus (Fig. 4G). These findings demonstrated that
the NF-κB and Wnt/β-catenin pathways were activated in
TNF-α-induced inflammatory responses in NCM460s cells.
NF-κB and Wnt/β-catenin pathways
cross-regulate each other in TNF-α-induced NCM460s cells
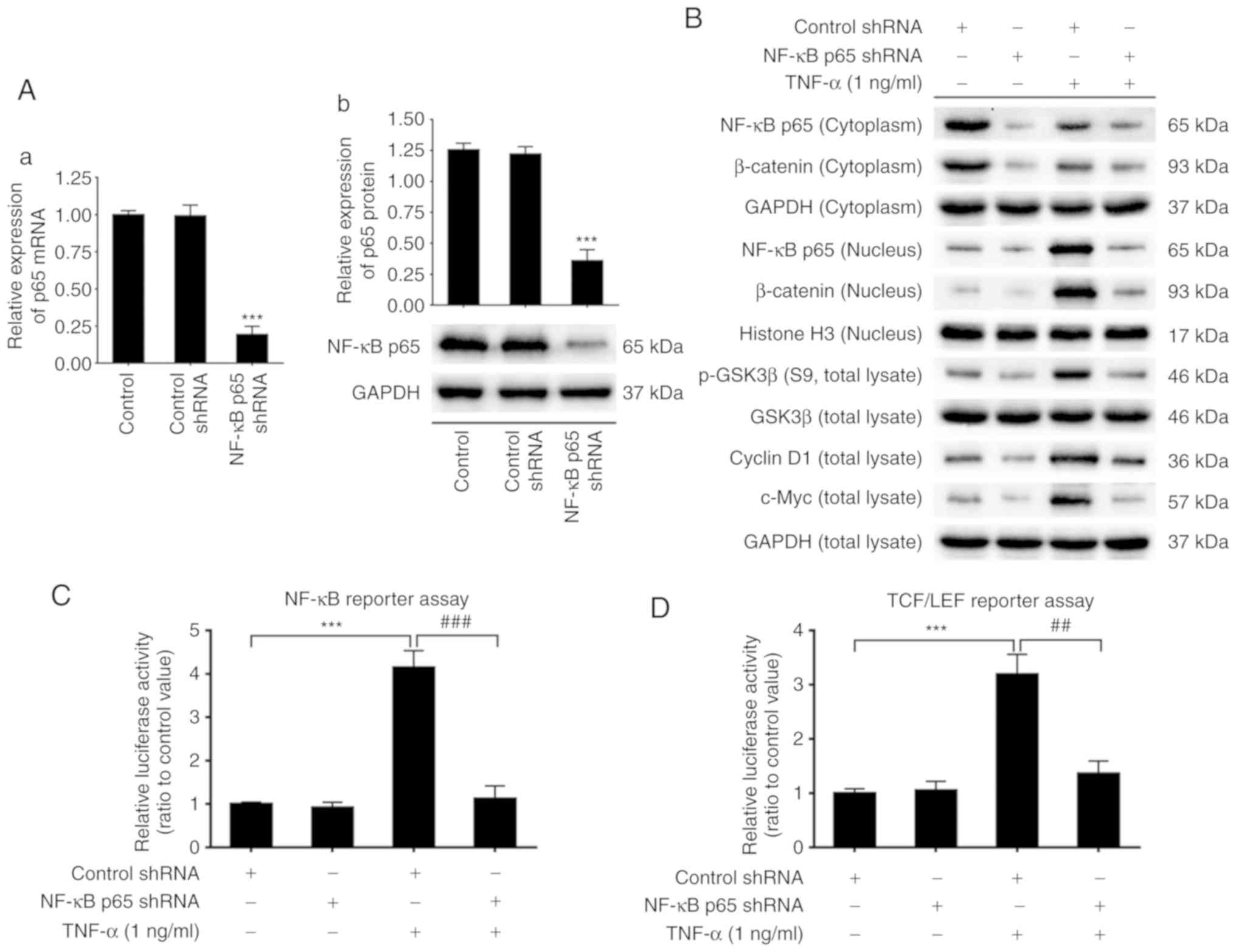
To elucidate the potential molecular mechanisms of
TNF-α-induced malignant transformation in NCM460s cells, p65 shRNA
was used to knockdown the activity of NF-κB signaling. The effect
of p65 shRNA transfection on p65 expression was evaluated using
qPCR and western blotting. The results revealed that the mRNA and
protein levels of total p65 were considerably suppressed by p65
shRNA transfection (Fig. 5A),
indicating that the p65 gene expression was successfully knocked
down.
Protein expression of p65 in the cytoplasm, which
was downregulated by TNF-α, remained at a low level by p65 shRNA.
Protein expression of p65 in the nucleus, which was upregulated by
TNF-α, was also downregulated by p65 shRNA (Fig. 5B). These results indicated that p65
knockdown blocked the protein expression of total p65 and
suppressed the nuclear translocation of p65, both of which were
promoted by TNF-α treatment. NF-κB promoter activity, which was
markedly increased by TNF-α, was also significantly downregulated
to almost basal levels by p65 shRNA (Fig. 5C). Notably, TNF-α-induced activation
of the Wnt/β-catenin pathway, including increased expression of
GSK3β phosphorylation, protein levels of c-Myc and cyclin D1 and
the translocation of β-catenin from the cytoplasm to the nucleus,
were all suppressed by p65 shRNA (Fig.
5B). TCF/LEF promoter activity, which was significantly
upregulated by TNF-α, was significantly suppressed to almost basal
levels by p65 shRNA (Fig. 5D).
Similarly, mRNA expression of proinflammatory cytokines IL-1β,
IL-6, IL-8 and TNF-α, which were significantly increased by TNF-α,
were also blocked by p65-knockdown (Fig. 5E-H).
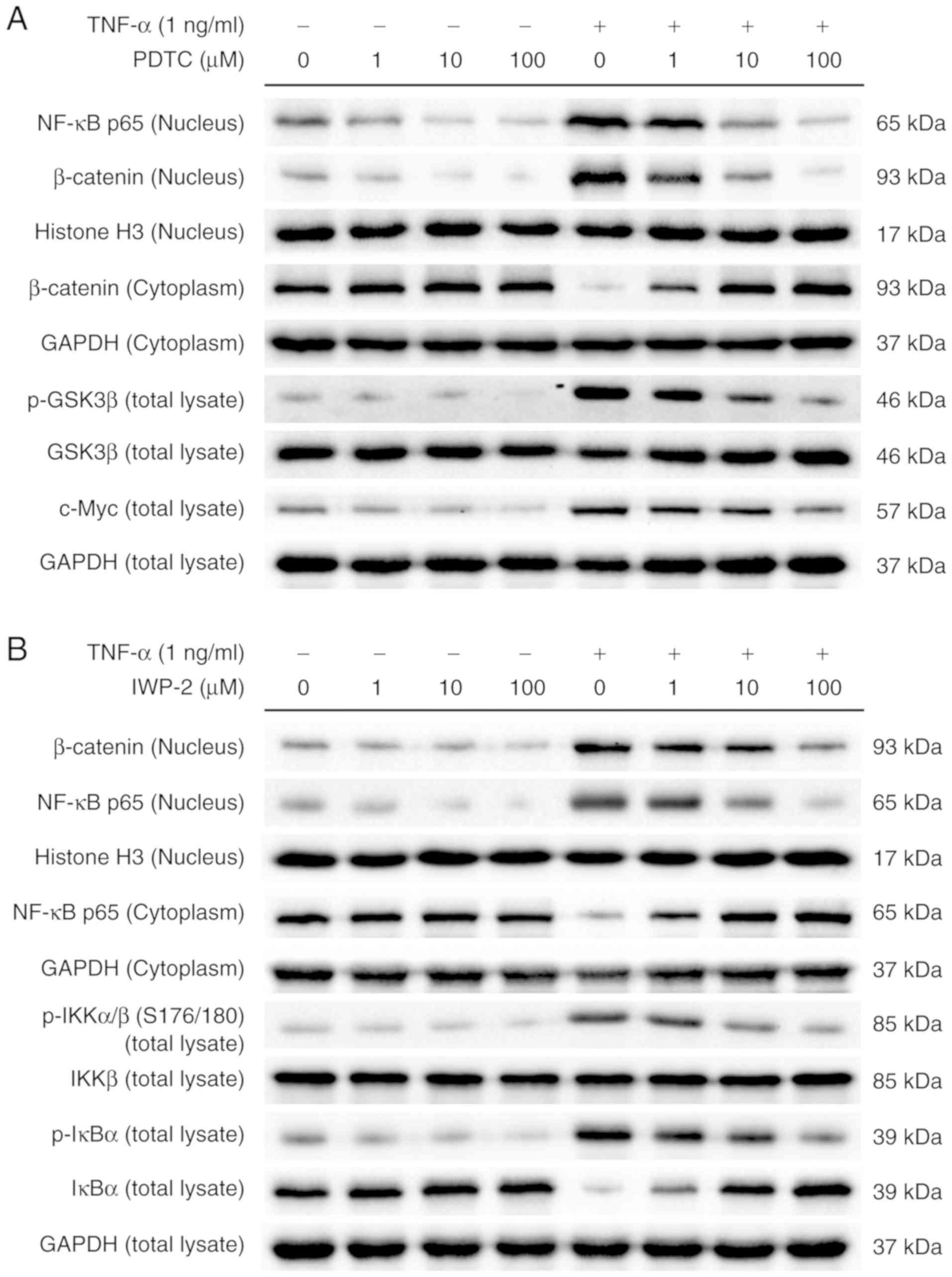
To further explore the molecular basis for the
cross-regulation between the NF-κB and Wnt/β-catenin pathways in
the TNF-α-induced malignant transformation of NCM460s cells, two
inhibitors were used to examine the protein levels of signaling
molecules associated with these two pathways. When NCM460s cells
were incubated with PDTC, an inhibitor of NF-κB signaling,
TNF-α-induced translocation of p65 from the cytoplasm to the
nucleus was reversed in a dose-dependent manner. Notably, PDTC
counteracted TNF-α-induced activation of the Wnt/β-catenin pathway
in NCM460s cells, including the increased phosphorylation of GSK3β
and β-catenin, as well as the increased protein levels of c-Myc and
nuclear translocation of β-catenin (Fig. 6A). Furthermore, as an inhibitor of
Wnt processing and secretion, IWP-2 prevented TNF-α-induced nuclear
translocation of β-catenin in a dose-dependent manner. Notably,
IWP-2 also counteracted TNF-α-induced activation of the NF-κB
pathway in NCM460s cells, including increased phosphorylation of
IKKα/β and IκBα, decreased protein levels of IκBα and nuclear
translocation of p65 (Fig. 6B).
Collectively, these results indicated that NF-κB and Wnt/β-catenin
pathways cross-regulated each other in TNF-α-induced NCM460s
cells.
Discussion
Stem cells are characterized by their self-renewal
capacity and potential for multilineage differentiation. Normal
stem cells serve a critical role in injury, disease and regular
maintenance. Unfortunately, genetic alterations of tumor-initiating
cells drive the acquisition of oncogenic mutations through the
interactions with abnormal environmental elements (12,16).
In most cases, one mutation is insufficient and at least four to
five genetic mutations are required for tumor initiation (25). It is imperative that oncogenic
mutations only accumulate in long-lived but quiescent stem cells
rather than in differentiated cells, which are rapidly eliminated
before the next mutation occurs (16,25).
Notably, dysregulation in the mechanisms underlying proliferation
and differentiation initiates the malignant transformation of
normal stem cells, leading to tumorigenesis (11). CSCs are responsible for tumor
initiation, progression, recurrence and metastasis, and can
differentiate into all cell types in cancer tissues (3,4). The
similar biological properties between normal stem cells and CSCs
are the basis of a recent hypothesis that CSCs originate from
normal stem cells. Furthermore, studies have demonstrated that ISCs
are the origin of intestinal neoplasia (8,12,13).
Barker et al (26) and Leung
et al (27), reported that
Lgr5-positive crypt base columnar cells, which are often located
adjacent to Paneth cells, could generate all epithelial lineages,
suggesting that Lgr5-positive crypt base columnar cells were stem
cells of the normal small intestine and colon. Using mouse models,
Lgr5-positive ISCs were identified as the origin cells of
intestinal neoplasia and were found to accelerate tumor progression
(13). Thus, ISCs are considered to
be the origin of intestinal CSCs and sustain tumorigenesis and
progression.
Recently, it was demonstrated that spheroid cells
derived from cancer cell lines in SFM under suspension conditions
had increased expression of stemness-associated genes (3,10,12,28).
As one effective strategy to enrich rare CSCs, serum-free
suspension culture has been widely used to isolate several types of
CSCs, including breast (6), ovarian
(11), colorectal (3,29) and
lung cancer (30). A number of
human colorectal cancer cell lines, including HT29, SW480, DLD-1
and HCT116, can form spheres under serum-free suspension culture
conditions (27,28). In this present study, NCM460 cells
formed spheres when cultured under SFM suspension conditions.
NCM460s cells exhibited higher mRNA and protein expression compared
with those in NCM460 adherent cells, such as stem cell genes Oct4,
Nanog, Sox2 and Lgr5, a marker gene of intestinal stem cells.
Furthermore, the proportion of CD133+ cells, a
colorectal CSC-specific surface marker, was significantly increased
in NCM460s cells compared with NCM460 adherent cells. Thus,
serum-free suspension culture is a highly efficient method to
enrich NCM460s cells with stem cell characteristics from the NCM460
normal intestinal epithelial cell line. In addition, other options
such as intestinal stem cells or organoids, which is the limitation
of the present study, but more convincing methods in the study on
CSCs, will be the next-step directions and methods to reveal the
cross-relationship of CSCs and tumorigenesis.
An increasing number of studies have demonstrated
that chronic inflammation plays an important role in tumorigenesis
and progression (14,20), especially in colorectal cancer
(16). Several inflammatory
cytokines are found in serum, stools and bowel mucosa of patients
with inflammatory bowel disease (31). Among these cytokines, TNF-α is a
potent inflammatory factor, which can activate NF-κB signaling,
upregulate the expression of other cytokines, chemokines, growth
factors and transcription factors and accelerate tumorigenesis
(31,32). Expression of TNF-α is notably
increased in various cancer types and is often correlated with poor
patient prognosis, thus TNF-α is regarded as a critical
pro-tumorigenic cytokine (16,32).
NF-κB activation induces innate and adaptive immune responses that
can influence the progression of cancer and inflammation (16). In line with these observations, the
present study reported that TNF-α treatment promoted cell
proliferation, migration and invasion, induced the EMT phenotype,
increased chemotherapy resistance and the ratio of
CD133+ cells in NCM460s cells, which are all
characteristics of malignant transformation. Certainly, it would be
preferable to study the effect of anti-TNF antibodies on the
indices, which is a potential limitation of the present study.
Furthermore, it was determined that TNF-α increased
the phosphorylation of IKK in NCM460s cells at 0.5 h after
stimulation, resulting in the phosphorylation and degradation of
IκBα and promoting the phosphorylation and nuclear translocation of
p65. As a crucial event of NF-κB signaling, the transcriptional
activity of the NF-κB p65 responsive promoter was also
significantly increased and maintained this increase up to 24 h
after stimulation. Subsequently, the expression of proinflammatory
cytokines IL-1β, IL-6, IL-8 and TNF-α were increased after
stimulation, thus establishing a positive feedback loop that
further enhanced activation of the NF-κB pathway and induced the
expression of proinflammatory cytokines. Moreover, it was
demonstrated that, in NCM460s cells, TNF-α also initiated the
sequential activation of Wnt/β-catenin signaling, which serves a
critical role in tumorigenesis and progression (33). The protein levels of APC, a negative
modulator of the Wnt/β-catenin pathway, were downregulated 0.5 h
after stimulation, while GSK3β phosphorylation, another negative
modulator, was upregulated. Then, the dephosphorylation,
accumulation and nuclear translocation of β-catenin, a central
mediator in the canonical Wnt/β-catenin cascade, was observed in
TNF-α-induced NCM460s cells. The luciferase reporter assay revealed
that TNF-α stimulation significantly increased the promoter
activity of TCF/LEF transcription factors in NCM460s cells. Thus,
the data revealed that TNF-α induced the activation of NF-κB and
Wnt/β-catenin pathways in NCM460s cells.
It has been demonstrated that activation of the
Wnt/β-catenin and NF-κB pathways upregulates the expression of
individual target genes through independent cascades during
tumorigenesis, modulating cell proliferation, cell survival,
apoptosis, metastasis and differentiation (14). In addition to these shared
functions, NF-κB signaling is more crucial for inflammation and
immune responses, whereas the Wnt/β-catenin pathway is mainly
responsible for development and tissue regeneration (16). However, studies have suggested that
these two pathways cross-regulate each other, extending their
functions for each individual pathway. NF-κB signaling influences
the activity of Wnt/β-catenin pathway, and the Wnt/β-catenin
pathway also regulates inflammatory responses via interaction with
NF-κB signaling (19,34).
Schwitalla et al (35), revealed that NF-κB signaling played
a crucial role in the expression of Wnt/β-catenin and the
intestinal epithelial cell-specific ablation of p65 suppressed the
expansion of crypt stem cells. Furthermore, upregulated NF-κB
signaling promoted the activation of the Wnt/β-catenin pathway and
induced dedifferentiation of non-stem cells that acquired
tumor-initiating capacity. Jang et al (34), revealed that Wnt/β-catenin signaling
modulated the NF-κB pathway in TNF-α-induced inflammatory
responses. In addition, NF-κB signaling can activate the expression
of mesenchymal markers N-cadherin, vimentin, Slug and Snail, which
downregulates the expression of adhesion protein E-cadherin,
leading to EMT (16). Furthermore,
nuclear β-catenin established a TCF/LEF/β-catenin complex via the
interaction with TCF/LEF transcription factors, which also induces
the EMT phenotype (33,36). Concurrently, the TCF/LEF/β-catenin
complex has been revealed to promote the expression of downstream
Wnt target genes c-Myc and cyclin D1 in TNF-α-induced
NCM460s cells (19). Other studies
have revealed that NF-κB activation upregulates the expression of
various cell cycle proteins, especially the Wnt signaling target
gene cyclin D1 (35,37,38).
Crosstalk between Wnt/β-catenin and NF-κB signaling has been
revealed to play a crucial role in inflammation-induced
tumorigenesis (19).
Notably, the present study also revealed that there
was a crosstalk between NF-κB and Wnt/β-catenin signaling in
TNF-α-induced inflammatory responses. The nuclear protein levels of
NF-κB p65 and the transcriptional activity of NF-κB p65 responsive
promoter, which were markedly increased by TNF-α, were both blocked
by p65-knockdown. Then, mRNA expression levels of proinflammatory
cytokines IL-1β, IL-6, IL-8 and TNF-α, which were significantly
upregulated by TNF-α, were also reduced to almost basal levels by
p65-knockdown in TNF-α-treated NCM460s cells. GSK3β phosphorylation
and nuclear protein levels of β-catenin were reduced by
p65-knockdown in TNF-α-treated NCM460s cells. Subsequently, TCF/LEF
promoter activity and expression of Wnt target genes c-Myc
and cyclin D1 were also suppressed by p65 knockdown. Furthermore,
PDTC, an inhibitor of NF-κB signaling, reversed the activation of
the NF-κB pathway in a dose-dependent manner in TNF-α-treated
NCM460s cells and counteracted TNF-α-induced activation of the
Wnt/β-catenin pathway. The same effect on the NF-κB pathway was
found following Wnt/β-catenin inhibitor IWP-2 application to
TNF-α-treated NCM460s cells. Therefore, these results indicated
that NF-κB and Wnt/β-catenin signaling cross-regulate each other in
TNF-α-induced malignant transformation of ISCs.
The present study demonstrated that serum-free
suspension culture is an effective strategy to enrich spheroid
cells with stem cell characteristics in normal intestinal
epithelial cell lines. The NF-κB and Wnt/β-catenin signaling
pathways were activated to promote the malignant transformation of
ISCs in TNF-α-induced inflammatory responses, in which these two
pathways cross-regulated each other. These findings may aid our
understanding of the underlying molecular mechanisms in intestinal
tumorigenesis and progression, and provide new insight to develop
more specific and effective treatments against
inflammation-associated cancer types.
Supplementary Material
Supporting Data
Acknowledgements
The authors greatly appreciate and thank the
colleagues at the Key Laboratory of Fertility Preservation and
Maintenance, Ministry of Education, of Ningxia Medical
University.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31660336) and West
China first-class Disciplines Basic Medical Sciences at Ningxia
Medical University (grant no. NXYLXK2017B07).
Availability of data and materials
All data used during this study are available from
the corresponding authors upon reasonable request. Supplementary
files related to this article can be found in the online
version.
Authors' contributions
XZ and LD were responsible for the acquisition and
analysis of all the experimental data, as well as the writing of
the paper. LM, XL and FX participated in the study design. DZ and
HZ were involved in the data interpretation and the final revision
of the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
ISCs
|
intestinal stem cells
|
|
SFM
|
serum-free medium
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olejniczak A, Szarynska M and Kmiec Z: In
vitro characterization of spheres derived from colorectal cancer
cell lines. Int J Oncol. 52:599–612. 2018.PubMed/NCBI
|
|
4
|
Zhao H, Yan C, Hu Y, Mu L, Huang K, Li Q,
Li X, Tao D and Qin J: Sphere-forming assay vs. organoid culture:
Determining long-term stemness and the chemoresistant capacity of
primary colorectal cancer cells. Int J Oncol. 54:893–904.
2019.PubMed/NCBI
|
|
5
|
Shah S, Pocard M and Mirshahi M: Targeting
the differentiation of gastric cancer cells (KATOIII) downregulates
epithelial-mesenchymal and cancer stem cell markers. Oncol Rep.
42:670–678. 2019.PubMed/NCBI
|
|
6
|
Zhu R, Gires O, Zhu L, Liu J, Li J, Yang
H, Ju G, Huang J, Ge W, Chen Y, et al: TSPAN8 promotes cancer cell
stemness via activation of sonic Hedgehog signaling. Nat Commun.
10:28632019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Celia-Terrassa T, Liu DD, Choudhury A,
Hang X, Wei Y, Zamalloa J, Alfaro-Aco R, Chakrabarti R, Jiang YZ,
Koh BI, et al: Normal and cancerous mammary stem cells evade
interferon-induced constraint through the miR-199a-LCOR axis. Nat
Cell Biol. 19:711–723. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cable J, Fuchs E, Weissman I, Jasper H,
Glass D, Rando TA, Blau H, Debnath S, Oliva A, Park S, et al: Adult
stem cells and regenerative medicine-a symposium report. Ann N Y
Acad Sci. 1462:27–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji R, Zhang X, Qian H, Gu H, Sun Z, Mao F,
Yan Y, Chen J, Liang Z and Xu W: miR-374 mediates the malignant
transformation of gastric cancer-associated mesenchymal stem cells
in an experimental rat model. Oncol Rep. 38:1473–1481. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastrana E, Silva-Vargas V and Doetsch F:
Eyes wide open: A critical review of sphere-formation as an assay
for stem cells. Cell Stem Cell. 8:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li SC, Lee KL, Luo J, Zhong JF and Loudon
WG: Convergence of normal stem cell and cancer stem cell
developmental stage: Implication for differential therapies. World
J Stem Cells. 3:83–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drost J, van Jaarsveld RH, Ponsioen B,
Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus
GJ, Begthel H, et al: Sequential cancer mutations in cultured human
intestinal stem cells. Nature. 521:43–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barker N, Ridgway RA, van Es JH, van de
Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR,
Sansom OJ and Clevers H: Crypt stem cells as the cells-of-origin of
intestinal cancer. Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shigdar S, Li Y, Bhattacharya S, O'Connor
M, Pu C, Lin J, Wang T, Xiang D, Kong L, Wei MQ, et al:
Inflammation and cancer stem cells. Cancer Lett. 345:271–278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang F, Wang Y, Hemmings BA, Rüegg C and
Xue G: PKB/Akt-dependent regulation of inflammation in cancer.
Semin Cancer Biol. 48:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zanotto-Filho A, Rajamanickam S, Loranc E,
Masamsetti VP, Gorthi A, Romero JC, Tonapi S, Goncalves RM, Reddick
RL, Benavides R, et al: Sorafenib improves alkylating therapy by
blocking induced inflammation, invasion and angiogenesis in breast
cancer cells. Cancer Lett. 425:101–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bloemendaal AL, Buchs NC, George BD and
Guy RJ: Intestinal stem cells and intestinal homeostasis in health
and in inflammation: A review. Surgery. 159:1237–1248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma B and Hottiger MO: Crosstalk between
Wnt/β-catenin and NF-κB signaling pathway during inflammation.
Front Immunol. 7:3782016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakiuchi N, Yoshida K, Uchino M, Kihara T,
Akaki K, Inoue Y, Kawada K, Nagayama S, Yokoyama A, Yamamoto S, et
al: Frequent mutations that converge on the NFKBIZ pathway in
ulcerative colitis. Nature. 577:1–6. 2020. View Article : Google Scholar
|
|
21
|
Kong FF, Li D, Yang H, Ma J, Pan X, Liu
HX, Huo JN and Ma XX: Preliminary identification of endometrial
cancer stem cells in vitro and in vivo. Biochem Biophys Res Commun.
490:506–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhai Y, Wei R, Sha S, Lin C, Wang H, Jiang
X and Liu G: Effect of NELL1 on lung cancer stemlike cell
differentiation. Oncol Rep. 41:1817–1826. 2019.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Yun CW, Han YS, Kim S, Jeong D,
Kwon HY, Kim H, Baek MJ and Lee SH: Melatonin and 5-fluorouracil
co-suppress colon cancer stem cells by regulating cellular prion
protein-Oct4 axis. J Pineal Res. 65:e125192018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ, et al: Identification of stem cells in small intestine
and colon by marker gene Lgr5. Nature. 449:1003–1007. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung C, Tan SH and Barker N: Recent
advances in Lgr5(+) stem cell research. Trends Cell Biol.
28:380–391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arab-Bafrani Z, Shahbazi-Gahrouei D,
Abbasian M, Saberi A, Fesharaki M, Hejazi SH and Manshaee S:
Culturing in serum-free culture medium on collagen type-I-coated
plate increases expression of CD133 and retains original phenotype
of HT-29 cancer stem cell. Adv Biomed Res. 5:592016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leng Z, Tao K, Xia Q, Tan J, Yue Z, Chen
J, Xi H, Li J and Zheng H: Kruppel-like factor 4 acts as an
oncogene in colon cancer stem cell-enriched spheroid cells. PLoS
One. 8:e560822013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu F, Li C, Zheng X, Zhang H, Shen Y, Zhou
L, Yang X, Han B and Zhang X: Lung adenocarcinoma resistance to
therapy with EGFR-tyrosine kinase inhibitors is related to
increased expression of cancer stem cell markers SOX2, OCT4 and
NANOG. Oncol Rep. 43:727–735. 2020.PubMed/NCBI
|
|
31
|
Chen X, Liu R, Liu X, Xu C and Wang X:
Protective role of Coxsackie-adenovirus receptor in the
pathogenesis of inflammatory bowel diseases. Biomed Res Int.
2018:72072682018.PubMed/NCBI
|
|
32
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kretzschmar K and Clevers H: Wnt/β-catenin
signaling in adult mammalian epithelial stem cells. Dev Biol.
428:273–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang J, Jung Y, Chae S, Chung SI, Kim SM
and Yoon Y: WNT/β-catenin pathway modulates the TNF-α-induced
inflammatory response in bronchial epithelial cells. Biochem
Biophys Res Commun. 484:442–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwitalla S, Fingerle AA, Cammareri P,
Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ,
Moreaux G, et al: Intestinal tumorigenesis initiated by
dedifferentiation and acquisition of stem-cell-like properties.
Cell. 152:25–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teo JL and Kahn M: The Wnt signaling
pathway in cellular proliferation and differentiation: A tale of
two coactivators. Adv Drug Deliv Rev. 62:1149–1155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ledoux AC and Perkins ND: NF-κB and the
cell cycle. Biochem Soc Trans. 42:76–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Chen H, Hou Y, Ma X, Ye M, Huang R,
Hu B, Cao H, Xu L, Liu M, et al: Adaptive EGF expression sensitizes
pancreatic cancer cells to ionizing radiation through activation of
the cyclin D1/P53/PARP pathway. Int J Oncol. 54:1466–1480.
2019.PubMed/NCBI
|