Introduction
Gastric cancer was the fifth most prevalent
malignancy in 2018 globally with an estimated 1 million new cases.
Korea has been listed first among the global GC cases, with stomach
cancer incidence rates in men of approximately 58 per 100,000 and
in women of 24 per every 100,000, which is an unfortunate reality
for many Koreans (1). Epigenetic
alterations, aberrant molecular signaling pathways, and multiple
genetic mutations are engaged in the development of GC (2). Substantial advances in diagnostic
techniques, chemotherapy and surgical approaches, treatment by
multidisciplinary teams and the advancement of novel therapeutic
agents have shown minor improvement in survival rate in recent
times (3). If we can identify the
biomarkers of drug treatment responses and their efficacy, the
unnecessary burden of adverse effects and noticeable toxicity from
chemotherapy on GC patients unlikely to respond can be averted
(4). It is imperative to identify
the specific biomarkers and unique molecular patterns of the tumor
to develop treatments that target the distinct tumor behavior. In
the standard treatment of GC, a significant number of novel
anticancer agents targeting dysfunctional molecular signaling
pathways has been reported already, whereas others remain elusive
(5,6). The global proteomic approach is one of
the computational technologies that have been improved in recent
times over many other complementary techniques that briskly change
our approach to cancer research (7). This empowers scientists to screen
substantial numbers of proteins within clinical discrete samples,
as well as different cell lines treated with specific drugs that
help to analyze and confirm drug targets, find new disease
biomarkers, design more adequate drugs, and estimate drug efficacy
(8). The conventional proteomics
approach was achieved by implementing a combination of 2-DE coupled
with differential image analysis and protein identification using
mass spectrometry (MALDI-TOF; matrix-assisted laser desorption/
ionization-time of flight) and bioinformatics to predominantly
identify and characterize proteins in the tissues and cells from
both in-vitro and in-vivo models (8,9).
Protein network, functional interpretation, and pathway analysis
tools can help to address the difficulties in the illustration of
the obtained proteomics data. To identify the activated pathway
element of functional proteomic data, the analysis of proteomic
data at the pathway level has become universally popular (10). The comparative proteomic analysis
could persuade the molecular characterization of cellular events
correlated with cancer developmental, signaling, and progression
phases that leads to the discovery of cancer-specific protein
markers, which provides the basis for understanding cancer
progression, carcinogenesis and targets of protein molecules for
anticancer agents (11).
Herbal products and their components have been
identified as exhibiting anticancer effects by targeting
dysregulated genes that contribute to carcinogenesis in several
cancer cell lines by multiple cell signaling pathways (12,13).
Flavonoids are natural polyphenolic compounds that are abundantly
present in plant parts, especially in leaves and fruits, and
previous studies have demonstrated several anticancer effects by
regulating multiple cellular mechanisms such as the PI3K/AKT/mTOR
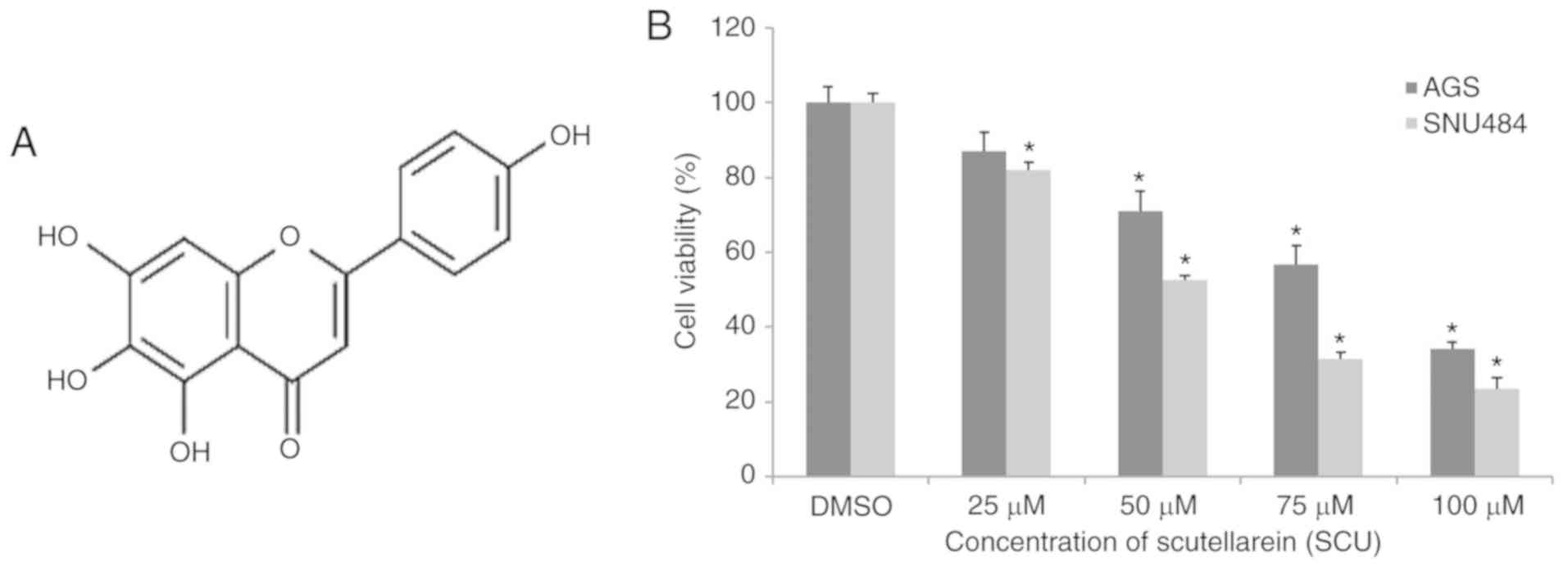
signaling pathway (13,14). Fig.
1A shows Scutellarein (SCU), a flavone, which belongs to the
family of flavonoids, that are abundantly present in perpetual
herbs, such as Scutellaria barbata and Scutellaria
baicalensis. Studies have demonstrated that SCU monomers, as
well as SCU-containing flavonoid extracts, cover an immense range
of biological applications, including anti-inflammatory,
antioxidant, and anticancer activity, by regulating different
biological activities (15). Our
previous study demonstrated that SCU induces apoptotic cell death
and inhibits cell proliferation via downregulation of MDM2 protein
expression which in turns activated the tumor-suppressor protein
p53; and the family of inhibitors of apoptosis proteins (cIAP1,
cIAP2, and XIAP) were downregulated leading to caspase-dependent
apoptosis in human GC cells (16).
However, despite elucidation of the anticancer effect of SCU on GC
cells, the global proteomic changes that encompass the cellular
response to this compound have remained elusive. In the present
study, we aimed to identify the novel protein biomarkers and
characterize the protein alterations that can predict the treatment
efficacy of SCU in GC cells. The comparative proteomic analysis may
facilitate future molecular research on the anticancer effects of
flavonoids.
Materials and methods
Cell lines, reagents and
antibodies
Human GC cell lines, AGS and SNU484, were procured
from the Korea Cell Line Bank (Seoul, Korea). Antibiotics
(penicillin/streptomycin), fetal bovine serum (FBS) and RPMI-1640
growth medium were purchased from Gibco, Thermo Fisher Scientific,
Inc. Compound Scutellarein was procured from Chengdu Biopurify
Phytochemicals Ltd. (product no. 611130). Chemicals and materials
utilized for electrophoresis were procured from Bio-Rad
Laboratories, Inc.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Duchefa Biochemie. Antibodies for CIP2A
(#148053) and PI3KCB (#3011S) were purchased from Cell Signaling
Technology, Inc. OFD1 (#ab222837), VCL (#ab129002), and HIP1R
(#ab226197) antibodies were purchased from Abcam. The β-actin
(#MABT825) antibody was purchased from Millipore.
Treatment of SCU and determination of
cell viability
Mycoplasma-free AGS and SNU484 cells were cultured
in RPMI-1640 medium containing 10% FBS (heat activated) and 1%
antibiotics (penicillin/streptomycin) in an incubator with 5%
CO2 at 37°C in a humidified condition. To confirm
mycoplasma contamination, we used the e-Myco™ Mycoplasma PCR
Detection kit (iNtRON Biotechnology). Cell viability assay was
performed using MTT assay. The cells were at a density of
5×105 cells per well in 24-well plates and grown
overnight. Subsequently treat the cells were treated with the
indicated concentrations of SCU (0, 25, 50, 75, and 100 µM) for 24
h at 37°C. An amount 50 µl of MTT solution (0.5 mg/ml) was added to
each well after 24 h, and incubation was carried out at 37°C for 3
h in a dark condition. DMSO (300 µl) was added to each well to
solubilize the formazan contained in the cells, and the absorbance
was measured at 540 nm using a microplate reader (BioTek
Instruments). Cell viability was expressed as a percentage of the
control and experiments were conducted in triplicates for each
assay condition.
Protein sample preparation for 2-DE
analysis
AGS and SNU484 cells were treated or untreated
(control) with SCU (75 µM) for 24 h. Whole proteins were extracted
from both groups of cells as previously described (17). Briefly, after incubation time, the
cells were harvested and lysed with lysis buffer on ice for 1 h.
The cell lysates were centrifuged at 20,000 × g at 4°C for 15 min
and the supernatants were collected. Proteins were precipitated and
extracted using the TCA precipitation method, followed by
dissolving the lyophilized protein with 200 µl of sample buffer for
further analysis. The proteins were quantified according to the
manufacturer's instructions using a BCA protein assay (Pierce™;
Thermo Fisher Scientific, Inc.), and the protein samples were
stored in a −80°C freezer.
Two-dimensional gel
electrophoresis
Proteins were separated using 2-DE, as reported in
our previous study (17). Briefly,
400 µg of protein per sample (equal quantity) were immobilized onto
18 cm (pH 4–7) DryStrip Gels (GE Healthcare Immobiline™; Amersham
Biosciences) for the first dimensional electrophoresis (isoelectric
focusing: IEF) using Ettan DALT II system (Amersham Biosciences).
After the isoelectric focusing, the strips were equilibrated twice;
first time with equilibration buffer containing 10 mg/ml
dithiothreitol (DTT) and the second time with equilibration buffer
containing 40 mg/ml iodoacetamide (IAA) for 15 min each. Proteins
in equilibrated strips were then separated, depending upon the
molecular weight, with the second dimension on 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Silver
nitrate staining methods were implemented as reported previously
(18) with slight changes, and gels
were prepared in triplicates for each assay condition. The
silver-stained gels were scanned for image analysis, and Progenesis
Samespots software version 4.0 Nonlinear Dynamics Ltd.) was used to
perform spot density-based image analysis. Those spots were
considered for further analysis depending on the difference in the
spot intensities with fold-change ≥1.5 and statistical significance
of P<0.05 in SCU-treated AGS and SNU484 cells, compared with the
untreated (DMSO) groups.
Trypsin digestion and mass
spectrometry analysis
Differentially expressed protein spots from the 2-DE
gel were excised manually, and digestion of protein and MS analysis
were performed as previously reported (19) with slight changes. Briefly, the
selected gel spots were trypsin digested (Promega Corp.) for mass
spectrometry analysis with 10–20 µl on ice for 45 min. After
incubation on ice, 10–20 µl of trypsin digestion buffer without
trypsin was added, and digestion was carried out overnight at 37°C.
Proteins were extracted by adding 10–20 µl of the extraction buffer
and incubated for 30 min at room temperature (RT) twice, and the
solution was collected onto siliconized e-tubes. Collected extracts
were lyophilized in a vacuum lyophilizer, and the pellets were
re-suspended in a mixture of 1 µl of extraction buffer and 1 µl of
matrix solution containing α-acyano-4-hydroxycinnamic acid (HCCA).
The suspension of matrix solution containing protein was targeted
onto a MALDI-TOF plate, and the targeted spots were read by mass
spectrometer (Voyager-DE STR; Applied Biosystems), supplied with
delay ion extraction. Mass spectra were selected over a mass range
of ≥3,000 Da.
Identification of protein from the MS
data
To identify the proteins from the MS data, the
peptide protein mass fingerprinting data were adopted to search
against NCBI non-redundant protein database using the
ProteinProspector (v 5.22.1) (http://www.prospector.ucsf.edu) peptide mass
fingerprinting (PMF) data (20),
and the SwissProt database (SwissPort.2017.11.01) was implemented
to find the matching proteins, as defined previously 38 (17). Database search was performed using
the following parameters: Homo sapiens (human) was used in
terms of Taxonomy, trypsin with 1 missed cleavage permitted was
used for digest specificity, peptide tolerance of less than 100 ppm
was used for fragment ions, carbamidomethyl (C) was used with fixed
modifications and oxidation (M) was used as a variable
modification. Protein MOWSE scores (P<0.05) were considered
statically significant.
Protein validation by
immunoblotting
For western blotting, both cell lines were cultured
in 6-well plates at 3×106 cells per well and after the
cells reached optimal confluence, both the cell lines were treated
with SCU (75 µM) or untreated (DMSO) for 24 h. Cells were harvested
after incubation, and lysed in ice-cold RIPA buffer containing
protease and phosphatase inhibitor. Total proteins were quantified
using BCA protein assay and 15 µg of proteins from each group were
separated by 10–12% SDS-PAGE, and the protein bands were
transferred onto a polyvinylidene difluoride (PVDF) membrane. The
membranes were blocked with 5% non-fat skim milk or BSA in
Tris-buffered saline containing 1% Tween 20 (TBS-T, pH 7.4) at room
temperature (RT) for 1 h, and incubated overnight at 4°C at a
1:1,000 dilution of the respected primary antibody. The membranes
were washed five times with TBS-T for 10 min each at RT, and
incubated with a 1:2,000 dilution of HRP-conjugated secondary
antibody for 3 h at RT. The membranes were then rewashed five times
with TBS-T. Blots were developed using the ECL detection system (GE
Healthcare Life Science). The bands were quantitatively analyzed
using the ImageJ software version 1.52a (National Institutes of
Health) (http://rsb.info.nih.gov). The
densitometry readings of the bands were normalized according to
β-actin expression.
Molecular docking analysis
In the present study, the binding affinity of
Scutellarein was evaluated through macromolecular docking studies
using Glide of Schrödinger-Maestro v.8.5 (in silico analysis)
(21). Initially, the structure of
the Scutellarein molecule was obtained and energy was minimized.
The 3D structure of protein targets was downloaded from the PDB
database (https://www.rcsb.org/). During docking,
the receptor grids were determined for processed proteins, such
that various ligand poses bind within the anticipated active site.
Final scoring was performed on energy-minimized poses and displayed
as Glide score. The best ligand binding pose with the least
Glide/IFD score or energy was chosen. For each ligand, the
best-docked pose with lowest Glide score value was recorded.
Binding energy was calculated by implementing Schrödinger Prime
based on molecular mechanics generalized born surface area
(MM-GBSA). The interacting amino acids of the protein with the
structure of SCU were viewed by the ligand-interaction diagram
constructed by LigPlot.
Gene Ontology (GO) and pathway
enrichment analysis
SwissProt identified proteins were further submitted
to Web Gestalt (http://www.webgestalt.org), to find the GO annotations
of the acquired differential proteins expressed in AGS and SNU
cells treated with SCU independently. Emerging annotation were
encapsulated based on the GOSlim set enrichments using a GOSlim
Viewer to allocate their biological process, cellular component,
and molecular function. The biological process identified from Web
Gestalt was further studied individually for each protein
annotations. A web-based tool GeneCodis (http://genecodis.cnb.csic.es) was used for comparative
protein analysis between AGS and SNU484 cells treated with SCU
(22). The significantly identified
proteins were subjected to pathway analysis by utilizing the
PANTHER (Protein Analysis Through Evolutionary Relationships,
version 9.0) database (http://www.pantherdb.org) in both the cell lines
(23). STRING database (https://string-db.org) was used to identify
protein-protein interaction among the differentially expressed
protein as well as for the construction of individual protein
clusters of commonly expressed proteins (24).
Statistical analysis
Statistical analysis was performed with the
Student's t-test using SPSS version 10.0 for Windows (SPSS, Inc.)
and a one-way ANOVA test was implemented followed by Tukey's test
for the comparison of multiple independent variables. A fold-change
≥1.5 and statistical significance of P<0.05 were considered for
the selection of differential expressed protein spots. Data were
considered statistically significant at P<0.05. All the results
are expressed as the mean ± standard deviation (SD) of
triplicates.
Results
Scutellarein (SCU) inhibits the cell
viability of GC cells
In our previous study, we reported that SCU was able
to inhibit the cell viability of AGS and SNU484 human GC cells via
inducing apoptotic cell death (16). Cell viability assay was performed
after treatment with different concentrations of SCU (0, 25, 50, 75
and 100 µM) for 24 h, as shown in Fig.
1B in AGS and SNU484 cells using MTT assay. SCU significantly
inhibited the cell viability in a dose-dependent manner in both
cell lines, when compared with the untreated control (DMSO only)
group of cells. The IC50 (50% inhibitory concentration)
value obtained for AGS cells was 62.88 µM and for SNU484 cells was
59.45 µM. Henceforth for the subsequent experiments, we used 0
(control) and 75 µM (Test) (most effective concentration) of SCU on
both the GC cell lines.
Protein identification by 2-DE and
MALDI/TOF-MS analysis
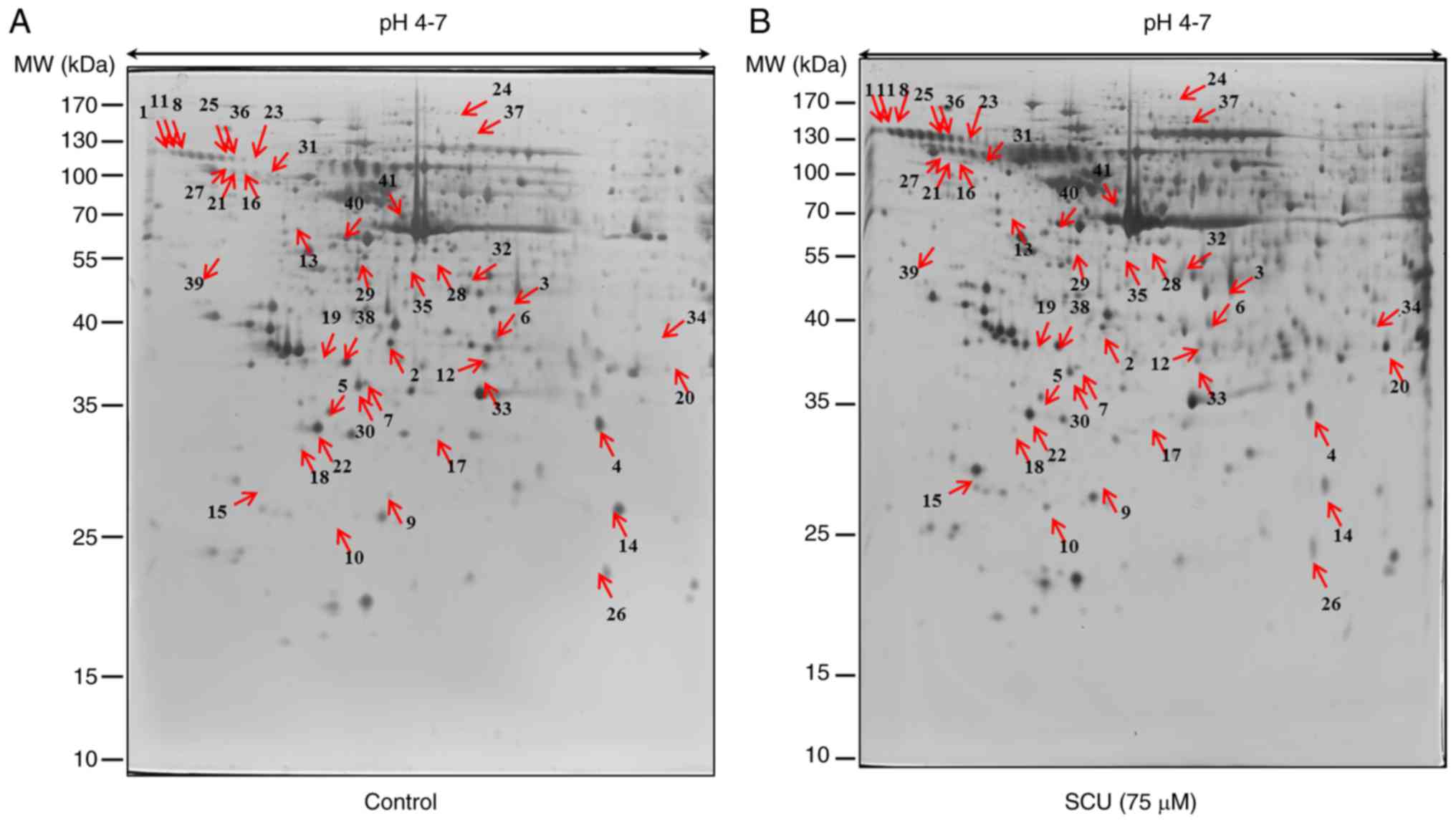
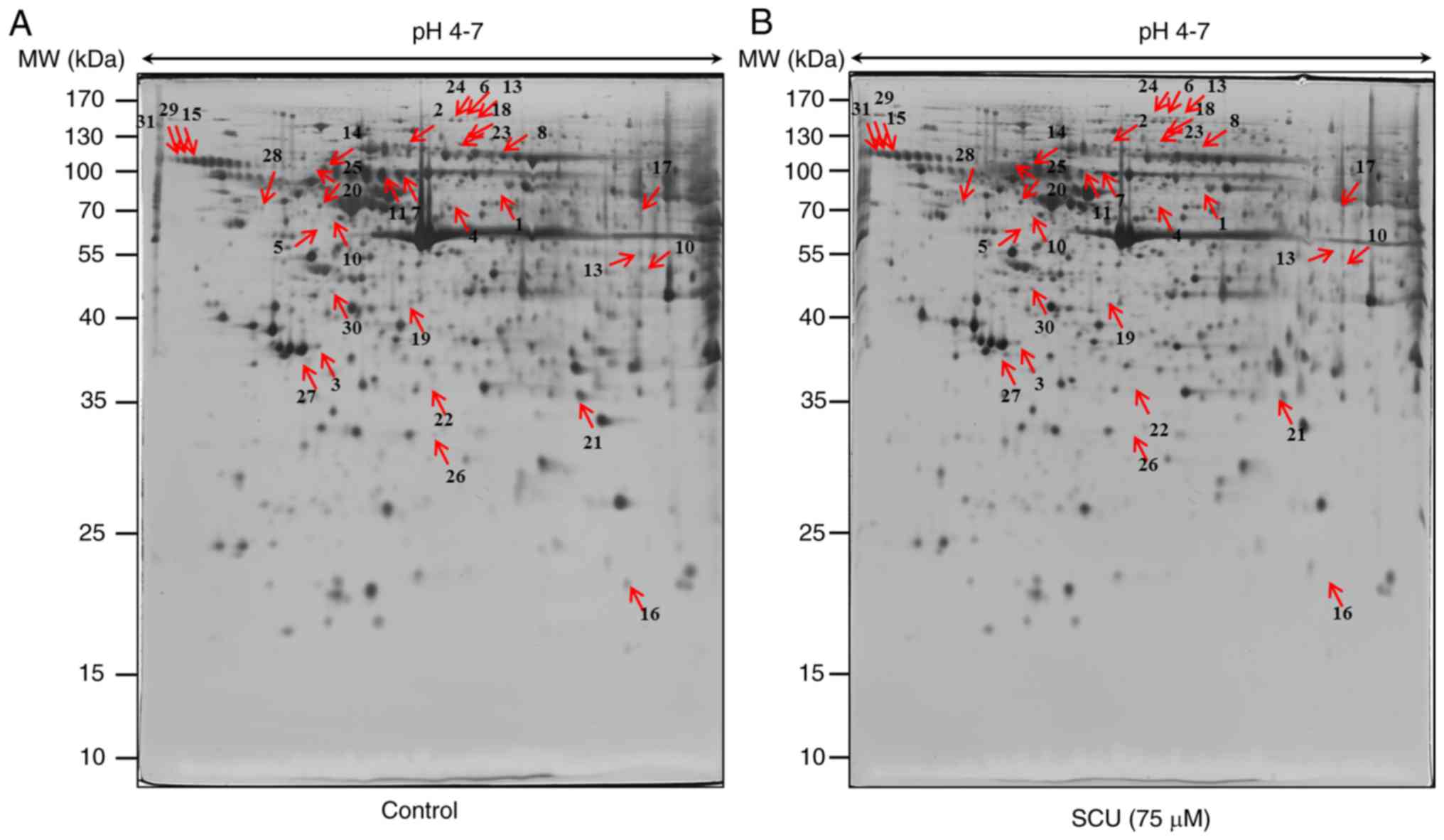
Proteomic analysis of AGS and SNU484 cells in
response to SCU treatment was conducted. To analyze the proteome
changes upon treatment with SCU to induce cell death in AGS and
SNU484 cells, 400 µg total proteins were separated by isoelectric
focusing (IEF) on 18 cm IPG strips in the first dimension, and
resolved by 2-DE, followed by silver staining for visualization.
Three gels per sample were analyzed simultaneously by confirming
the representative 2-DE patterns of protein spots from the control
and SCU-treated (75 µM) AGS and SNU484 cells (Figs. 2A and B and 3A and B respectively). After automatic
spot identification, background subtraction, and volume
normalization in AGS and SNU484 cells, a total of 58 and 43 spots
were found to be differentially expressed between the untreated and
SCU-treated cells (fold change ≥1.5; P<0.05) by using Progenesis
Samespots image analysis software, respectively. After
MALDI-TOF-MS, 41 (AGS) and 31 (SNU484) proteins were successfully
identified upon database search (http://www.prospector.ucsf.edu). Among the identified
proteins, 17 proteins were upregulated and 24 proteins were
downregulated in the AGS cells treated with SCU, compared with the
untreated group of cells and 18 proteins were upregulated and 13
proteins were downregulated in SNU484 cells treated with SCU
compared with the untreated group of cells. Tables I (AGS) and II (SNU484) show the
characterization of the identified proteins with their respective
accession number, fold change, analytical isoelectric point,
analytical molecular weight, and MOWSE score, and the sequence
coverage and number of peptide matches, respectively.
 | Table I.List of differentially expressed
proteins in AGS cells treated with SCU, as identified using
MALDI-TOF/TOF-MS analysis. |
Table I.
List of differentially expressed
proteins in AGS cells treated with SCU, as identified using
MALDI-TOF/TOF-MS analysis.
| Spot no. | Uniprot ID | Protein name | Accession no. | MOWSE score | Sequence coverage
(%)/peptides matched | Protein MW
(Da) | pI value | Fold change | Up/Down |
|---|
| 1 | OGT1_HUMAN |
UDP-N-acetylglucosamine-peptide
N-acetylglucosaminyltransferase 110 kDa subunit | O15294 | 9.02E+06 | 24.7/20 | 116926 | 6.2 | 3.2 | ↑ |
| 2 | KIF11_HUMAN | Kinesin-like
protein KIF11 | P52732 | 2.07E+16 | 28.6/10 | 119160 | 5.5 | 2.1 | ↓ |
| 3 | KI20A_HUMAN | Kinesin-like
protein KIF20A | O95235 | 2.65E+08 | 11.7/11 | 100279 | 6.5 | 1.9 | ↓ |
| 4 | NALP7_HUMAN | NACHT, LRR and PYD
domains-containing protein 7 | Q8WX94 | 1.14E+12 | 12.6/13 | 111808 | 5.9 | 2 | ↓ |
| 5 | CPSF2_HUMAN | Cleavage and
polyadenylation specificity factor subunit 2 | Q9P2I0 | 1.94E+06 | 21.1/20 | 88488 | 5 | 1.6 | ↓ |
| 6 | IF4G2_HUMAN | Eukaryotic
translation initiation factor 4 γ 2 | P78344 | 3.07E+09 | 16.8/11 | 102363 | 6.7 | 1.8 | ↓ |
| 7 | CCD57_HUMAN | Coiled-coil
domain-containing protein 57 | Q2TAC2 | 5.16E+08 | 18.4/17 | 103168 | 6.1 | 2 | ↓ |
| 8 | GG6L6_HUMAN | Golgin subfamily A
member 6-like protein 6 | A8MZA4 | 6.36E+06 | 11.5/13 | 90953 | 5.1 | 1.5 | ↑ |
| 9 | KTU_HUMAN | Protein
kintoun | Q9NVR5 | 2.60E+06 | 19.4/27 | 91115 | 5.1 | 6.5 | ↓ |
| 10 | FETA_HUMAN | α-fetoprotein | P02771 | 7.09E+06 | 28.7/31 | 68678 | 5.5 | 5.1 | ↑ |
| 11 | AT1A2_HUMAN |
Sodium/potassium-transporting ATPase
subunit α-2 | P50993 | 1.05E+06 | 35.9/28 | 112266 | 5.5 | 1.6 | ↑ |
| 12 | GCR_HUMAN | Glucocorticoid
receptor | P04150 | 1.53E+09 | 12.8/13 | 85660 | 6 | 1.6 | ↓ |
| 13 | VPS35_HUMAN | Vacuolar protein
sorting-associated protein 35 | Q96QK1 | 2.43E+06 | 18.2/29 | 91708 | 5.3 | 1.7 | ↓ |
| 14 |
OFD1_HUMAN |
Oral-facial-digital syndrome 1
protein | O75665 |
1.30E+09 | 18.5/12 | 116672 | 5.8 | 2.3 | ↓ |
| 15 | DREB_HUMAN | Drebrin | Q16643 | 2.61E+07 | 22.8/22 | 71430 | 4.4 | 10.6 | ↑ |
| 16 | DNM3A_HUMAN | DNA
(cytosine-5)-methyltransferase 3A | Q9Y6K1 | 6.32E+08 | 15.8/14 | 101859 | 6.2 | 2.1 | ↑ |
| 17 | UBA6_HUMAN | Ubiquitin-like
modifier-activating enzyme 6 | A0AVT1 | 4.37E+07 | 38.1/48 | 117971 | 5.8 | 2.4 | ↓ |
| 18 |
VINC_HUMAN |
Vinculin | P18206 |
9.87E+06 | 33.9/19 | 123800 | 5.5 | 1.9 | ↓ |
| 19 | ITB1_HUMAN | Integrin β-1 | P05556 | 1.07E+06 | 26.3/25 | 88416 | 5.3 | 5.7 | ↑ |
| 20 | TAXB1_HUMAN | Tax1-binding
protein 1 | Q86VP1 | 1.61E+07 | 11.9/9 | 90878 | 5.3 | 6 | ↑ |
| 21 |
CA2D1_HUMAN |
Voltage-dependent calcium channel
subunit α-2/δ-1 | P54289 |
1.15E+06 | 19.5/29 | 124569 | 5.1 | 1.5 | ↑ |
| 22 | HSP74_HUMAN | Heat shock 70 kDa
protein 4 | P34932 | 1.30E+07 | 17/27 | 94332 | 5.1 | 1.5 | ↓ |
| 23 |
HIP1R_HUMAN |
Huntingtin-interacting protein
1-related protein | O75146 |
7.97E+08 | 21.4/12 | 119389 | 6.2 | 3.7 | ↑ |
| 24 | KIF5C_HUMAN | Kinesin heavy chain
isoform 5C | O60282 | 3.50E+09 | 33.3/18 | 109496 | 5.9 | 1.9 | ↑ |
| 25 |
VAV_HUMAN | Proto-oncogene
vav | P15498 |
5.75E+10 | 15.3/17 | 98315 | 6.2 | 1.6 | ↑ |
| 26 |
PK3CB_HUMAN |
Phosphatidylinositol 4,5-bisphosphate
3-kinase catalytic subunit β isoform | P42338 |
1.18E+12 | 30.2/29 | 122763 | 6.7 | 1.8 | ↓ |
| 27 | UBP29_HUMAN | Ubiquitin
carboxyl-terminal hydrolase 29 | Q9HBJ7 | 5.67E+06 | 16.3/13 | 104157 | 5.6 | 1.9 | ↑ |
| 28 | BUB1B_HUMAN | Mitotic checkpoint
serine/threonine-protein kinase BUB1 β | O60566 | 4.19E+07 | 15.9/9 | 119546 | 5.2 | 1.9 | ↓ |
| 29 | PK3CA_HUMAN |
Phosphatidylinositol 4,5-bisphosphate
3-kinase catalytic subunit α isoform | P42336 | 1.11E+20 | 25.1/14 | 124285 | 6.9 | 1.7 | ↓ |
| 30 | AKIB1_HUMAN | Ankyrin repeat and
IBR domain-containing protein 1 | Q9P2G1 | 5.67E+06 | 27.9/26 | 122003 | 5 | 1.8 | ↓ |
| 31 | ANPRA_HUMAN | Atrial natriuretic
peptide receptor 1 | P16066 | 4.88E+07 | 19/19 | 118920 | 6.2 | 1.9 | ↑ |
| 32 | STK31_HUMAN |
Serine/threonine-protein kinase 31 | Q9BXU1 | 1.28E+07 | 47.8/53 | 115695 | 5 | 1.6 | ↓ |
| 33 | PGFRA_HUMAN | Platelet-derived
growth factor receptor α | P16234 | 1.44E+07 | 13.3/11 | 122671 | 5.1 | 2.2 | ↓ |
| 34 | DNM1L_HUMAN | Dynamin-1-like
protein | O00429 | 1.12E+06 | 24.2/23 | 81878 | 6.4 | 2.5 | ↑ |
| 35 | ARHG2_HUMAN | Rho guanine
nucleotide exchange factor 2 | Q92974 | 1.05E+10 | 21.7/18 | 111544 | 6.9 | 2.2 | ↓ |
| 36 | DISC1_HUMAN | Disrupted in
schizophrenia 1 protein | Q9NRI5 | 3.96E+08 | 26.3/30 | 93611 | 6 | 2.1 | ↑ |
| 37 | MMS19_HUMAN | MMS19 nucleotide
excision repair protein homolog | Q96T76 | 3.32E+07 | 25/23 | 113291 | 5.9 | 2.3 | ↑ |
| 38 |
SYCP1_HUMAN | Synaptonemal
complex protein 1 | Q15431 |
1.44E+07 | 15/12 | 114193 | 5.8 | 1.6 | ↓ |
| 39 | APOBR_HUMAN | Apo lipoprotein B
receptor | Q0VD83 | 1.00E+06 | 33.4/21 | 114875 | 4.4 | 6.6 | ↓ |
| 40 | AKT3_HUMAN | RAC-gamma
serine/threonine-protein kinase | Q9Y243 | 4.93E+09 | 20.5/13 | 55775 | 5.7 | 1.6 | ↓ |
| 41 | ITA4_HUMAN | Integrin α-4 | P13612 | 8270000 | 26/28.5 | 114901 | 6 | 1.6 | ↓ |
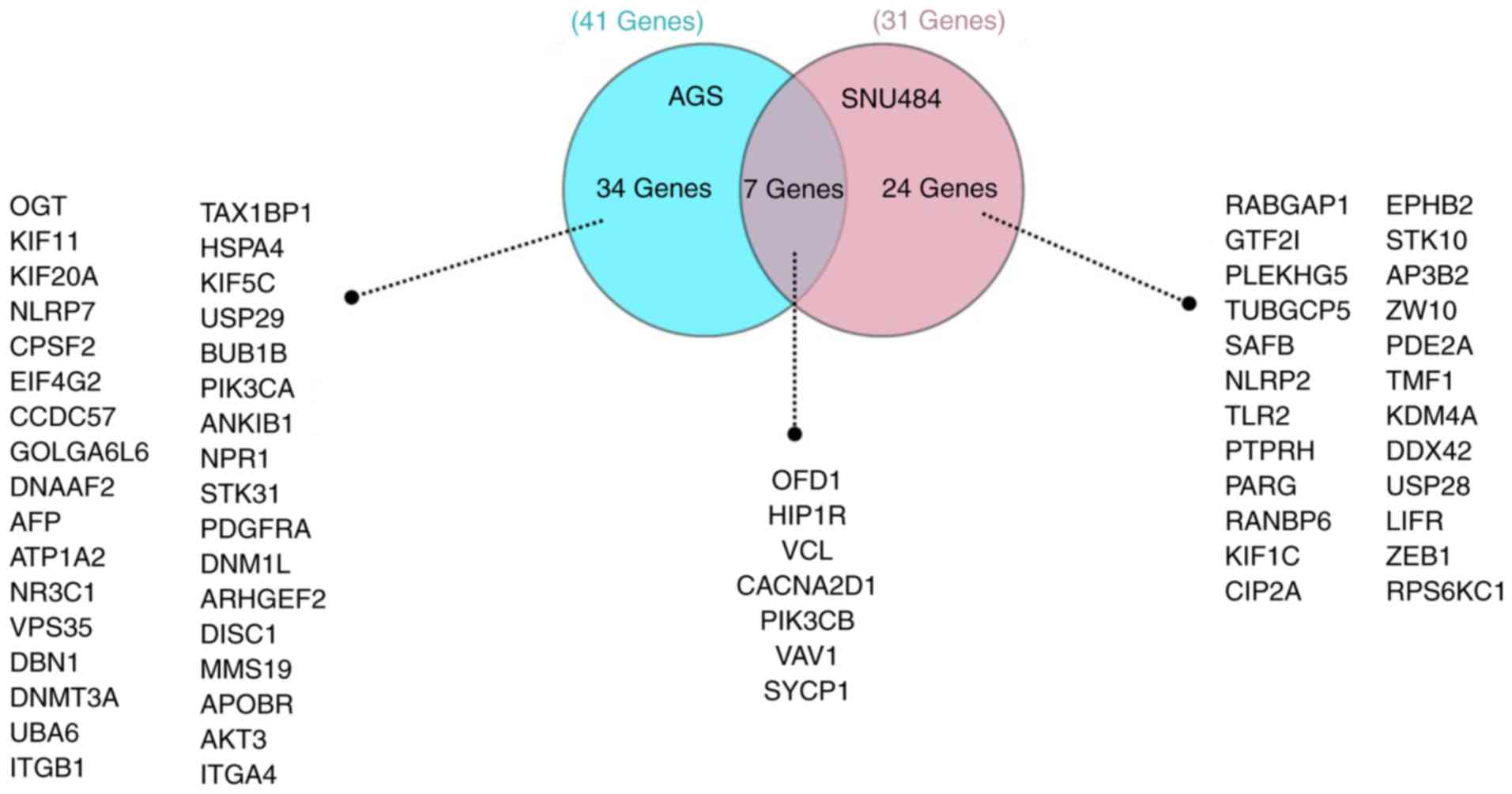
Comparative analysis of the functional
enrichment of the differentially expressed proteins
Oral-facial-digital syndrome 1 protein (OFD1),
vinculin (VINC), voltage-dependent calcium channel subunit α-2/δ-1
(CACNA2D1), Huntingtin-interacting protein 1-related protein
(HIP1R), proto-oncogene vav 1 (VAV1), phosphatidylinositol
4,5-bisphosphate 3-kinase catalytic subunit β isoform (PIK3CB) and
synaptonemal complex protein 1 (SYCP1) were found to be the 7
proteins commonly expressed in both GC cells treated with SCU by
the comparative analysis using GeneCodis (http://genecodis.cnb.csic.es) a web-based tool. The
remaining 34 from AGS and 24 from SNU484 proteins were uniquely
differentially expressed when treated with SCU as shown in Fig. 4. OFD1, VINC, HIP1R, and PIK3CB were
downregulated in both cell lines, whereas CA2D1, VAV1 and SYCP1
protein expression were elevated in both cell lines treated with
SCU compared with the control group of cells. Fig. S1 shows the protein clusters of the
individual proteins (A) OFD1, (B) VINC, (C) HIPR1, (D) CA2D1, (E)
PIK3CB, (F) VAV1 and (G) CIP2A by STRING database. Table IV (AGS) and Table V (SNU) show that the OFD1 protein
cluster contains biological processes: Regulation of the G2/M
transition of the mitotic cell cycle (GO:0010389), and cell
differentiation (GO:0030154). VINC protein cluster contains
biological processes: Negative regulation of apoptotic process
(GO:0043066), regulation of cellular process (GO:0050794), positive
regulation of cellular metabolic process (GO:0031325), and positive
regulation of phosphatidylinositol 3-kinase activity (GO:0043552).
CA2D1 protein cluster contains biological processes: Positive
regulation of high voltage-gated calcium channel activity
(GO:1901843), regulation of protein transport (GO:0051223), and
homeostatic process (GO:0042592). HIP1R protein cluster contains
biological processes: Negative regulation of apoptotic process
(GO:0043066), negative regulation of cellular process (GO:0048523),
and regulation of cytoskeleton organization (GO:0051493). PIK3CB
protein cluster contains biological processes: Negative regulation
of apoptotic process (GO:0043066), positive regulation of cellular
metabolic process (GO:0031325), regulation of DNA-binding
transcription factor activity (GO:0051090), and regulation of
protein ubiquitination (GO:0031396). VAV1 protein cluster contains
biological processes: Immune response-activating cell surface
receptor signaling (GO:0002429), integrin-mediated signaling
pathway (GO:0007229), and regulation of cell adhesion (GO:0030155).
In addition to these proteins, we were interested in one more
protein, which was found to be expressed in our previous studies
and is related to proteomic analysis in GC cells treated with
flavonoids (17). We found that
protein CIP2A (CIP2A) was downregulated in SCU-treated SNU484 cells
compared to the untreated group of cells. Protein cluster of CIP2A
contains biological processes: Regulation of DNA metabolic process
(GO:0051052), regulation of apoptotic process (GO:0042981),
negative regulation of stress-activated MAPK cascade (GO:0032873)
and mitotic cell cycle process (GO:1903047).
 | Table IV.List of differentially expressed
proteins involved in different biological processes in AGS cells
treated with Scutellarein. |
Table IV.
List of differentially expressed
proteins involved in different biological processes in AGS cells
treated with Scutellarein.
| AGS cell line |
|---|
|
|---|
| Sr. No. | Protein | Description | Biological
process |
|---|
| 1 | VCL | Vinculin | GO:0030168:
platelet |
|
| VAV1 | av guanine
nucleotide exchange factor 1 | activation |
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| PDGFRAV | Platelet derived
growth factor receptor α |
|
| 2 | ITGB1 | Integrin subunit β
1 | GO:0051897:
positive |
|
| VAV1 | Vav guanine
nucleotide exchange factor 1 | regulation of
protein |
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β | kinase B
signaling |
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| PDGFRA | Platelet derived
growth factor receptor α |
|
| 3 | DBN1 | Drebrin 1 | GO:0048667:
cell |
|
| VCL | Vinculin | morphogenesis
involved |
|
| ITGB1 | Integrin subunit β
1 | in neuron
differentiation |
|
| KIF5C | Kinesin family
member 5C |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| DNM1L | Dynamin 1 like |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 4 | ATP1A2 | ATPase Na+/K+
transporting subunit α 2 | GO:0098657:
import |
|
| ITGB1 | Integrin subunit β
1 | into cell |
|
| CACNA2D1 | Calcium
voltage-gated channel auxiliary subunit α2δ1 |
|
|
| HIP1R | Huntingtin
interacting protein 1 related |
|
|
| VAV1 | Vav guanine
nucleotide exchange factor 1 |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| DNM1L | Dynamin 1 like |
|
|
| APOBR | Apolipoprotein B
receptor |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 5 | DNAAF2 | Dynein axonemal
assembly factor 2 | GO:0120036:
plasma |
|
| VPS35 | VPS35, retromer
complex component | membrane bounded
cell |
|
| OFD1 | OFD1, centriole and
centriolar satellite protein | projection
organization |
|
| DBN1 | Drebrin 1 |
|
|
| UBA6 | Ubiquitin like
modifier activating enzyme 6 |
|
|
| VCL | Vinculin |
|
|
| ITGB1 | Integrin subunit β
1 |
|
|
| KIF5C | Kinesin family
member 5C |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| DNM1L | Dynamin 1 like |
|
|
| DISC1 | DISC1 scaffold
protein |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 6 | KIF11 | Kinesin family
member 11 | GO:0006928:
movement |
|
| KIF20A | Kinesin family
member 20A | of cell or
subcellular |
|
| DNAAF2 | Dynein axonemal
assembly factor 2 | component |
|
| ATP1A2 | ATPase Na+/K+
transporting subunit α 2 |
|
|
| OFD1 | OFD1, centriole and
centriolar satellite protein |
|
|
| VCL | Vinculin |
|
|
| ITGB1 | Integrin subunit β
1 |
|
|
| CACNA2D1 | Calcium
voltage-gated channel auxiliary subunit α2δ1 |
|
|
| KIF5C | kinesin family
member 5C |
|
|
| VAV1 | Vav guanine
nucleotide exchange factor 1 |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| PDGFRA | Platelet derived
growth factor receptor α |
|
|
| ARHGEF2 | Rho/Rac guanine
nucleotide exchange factor 2 |
|
|
| DISC1 | DISC1 scaffold
protein |
|
|
| AKT3 | AKT
serine/threonine kinase 3 |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 7 | DNAAF2 | Dynein axonemal
assembly factor 2 | GO:0030030:
cell |
|
| VPS35 | VPS35, retromer
complex component | projection
organization |
|
| OFD1 | OFD1, centriole and
centriolar satellite protein |
|
|
| DBN1 | Drebrin 1 |
|
|
| UBA6 | Ubiquitin like
modifier activating enzyme 6 |
|
|
| VCL | Vinculin |
|
|
| ITGB1 | Integrin subunit β
1 |
|
|
| KIF5C | Kinesin family
member 5C |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| DNM1L | Dynamin 1 like |
|
|
| DISC1 | DISC1 scaffold
protein |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 8 | DBN1 | Drebrin 1 | GO:0048699:
generation |
|
| DNMT3A | DNA
methyltransferase 3 α | of neurons |
|
| UBA6 | Ubiquitin like
modifier activating enzyme 6 |
|
|
| VCL | Vinculin |
|
|
| ITGB1 | Integrin subunit β
1 |
|
|
| KIF5C | Kinesin family
member 5C |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| DNM1L | Dynamin 1 like |
|
|
| ARHGEF2 | Rho/Rac guanine
nucleotide exchange factor 2 |
|
|
| DISC1 | DISC1 scaffold
protein |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 9 | DNAAF2 | Dynein axonemal
assembly factor 2 |
|
|
| ATP1A2 | ATPase Na+/K+
transporting subunit α 2 |
|
|
| VPS35 | VPS35, retromer
complex component |
|
|
| VCL | Vinculin |
|
|
| ITGB1 | Integrin subunit β
1 |
|
|
| KIF5C | Kinesin family
member 5C |
|
|
| VAV1 | Vav guanine
nucleotide exchange factor 1 |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| PDGFRA | Platelet derived
growth factor receptor α |
|
|
| ARHGEF2 | Rho/Rac guanine
nucleotide exchange factor 2 |
|
|
| DISC1 | DISC1 scaffold
protein |
|
|
| AKT3 | AKT
serine/threonine kinase 3 |
|
|
| ITGA4 | Integrin subunit α
4 |
|
| 10 | OGT | O-linked
N-acetylglucosamine (GlcNAc) transferase | GO:0006915:
apoptotic |
|
| NR3C1 | Nuclear receptor
subfamily 3 group C member 1 | process |
|
| VPS35 | VPS35, retromer
complex component |
|
|
| DNMT3A | DNA
methyltransferase 3 α |
|
|
| ITGB1 | Integrin subunit β
1 |
|
|
| TAX1BP1 | Tax1 binding
protein 1 |
|
|
| HIP1R | Huntingtin
interacting protein 1 related |
|
|
| VAV1 | Vav guanine
nucleotide exchange factor 1 |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit β |
|
|
| BUB1B | BUB1 mitotic
checkpoint serine/threonine kinase B |
|
|
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α |
|
|
| PDGFRA | Platelet derived
growth factor receptor α |
|
|
| DNM1L | Dynamin 1 like |
|
|
| ARHGEF2 | Rho/Rac guanine
nucleotide exchange factor 2 |
|
|
| ITGA4 | Integrin subunit α
4 |
|
 | Table V.List of differentially expressed
proteins involved in different biological processes in SNU484 cells
treated with Scutellarein. |
Table V.
List of differentially expressed
proteins involved in different biological processes in SNU484 cells
treated with Scutellarein.
| SNU484 cell
line |
|---|
|
|---|
| Sr. No. | Protein | Description | Biological
process |
|---|
| 1 | TLR2 | Toll like receptor
2 | GO:0036005:
response to |
|
| PDE2A | Phosphodiesterase
2A | macrophage
colony- |
|
|
|
| stimulating
factor |
| 2 | TLR2 | Toll like receptor
2 | GO:0036006:
cellular response to |
|
| PDE2A | Phosphodiesterase
2A | macrophage
colony-stimulating |
|
|
|
| factor
stimulus |
| 3 | HIP1R | Huntingtin
interacting protein 1 related | GO:2000369:
regulation of |
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase | clathrin-dependent
endocytosis |
|
|
| catalytic subunit
β |
|
| 4 | SYCP1 | Synaptonemal
complex protein 1 | GO:0007289:
spermatid nucleus |
|
| TMF1 | TATA element
modulatory factor 1 |
differentiation |
| 5 | TUBGCP5 | Tubulin gamma
complex associated protein 5 | GO:0090307: mitotic
spindle |
|
| OFD1 | OFD1, centriole and
centriolar satellite protein | assembly |
|
| PIBF1 | Progesterone
immunomodulatory binding factor 1 |
|
| 6 | TUBGCP5 | Tubulin γ complex
associated protein 5 | GO:1902850:
microtubule |
|
| OFD1 | OFD1, centriole and
centriolar satellite protein | cytoskeleton
organization involved |
|
| ZW10 | zw10 kinetochore
protein | in mitosis |
|
| PIBF1 | Progesterone
immunomodulatory binding factor 1 |
|
| 7 | TUBGCP5 | Tubulin γ complex
associated protein 5 | GO:0007052: mitotic
spindle |
|
| OFD1 | OFD1, centriole and
centriolar satellite protein | organization |
|
| PIBF1 | Progesterone
immunomodulatory binding factor 1 |
|
| 8 | TUBGCP5 | Tubulin γ complex
associated protein 5 | GO:0051225: spindle
assembly |
|
| OFD1 | OFD1, centriole and
centriolar satellite protein |
|
|
| PIBF1 | Progesterone
immunomodulatory binding factor 1 |
|
| 9 | SYCP1 | Synaptonemal
complex protein 1 | GO:0000280: nuclear
division |
|
| TUBGCP5 | Tubulin γ complex
associated protein 5 |
|
|
| OFD1 | OFD1, centriole and
centriolar satellite protein |
|
|
| ZW10 | zw10 kinetochore
protein |
|
|
| PIBF1 | Progesterone
immunomodulatory binding factor 1 |
|
| 10 | PLEKHG5 | Pleckstrin homology
and RhoGEF domain containing G5 | GO:0006915:
apoptotic process |
|
| NLRP2 | NLR family pyrin
domain containing 2 |
|
|
| TLR2 | Toll like receptor
2 |
|
|
| PTPRH | Protein tyrosine
phosphatase, receptor type H |
|
|
| HIP1R | Huntingtin
interacting protein 1 related |
|
|
| PIK3CB |
Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic |
|
|
|
| subunit β |
|
|
| EPHB2 | EPH receptor
B2 |
|
|
| TMF1 | TATA element
modulatory factor 1 |
|
|
| DDX42 | DEAD-box helicase
42 |
|
|
| USP28 | Ubiquitin specific
peptidase 28 |
|
Validation of selected proteins by
immunoblotting analysis
To verify the identified proteins from 2-DE, the
immunoblotting analysis was performed in the SCU-treated AGS and
SNU484 cells for a few selected important proteins. Fig. 5 shows that the western blot analysis
revealed significantly decreased protein expression of OFD1, VCL,
PIK3CB, HIP1R, and CIP2A in both the SCU-treated GC cell lines, in
comparison with the control groups (P<0.05). These data
confirmed that the results of the immunoblotting were consistent
with those of the 2-DE outcome. The comparative proteomic analysis
and the confirmation of these proteins by western blot analysis
increased the confidence in the obtained results from the 2-DE
analysis to consider these proteins as biomarker candidates.
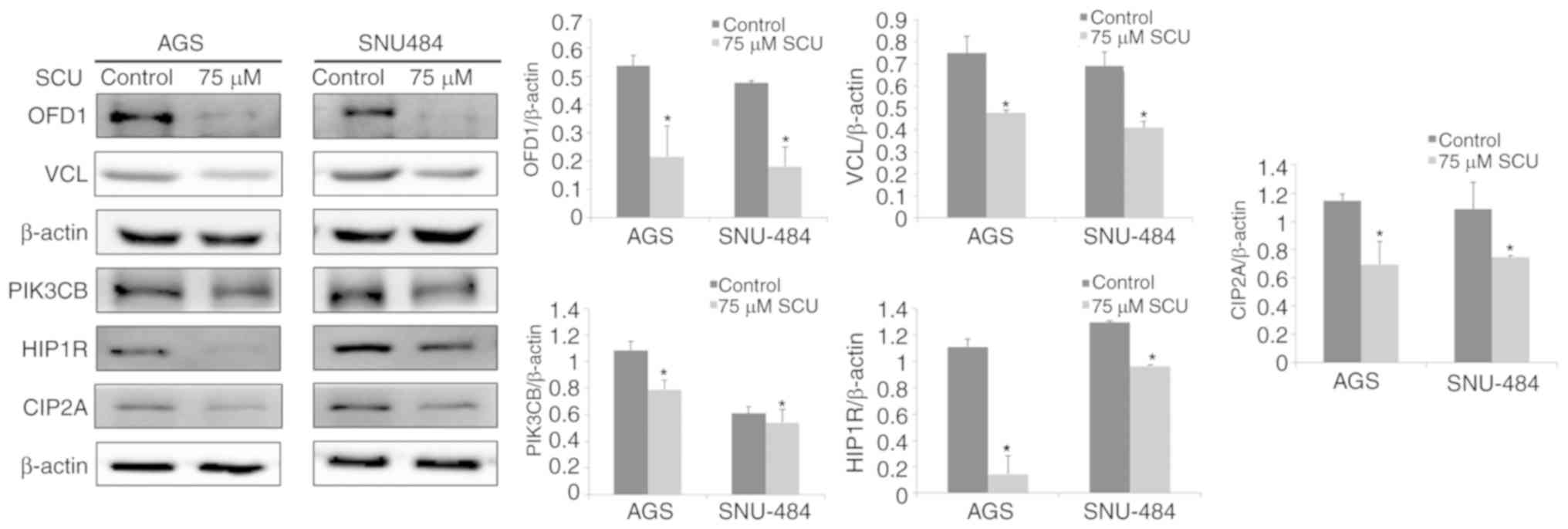 | Figure 5.Western blot analysis confirmation of
differentially expressed proteins. (A) AGS and (B) SNU484 GC cell
lines were treated with control (DMSO) or SCU (75 µM), incubated
for 24 h and protein samples were prepared and separated on 10–12%
SDS-PAGE. OFD1, VCL, PIK3CB, HIP1R and CIP2A proteins were assessed
using the respective antibodies. For the loading control β-actin
was used and normalized to measure the expression changes. The
bands are representative of three independent experiments
[*P<0.05, significant difference vs. the control (DMSO)]. GC,
gastric cancer; SCU, Scutellarein; OFD1, oral-facial-digital
syndrome 1 protein; VCL, vinculin; PIK3CB, phosphatidylinositol
4,5-bisphosphate 3-kinase catalytic subunit β isoform; HIP1R,
Huntingtin-interacting protein 1-related protein; CIP2A, cancerous
inhibitor of protein phosphatase 2A. |
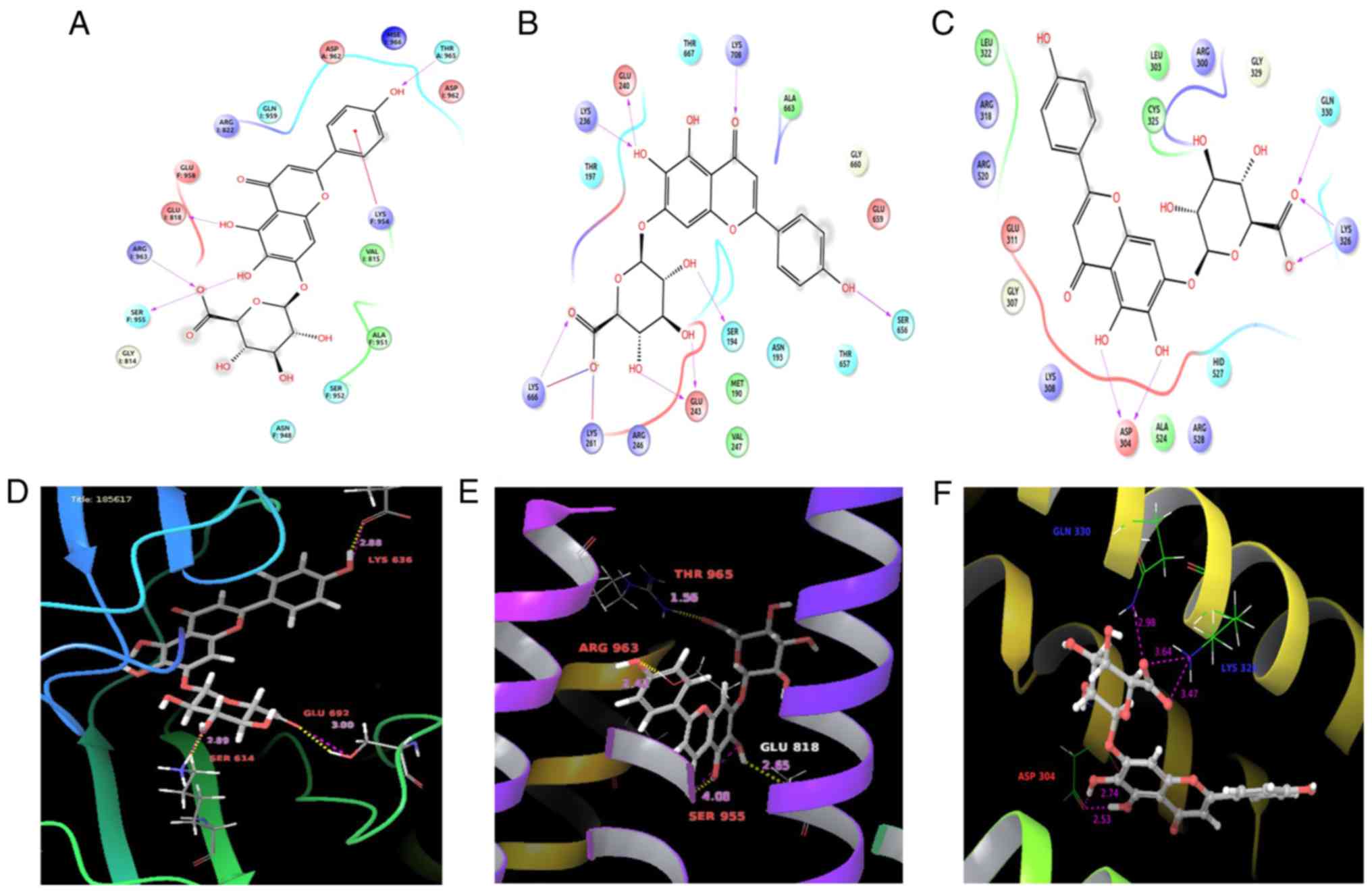
Molecular docking studies confirm the
binding affinity of SCU with biomarker candidates
Molecular docking analysis against Scutellarein
revealed that all the significant protein macromolecules chosen
form a stable H-bond with Scutellarein. Table III shows the Glide parameters,
such as Glide G-Score and Glide energy of the significant protein
molecules with the structure of SCU along with the number of
H-bonds, interacting with the amino acids. The Glide score
describes the perfect fit for a ligand in the active site of the
target molecule, and gives the efficiency of the molecular binding.
The Glide score of the target molecules, PIK3CB, HIPR1, CIP2A, VAV1
and VINC, were found to be −8.767, −4.678, −2.952, −6.666, and
−6.049), respectively. Fig. 6 shows
the molecular binding models of the three significant target
molecules, PIK3CB, HIPR1 and VINC, with their ligand interaction
diagram. The LigPlot shows the binding with their appropriate
interacting amino acids with stable Vander Waals force and
H-bonding. PIK3CB interacted with SCU by forming five stable
hydrogen bonds with amino acids: Glu692, Ile685, Gln683, Lys636,
and Ser614. Vinculin formed interactions with the following
residues: Lys236, Glu240, Lys708, Arg246, Glu243, Ser656, Lys261,
and Lys666; and HIP1R interacts with ThrA965, GluI818, ArgI963, and
SerF:955. With consideration of the Glide parameters of all the
target molecules with SCU, the interaction of PIK3CB has shown more
significant binding with a Glide score of −8.767 forming stable
affinity, and is considered as the best fit among our proteins in
the study. Together with this, Fig.
S2 shows the molecular binding models of the proteins CIP2A and
VAV with their ligand interaction with SCU. CIP2A shows interaction
with SCU by forming five hydrogen bonds, interacting with amino
acids: Glu34, Val35, Gln82, and AspB6; whereas, protein VAV showed
interaction with only a few amino acids: Tyr56, Ser129, and Thr75
compared with the other structures.
 | Table III.Number of interacting amino acid
residues with the different Glide parameters of selected
macromolecules with Scutellarein. |
Table III.
Number of interacting amino acid
residues with the different Glide parameters of selected
macromolecules with Scutellarein.
| Sr. No. | Macromolecule | Interacting
residues | No of H-bonds | Glide G-Score | Glide energy |
|---|
| 1 | CIP2A | Glu34; Val35;
Gln82; AspB6 | 5 | −2.952 | −29.625 |
| 2 | PIK3CB | Glu692; Ile685;
Gln683; Lys636; Ser614 | 5 | −8.767 | −58.531 |
| 3 | VINC | Lys236; Glu240;
Lys708; Arg246; Glu243; Ser656; Lys261; Lys666 | 5 | −6.049 | −54.679 |
| 4 | HIPR1 | ThrA:965; GluI:818;
ArgI:963; SerF:955 | 4 | −4.678 | −47.516 |
| 5 | VAV | Tyr56; Ser129;
Thr75 | 6 | −6.666 | −59.988 |
Functional classifications of the
identified differentially expressed proteins
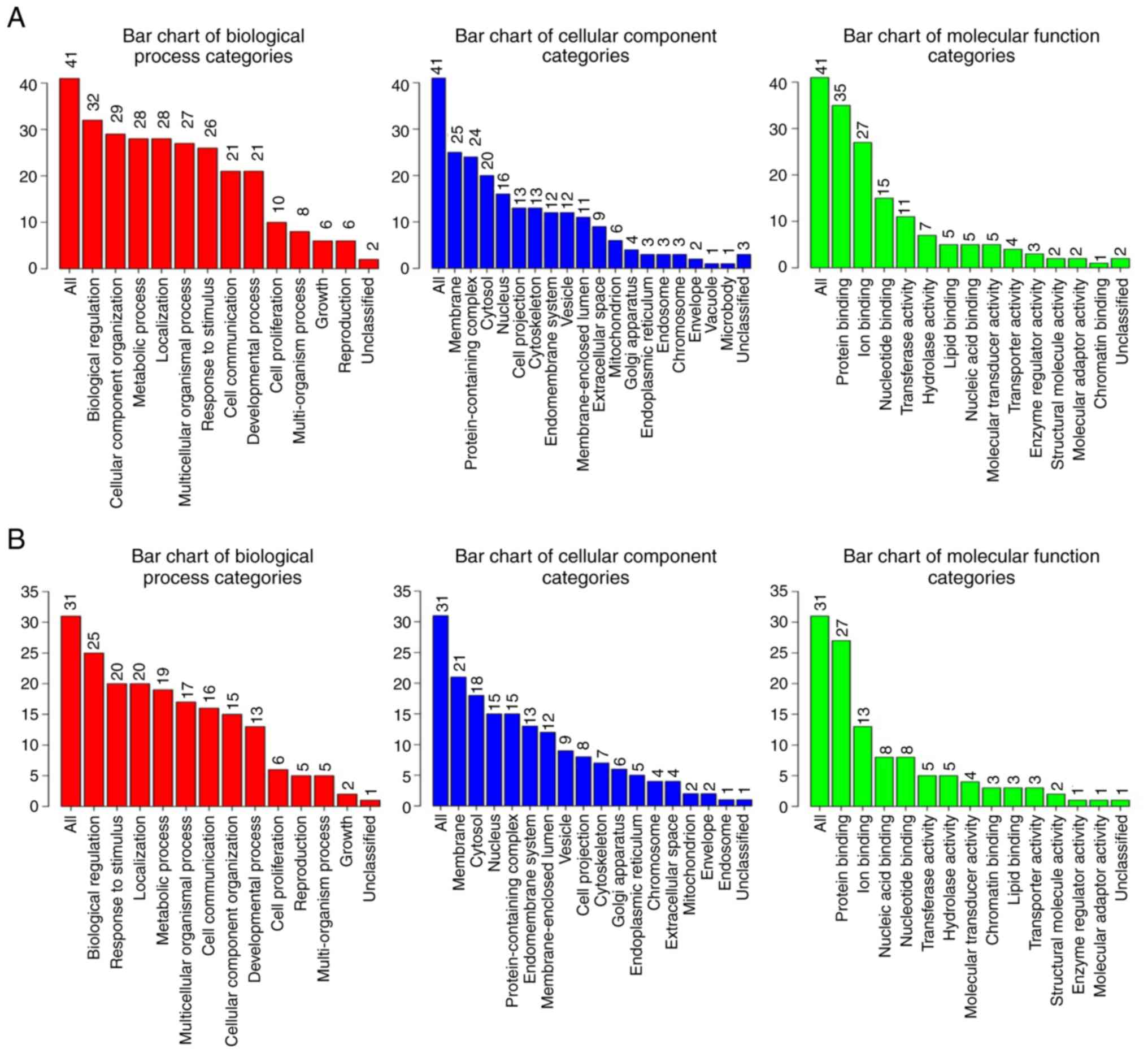
The Gene Ontology bioinformatics tool was useful to
facilitate the interpretation of the proteomics data. WebGestalt
(http://www.webgestalt.org) tool was used
to analyze the differentially identified proteins from both cell
lines treated with SCU in terms of biological process, cellular
component and molecular function by GOSlim view. Fig. 7A (AGS) and Fig. 7B (SNU484) show the distribution of
the number of proteins to several categories of the functional
annotation. Among the GO categories, biological processes
enrichment of these differentially protein from both cell lines
were viewed independently with WebGestalt with the condition of
P-value <0.05. Table IV (AGS)
and Table V (SNU) show that a total
of 10 biological processes were significant in both the cell lines
treated with SCU. Apoptotic process (GO:0006915) was found to be
the leading biological process in both cell lines treated with SCU,
with 15 proteins in AGS cells and 11 proteins in SNU484 cells being
involved. GO analysis showed that SCU could affect proteins that
regulate a broad range of molecular functions, such as protein
binding and ion binding. We uploaded 41 (AGS) and 31 (SNU484)
differentially expressed proteins on to the PANTHER database for
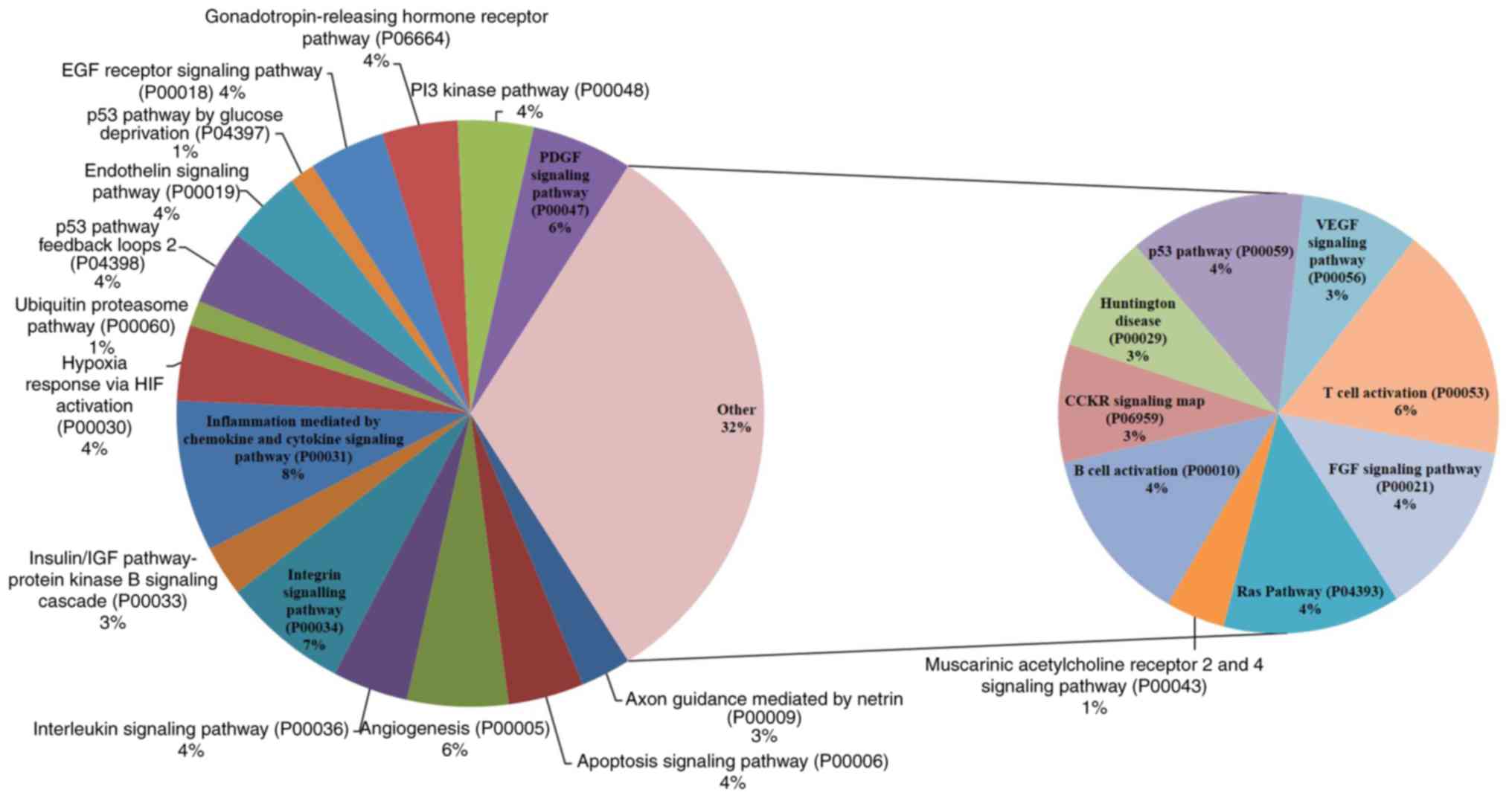
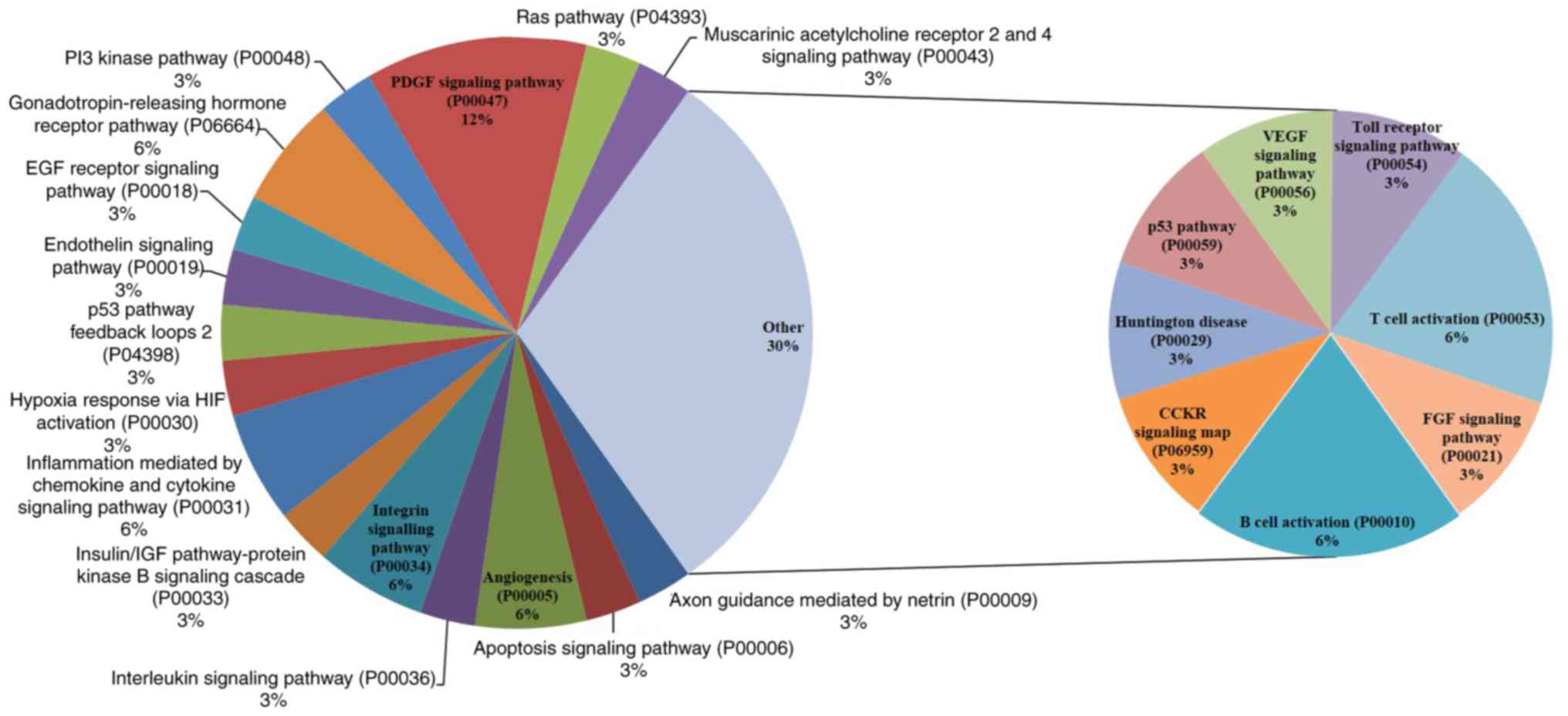
pathway enrichment, which identified 25 and 24 pathways with
signaling mechanisms that are concerned with the effect of SCU on
AGS and SNU484 cancer cells, respectively as shown in Figs. 8 (AGS) and 9 (SNU484). Along with the commonly
differentiated proteins in both the cell lines AGS and SNU484,
there are three additional proteins (AKT3, ITGB1, and PIK3CA)
expressed only in AGS which is found to be vital in contributing to
the cellular mechanisms of the enriched pathways. Tables SI and SII shows the detailed individual pathways
with protein distribution in AGS and SNU484 cell lines.
Discussion
Recent developments in proteome comparative analysis
are intermittently used in the identification of protein expression
alterations upon drug treatment of cancer cells (9). These strands of evidence could provide
clues for the investigation of the effects of drug treatment and
further provide better knowledge of the molecular mechanism of
action of agents. In the current study, two gastric cancer (GC)
cell lines were used as in vitro models; the data presented
in our present and previous studies showed that Scutellarein (SCU)
significantly inhibited cell proliferation and induced apoptosis in
both GC cell lines. A higher proportion of the proteome is expected
to be involved in this comparative proteomic approach thereby
increasing the opportunities for the discovery of protein
biomarkers in GC cell response to SCU treatment. To analyze the
alterations at the protein level, a comparative proteomic technique
using 2-DE coupled with mass spectrometry was implemented to find
the modified proteins in GC cells in response to SCU treatment. In
AGS cells, a total of 41 differentially expressed proteins, and in
SNU484, a total of 31 differentially expressed proteins were
identified successfully by MALDI/TOF-MS analysis. All the proteins
spots were not identified successfully because of the limitations
and relatively low concentrations in mass spectrometry. Among the
differentially expressed proteins, 5 critical proteins were
confirmed by immunoblotting by commercially available antibodies,
and 5 of these (PIK3CB, HIPR1, VINC, CIP2A and VAV) were studied
for their binding affinity with SCU using molecular simulation. The
Glide scores and energy values showed optimum values, and
significantly confirmed the efficiency of the compound SCU to bind
with the significantly expressed proteins in the present study. Of
note, structurally, the presence of four hydroxyl groups in the
compound SCU makes it more stable for binding and affinity to form
hydrogen bonding interactions with the molecular targets (25). The identified proteins were found to
be predominantly involved in the process of tumor growth, cell
cycle regulation, and apoptosis in cancer cells. Implementation of
comparative proteomics analysis yielded 7 [oral-facial-digital
syndrome 1 protein (OFD1), vinculin (VINC), voltage-dependent
calcium channel subunit α-2/δ-1 (CACNA2D1), Huntingtin-interacting
protein 1-related protein (HIP1R), proto-oncogene vav 1 (VAV1),
phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit β
isoform (PIK3CB) and synaptonemal complex protein 1 (SYCP1)]
commonly expressed proteins among the differentially expressed
proteins between AGS and SNU484 cells treated with SCU along with
CIP2A (SNU484), which attracted our interest since dysregulation of
its function and expression is correlated with cancer progression,
tumorigenesis, and apoptosis (26,27).
To the best of our knowledge, the anticancer effects of SCU on
expression of these genes have not previously been reported in GC
cells. GO biological process and PANTHER pathway analysis revealed
that all 7 proteins appear to participate in major biological
processes such as apoptosis, cellulare movement or subcellular
component, and also appear in several pathways stimulated by SCU in
both cell lines.
Initially, OFD1 was found to be expressed in
oral-facial-digital syndrome, and is subject to ciliopathies such
as retinitis pigmentosa, and Simpson-Golabi-Behmel syndrome type 2
(28). OFD1 is essential for
primary cilia formation, and depletion of OFD1 results in the loss
of primary cilia. The contribution of primary cilium function to
tumorigenesis is complex; studies recommend that dissecting the
regulatory mechanisms of OFD1 will provide insight into the
tumorigenesis functions and offer potential new therapeutic tools
for the treatment of cancers. In our present results, OFD1 was
downregulated in both GC cell lines treated with SCU, and
functional analysis showed its involvement in mitotic spindle
assembly, nuclear division, and microtubule cytoskeleton
organization involved in mitosis. PIK3CB, an isoform of the
catalytic subunit of phosphoinositide 3-kinase (PI3K), is expressed
to be oncogenic in their wild-type form. The depletion of PIK3CB
isoform encodes for p110, which was found to inactivate the PTEN
pathway, followed by the inhibition of growth in both in
vitro and in vivo environments in cancer models
(29). PI3Kβ activation is
responsible for the alteration in PIK3CB expression, which may lead
to an elevation in cancer survival and cellular proliferation.
PI3Kβ may be involved directly in cell invasion and migration
through interactions with other proteins, clathrin and integrins,
which play key roles in assisting cell motility function (30,31).
In the present study, the results revealed that SCU abated the
PIK3CB protein expression in both GC cell lines, when compared to
the control cell group. The decreased expression of PIK3CB may be
explained by its binding affinity with SCU. GO analysis results
showed the greater role of PIK3CB involvement in the maximum
category of the biological process as well as being a prominent
candidate of all the pathways identified by PANTHER analysis in
both cell lines. From our previous study, GC cells treated with
Pectolinarigenin, a natural flavonoid, also showed downregulation
of PIK3CB protein expression accompanied by a distinct group of
cellular functions, including cell growth and proliferation
(17). HIP1R is known as an
endocytic adaptor with its functional roles in vesicle trafficking
and clathrin-mediated endocytosis. Studies have shown that the
downregulation of HIP1R in aggressive prostate cancer cell lines
recapped the anti-metastatic effects of miR-23b/miR-27b, including
decreased migration and anchorage-independent growth (32). The present results indicate that SCU
downregulated the expression of HIP1R protein in AGS and SNU484
cells compared with the untreated group of cells. GO analysis of
differentially expressed proteins from both cell lines showed the
involvement of HIP1R proteins in different biological processes
such as import into the cell, regulation of clathrin-dependent
endocytosis, and apoptotic process. VINC is a well-known actin
filament binding protein that is involved in the process of
cell-cell adhesion, cell-matrix adhesion, and it alters E-cadherin
expression on the cell surface. VINC also plays vital roles in cell
locomotion and morphology, and downregulation of VINC was found to
significantly inhibit pancreatic cancer cell migration (33). Further VINC was also found to
stimulate tumor progression by increasing PI3K activation of
phosphatidylinositol (3,4,5)-triphosphate in cancer cells (34). VCL gene expression was found
to be significantly elevated in GC tissues, indicating that VCL may
contribute to GC progression by stimulation of tumor malignancy and
its invasiveness (35). Targeting
the depletion of VCL by anticancer agents may help to overcome cell
metastasis and invasion. VINC was downregulated in both GC cell
lines treated with SCU, and the biological process of
differentially expressed proteins from the current results indicate
the involvement of VCL in the locomotion of the cell or its
subcellular component and cell projection organization in the
SCU-treated GC cells. VAV1, a Dbl superfamily of the Rho/Rac
guanine nucleotide exchange factors whose members are predominantly
found in the hematopoietic lineage, is well known as a central
regulator in the rearrangements of actin cytoskeletal filaments
during cell activation. Vav1 acts as a pro-apoptotic factor in
breast cancer cells when the expression of p53 is lacking. Studies
have targeted VAV1 for its unregulated levels, which could be a
strategy to improve breast cancer outcomes by regulating p-Akt
(36). VAV1 was elevated in AGS and
SNU484 cells treated with SCU, and GO revealed its involvement in
the movement of a cell or subcellular component, the regulation of
protein kinase B signaling, locomotion and apoptotic process. The
current study results of the upregulation of VAV1 may support our
previous results that show that SCU induces apoptotic cell death by
regulating p53 in GC cells. Pathway analysis showed its involvement
in the activation of B cells, and inflammation mediated by cytokine
and chemokine signaling pathways, PDGF signaling pathway and
activation of T cells.
BUB1B, DNMT3A, PIK3CA, PDGFRA, DNM1L, ARHGEF2, TLR2,
and AKT3 are critical proteins that participate in a majority of
biological processes from the GO analysis of the differentially
expressed proteins from SCU-treated GC cells. The mitotic
checkpoint serine/threonine-protein kinase that involves kinases
(BUB1B) is responsible for chromosome segregation, and plays a key
role in spindle checkpoint operation. BUB1B is bound to the
kinetochore, and helps inhibition at the anaphase-promoting
complex, which tends to procrastinate the initiation of anaphase
and provides better chromosome segregation (37). Impaired spindle checkpoint operation
has been identified in many forms of cancer. BUB1B is overexpressed
and highly correlated with survival time, outperforming markers in
the cancer cell, and is a prime therapeutic target in glioblastoma
(38). In our study, BUB1B was
downregulated by SCU in AGS cells and GO analysis showed its
involvement in the apoptotic process. DNA methylation plays a key
role in the initiation, as well as the progression of human
cancers, providing captivating biomarkers and targets for
diagnostic and therapeutic purposes (39). Promoter hypermethylation and
deacetylation of tumor-suppressor genes play major roles in cancer
induction, through transcriptional silencing of these genes
(40). DNA hypermethylation is
carried out by a family of DNMTs including DNMT3A. In
hepatocellular carcinoma, a positive correlation between the
overexpression of these genes and cancer induction has been
reported to be significant (41).
DNMT3A protein expression was depleted in AGS cells treated with
SCU, and functional analysis indicated its role in the apoptotic
process by GO. Platelet-derived growth factor receptor α (PDGFRA)
encodes a receptor tyrosine kinase (RTK) that activates key
pathways such as PI3K/AKT/mTOR that are involved in many types of
cancers, and it plays a role in several cell functions that include
migration, cell proliferation, and angiogenesis (42). Increased PDGFRA expression was found
to enhance cell proliferation, migration and reduce apoptosis by
the downregulation of miR-140-5p in ovarian cancer (43). The present study result revealed
that downregulation of PDGFRA protein expression was observed in
SCU-treated AGS cells participating in a major biological process
regulated by differentially expressed proteins such as positive
regulation of protein kinase B signaling, platelet activation,
apoptotic process, and pathways including angiogenesis and the PDGF
signaling pathway. Rho guanine exchange factor (ARHGEF2) a
microtubule-associated Dbl family member of guanine exchange
factors with distinct transaction activity for RHOA, provides cell
growth and survival in RAS-transformed cells. ARHGEF2 promotes cell
motility via the activation of RhoA signaling in hepatocellular
carcinoma in an amplified condition (44). The current results indicate that SCU
treatment downregulated the ARHGEF2 protein expression in AGS
cells, and ARHGEF2 was found to participate in biological
processes, such as cell movement or subcellular component,
locomotion, and apoptotic process. Toll-like receptor 2 (TLR2) is a
major regulator of the responses of the innate immune system, and
alteration of this protein leads to inflammation-related
malignancies in GC, where the expression levels at both the mRNA
and protein are elevated by more than 50% in GC patient tumors. The
TLR2-promoted growth receptivity of human GC cells coincides with
the elevation of anti-apoptotic proteins, such as BCL2, BCL2A1,
TNFAIP3, BIRC3, CFLAR, and IER3 (45). Expression of TLR2 and TLR4 is
elevated in H. pylori-positive gastritis patients, and their
gene polymorphisms are correlated with an elevated risk of GC; thus
selectively blocking TLR2 may be a therapeutic approach to the
suppression of tumorigenesis (46).
SCU treatment showed depletion of TLR2 protein expression in AGS
cells, and GO analysis revealed it to participate in biological
processes, such as response to macrophage colony-stimulating factor
and apoptotic process. AKT3 is a member of the AKT family proteins,
and a central protein for the signal mediation from receptor
tyrosine kinases, and phosphatidylinositol 3-kinase (PI3K). AKT3
regulates multiple biological processes, such as cell cycle
progression, cell proliferation, apoptosis, migration, and invasion
(47). Knockdown of AKT3 was found
to induce apoptosis by activating caspase-9 and caspase-3 in human
glioblastoma cell lines U87MG and T98G (48). AKT3 was found to be upregulated in
GC tissues compared to that in para-carcinoma tissues at the mRNA
level, whereas suppression of AKT3 expression partially by
miR-582-5p suppressed tumorigenesis of GC, by promoting cell
apoptosis and G0/G1 arrest (49).
SCU downregulated the expression of AKT3 in AGS cells, which may
support our previous results that demonstrated that activation of
caspase-9 and caspase-3 in GC cells leads to apoptosis (16). GO analysis of differentially
expressed proteins from AGS cells treated with SCU also showed the
involvement of AKT3 in biological processes, including cell
movement or subcellular component and locomotion, and pathway
analysis showed that it participates in angiogenesis, the EGF
receptor signaling and apoptosis signaling pathways, exerts hypoxia
via HIF activation, participates in the p53 pathway, inflammation
mediated by the chemokine and cytokine signaling pathway and PI3
kinase pathway. Apart from all these proteins, another protein that
gained our attention which is differentially expressed in SNU484
cells treated with SCU, is CIP2A (cancerous inhibitor of protein
phosphatase 2A). As confirmed by immunoblotting, CIP2A protein
expression was downregulated after the treatment with SCU in AGS
and SNU484 cells. CIP2A gained our attention, due to similar
results observed in our previous study where the flavonoid,
Pectolinarigenin significantly decreased the protein expression of
CIP2A in AGS and MKN28 human GC cells. CIP2A is an oncoprotein that
affects cancer cell proliferation, anchorage-independent cell
growth, and apoptotic resistance in human cancer cells (26). The inactivation of protein
phosphatase 2A (PP2A) occurs by CIP2A depletion and also the
phosphorylation of Akt which sustains c-Myc oncogene product in
cancer cells. CIP2A has been identified to be elevated at both mRNA
and protein levels in GC tissues. Gene silencing approach of CIP2A
protein in GC cells was found to reduce the colony-forming
potential and proliferation rate of cancer cells, which is a great
strategy for the therapy of GC (50). Research has revealed that
pharmacological decrease of CIP2A resulted in inhibition of cell
proliferation and induced apoptosis in breast cancer cells
(51).
Study limitations and further study
The present study consists of the preliminary data
of altered proteins upon treatment of GC cells with SCU;
in-vivo or solid tumor data were included. In the present
study we have selected to validate a subset of critical proteins
depending upon the correlation to the current and to our previous
study using western blot analysis, and further validation of
remaining critical proteins will help to understand the molecular
mechanism. Further experiments, such as data using cell lines in
vivo with knockdown and/or overexpression experimental models
of these biomarker candidates will be conducted, to narrow down the
valid biomarker candidate proteins for the treatment of SCU in
GC.
In conclusion, the current study highlights an
innovative strategy to investigate physiologically relevant targets
of SCU treatment, with an emphasis on its functional effects. The
synergistic analyses of proteomics data were successful in
identifying a unique set of proteins regulated by SCU to induce
apoptosis in both treated GC cell lines. Proteins such as PIK3CB,
OFD1, CIP2A, BUB1B, ARHGEF2, VCL, EPHB2, IF4G2, CACNA2D1, HIP1R,
VAV, and TLR2 have been previously reported to be associated with
apoptosis, cell cycle arrest and tumor suppressors induced by
another flavonoid in GC cell lines (17). Previous and current data showed that
these protein candidates can be altered upon treatment with
flavonoids in GC cells. We can consider these proteins for further
investigation for their applicability as treatment markers of
apoptosis, especially in the context of evaluating the intensity to
which GC cells have undergone apoptosis following chemotherapy
using flavonoids. In this aspect, the current results may be of
clinical utility in the estimation of the GC response to
flavonoids, and may aid in their future development as novel
clinical therapeutic agents with which to treat GC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea funded by the Ministry of Science and
ICT (grant nos. 2012M3A9B8019303 and 2020R1A2B5B01001807).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
VVGS and GSK conceived and designed the experiments,
performed the experiments, organized focus group discussion,
collected, analyzed all study data and prepared the final
manuscript. PV, RM and HJL carried out the bioinformatics analysis,
contributed to the statistical analysis and editing of the
manuscript. SMK, SEH, JDH and EHK participated in focus group
discussion and revised the study design, revised the results and
final revision of the manuscript for publication. All authors read
and approved the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shin A, Kim J and Park S: Gastric cancer
epidemiology in Korea. J Gastric Cancer. 11:135–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivas-Ortiz CI, Lopez-Vidal Y,
Arredondo-Hernandez LJR and Castillo-Rojas G: Genetic alterations
in gastric cancer associated with helicobacter pylori infection.
Front Med (Lausanne). 4:472017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilson DH: Advances in the treatment of
gastric cancer. Curr Opin Gastroenterol. 34:465–468. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S and Yuan L: Predictive biomarkers
for targeted and cytotoxic agents in gastric cancer for
personalized medicine. Biosci Trends. 10:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An W, Lai H, Zhang Y, Liu M, Lin X and Cao
S: Apoptotic pathway as the therapeutic target for anticancer
traditional chinese medicines. Front Pharmacol. 10:7582019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pavet V, Portal MM, Moulin JC, Herbrecht R
and Gronemeyer H: Towards novel paradigms for cancer therapy.
Oncogene. 30:1–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alessandro R, Fontana S, Kohn E and De Leo
G: Proteomic strategies and their application in cancer research.
Tumori. 91:447–455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ludwig JA and Weinstein JN: Biomarkers in
cancer staging, prognosis and treatment selection. Nat Rev Cancer.
5:845–856. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanash SM, Madoz-Gurpide J and Misek DE:
Identification of novel targets for cancer therapy using expression
proteomics. Leukemia. 16:478–485. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Chen L, Kong X, Huang T and Cai
YD: Analysis of tumor suppressor genes based on gene ontology and
the KEGG pathway. PLoS One. 9:e1072022014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HH, Lim CA, Cheong YT, Singh M and Gam
LH: Comparison of protein expression profiles of different stages
of lymph nodes metastasis in breast cancer. Int J Biol Sci.
8:353–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agbarya A, Ruimi N, Epelbaum R, Ben-Arye E
and Mahajna J: Natural products as potential cancer therapy
enhancers: A preclinical update. SAGE Open Med.
2:20503121145469242014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chahar MK, Sharma N, Dobhal MP and Joshi
YC: Flavonoids: A versatile source of anticancer drugs. Pharmacogn
Rev. 5:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Venkatarame Gowda Saralamma V, Kim
SM, Ha SE, Raha S, Lee WS, Kim EH, Lee SJ, Heo JD and Kim GS:
Pectolinarigenin induced cell cycle arrest, autophagy, and
apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling
pathway. Nutrients. 10:10432018. View Article : Google Scholar
|
|
15
|
Sang Eun H, Seong Min K, Ho Jeong L,
Vetrivel P, Venkatarame Gowda Saralamma V, Jeong Doo H, Eun Hee K,
Sang Joon L and Gon Sup K: Scutellarein induces fas-mediated
extrinsic apoptosis and G2/M cell cycle arrest in Hep3B
hepatocellular carcinoma cells. Nutrients. 11:2632019. View Article : Google Scholar
|
|
16
|
Gowda Saralamma VV, Lee HJ, Raha S, Lee
WS, Kim EH, Lee SJ, Heo JD, Won C, Kang CK and Kim GS: Inhibition
of IAP's and activation of p53 leads to caspase-dependent apoptosis
in gastric cancer cells treated with scutellarein. Oncotarget.
9:5993–6006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HJ, Venkatarame Gowda Saralamma V, Kim
SM, Ha SE, Vetrivel P, Kim EH, Lee SJ, Heo JD, Rampogu S, Lee KW
and Kim GS: Comparative proteomic profiling of tumor-associated
proteins in human gastric cancer cells treated with
pectolinarigenin. Nutrients. 10:15962018. View Article : Google Scholar
|
|
18
|
Swain M and Ross NW: A silver stain
protocol for proteins yielding high resolution and transparent
background in sodium dodecyl sulfate-polyacrylamide gels.
Electrophoresis. 16:948–951. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shevchenko A, Wilm M, Vorm O and Mann M:
Mass spectrometric sequencing of proteins silver-stained
polyacrylamide gels. Anal Chem. 68:850–858. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baker PR and Chalkley RJ: MS-viewer: A
web-based spectral viewer for proteomics results. Mol Cell
Proteomics. 13:1392–1396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halgren TA, Murphy RB, Friesner RA, Beard
HS, Frye LL, Pollard WT and Banks JL: Glide: A new approach for
rapid, accurate docking and scoring. 2. Enrichment factors in
database screening. J Med Chem. 47:1750–1759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tabas-Madrid D, Nogales-Cadenas R and
Pascual-Montano A: GeneCodis3: A non-redundant and modular
enrichment analysis tool for functional genomics. Nucleic Acids
Res. 40:W478–W483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thomas PD, Campbell MJ, Kejariwal A, Mi H,
Karlak B, Daverman R, Diemer K, Muruganujan A and Narechania A:
PANTHER: A library of protein families and subfamilies indexed by
function. Genome Res. 13:2129–2141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Starovoytov ON, Liu Y, Tan L and Yang S:
Effects of the hydroxyl group on phenyl based ligand/ERRγ protein
binding. Chem Res Toxicol. 27:1371–1379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen KF, Liu CY, Lin YC, Yu HC, Liu TH,
Hou DR, Chen PJ and Cheng AL: CIP2A mediates effects of bortezomib
on phospho-Akt and apoptosis in hepatocellular carcinoma cells.
Oncogene. 29:6257–6266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao F, Xu T, Wang X, Zhong S, Chen S,
Zhang M, Zhang X, Shen Y, Wang X, Xu C and Shen Z: CIP2A mediates
fibronectin-induced bladder cancer cell proliferation by
stabilizing beta-catenin. J Exp Clin Cancer Res. 36:702017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feather SA, Woolf AS, Donnai D, Malcolm S
and Winter RM: The oral-facial-digital syndrome type 1 (OFD1), a
cause of polycystic kidney disease and associated malformations,
maps to Xp22.2-Xp22.3. Hum Mol Genet. 6:1163–1167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wee S, Wiederschain D, Maira SM, Loo A,
Miller C, deBeaumont R, Stegmeier F, Yao YM and Lengauer C:
PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA.
105:13057–13062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riquelme I, Tapia O, Leal P, Sandoval A,
Varga MG, Letelier P, Buchegger K, Bizama C, Espinoza JA and Peek
RM: miR-101-2, miR-125b-2 and miR-451a act as potential tumor
suppressors in gastric cancer through regulation of the
PI3K/AKT/mTOR pathway. Cell Oncol (Dordr). 39:23–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rice MA, Ishteiwy RA, Magani F, Udayakumar
T, Reiner T, Yates TJ, Miller P, Perez-Stable C, Rai P, Verdun R,
et al: The microRNA-23b/-27b cluster suppresses prostate cancer
metastasis via Huntingtin-interacting protein 1-related. Oncogene.
35:4752–4761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ai J, Jin T, Yang L, Wei Q, Yang Y, Li H
and Zhu Y: Vinculin and filamin-C are two potential prognostic
biomarkers and therapeutic targets for prostate cancer cell
migration. Oncotarget. 8:82430–82436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rubashkin MG, Cassereau L, Bainer R,
DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY and Weaver
VM: Force engages vinculin and promotes tumor progression by
enhancing PI3K activation of phosphatidylinositol
(3,4,5)-triphosphate. Cancer Res. 74:4597–4611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu W, Zhao Y and Wu J: Gene expression
profile analysis of the progression of carotid atherosclerotic
plaques. Mol Med Rep. 17:5789–5795. 2018.PubMed/NCBI
|
|
36
|
Grassilli S, Brugnoli F, Lattanzio R,
Marchisio M, Perracchio L, Piantelli M, Bavelloni A, Capitani S and
Bertagnolo V: Vav1 downmodulates Akt in different breast cancer
subtypes: A new promising chance to improve breast cancer outcome.
Mol Oncol. 12:1012–1025. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davenport JW, Fernandes ER, Harris LD,
Neale GA and Goorha R: The mouse mitotic checkpoint gene bub1b, a
novel bub1 family member, is expressed in a cell cycle-dependent
manner. Genomics. 55:113–117. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Q, Liu Y, Shang L, Yu J and Qu Q: The
FOXM1/BUB1B signaling pathway is essential for the tumorigenicity
and radioresistance of glioblastoma. Oncol Rep. 38:3367–3375.
2017.PubMed/NCBI
|
|
39
|
Fort L, Batista JM, Thomason PA, Spence
HJ, Whitelaw JA, Tweedy L, Greaves J, Martin KJ, Anderson KI, Brown
P, et al: Fam49/CYRI interacts with Rac1 and locally suppresses
protrusions. Nat Cell Biol. 20:1159–1171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sakata M, Kitamura YH, Sakuraba K, Goto T,
Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y and
Hibi K: Methylation of HACE1 in gastric carcinoma. Anticancer Res.
29:2231–2233. 2009.PubMed/NCBI
|
|
41
|
Sanaei M, Kavoosi F, Roustazadeh A and
Golestan F: Effect of genistein in comparison with trichostatin a
on reactivation of dnmts genes in hepatocellular carcinoma. J Clin
Transl Hepatol. 6:141–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ravichandran K and Edelstein CL:
Polycystic kidney disease: A case of suppressed autophagy? Semin
Nephrol. 34:27–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang Z, Lin MG, Stowe TR, Chen S, Zhu M,
Stearns T, Franco B and Zhong Q: Autophagy promotes primary
ciliogenesis by removing OFD1 from centriolar satellites. Nature.
502:254–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kent OA, Sandi MJ, Burston HE, Brown KR
and Rottapel R: An oncogenic KRAS transcription program activates
the RHOGEF ARHGEF2 to mediate transformed phenotypes in pancreatic
cancer. Oncotarget. 8:4484–4500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
West AC, Tang K, Tye H, Yu L, Deng N,
Najdovska M, Lin SJ, Balic JJ, Okochi-Takada E, McGuirk P, et al:
Identification of a TLR2-regulated gene signature associated with
tumor cell growth in gastric cancer. Oncogene. 36:5134–5144. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Oliveira JG and Silva AE: Polymorphisms
of the TLR2 and TLR4 genes are associated with risk of gastric
cancer in a Brazilian population. World J Gastroenterol.
18:1235–1242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li L and Ma L: Upregulation of miR-582-5p
regulates cell proliferation and apoptosis by targeting AKT3 in
human endometrial carcinoma. Saudi J Biol Sci. 25:965–970. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Turner KM, Sun Y, Ji P, Granberg KJ,
Bernard B, Hu L, Cogdell DE, Zhou X, Yli-Harja O, Nykter M, et al:
Genomically amplified Akt3 activates DNA repair pathway and
promotes glioma progression. Proc Natl Acad Sci USA. 112:3421–3426.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jin Y, Tao LP, Yao SC, Huang QK, Chen ZF,
Sun YJ and Jin SQ: MicroRNA-582-5p suppressed gastric cancer cell
proliferation via targeting AKT3. Eur Rev Med Pharmacol Sci.
21:5112–5120. 2017.PubMed/NCBI
|
|
50
|
Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia
J and Xu D: CIP2A is overexpressed in gastric cancer and its
depletion leads to impaired clonogenicity, senescence, or
differentiation of tumor cells. Clin Cancer Res. 14:3722–3728.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Niemela M, Kauko O, Sihto H, Mpindi JP,
Nicorici D, Pernilä P, Kallioniemi OP, Joensuu H, Hautaniemi S and
Westermarck J: CIP2A signature reveals the MYC dependency of
CIP2A-regulated phenotypes and its clinical association with breast
cancer subtypes. Oncogene. 31:4266–4278. 2012. View Article : Google Scholar : PubMed/NCBI
|