Introduction
Renal cell carcinoma (RCC) is a common malignant
tumor of the urinary system in adults, accounting for 3% of all
cancer cases (1). In recent years,
the incidence of RCC has been increasing annually; its degree of
malignancy is extremely high, and it is difficult to detect in the
early stage (2). By the time it is
first diagnosed, 30% of RCC patients present with tumor metastases
(3), and the survival rate of
patients with metastatic RCC can be as low as 9.5% (4). Renal cancer is not sensitive to
conventional chemo-radiotherapy and the effectiveness of molecular
targeted therapy is low. Therefore, exploring effective treatment
methods for RCC is of great significance for improving the survival
rate of patients with RCC.
Cytotoxic T lymphocytes (CTLs) are specific immune
defense effector cells of the immune system. Several factors affect
their killing effects on tumors, such as the secretion of
interferon (IFN)-γ and tumor necrosis factor (TNF) and the distance
between CTLs and tumors (5).
Clinical results have revealed that T-cell persistence can improve
therapeutic responses, and immune memory can enhance T-cell
persistence (6,7). As a result, immune memory is essential
for therapeutic responses. Preclinical animal models have also
confirmed that memory T cells are key to antitumor effects,
including central memory T (TCM) and effector memory T
(TEM) cells (8).
CD4+T cells are indispensable for functional
CD8+T cell memory formation (9). In addition, CD4+T cells
play several roles in cancer treatment, including enhancing the
activation, recruitment, proliferation and cytotoxic function of
CD8+T cells and directly inhibiting tumor growth
(10,11). Given the functions of
CD4+T cells, these T cells were used in the present
experiments. CTLL-2 cells isolated from C57/BL/6 inbred mice are
mouse-derived cytotoxic T cells, and the signaling pathway in
CTLL-2 cells can be used as a model in CTL cells (12).
As an important tumor treatment method, chemotherapy
can kill cancer cells in the rapid proliferation stage. However,
the dose and duration of the effects of chemotherapeutic drugs are
limited by acute and cumulative toxicity in normal tissues
(13,14). Hence, it is urgent to identify
anticancer treatments with natural properties, low toxicity, and
high efficacy. Low-dose chemotherapy not only has no significant
side effects or inhibitory effects on the immune system, but can
also improve the immune activity of some effector cells (15). In the present study,
diaminedichloroplatinum (DDP) and mitomycin C (MMC), two
traditional broad-spectrum chemotherapeutic drugs used for various
types of tumors (16), were
selected to study the toxicity of low-dose drugs in tumor cells.
DDP is a nonspecific chemotherapeutic drug that can inhibit
proliferation and promote apoptosis in tumor cells (17), and it has been used for several
years in clinical tumor treatment (18). MMC is also widely used for the
treatment of various types of cancer, such as bladder, stomach and
cervical cancer (19). These two
drugs were selected with the purpose of extending this approach to
more tumors than just renal cancer.
The rapid development of immunotherapy has generated
a fourth therapy for cancers, which can be used in parallel with
surgery, chemotherapy and radiotherapy (20). Cellular immunotherapy can recognize
a small number of tumor cells and has powerful lethal and targeting
effects (21). Therefore, low-dose
chemotherapy combined with cellular immunotherapy is of great
significance for clearing small lesions and reducing recurrence
rate in patients. In the present study, the synergistic killing
effect of low dose-chemotherapy combined with T cells on RCC cells
in vitro was observed and the possible underlying
synergistic mechanisms were explored. The present results provided
a new concept and an experimental basis for the comprehensive
treatment of RCC.
Materials and methods
Cell culture and chemotherapy
treatment
The RCC cell lines RENCA and ACHN and the
mouse-derived cytotoxic T cells CTLL-2 were obtained from the
Jiangsu Cancer Biotherapy Institute, Xuzhou Medical University
(Xuzhou, China). RENCA and CTLL-2 cells were cultured in RPMI-1640
medium and ACHN cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Life Technologies; Thermo Fisher Scientific,
Inc.). All media were supplemented with 10% fetal bovine serum
(FBS; Gibco; Life Technologies; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin (Sangon Biotech Co., Ltd.). RCC cells
were detached using 0.25% trypsin (Beyotime Institute of
Biotechnology). All cells were maintained in incubators (Thermo,
Fisher Scientific, Inc.) with 95% air and 5% CO2 at
37°C.
DDP and MMC were purchased from Jiangsu Hansoh
Pharmaceutical Group Co., Ltd. and Vicmed, respectively. The
concentration of the DDP storage solution was 5 mg/ml. Two
milligrams of MMC powder was dissolved in phosphate-buffered saline
(PBS) to prepare a 2 mg/ml stock solution. For chemotherapy
treatment and antitumor analysis, renal cancer cells were treated
with serially diluted doses of DDP or MMC for 24 h. In order to
avoid the cumulative toxicity of drugs, tumor cells treated with
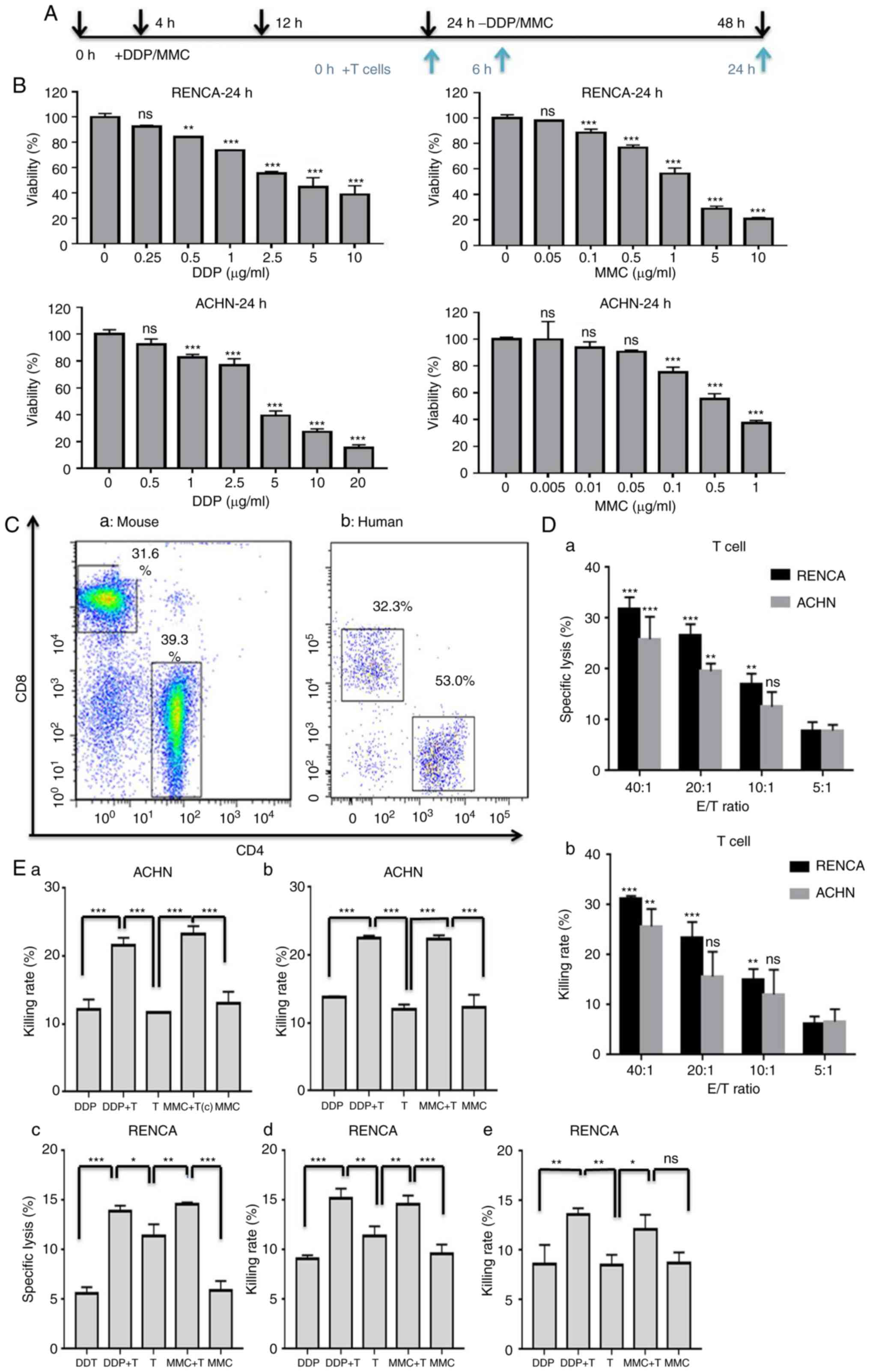
drugs for 24 h were used to co-incubate with T cells (Fig. 1A).
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 detection kit (Nanjing KeyGen Biotech Co.,
Ltd.) was used to select a low-dose drug concentration with an
inhibition rate of <30% that kills tumor cells and avoids toxic
effects on normal tissues (22) and
to evaluate the sensitivity of tumor cells to chemotherapy drugs.
Briefly, the two cell lines were inoculated into 96-well plates at
4.0×103 cells/well for 24 h, and then the cells were
treated with drugs for another 24 h. RENCA cells were exposed to
various concentrations of DDP diluent (0, 0.25, 0.5, 1, 2.5, 5 and
10 µg/ml) or MMC diluent (0, 0.05, 0.1, 0.5, 1, 5 and 10 µg/ml),
ACHN cells were exposed to various concentrations of DDP diluent
(0, 0.5, 1, 2.5, 5, 10 and 20 µg/ml) or MMC diluent (0, 0.005,
0.01, 0.05, 0.1, 0.5 and 2 µg/ml). Subsequently, the cells were
incubated in 100 µl serum-free medium with 10 µl CCK-8 solution at
37°C for 2 h. The relative cell viability was determined by
measuring the absorbance of the converted dye at 450 nm. RENCA and
ACHN cells were exposed to 1 µg/ml DDP and 0.1 µg/ml MMC diluent
separately for 0, 24, 48 and 72 h to detect tumor cell
proliferation.
Preparation of T cells
Ten 4-week-old female BALB/c mice weighing 16 g
(Huafukang Bioscience) were raised with the feed and sterile water
for 1 week at our well-ventilated animal facility with 25°C,
humidity of 60% and a 12-h light/dark cycle before testing was
initiated. The Animal Experimental Ethics Committee of Xuzhou
Medical University approved all the animal studies. The mice were
sacrificed by cervical dislocation and the disappearance of a blink
reflex indicated the death of the mice. T cells were isolated from
the lymph nodes and spleen of mice with an EasySep™ Mouse T Cell
Isolation Kit (STEMCELL Technologies, Inc.). T cells were cultured
in RPMI-1640 complete medium at a cell density of 1×106
/ml and activated by CD3 antibody (5 µg/ml) and CD28 antibody (2
µg/ml; both from Sigma-Aldrich; Merck KGaA). Peripheral blood
mononuclear cells (PBMCs) from a 25-year-old female healthy blood
donor who agreed to use her samples in scientific research were
collected and isolated by density gradient centrifugation at our
institute. The inclusion of human subjects in our study was
approved by the Ethics Committee of the First People's Hospital of
Lianyungang and the informed consent was obtained from the blood
donor. T cells were isolated with an EasySep™ Human T Cell
Isolation Kit (STEMCELL Technologies, Inc.) and amplified using a
Dynabeads® Human T-Expander CD3/CD28 Kit (Thermo, Fisher
Scientific, Inc.). T cells were activated in RPMI-1640 complete
medium with 300 IU/ml interleukin (IL)-2 (SL PHARM) at a cell
density of 1×106 /ml. After 48 h of activation, the T
cells were used for subsequent experiments.
Flow cytometric analysis
Flow cytometry was used to detect the surface
expression of CD4 and CD8 in T cells. The antibodies used for
staining were purchased from BD Biosciences and included anti-mouse
CD4-fluorescein isothiocyanate (FITC; cat. no. 557667), anti-mouse
CD8-peridinin chlorophyll protein-cyanin 5.5 (PerCP-Cy5.5; cat. no.
551162), anti-human CD4-FITC (cat. no. 557695) and anti-human
CD8-phycoerythrin (PE; cat. no. 557086). A total of
1×106 T cells were incubated in 100 µl PBS with 0.5 µl
fluorescence-labeled antibody for 30 min at room temperature in the
dark, washed, and then analyzed using a FACS instrument (FACSCanto
II; BD Biosciences).
An FITC-Annexin V Apoptosis Detection Kit (Nanjing
KeyGen Biotech Co., Ltd.) was used to detect the effects of
chemotherapeutic drugs on apoptosis in RCC cells. RENCA and ACHN
cells, which had been exposed to 1 µg/ml DDP or 0.1 µg/ml MMC for
24 h, were stained with 5 µl Annexin V-FITC and 5 µl propidium
iodide in 500 µl binding buffer for 15 min at room temperature in
the dark, washed, and then analyzed with a FACS instrument. T cells
were mixed with RCC cells that had been treated with DDP and MMC in
a 10:1 ratio and cultured in a 24-well plate for 24 h. After
removing the T cells, 1×105 RCC cells were stained for
analysis.
The effects of chemotherapeutic drugs on T cell
phenotypes were detected using flow cytometry. RENCA cells were
exposed to 1 µg/ml DDP or 0.1 µg/ml MMC for 24 h, and T cells were
then mixed with the RENCA cells at a 10:1 ratio and cultured in a
24-well plate for 24 h. The antibodies used for staining were
purchased from BD Biosciences and included anti-CD8-PerCP-Cy5.5
(cat. no. 551162), anti-CD62L-allophycocyanin (APC; cat. no.
561919), anti-CD44-PE (cat. no. 561860), and anti-programmed death
factor 1 receptor/ligand (PD-L1)-APC (cat. no. 564715). A total of
1×106 T cells were stained with 0.5 µl
fluorescence-labeled antibody in 100 µl PBS for 30 min at room
temperature in the dark, and then the stained T cells were detected
using a flow cytometer.
Cytotoxicity assay
T cells were cultured with target cells (RENCA/ACHN)
in 96-well plates at effector-to-target (E/T) ratios of 40:1, 20:1,
10:1 and 5:1 for 6 h (6,23). The released lactate dehydrogenase
(LDH) in the supernatants was assessed using the CytoTox
96® Non-Radioactive Cytotoxicity Test Kit (Promega
Corporation) according to the manufacturer's recommendations. The
specific cytotoxicity was calculated according to the formula: %
Cytotoxicity=100× [(experimental release-effector spontaneous
release-target spontaneous release)/(target maximal release-target
spontaneous release)]. The CCK-8 method was also utilized to
evaluate the killing effect of T cells on RCC cells. Before
testing, the T cells were removed, and RCC cells were then
incubated in 100 µl serum-free medium with 10 µl CCK-8 solution at
37°C for 2 h. The viability was determined by measuring the
absorbance of the converted dye at 450 nm.
RCC cells (RENCA/ACHN) were exposed to 1 µg/ml DDP
or 0.1 µg/ml MMC for 24 h. T cells were mixed with RCC cells at a
10:1 ratio and cultured in 96-well plates for 6 h. LDH and CCK-8
assays were used to explore the cell toxicity effects induced by T
cells combined with chemotherapy. Additionally, luciferase
transfected RCC cell lines preserved in our laboratory were used to
determine the cell killing rate by measuring the absorbance at 135
nm within 15 min after adding fluorescein (TCI) to the 96-well
plates in the dark as previously described (24).
Enzyme-linked immunosorbent assay
(ELISA)
T cells were mixed with RCC cells that had been
treated with 1 µg/ml DDP or 0.1 µg/ml MMC for 24 h at an E/T ratio
of 10:1 in a 96-well plate for 6 or 24 h. The IFN-γ level in the
supernatant was analyzed by ELISA kits [MultiSciences (Lianke)
Biotech, Co., Ltd.] according to the manufacturer's
recommendations.
RCC cells (RENCA/ACHN) were exposed to 1 µg/ml DDP
or 0.1 µg/ml MMC for 24 h, and then the TGF-β in the supernatant
was analyzed. RCC cells that had been exposed to 1 µg/ml DDP or 0.1
µg/ml MMC for 24 h were cultured in medium for another 24 h. The
TGF-β level in the supernatant was assayed by an ELISA kit
[MultiSciences (Lianke) Biotech, Co., Ltd.] according to the
manufacturer's recommendations.
Cell chemotaxis analysis
The effects of drugs (DDP/MMC) on the chemotaxis of
T cells were assessed using Transwell chambers (5-µm polycarbonate
membrane; Costar; Corning, Inc). Briefly, the top chamber was
loaded with 0.3 ml 2×106 T cells in media, and the
bottom chamber was loaded with 0.5 ml 2×105 RCC cells in
medium. After 6 and 24 h of treatment, the penetrating T cells were
collected, stained with a 0.4% trypan blue solution (Sigma-Aldrich;
Merck KGaA), and counted under a light microscope.
Western blot analysis
After 24 h of treatment with DDP or MMC, the drugs
were removed and the RCC cells were cultured in medium for another
24 h. RCC cells that had been treated with drugs for 4, 12, and 48
h were collected, and T cells were collected after a 24-h
incubation with RCC cells treated with DDP or MMC at a 10:1 ratio.
Protein from these cells was extracted in lysis buffer (Beyotime
Institute of Biotechnology). Equal amounts of protein (20 µg) after
protein concentration determination by BCA on a microplate reader
(ELx800™; BioTek USA) were loaded, separated on 10% sodium dodecyl
sulfate (SDS) gels and transferred onto nitrocellulose membranes
(Amersham; GE Healthcare). Then, the membranes were blocked with 5%
nonfat milk at room temperature for 2 h and immunoblotted with
anti-TGF-βR1 (3712), Smad2 antibody (5339; 1:1,000; Cell Signaling
Technology, Inc.) or β-actin antibody (4967; 1:1,000; Cell
Signaling Technology, Inc.) overnight at 4°C. A horseradish
peroxidase (HRP)-conjugated anti-rabbit or mouse antibody (BA1056;
1:10,000; Wuhan Boster Biological Technology, Ltd.) was reacted
with the membrane at room temperature for 2 h. The immobilized
antibodies were then detected by an ECL chemiluminescent reagent
(EMD Millipore) and visualized with the ChemiDoc XRS system
(Bio-Rad laboratories, Inc.).
Statistical analysis
The data are presented as the mean ± standard
deviation (SD) of three independent experiments. The data were
analyzed using SPSS statistical software version 18.0 (SPSS, Inc.).
Comparisons between two groups were performed using a two-tailed
Student's t-test. One-way ANOVA and Tukey's post hoc test were used
when more than two groups were compared. Differences with P<0.05
were considered statistically significant.
Results
Killing effects of T cells combined
with low dose chemotherapy on renal cancer cells in vitro
CCK-8 assays were performed to determine the
concentrations of DDP and MMC with an inhibitory rate of <30% in
the early stage (24 h). The inhibitory effects of DDP or MMC on RCC
cells were revealed to be dose-dependent. As drug concentrations
increased, the survival rate was obviously decreased in RCC cells
when compared with untreated cells (Fig. 1B). After 24 h that of treatment with
drugs, the viabilities of RENCA and ACHN cells were 73.85±0.35 and
82.89±2.07%, respectively, in the DDP (1 µg/ml) group and
88.58±2.68 and 75.35±3.75%, respectively, in the MMC (0.1 µg/ml)
group (Table I). Follow-up
experiments were carried out with 1 µg/ml DDP and 0.1 µg/ml
MMC.
 | Table I.Effects of DDP and MMC on the
viability of RCC cells (mean ± SD). |
Table I.
Effects of DDP and MMC on the
viability of RCC cells (mean ± SD).
| RENCA cells | ACHN cells |
|---|
|
|
|---|
| Drug concentration
(µg/ml) | Viability at 24 h
(%) | Drug concentration
(µg/ml) | Viability at 24 h
(%) |
|---|
| DDP |
|
|
|
| 0 | 100.00±2.95 | 0 | 100.00±3.35 |
|
0.25 |
92.52±1.07a | 0.5 |
92.49±3.94a |
|
0.5 |
84.28±0.01b | 1 |
82.89±2.07b |
| 1 |
73.85±0.35b | 2.5 |
77.07±4.72b |
|
2.5 |
55.42±1.58b | 5 |
39.38±3.44b |
| 5 |
44.85±7.32b | 10 |
27.25±2.29b |
| 10 |
38.86±6.87b | 20 |
15.57±2.07b |
| MMC |
|
|
|
| 0 | 100.00±2.69 | 0 | 100.00±1.49 |
|
0.05 |
97.99±0.41c | 0.005 |
99.84±13.33c |
|
0.1 |
88.58±2.68d | 0.01 |
93.84±4.25c |
|
0.5 |
76.82±2.00d | 0.05 |
90.56±1.32c |
| 1 |
56.28±4.47d | 0.1 |
75.35±3.75d |
| 5 |
28.78±2.05d | 0.5 |
55.46±3.86d |
| 10 |
20.97±1.06d | 1 |
37.55±1.69d |
FACS was used to assess the surface expression of
CD4 and CD8 in purified mouse or human T cells. The percentages of
CD4 and CD8 cells were 38.1±0.14 and 31.7±0.42%, respectively, in
mouse T cells and 52.1±1.27 and 32.65±0.49%, respectively, in human
T cells (Fig. 1C). To evaluate the
cytotoxic effects of T cells against renal cancer cells, lactate
dehydrogenase (LDH) release assays and CCK-8 assays were performed.
As revealed in Fig. 1D, with the
increase in the E/T ratio, the killing activity of T cells against
RENCA and ACHN cells was enhanced compared to that in the 5:1 E/T
ratio group. Therefore, an E/T ratio of 10:1 was used for all
subsequent in vitro studies.
To explore whether DDP or MMC treatment could
increase the sensitivity of RCC cells to T cell-mediated lysis,
RENCA and ACHN cells were treated with drugs for 24 h and then used
as target cells in LDH release assays, CCK-8 assays, and Luc
assays. The results revealed that DDP and MMC significantly
increased the sensitivity of RENCA and ACHN cells to the effects of
T cells (Fig. 1E). Collectively,
the results revealed that DDP and MMC was capable of altering renal
cancer cells to render them more amenable to T cell-mediated
attack, and low-dose chemotherapy and the T cells exhibited a
synergistic killing effect on renal cancer cells in
vitro.
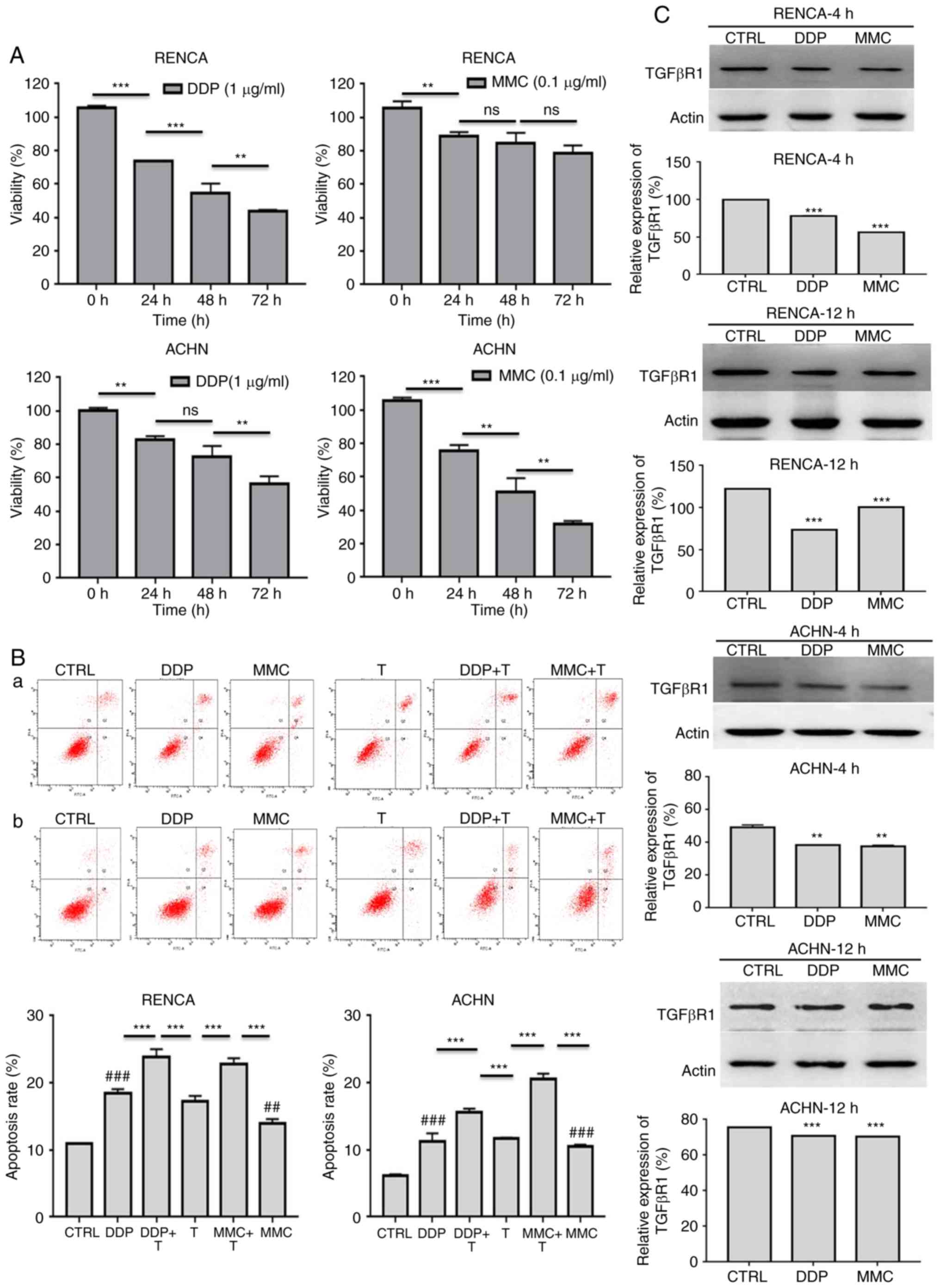
Effect of low-dose chemotherapy on
renal cancer cells in vitro
First, the CCK-8 method was utilized to assay the
effect of DDP and MMC on the proliferation of RENCA and ACHN cells.
RENCA and ACHN cells were exposed to 1 µg/ml DDP or 0.1 µg/ml MMC
for 0, 24, 48, or 72 h, and the viability of RENCA and ACHN cells
was significantly decreased as the reaction time increased
(Fig. 2A; Table II). Then, FACS was used to assess
the apoptosis rates of RENCA and ACHN cells treated with
chemotherapy drugs (24 h) or chemotherapy combined with T cells (48
h). Compared with those of untreated cells, the apoptosis rates of
RCC cells treated with DDP or MMC were significantly increased
(Fig. 2B; Table III). These data also revealed that
low-dose chemotherapy and T cells had a synergistic apoptotic
effect on renal cancer cells in vitro. Finally, western blot
analysis was performed to detect the expression of transforming
growth factor-β receptor 1 (TGF-βR1) in renal cancer cells treated
with DDP or MMC for 4 h and 12 h. The results revealed that DDP and
MMC significantly decreased the expression of TGF-βR1 compared with
untreated cells, indicating that chemotherapy drugs could inhibit
the expression of immune-related proteins in the early stage of
treatment to enhance the anti-tumor effect of T cells (Fig. 2C).
 | Table II.Effects of DDP and MMC on the
proliferation of RCC cells. |
Table II.
Effects of DDP and MMC on the
proliferation of RCC cells.
|
|
| Viability (%, mean
± SD) |
|---|
|
|
|
|
|---|
| Group | Drug concentration
(µg/ml) | 0 h | 24 h | 48 h | 72 h |
|---|
| RENCA | DDP (1) | 105.70±1.16 |
73.85±0.35a |
54.71±5.63b |
43.51±1.11d |
|
| MMC (0.1) | 105.65±4.03 |
88.58±2.68a |
84.44±6.39c |
78.58±4.64e |
| ACHN | DDP (1) | 100.20±1.71 |
82.89±2.07a |
72.69±6.39c |
56.31±4.63d |
|
| MMC (0.1) | 105.60±1.85 |
75.35±3.75a |
51.10±8.21b |
31.65±1.95d |
 | Table III.Apoptosis of RCC cells treated with
drugs or combined with T cells (%, mean ± SD). |
Table III.
Apoptosis of RCC cells treated with
drugs or combined with T cells (%, mean ± SD).
| Group | RENCA | ACHN |
|---|
| CTRL | 10.90±0.14 | 6.15±0.21 |
| DDP + T | 23.90±1.13 | 15.65±0.49 |
| MMC + T | 22.80±0.85 | 20.55±0.78 |
| DDP |
18.50±0.57a,b |
11.20±1.27a,b |
| MMC |
13.90±0.17a,c |
10.50±0.28a,c |
| T |
17.25±0.78b,c |
11.70±0.14b,c |
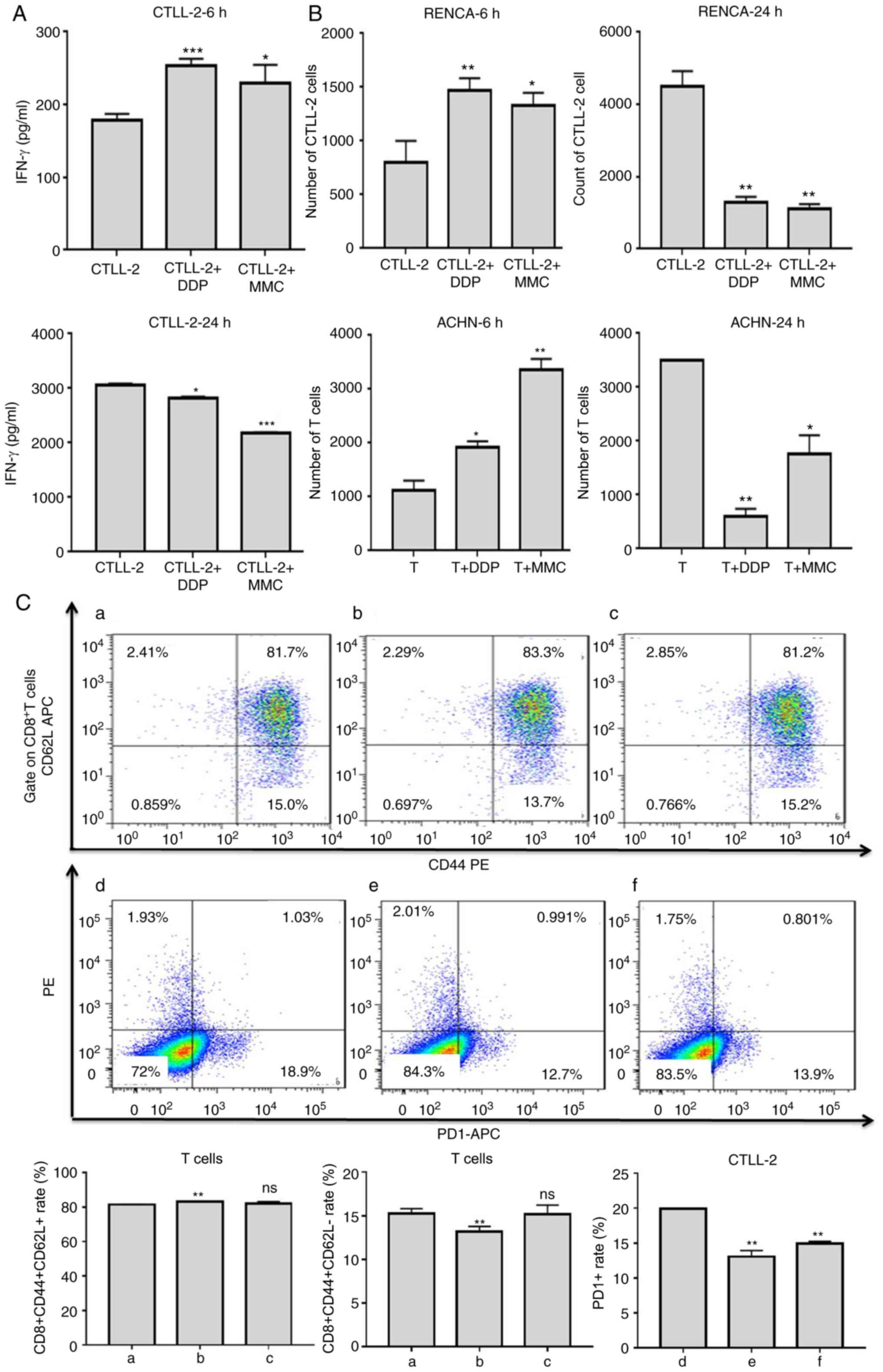
Effect of low-dose chemotherapy on T
cells in vitro
To investigate whether chemotherapy drugs could
enhance the specificity and activation of T cells, cytokine release
assays were performed. T cells were co-cultured with RCC cells that
had been treated with DDP or MMC for 24 h at an E/T ratio of 10:1.
After 6 h of incubation, the IFN-γ cytokine expression of
CTLL-2 cells in the drug groups was significantly higher than that
in the CTLL-2 untreated group (Fig.
3A). These results indicated that the DDP and MMC could
specifically promote IFN-γ cytokine expression to enhance
the killing effect of T cells in early stages. However, the level
of the cytokine IFN-γ in the drug-treated group was
significantly reduced after 24 h of incubation (Fig. 3A).
Cell migration assays were performed using a
Transwell system. As revealed in Fig.
3B, a significantly higher number of CTLL-2 or T cells passed
through the membrane into the lower chamber in the drug group than
in the untreated group after 6 h of incubation with RCC cells.
These results indicated that DDP and MMC could promote the
migration of T cells to tumor sites to enhance the killing effect
of T cells in the early stages. However, the migration of T cells
in the drug group was significantly inhibited, and even the number
of T cells was decreased compared to that in the untreated CTLL-2
or T cell group after 24 h of incubation (Fig. 3B).
It has been previously reported that chemotherapy
can alter the phenotype of T cells to render them more sensitive to
tumor cells (25). To determine
whether DDP or MMC could regulate the cell surface marker
expression, T cells were co-cultured with RENCA cells that had been
treated with DDP or MMC for 24 h, and then the T cells were stained
and analyzed by flow cytometry. DDP increased the percentage of
CD8+CD44+CD62L+T cells and
decreased the percentage of
CD8+CD44+CD62L−T cells compared
with the untreated cell group (Fig.
3C; Table IV). These data
indicated that low-dose DDP could promote the transformation of
effector memory T cells into central memory T cells to enhance the
cytotoxicity of T cells toward tumor cells. Compared with the
untreated cell group, DDP and MMC also significantly decreased PD1
expression in T cells (Fig. 3C;
Table IV). The altered expression
of these markers may aid in T cell cancer-killing effects.
 | Table IV.Effects of DDP and MMC on T cell
subtypes (%, mean ± SD). |
Table IV.
Effects of DDP and MMC on T cell
subtypes (%, mean ± SD).
| Groups | CD8+
(CD44+CD62L+) | CD8+
(CD44+CD62L−) | PD1 |
|---|
| T | 81.55±0.21 | 15.23±0.59 | 19.93±0.01 |
| T+DDP |
83.25±0.07a |
13.17±0.61a |
13.07±0.88a |
| T+MMC |
82.05±1.20b |
15.17±1.05b |
14.93±0.32a |
Possible causes of the functional
inhibition of T cells in vitro
To explore the causes of the decreased secretion of
T-cell immune factors and the inhibition of T-cell migration toward
tumor sites at 24 h, cytokine release assays and western blot
analyses were performed to detect the expression of the cytokine
TGF-β and the TGF-βR1 and Smad2 proteins in renal cancer cells.
Compared with those in the control (CTRL) group, RCC cells in the
DDP and MMC groups had stronger expression of TGF-β cytokine and
TGF-βR1 and Smad2 proteins, although the increase in TGF-βR1
expression in RENCA cells in the DDP group was not statistically
significant (Fig. 4A and B;
Table V). TGF-βR1 and Smad2 protein
expression in CTLL-2 and T cells was also detected. In the DDP and
MMC groups, TGF-βR1 and Smad2 protein expression was significantly
higher than that in the control group (Fig. 4C). These results indicated that the
TGF-β signaling pathway may be involved in inhibiting T-cell
function.
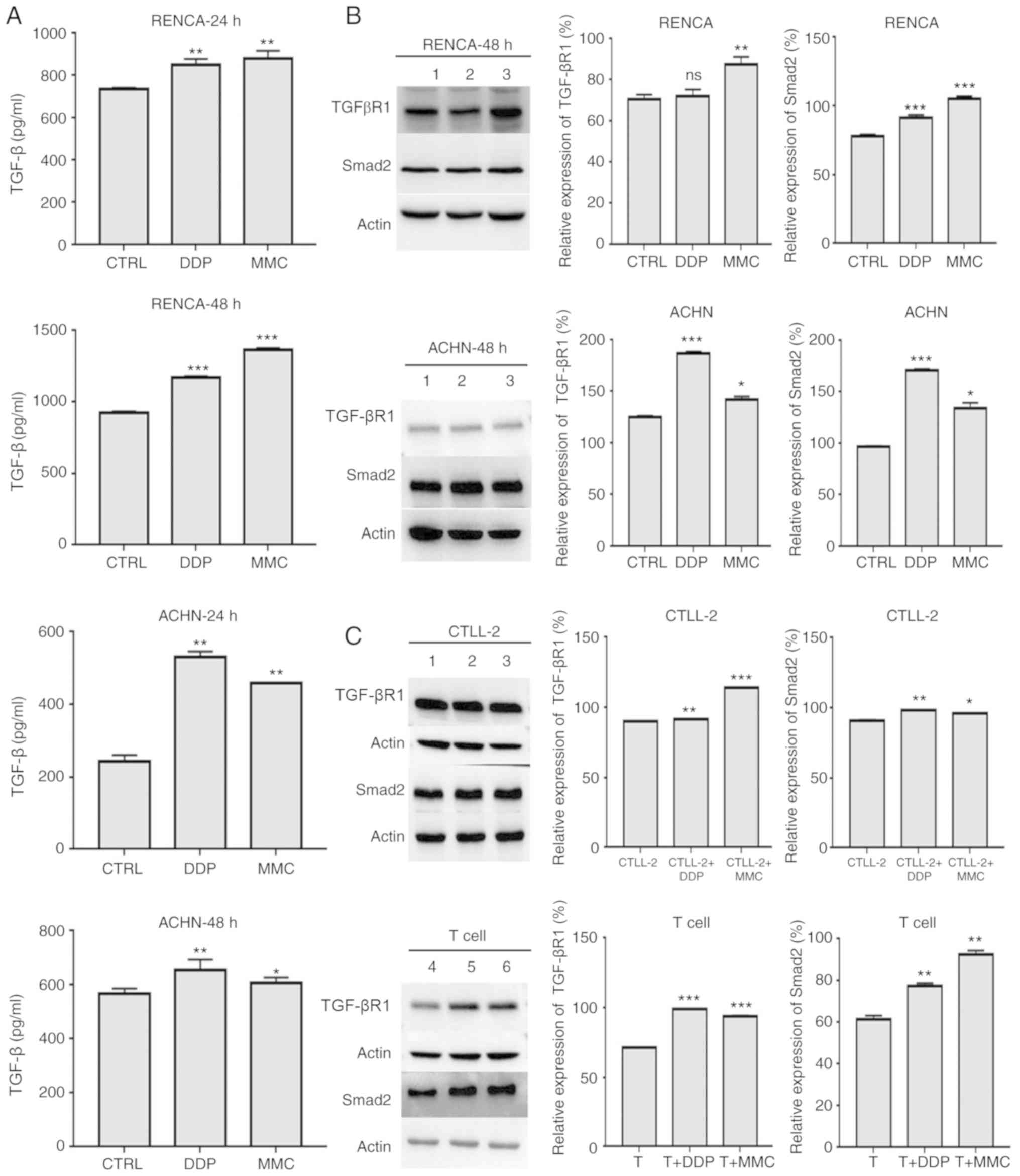 | Figure 4.Possible causes of the functional
inhibition of T cells in vitro. (A) TGF-β release by RCC
cells treated with DDP and MMC. RENCA and ACHN cells were treated
with 1 µg/ml DDP or 0.1 µg/ml MMC for 24 h and then incubated with
medium for another 24 h. The levels of the cytokine TGF-β released
by RENCA and ACHN cells were measured by ELISA. (B) TGF-βR1 and
Smad2 expression in RCC cells treated with DDP and MMC. RENCA and
ACHN cells were treated with 1 µg/ml DDP or 0.1 µg/ml MMC for 24 h
and then incubated with medium for another 24 h. TGF-βR1 and Smad2
expression in RENCA and ACHN cells was assessed by western blot
analysis. Actin was used to normalize the protein content. 1, CTRL
group; 2, DDP group; 3, MMC group. (C) TGF-βR1 and Smad2 expression
in T cells combined with DDP or MMC in RCC. RENCA and ACHN cells
treated with 1 µg/ml DDP or 0.1 µg/ml MMC for 24 h were coincubated
with CTLL-2 and T cells for another 24 h. TGF-βR1 and Smad2
expression in CTLL-2 and T cells was assessed by western blot
analysis. Actin was used to normalize the protein content. 1,
CTLL-2-cell group; 2, CTLL-2 cells combined with the DDP group; 3,
CTLL-2 cells combined with the MMC group; 4, T-cell group; 5, T
cells combined with the DDP group; 6, T cells combined with the MMC
group. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
RCC, renal cell carcinoma; DDP, diaminedichloroplatinum; MMC,
mitomycin C; ELISA, enzyme-linked immunosorbent assay; TGF-β,
transforming growth factor β; TGF-βR1, transforming growth factor β
receptor 1. |
 | Table V.Effects of DDP and MMC on TGF-β
secretion of RCC cells (%, mean ± SD). |
Table V.
Effects of DDP and MMC on TGF-β
secretion of RCC cells (%, mean ± SD).
|
| RENCA | ACHN |
|---|
|
|
|
|
|---|
| Drug concentration
(µg/ml) | 24 h | 48 h | 24 h | 48 h |
|---|
| CTRL (0) | 734.29±7.56 | 922.14±9.20 | 242.31±18.20 | 1,566.4±20.50 |
| DDP (1) |
851.97±26.22a |
1,172.8±6.70a |
530.13±16.16a |
657.25±36.20a |
| MM (0.1) |
880.53±36.8a |
1,363.90±16.0a |
459.38±0.00a |
606.27±21.87a |
Discussion
RCC is a malignant tumor that seriously affects
human health. Thirty percent of RCC patients have metastatic
lesions at the time of diagnosis. At present, there are no
effective drugs for patients with metastasis (4,26).
Thus, the treatment of RCC represents an unmet clinical need and
the discovery of innovative approaches for this purpose is urgently
required. In the present study, low-dose chemotherapy combined with
T cell immunotherapy, and the combination of the two methods had
synergistic effects on renal cancer cell treatment in vitro.
However, the tumor microenvironment (TME) in the body is a complex
system composed of numerous cells that regulate different immune
responses (27). The development of
standardized, individualized chemotherapy combined with
immunotherapy with few side effects is the main direction of future
research.
DDP and MMC are two of the earliest discovered
chemotherapeutic agents and are the most widely used drugs
(28). To avoid long-term and
delayed side effects, low-dose chemotherapy drugs combined with T
cell therapy was used to treat RCC. One of the main methods of
cancer treatment is to induce apoptosis in cancer cells (29). Most chemotherapy drugs rely on the
induction of apoptosis for efficacy (28). It has been reported that MMC and DDP
can effectively inhibit tumor growth or significantly increase
tumor apoptosis in rapidly proliferating cell populations (15,28,30).
The present study revealed that low-dose chemotherapy could also
inhibit proliferation and induce apoptosis in renal cancer cells.
When combined with T cells, low-dose chemotherapy had a synergistic
effect in inducing the apoptosis of renal cancer cells. Several
studies have revealed that some chemotherapeutic drugs, such as DDP
or MMC, in addition to their direct cytotoxic effects on tumor
cells, can also regulate the antitumor immune response (25,31,32).
In the present study it was revealed that DDP and MMC inhibited the
expression of the immune-related protein TGF-βR1 in renal cancer
cells in the early stage (4 or 12 h). TGF-βR1 is an important
element of the TGF-β/SMAD signaling pathway, which has emerged as a
central mediator of cancer progression due to its capacity to
regulate cell growth, differentiation, and migration (33,34).
Adoptive T cell therapy provides a new treatment
option for advanced cancer patients. Chemotherapeutic drugs can
effectively induce immune responses by increasing the
cross-presentation of tumor antigens, influencing the expression of
cytokines and the migration of effector T cells, affecting
production of immune memory, and reducing the number of
immunosuppressive cells (35,36).
The present study revealed that the combination of T cells with DDP
or MMC increased the killing effect on renal cancer cells.
Cytokines are important in maintaining or enhancing the function of
T cells, and IFN-γ secretion was detected in T cells. The results
revealed that low doses of the chemotherapeutic drugs DDP and MMC
promoted IFN-γ secretion in the early stage (6 h) to enhance the
antitumor effect. T cells must penetrate tumor sites to play their
antitumor role effectively. Therefore, the effect of
chemotherapeutic drugs on T-cell chemotaxis was examined. The
results revealed that DDP and MMC could promote T cell infiltration
into tumor cell sites at the early stage (6 h) to enhance the
antitumor effect of T cells. CD8+ (CD44+
CD62L+) TCMs, with greater proliferation and
survival potential than with CD8+ (CD44+
CD62L−) TEM, are pivotal for antitumor
efficacy (6,8). The present results revealed that DDP
could induce the transformation of T cells from effector memory T
cells into central memory T cells, which is of great significance
for T cells in exerting sustainable killing effects on tumor cells.
Programmed death factor 1 receptor/ligand (PD1/PD-L1) plays an
important role in regulating T cell immunity and has become a
research hotspot in immunotherapy in recent years. PD1/PD-L1 can
inhibit T-cell activation and proliferation, weaken the antitumor
immune response, and negatively regulate immune responses (37). PD1 is expressed at various levels
mainly on the surface of activated T cells (38). The present results revealed that
chemotherapeutic drugs (DDP/MMC) could reduce PD1 expression in T
cells to improve the persistence of T cells. A limitation of the
present study was that FACS did not contain an isotype control.
However, the antitumor effects of T cells depend on their
activation, which is limited by a dual-signal system (39). Recently, chimeric antigen
receptor-modified T (CAR-T) cells, which can transcend the dual
signaling pathway and are not restricted by the major
histocompatibility complex (MHC), have attracted wide attention
(40,41). The present study provided a
theoretical basis for the use of CAR-T cells combined with low-dose
chemotherapy in the future.
In the later stage (24 h), it was revealed that
chemotherapeutic drugs (DDP/MMC) inhibited IFN-γ secretion and
T-cell chemotaxis, which may be related to the TGF-β signaling
pathway. TGF-β/Smad2 signaling is a strong immunosuppressive
factor. TGF-β can effectively inhibit the proliferation,
activation, and infiltration of T cells and induce T-cell apoptosis
to attenuate the killing activity of T cells in tumors (42–44).
To study the possible causes, the expression of TGF-β, TGF-βR1, and
Smad2 was examined in renal cancer cells and the expression of
TGF-βR1 and Smad2 in T cells. It was determined that the expression
of TGF-β, TGF-βR1, and Smad2 in renal cancer cells was
significantly increased following DDP and MMC exposure in the later
stage (48 h), and the expression of TGF-βR1 and Smad2 in T cells
was also significantly increased. Therefore, it was theorized that
the decreased secretion of immune factors and the inhibitory
infiltration of T cells may be related to the TGF-β signaling
pathway. It has been reported that the inhibitory effects of TGF-β
on T-cell function could be reversed by using a small molecule
inhibitor of TGF-βR1 signaling, and this molecule may function as
an antitumor drug in the future (44). However, whether TGF-βR1 silencing
could reverse the effects of DDP and MMC on renal cells was not
explored, which is a limitation of the present study.
In conclusion, the present results revealed that
low-dose chemotherapy drugs (DDP and MMC) have the potential to
kill RCC cells, and treatment with DDP or MMC can inhibit
proliferation and promote apoptosis in RCC. Low-dose chemotherapy
drugs (DDP and MMC) can synergistically enhance the
immunotherapeutic effects of T cells on RCC in vitro by
inhibiting TGF-βR1 expression in tumor cells, promoting IFN-γ
secretion, chemotaxis and phenotype changes of T cells and
inhibiting PD1 expression. It was also revealed that the TGF-β
signaling pathway may be involved in inhibiting the function of T
cells by decreasing immune factor secretion, preventing chemotaxis
toward tumor cells and even promoting the apoptosis of T cells. The
targeted inhibition of TGF-β signals may be a promising therapeutic
strategy for the treatment of renal cancer. The present study was
conducted in RCC cell lines in vitro. Future studies of this
therapeutic strategy need to be performed in vivo. The
present study provided a novel therapeutic option for sequential
immune chemotherapy to enhance the therapeutic efficacy of T
cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jiangsu Planned
Projects for Postdoctoral Research Funds (grant no. 1501060A) and
Jiangsu Overseas Research and Training Program for University
Prominent Young and Middle-aged Teachers and Presidents.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NL and XJF conceived and designed the project. DDX,
PT, YYC, WYG and YL performed the experiments. DDX and MD drafted
and revised the manuscript. DDX, PT, YYC and MD analyzed and
interpreted the data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All applicable international, national, and
institutional guidelines for the care and use of animals were
followed. The inclusions of mouse and human subjects in the present
study were approved by the Animal Experimental Ethics Committee of
Xuzhou Medical University and the Ethics Committee of the First
People's Hospital of Lianyungangand, respectively. Written informed
consent was obtained from the blood donor.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gansler T, Ganz PA, Grant M, Greene FL,
Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR Jr, Thun MJ, et
al: Sixty years of CA: A cancer journal for clinicians. CA Cancer J
Clin. 60:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Escudier B, Porta C, Schmidinger M,
Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S and
Horwich A; ESMO Guidelines Committee. Electronic address, :
Clinicalguidelines@esmo.org: Renal cell carcinoma: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 30:706–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russo P: Delayed systemic treatment in
metastatic renal-cell carcinoma. Lancet Oncol. 17:1187–1189. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farhood B, Najafi M and Mortezaee K:
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A
review. J Cell Physiol. 234:8509–8521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaartinen T, Luostarinen A, Maliniemi P,
Keto J, Arvas M, Belt H, Koponen J, Mäkinen PI, Loskog A, Mustjoki
S, et al: Low interleukin-2 concentration favors generation of
early memory T cells over effector phenotypes during chimeric
antigen receptor T-cell expansion. Cytotherapy. 19:689–702. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive T
cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gattinoni L, Klebanoff CA, Palmer DC,
Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR,
Rosenberg SA and Restifo NP: Acquisition of full effector function
in vitro paradoxically impairs the in vivo antitumor efficacy of
adoptively transferred CD8+ T cells. J Clin Invest. 115:1616–1626.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janssen EM, Lemmens EE, Wolfe T, Christen
U, von Herrath MG and Schoenberger SP: CD4+ T cells are required
for secondary expansion and memory in CD8+ T lymphocytes. Nature.
421:852–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ho PC, Meeth KM, Tsui YC, Srivastava B,
Bosenberg MW and Kaech SM: Immune-based antitumor effects of BRAF
inhibitors rely on signaling by CD40L and IFNү. Cancer Res.
74:3205–3217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bos R and Sherman LA: CD4+ T-cell help in
the tumor milieu is required for recruitment and cytolytic function
of CD8+ T lymphocytes. Cancer Res. 70:8368–8377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukushima K and Yamashita K: Interleukin-2
carbohydrate recognition modulates CTLL-2 cell proliferation. J
Biol Chem. 276:7351–7356. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou XL, Wang WW, Zhu WG, Yu CH, Tao GZ,
Wu QQ, Song YQ, Pan P and Tong YS: High expression of long
non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor
prognosis in patients with esophageal squamous cell carcinoma
treated with definitive chemoradiotherapy. Mol Carcinog.
55:2095–2105. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
So B, Marcu L, Olver I, Gowda R and Bezak
E: Oesophageal cancer: Which treatment is the easiest to swallow? A
review of combined modality treatments for resectable carcinomas.
Crit Rev Oncol Hematol. 113:135–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han SZ, Liu HX, Yang LQ, Cui LD and Xu Y:
Piperine (PP) enhanced mitomycin-C (MMC) therapy of human cervical
cancer through suppressing Bcl-2 signaling pathway via inactivating
STAT3/NF-KB. Biomed Pharmacother. 96:1403–1410. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan XY: Comparison of the cytotoxic effect
of fresh garlic, diallyl trisulfide, 5-fluorouracil (5-FU),
mitomycin C (MMC) and Cis-DDP on two lines of gastric cancer cells.
Zhonghua Zhong Liu Za Zhi. 7:103–105. 1985.(In Chinese). PubMed/NCBI
|
|
17
|
Hu X, Ma J, Vikash V, Li J, Wu D, Liu Y,
Zhang J and Dong W: Thymoquinone augments cisplatin-induced
apoptosis on esophageal carcinoma through mitigating the activation
of JAK2/STAT3 Pathway. Dig Dis Sci. 63:126–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomyo Y, Sasaki J, Branch C, Roth JA and
Mukhopadhyay T: 5-aza-2′-deoxycytidine upregulates caspase-9
expression cooperating with p53-induced apoptosis in human lung
cancer cells. Oncogene. 23:6779–6787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inman BA, Stauffer PR, Craciunescu OA,
Maccarini PF, Dewhirst MW and Vujaskovic Z: A pilot clinical trial
of intravesical mitomycin-C and external deep pelvic hyperthermia
for non-muscle-invasive bladder cancer. Int J Hyperthermia.
30:171–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maus MV, Fraietta JA, Levine BL, Kalos M,
Zhao Y and June CH: Adoptive immunotherapy for cancer or viruses.
Annu Rev Immunol. 32:189–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inamura SO, Ito H, Taga M, Tsuchiyama K,
Hoshino H, Kobayashi M and Yokoyama O: Low-dose docetaxel enhanced
the anticancer effect of temsirolimus by overcoming autophagy in
prostate cancer cells. Anticancer Res. 39:5417–5425. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Q, Tian K, Xu J, Zhang H, Li L, Fu
Q, Chai D, Li H and Zheng J: Synergistic effects of cabozantinib
and EGFR-Specific CAR-NK-92 cells in renal cell carcinoma. J
Immunol Res. 2017:69159122017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Y, Tang Y and Cheng YS: MiR-34a
inhibits pancreatic cancer progression through Snail1-mediated
epithelial–mesenchymal transition and the Notch signaling pathway.
Sci Rep. 7:382322017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zitvogel L, Apetoh L, Ghiringhelli F and
Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev
Immunol. 8:59–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mbeunkui F and Johann DJ Jr: Cancer and
the tumor microenvironment: A review of an essential relationship.
Cancer Chemother Pharmacol. 63:571–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo XH, Ni J, Xue JL and Wang X:
Phyllanthus emblica Linn. fruit extract potentiates the anticancer
efficacy of mitomycin C and cisplatin and reduces their
genotoxicity to normal cells in vitro. J Zhejiang Univ Sci B.
18:1031–1045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye LY, Hu S, Xu HE, Xu RR, Kong H, Zeng
XN, Xie WP and Wang H: The effect of tetrandrine combined with
cisplatin on proliferation and apoptosis of A549/DDP cells and A549
cells. Cancer Cell Int. 17:402017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X, Chen YT, Song HZ, Huang GC and
Chen LB: Cisplatin pretreatment enhances anti-tumor activity of
cytokine-induced killer cells. World J Gastroenterol. 17:3002–3011.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bergmann-Leitner ES and Abrams SI:
Treatment of human colon carcinoma cell lines with anti-neoplastic
agents enhances their lytic sensitivity to antigen-specific CD8+
cytotoxic T lymphocytes. Cancer Immunol Immunother. 50:445–455.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vollebergh MA, Kappers I, Klomp HM,
Buning-Kager JC, Korse CM, Hauptmann M, de Visser KE, van den
Heuvel MM and Linn SC: Ligands of epidermal growth factor receptor
and the insulin-like growth factor family as serum biomarkers for
response to epidermal growth factor receptor inhibitors in patients
with advanced non-small cell lung cancer. J Thorac Oncol.
5:1939–1948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heuer JG, Harlan SM, Yang DD, Jaqua DL,
Boyles JS, Wilson JM, Heinz-Taheny KM, Sullivan JM, Wei T, Qian HR,
et al: Role of TGF-alpha in the progression of diabetic kidney
disease. Am J Physiol Renal Physiol. 312:F951–F962. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nowak AK, Lake RA, Marzo AL, Scott B,
Heath WR, Collins EJ, Frelinger JA and Robinson BW: Induction of
tumor cell apoptosis in vivo increases tumor antigen
cross-presentation, cross-priming rather than cross-tolerizing host
tumor-specific CD8 T cells. J Immunol. 170:4905–4913. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang X, Guan D, Shu YQ, Liu LK and Ni F:
Effect of cisplatin on the frequency and immuno-inhibitory function
of myeloid-derived suppressor cells in a375 melanoma model. Asian
Pac J Cancer Prev. 16:4329–4333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu J, Zhong Y, Peng S, Zhou X and Gan X:
Efficacy and safety of PD1/PDL1 blockades versus docetaxel in
patients with pretreated advanced non-small-cell lung cancer: A
meta-analysis. Onco Targets Ther. 11:8623–8632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan TSY, Li J, Loong F, Khong PL, Tse E
and Kwong YL: PD1 blockade with low-dose nivolumab in NK/T cell
lymphoma failing L-asparaginase: Efficacy and safety. Ann Hematol.
97:193–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roybal KT, Rupp LJ, Morsut L, Walker WJ,
McNally KA, Park JS and Lim WA: Precision tumor recognition by T
cells with combinatorial antigen-sensing circuits. Cell.
164:770–779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jackson HJ, Rafiq S and Brentjens RJ:
Driving CAR T-cells forward. Nat Rev Clin Oncol. 13:370–383. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang J, Wahdan-Alaswad R and Danielpour D:
Critical role of Smad2 in tumor suppression and transforming growth
factor-beta-induced apoptosis of prostate epithelial cells. Cancer
Res. 69:2185–2190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
di Bari MG, Lutsiak ME, Takai S, Mostböck
S, Farsaci B, Semnani RT, Wakefield LM, Schlom J and Sabzevari H:
TGF-beta modulates the functionality of tumor-infiltrating CD8+ T
cells through effects on TCR signaling and Spred1 expression.
Cancer Immunol Immunother. 58:1809–1818. 2009. View Article : Google Scholar : PubMed/NCBI
|