Introduction
Colon cancer is a common malignant tumor of the
digestive tract located in the colon, which mainly occurs at the
junction of the rectum and the sigmoid colon. Statistics show that
the highest incidence of colon cancer is in the age group of 40–50
years, the ratio of male to female is 2–3:1, and the incidence of
colon cancer ranks third among all cases of gastrointestinal tumors
(1,2). The 5-year survival rate of patients
with colon cancer is approximately 64.9%, yet the 5-year survival
rate of patients with advanced stage disease is as low as 12.5%.
Since the early symptoms of patients with colon cancer are not
obvious, only about 40% of patients can be diagnosed at the early
stage of the disease (3,4). Chemotherapy is one of the most
important treatments for patients with advanced colon cancer, of
which 5-fluorouracil (5-FU) is the most widely used. 5-FU inhibits
the proliferation, invasion and migration of tumor cells by
interfering with the nucleic acid metabolism of tumor cells, but it
is also toxic to normal cells, causing serious adverse reactions,
even endangering the life safety of patients, severely limiting its
clinical application (5,6). Previous research has shown that 5-FU
combined with other agents may reduce the required dosage of 5-FU
consequently reducing the adverse reactions caused by 5-FU without
affecting or even improving the efficacy of chemotherapy (7,8).
Compared with chemical drugs and biopharmaceuticals,
multi-component, multi-target, and less adverse reactions are
unique advantages of traditional Chinese medicine for the treatment
of diseases. In patients with colon cancer, Chinese medicine can
improve patient immunity, reduce the side effects of radiotherapy
and chemotherapy or enhance drug sensitivity. Inhibiting the
expression of oncogenes helps to inhibit the migration of cancer
cells and has a good effect on the treatment of colon cancer
(9,10). Ginsenoside Rg3 (Rg3), an active
ingredient isolated from ginseng, is a tetracyclic triterpenoid
saponin that inhibits neovascularization, induces tumor cell
apoptosis, and selectively inhibits tumor cell metastasis and
enhances immune function (11,12).
Previous studies have shown that Rg3 exhibits an inhibitory effect
on proliferation, invasion and migration of human tumor cells, such
as lung cancer (13,14), gastric carcinoma (15) and prostate cancer (16). In colon cancer, Rg3 was found to
activate the AMPK signaling pathway to accelerate apoptosis in
colon cancer cell line HT-29 in vitro, and also to block
colon cancer progression by targeting inhibition of cancer stem
cells and tumor angiogenesis in vivo (17). Although numerous studies have shown
that Rg3 increases the efficacy and decreases the toxicity of
chemotherapeutic drugs and suppresses the chemotherapeutic
resistance in cancer (18,19), its combination with chemotherapeutic
agent 5-FU to achieve extra benefits in anti-colon cancer treatment
warrants detailed investigation.
In the present study, the effects of a combined
treatment of Rg3 and 5-FU on the biological properties of SW620 and
LOVO cells were investigated in vivo and in vitro. We
found that a combined treatment of Rg3 and 5-FU not only enhanced
the inhibition of colon cancer cell proliferation, migration and
invasion, but also promoted apoptosis of colon cancer cells and
arrested the cells in the G0/G1 phase. In addition, it was also
found that Rg3 could synergize the capacity of 5-FU to inhibit the
growth of human colon cancer xenografts in nude mouse, and the
combined treatment of Rg3 and 5-FU enhanced the inhibition of the
PI3K/AKT pathway in colon cancer cells.
Materials and methods
Cell lines and agents
SW620 (CCL-227; ATCC, American Type Culture
Collection, Manassas, VA, USA) and LOVO (CCL-229; ATCC) cell lines
were cultured with DMEM medium (cat. no. 12491-15; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
cat. no. 10100-147; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (cat. no. 15640055, Thermo Fisher
Scientific, Inc.). The cell lines used in the present study were
cultured at 37°C with 5% CO2.
Rg3 (cat. no. 64139; Sigma-Aldrich; Merck KGaA) and
5-FU (cat. no. 04541, Sigma-Aldrich; Merck KGaA) were dissolved in
DMSO. For the cell experiments, the diluted culture solution of Rg3
or 5-FU was dissolved in DMSO to achieve the experimental
concentration and was administered to the cells for 48 h. For
animal experiments, PBS diluted Rg3 or 5-FU was dissolved in DMSO
to the experimental concentration. The experiments were approved by
the Ethics Committee of The Quanzhou First Hospital Affiliated to
Fujian Medical University (Quanzhou, Fujian, China).
MTT assay
A total of 2×103 cells/well were
inoculated in a 96-well culture plate containing the indicated
medium (DMEM plus 10% FBS). We evaluated the viability of the SW620
and LOVO cells by MTT assay. In short, after 4 h of culture, MTT
(10 µl, 10 mg/ml), which was dissolved in DMSO, was added to the
cells and incubated. The cell supernatant was removed and then 100
µl DMSO was added. After 30 min, the optical density (OD570) was
determined using a plate reader (ELx808; Bio-Tek Instruments).
Cell colony formation assay
A total of 2×103 cells/ml were seeded in
6-well plates with 2 ml medium/well, and medium was exchanged once
every 3 days. Cells were routinely cultured for about 3 weeks. When
visible clones appeared in the well, the culturing was stopped. The
supernatant culture medium was drawn, washed with PBS 2 times, and
fixed with 4% formaldehyde for 15 min. The supernatant was drawn,
stained with 0.25% crystal violet for 25 min, and slowly rinsed
with sterile water. Plates were placed in a sterile purification
table and images were captured after drying. The relative
proliferation was measured by measuring the absorbance at 595 nm
using a plate reader (ELx808; Bio-Tek Instruments).
Western blot analysis
RIPA lysate buffer (cat. no. P0013C; Beyotime
Institute of Biotechnology, Shanghai, China) was used to extract
total cellular protein, and the BCA kit (cat. no. P0009; Beyotime
Institute of Biotechnology) was used to determine the protein
concentration. Then cell lysates of SW20 and LOVO cells were
separated by 10% SDS-page with 50 µg total protein and transferred
to a PVDF membrane. The following primary antibodies were selected
as follows: Anti-N-cadherin antibody (ab18203, dilution 1:1,000),
anti-E-cadherin antibody (ab1416, dilution 1:50), anti-MMP-9
(ab38898, dilution 1:1,000), anti-active-caspase-9 antibody
(ab2324, dilution 1:500), anti-active-caspase-3 antibody (ab2302,
dilution 1:500), anti-Apaf1 antibody (ab2324, dilution 1:1,000),
anti-PI3K-p85 antibody (ab191606, dilution 1:1,000), anti-PI3K-110β
antibody (ab32569, dilution 1:1,000), anti-pan-AKT (phospho T308)
antibody (ab38449, dilution 1:500), Anti-pan-AKT antibody (ab8805,
dilution 1:500), anti-PDK1 antibody (ab52893, dilution 1:1,000) and
anti-GAPDH (ab9484, dilution 1:3,000). The secondary antibody was
selected as follows: Goat anti-rabbit (ab150077, dilution 1:1,000),
or goat anti-rat (ab150117, dilution 1:1,000). The blocking
protocol was with 5% skim milk for 1 h at room temperature. The
primary antibody was incubated overnight at 4°C and the secondary
antibody was incubated for 1 h at room temperature. The BeyoECL
Plus kit (cat. no. P0018S, Beyotime) was used for the chromogenic
protein bands with Beckman Coulter Immunoassay System (UniCel DxI
800; Beckman Coulter), and ImageJ (v2.1.4.7; National Institutes of
Health) was used for the densitometric analysis of protein bands.
All antibodies were purchased from Abcam unless otherwise
stated.
Transwell invasion experiment
The cell density was adjusted to 0.5×106
cells/ml and then the cells were added to a 24-well Transwell upper
chamber (Corning, Corning, NY, USA). Medium containing 20% FBS
(Gibco; Thermo Fisher Scientific, Inc.) was added into the lower
Transwell chamber and the Transwell was incubated at 37°C for 24 h.
The Transwell was taken out and the medium was removed. It was
washed twice with PBS, methanol was added, and dried after being
fixed for 30 min. After the membrane was dried, it was stained with
crystal violet for 20 min, and the relative migration was
determined by measuring the absorbance at 595 nm using a plate
reader (ELx808; Bio-Tek Instruments, Inc.).
Cell scratch test
A total of 5×105 cells were placed in a
6-well plate (2 ml/well). A scratch was made as far as possible
perpendicular to the back of a horizontal line by using tips
against a ruler (tips should be vertical and cannot be tilted). The
cells were washed with PBS for three times and the scratched cells
were removed, and serum-free DMEM was added. Cells were cultured at
37°C in a 5% CO2 incubator for 24 h, and images were
captured in 0 and 24 h using an CKX41 Olympus inverted microscope
(magnification, ×100; Olympus Corp.).
Flow cytometric analysis
Cells that had been treated in different manners
were collected and 70% pre-cooled ethanol (pre-chilled PBS and
water-free configuration) was added at 4°C overnight. Then the
cells were washed with PBS and stained with propidium iodine (PI)
(for cell cycle). MACSQuant® Analyzer 10 Flow cytometer
(Miltenyi Biotec=) was used to detect the cell cycle, and the
Annexin V FITC/PI kit (Invitrogen; Thermo Fisher Scientific, Inc.)
was used for flow cytometry to detect apoptosis.
Animal experiment
Human colon cancer cells (5×106/0.2 ml)
in the logarithmic phase were selected. A total of 20 female nude
mice (5–6 weeks of age, 18–25 g; Shanghai Lingchang Biological
Technology Co., Ltd.) that were adaptive for feeding [room
temperature of 20–24°C half day (light) and night (dark) cycle, air
humidity of 60%] for one week were selected. Mice were anesthetized
[3% sodium pentobarbital, 50 mg/kg, intraperitoneal (i.p.)], and
then the lateral skin of the nude mice was selected as a cell
inoculation site to inoculate 5×106/0.2 ml human colon
cancer cells (at the logarithmic phase of growth). When the tumor
tissue grew to a volume of approximately 50 mm3, then
the mouse were randomly assigned to the Solvent group (equal amount
of PBS + DMSO), Rg3 group (200 mg/kg, gavage administration once
every two days), 5-FU group (20 mg/kg, i.p. injection once every
two days) and Rg3+5-FU group (combined Rg3 and 5-FU group
administration). After 3 weeks of treatment, the mice were
sacrificed using cervical dislocation and breathing and heartbeat
for 3 min were observed to determine death, and tumor tissues were
extracted and weighed with an analytical balance (BSA124S; Beijing
Sartorius Instruments Ltd., Beijing, China). All animal experiments
were approved by the Ethics Committee of Quanzhou First Hospital
Affiliated to Fujian Medical University.
Statistical analysis
All data are expressed as mean ± standard deviation,
and SPSS 20.0 (IBM Corp.) was used to analyze the data. Student's
t-test was used to compare differences between two groups, and
multiple groups were compared with one-way ANOVA followed by Duncan
test as a post hoc test. P<0.05 was assigned to indicate that a
difference was statistically significant.
Results
Combined treatment of Rg3 and 5-FU
enhances inhibition of cell proliferation
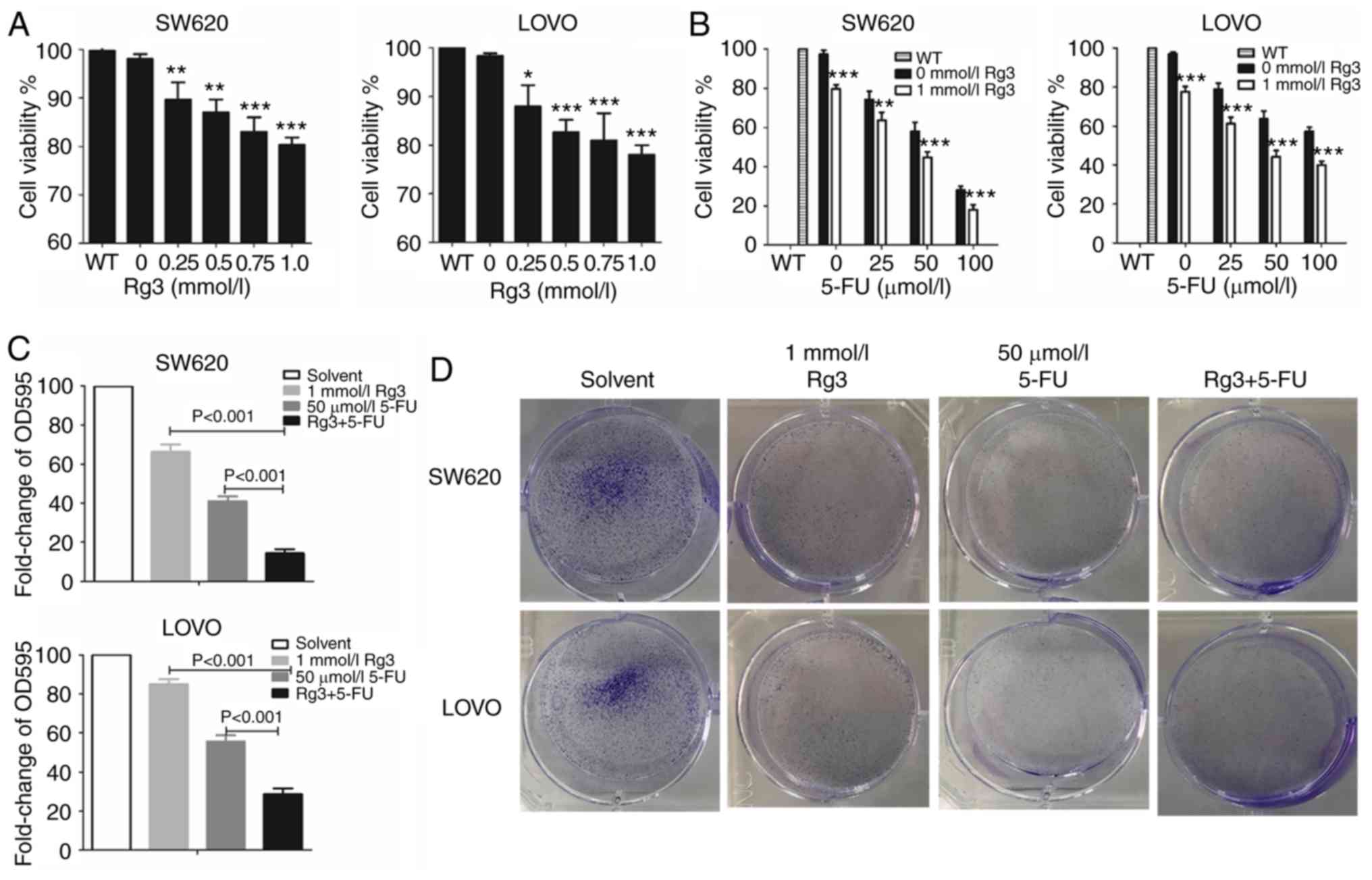
After treatment with different doses of Rg3 or 5-FU,
MTT assay was used to measure the cell viability. The results
revealed that the cell viability of SW620 and LOVO cells was
significantly and gradually decreased with an increasing dose of
Rg3. Thus, we chose 1.0 mmol/l Rg3 for subsequent experiments
(Fig. 1A). As shown in Fig. 1B, the proliferative activity of the
colon cancer cells in the combined treatment group of Rg3 and 5-FU
was significantly lower than that of the 5-FU treatment alone
group. In addition, the cell viability of SW620 and LOVO cells
gradually decreased with the increasing dose of 5-FU. However,
after treatment with the combination of 1 mmol/l Rg3 and 100 µmol/l
5-FU for 48 h, the cell viability of SW620 cells was only 10–20%
which was not conducive to subsequent protein detection
experiments. Thus, 1 mmol/l Rg3 and 50 µmol/l 5-FU were chosen for
subsequent experimentation.
Cell clone formation assays were also used to detect
in vitro proliferation of colon cancer cells. As shown in
Fig. 1C and D, the number of
colonies formed by the colon cancer cells treated with Rg3 and 5-FU
was significantly lower than that of Rg3 or 5-FU alone. These
findings indicated that combined treatment of Rg3 and 5-FU enhanced
the inhibition of colon cancer cell proliferation in
vitro.
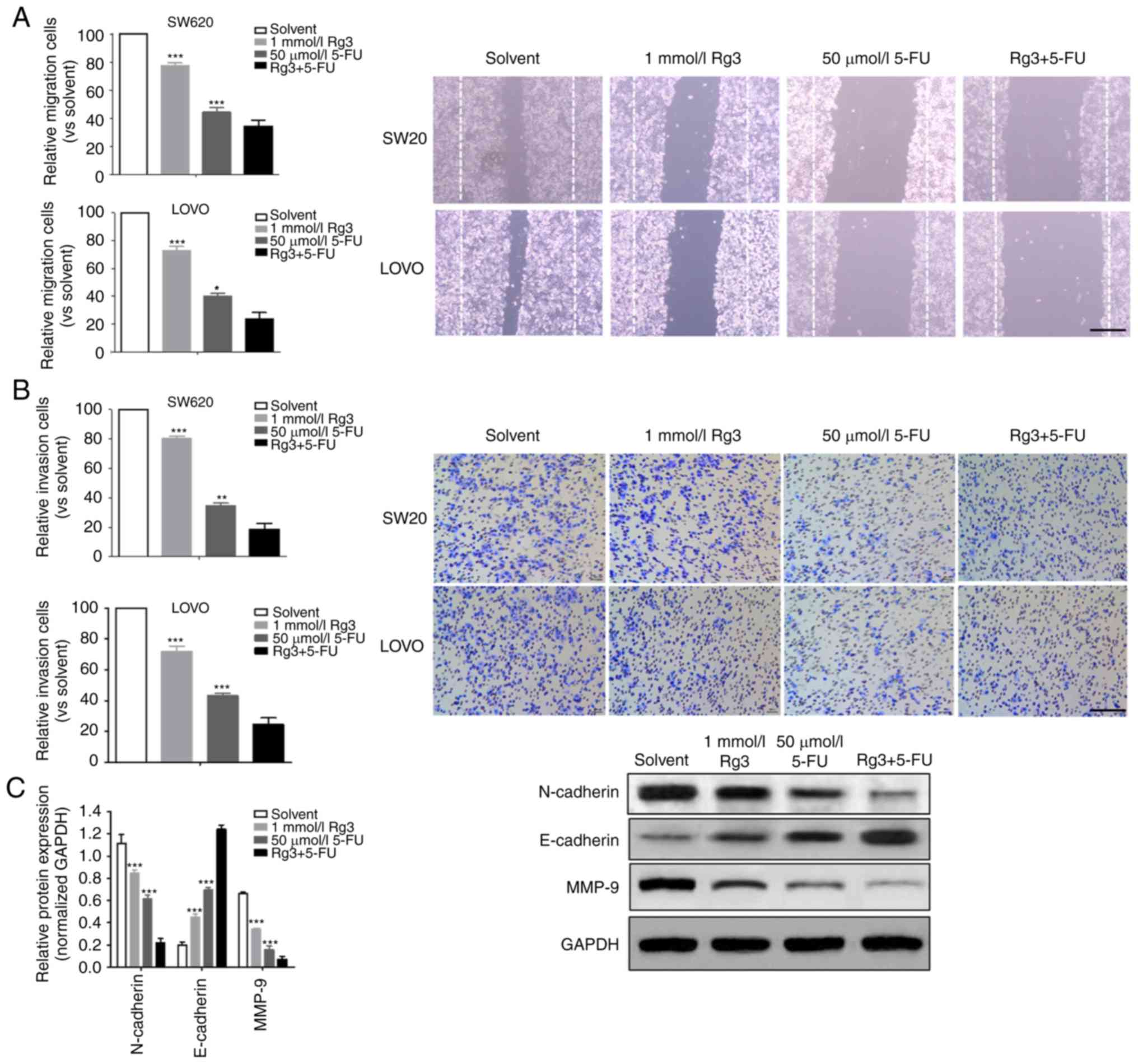
Combined treatment of Rg3 and 5-FU
enhances the inhibition of cell migration and invasion
The ability of tumor cells to invade and migrate is
the key to tumor progression. In the present study, we compared the
effects of different treatment conditions on the invasion and
migration of colon cancer cells. It was demonstrated that the
invasion and migration ability of the colon cancer cells treated
with Rg3 combined with 5-FU was significantly lower than that of
Rg3 or 5-FU alone (Fig. 2A and B).
Epithelial-mesenchymal transition (EMT) is the source of tumor cell
ability to acquire higher invasion and migration capacity. Thus, we
determined the levels of three EMT-related proteins (N-cadherin,
E-cadherin and MMP-9) and found that the expression of N-cadherin
and MMP-9 protein in the Rg3+5-FU group was significantly lower
than that of Rg3 or 5-FU alone group, but the expression of
E-cadherin protein was significantly higher (Fig. 2C).
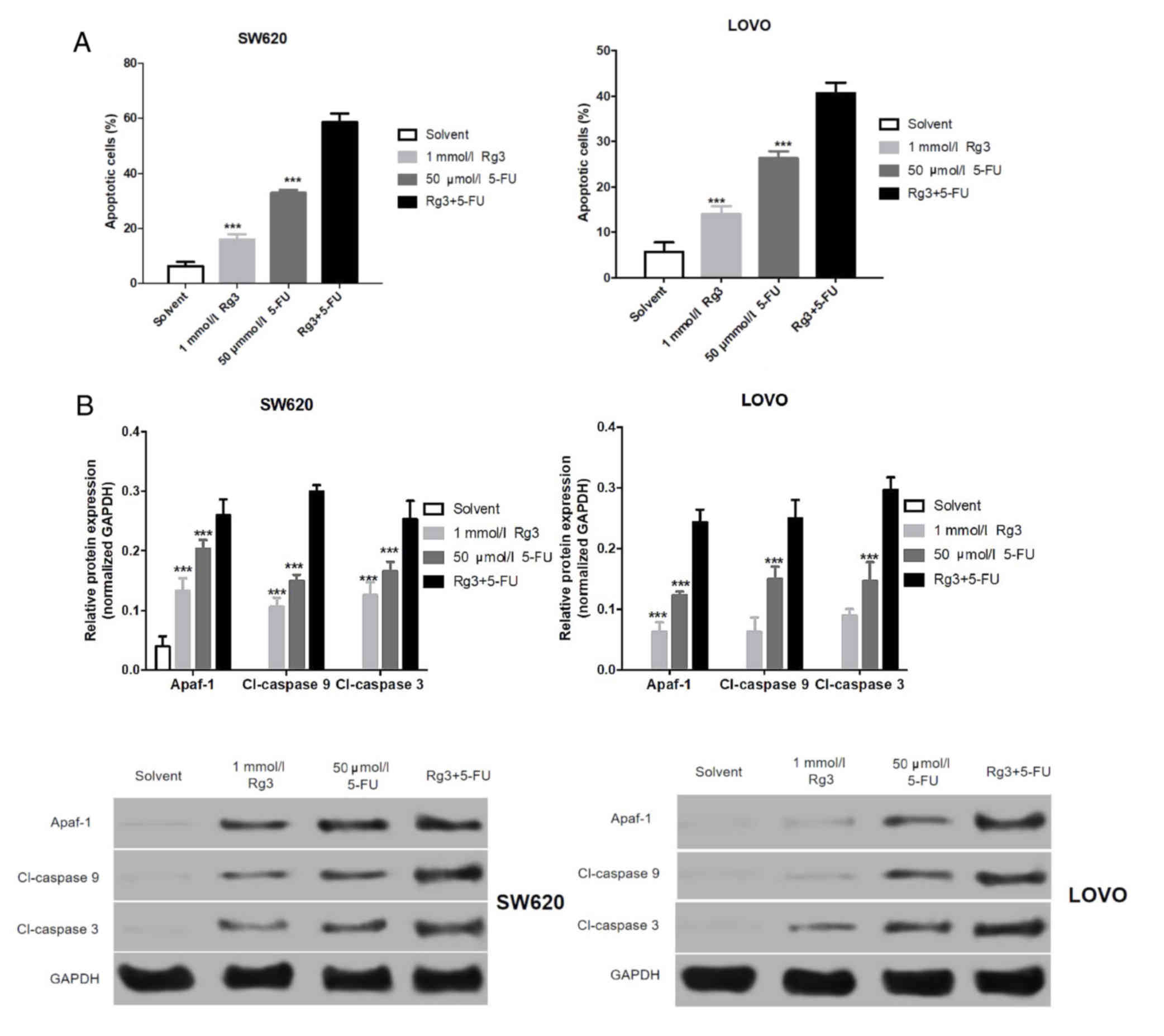
Combined treatment of Rg3 and 5-FU
promotes apoptosis of colon cancer cells
Fist, we found that the apoptosis of the colon
cancer cells treated with Rg3 combined with 5-FU was significantly
higher than that of Rg3 or 5-FU alone (Fig. 3A). The levels of apoptosis-related
proteins in the SW620 and LOVO cells were assessed by western blot
analysis. As shown in Fig. 3B,
expression levels of Apaf-1, cleaved (Cl)-caspase 9 and Cl-caspase
3 protein in colon cancer cells (SW620 and LOVO) treated with Rg3
were significantly increased, and the expression of these
apoptosis-related protein in colon cancer cells following 5-FU
treatment was significantly higher than that treated with Rg3. More
importantly, expression levels of these apoptosis-related proteins
in colon cancer cells treated with the combination of Rg2 and 5-FU
were significantly higher than levels treated with Rg3 or 5-FU
alone.
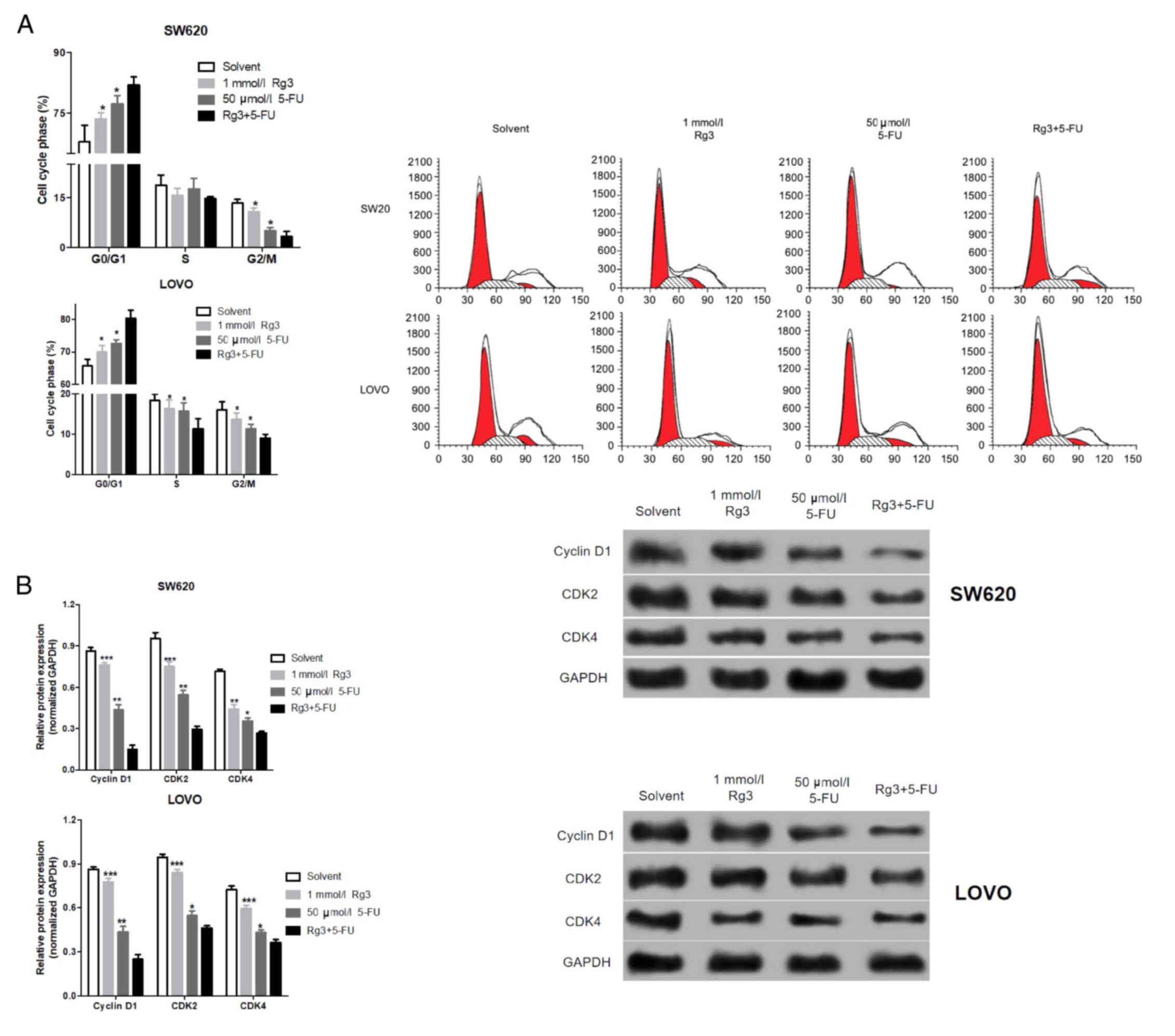
We analyzed the cell cycle distribution of the colon
cancer cells after treatment with the different agents. As shown in
Fig. 4A, the percentages of colon
cancer cells in the G0/G1 phase treated with the Rg3 and 5-FU
combination were significantly higher than the percentages
following Rg3 or 5-FU alone. Similarly, we also detected cell
cycle-associated protein by western blot analysis. As shown in
Fig. 4B, the expression levels of
cyclin D1, CDK2 and CDK4 protein in colon cancer cells which were
treated with the Rg3 and 5-FU combination were significantly lower
than levels following treatment with Rg3 or 5-FU alone.
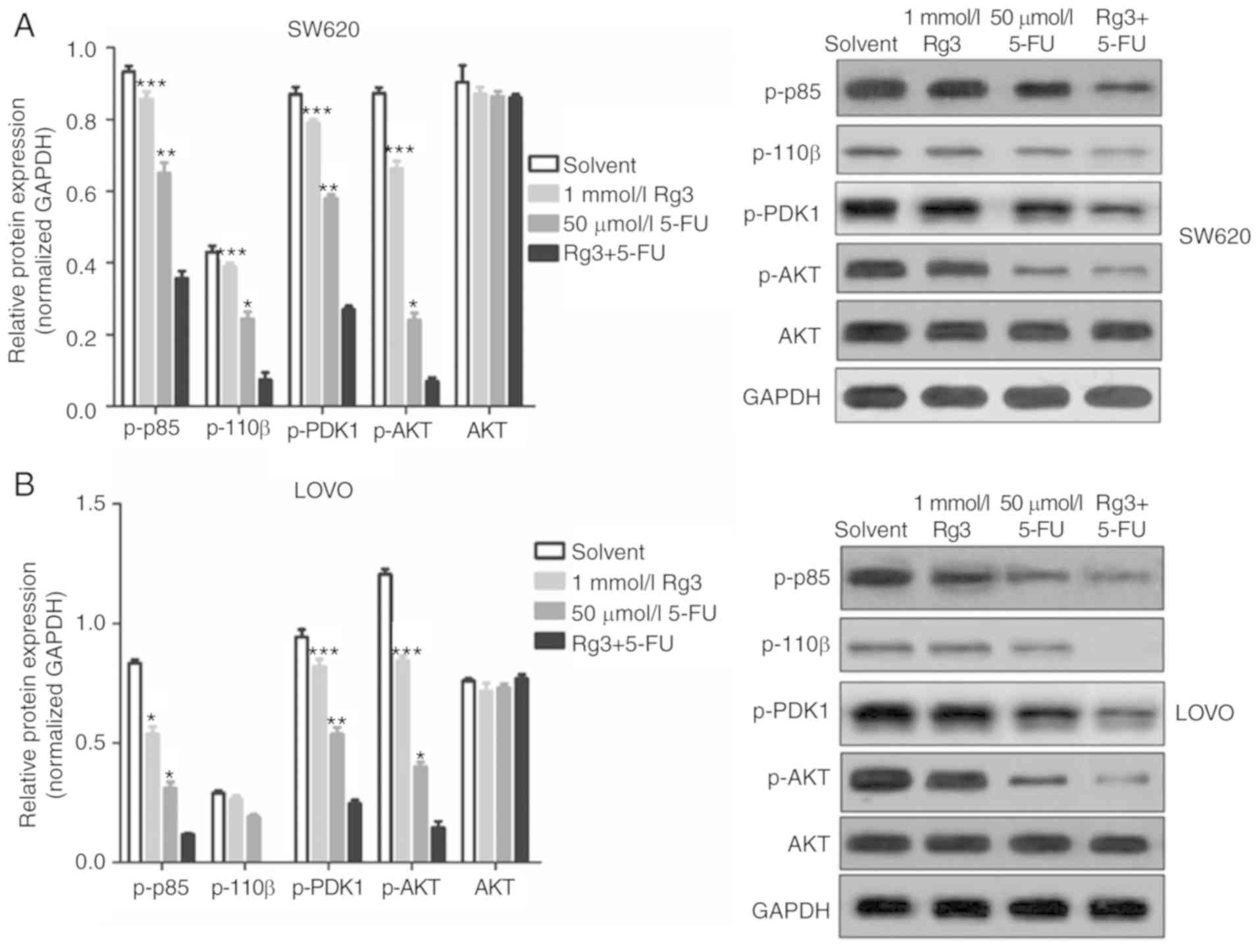
Combined treatment of Rg3 and 5-FU
suppresses PI3K/AKT signaling in colon cancer cells
The PI3K/AKT signaling pathway is a signaling
pathway involved in cancer cell proliferation, invasion and
migration, and its abnormal activation can confer high
proliferation, invasion and migration ability of cancer cells. In
the present study, we found that the expression levels of p-p85,
p-110β, p-PDK1 and p-AKT protein in the colon cancer cells which
was treated with Rg3 and 5-FU combination were significantly lower
than levels in the cells treated with Rg3 or 5-FU alone (Fig. 5). These results indicated that the
combined treatment of Rg3 and 5-FU enhanced the inhibition of the
PI3K/AKT signaling pathway in colon cancer cells in
vitro.
Combined treatment of Rg3 and 5-FU
suppresses tumor growth in nude mice
Based on the results of in vitro studies, we
further investigated the effects of the Rg3 and 5-FU combination on
colon cancer cell proliferation and protein expression in nude
mice. SW620 cells were injected into the armpits of nude mice.
After 3 weeks of treatment, the mice were sacrificed, and the
weight and volume of tumor tissues were measured. It was found that
the weight and volume of tumor tissues in the Rg3+5-FU group were
significantly lower than these parameters in the groups treated
with Rg3 or 5-FU alone (Fig. 6A and
B).
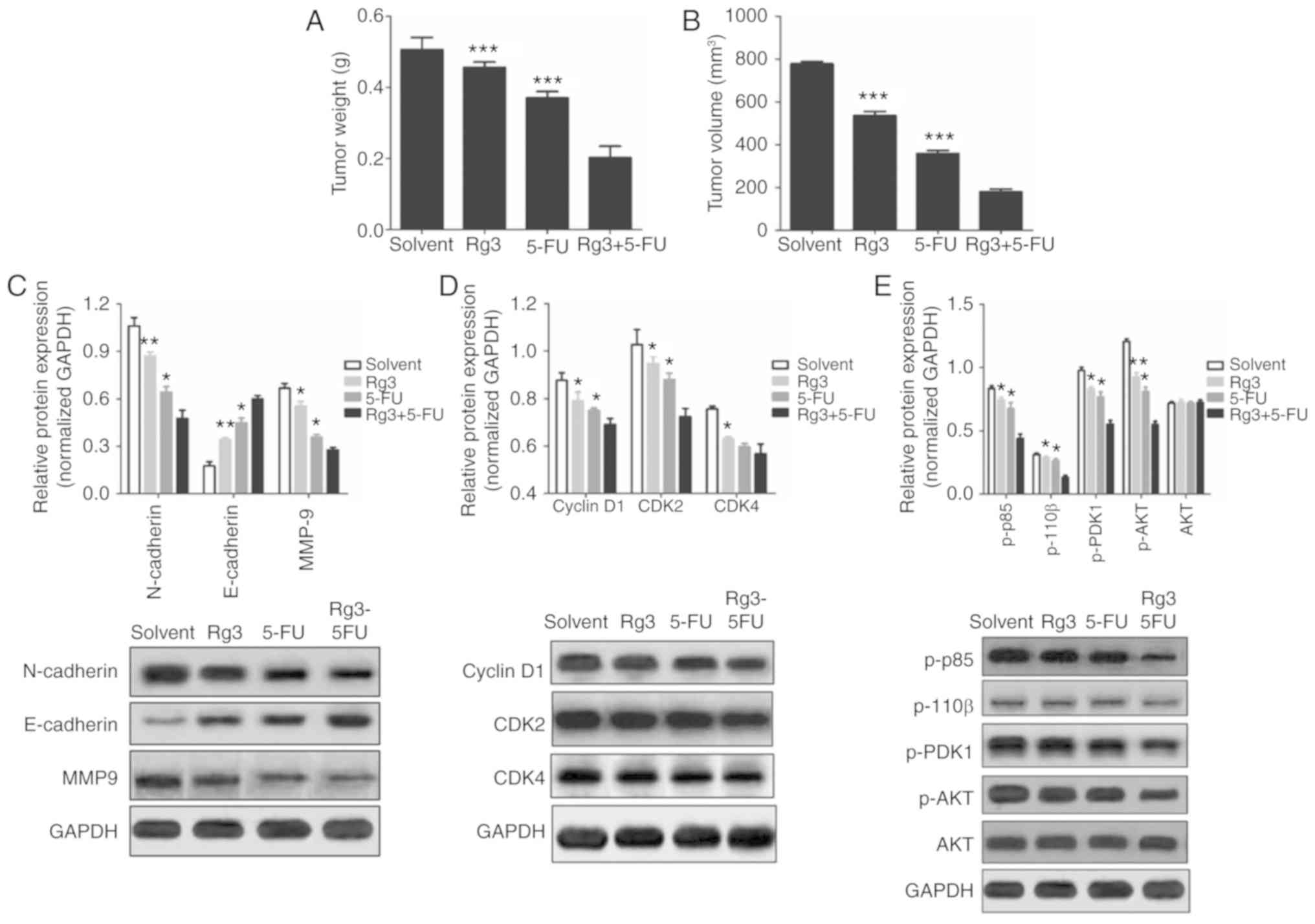 | Figure 6.Effects of the combined treatment of
Rg3 and 5-FU on tumor growth and protein expression of colon cancer
cells in vivo. After 3 weeks of treatment, the mice were
sacrificed, tumor tissues were excised, and the weight (A) and
volume (B) of tumor tissues were measured. (C-E) Total protein was
extracted from the colon cancer tumor tissues, and the expression
of proteins was detected by western blot analysis. Five nude mice
in each group, and at least 3 tumor tissues were used to evaluate
protein expression., *P<0.05, **P<0.01 and ***P<0.001,
compared with the Rg3+5-FU group. 5-FU, 5-fluorouracil; Rg3,
ginsenoside Rg3. |
Moreover, western blot analysis was used to detect
the expression of EMT-related proteins, cell cycle-related proteins
and key proteins in the PI3K/AKT signaling pathway. It was
found that although the effects of the Rg3 and 5-FU combination
were not as obvious as the in vitro results compared with
Rg3 of 5-FU alone, the overall trend in protein expression was
consistent (Fig. 6C-E). These
results demonstrated that Rg3 synergizes the effect of 5-FU to
inhibit the growth of human colon cancer xenografts in nude
mice.
Discussion
The anticancer effect of 5-fluorouracil (5-FU) is
exerted mainly by interfering with tumor cell DNA replication, and
it is a commonly used antitumor agent for the treatment of advanced
colon cancer (20,21). However, since 5-FU displays
non-specific cytotoxicity, it also causes damage to normal cells,
causing irreversible renal dysfunction and severe gastrointestinal
reactions. These adverse effects limit its clinical application and
further improvements in the efficacy of chemotherapy are needed
(20,22). Therefore, it is urgent to discover a
drug that can enhance the chemotherapeutic effects of 5-FU and
reduce the 5-FU toxicity when used in combination with 5-FU.
Ginsenoside Rg3 (Rg3) is one of the main active
ingredients extracted from ginseng. Research has shown that
ginsenoside Rg3 has certain inhibitory effects on lung cancer,
breast and prostate cancer. The antitumor mechanism of Rg3 was that
Rg3 reduced the neovascularization, probability of tumor
recurrence, proliferation and metastasis in tumors by inhibiting
KDR/VEGF protein expression and blocking HIF-1α/COX2/VEGF pathway
(12). In the present study, we
found that the combined treatment of Rg3 and 5-FU promoted the
inhibition of colon cancer cell proliferation in vivo and
in vitro. Tumor growth, development and metastasis are
closely related to cell proliferation. The previous study found
that Rg3 inhibits the proliferation of tumor cells, such as
Rg3-induced EGFR/MAPK pathway deactivation was found to
inhibit melanoma cell proliferation by decreasing FUT4/LeY
expression (23). Rg3 was found to
inhibit the proliferation of multiple myeloma cells by inducing the
secretion of IGF-1 (24).
Promoting tumor cell apoptosis is also a method of
inhibiting tumor cell proliferation. In the present study, we found
that the combined treatment of Rg3 and 5-FU significantly enhanced
the apoptosis of colon cancer cells by activating the
Apaf1/caspase 9/caspase 3 pathway. In the mitochondrial
pathway of apoptosis, apoptosis-related signals release cytochrome
c by stimulating the mitochondrial outer membrane.
Cytochrome c enters the cytoplasm which activates caspase-9
by binding with apaf-1. Activation of caspase-9 further activates
caspase-3, while the activated caspase-3 can activate caspase-6/7/8
leading to apoptosis (25,26). In addition, we also found that the
Rg3 and 5-FU combination enhanced the number of G0/G1 phase colon
cancer cells and decreased expression of Cyclin D1, CDK2 and CDK4.
The cell cycle refers to the whole process that the cell undergoes
from the completion of one division to the end of the next
division, and the regulation of the cell cycle is mainly achieved
by the retention of the G1 phase. When a cell is in the G1 phase,
there is an important node regulating the cell cycle, the R point.
When the cell cycle is before the R point, the cell needs the
external growth factor to achieve the normal operation of the cell
cycle. After the cell cycle crosses the R point, the cell cycle
becomes a process that is controlled autonomously by the cell and
no longer depends on the presence of external cytokines (27,28).
Cyclin D1 is a G1/S-specific cyclin, and its main function is to
promote the cell cycle from G1 to S by binding and activating the
cyclin-dependent kinase CDK2/4, a unique cyclin-dependent
kinase of G1, so as to promote cell proliferation (29).
Invasion and migration of tumor cells are the most
important features of malignant tumors and the important causes of
death in patients with malignant tumors. N-cadherin, E-cadherin and
MMP-9 are three proteins that play important roles in cell
epithelial-mesenchymal transition (EMT), whereas EMT provides cells
the ability to transfer and invade. Promoting tumor cell EMT can
inhibit the expression of intercellular junction protein, resulting
in decreased intercellular connectivity, which is beneficial to the
invasion and migration of tumor cells to surrounding healthy
tissues (30,31). Previous studies have found that Rg3
not only inhibits metastasis and invasion of lung cancer cells by
inhibiting EMT induced by transforming factor β1 (32), but also inhibited the metastasis of
prostate PC-3M cells by downregulating the expression of AQP1
(33). By downregulating
MMP-13, Rg3 affected the metastasis and invasion ability of
melanoma cells (34). The present
study demonstrated that the combined treatment of Rg3 and 5-FU
significantly suppressed the invasion and migration ability of
human colon cancer cell in vitro by altering EMT-related
protein.
Furthermore, we also found that Rg3 and 5-FU
combination inhibited the conduction of the PI3K/AKT
signaling pathway in vivo and in vitro. Many studies
have shown that the occurrence and development of tumors are the
result of multi-factor, multi-gene, and multi-pathway processes,
and the cell signal transduction pathway is crucial in the process
of tumor development, invasion and metastasis. The
phosphatidylinositol 3-kinase/serine/threonine kinase B
(PI3K/Akt) signaling pathway plays an important role in the
regulation of solid tumors [e.g., liver cancer (35), breast cancer (36), colon cancer (37), gastric cancer (38), neuroblastoma (39)] and blood tumors [e.g., leukemia
(40)]. PI3K acts as a
bridge molecule for the relationship between extracellular signals
and cellular responses, under the influence of a series of upstream
or bypass signaling molecules. It acts on the downstream of the
effects of a variety of molecules, thus promotes cell migration,
inhibits cell apoptosis, accelerates the process of the cell cycle
and promotes cell proliferation (41). Many previous studies have shown that
traditional Chinese medicine or traditional Chinese medicine
monomers can play an antitumor role by inhibiting the
PI3K/Akt signaling pathway (42,43).
In conclusion, Rg3 enhances 5-FU inhibiting
proliferation, invasion and migration of colorectal cancer cells,
and helps 5-FU block G1 phase induced apoptosis in more colorectal
cells. All in all, our study found that Rg3 enhanced the
anti-cancer effect of 5-FU on colon cancer cell via PI3K/Akt
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XC made substantial contributions to the conception
and design of the study and critically revised it for important
intellectual content. SH contributed to the acquisition of the
data. WC, ZH, YW, XM, YH and ZL analyzed and interpreted the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal and cell experiments were approved by the
Ethics Committee of The Quanzhou First Hospital Affiliated to
Fujian Medical University (Quanzhou, Fujian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Siegel RL, Ward EM and Jemal A: Trends in
colorectal cancer incidence rates in the United States by tumor
location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev.
21:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 60:277–300. 2015.
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Zhang S, Zeng H, Zuo T,
Xia C, Yang Z and He J: Cancer incidence and mortality in China in
2013: An analysis based on urbanization level. Chin J Cancer Res.
29:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanoff HK, Carpenter WR, Freburger J, Li
L, Chen K, Zullig LL, Goldberg RM, Schymura MJ and Schrag D:
Comparison of adverse events during 5-fluorouracil versus
5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III
colon cancer: A Population-based analysis. Cancer. 118:4309–4320.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheah KY, Howarth GS, Bindon KA, Kennedy
JA and Bastian SEP: Low Molecular weight procyanidins from grape
seeds enhance the impact of 5-fluorouracil chemotherapy on Caco-2
human colon cancer cells. PLoS One. 9:e989212014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang
Z, Li Z, Feng X, Hao J, Zhang K, et al: Melatonin synergizes the
chemotherapeutic effect of 5-fluorouracil in colon cancer by
suppressing PI3K/AKT and NF-B/iNOS signaling pathways. J Pineal
Res. 622017.doi: 10.1111/jpi.12380.
|
|
9
|
Wang SF, Wu MY, Cai CZ, Li M and Lu JH:
Autophagy modulators from traditional Chinese medicine: Mechanisms
and therapeutic potentials for cancer and neurodegenerative
diseases. J Ethnopharmacol. 194:861–876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ernst E: Traditional Chinese medicine for
cancer? Br J Cancer. 107:4052012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun HY, Lee JH, Han YS, Yoon YM, Yun CW,
Kim JH, Song YS and Lee SH: Pivotal roles of ginsenoside Rg3 in
tumor apoptosis through regulation of reactive oxygen species.
Anticancer Res. 36:4647–4654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY,
Gong FR, Shen M, Liu L, Tao M, Shen B, et al: Ginsenoside Rg3
targets cancer stem cells and tumor angiogenesis to inhibit
colorectal cancer progression in vivo. Int J Oncol.
52:127–138. 2018.PubMed/NCBI
|
|
13
|
Wang J, Tian L, Khan MN, Zhang L, Chen Q,
Zhao Y, Yan Q, Fu L and Liu J: Ginsenoside Rg3 sensitizes hypoxic
lung cancer cells to cisplatin via blocking of NF-κB mediated
epithelial-mesenchymal transition and sternness. Cancer Lett.
415:73–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joo E, Ha YW and Kim YS: Abstract #LB-23:
Molecular mechanisms of ginsenoside Rg3 related to apoptosis in
human lung and pancreatic adenocarcinomas. Cancer Res:. 69:LB–23.
2009.
|
|
15
|
Kim BJ, Nah SY, Jeon JH, So I and Kim SJ:
Transient receptor potential Melastatin 7 channels are involved in
Ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic
Clin Pharmacol. 109:233–239. 2011. View Article : Google Scholar
|
|
16
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon DY, Oh KW, Han SB and Hong JT: Combination of
ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappa B. Eur J
Pharmacol. 631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.PubMed/NCBI
|
|
18
|
Liu TG, Huang Y, Cui DD, Huang XB, Mao SH,
Ji LL, Song HB and Yi C: Inhibitory effect of ginsenoside Rg3
combined with gemcitabine on angiogenesis and growth of lung cancer
in mice. BMC Cancer. 9:2502009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun MY, Ye Y, Xiao L, Duan XY, Zhang YM
and Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J
Mol Med. 39:507–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hokmabady L, Raissi H and Khanmohammadi A:
Interactions of the 5-fluorouracil anticancer drug with DNA
pyrimidine bases: A detailed computational approach. Struct Chem.
27:487–504. 2016. View Article : Google Scholar
|
|
22
|
Rateesh S, Luis SA, Luis CR, Hughes B and
Nicolae M: Myocardial infarction secondary to 5-fluorouracil: Not
an absolute contraindication to rechallenge? Int J Cardiol.
172:e331–e333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan X, Aziz F, Tian LL, Wang XQ, Yan Q
and Liu JW: Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation
inhibits melanoma cell proliferation by decreasing FUT4/LeY
expression. Int J Oncol. 46:1667–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Y, Zhang P, Zeng HQ, Lou SF and Wang
DX: Ginsenoside Rg3 induces apoptosis in human multiple myeloma
cells via the activation of Bcl-2-associated X protein. Mol Med
Rep. 12:3557–3562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boward B, Wu TM and Dalton S: Concise
review: Control of cell fate through cell cycle and pluripotency
networks. Stem Cells. 34:1427–1436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harashima H, Dissmeyer N and Schnittger A:
Cell cycle control across the eukaryotic kingdom. Trends in Cell
Biol. 23:345–356. 2013. View Article : Google Scholar
|
|
29
|
Lamb J, Ramaswamy S, Ford HL, Contreras B,
Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR and Ewen
ME: A mechanism of cyclin D1 action encoded in the patterns of gene
expression in human cancer. Cell. 114:323–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K,
Kim TJ, Ham J, Jang HJ, Kang KS and Ko H: Stereospecific effects of
ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal
transition and suppresses lung cancer migration, invasion and
anoikis resistance. Toxicology. 322:23–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan XY, Guo H, Han J, Hao F, An Y, Xu Y,
Xiaokaiti Y, Pan Y and Li XJ: Ginsenoside Rg3 attenuates cell
migration via inhibition of aquaporin 1 expression in PC-3M
prostate cancer cells. Eur J Pharmacol. 683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SG, Kang YJ and Nam JO:
Anti-metastasis effects of Ginsenoside Rg3 in B16F10 cells. J
Microbiol Biotechnol. 25:1997–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng L, Luo S, Jin C, Ma H, Zhou H and
Jia L: FUT family mediates the multidrug resistance of human
hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell
Death Dis. 4:e9232013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams JR, Schachter NF, Liu JC,
Zacksenhaus E and Egan SE: Elevated PI3K signaling drives multiple
breast cancer subtypes. Oncotarget. 2:435–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang QG, Li TY, Liu DN and Zhang HT:
PI3K/Akt pathway involving into apoptosis and invasion in human
colon cancer cells LoVo. Mol Biol Rep. 41:3359–3367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu XQ, Feng JR, Zeng D, Ding Y, Yu CS and
Yang B: PAK4 confers cisplatin resistance in gastric cancer cells
via PI3K/Akt- and MEKERK-dependent pathways. Biosci Rep.
34:e000942014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stafman LL and Beierle EA: Cell
proliferation in neuroblastoma. Cancers (Basel). 8:132016.
View Article : Google Scholar
|
|
40
|
Ma H, Cheng L, Hao K, Li Y, Song X, Zhou H
and Jia L: Reversal effect of ST6GAL 1 on multidrug resistance in
human leukemia by regulating the PI3K/Akt pathway and the
expression of P-gp and MRP1. PLoS One. 9:e851132014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hennessy BT, Smith DL, Ram PT, Lu YL and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Disc. 4:988–1004. 2005. View Article : Google Scholar
|
|
42
|
Shi F, Liao X, Yao LI, et al: Plant
polyphenols exert anti-tumor activity by the PI3K/Akt signaling
pathway: A Review. 2016.
|
|
43
|
Wang H, Zhao L, Zhu LT, Wang Y, Pan D, Yao
J, You QD and Guo QL: Wogonin reverses hypoxia resistance of human
colon cancer HCT116 cells via downregulation of HIF-1α and
Glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol Carcinog.
53 (Suppl 1):E107–E118. 2014. View Article : Google Scholar : PubMed/NCBI
|