Introduction
Worldwide, oral cancer accounts for >650,000
cases and 330,000 deaths annually (1). Oral squamous cell carcinoma (OSCC)
comprises >90% of all types of oral cancer. Despite recent
therapeutic advances, OSCC has a significant recurrence rate and
metastasizes to cervical lymph nodes in ~40% of the patients
(2). Although chemotherapeutic
agents including 5-fluorouracil (5-FU) and
cis-diamminedichloroplatinum (CDDP) are frequently used as
induction, adjuvant, neoadjuvant or palliative therapy in patients
with advanced-stage OSCC, achieving complete remission by
chemotherapy alone is extremely difficult. Additionally, in certain
patients, an initial decrease in tumor size is followed by a
gradual progression to an aggressive behavior and worse clinical
outcome through the acquisition of chemoresistance by tumor cells
(3). However, the molecular
mechanisms underlying the acquisition of chemoresistance in OSCC
cells are not fully understood.
Several studies have reported that stromal cells
within the tumor microenvironment including macrophages and
fibroblasts are involved in the acquisition of resistance to
chemotherapeutic agents by cancer cells (4–6).
Tumor-associated macrophages (TAMs) infiltrate and accumulate in
the tumor microenvironment to promote tumor growth through the
induction of angiogenesis and extracellular remodeling; TAMs also
suppress antitumor immunity through the secretion of various
factors, including growth and pro-angiogenic factors,
matrix-degrading enzymes and immunosuppressive cytokines (7). Additionally, previous studies have
reported that TAMs also induce chemoresistance against 5-FU and
CDDP through the release of cytokines, chemokines, microRNAs and
inorganic compounds (8–12). The frequently observed TAM
infiltration of the OSCC tissue specimens and the reported
relationship between TAMs and cancer progression (13–15),
suggest that TAMs might also regulate the chemosensitivity of OSCC
cells.
Exosomes, which are small membrane vesicles released
by a variety of mammalian cells into the extracellular space, are
taken up by the recipient cells affecting their function and
activity through their cargo, which include lipids, proteins and
nucleic acids that come from the cell of origin (16–19).
We also previously reported that OSCC cell-derived exosomes can be
taken up by the OSCC cells themselves promoting tumor progression
through the activation of the PI3K/AKT, mitogen-activated protein
kinase/extracellular signal-regulated kinase (ERK) and c-jun
N-terminal kinase 1/2 pathways (20). These reports suggest that within the
tumor microenvironment, exosomes secreted from the stromal cells,
including those released from the TAMs, may also be involved in
chemotherapy failure in OSCC (16–20).
However, the role of TAM-derived exosomes in the regulation of
chemoresistance in OSCC has not been fully elucidated. The present
study investigated the influence of TAM-derived exosomes on the
malignant potential and chemosensitivity of OSCC cells, as well as
the mechanisms underlying the contribution of TAM-derived exosomes
in the progression of OSCC in order to elucidate molecular
mechanisms underlying the acquisition of chemoresistance in OSCC
cells.
Materials and methods
Cell culture and reagents
The OSC-4 cells, which were established in our
laboratory using a sample from a patient with tongue cancer, were
cultured in Dulbecco's modified Eagle's medium (DMEM) (Nissui
Pharmaceutical Co., Ltd.) supplemented with 10% (v/v) fetal bovine
serum (FBS), 10 mM glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a humidified 5% CO2 air atmosphere (21). This patient provided written
informed consent at the time when the original study and
establishment of the cell line was conducted. The human monocytic
THP-1 cells, derived from a patient with acute monocytic leukemia,
were obtained from the American Type Culture Collection and
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(Nissui Pharmaceutical Co., Ltd.) supplemented with 10% (v/v) FBS,
10 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C in a humidified 5% CO2 air atmosphere. THP-1 cells
(1×106 cells/ml) were differentiated into macrophages by
treatment with 200 ng/ml phorbol 12-myristate 13-acetate (PMA)
(Sigma Aldrich; Merck KGaA) for 24 h at 37°C. Peripheral blood
mononuclear cells (PBMC) were isolated from peripheral blood of
eight healthy adults from Kochi University Hospital (Nankoku,
Japan) between October 2018 and March 2020 using Ficoll-Paque Plus
(GE Healthcare Life Sciences), according to the manufacturer's
instructions. The mean age was 32.2±8.1 years, 50% were female and
50% were male. Primary human monocytes (PHMs) from human PBMCs were
isolated using anti-CD14 monoclonal antibody-coated microbeads
(Miltenyi Biotec, Inc.), and were differentiated into macrophages
through culture in RPMI-1640 medium supplemented with 5 ng/ml
granulocyte-macrophage colony-stimulating factor (PeproTech, Inc.)
for 7 days. The 5-FU and CDDP were obtained from Sigma-Aldrich;
Merck KGaA. LY294002 and MK-2206 were purchased from ChemScene,
LLC. OSC-4 cells were treated with 10 µM LY294002 or 10 µM MK-2206
for 24 h in each experiment.
Exosome isolation
THP-1 cells and PHMs were cultured in RPMI-1640
medium supplemented with 5% (v/v) exosome-depleted FBS (System
Biosciences, LLC) for 48 h, and the exosomes were isolated using
the Total Exosome Isolation kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, the cell culture supernatants were collected and
centrifuged at 2,000 × g for 30 min at room temperature to remove
cells and cell debris. Next, the total exosome isolation kit
reagent was added to the supernatants and the mixture was
refrigerated at 4°C overnight. The mixture was centrifuged at
10,000 × g for 60 min at 4°C to remove the supernatants. The
pellets containing the exosomes were resuspended in
phosphate-buffered saline (PBS), and the protein concentrations
were determined using the bicinchoninic acid (BCA) assay.
Exosome labeling and cellular
uptake
The purified exosomes were labeled with PKH26 or
PKH67 (Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Briefly, 1 µl PKH26 or PKH67 was added to 100 µg of the
exosome pellets in a total volume of 400 µl diluent C and incubated
for 5 min at room temperature. The labeling reaction was stopped by
adding an equal volume of 1% bovine serum albumin (Sigma Aldrich;
Merck KGaA), and the samples were ultra-centrifuged at 10,000 × g
for 60 min at 4°C. The supernatants were removed, and the pellets
were resuspended in PBS.
To assess cellular uptake of the PKH26-labeled
exosomes by confocal laser microscopy, a total of 1×104
cells/well OSC-4 cells were first cultured in Nunc Lab-Tek 8-well
chamber slides (Thermo Fisher Scientific, Inc.) for 24 h to achieve
complete adhesion. Next, the cells were treated with 10 µg/ml
PKH26-labeled exosomes for 24 h. After the incubation, cells were
washed twice with PBS and mounted with SlowFade Diamond Antifade
Mountant with DAPI. The images were captured using a Fluoview
FV-1000D confocal laser scanning microscope at ×400
magnification.
To assess cellular uptake of the exosomes by flow
cytometry, a total of 1×105 cells/well OSC-4 cells were
cultured in 12-well microplates for 24 h to achieve complete
adhesion. Next, the cells were treated with 10 µg/ml PKH67-labeled
exosomes for 0, 2, 4, 8, 12 and 24 h at 37°C. After the incubation,
cells were washed twice with PBS and analyzed on a FACScan
cytometer using FlowJo software (version 10; BD Biosciences). Each
experiment was performed in triplicate.
Protein extraction and western blot
analysis
OSC-4 cells were treated with 50 µg/ml THP-1- or
PHM-derived exosomes in the presence or absence of 100 µM
chemotherapeutic drugs, 10 µM LY294002 or MK-2206 for 24 h. Then,
the total cell lysates from cell cultures and proteins from the
THP-1- and PHM-derived exosomes were collected by
radioimmunoprecipitation assay buffer (50 mM; Tris pH 7.4, 150 mM
NaCI, 1 mM EDTA, 0.25% sodium deoxycholate, 1.0% NP-40 and protease
inhibitors). The protein concentrations were determined using a BCA
assay. The extracted proteins (50 µg/lane) were separated by
SDS-PAGE and transferred to PVDF membranes. The membranes were
blocked in Tris-buffered saline containing 5% (w/v) skimmed milk
powder and 0.1% (v/v) Tween-20 at 4°C overnight. The membranes were
then probed with the primary antibodies against CD9 (1:1,000; cat.
no. ab92726; Abcam), CD63 (1:1,000; cat. no. sc-5275; Santa Cruz
Biotechnology, Inc.), Ras-related protein Rab-5B (Rab5B; 1:1,000;
cat. no. sc-598; Santa Cruz Biotechnology, Inc.), calnexin (1:200;
cat. no. MAB3126; EMD Millipore), cytochrome C (1:1,000; cat. no.
556433; BD Biosciences), phosphorylated (Ser473) Akt (p-AKT; 1:200;
cat. no. cs4060; Cell Signaling Technology, Inc.), AKT (1:500; cat.
no. cs4691; Cell Signaling Technology, Inc.), phosphorylated
(Ser21/9) glycogen synthase kinase-3β (GSK-3β) (p-GSK-3β; 1:500;
cat. no. cs9327; Cell Signaling Technology, Inc.), GSK-3β (1:500;
cat. no. cs9315; Cell Signaling Technology, Inc.), and β-actin
(1:500; cat. no. ab8226; Abcam). The signals were incubated for 1 h
at room temperature with horseradish peroxidase (HRP)-conjugated
anti-mouse IgG secondary antibody (cat. no. NA9310) or
HRP-conjugated anti-rabbit IgG secondary antibody (cat. no. NA9340)
(both 1:2,000; both from GE Healthcare). The signal detection was
performed using and enhanced chemiluminescence system (GE
Healthcare).
Proteome profiling
The Human Chemokine Array C1 (RayBiotech, Inc.) was
used to measure chemokine levels within the exosomes. Briefly, 100
µg exosomes were lysed in lysis buffer and analyzed according to
the manufacturer's instructions. The densitometric analysis of the
arrays was performed using the Multi Gauge (version 3.11; Fuji
Film).
Cell proliferation assay
Cell proliferation was analyzed using standard cell
counting and the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay. For cell counting, a total of
5×104 cells/well OSC-4 cells were seeded in 12-well
culture plates and cultured for 24 h at 37°C, followed by
incubation with 50 µg/ml THP-1- or PHM-derived exosomes for 24, 48
and 72 h. Thereafter, cells were trypsinized and counted using a
hemocytometer. For the CCK-8 assay, a total of 5×103
cells/well OSC-4 cells were seeded in 96-well culture plates and
cultured for 24 h, followed by incubation with THP-1 and
PHM-derived conditioned medium (CM) (1:10 ratio, v/v) or increasing
concentrations of THP-1- or PHM-derived exosomes in the presence or
absence of 100 µM 5-FU or CDDP for 24 h. Then, 10 µl of CCK-8
solution was added to each well and the cells were incubated for an
additional 2 h at 37°C and the absorbance was measured at 450 nm
using a microplate reader. Each experiment was performed in
triplicate.
Migration assay
The migratory potential of the cells was examined
using the CytoSelect 24-well cell migration assay (Cell Biolabs,
Inc.). Briefly, a total of 2.5×105 cells/well OSC-4
cells were seeded in 24-well plates containing proprietary treated
plastic inserts and maintained in culture for 24 h. The inserts
were then removed, and the cells were treated with increasing
concentrations of THP-1- or PHM-derived exosomes for 10 h at 37°C.
After staining with 0.5% crystal violet in 10% ethanol for 10 mins
at room temperature, the percentage of closure in the wound field
was determined by light microscopy at ×40 magnification. Each
experiment was performed in triplicate.
Invasion assay
The invasive potential of the cells was evaluated
using the BioCoat Matrigel Invasion Chamber kit (BD Biosciences).
Briefly, the OSC-4 cells at a density of 7.5×104
cells/500 µl were added to the Transwell insert chamber containing
a filter coated with Matrigel. In the lower compartment, 750 µl
DMEM containing 10% FBS was used as the chemoattractant. The OSC-4
cells were incubated with increasing concentrations of THP-1- or
PHM-derived exosomes for 24 h at 37°C. The inserts were removed and
non-invading cancer cells remaining on the upper side of the filter
were scraped off. Cells that invaded the lower side of the filter
were then stained with the Diff-Quick solution (Sysmex Corporation)
at room temperature for 10 mins and light microscopically observed
and counted in five randomly selected fields, at ×200
magnification. Each experiment was performed in triplicate.
Apoptosis assay
The OSC-4 cells were incubated with 50 µg/ml THP-1-
or PHM-derived exosomes in the presence or absence of 100 µM 5-FU
or CDDP for 24 h at 37°C. Next, the cells were stained with
propidium iodide (PI) and FITC-conjugated annexin V and analyzed on
a FACScan cytometer using FlowJo software (version 10; BD
Biosciences). Each experiment was performed in triplicate.
Cell cycle analysis
The cell cycle status was determined by flow
cytometric analysis of PI-stained cells. The cells were treated
with 50 µg/ml THP-1- or PHM-derived exosomes in the presence or
absence of 100 µM 5-FU or CDDP for 24 h at 37°C, fixed with 70%
ethanol for 1 h at 4°C, and treated with 0.5 mg/ml RNase A for 30
min at 37°C. Next, the cells were stained with 20 µg/ml PI and
analyzed on a FACScan cytometer using FlowJo. Each experiment was
performed in triplicate.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical differences among the experimental
conditions were performed using one-way analysis of variance
(ANOVA) or two-way ANOVA followed by Tukey's multiple comparisons
test. All statistical analyses were performed using BellCurve for
Excel (Social Survey Research Information Co., Ltd.) and P<0.05
was considered to indicate a statistically significant
difference.
Results
THP-1- and PHM-derived exosomes are
taken up by the OSC-4 cells
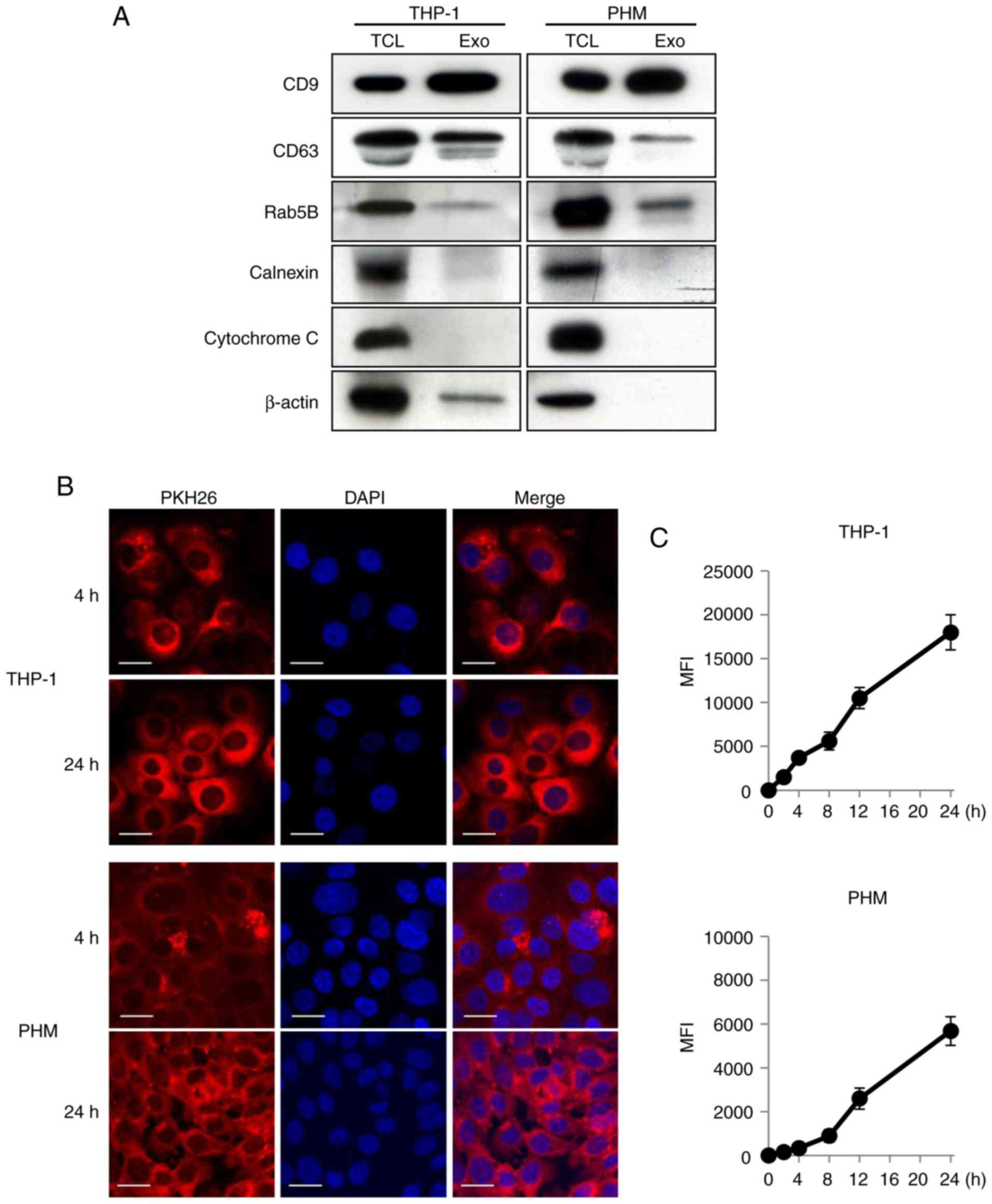
First, a western blot analysis was performed in
order to characterize the THP-1- and PHM-derived exosomes. CD9,
CD63 and Rab5B, which are used as exosomal markers, were expressed
on the THP-1- and PHM-derived exosomes (Fig. 1A). Conversely, calnexin and
cytochrome C were not detectable in the exosomal lysates (Fig. 1A). Next, the THP-1 and PHM-derived
exosomes were treated with PKH26 and PKH67, two fluorescent dyes
with long aliphatic tails that are incorporated into the lipid
membrane of exosomes, to study the uptake of the isolated exosomes.
Following the incubation of OSC-4 cells with the PKH26-labeled
THP-1- or PHM-derived exosomes, the presence of PKH26-positive
granules in the cytoplasm of OSC-4 cells was observed by confocal
laser microscopy; the granules were more diffuse at 24 h compared
with their distribution at 4 h after the addition (Fig. 1B). The flow cytometric analysis also
revealed that there was a time-dependent increase in the uptake of
both the PKH67-labeled THP-1- and the PHM-derived exosomes by the
OSC-4 cells (Fig. 1C). These data
suggested that the macrophage-derived exosomes could be taken up by
the OSC-4 cells.
Differential effects of THP-1- and
PHM-derived exosomes on proliferation, migration and invasion of
the OSC-4 cells
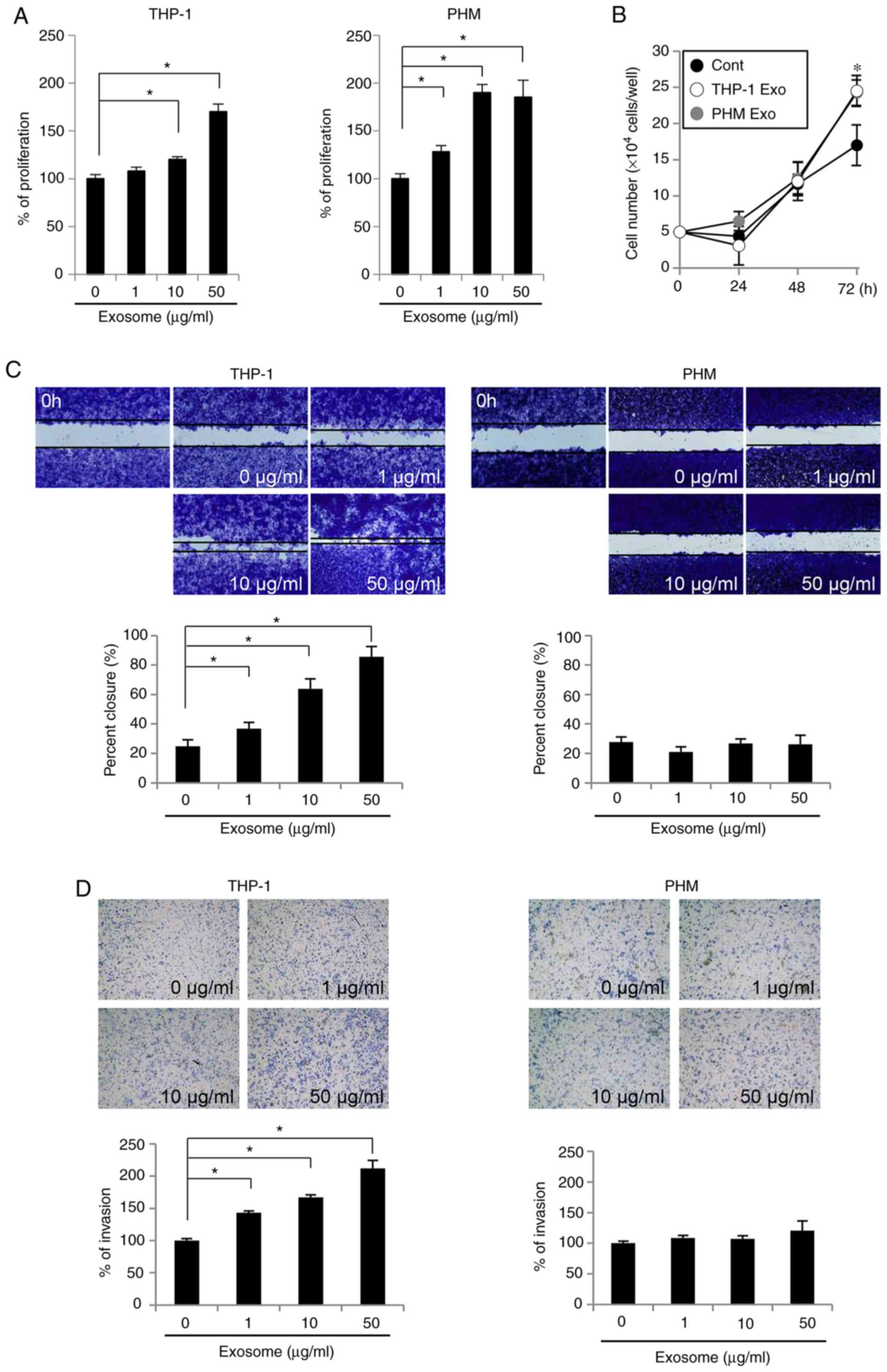
In order to determine the effects of THP-1- and
PHM-derived exosomes on the malignant potential of OSCC cells,
their effects on the proliferation, migration and invasion of OSC-4
cells were assessed. Both the THP-1- and PHM-derived exosomes
facilitated proliferation of OSC-4 cells (Fig. 2A). In addition, the cell counting
assay demonstrated a significant cell number increase in OSC-4
cells treated with these exosomes at 72 h, compared with the
control untreated cells (Fig. 2B).
THP-1-derived exosomes also promoted the dose-dependent migration
of OSC-4 cells; treatment with 50 µg/ml THP-1-derived exosomes led
to approximately 90% closure of the wound area in the migration
assay (Fig. 2C). Furthermore, the
increase in the number of OSC-4 cells invading the Matrigel was
dependent on the concentration of the THP-1-derived exosomes
(Fig. 2D). Conversely, the
PHM-derived exosomes did not exhibit a significant effect on the
migration or the invasion of OSC-4 cells, suggesting that the
THP-1-derived exosomes were more likely to promote malignancy in
the OSC-4 cells compared with the PHM-derived exosomes.
Differences in the protein content of
the THP-1- and PHM-derived exosomes
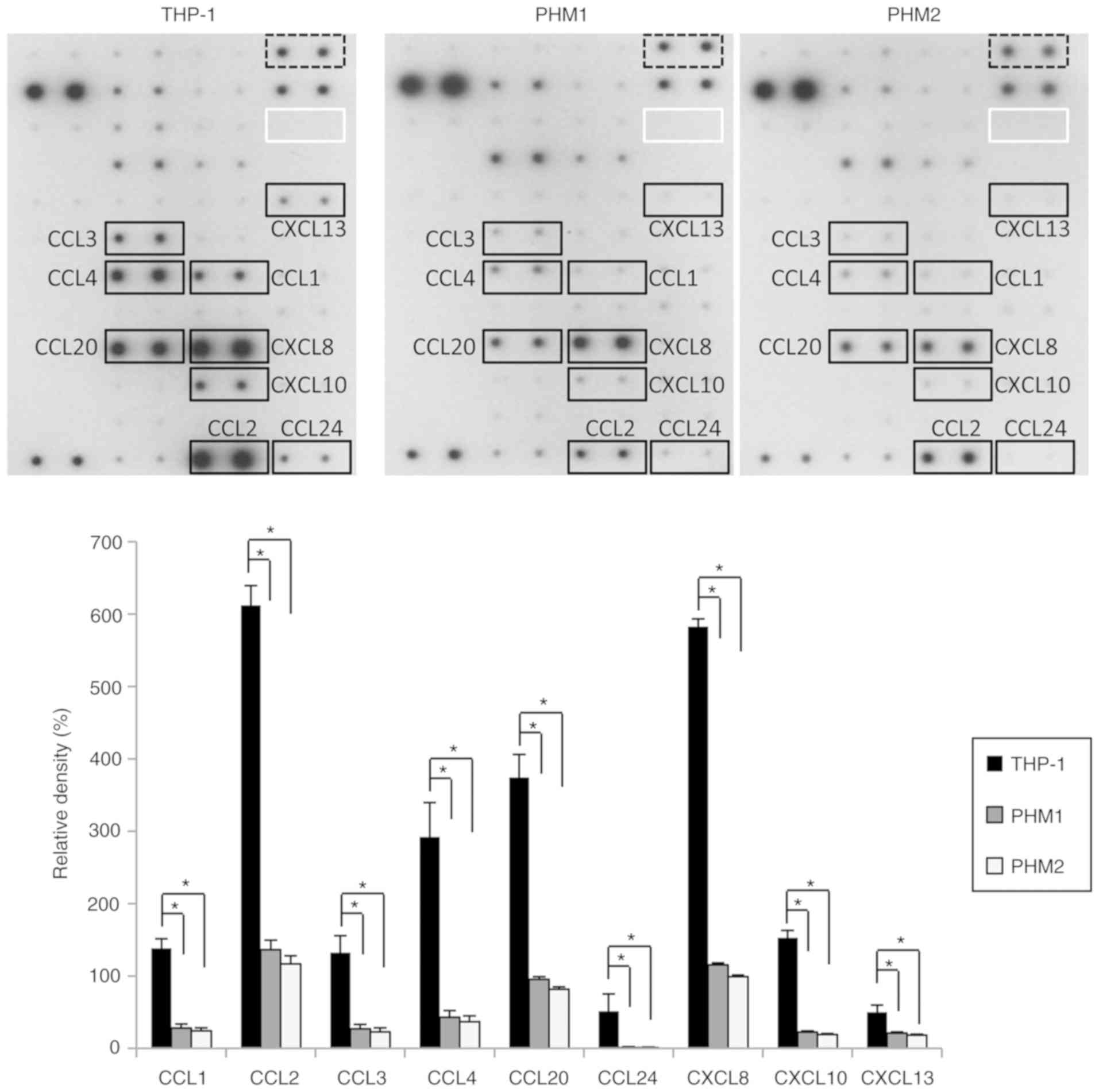
To determine the mechanism underlying the promotion
of OSC-4 cell migration and invasion by the THP-1-derived exosomes
but not the PHM-derived exosomes, the protein content of the
exosomes obtained from the THP-1 cells and PHMs was examined. The
expression levels of the chemokines including C-C motif chemokine
ligand (CCL)-1, CCL-2, CCL-3, CCL-4, CCL-20, CCL-24, C-X-C motif
chemokine ligand (CXCL)-8, CXCL-10 and CXCL-13 were >1.5 fold
higher in the exosomes derived from the THP-1 cells compared with
those derived from the PHMs of healthy donors (Fig. 3). These results suggested that the
THP-1-derived exosomes contained higher levels of chemokines than
the PHM-derived exosomes and that these chemokines might promote
the migration and invasion of OSC-4 cells.
Sensitivity of OSC-4 cells to 5-FU and
CDDP is decreased by THP-1- and PHM-derived exosomes
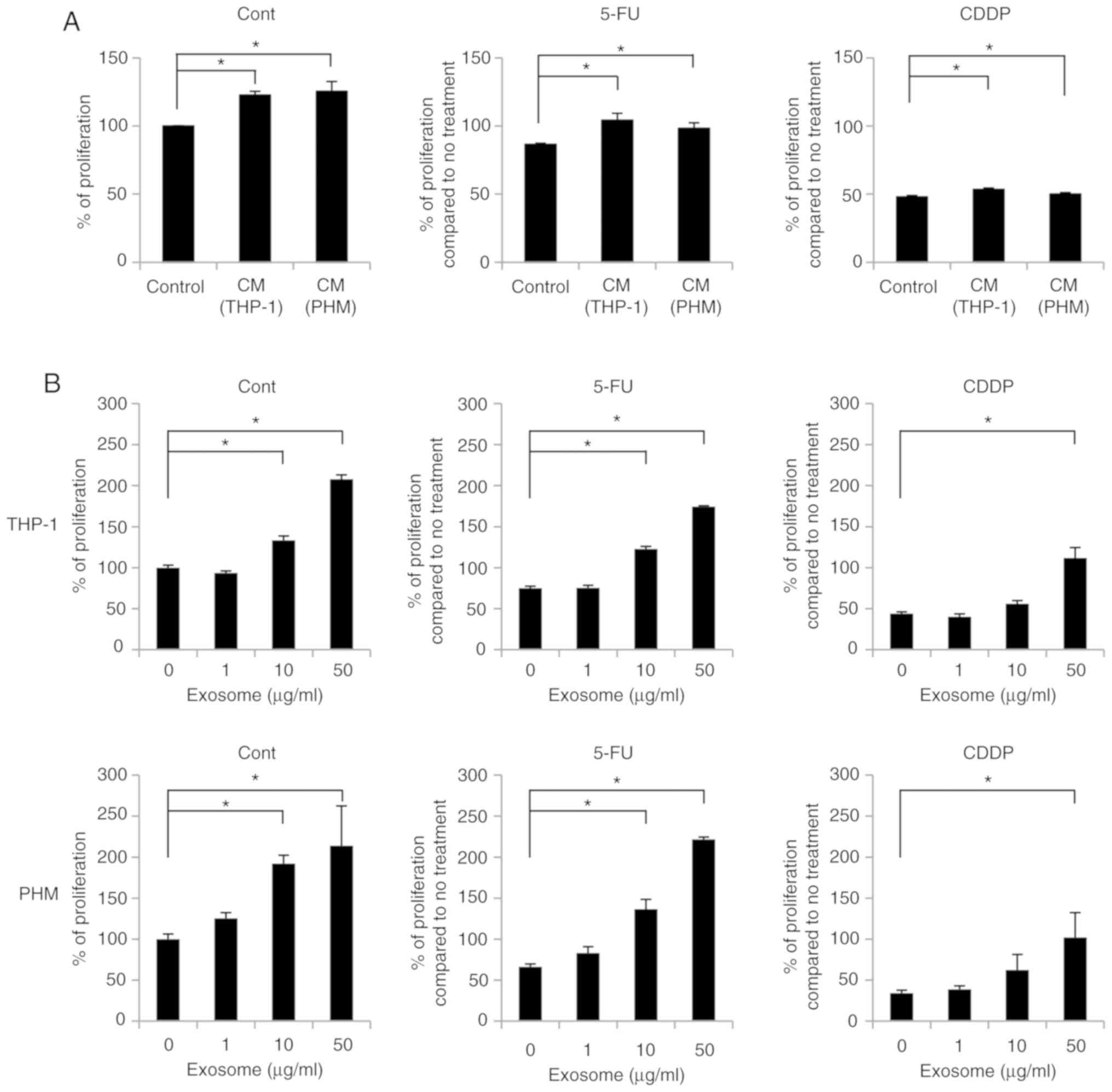
To evaluate the effect of THP-1- and PHM-derived
exosomes on the sensitivity of OSC-4 cells towards chemotherapeutic
agents, cell proliferation was measured. OSC-4 cells were treated
with the THP-1 and PHM culture supernatants containing exosomes and
cells were incubated for 24 h in the presence or absence of 5-FU or
CDDP before performing the CCK-8 assay. The proliferation-promoting
effect of THP-1- and PHM-derived isolated exosomes was higher than
the culture supernatants (Fig. 4A and
B).
Both the THP-1 and PHM culture supernatants
attenuated the 5-FU- or CDDP-mediated inhibition of OSC-4
proliferation significantly (Fig.
4A). Similarly, the CCK-8 assay revealed that both the THP-1-
and PHM-derived exosomes also suppressed the 5-FU- and
CDDP-mediated inhibition of OSC-4 proliferation in a dose-dependent
manner and that the treatment of OSC-4 cells with 50 µg/ml exosomes
abolished the inhibitory effect of 5-FU and CDDP (Fig. 4B).
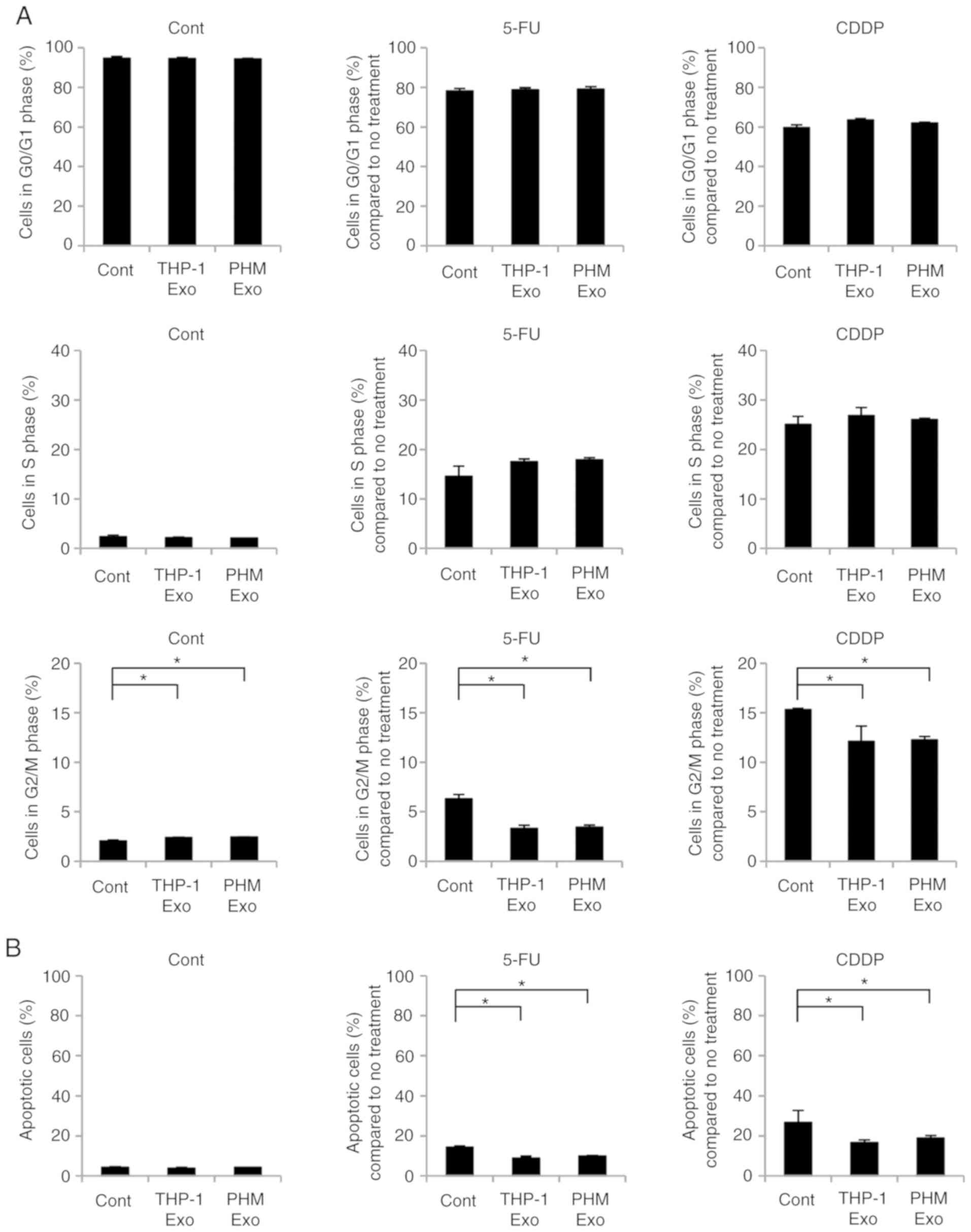
Effects of THP-1- and PHM-derived
exosomes on cell cycle and apoptosis of OSC-4 cells
In order to determine the effect of THP-1- and
PHM-derived exosomes on the cell cycle regulation of OSC-4 cells
treated with chemotherapeutic drugs, PI staining was utilized to
assess the prevalence of each cell cycle stage. Treatment with 5-FU
and CDDP was observed to induce the accumulation of OSC-4 cells in
the S and G2/M phases, whereas the addition of the
THP-1- or PHM-derived exosomes inhibited the transition of the
cells into the G2/M phase (Figs. 5A
and S1). Furthermore, the
treatment of OSC-4 cells with 5-FU or CDDP increased the percentage
of apoptotic cells, which was inhibited by the addition of the
THP-1- or PHM-derived exosomes to the cell culture (Figs. 5B and S2). These results suggested that the
macrophage-derived exosomes decreased the sensitivity of OSC-4
cells to 5-FU and CDDP through the attenuation of the effects of
the chemotherapeutic agents on cell cycle regulation and
apoptosis.
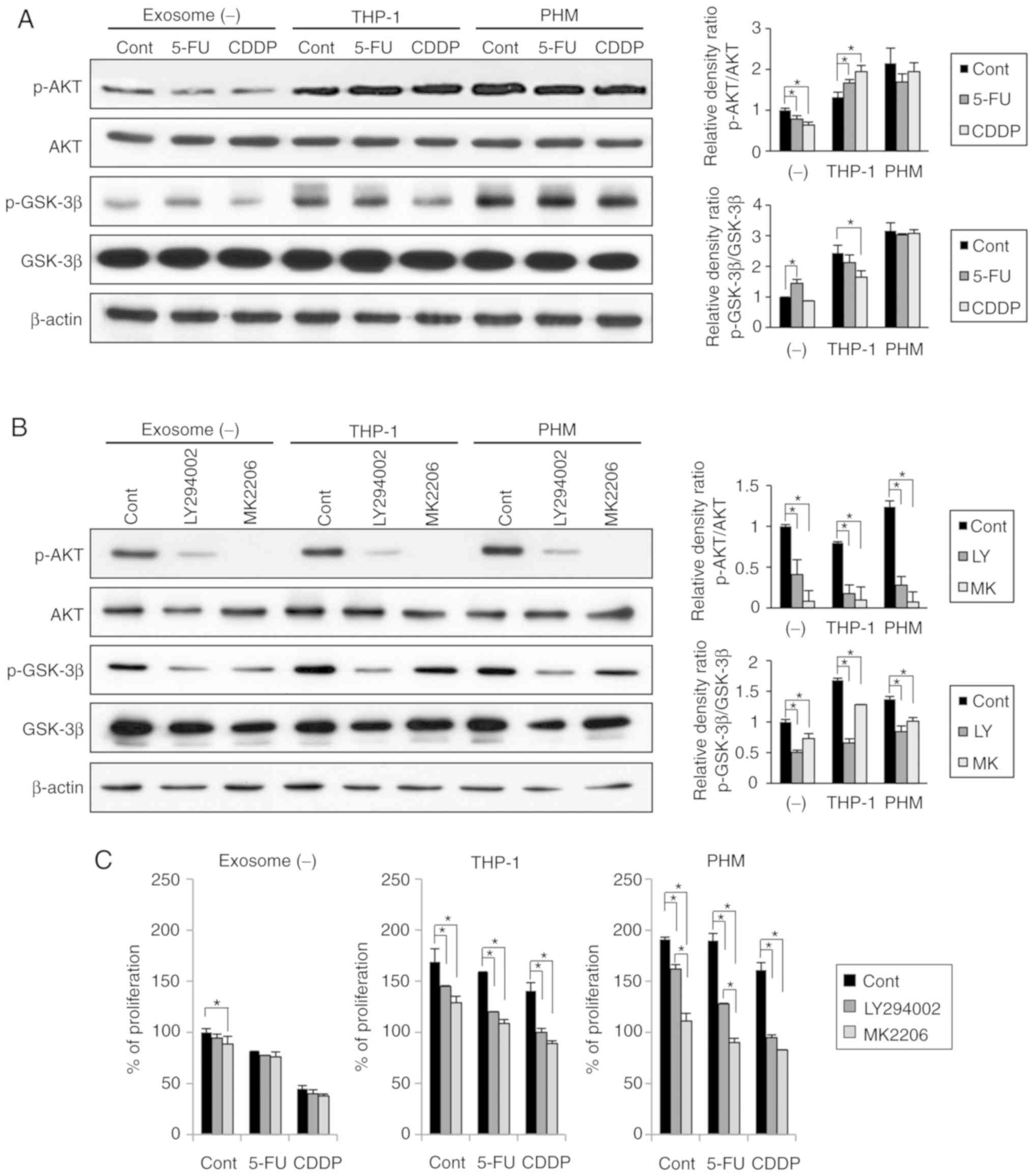
Effects of THP-1- and PHM-derived
exosomes on the AKT/GSK-3β signaling pathway in OSC-4 cells
To elucidate the mechanism underlying the
attenuation of OSC-4 sensitivity to chemotherapeutic agents by
macrophage-derived exosomes, the effects of THP-1- and PHM-derived
exosomes on the AKT/GSK-3β signaling pathway were examined. Both,
THP-1- and PHM-derived exosomes were found to induce the
phosphorylation of AKT as well as GSK-3β in the OSC-4 cells
(Fig. 6A), whereas the treatment
with 5-FU or CDDP slightly decreased the phosphorylation levels of
AKT and GSK-3β. Notably, both the THP-1- and PHM-derived exosomes
abolished the suppressive effects of 5-FU and CDDP (Fig. 6A). Furthermore, to examine whether
these changes could be reversed by PI3K/AKT inhibition, two
inhibitors were utilized: LY294002, a PI3K inhibitor and MK-2206,
an AKT inhibitor. Both inhibitors were found to decrease the
phosphorylation levels of both AKT and GSK-3β in the presence of
THP-1- and PHM-derived exosomes (Fig.
6B). In addition, both inhibitors were able to increase the
sensitivity of OSC-4 cells to both 5-FU and CDDP drugs in the
presence of THP-1- and PHM-derived exosomes (Fig. 6C). These results suggested that the
THP-1- and PHM-derived exosomes might reduce the sensitivity of
OSC-4 cells to 5-FU and CDDP through the activation of the
AKT/GSK-3β signaling pathway.
Discussion
Infiltrating macrophages in the tumor
microenvironment have been reported to promote tumor progression
through their interaction with the surrounding cells such as tumor,
stromal and immune cells, mediated by the secretion of paracrine
factors (4–6). Specifically, TAM infiltration has been
reported to be associated with tumor progression and poor prognosis
in patients with OSCC (13–15). THP-1 cells differentiated with PMA
are widely used as a model to study the function and biology of
human macrophages (22). However,
THP-1 cells are malignant tumor cells and their characteristics are
different from normal macrophages (23). Therefore, the present study used two
kinds of macrophages and examined the influence of these
cells-derived exosomes on malignant potentials and chemosensitivity
of OSCC cells.
The THP-1- and PHM-derived exosomes were rapidly
internalized by the OSC-4 cells after their addition to the culture
medium and their uptake was time-dependent, which suggested that
macrophage-derived exosomes could interact with OSCC cells
intimately and could be incorporated into OSCC cells in a very
short period of time. Subsequently, to examine the effect of
macrophage-derived exosomes on the malignant potential of OSC-4
cells, proliferation, migration and invasion assay were performed.
The results demonstrated that both the THP-1- and PHM-derived
exosomes promoted the proliferation of OSC-4 cells in a
dose-dependent manner. These results coincide with previous studies
that reported that macrophage-derived exosomes promoted the
progression of various tumors including pancreatic ductal
adenocarcinoma, colon cancer and gastric cancer (24–26).
However, the exosomes used in those studies were derived from the
M2-polarized macrophages induced by exposure to THP-1 cells or
primary monocytes treated with T helper type (Th)2 cytokines
(interleukin (IL)-4 and IL13). In the present study, THP-1 cells or
PHMs were not treated with Th1 or Th2 cytokines. Therefore,
exosomes derived from macrophages not polarized in M2 may also be
able to promote OSCC progression.
Although the THP-1-derived exosomes promoted the
migration and invasion of OSC-4 cells, a similar effect was not
observed following the exposure of OSC-4 cells to the PHM-derived
exosomes. Therefore, the expression levels of chemokines between
the THP-1- and PHM-derived exosomes was analyzed in the present
study. A variety of chemokines, including CCL-1, CCL-2, CCL-3,
CCL-4, CCL-20, CCL-24, CXCL-8, CXCL-10 and CXCL-13 were highly
expressed in the THP-1-derived exosomes compared with the
PHM-derived exosomes. Among these chemokines, the expression level
of CCL-2 was the highest in the THP-1-derived exosomes. CCL-2 was
reported to be secreted by cancer-associated fibroblasts and
tumor-associated neutrophils into the tumor microenvironment and
was shown to promote the migration and invasion of OSCC cells
following bone invasion and lymph node metastasis through the
CCL-2/CCR-2 axis (27–31). The stimulatory effects on migration
and invasion were also reported for the other chemokines that were
highly expressed in the THP-1-derived exosomes, including CCL-4,
CCL-20 and CXCL-8 (32–34). From these findings, certain
chemokines, contained in large quantities in the THP-1-derived
exosomes, may be taken up by OSCC cells and regulate their
migration and invasion.
In agreement with studies reporting that TAMs could
regulate chemoresistance (7–12), the
present study demonstrated the THP-1- and PHM-conditioned medium,
as well as the THP-1- and PHM-derived exosomes, were able to
decrease the sensitivity of OSC-4 cells to 5-FU and CDDP through
the promotion of proliferation, regulation of cell cycle and
suppression of apoptosis. These results suggest that, among the
TAM-secreted factors, exosomes can be internalized by the OSCC
cells to specifically decrease their chemosensitivity. Conversely,
previous studies have shown that tumor cell-derived exosomes can
also be taken up by macrophages and induce their polarization to an
immunosuppressive, M2-like phenotype; upregulate their expression
of programmed cell death 1; lead to their accumulation in the tumor
microenvironment; promote their secretion of protumoral, bioactive
molecules, such as vascular endothelial growth factor, monocyte
chemoattractant protein-1, IL-6, IL-1β, matrix metalloproteinase-9
and tumor necrosis factor α; and lead to altered phagocytosis
(35–40). Thus, the communication between
macrophages and tumor cells through exosomes may facilitate the
progression and metastasis of OSCC.
The AKT/GSK-3β signaling pathway is involved in
chemoresistance, epithelial-to-mesenchymal transition (EMT), and
cancer stemness (41–43). In the present study, THP-1- and
PHM-derived exosomes were demonstrated to result in a marked
increase in the levels of p-AKT and p-GSK-3β, suggesting that the
mechanism underlying the malignant potential and chemoresistance of
OSC-4 cells to 5-FU and CDDP potentiated by the THP-1- and
PHM-derived exosomes might involve the activation of the AKT/GSK-3β
signaling pathway. Some highly expressed chemokines contained in
the THP-1 and PHM-derived exosomes are known to be involved in the
promotion of cancer progression through the stimulation of the
PI3K/AKT signaling pathway. For example, CCL-2 secretion by TAM
activated the PI3K/AKT pathway in tumor cells to promote
chemoresistance of breast cancer and migration of prostate cancer
cells (44,45). CCL-20 secreted by TAM-activated EMT
and the migratory ability of renal cell carcinoma cells via AKT
activation (46). CXCL-8 derived
from macrophages promoted the migration and invasion of esophageal
squamous cell carcinoma cells via the phosphorylation of AKT and
ERK1/2 through C-X-C motif chemokine receptor (CXCR)-1 and CXCR-2
in vitro (47). These
chemokines contained in macrophages-derived exosomes may be
associated with the activation of the AKT/GSK-3β signaling pathway
in OSCC cells. To support this mechanism, PI3K/AKT inhibitors were
found to exert significant anticancer effects in the presence of
macrophage-derived exosomes. Furthermore, the PI3K/AKT inhibitors
LY294002 and wortmannin were previously shown to enhance the CDDP-,
5-FU- and docetaxel-induced apoptosis in OSCC cells (48). The results from the present study
suggest that combination therapies, including PI3K/AKT inhibitors
and traditional chemotherapeutic agents, may improve the prognosis
of patients with OSCC not only by augmenting the OSCC cell
apoptosis but also by blocking the inhibitory effects of the
TAM-derived exosomes.
In conclusion, the present study provided evidence
that macrophage-derived exosomes incorporated into OSCC cells may
facilitate chemoresistance through the activation of the AKT/GSK-3β
signaling pathway. Further studies are necessary to determine
whether TAM-derived exosomes in the tumor microenvironment of OSCC
may be a significant factor that can predict chemosensitivity of
patients and whether the inhibition of TAM-derived exosomes might
be an effective therapeutic strategy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was partly funded by Grants-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (grant no. 26463012).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ES conceived and designed the study. RT and AT
performed the experiments. RT wrote the manuscript. TY participated
in analyzing the data, performing the statistical analysis and
drafting the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the local Ethics
Committee on Medical Research at Kochi Medical School (approval no.
30-107). All blood samples were obtained strictly with the explicit
informed consent of all participants of the study. Written informed
consent was also obtained from the patient with tongue cancer at
the time the sample was taken, and the cell line established.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okura M, Aikawa T, Sawai NY, Iida S and
Kogo M: Decision analysis and treatment threshold in a management
for the N0 neck of the oral cavity carcinoma. Oral Oncol.
45:908–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castilho RM, Squarize CH and Almeida LO:
Epigenetic modifications and head and neck cancer: Implications for
tumor progression and resistance to therapy. Int J Mol Sci.
18:15062017. View Article : Google Scholar
|
|
4
|
Kulkarni B, Kirave P, Gondaliya P, Jash K,
Jain A, Tekade RK and Kalia K: Exosomal miRNA in chemoresistance,
immune evasion, metastasis and progression of cancer. Drug Discov
Today. 24:2058–2067. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ireland LV and Mielgo A: Macrophages and
fibroblasts, key players in cancer chemoresistance. Front Cell Dev
Biol. 6:1312018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Senthebane DA, Rowe A, Thomford NE,
Shipanga H, Munro D, Al Mazeedi MAM, Almazyadi HAM, Kallmeyer K,
Dandara C, Pepper MS, et al: The role of tumor microenvironment in
chemoresistance: To survive, keep your enemies closer. Int J Mol
Sci. 18:15862017. View Article : Google Scholar
|
|
7
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Chen Y, Hao L, Hou A, Chen X, Li
Y, Wang R, Luo P, Ruan Z, Ou J, et al: Macrophages induce
resistance to 5-fluorouracil chemotherapy in colorectal cancer
through the release of putrescine. Cancer Lett. 381:305–313. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Y, Yao S, Hu Y, Feng Y, Li M, Bian Z,
Zhang J, Qin Y, Qi X, Zhou L, et al: The immune-microenvironment
confers chemoresistance of colorectal cancer through
macrophage-derived IL6. Clin Cancer Res. 23:7375–7387. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X and Xiong B: M2 macrophages confer resistance to 5-fluorouracil
in colorectal cancer through the activation of CCL22/PI3K/AKT
signaling. Onco Targets Ther. 12:3051–3063. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perrotta C, Cervia D, Di Renzo I, Moscheni
C, Bassi MT, Campana L, Martelli C, Catalani E, Giovarelli M,
Zecchini S, et al: Nitric oxide generated by tumor-associated
macrophages is responsible for cancer resistance to cisplatin and
correlated with syntaxin 4 and acid sphingomyelinase inhibition.
Front Immunol. 9:11862018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Troiano G, Caponio VCA, Adipietro I,
Tepedino M, Santoro R, Laino L, Lo Russo L, Cirillo N and Lo Muzio
L: Prognostic significance of CD68+ and
CD163+ tumor associated macrophages in head and neck
squamous cell carcinoma: A systematic review and meta-analysis.
Oral Oncol. 93:66–75. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kubota K, Moriyama M, Furukawa S, Rafiul
HASM, Maruse Y, Jinno T, Tanaka A, Ohta M, Ishiguro N, Yamauchi M,
et al: CD163+ CD204+ tumor-associated
macrophages contribute to T cell regulation via interleukin-10 and
PD-L1 production in oral squamous cell carcinoma. Sci Rep.
7:17552017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, He MY, Zhu LF, Yang CC, Zhou ML,
Wang Q, Zhang W, Zheng YY, Wang DM, Xu ZQ, et al: Tumor-associated
macrophages correlate with the clinicopathological features and
poor outcomes via inducing epithelial to mesenchymal transition in
oral squamous cell carcinoma. J Exp Clin Cancer Res. 35:122016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bang C and Thum T: Exosomes: New players
in cell-cell communication. Int J Biochem Cell Biol. 44:2060–2064.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sento S, Sasabe E and Yamamoto T:
Application of a persistent heparin treatment inhibits the
malignant potential of oral squamous carcinoma cells induced by
tumor cell-derived exosomes. PLoS One. 11:e01484542016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Osaki T, Tatemoto Y, Yoneda K and Yamamoto
T: Tumorigenicity of cell lines established from oral squamous cell
carcinoma and its metastatic lymph nodes. Eur J Cancer B Oral
Oncol. 30B:296–301. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chanput W, Mes JJ and Wichers HJ: THP-1
cell line: An in vitro cell model for immune modulation approach.
Int Immunopharmacol. 23:37–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Daigneault M, Preston JA, Marriott HM,
Whyte MK and Dockrell DH: The identification of markers of
macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PLoS One. 5:e86682010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan
J, Zou Y and Chen S: Macrophage-derived exosomal microRNA-501-3p
promotes progression of pancreatic ductal adenocarcinoma through
the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res.
38:3102019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan J, Sun L, Xu F, Liu L, Hu F, Song D,
Hou Z, Wu W, Luo X, Wang J, et al: M2 macrophage-derived exosomes
promote cell migration and invasion in colon cancer. Cancer Res.
79:146–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng P, Luo Q, Wang W, Li J, Wang T, Wang
P, Chen L, Zhang P, Chen H, Liu Y, et al: Tumor-associated
macrophages-derived exosomes promote the migration of gastric
cancer cells by transfer of functional apolipoprotein E. Cell Death
Dis. 9:4342018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Xu Q, Wu Y, Li J, Tang D, Han L and
Fan Q: A CCL2/ROS autoregulation loop is critical for
cancer-associated fibroblasts-enhanced tumor growth of oral
squamous cell carcinoma. Carcinogenesis. 35:1362–1370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT
and Chen YL: Targeting galectin-1 in carcinoma-associated
fibroblasts inhibits oral squamous cell carcinoma metastasis by
downregulating MCP-1/CCL2 expression. Clin Cancer Res.
17:1306–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quan J, Morrison NA, Johnson NW and Gao J:
MCP-1 as a potential target to inhibit the bone invasion by oral
squamous cell carcinoma. J Cell Biochem. 115:1787–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita S and Ikeda T: The CCL2-CCR2 axis
in lymph node metastasis from oral squamous cell carcinoma: An
immunohistochemical study. J Oral Maxillofac Surg. 75:742–749.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ku WT, Tung JJ, Lee TJ and Lai KC:
Long-term exposure to Oroxylin A inhibits metastasis by suppressing
CCL2 in oral squamous cell carcinoma cells. Cancers (Basel).
11:E3532019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu H, Wu B, Ma G, Zheng D, Song R, Huang
E, Mao M and Lu B: Melatonin represses oral squamous cell carcinoma
metastasis by inhibiting tumor-associated neutrophils. Am J Transl
Res. 9:5361–5374. 2017.PubMed/NCBI
|
|
33
|
Chen CH, Chuang HC, Lin YT, Fang FM, Huang
CC, Chen CM, Lu H and Chien CY: Circulating CD105 shows significant
impact in patients of oral cancer and promotes malignancy of cancer
cells via CCL20. Tumour Biol. 37:1995–2005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Christofakis EP, Miyazaki H, Rubink DS and
Yeudall WA: Roles of CXCL8 in squamous cell carcinoma proliferation
and migration. Oral Oncol. 44:920–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Linton SS, Abraham T, Liao J, Clawson GA,
Butler PJ, Fox T, Kester M and Matters GL: Tumor-promoting effects
of pancreatic cancer cell exosomes on THP-1-derived macrophages.
PLoS One. 13:e02067592018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Zhou J, Li X and Wang X, Lin Y and
Wang X: Exosomes derived from hypoxic epithelial ovarian cancer
cells deliver microRNAs to macrophages and elicit a tumor-promoted
phenotype. Cancer Lett. 435:80–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsieh CH, Tai SK and Yang MH:
Snail-overexpressing cancer cells promote M2-like polarization of
tumor-associated macrophages by delivering MiR-21-abundant
exosomes. Neoplasia. 20:775–788. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Luo G, Zhang K, Cao J, Huang C,
Jiang T, Liu B, Su L and Qiu Z: Hypoxic tumor-derived exosomal
miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to
promote pancreatic cancer metastasis. Cancer Res. 78:4586–4598.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang
P, Yang J, He W, Chen H, Jiao Z and Li Y: Tumor-derived exosomes
induce PD1+ macrophage population in human gastric
cancer that promotes disease progression. Oncogenesis. 7:412018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Piao YJ, Kim HS, Hwang EH, Woo J, Zhang M
and Moon WK: Breast cancer cell-derived exosomes and macrophage
polarization are associated with lymph node metastasis. Oncotarget.
9:7398–7410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Namba T, Kodama R, Moritomo S, Hoshino T
and Mizushima T: Zidovudine, an anti-viral drug, resensitizes
gemcitabine-resistant pancreatic cancer cells to gemcitabine by
inhibition of the Akt-GSK3β-Snail pathway. Cell Death Dis.
6:e17952015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsumoto T, Yokoi A, Hashimura M, Oguri
Y, Akiya M and Saegusa M: TGF-β-mediated LEFTY/Akt/GSK-3β/Snail
axis modulates epithelial-mesenchymal transition and cancer stem
cell properties in ovarian clear cell carcinomas. Mol Carcinog.
57:957–967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meng Q, Shi S, Liang C, Liang D, Hua J,
Zhang B, Xu J and Yu X: Abrogation of glutathione peroxidase-1
drives EMT and chemoresistance in pancreatic cancer by activating
ROS-mediated Akt/GSK3β/Snail signaling. Oncogene. 37:5843–5857.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li D, Ji H, Niu X, Yin L, Wang Y, Gu Y,
Wang J, Zhou X, Zhang H and Zhang Q: Tumor-associated macrophages
secrete CC-chemokine ligand 2 and induce tamoxifen resistance by
activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 111:47–58.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maolake A, Izumi K, Shigehara K,
Natsagdorj A, Iwamoto H, Kadomoto S, Takezawa Y, Machioka K,
Narimoto K, Namiki M, et al: Tumor-associated macrophages promote
prostate cancer migration through activation of the CCL22-CCR4
axis. Oncotarget. 8:9739–9751. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kadomoto S, Izumi K, Hiratsuka K, Nakano
T, Naito R, Makino T, Iwamoto H, Yaegashi H, Shigehara K, Kadono Y,
et al: Tumor-associated macrophages induce migration of renal cell
carcinoma cells via activation of the CCL20-CCR6 axis. Cancers
(Basel). 12:892019. View Article : Google Scholar
|
|
47
|
Hosono M, Koma YI, Takase N, Urakawa N,
Higashino N, Suemune K, Kodaira H, Nishio M, Shigeoka M, Kakeji Y
and Yokozaki H: CXCL8 derived from tumor-associated macrophages and
esophageal squamous cell carcinomas contributes to tumor
progression by promoting migration and invasion of cancer cells.
Oncotarget. 8:106071–106088. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Iwase M, Yoshiba S, Uchid M, Takaoka S,
Kurihara Y, Ito D, Hatori M and Shintani S: Enhanced susceptibility
to apoptosis of oral squamous cell carcinoma cells subjected to
combined treatment with anticancer drugs and phosphatidylinositol
3-kinase inhibitors. Int J Oncol. 31:1141–1147. 2007.PubMed/NCBI
|