Introduction
Ovarian cancer is the most fatal type of tumor of
the female genital tract (1).
Although surgical techniques and chemotherapy have been improved in
recent years, the prognosis for this disease remains poor.
According to the GLOBOCAN statistics for 2018, the mortality rate
(4.4%) was higher than the rate of new cases (3.4%) in 185
countries (2). This may be due to
recurrence and chemotherapy resistance. Therefore, it is important
to explore the mechanisms of drug resistance.
The Hedgehog (Hh) gene was first reported in 1980
(3). There are three highly
conserved ligand proteins: Sonic Hh (Shh), Indian Hh and Desert Hh
(4). The glioma-associated oncogene
1 (Gli) gene family comprises three members: Gli1, Gli2 and Gli3.
Gli1 is a target gene of Gli2. The Hh signaling pathway is
suppressed in the absence of ligand binding to the transmembrane
protein receptor Patched (Ptch). Ligand binds to Ptch, releasing
Ptch-mediated suppression of Smoothened (Smo), which translocates
into the primary cilium (PC) with Gli, Suppressor of Fused (Sufu)
and kinesin family member 7; then, activated Smo induces Sufu to
release Gli, which results in Hh signaling pathway activation
(5). The Hh signaling pathway has
an important role in embryogenesis, as well as in the initiation
and development of basal-cell carcinoma (6), lung cancer (7) and ovarian cancer (8).
Several studies have indicated that the Hh signaling
pathway correlates with chemotherapy resistance. Song et al
(9) revealed widespread expression
of Smo, Gli1 and Ptch in ovarian tumors. In addition, the
expression of Smo and Gli1 in the cisplatin (DDP)-resistant A2780
cells (A2780/DDP) was significantly higher than that in native
A2780 cells. Steg et al (10) suggested that smoothened antagonists
are able to reverse taxane resistance in ovarian cancer. LDE-225,
an Smo inhibitor, increased the sensitivity of
chemotherapy-resistant ovarian cancer cells to paclitaxel by
downregulating multidrug resistance protein 1 (MDR1) expression. In
addition, Bcl2 (11,12) and forkhead box M1 (13,14),
which are target genes of the Hh signaling pathway, are
significantly associated with DDP resistance in ovarian cancer and
result in poor outcomes. Furthermore, the Hh signaling pathway
induces drug resistance via DNA damage repair (15), DNA methylation (16) and epithelial-mesenchymal transition
(17). Cancer stem cells (CSCs) and
the stem cell signaling pathway are associated with chemotherapy
resistance (18). However, the
mechanisms of the chemoresistance associated with the Hh signaling
pathway have remained elusive. Therefore, it is important to
explore the mechanistic roles of the Hh signaling pathway in the
drug resistance of ovarian cancer.
MDR1/P-glycoprotein, which is encoded by the
ATP-binding cassette (ABC) subfamily B member 1 gene, is a member
of the ABC transporter family. This molecular efflux pump
eliminates drugs from cancer cells to reduce xenobiotic molecule
accumulation, resulting in drug resistance (19). A large number of studies have
revealed that MDR1 expression is positively correlated with poor
prognosis and drug resistance (20,21).
In addition, MDR1 knockdown reverses paclitaxel resistance in
ovarian CSCs. Cui et al (22) suggested that blocking the Hh pathway
increases glioma cell sensitivity to chemotherapy by downregulating
the expression of MDR1, multidrug resistance protein 1, major vault
protein, O6-methylguanine-DNA methyltransferase, Bcl-2 and survivin
genes.
In the present study, the role of the Hh signaling
pathway in DDP resistance in ovarian cancer was investigated. Steg
et al (18) revealed that
knockdown of Gli2 diminished the viability of ES2 cells. Increased
sensitivity to DDP was noted in ES2 cells transfected with Gli2
small interfering (si)RNAs, but not Gli1 siRNAs. This may indicate
Gli2 has an important role in drug resistance in ovarian cancer. A
complementary (c)DNA microarray revealed that MDR1 may be a target
gene. To date, no previous study has assessed the promotion of DDP
resistance in ovarian cancer via the Hh pathway based on the
transcription factor Gli2 and MDR1, to the best of our knowledge.
Thus, the present study focused on Gli2, which has been indicated
to influence the proliferation and DNA damage repair and correlate
with resistance to chemotherapy in ovarian cancer. The present
study explored whether MDR1 is regulated by Gli2 to promote drug
resistance in ovarian cancer.
Materials and methods
Reagents and antibodies
MTT, DMSO and protease inhibitor (cat. no. P8340)
were purchased from Sigma-Aldrich (Merck KGaA). Puromycin (cat. no.
P8230-25) was purchased from Solarbio. Doxycycline was purchased
from Sangon Biotech. Lipofectamine 2000 (cat. no. 11668-019),
TRIzol (cat. no. 15596016), penicillin/streptomycin (cat. no.
15140122) and a bicinchoninic acid (BCA) protein assay kit (cat.
no. 23225) were purchased from Thermo Fisher Scientific, Inc.
Cyclopamine (cat. no. S1146) was purchased from Selleck and
Gli-antagonist 61 (GANT61; cat. no. HY-13901) was purchased from
MedChem Express. Anti-Gli1 primary antibody (cat. no. 2643S) was
purchased from Cell Signaling Technology, Inc. (CST) and anti-MDR1
primary antibody (cat. no. ab170904) was obtained from Abcam.
Anti-phospho-histone H2A.X (Ser139) primary antibody (cat. no.
2577) was purchased from CST and anti-GAPDH antibody (cat. no.
MAB374) was purchased from EMD Millipore. Verapamil, an MDR1
inhibitor, was purchased from Sigma Aldrich (Merck KGaA).
Cell lines and culture
The human ovarian cancer cell lines SK-OV-3 and ES-2
were purchased from the Cell Bank of the Chinese Academy of
Sciences between 2010 and 2013. The SK-OV-3 cell line, a
hypodiploid cell line, was established from an adenocarcinoma
tumor. The ES-2 cell line with a complex hyperdiploid karyotype of
66XX to 88XX was established from a surgical clear-cell carcinoma
specimen. The cells were authenticated in December 2017. 293T cells
were purchased from the American Type Culture Collection between
2010 and 2015. SK-OV-3 and 293T cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; cat. no. C11995500BT) supplemented
with 10% FBS (cat. no. 04-001-1A; Biological Industries) and 1X
penicillin/streptomycin. ES-2 cells were cultured in McCoy's 5A
medium (cat. no. 01-075-1AS; BI) supplemented with 10% FBS and 1X
penicillin/streptomycin. All cells were cultured in a 37°C in an
atmosphere with 5% CO2. Transient cell transfection was
performed with Lipofectamine 2000 or PEI according to the
manufacturer's protocol.
Complementary (c)DNA microarray
analysis
SKOV3 cells were treated with GANT61 or DMSO. RNA
was isolated with TRIzol. Double-stranded cDNA and biotin-labeled
cRNA were synthesized from RNA (300 ng) using an IlluminaH
TotalPrep RNA Amplification Kit (Ambion Inc.) according to the
manufacturer's protocol. Each biotinylated cRNA (750 ng) was
hybridized to an Illumina Human HT expression BeadChip V4 (Illumina
Inc.). Following standard washing steps and staining with
StreptavidinCy3, the arrays were scanned using an IlluminaH
BeadArray Reader. Genes with a DiffScore of <200 or >20 (i.e.
P<0.01) were considered as the differentially expressed genes
(23).
Lentivirus (LV) transfection
A Lenti-X-small hairpin (sh)RNA Tet-On system
(pGV307-red fluorescent protein) comprising shRNA-Gli2 targeting
the sequence 5′-TCCTGAACATGATGACCTA-3′ of Gli2 and an LV (SL)
system (GV356-enhanced green fluorescence protein) comprising
LV-activated Gli2 (Gli2A; GenBank accession no. NM_005270) were
constructed and packaged by GeneChem. LV-shRNA-control (shControl)
targeted the sequence 5′-TTCTCCGAACGTGTCACGT-3′. SK-OV-3 cells
seeded in a 24-well dish at 30% density were infected by shGli2 LV
[1×108 transfection units (TU)/ml] or shControl LV
(1×108 TU/ml), which were diluted by enhanced infection
solution (GeneChem), and 5 µg/ml of polybrene (GeneChem) was used
to enhance the transfection efficiency. After 12 h of transfection,
the medium was replaced with fresh medium. After 48 h, the cells
were cultured in a 12-well dish. ES-2 cells seeded in a 24-well
dish at 30% density were infected with LV-Gli2A (5×108
TU/ml) or LV-control (8×108 TU/ml), which were diluted
by enhanced infection solution (GeneChem), and 5 µg/ml of polybrene
(GeneChem) was used to enhance the transfection efficiency. After
12 h of transfection, the medium was replaced by fresh medium.
After 48 h, the cells were cultured in a 12-well dish. Cells were
selected with puromycin (2 µg/ml; Sangon Biotech) and doxycycline
(2 µg/ml; Sangon Biotech). The cell overexpression/knockdown
efficiency was confirmed by reverse transcription-quantitative PCR
(RT-qPCR) and western blot analyses (Fig. S1A and B) (24).
Cell transfection
pCMV6-Entry-Gli2-myc (cat. no. RC217291) containing
human Gli2 mRNA (GenBank accession no. NM_005270) and empty vector
(cat. no. PS100001) were obtained from OriGene. The first 984 bases
of Gli2 mRNA were deleted to generate an active form of Gli2, Gli2A
(25). pUB6/V5-hisB-Gli1 was
obtained from Dr Shiwen Luo and Dr Yong Li (Center for Experimental
Medicine, the First Affiliated Hospital of Nanchang University,
Nanchang, China) who constructed it according to a published
protocol (26). pUB6/V5-hisB vector
(cat. no. V25020) was obtained from Invitrogen (Thermo Fisher
Scientific, Inc.). SK-OV-3 cells were plated in 6-well plates at a
confluency of 60% and cultured overnight. 2 µg of
pCMV6-Entry-Gli2-myc plasmid, pUB6/V5-hisB-Gli1 plasmid or empty
vector was transfected into SK-OV-3 cells using Lipofectamine 2000
(cat. no. 11668-019; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The medium was replaced prior to
transfection. After 3–6 h of transfection, the medium containing
Lipofectamine was replaced by fresh medium. After 24–48 h, the
cells were harvested for further validation and investigations.
Secretory Flag-tagged N-terminal Shh
domain (N-Shh)-conditioned medium
An expression plasmid encoding the N-Shh, the
secreted segment of the Shh ligand with ligand activity, was
constructed as previously described by inserting the amino-terminal
signaling domain of a human Shh cDNA (cat. no. aa26-184; GenBank
accession no. NM_000193.2) into a pFlag-cytomegalovirus (CMV)1
vector (cat. no. E7273; Sigma-Aldrich; Merck KGaA) and the
pCMV-Flag1 vector was used as a control (23). 293T cells at 25% density were
transfected with the Flag-N-Shh plasmid in a 10-cm dish; 293T cells
at 25% density in another 10-cm dish were transfected with the
pCMV-Flag1 vector. After ~12 h, the medium was replaced with DMEM
supplemented with 2% FBS. All cells were cultured for an additional
24 h and the medium containing secreted N-Shh was then harvested
and stored at 4°C (27). Western
blot analysis confirmed the presence of N-Shh in the conditioned
medium (Fig. S1C). When culturing
ES-2 and SK-OV-3 cells, the conditioned Shh medium or the control
medium was mixed with an equivalent volume of fresh culture medium
supplemented with 5% FBS (24).
Induction of SK-OV-3/DDP cells
SK-OV-3 cells were cultured with different
concentrations of DDP for 72 h and the IC50 of DDP was
determined using the MTT assay. The cells were then cultured in the
presence of DDP at the IC50 value. Every month, a new
IC50 was detected. When the new IC50 value
was significantly greater than the previous one, the cells were
cultured under DDP at the new IC50 value. After 6
months, SK-OV-3/DDP cells were fully induced by DDP from SK-OV-3
cells. The resistance index was calculated as the IC50
value of the resistant cells divided by the IC50 value
of the native cells. The resistance index of the SK-OV-3/DDP cells
was 3.0 (28) and the
characteristics of resistance were maintained by continuous culture
with 1.25 µmol/l DDP.
RT-qPCR
Total RNA was isolated from cultured cells using
TRIzol reagent. A total of 2 micrograms of RNA was
reverse-transcribed to cDNA using a PrimeScript RT Reagent kit
(cat. no. RRO47A; Takara Bio, Inc.) according to the manufacturer's
protocols. Gene expression was then analyzed in these samples using
the Applied Biosystems Step One Plus™ Real-Time PCR Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
PCR mixture contained the following: SYBR® Premix Ex
Taq™ II (2X) 2 µl, PCR forward primer (10 µM) 0.8 µl, PCR reverse
primer (10 µM) 0.8 µl, ROX reference dye (50X) 0.4 µl, cDNA (taken
from the RT product mixture) 2 µl, deionized H2O 6 µl.
The real-time PCR conditions were as follows: Activation at 95°C
for 30 sec, followed by 40 cycles of denaturation at 95°C for 5
sec, primer annealing and extension at 60°C for 30 sec and a ramp
up back to 95°C. The quantification cycle (Cq) values for each
gene, normalized to the expression levels of GAPDH, were calculated
by the 2−∆∆Cq method (29). The respective forward and reverse
primer sequences for real-time PCR were as follows: MDR1,
5′-TGCTCAGACAGGATGTGAGTTG-3′ and 5′-TAGCCCCTTTAACTTGAGCAGC-3′;
GAPDH, 5′-CAGGGCTGCTTTTAACTCTG-3′ and 5′-GATTTTGGAGGGATCTCGC-3′;
Gli2, 5′-CTCAAGGAAGATCTGGACAGG-3′ and 5′-GATGTGCTCGTTGTTGATGTG-3′;
and Gli1, 5′-AGCGTGAGCCTGAATCTGTG-3′ and
5′-CAGCATGTACTGGGCTTTGAA-3′.
Western blot analysis
Total protein was extracted using a buffer [3 M
NaCl, 10% Nonidet P-40, 0.5 M EDTANa2, 1 M Tris-HCl (pH
7.5) and 1% protease inhibitor] and the protein concentrations were
detected by a BCA protein assay kit (cat. no. 23225; Thermo Fisher
Scientific, Inc.). The proteins (20–100 µg) were separated by 8–10%
SDS-PAGE for 4 h and then transferred to nitrocellulose membranes
(HATF-V0010; EMD Millipore). The following primary antibodies were
used: Gli1 (1:500 dilution), MDR1 (1:500 dilution), γ-H2AX (1:1,000
dilution) and GAPDH (1:2,000 dilution). Incubation was performed
for 12–16 h at 4°C. The blots were then incubated with a goat
anti-rabbit secondary antibody (cat. no. 31460; 1:1,000 dilution;
Thermo Fisher Scientific, Inc.) to detect MDR1, Gli1 and γ-H2AX,
and with a goat anti-mouse secondary antibody (cat. no. 31430;
1:2,000 dilution; Thermo Fisher Scientific, Inc.) to detect GAPDH;
incubation was performed for 4 h at 4°C. The secondary antibodies
were conjugated with horseradish peroxidase. Images of the
immunoblot bands were captured on X-ray film (Kodak) and then
scanned with an Epson scanner (Epson Perfection V700 Photo).
Quantified results obtained by densitometric analysis using ImageJ
software (v1.48; National Institutes of Health).
MTT and colony formation assays
SK-OV-3 cells were seeded in 96-well plates at 2,000
cells per well. Subsequently, they were treated with different
concentrations of DDP (0, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20 and 50
µmol/l) with or without cyclopamine (30 µmol/l) for 72 h. Next, the
cell viability was assessed using an MTT assay according to the
manufacturer's protocol with the optical density measured at 570
mm. SK-OV-3 cells were transfected with shRNA-Gli2 or shControl.
After determining stable expression via detection of red
fluorescence, the cells were replated in 96-well plates at 2,000
cells per well. The cells were then treated with different
concentrations of DDP (0, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20 and 50
µmol/l) for 72 h and subsequently, cell viability was assessed
using an MTT assay according to the manufacturer's protocol with
the optical density measured at 570 mm. Next, the cells were plated
in 6-well plates at 500 cells per well. ES-2 cells were treated
with 0, 0.1 and 0.2 µmol/l DDP, and SK-OV-3 cells were treated with
0, 0.2, 0.5, 2 and 5 µmol/l DDP; all cells were then cultured for
10–14 days and colonies were counted.
Statistical analysis
All experiments were performed three times.
Statistical analysis and figure plotting were performed with
GraphPad Prism 8 (GraphPad Software, Inc.). Values are expressed as
the mean ± the standard deviation. The results of the MTT assay
were analyzed by analysis of variance for repeated measurements.
Multi-group comparisons were performed by analysis of variance and
post-hoc tests (least-significant differences test). Two different
groups of quantitative data were compared by an unpaired
t-test.
Results
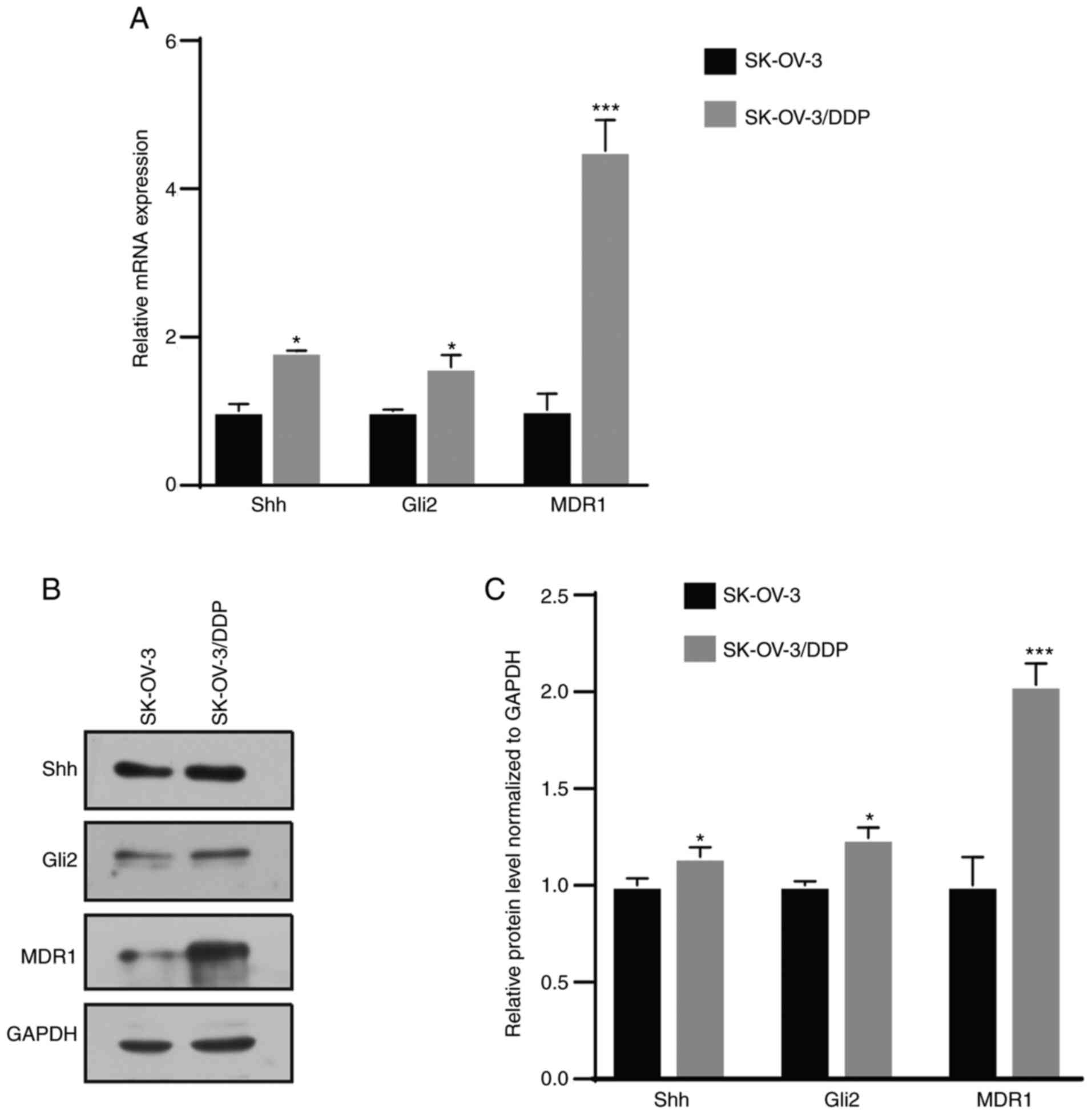
MDR1, Gli2 and Shh expression is
elevated in resistant cells
The cDNA microarray revealed that MDR1 was
downregulated by GANT61, a Gli inhibitor, in SK-OV-3 cells
(Fig. S1D). MDR1 is a
well-reported drug resistance gene in ovarian cancer (20). Next, it was attempted to determine
whether there was any association between Hh signaling and drug
resistance in ovarian cancer. Western blot and RT-qPCR assays
revealed that Shh, Gli2 and MDR1 expression was significantly
higher in SK-OV-3/DDP cells than in SK-OV-3 cells (Fig. 1). This result indicated that the Hh
signaling pathway was aberrantly activated in chemoresistant
cells.
Inhibition of Hh signaling or
knockdown of Gli2 enhances DDP sensitivity of ovarian cancer
cells
In clinical treatment, most patients are sensitive
to DDP during the first chemotherapy, while drug resistance usually
occurs after several treatments. Furthermore, SK-OV-3/DDP cells are
artificial cells induced by SK-OV-3. Various resistance studies
have been performed with native cells (18,30,31).
Therefore, native cells were chosen to explore the roles and
mechanisms of the Hh pathway in the effectiveness of DDP in ovarian
cancer. To explore the association between the Hh signaling pathway
and DDP resistance in ovarian cancer, ES-2 cells were treated with
cyclopamine (30 µmol/l), an Smo inhibitor, or DMSO (30 µmol/l) and
the cell viability was detected using the MTT assay after
increasing the DDP concentration. Cells were also treated with
GANT61 (5 µmol/l), a Gli antagonist, or DMSO (5 µmol/l). The
combination of DDP and cyclopamine or GANT61 resulted in
sensitivity to chemotherapy (P<0.001; Fig. 2A and B). Furthermore, treatment of
SK-OV-3 cells with GANT61 in combination with DDP was more
effective than treatment with either of the drugs alone (Fig. 2C), particularly at 0.5, 1 and 5
µmol/l DDP. These results indicated that targeting the Hh signaling
pathway may increase the sensitivity of ovarian cancer cells to
DDP. Gli2 expression was much higher in SK-OV-3/DDP cells than in
SK-OV-3 cells. As Gli2 is one of the most important transcription
factors, its role in DDP resistance was explored. As presented in
Fig. 2D, a significant increase in
cell death under DDP treatment was observed after knocking down
Gli2 in SK-OV-3 cells (P<0.01). By contrast, DDP resistance was
observed in ES-2 cells overexpressing Gli2 (P<0.001; Fig. 2E). Similar results were also
obtained in the colony formation assays. As compared to the control
conditions, Gli2 overexpression in ES-2 cells resulted in a
significantly greater number of colonies under the same DDP
concentration (Fig. 3A and B),
while knockdown of Gli2 in SK-OV-3 cells resulted in fewer colonies
under the same DDP concentration (Fig.
3C and D). These results indicated that the Hh signaling
pathway has a significant role in DDP resistance.
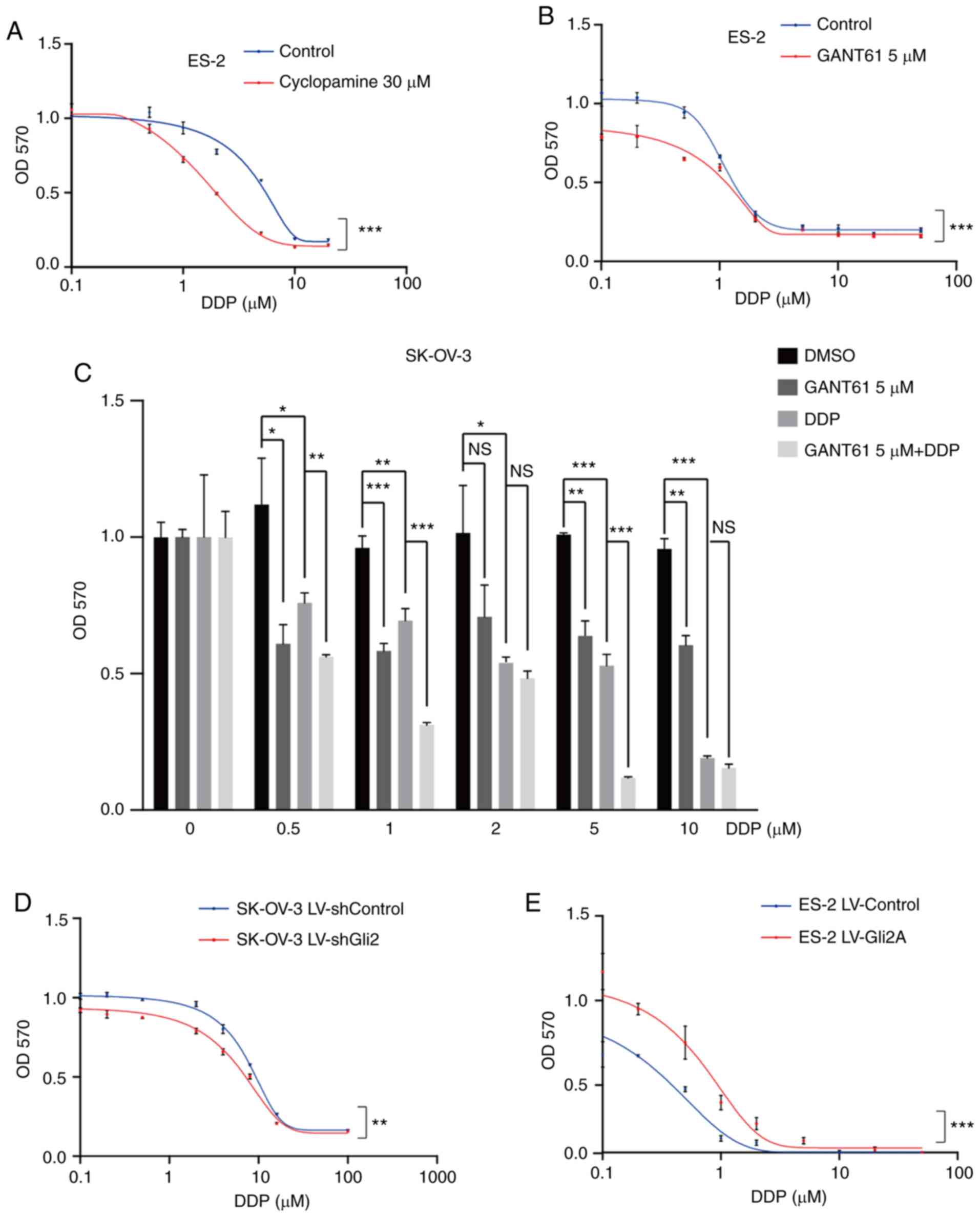 | Figure 2.Hh signaling pathway inhibition or
Gli2 knockdown enhance the effect of DDP on ovarian cancer cells.
(A and B) MTT assays revealed that inhibiting the Hh signaling
pathway with (A) cyclopamine (30 µmol/l) and (B) GAT61 (5 µmol/l)
increased cell death induced by cisplatin in ES-2 cells. (C) MTT
assays indicated that treatment with GANT61 in combination with DDP
was more effective than treatment with each drug alone in SK-OV-3
cells. (D) MTT assays indicated that Gli2 knockdown decreased the
proliferation of SK-OV-3 cells under DDP treatment. (E) Gli2
overexpression increased ES-2 cell viability under cisplatin
treatment, as determined by the MTT assay. GraphPad Prism 8
software was used for nonlinear regression (curve fit)
measurements. Experiments were performed three times. Data were
analyzed by repeated-measures two-factor ANOVA in A, B, D and E,
and by ANOVA and post-hoc tests (least-significant differences) in
C. *P<0.05, **P<0.01, ***P<0.001. NS, no significance;
ANOVA, analysis of variance; OD570, optical density at 570 nm; Hh,
Hedgehog; DDP, cisplatin; SK-OV-3 LV-shControl, SK-OV-3 cells
transfected with LV plasmid expressing control small hairpin RNA;
SK-OV-3 LV-shGli2, SK-OV-3 cells transfected with LV plasmid
expressing small hairpin RNA targeting Gli2; Gli2,
glioma-associated oncogene 2; LV, lentivirus; GANT61,
Gli-antagonist 61. |
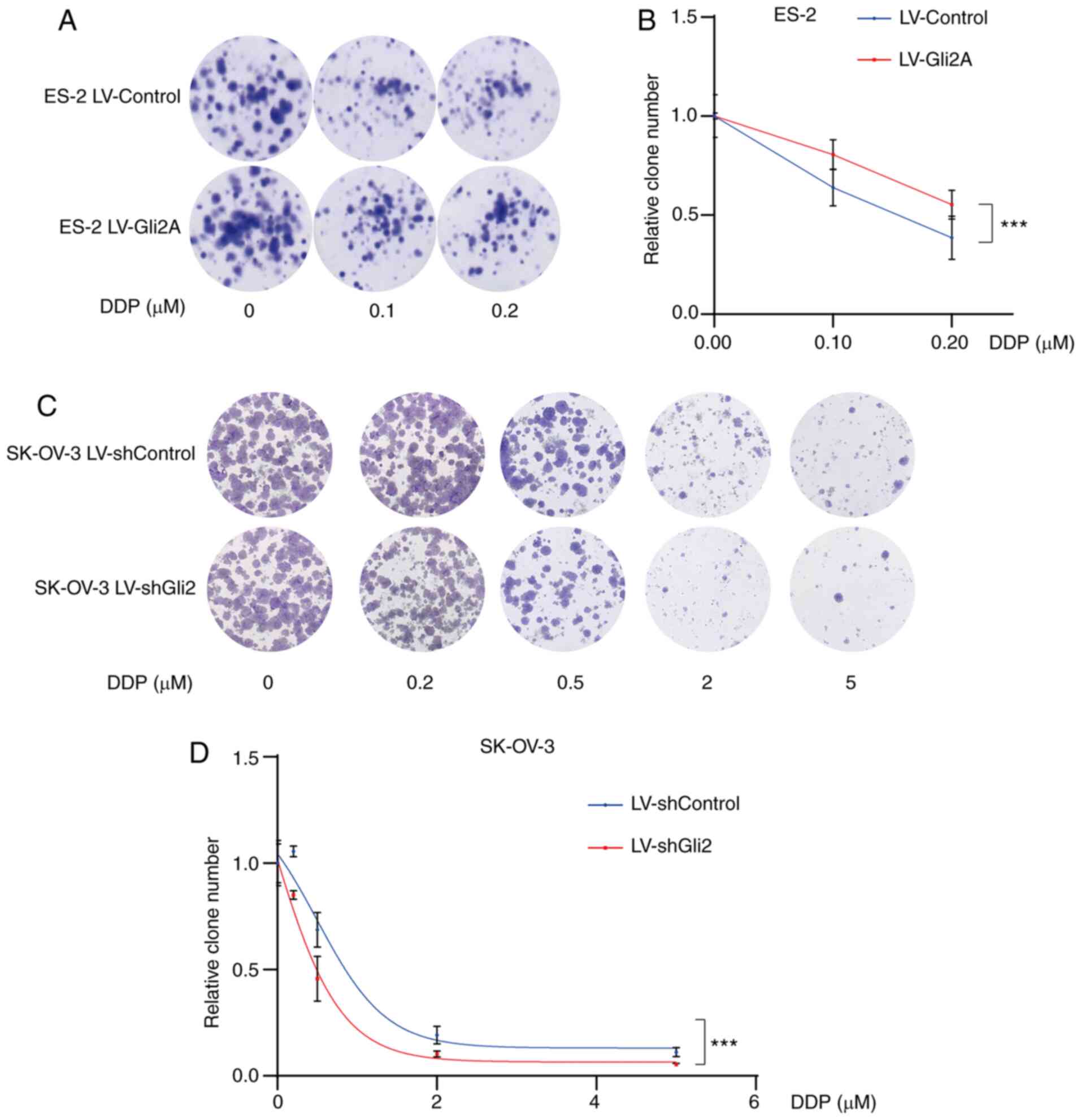 | Figure 3.Targeting Gli2 affects DDP
sensitivity in ovarian cancer cells. (A and B) Colony-formation
assays demonstrated a significantly increased number of colonies of
ES-2 LV-Gli2 cells compared to control ES-2 cells under cisplatin
treatment (0.1, 0.2 µmol/l). (A) Representative images of colonies
and (B) quantification of colonies using ImageJ software. There was
a statistically significant difference in the number of colonies
formed between the Gli2 overexpression and control groups
(F=45.995, P<0.05) and between the different doses of DDP
(F=62.158, P<0.0001), while the interaction between these terms
was not significant. A post-hoc test revealed significant pairwise
differences between shControl vs. shGli2, between cisplatin 0 and
0.1 µM, between cisplatin 0 and 0.2 µM, and between cisplatin 0.1
and 0.2 µM. (C and D) Colony formation assays indicated
significantly decreased numbers of colonies of SK-OV-3 LV-shGli2
cells compared to control SK-OV-3 cells. (C) Representative images
of colonies and (D) quantification of colonies using ImageJ
software. There was a statistically significant difference in the
number of colonies formed between the Gli2 knockdown and control
groups (F=17.653, P<0.001) and between the different doses of
DDP (F=139.978, P<0.0001), but the interaction between these
terms was not significant. A post-hoc test revealed that all
comparisons were significant except for the comparison between DDP
2 and 5 µM. Data were analyzed by two-way analysis of variance.
***P<0.001. DDP, cisplatin; SK-OV-3 LV-shControl, SK-OV-3 cells
transfected with LV plasmid expressing control small hairpin RNA;
SK-OV-3 LV-shGli2, SK-OV-3 cells transfected with LV plasmid
expressing small hairpin RNA targeting Gli2; Gli2,
glioma-associated oncogene 2; LV, lentivirus. |
Targeting the Hh signaling pathway
increases the DNA damage effect of DDP
DDP is commonly used to treat ovarian cancer. The
mechanism of its anticancer action is cytotoxicity by binding to
DNA molecules to form a platinum-DNA adduct (32). γ-H2AX is a DNA damage marker.
Therefore, in the present study, changes in the levels of γ-H2AX
were detected and the levels were indicated to be increased in a
concentration-dependent manner after DDP treatment for 72 h
(Fig. 4A and B). An increase in
γ-H2AX was observed in SK-OV-3 cells after treatment with GANT61 (5
µmol/l) combined with DDP (1 or 2 µmol/l) for 72 h compared with
DDP only (1 or 2 µmol/l) (Fig.
4C-F). After Gli2 was knocked down in SK-OV-3 cells, a further
elevation of γ-H2AX under DDP treatment still occurred, but MDR1
expression decreased (Fig. 4G-J).
These results indicated that targeting the Hh signaling pathway or
Gli2 promoted DNA damage, consequently enhancing the sensitivity of
ovarian cancer cells to DDP. Previous studies suggested that MDR1
is a candidate downstream gene of the Hh signaling pathway. In
various studies, MDR1 has been significantly correlated with the
chemoresistance of ovarian cancer (20,21,33).
In the present study, suppression of MDR1 expression was observed
(Fig. 4G-J). Therefore, the
possible relationship between the Hh signaling pathway and MDR1
expression was further explored.
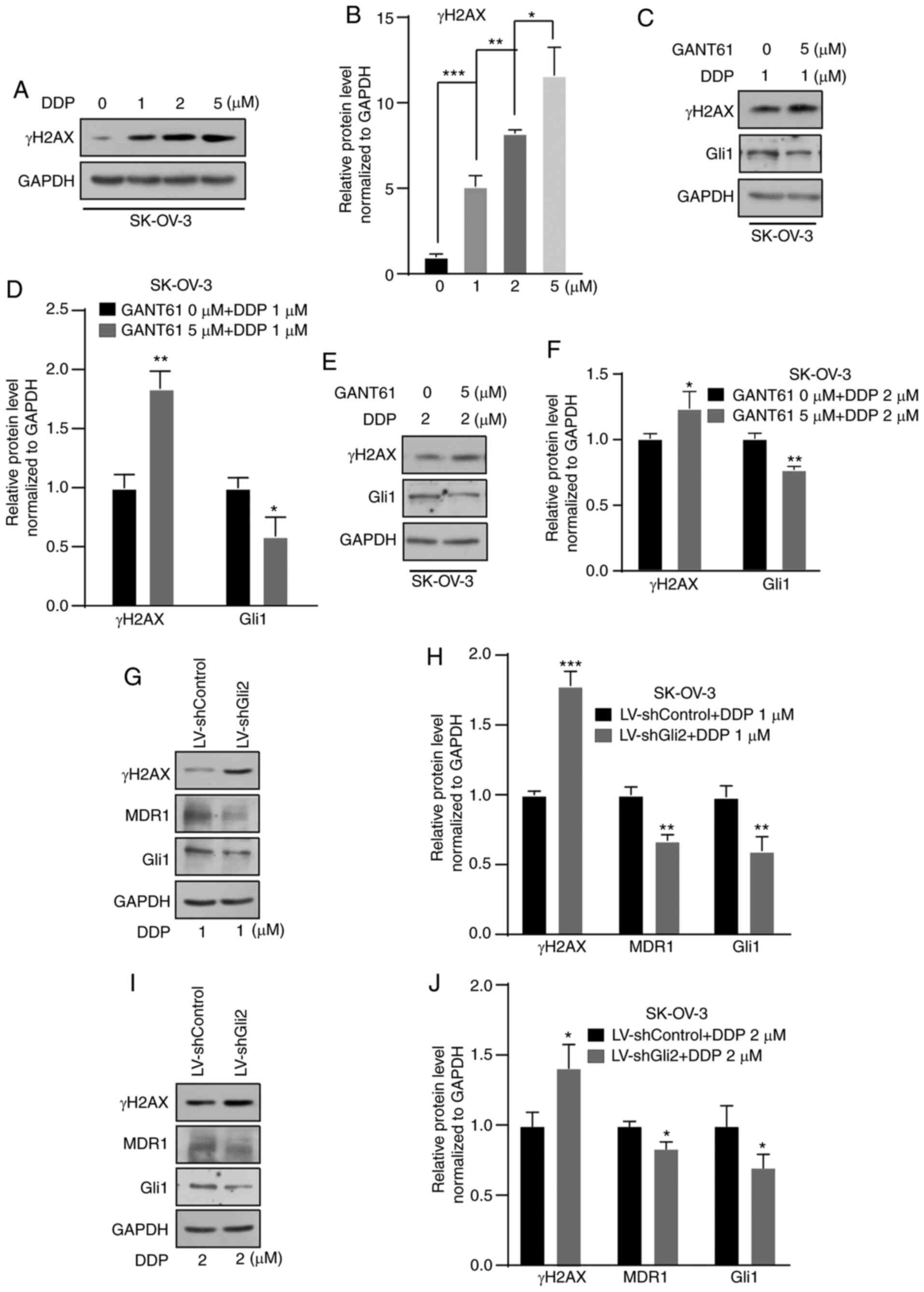 | Figure 4.Suppression of the Hedgehog signaling
pathway increases DNA damage resulting from DDP treatment. (A and
B) Western blot analysis indicated an increase in γ-H2AX after
treatment with 1, 2 and 5 µmol/l DDP in SK-OV-3 cells. (A)
Representative western blot image and (B) quantified results
obtained by densitometric analysis using ImageJ software. (C and D)
Higher levels of γ-H2AX after treatment with 1 µmol/l DDP in
combination with GANT61 (5 µmol/l) was observed in SK-OV-3 cells by
western blot analysis. (C) Representative western blot image and
(D) quantified results obtained by densitometric analysis. (E and
F) Higher levels of γ-H2AX after treatment with 2 µmol/l DDP in
combination with GANT61 (5 µmol/l) was observed by western blot
assay in SK-OV-3 cells. (E) Representative western blot image and
(F) quantified results obtained by densitometric analysis. (G and
H) Gli2 knockdown in combination with 1 µmol/l DDP treatment
increased γ-H2AX levels, decreased MDR1 levels in SK-OV-3 cells.
(G) Representative western blot image and (H) quantified results
obtained by densitometric analysis. (I and J) Gli2 knockdown in
combination with 2 µmol/l DDP treatment increased γ-H2AX levels,
decreased MDR1 levels in SK-OV-3 cells. (I) Representative western
blot image and (J) quantified results obtained by densitometric
analysis. Gli1 was used as a positive control. Values are expressed
as the mean ± standard deviation (n=3). Data were analyzed by
unpaired t-tests or analysis of variance and post-hoc tests.
*P<0.05, **P<0.01, ***P<0.001. DDP, cisplatin; SK-OV-3
LV-shControl, SK-OV-3 cells transfected with LV plasmid expressing
control small hairpin RNA; SK-OV-3 LV-shGli2, SK-OV-3 cells
transfected with LV plasmid expressing small hairpin RNA targeting
Gli2; Gli2, glioma-associated oncogene 2; γ-H2AX, γ-phosphorylated
H2A.X variant histone; MDR1, multidrug resistance protein 1; LV,
lentivirus.; GANT61, Gli-antagonist 61. |
MDR1 expression is regulated by the Hh
signaling pathway via Gli2
In previous studies by our group, several target
genes were explored using a cDNA microarray and it was demonstrated
that aberrant activation of the Hh signaling pathway promotes
ovarian cancer invasion and migration via target genes such as CD24
(34) and MMP7 (24). The present study focused on MDR1,
which is considered a candidate gene. First, ES-2 cells were
treated with N-Shh-conditioned media or control media and a
time-dependent increase in MDR1 protein levels was observed by
western blot analysis (Fig. 5A and
B). Furthermore, a time-dependent increase in MDR1 protein
expression in SK-OV-3 cells was observed after N-Shh treatment
(Fig. S2A and B). ES-2 and SK-OV-3
cells were then treated with cyclopamine and the results indicated
that the protein expression of MDR1 was suppressed in a
time-dependent manner in ES-2 cells (Fig. 5C and D), and the mRNA level was
decreased in SK-OV-3 cells (Fig.
5E). Furthermore, the protein and mRNA levels of MDR1 were
decreased in ES-2 and SK-OV-3 cells after GANT61 treatment
(Fig. 5F-H; Fig. S2C). Simultaneously, MDR1 expression
was downregulated in SK-OV-3 cells after Gli2 knockdown (Fig. 5I and J). By contrast, there was an
increase in the level of MDR1 mRNA after transfecting SK-OV-3 cells
with the Gli2 overexpression plasmids (Fig. 5K). Regardless, there was no increase
in the level of MDR1 mRNA after transfecting SK-OV-3 cells with
Gli1 overexpression plasmid (Fig.
S2D). These results revealed that MDR1 is regulated by the Hh
signaling pathway via Gli2. To define the mechanism by which the Hh
pathway promotes DDP resistance via MDR1, ES-2 cells with or
without overexpression of Gli2 were treated with verapamil (5
µmol/l), an MDR1 inhibitor, and the cell viability was detected by
the MTT assay after increasing the DDP concentration. An increase
in cell viability was observed in ES-2 cells overexpressing Gli2
under DDP treatment, but there was no increase in the viability of
Gli2-overexpressing ES-2 cells after treatment with verapamil
(Fig. S3A). Thus, the Hh signaling
pathway promotes chemotherapy resistance via MDR1.
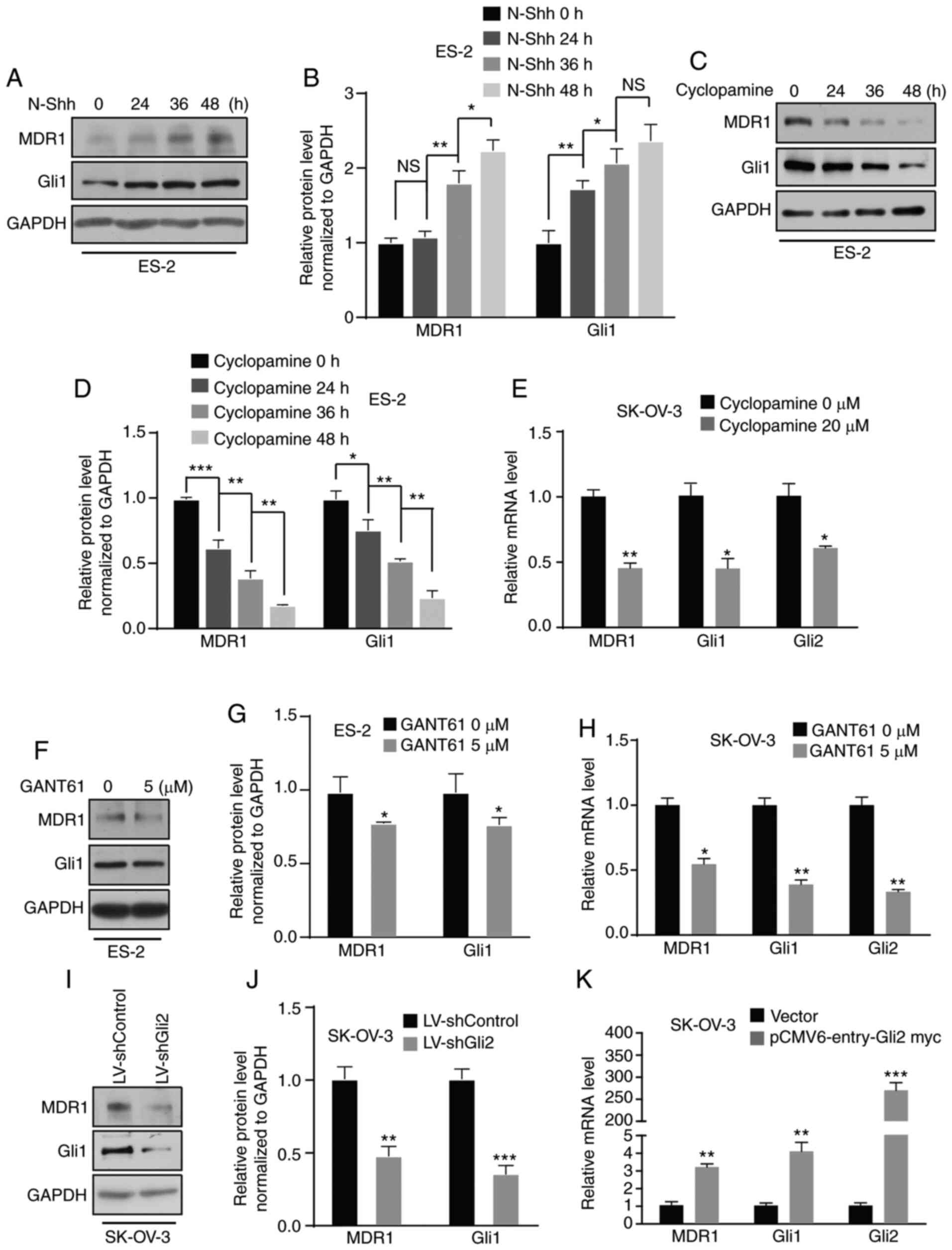 | Figure 5.MDR1 is regulated by the Hedgehog
signaling pathway. (A and B) ES-2 cells were incubated with N-Shh
for 0, 24, 36 and 48 h. Western blot analysis revealed that MDR1
expression was upregulated. Gli1 was used as a positive control.
(A) Representative western blot image and (B) quantified results
obtained by densitometric analysis using ImageJ software. (C and D)
ES-2 cells were treated with cyclopamine (20 µmol/l) for 0, 24, 36
and 48 h. Western blot assays indicated that MDR1 expression was
downregulated. (C) Representative western blot image and (D)
quantified results obtained by densitometric analysis. (E) RT-qPCR
indicated a reduction in the mRNA level of MDR1 in SK-OV-3 cells
treated with cyclopamine (20 µmol/l). Gli1 and Gli2 were used as
positive controls. (F and G) ES-2 cells were treated with GANT61 (5
µmol/l). Western blot assays indicated that MDR1 expression was
downregulated. (F) Representative western blot image and (G)
quantified results obtained by densitometric analysis. (H) RT-qPCR
revealed a decrease in the mRNA level of MDR1 in SK-OV-3 cells
treated with GANT61 (5 µmol/l). Gli1 and Gli2 were used as positive
controls. (I and J) Gli2 knockdown in SK-OV-3 cells resulted in a
decrease in MDR1 protein expression. (I) Representative western
blot image and (J) quantified results obtained by densitometric
analysis. (K) RT-qPCR revealed that Gli2 overexpression in SK-OV-3
cells induced an increase in MDR1 mRNA levels. Values are expressed
as the mean ± standard deviation (n=3). Data were analyzed by
unpaired t-tests or analysis of variance and post-hoc tests
(least-significant difference). *P<0.05, **P<0.01,
***P<0.001 vs. control. NS, no significance; DDP, cisplatin;
SK-OV-3 LV-shControl, SK-OV-3 cells transfected with LV plasmid
expressing control small hairpin RNA; SK-OV-3 LV-shGli2, SK-OV-3
cells transfected with LV plasmid expressing small hairpin RNA
targeting Gli2; Gli2, glioma-associated oncogene 2; N-Shh,
N-terminal ‘Hedge’ domain; MDR1, multidrug resistance protein 1;
RT-qPCR, reverse transcription-quantitative PCR; LV, lentivirus;
GANT61, Gli-antagonist 61. |
Discussion
Ovarian cancer has been indicated to be the leading
cause of cancer-related death among cancers of the female genital
tract (1). DDP and other
chemotherapeutic drugs are commonly used to treat ovarian cancer,
but the prognosis is poor due to chemotherapy resistance (35). The present study sought to determine
ways to reverse DDP resistance.
Hh was first reported in Drosophila
melanogaster in 1980 and has an important role in embryogenesis
(36). The Hh signaling pathway
participates in the invasion, metastasis and drug resistance of a
variety of tumor types, including ovarian cancer (37), hepatoma (38), cervical cancer (39) gliomas (22) and gastric cancer (40). Zahreddine et al (41) suggested that Gli1 alone should be
sufficient to facilitate UGT1A-dependent glucuronidation of
cytarabine and ribavirin and ultimately drug resistance. A Gli1
inhibitor was able to restore drug sensitivity and thereby provide
therapeutic benefits. Steg et al (37) indicated that proteasome inhibition,
through alteration of microtubule dynamics and Hh signaling, was
able to reverse taxane-mediated chemoresistance. Another study
revealed that Gli1, Gli2 or Smo knockdown enhances taxane
sensitivity (10), which was
consistent with the results of the present study. The protein and
RNA levels of Gli2, Shh and MDR1 are higher in chemoresistant cell
lines than in wild-type ovarian cancer cells, indicating that the
Hh signaling pathway may be activated in chemotherapy-resistant
ovarian cancer. MTT and colony formation assays confirmed that
treatment with a Gli inhibitor (GANT61), treatment with an Smo
antagonist (cyclopamine) or Gli2 knockdown increased the
sensitivity of ovarian cancer cell lines to DDP. By contrast, Gli2
overexpression promoted the resistance of ES-2 cells to DDP.
However, after inhibiting MDR1, overexpression of Gli2 in ES-2
cells did not increase cell viability under DDP. Therefore, it is
indicated that the Hh signaling pathway is abnormally activated in
chemoresistant ovarian cancer cell lines and that targeting the Hh
signaling pathway, Gli2 or MDR1 is able to reverse chemoresistance
to DDP.
The mechanisms of DDP resistance are associated with
DNA repair alterations and cellular accumulation and drug
inactivation (42). Platinum
compounds induce cytotoxicity by binding to DNA molecules to form a
platinum-DNA adduct, which may be removed by nucleotide excision
repair (NER). ERCC excision repair 1, endonuclease non-catalytic
subunit (ERCC1), which is regarded as one of the most potent
biomarkers for DDP resistance, is associated with the removal of
DNA-platinum adducts and results in DDP resistance (43). Targeting Gli1 alters the expression
of NER-associated genes (e.g., c-JUN, ERCC1, and X-ray repair
cross-complementing 1) and increases DDP sensitivity (44–46).
Therefore, it may be speculated that the Hh signaling pathway is
involved in DNA damage repair to promote chemotherapy resistance in
ovarian cancer. γ-H2AX is a DNA damage marker. GANT61, an inhibitor
of Gli, increases γ-H2AX expression in glioma cells in response to
temozolomide treatment (47). In
the present study, an increase in γ-H2AX protein expression, along
with a decrease in MDR1, was detected in ovarian cancer cells at 72
h following DDP treatment combined with GANT61 treatment or Gli2
knockdown. At such a time-point, DNA damage is more common than DNA
damage repair (48). It has been
confirmed that there is an increase in DNA damage under DDP
treatment combined with Hh signaling pathway targeting. Further
exploration of the mechanism of the Hh signaling pathway in DNA
damage or DNA damage repair may provide strategies to reverse
chemotherapy resistance in ovarian cancer.
Targeting the Hh signaling pathway in ovarian cancer
alters cell viability and the DNA damage response to treatment with
DDP, which indicates that the Hh signaling pathway has an important
role in ovarian cancer cell resistance to DDP. However, the
mechanisms underlying this process remain to be fully elucidated.
As a drug resistance gene, MDR1 has been demonstrated to be a cause
of drug resistance. It has been revealed that Hh signaling
increases resistance to multiple chemotherapeutic agents in part by
promoting drug efflux through regulation of MDR1 expression
(19). A gene microarray suggested
that MDR1 is a candidate target gene of the Hh signaling pathway
after treatment of SK-OV-3 cells with GANT61. Western blot and
RT-qPCR assays demonstrated that MDR1 is a target gene of the Hh
signaling pathway. There was a decrease in the expression of MDR1
along with an increase in γ-H2AX, increase of DNA damage and
reduction of MDR1 expression after DDP treatment combined with
targeting of the Hh signaling pathway.
In conclusion, targeting the Hh signaling pathway
increases DDP sensitivity in ovarian cancer. MDR1 is a target gene
of the Hh signaling pathway. The Hh signaling pathway may affect
DDP chemoresistance in ovarian cancer via MDR1.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Dr Shiwen Luo and Dr Yong Li
(Center for Experimental Medicine, The First Affiliated Hospital of
Nanchang University, Nanchang, China) for producing and providing
the plasmid.
Funding
This work was supported in part by grants from the
National Natural Science Foundation of China (grant no. 81960470)
and the Key R&D Project of Jiangxi Science and Technology
Department (grant no. 20171BBG70008), as well as the Graduate
Innovation Foundation of Nanchang University (grant no.
CX2018195).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and LH performed the MTT assays and colony
formation assays; HZ analyzed and interpreted the data; LH drafted
the initial manuscript; MC revised the manuscript and performed
statistical analysis; QW and XH performed the western blot and
RT-qPCR analyses; QC provided the experimental design, managed the
study, reviewed the manuscript and provided funding support. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nüsslein-Volhard C and Wieschaus E:
Mutations affecting segment number and polarity in
Drosophila. Nature. 287:795–801. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development_ paradigms and princip. Genes Dev.
15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hui CC and Angers S: Gli proteins in
development and disease. Annu Rev Cell Dev Biol. 27:513–537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bakshi A, Chaudhary SC, Rana M, Elmets CA
and Athar M: Basal cell carcinoma pathogenesis and therapy
involving hedgehog signaling and beyond. Mol Carcinog.
56:2543–2557. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park KS, Martelotto LG, Peifer M, Sos ML,
Karnezis AN, Mahjoub MR, Bernard K, Conklin JF, Szczepny A, Yuan J,
et al: A crucial requirement for hedgehog signaling in small cell
lung cancer. Nat Med. 17:1504–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Li J and Feng L: Hedgehog signaling
pathway as a therapeutic target for ovarian cancer. Cancer
Epidemiol. 40:152–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song X, Yan L, Lu C, Zhang C, Zhu F, Yang
J, Jing H, Zhang Y, Qiao J and Guo H: Activation of hedgehog
signaling and its association with cisplatin resistance in ovarian
epithelial tumors. Oncol Lett. 15:5569–5576. 2018.PubMed/NCBI
|
|
10
|
Steg AD, Katre AA, Bevis KS, Ziebarth A,
Dobbin ZC, Shah MM, Alvarez RD and Landen CN: Smoothened
antagonists reverse taxane resistance in ovarian cancer. Mol Cancer
Ther. 11:1587–1597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao X, Siu MK, Au CW, Wong ES, Chan HY,
Ip PP, Ngan HY and Cheung AN: Aberrant activation of hedgehog
signaling pathway in ovarian cancers: Effect on prognosis, cell
invasion and differentiation. Carcinogenesis. 30:131–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben-Hamo R, Zilberberg A, Cohen H,
Bahar-Shany K, Wachtel C, Korach J, Aviel-Ronen S, Barshack I,
Barash D, Levanon K and Efroni S: Resistance to paclitaxel is
associated with a variant of the gene BCL2 in multiple tumor types.
NPJ Precis Oncol. 3:122019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Li ZY, Duan XJ, Fan XM, Liu WN
and Li YH: Clinical significance of FOXM1 and gli-1 protein
expression in high-grade ovarian serous carcinoma. Zhonghua Zhong
Liu Za Zhi. 38:904–908. 2016.(In Chinese). PubMed/NCBI
|
|
14
|
Tassi RA, Todeschini P, Siegel ER, Calza
S, Cappella P, Ardighieri L, Cadei M, Bugatti M, Romani C, Bandiera
E, et al: FOXM1 expression is significantly associated with
chemotherapy resistance and adverse prognosis in non-serous
epithelial ovarian cancer patients. J Exp Clin Cancer Res.
36:632017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng E, Hanna A, Samant RS and Shevde LA:
The impact of hedgehog signaling pathway on DNA repair mechanisms
in human cancer. Cancers (Basel). 7:1333–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang RL, Gu F, Kirma NB, Ruan J, Chen CL,
Wang HC, Liao YP, Chang CC, Yu MH, Pilrose JM, et al: Comprehensive
methylome analysis of ovarian tumors reveals hedgehog signaling
pathway regulators as prognostic DNA methylation biomarkers.
Epigenetics. 8:624–634. 2014. View Article : Google Scholar
|
|
17
|
Ke Z, Caiping S, Qing Z and Xiaojing W:
Sonic hedgehog-gli1 signals promote epithelial-mesenchymal
transition in ovarian cancer by mediating PI3K/AKT pathway. Med
Oncol. 32:3682015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steg AD, Bevis KS, Katre AA, Ziebarth A,
Dobbin ZC, Alvarez RD, Zhang K, Conner M and Landen CN: Stem cell
pathways contribute to clinical chemoresistance in ovarian cancer.
Clin Cancer Res. 18:869–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bossennec M, Di Roio A, Caux C and
Ménétrier-Caux C: MDR1 in immunity: Friend or foe? Oncoimmunology.
7:e14993882018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu L, Katsaros D, Wiley A, de la Longrais
IA, Puopolo M and Yu H: Expression of MDR1 in epithelial ovarian
cancer and its association with disease progression. Oncol Res.
16:395–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaidyanathan A, Sawers L, Gannon AL,
Chakravarty P, Scott AL, Bray SE, Ferguson MJ and Smith G: ABCB1
(MDR1) induction defines a common resistance mechanism in
paclitaxel- and olaparib-resistant ovarian cancer cells. Br J
Cancer. 115:431–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui D, Xu Q, Wang K and Che X: Gli1 is a
potential target for alleviating multidrug resistance of gliomas. J
Neurol Sci. 288:156–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Q, Xu R, Zeng C, Lu Q, Huang D, Shi
C, Zhang W, Deng L, Yan R, Rao H, et al: Down-Regulation of gli
transcription factor leads to the inhibition of migration and
invasion of ovarian cancer cells via integrin beta4-mediated FAK
signaling. PLoS One. 9:e883862014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Wang Y, Chen T, Zhang Y, Xu R,
Wang W, Cheng M and Chen Q: Aberrant activation of hedgehog
signalling promotes cell migration and invasion via matrix
metalloproteinase-7 in ovarian cancer cells. J Cancer. 10:990–1003.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roessler E, Ermilov AN, Grange DK, Wang A,
Grachtchouk M, Dlugosz AA and Muenke M: A previously unidentified
amino-terminal domain regulates transcriptional activity of
wild-type and disease-associated human GLI2. Hum Mol Genet.
14:2181–2188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang D, Hu G, Du Y, Zhang C, Lu Q, Lv N
and Luo S: Aberrant activation of hedgehog signaling promotes cell
proliferation via the transcriptional activation of forkhead Box M1
in colorectal cancer cells. J Exp Clin Cancer Res. 36:232017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan R, Peng X, Yuan X, Huang D, Chen J, Lu
Q, Lv N and Luo S: Suppression of growth and migration by blocking
the hedgehog signaling pathway in gastric cancer cells. Cell Oncol
(Dordr). 36:421–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo P, Peng D, Xiong X and Zhang S:
Expression of microRNA-100 and its correlation with drug resistance
in human ovarian cancer SKOV3/DDP cells. Nan Fang Yi Ke Da Xue Xue
Bao. 35:1624–1627. 2015.(In Chinese). PubMed/NCBI
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative CT method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Q, Shi YL, Zhou K, Wang LL, Yan ZX,
Liu YL, Xu LL, Zhao SW, Chu HL, Shi TT, et al: PIK3CA mutations
confer resistance to first-line chemotherapy in colorectal cancer.
Cell Death Dis. 9:7392018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao ZJ, Yuan WD, Yuan JQ, Yuan K and Wang
Y: Downregulation of HIF-2alpha reverse the chemotherapy resistance
of lung adenocarcinoma A549 cells to cisplatin. Med Sci Monit.
24:1104–1111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reed E, Larkins TL, Chau CH and Figg WD:
DNA repair: ERCC1, nucleotide excision repair, and platinum
resistance. Handbook of Anticancer Pharmacokinetics and
Pharmacodynamics. Cancer Drug Discovery and Development. Rudek MA,
et al: Springer Science and Business Media; New York: DOI
10.1007/978-1-4614-9135-4_18; pp. 333–349. 2014, View Article : Google Scholar
|
|
33
|
Ding Y, Niu W, Zhang T, Wang J, Cao J,
Chen H, Wang R and An H: Levistolide A synergistically enhances
doxorubicininduced apoptosis of k562/dox cells by decreasing MDR1
expression through the ubiquitin pathway. Oncol Rep. 41:1198–1208.
2018.PubMed/NCBI
|
|
34
|
Zeng C, Chen T, Zhang Y and Chen Q:
Hedgehog signaling pathway regulates ovarian cancer invasion and
migration via adhesion molecule CD24. J Cancer. 8:786–792. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiechert A, Saygin C, Thiagarajan PS, Rao
VS, Hale JS, Gupta N, Hitomi M, Nagaraj AB, DiFeo A, Lathia JD and
Reizes O: Cisplatin induces stemness in ovarian cancer. Oncotarget.
7:30511–30522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barakat MT, Humke EW and Scott MP:
Learning from jekyll to control hyde: Hedgehog signaling in
development and cancer. Trends Mol Med. 16:337–348. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steg AD, Burke MR, Amm HM, Katre AA,
Dobbin ZC, Jeong DH and Landen CN: Proteasome inhibition reverses
hedgehog inhibitor and taxane resistance in ovarian cancer.
Oncotarget. 5:7065–7080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding J, Zhou XT, Zou HY and Wu J: Hedgehog
signaling pathway affects the sensitivity of hepatoma cells to drug
therapy through the ABCC1 transporter. Lab Invest. 97:819–832.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chaudary N, Pintilie M, Hedley D, Hill RP,
Milosevic M and Mackay H: Hedgehog inhibition enhances efficacy of
radiation and cisplatin in orthotopic cervical cancer xenografts.
Br J Cancer. 116:50–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu B, Gu D, Zhang X, Li J, Liu B and Xie
J: GLI1-Mediated regulation of side population is responsible for
drug resistance in gastric cancer. Oncotarget. 8:27412–27427. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zahreddine HA, Culjkovic-Kraljacic B,
Assouline S, Gendron P, Romeo AA, Morris SJ, Cormack G, Jaquith JB,
Cerchietti L, Cocolakis E, et al: The sonic hedgehog factor GLI1
imparts drug resistance through inducible glucuronidation. Nature.
511:90–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Q, Yu JJ, Mu C, Yunmbam MK, Slavsky D,
Cross CL, Bostick-Bruton F and Reed E: Association between the
level of ERCC-1 expression and the repair of cisplatin-induced DNA
damage in human ovarian cancer cells. Anticancer Res. 20:645–652.
2000.PubMed/NCBI
|
|
44
|
Laner-Plamberger S, Kaser A, Paulischta M,
Hauser-Kronberger C, Eichberger T and Frischauf AM: Cooperation
between GLI and JUN enhances transcription of JUN and selected GLI
target genes. Oncogene. 28:1639–1651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kudo K, Gavin E, Das S, Amable L, Shevde
LA and Reed E: Inhibition of gli1 results in altered c-Jun
activation, inhibition of cisplatin-induced upregulation of ERCC1,
XPD and XRCC1, and inhibition of platinum-DNA adduct repair.
Oncogene. 31:4718–4724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Agyeman A, Mazumdar T and Houghton JA:
Regulation of DNA damage following termination of hedgehog (HH)
survival signaling at the level of the GLI genes in human colon
cancer. Oncotarget. 3:854–868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Cai J, Zhao S, Yao K, Sun Y, Li Y,
Chen L, Li R, Zhai X, Zhang J and Jiang C: GANT61, a GLI inhibitor,
sensitizes glioma cells to the temozolomide treatment. J Exp Clin
Cancer Res. 35:1842016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kuo LJ and Yang LX: γ-H2AX-a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–310.
2008.PubMed/NCBI
|