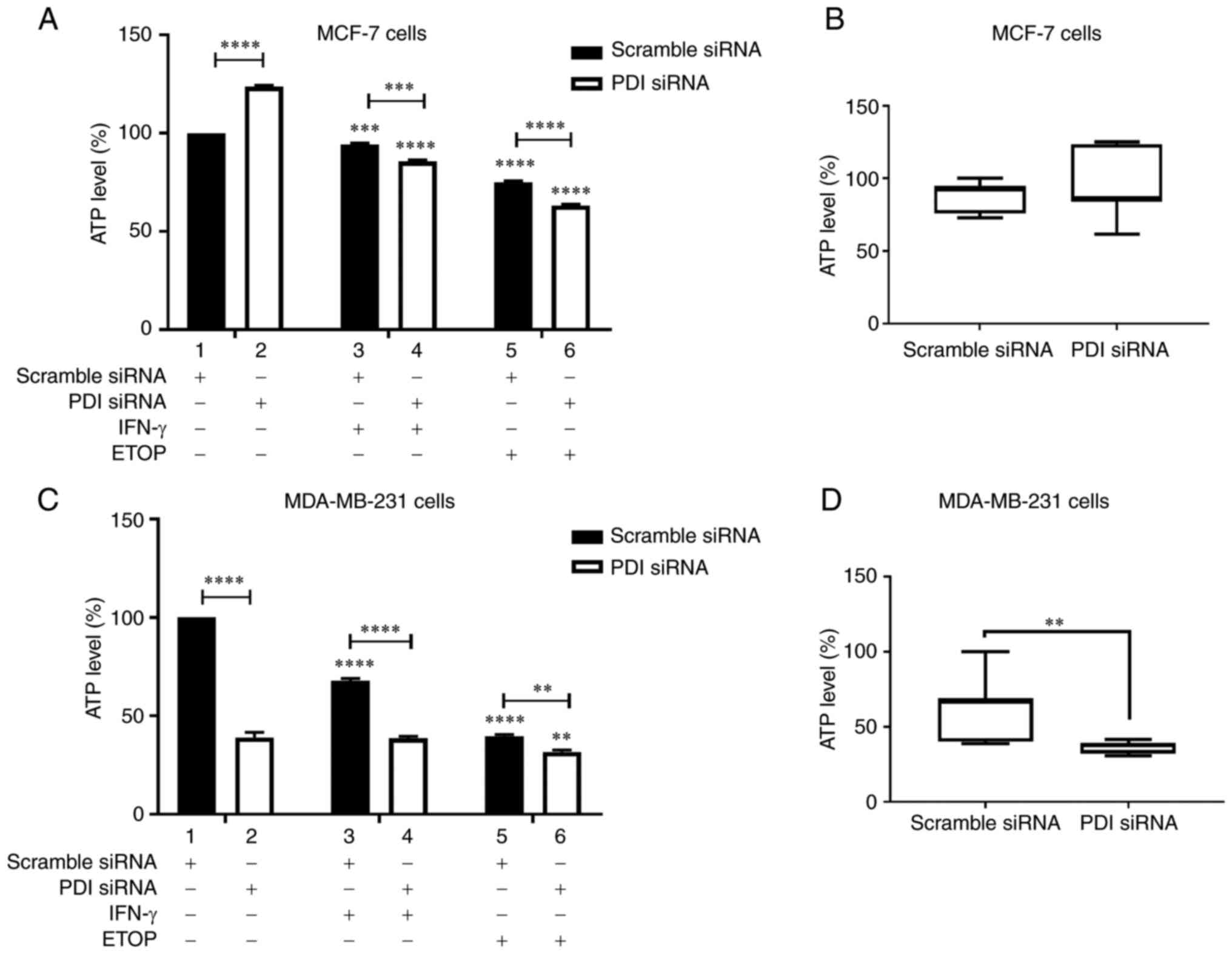
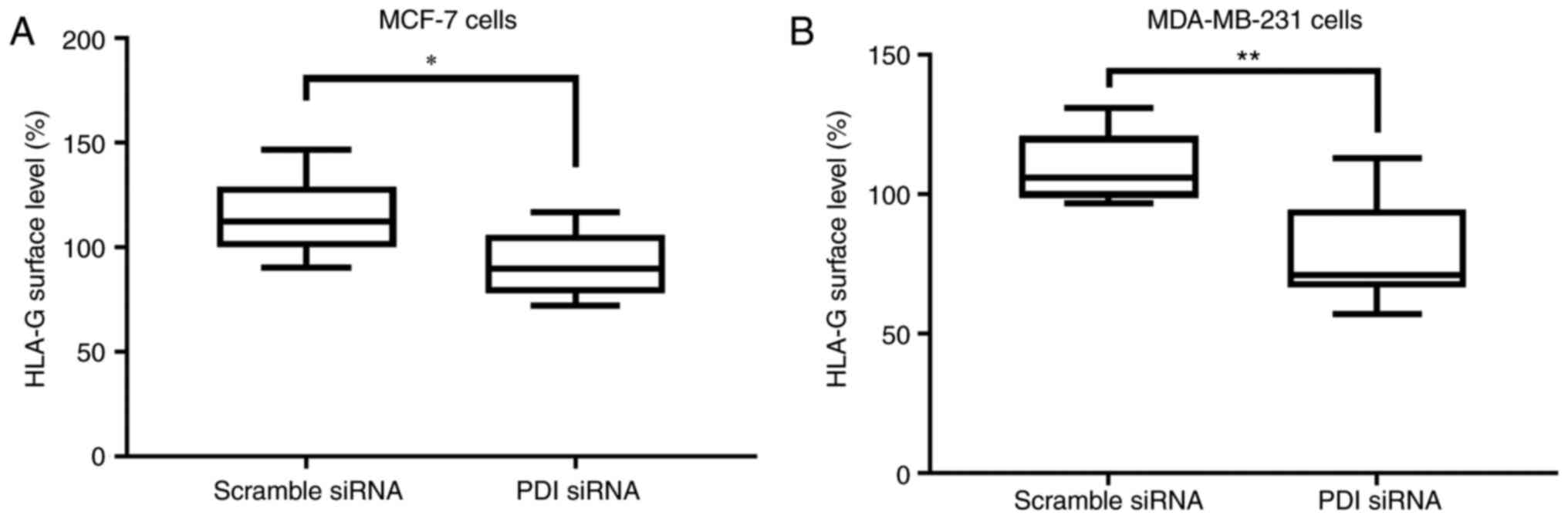
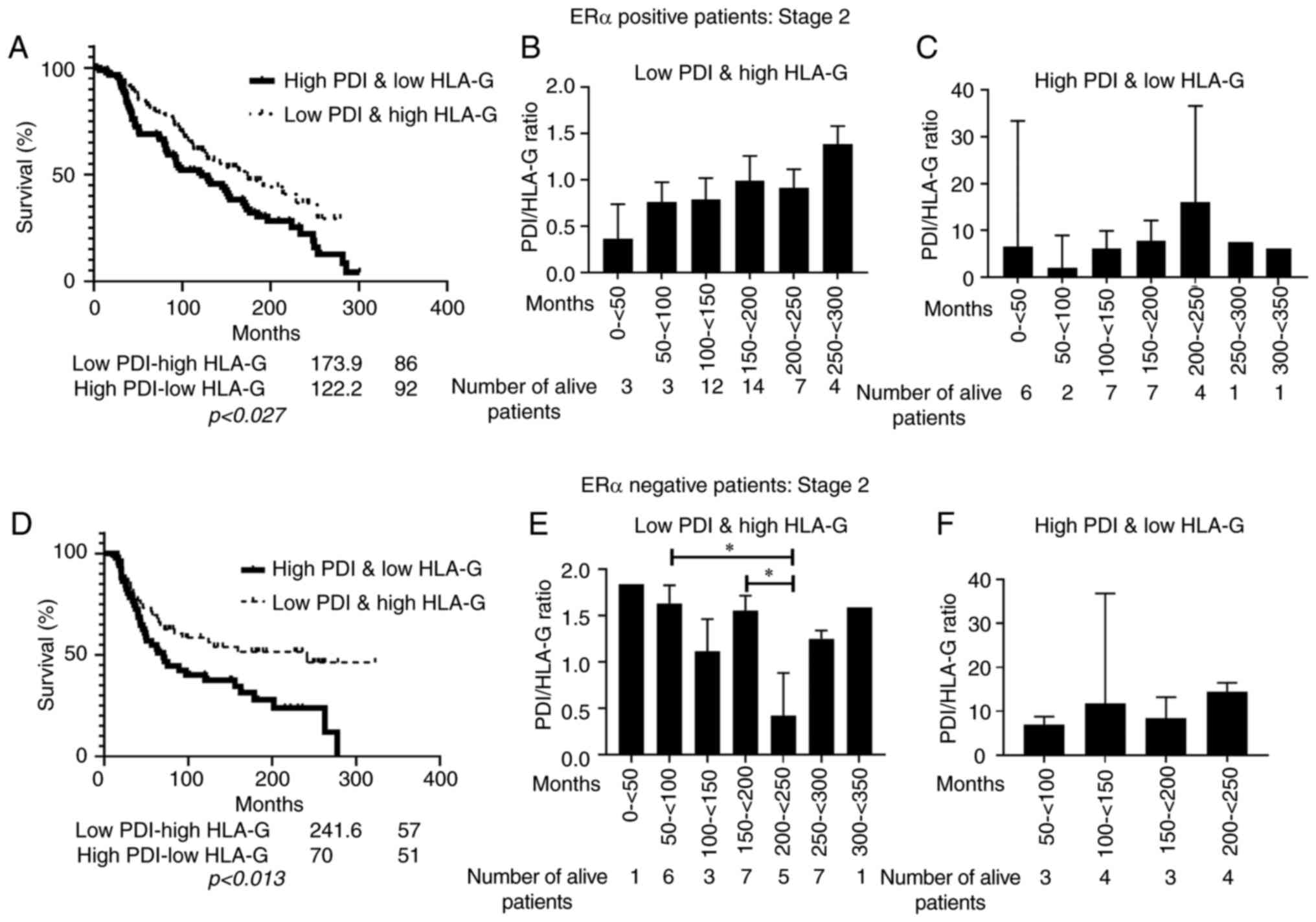
|
1
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eletto D, Chevet E, Argon Y and
Appenzeller-Herzog C: Redox controls UPR to control redox. J Cell
Sc. 127:3649–3658. 2014. View Article : Google Scholar
|
|
4
|
Almanza A, Carlesso A, Chintha C,
Creedican S, Doultsinos D, Leuzzi B, Luis A, McCarthy N,
Montibeller L, More S, et al: Endoplasmic reticulum stress
signalling-from basic mechanisms to clinical applications. FEBS J.
286:241–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oakes SA: Endoplasmic reticulum
proteostasis: A key checkpoint in cancer. Am J Physiol Cell
Physiol. 312:C93–C102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Zhang L, Zhou L, Lei Y, Zhang Y
and Huang C: Redox signaling and unfolded protein response
coordinate cell fate decisions under ER stress. Redox Biol.
25:1010472018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kranz P, Neumann F, Wolf A, Classen F,
Pompsch M, Ocklenburg T, Baumann J, Janke K, Baumann M, Goepelt K,
et al: PDI is an essential redox-sensitive activator of PERK during
the unfolded protein response (UPR). Cell Death Dis. 8:e29862017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada K, Fukui M and Zhu BT: Protein
disulfide isomerase mediates glutathione depletion-induced
cytotoxicity. Biochem Biophys Res Commun. 477:495–502. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grek C and Townsend DM: Protein disulfide
isomerase superfamily in disease and the regulation of apoptosis.
Endoplasmic Reticulum Stress Dis. 1:4–17. 2014.PubMed/NCBI
|
|
10
|
Ali Khan H and Mutus B: Protein disulfide
isomerase a multifunctional protein with multiple physiological
roles. Front Chem. 2:702014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Li W, Ren J, Fang J, Ke H, Gong W,
Feng W and Wang CC: Structural insights into the redox-regulated
dynamic conformations of human protein disulfide isomerase.
Antioxid Redox Signal. 19:36–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu J, Li T, Liu Y, Wang X, Zhang J, Shi G,
Lou J and Wang L, Wang CC and Wang L: Phosphorylation switches
protein disulfide isomerase activity to maintain proteostasis and
attenuate ER stress. EMBO J. 39:e1038412020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turano C, Coppari S, Altieri F and Ferraro
A: Proteins of the PDI family: Unpredicted non-ER locations and
functions. J Cell Physiol. 193:154–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parakh S and Atkin JD: Novel roles for
protein disulphide isomerase in disease states: A double edged
sword? Front Cell Dev Biol. 3:302015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao G, Lu H and Li C: Proapoptotic
activities of protein disulfide isomerase (PDI) and PDIA3 protein,
a role of the Bcl-2 protein Bak. J Biol Chem. 290:8949–8963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghanbari Movahed Z, Rastegari-Pouyani M,
Mohammadi MH and Mansouri K: Cancer cells change their glucose
metabolism to overcome increased ROS: One step from cancer cell to
cancer stem cell? Biomed Pharmacother. 112:1086902019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higuchi T, Watanabe Y and Waga I: Protein
disulfide isomerase suppresses the transcriptional activity of
NF-kappaB. Biochem Biophys Res Commun. 318:46–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goplen D, Wang J, Enger PO, Tysnes BB,
Terzis AJ, Laerum OD and Bjerkvig R: Protein disulfide isomerase
expression is related to the invasive properties of malignant
glioma. Cancer Res. 66:9895–9902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reverendo M, Mendes A, Arguello RJ, Gatti
E and Pierre P: At the crossway of ER-stress and proinflammatory
responses. FEBS J. 286:297–310. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Obacz J, Avril T, Rubio-Patino C,
Bossowski JP, Igbaria A, Ricci JE and Chevet E: Regulation of
tumor-stroma interactions by the unfolded protein response. FEBS J.
286:279–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamers M, Berlin I and Neefjes J: Antigen
presentation: Visualizing the MHC Class I peptide-loading
bottleneck. Curr Biol. 28:R83–R86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang K, Park B, Oh C, Cho K and Ahn K: A
role for protein disulfide isomerase in the early folding and
assembly of MHC class I molecules. Antioxid Redox Signal.
11:2553–2561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park B, Lee S, Kim E, Cho K, Riddell SR,
Cho S and Ahn K: Redox regulation facilitates optimal peptide
selection by MHC class I during antigen processing. Cell.
127:369–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho K, Cho S, Lee SO, Oh C, Kang K, Ryoo
J, Lee S, Kang S and Ahn K: Redox-regulated peptide transfer from
the transporter associated with antigen processing to major
histocompatibility complex class I molecules by protein disulfide
isomerase. Antioxid Redox Signal. 15:621–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee S, Park B, Kang K and Ahn K:
Redox-regulated export of the major histocompatibility complex
class I-peptide complexes from the endoplasmic reticulum. Mol Biol
Cell. 20:3285–3294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soares Moretti AI and Martins Laurindo FR:
Protein disulfide isomerases: Redox connections in and out of the
endoplasmic reticulum. Arch Biochem Biophys. 617:106–119. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guazzelli A, Meysami P, Bakker E,
Demonacos C, Giordano A, Krstic-Demonacos M and Mutti L: BAP1
status determines the sensitivity of malignant mesothelioma cells
to gemcitabine treatment. Int J Mol Sci. 20:4292019. View Article : Google Scholar
|
|
29
|
Forkink M, Smeitink JA, Brock R, Willems
PH and Koopman WJ: Detection and manipulation of mitochondrial
reactive oxygen species in mammalian cells. Biochim Biophys Acta.
1797:1034–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cereda M, Gambardella G, Benedetti L,
Iannelli F, Patel D, Basso G, Guerra RF, Mourikis TP, Puccio I,
Sinha S, et al: Patients with genetically heterogeneous synchronous
colorectal cancer carry rare damaging germline mutations in
immune-related genes. Nat Commun. 7:120722016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barrey E, Saint-Auret G, Bonnamy B, Damas
D, Boyer O and Gidrol X: Pre-microRNA and mature microRNA in human
mitochondria. PLoS One. 6:e202202011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zibara K, Zeidan A, Bjeije H, Kassem N,
Badran B and El-Zein N: ROS mediates interferon gamma induced
phosphorylation of Src, through the Raf/ERK pathway, in MCF-7 human
breast cancer cell line. J Cell Commun Signal. 11:57–67. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oh SY, Sohn YW, Park JW, Park HJ, Jeon HM,
Kim TK, Lee JS, Jung JE, Jin X, Chung YG, et al: Selective cell
death of oncogenic Akt-transduced brain cancer cells by etoposide
through reactive oxygen species mediated damage. Mol Cancer Ther.
6:2178–2187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hodny Z, Reinis M, Hubackova S, Vasicova P
and Bartek J: Interferon gamma/NADPH oxidase defense system in
immunity and cancer. Oncoimmunology. 5:e10804162016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laurindo FR, Pescatore LA and Fernandes
Dde C: Protein disulfide isomerase in redox cell signaling and
homeostasis. Free Radic Biol Med. 52:1954–1969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hegedűs C, Kovács K, Polgár Z, Regdon Z,
Szabó É, Robaszkiewicz A, Forman HJ, Martner A and Virág L: Redox
control of cancer cell destruction. Redox Biol. 16:59–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Traverso N, Ricciarelli R, Nitti M,
Marengo B, Furfaro AL, Pronzato MA, Marinari UM and Domenicotti C:
Role of glutathione in cancer progression and chemoresistance. Oxid
Med Cell Longev. 2013:9729132013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bulleid NJ: Disulfide bond formation in
the mammalian endoplasmic reticulum. Cold Spring Harb Perspect
Biol. 4:a0132192012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herrera-Cruz MS and Simmen T: Cancer:
Untethering mitochondria from the endoplasmic reticulum? Front
Oncol. 7:1052017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koczian F, Naglo O, Vomacka J, Vick B,
Servatius P, Zisis T, Hettich B, Kazmaier U, Sieber SA, Jeremias I,
et al: Targeting the endoplasmic reticulum-mitochondria interface
sensitizes leukemia cells to cytostatics. Haematologica.
104:546–555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchi S, Patergnani S and Pinton P: The
endoplasmic reticulum-mitochondria connection: One touch, multiple
functions. Biochim Biophys Acta. 1837:461–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kerkhofs M, Bittremieux M, Morciano G,
Giorgi C, Pinton P, Parys JB and Bultynck G: Emerging molecular
mechanisms in chemotherapy: Ca(2+) signaling at the
mitochondria-associated endoplasmic reticulum membranes. Cell Death
Dis. 9:3342018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoboue ED, Sitia R and Simmen T: Redox
crosstalk at endoplasmic reticulum (ER) membrane contact sites
(MCS) uses toxic waste to deliver messages. Cell Death Dis.
9:3312018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wacquier B, Combettes L and Dupont G:
Cytoplasmic and mitochondrial calcium signaling: A two-way
relationship. Cold Spring Harb Perspect Biol. 11:a0351392019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
So JS: Roles of endoplasmic reticulum
stress in immune responses. Mol Cells. 41:705–716. 2018.PubMed/NCBI
|
|
46
|
Kukita K, Tamura Y, Tanaka T, Kajiwara T,
Kutomi G, Saito K, Okuya K, Takaya A, Kanaseki T, Tsukahara T, et
al: Cancer-associated oxidase ERO1-α regulates the expression of
MHC class I molecule via oxidative folding. J Immunol.
194:4988–4996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim Y, Kang K, Kim I, Lee YJ, Oh C, Ryoo
J, Jeong E and Ahn K: Molecular mechanisms of MHC class I-antigen
processing: Redox considerations. Antioxid Redox Signal.
11:907–936. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Binder RJ: Functions of heat shock
proteins in pathways of the innate and adaptive immune system. J
Immunol. 193:5765–5771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Allen RL: Non-classical immunology. Genome
Biol. 2:REPORTS40042001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
da Silva GB, Silva TG, Duarte RA, Neto NL,
Carrara HH, Donadi EA, Goncalves MA, Soares EG and Soares CP:
Expression of the classical and nonclassical HLA molecules in
breast cancer. Int J Breast Cancer. 2013:2504352013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sivori S, Vacca P, Del Zotto G, Munari E,
Mingari MC and Moretta L: Human NK cells: Surface receptors,
inhibitory checkpoints, and translational applications. Cell Mol
Immunol. 16:430–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Morandi F, Rizzo R, Fainardi E,
Rouas-Freiss N and Pistoia V: Recent advances in our understanding
of HLA-G biology: Lessons from a wide spectrum of human diseases. J
Immunol Res. 2016:43264952016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Niu XL, Wang Y, Yao Z, Duan H, Li Z, Liu
W, Zhang H and Deng WM: Autocrine interferon-gamma may affect
malignant behavior and sensitivity to tamoxifen of MCF-7 via
estrogen receptor β subtype. Oncol Rep. 34:3120–3130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schultz-Norton JR, McDonald WH, Yates JR
and Nardulli AM: Protein disulfide isomerase serves as a molecular
chaperone to maintain estrogen receptor alpha structure and
function. Mol Endocrinol. 20:1982–1995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hashimoto S and Imaoka S:
Protein-disulfide isomerase regulates the thyroid hormone
receptor-mediated gene expression via redox factor-1 through thiol
reduction-oxidation. J Biol Chem. 288:1706–1716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mancini A, Bruno C, Vergani E, Guidi F,
Angelini F, Meucci E and Silvestrini A: Evaluation of oxidative
stress effects on different macromolecules in adult growth hormone
deficiency. PLoS One. 15:e02363572020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Boudreau HE, Casterline BW, Burke DJ and
Leto TL: Wild-type and mutant p53 differentially regulate NADPH
oxidase 4 in TGF-β-mediated migration of human lung and breast
epithelial cells. Br J Cancer. 110:2569–2582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Trevelin SC and Lopes LR: Protein
disulfide isomerase and Nox: New partners in redox signaling. Curr
Pharm Des. 21:5951–5963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lappi AK and Ruddock LW: Reexamination of
the role of interplay between glutathione and protein disulfide
isomerase. J Mol Biol. 409:238–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hudson DA, Gannon SA and Thorpe C:
Oxidative protein folding: From thiol-disulfide exchange reactions
to the redox poise of the endoplasmic reticulum. Free Radic Biol
Med. 80:171–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Coppola S and Ghibelli L: GSH extrusion
and and the mitochondrial pathway of apoptotic signalling. Biochem
Soc Trans. 28:56–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pedram A, Razandi M, Wallace DC and Levin
ER: Functional estrogen receptors in the mitochondria of breast
cancer cells. Mol Biol Cell. 17:2125–2137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fan Y and Simmen T: Mechanistic
connections between endoplasmic reticulum (ER) redox control and
mitochondrial metabolism. Cells. 8:10712019. View Article : Google Scholar
|
|
64
|
Gutierrez T and Simmen T: Endoplasmic
reticulum chaperones tweak the mitochondrial calcium rheostat to
control metabolism and cell death. Cell Calcium. 70:64–75. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dikalov S: Cross talk between mitochondria
and NADPH oxidases. Free Radic Biol Med. 51:1289–1301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kozieł R, Pircher H, Kratochwil M, Lener
B, Hermann M, Dencher NA and Jansen-Dürr P: Mitochondrial
respiratory chain complex I is inactivated by NADPH oxidase Nox4.
Biochem J. 452:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kochan G, Escors D, Breckpot K and
Guerrero-Setas D: Role of non-classical MHC class I molecules in
cancer immunosuppression. Oncoimmunology. 2:e264912013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu S, Sankar S and Neamati N: Protein
disulfide isomerase: A promising target for cancer therapy. Drug
Discov Today. 19:222–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee E and Lee DH: Emerging roles of
protein disulfide isomerase in cancer. BMB Rep. 50:401–410. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rosenberg N, Mor-Cohen R, Sheptovitsky VH,
Romanenco O, Hess O and Lahav J: Integrin-mediated cell adhesion
requires extracellular disulfide exchange regulated by protein
disulfide isomerase. Exp Cell Res. 381:77–85. 2019. View Article : Google Scholar : PubMed/NCBI
|