Introduction
Oral mucositis is one of the typical
treatment-limiting side effects of chemotherapy or
chemoradiotherapy in patients with cancer. The condition is
characterized by inflammation, ulceration, lesioning, and bleeding
of oral mucosa (1). Oral mucositis
can cause several problems, including acute oral pain, difficulty
in swallowing, reduced nutritional intake, and malnutrition, all of
which can significantly affect the oral hygiene and quality of life
of patients (2,3). Severe oral mucositis can be a costly
and dose-limiting side effect of intensive treatment for cancer,
which may lead to the discontinuation of cancer therapy and limit
the completion of treatment, thereby adversely affecting the
prognosis and survival rates of patients (1,4–6). There
may be several factors responsible for the onset and increase of
oral mucositis in patients with cancer who are receiving
chemotherapy or chemoradiotherapy. However, the detailed mechanisms
and the complex pathobiology underlying oral mucositis are not yet
fully understood.
Chemotherapeutic agents, including 5-fluorouracil
(5-FU), can damage the mouth epithelium, which is a key factor in
the development of mucositis. Sometimes, the basal cell layers of
epithelia are most affected, leading to the loss of epithelial
renewal capacity, which may result in ulceration. Chemotherapeutic
agents can also harm rapidly dividing, immature keratinocytes and
stem cells (1,7–9). There
are various treatments available for chemotherapy-induced oral
mucositis, which can reduce the frequency and severity of the
disease. However, most of these treatments are only partially
effective against established lesions and do not prevent the
development of new lesions. Therefore, the efficacy of these
treatments is limited (6,10–18).
Elental® (EA Pharma Co., Ltd.) is an
elemental diet (ED) that includes a high proportion of L-glutamine
and has been used in Japan as a treatment for patients who are
malnourished. Elental® is cost-effective, clinically
safe, and easily digestible. It contains a mixture of amino acids,
carbohydrates, vitamins, minerals, and a small amount of fat
(19,20). Elental® has been
successfully used in the treatment of Crohn's disease (21–24),
as well as in the management of chemotherapy-induced mucositis in
patients with cancer (25,26). Previous clinical studies revealed
the efficacy of Elental® for the treatment of
chemotherapy-induced oral mucositis in patients with oral cancer
(27,28). In addition, Elental® may
accelerate the healing process of 5-FU-induced oral mucositis and
dermatitis by promoting the production of fibroblast growth factor
and by suppressing the expression of pro-inflammatory cytokines
(TNF-α, IL-1β and IL-6) via the suppression of NF-κB in
keratinocytes (29,30). However, the exact mechanism of its
action remains unknown.
It is assumed that any nutritional supplement taken
by patients with oral cancer might not only provide an extra source
of nutrition for healthy (non-cancerous) cells but also for cancer
cells, which could promote the development of cancer (31,32).
Therefore, it is essential to clarify whether the beneficial effect
of an ED is sufficient to negate its possible harmful effects in
patients with oral cancer who are suffering from mucositis or
dermatitis.
In the present study, the effects of
Elental® were compared between human oral keratinocytes
(HOK) and human oral squamous cell carcinoma (OSCC) cell lines and
the effectiveness of Elental® in the treatment of
malnourished or damaged cells was investigated. In addition, the
mechanism of action of Elental® was analyzed in
different cell types, specifically healthy HOK and OSCC cell
lines.
Materials and methods
Cell lines and cell culture
Human oral keratinocyte cell line, HOK, was
purchased from ScienCell Research Laboratories, and cells of the
human OSCC cell lines, HSC2, HSC3 and HSC4 were purchased from Cell
Bank, Riken BioResource Center. HOK cells were cultured in Oral
Keratinocyte Medium-New Zealand Origin BPE medium (OKM-NZ, cat. no.
2611-NZ), containing OKM basal medium supplemented with 1% oral
keratinocyte growth supplement, New Zealand Origin BPE (OKGS-NZ,
cat. no. 2652NZ) and 1% penicillin/streptomycin solution (cat. no.
0503), referred to hereafter as complete medium for HOK. HSC2, HSC3
and HSC4 cells were cultured in Dulbecco's modified Eagle's medium
(D-MEM)/Ham's F-12 (Sigma-Aldrich) supplemented with 10% fetal
bovine serum (FBS) and 100 µg/ml streptomycin/100 U/ml penicillin
(Thermo Fisher Scientific), which will be referred to as complete
medium for OSCC cells. All the cells were cultured and maintained
in a humidified atmosphere containing 5% CO2 at
37°C.
Cell proliferation assay
Cells (5×103 cells per well) were seeded
in 96-well plates (Becton-Dickinson Labware) in OKM-NZ basal medium
with 1% OKGS-NZ (complete medium for HOK) or D-MEM/Ham's F-12
medium with 10% FBS (complete medium for HSC2 cells). The next day,
for HOK cells, the above medium was replaced with OKM basal medium
containing 0% OKGS-NZ growth supplement (low nutrition medium), OKM
basal medium with 1% OKGS-NZ growth supplement (complete medium),
or OKM basal medium containing 1% OKGS-NZ and 5-FU (final
concentration 2 µg/ml). Similarly, for OSCC cells, the medium was
replaced with D-MEM/Ham's F-12 medium with 0% or 1% FBS (low
nutrition medium), or D-MEM/Ham's F-12 with 10% FBS plus 5-FU (2
µg/ml). After 24 h, the cells were treated with different
concentrations of Elental® (0, 0.1, 0.5, 1, 5, 10, 50
and 100 µg/ml), which was dissolved in OKM basal medium containing
0% OKGS-NZ (low nutrition medium) or 1% OKGS-NZ (complete medium),
or D-MEM/Ham's F-12 medium with 0% or 1% FBS (low nutrition
medium). After 24 h, 3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT, 25 µl/well) was added to the
96-well plates and incubated for 4 h at 37°C. Next, the culture
medium containing MTT was removed and replaced with dimethyl
sulfoxide (100 µl/well), and the absorbance was measured using a
spectrophotometer (BioRad Laboratories) at 490 nm. Growth
stimulating effects were compared among the treatment groups. All
assays were run in triplicate.
Another protocol for MTT was used to determine the
effect of Elental® sequential treatment with anticancer
agents on OSCC cell proliferation. Cells (5×103 cells
per well) were seeded in 96-well plates (Becton-Dickinson Labware)
in D-MEM/Ham's F-12 supplemented with 10% FBS. After 24 h, the
cells were subjected to single or sequential treatments with
Elental® (5.0 µg/ml), 5-FU (1.0 µg/ml) and/or DOC
(docetaxel; 1.0 ng/ml). In case of untreated controls, the cells
were cultured in D-MEM/Ham's F-12 supplemented with 10% FBS for 48
h without any treatment. In case of single treatments, the cells
were cultured without any treatment; i.e., no treatment (trt.) for
24 h followed by Elental® (No trt.→ Ele), or 5-FU (No
trt.→ 5-FU), or DOC (No trt.→ DOC) for 24 h. In case of sequential
treatments, the cells were treated with Elental® for 24
h followed by 5-FU for 24 h (Ele → 5-FU), Elental® for
24 h followed by DOC for 24 h (Ele → 5-FU), 5-FU for 24 h followed
by Elental® for 24 h (5-FU→ Ele), or DOC for 24 h
followed by Elental® for 24 h (DOC→ Ele). D-MEM/Ham's
F-12 supplemented with 10% FBS was used with 5-FU or DOC; and
D-MEM/Ham's F-12 supplemented with 0% FBS was used with
Elental®. Then, MTT was added followed by DMSO, and the
absorbance was measured using the same procedure described above.
All assays were run in triplicate.
Cell migration assay
Cell migration assays were performed using a Boyden
chamber, according to the manufacturer's instructions (Neuro
Probe). Briefly, 25 µl OKM basal medium (containing 0 or 1%
OKGS-NZ) or D-MEM/Ham's F-12 (with 0 or 10% FBS) plus different
concentrations of Elental® (0, 0.1, 0.5, 1, 5, 10, 50
and 100 µg/ml) was added to the lower chamber as a chemoattractant.
Next, 50 µl OKM basal medium with 0% OKGS-NZ or D-MEM/Ham's F-12
medium with 0% FBS containing 5×103 cells was seeded on
a gelatin-coated polycarbonate membrane in the upper chamber. The
cells were incubated for 24 h at 37°C in a 5% CO2
atmosphere, then the membrane was washed with PBS, and the cells on
the top surface were removed using a cotton swab. Cells adhering to
the lower surface were fixed with methanol, stained with
hematoxylin solution, and counted under a microscope in five
predetermined fields (magnification, ×200). All assays were
independently repeated at least three times.
Whole transcriptome analysis
Total RNA was isolated from samples using an RNeasy
Mini Kit (Qiagen). The isolated RNA was examined with an Agilent
2100 Bioanalyzer (Agilent) using an RNA 6000 Nano Kit (Agilent),
once the concentrations had been determined using a Qubit (Thermo
Fisher Scientific). The RNA integrity number (RIN) values were
>7 for all samples, indicating that the samples contained
high-quality RNAs. The expression libraries were produced using an
Ion Ampliseq Transcriptome Human Gene Expression kit (Thermo Fisher
Scientific). Total RNAs (70 ng) were reverse-transcribed to cDNAs
and then amplified using the primer set of the Ion Amplicon
Transcriptome Human Gene Expression Core Panel under the following
PCR conditions: 11 cycles of denaturation for 15 sec at 99°C, then
annealing and extension for 16 min at 60°C. Barcodes were inserted
into the amplicons and adaptor sequences were inserted into both
ends using an Ion Barcode Adaptor 17-32 Kit (Thermo Fisher
Scientific). The libraries were amplified by PCR reactions of five
cycles of denaturation for 15 sec at 98°C and then annealing and
extension for 1 min at 64°C. The products were determined using a
quantitative PCR method, and the qualities were confirmed with a
Bioanalyzer (Agilent). Following emulsion PCR using the Ion Chef
system (Thermo Fisher Scientific), the sequence reaction was
carried out using Ion Proton with an Ion PI Hi-Q Chef Kit and an
Ion PI Chip Kit v3 BC (Thermo Fisher Scientific); approximately 93
million reads and 10.5 gigabases were detected from the reaction.
The reads were trimmed and mapped with Torrent Suit (Thermo Fisher
Scientific) using hg19 AmpliSeq Transcriptome ERCCv1 as the
reference sequences. The mapping ratio was >99.7%, and the read
count in each sample was >10 million.
Ingenuity network analysis
The gene sets significantly increased or decreased
by co-relation analysis using Prism8 were subjected to a network
analysis using Ingenuity Pathways Analysis software (IPA version
8.6; Qiagen). IPA indicates molecular and cellular functions and
canonical pathways on the basis of data from millions of molecular
interactions reported in the literature; this software is updated
weekly. IPA uses Fisher's exact test to determine whether the input
genes are significantly related to pathways by comparison with the
entire ingenuity knowledge base.
Western blotting
Cells (2.0×106 cells in a 100-mm dish)
were treated with different concentrations of Elental®
(0, 0.1, 0.5, 1, 5, 10, 50 and 100 µg/ml) dissolved in OKM basal
medium containing 0% OKGS-NZ or D-MEM/Ham's F-12 medium with 0%
FBS. For time-dependent experiments, the same number of cells were
treated with 5 µg/ml Elental® dissolved in OKM basal
medium containing 0% OKGS-NZ or D-MEM/Ham's F-12 medium with 0% FBS
for 0, 12, 24, 36, 48 or 60 h. The cells were lysed with RIPA
(radioimmunoprecipitation) buffer (Thermo Fisher Scientific).
Whole-cell lysates were subjected to electrophoresis on 10%
SDS-polyacrylamide gels (Thermo Fisher Scientific) and then
transferred to a PVDF membrane (Thermo Fisher Scientific). After
blocking, the membranes were incubated at 4°C overnight with
anti-ERK rabbit polyclonal antibody (dilution 1:500, cat. no. 4695;
Cell Signaling Technology), anti-p-ERK rabbit polyclonal antibody
(dilution 1:250, cat. no. 9101; Cell Signaling Technology),
anti-BiP mouse monoclonal antibody (dilution 1:500, cat. no.
66574-1-Ig; Proteintech Group, Inc.), anti-GRP94 rabbit polyclonal
antibody (dilution 1:250, cat. no. 20292; Cell Signaling
Technology, Inc.), or anti-α-tubulin mouse monoclonal antibody
(dilution 1:500, cat. no. sc-5286; Santa Cruz Biotechnology, Inc.).
Then the membranes were washed with a buffer and incubated with
Novex® alkaline-phosphatase conjugated (goat)
anti-rabbit immunoglobulin G (IgG) secondary antibody (no dilution,
cat. no. WB20007; Thermo Fisher Scientific) or (goat) anti-mouse
IgG secondary antibody (no dilution, cat. no. WB20006; Thermo
Fisher Scientific). The antibodies were detected using a
chromogenic immunodetection system, WesternBreeze (Thermo Fisher
Scientific), according to the manufacturer's instructions.
Quantification of protein bands was performed using ImageJ v1.51h
software available at http://rsb.info.nih.gov/ij/. The fold change of
expression of each protein of interest was calculated relative to
the internal control and expressed as a percentage.
TUNEL (terminal deoxynucleotidyl
transferase (Tdt)-mediated nick end labeling) assay
To detect apoptotic cells, a TUNEL assay was
performed by labeling 3′-OH DNA ends generated by DNA
fragmentation. Cells (5×103 cells per well) were seeded
on chamber slides (Iwaki & Co., Ltd.) in D-MEM/Ham's F-12
containing 10% FBS. After incubation at 37°C for 24 h, the cells
were subjected to single or sequential treatments with
Elental® (5.0 µg/ml), 5-FU (1.0 µg/ml) and/or DOC
(docetaxel; 1.0 ng/ml). In case of untreated controls, the cells
were cultured in D-MEM/Ham's F-12 supplemented with 10% FBS for 48
h without any treatment. In case of single treatments, the cells
were cultured without any treatment for 24 h followed by
Elental® (No trt.→ Ele), or 5-FU (No trt.→ 5-FU), or DOC
(No trt.→ DOC) for 24 h. In case of sequential treatments, cells
were treated with Elental® for 24 h followed by 5-FU for
24 h (Ele→ 5-FU) or DOC for 24 h (Ele→ 5-FU), 5-FU for 24 h
followed by Elental® for 24 h (5-FU→ Ele), or DOC for 24
h followed by Elental® for 24 h (DOC→ Ele). D-MEM/Ham's
F-12 supplemented with 10% FBS was used with 5-FU or DOC; and
D-MEM/Ham's F-12 supplemented with 0% FBS was used with
Elental®.
The treated cells were washed twice with
phosphate-buffered saline (PBS), air dried, and fixed in 4%
paraformaldehyde at room temperature for 30 min. The TUNEL assay
was performed using a DeadEnd™ Colorimetric TUNEL System according
to the manufacturer's instructions (Promega Corporation). Briefly,
the cells on the cover glass were immersed in 0.2%
Triton® X-100 in PBS for 5 min. After being washed with
PBS, the cells were incubated with equilibration buffer (0.05 M
phosphate buffer containing 0.145 M sodium chloride, pH 7.4) and
then Tdt enzyme in a humidified chamber at 37°C for 90 min. The
cells were subsequently put into pre-warmed working strength stop
wash buffer for 15 min. After being rinsed in PBS, the cells were
incubated with antidigoxigenin-peroxidase conjugate for 30 min.
Peroxidase activity in each cell was demonstrated by the
application of 3,3′-diaminobenzidine. The number of TUNEL-positive
cells (apoptotic cells) was counted under a microscope in three
random fields, and the result was expressed as a percentage of
TUNEL-positive cells.
Statistical analysis
Data were expressed as means ± SD. The significance
of the experimental results was determined using one-way analysis
of variance (ANOVA) and Tukey-Kramer multiple comparison tests. The
significance of the TUNEL assay data was determined by Mann-Whitney
U test. The differences were considered statistically significant
when P<0.05. The datasets measured by whole transcriptome
analysis were normalized by each transcripts per million (TPM) in
GAPDH and were analyzed using a correlation assay and principal
component analysis.
Results
Effect of Elental® on cell
morphology
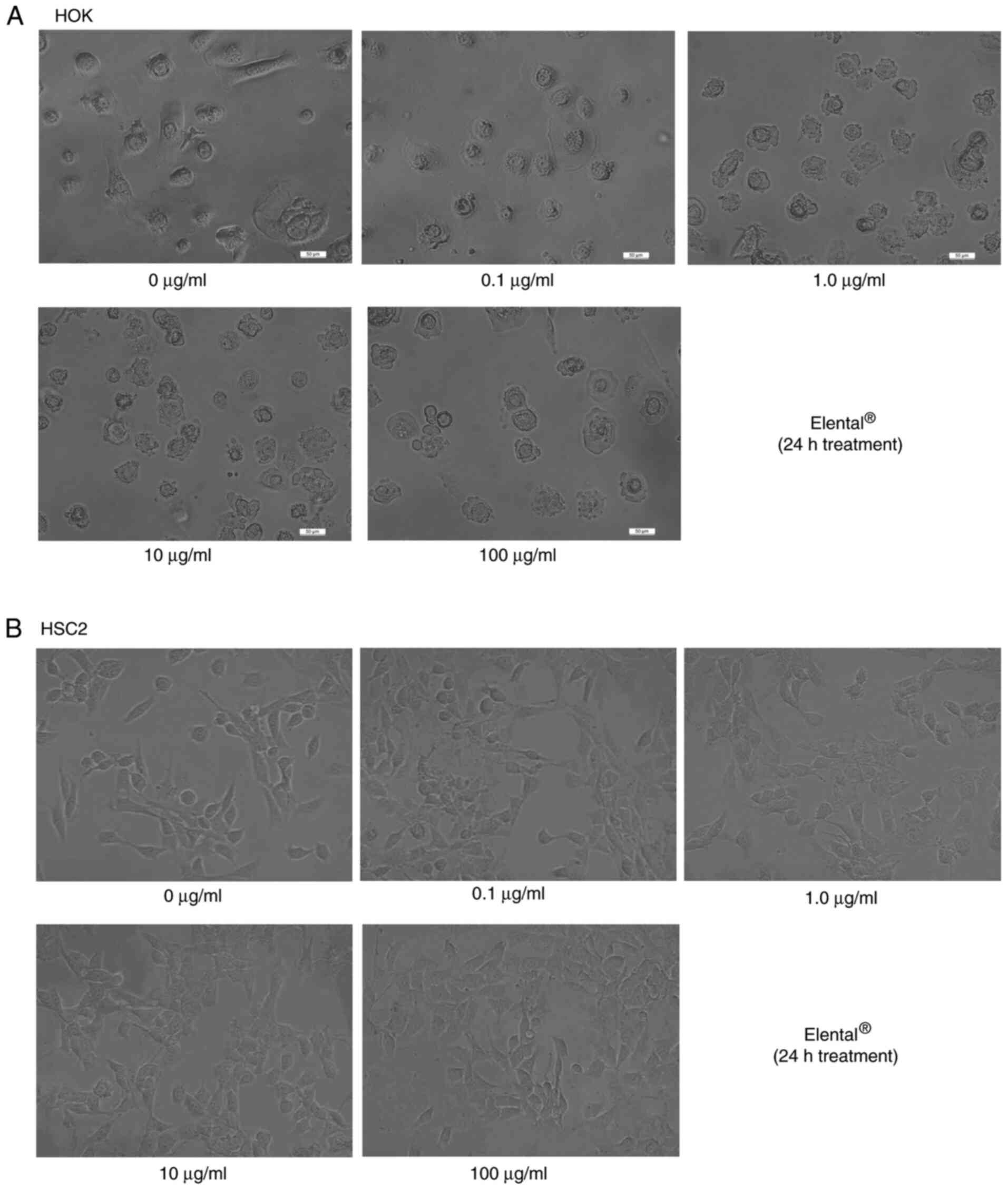
We did not observe any differences between the
morphology of untreated HOK and Elental®-treated HOK. As
shown in Fig. 1A, the majority of
cells in the two groups exhibited the same round shape. There was a
slight difference between the morphology of untreated HSC2 and
Elental®-treated HSC2 cells. Briefly,
Elental® treatment increased granular vesicles in
cytoplasm slightly (Fig. 1B).
Essentially, however, Elental® had almost no effect on
the morphology of HOK or HSC2 cells. Similarly, the morphology of
HSC3 and HSC4 cells remained almost unchanged after
Elental® treatment (data not shown).
Elental® affects the cell
proliferation ability of HOKs differently to that of OSCC cell
lines
MTT assays were used to measure the growth rate of
Elental®-treated and untreated HOKs or OSCC cell lines
(HSC2, HSC3 and HSC4). Fig. 2A
summarizes the experimental methodology used for the MTT assay.
Briefly, healthy cells or 5-FU-damaged cells were treated with
Elental® dissolved in low-nutrition culture medium (OKM
basal medium containing 0% OKGS-NZ, or D-MEM/Ham's F-12 with 0 or
1% FBS medium) or in complete medium (OKM basal medium containing
1% OKGS-NZ).
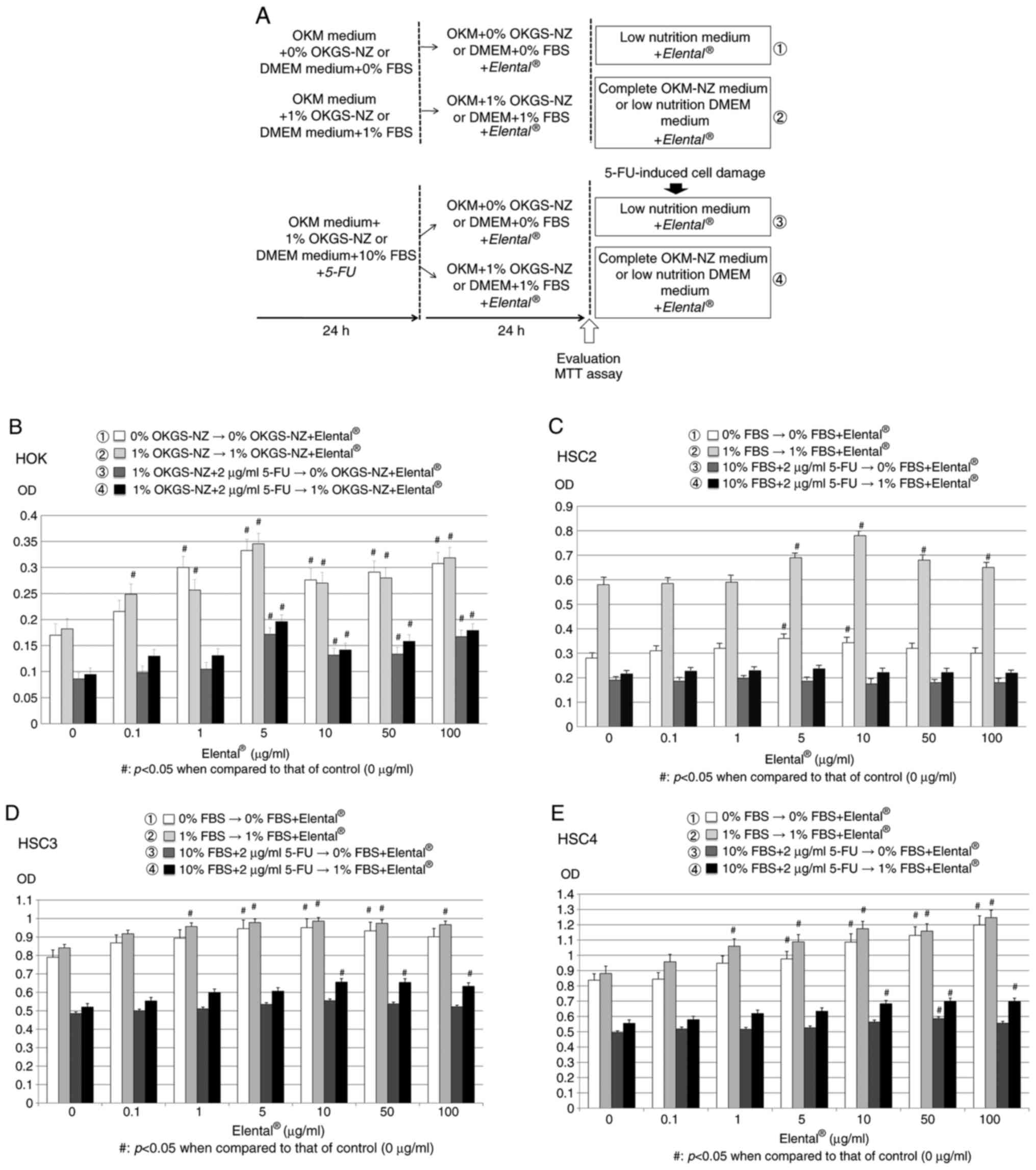 | Figure 2.Effect of Elental® on cell
proliferation. (A) Experimental methodology of the MTT assay.
Twenty-four hours after cell seeding, HOK were cultured in OKM
basal medium containing 0% OKGS-NZ (low nutrition medium) or 1%
OKGS-NZ growth supplement (complete medium), or 1% OKGS-NZ and 2
µg/ml 5-FU; HSC2 cells were cultured in D-MEM/Ham's F-12 medium
with 0% or 1% FBS (low nutrition medium), or D-MEM/Ham's F-12 with
10% FBS plus 5-FU (2 µg/ml). Then, both 5-FU-treated and -untreated
HOK cells were cultured in OKM basal medium containing 0% OKGS-NZ
(low nutrition medium) or 1% OKGS-NZ growth supplement (complete
medium) plus different concentrations of Elental® (0,
0.1, 0.5, 1, 5, 10, 50 and 100 µg/ml). Similarly, 5-FU-treated and
-untreated OSCC cells were cultured in D-MEM/Ham's F-12 medium with
0% or 1% FBS (low nutrition medium). After 24 h, the proliferation
ability of the cells was evaluated by an MTT assay. (B)
Elental® exerted a growth-stimulating effect on HOK even
under poor nutritional conditions (OKM basal medium containing 0%
OKGS-NZ) or in damaged HOK (5-FU-treated HOK). (C)
Elental® improved the growth ability in malnourished
HSC2 cells under poor nutritional conditions (D-MEM/Ham's F-12
medium with 0% or 1% FBS), but did not affect the growth ability of
5-FU-damaged HSC2 cells. (D and E) Elental® (1-100
µg/ml) improved the growth rate of HSC3 and HSC4 in poor
nutritional condition, and only D-MEM/Ham's F-12 medium with 1% FBS
Elental® (10-100 µg/ml) could increase the growth rate
of 5-FU-treated HSC3 and HSC4, but medium with 0% FBS could not.
#P<0.05 when compared with the control (one-way
analysis of variance and Tukey-Kramer multiple comparisons tests).
5-FU, 5-fluorouracil. |
Both in nutritionally poor conditions (medium with
0% OKGS-NZ growth supplement) and substantial nutrient conditions
(complete medium with 1% OKGS-NZ), the growth rate of
Elental® (0.1-100 µg/ml)-treated HOK was significantly
higher than that of untreated HOK. In addition, Elental®
(5-100 µg/ml) could stimulate the proliferation of 5-FU (2
µg/ml)-pretreated HOK even under poor nutritional conditions
(medium with 0% OKGS-NZ). Elental® improves cell growth
ability in malnourished or damaged HOK (Fig. 2B).
In the case of HSC2 cells, the growth rate of
Elental® (5-100 µg/ml)-treated HSC2 cells was higher
than that of untreated HSC2 cells after 48 h in poor nutritional
conditions (medium with 0 or 1% FBS). However, Elental®
(0.1-100 µg/ml) did not significantly affect the rate of
proliferation of 5-FU (2 µg/ml)-pretreated damaged HSC2 cells in
poor nutrition conditions. In short, Elental® improved
the growth ability of malnourished HSC2 cells, but did not affect
growth ability in damaged HSC2 cells (Fig. 2C). On the other hand, the growth
rates of Elental® (1-100 µg/ml)-treated HSC3 and HSC4
were higher than that of untreated HSC3 and HSC4 at 48 h in poor
nutritional condition (medium with 0-1% FBS). In addition,
Elental® (10-100 µg/ml) could increase the growth rate
of 5-FU (2 µg/ml)-pretreated HSC3 and HSC4 only in medium with 1%
FBS (poor nutritional condition), whereas the medium with 0% FBS
could not. In short, a high dosage of Elental®
significantly improved cell growth ability in malnourished or
damaged HSC3 and HSC4; however, the proliferation rate was still
low compared to HOK cells in poor nutritional condition (Fig. 2D and E).
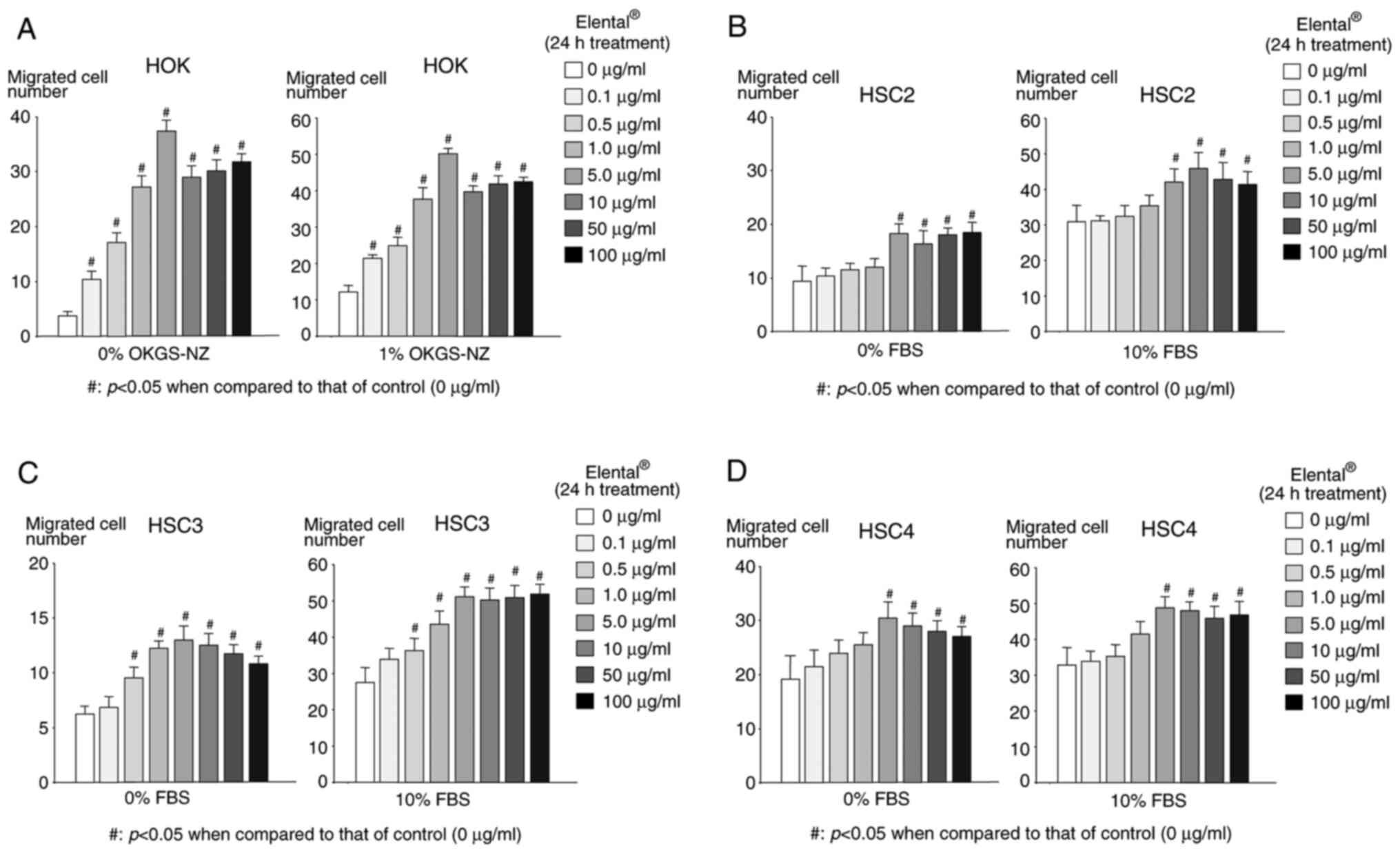
Elental® has different
effects on the ability of HOK and OSCC cell lines to migrate
The migration activity of
Elental®-treated HOK and OSCC cells was measured using a
Boyden chamber. Fig. 3A shows that
Elental® (0.1-100 µg/ml)-treated HOK had a significantly
higher migration ability compared with that of untreated HOK,
regardless of the nutritional conditions, with a concentration of 5
µg/ml Elental® showing the most noticeable effect on
migration. In the case of HSC2 and HSC4, the migration activity of
Elental® (5.0-100 µg/ml)-treated cells was significantly
higher compared with that of untreated HSC2 or HSC4 cells, both in
poor nutritional conditions (medium with 0% FBS) and substantial
nutrient conditions (complete medium with 10% FBS) (Fig. 3B and D). Similar results were
observed in the case of Elental® (1.0-100 µg/ml)-treated
HSC3 cells, both in poor nutritional and substantial nutrient
conditions (Fig. 3C). Briefly,
Elental® improved the migration ability of HOK and OSCC
cell lines, although low concentrations of Elental®
(0.1-0.5 µg/ml) did not improve the migration ability of OSCC cell
lines, regardless of the nutritional condition.
Mechanism of action of
Elental® in HOK and HSC2 cells
Our cell proliferation and migration assay results
showed that Elental® affected the growth and migration
ability of HOK more effectively than that of OSCC cell lines,
particularly HSC2 cells. To clarify the reasons behind this
difference in the action of Elental® in HOK and HSC2
cells, we examined the mechanism of action of Elental®
in these two cell types. Whole transcriptome analysis was performed
followed by network analysis (IPA) using differentially expressed
genes identified between Elental® (5 µg/ml)-treated
cells and untreated cells. We supplied the results of Heatmap and
Whole transcriptome analysis as supplement data (Fig. S1). An interesting pathway was found
in the first pathway of comparison between Elental® HOK
treated for 12 h and untreated HOK (0 h), i.e., a pathway via the
integrin-mediated activation of ERK (Fig. 4A). In addition, ERK may be
one of the genes of focus as detected from the first network
analysis comparing HOK treated for 12 h with Elental®
and untreated HOK (Fig. 4B). An
interesting pathway was also found in the first pathway of
comparison between HSC2 cells treated for 12 h with
Elental® and untreated HSC2 cells. This pathway showed
the possibility of activation of endoplasmic reticulum response
marker genes, including BiP and GRP94, which may lead
to the induction of apoptosis (Fig.
4C). The activation of HSP, as well as BiP, GRP94
and other genes, was also found further downstream, which suggests
that Elental® treatment could be involved in the
induction of apoptosis by adding a great deal of stress to HSC2
cells (Fig. 4C). Furthermore,
Akt and HSP may be genes of focus as indicated from
the first network analysis data when comparing HSC2 cells treated
for 12 h with Elental® and untreated HSC2 cells. This
suggests that Elental® treatment may not only cause
stress but also partially activate survival signals (Fig. 4D).
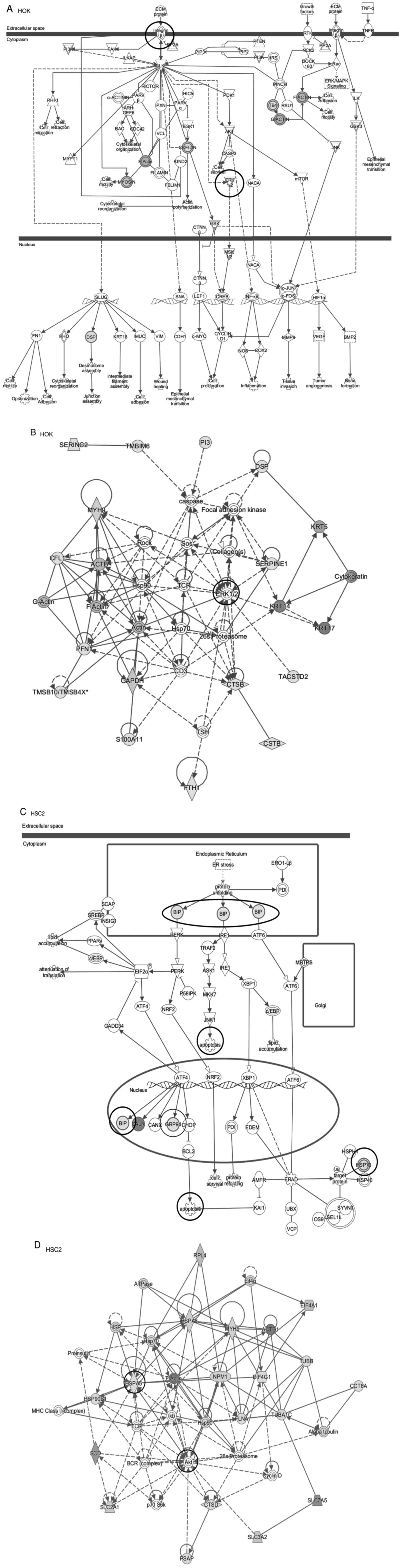 | Figure 4.Pathway analysis by IPA was performed
using the differentially expressed genes identified in
Elental® (5 µg/ml)-treated cells versus untreated cells.
(A) A pathway via the integrin-mediated activation of ERK
was detected in the first pathway of comparison between
Elental® 12 h-treated HOK and untreated HOK (0 h). IPA,
Ingenuity Pathways Analysis; HOK, human oral keratinocyte. Pathway
analysis by IPA was performed using the differentially expressed
genes identified in Elental® (5 µg/ml)-treated cells
versus untreated cells. (B) ERK could be the gene of focus
as found from the first network analysis when comparing
Elental® 12 h-treated HOKs and untreated HOK. IPA,
Ingenuity Pathways Analysis; HOK, human oral keratinocyte. Pathway
analysis by IPA was performed using the differentially expressed
genes identified in Elental® (5 µg/ml)-treated cells
versus untreated cells. (C) The pathway of comparison between
Elental® 12-h treated HSC2 cells and untreated HSC2
cells (0 h) showed the possible activation of endoplasmic reticulum
stress markers, including BiP, which may induce apoptosis.
(C) Activation of HSP, BiP, GRP94 and other genes was
detected further downstream, which suggests that
Elental® treatment may be involved in the induction of
apoptosis by subjecting HSC2 cells to considerable stress. IPA,
Ingenuity Pathways Analysis; HOK, human oral keratinocyte. Pathway
analysis by IPA was performed using the differentially expressed
genes identified in Elental® (5 µg/ml)-treated cells
versus untreated cells. (D) Akt and HSP could be the
genes of focus as found from the first network analysis comparing
HSC2 cells treated with Elental® for 12 h with untreated
HSC2 cells, which suggests that Elental® treatment not
only exerted stress but also partially induced survival signals.
IPA, Ingenuity Pathways Analysis; HOK, human oral keratinocyte. |
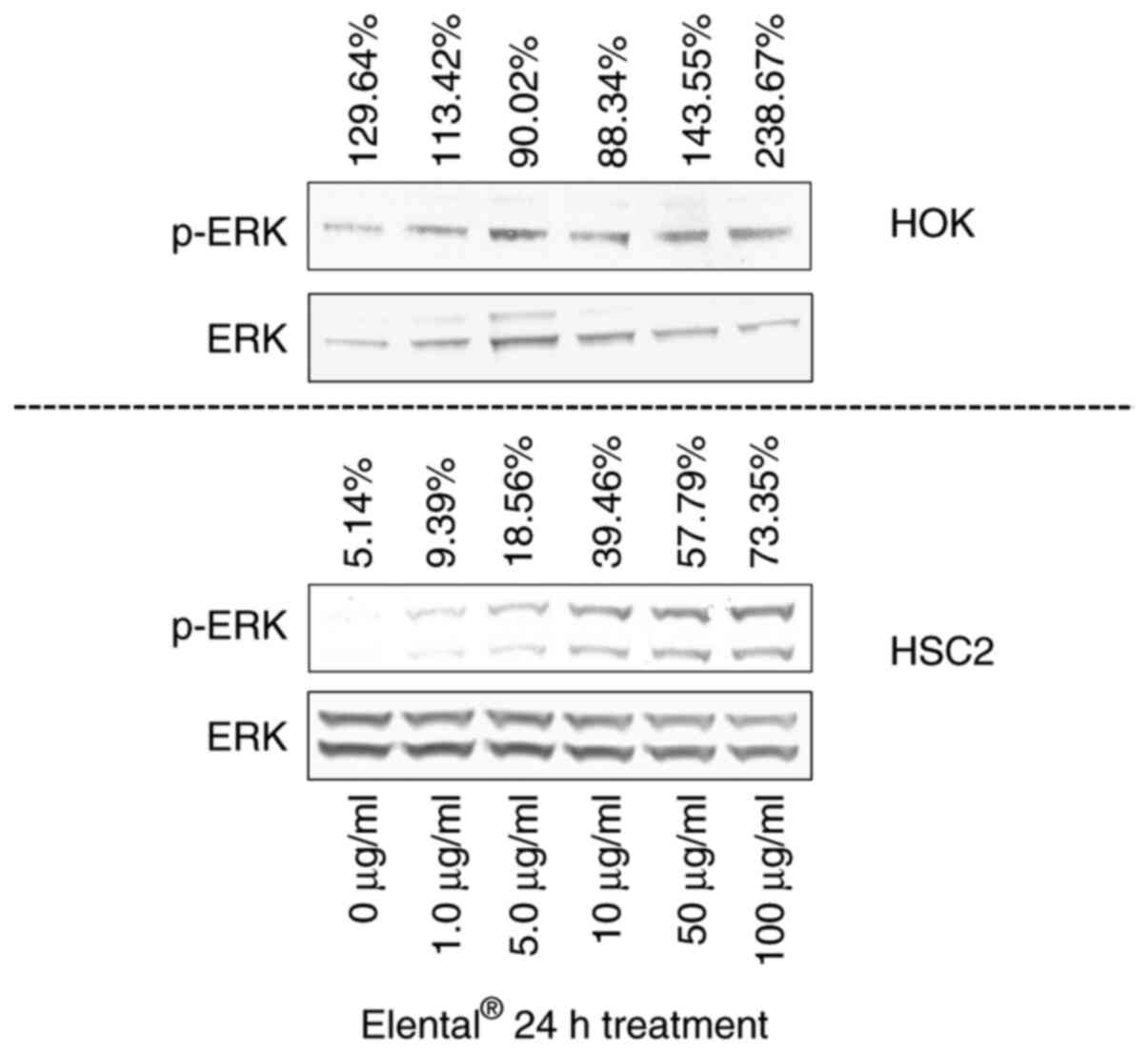
Expression of ERK in
Elental®-treated cells
To confirm the results of whole transcriptome
analysis and IPA, we examined the expression of ERK in HOK and HSC2
cells using western blot analysis. Fig.
5 shows that Elental® (50-100 µg/ml) enhanced the
expression of p-ERK in HOK compared with its expression in
untreated HOK. On the other hand, we could only detect faint bands
of p-ERK in Elental® (1.0-5.0 µg/ml)-treated HSC2 cells.
Although the p-ERK expression was a bit higher in treated cells
than in untreated HSC2 cells, the expression level of p-ERK/ERK in
HSC2 was low compared with its expression level in HOK (Fig. 5). Briefly, Elental®
induced p-ERK expression in HOK, but not in HSC2 cells.
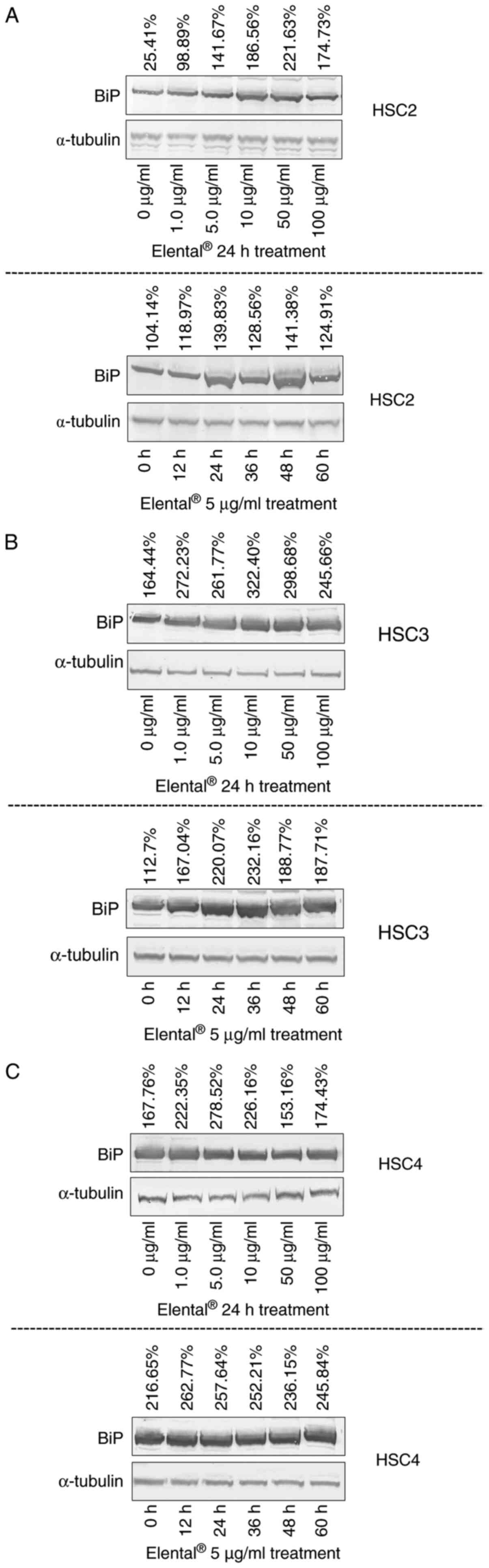
Expression of BiP and GRP94 in
Elental®-treated cells
Western blot analysis was used to examine the
expression of BiP and glucose-regulated protein 94 (GRP94) in HSC2
cells and further check the results of the whole transcriptome
analysis and IPA. Fig. 6A shows
that Elental® (1.0-100 µg/ml) enhanced the expression of
BiP in HSC2 cells after 24 h of treatment than in untreated cells.
Elental® (1.0-100 µg/ml) also induced BiP expression in
HSC3 and HSC4 (Fig. 6B and C).
Additionally, Elental® (5.0 µg/ml) enhanced the
expression of BiP in treated cells after 12=60 h of treatment
compared with its expression in untreated cells. Therefore,
Elental® could induce BiP expression in OSCC cells.
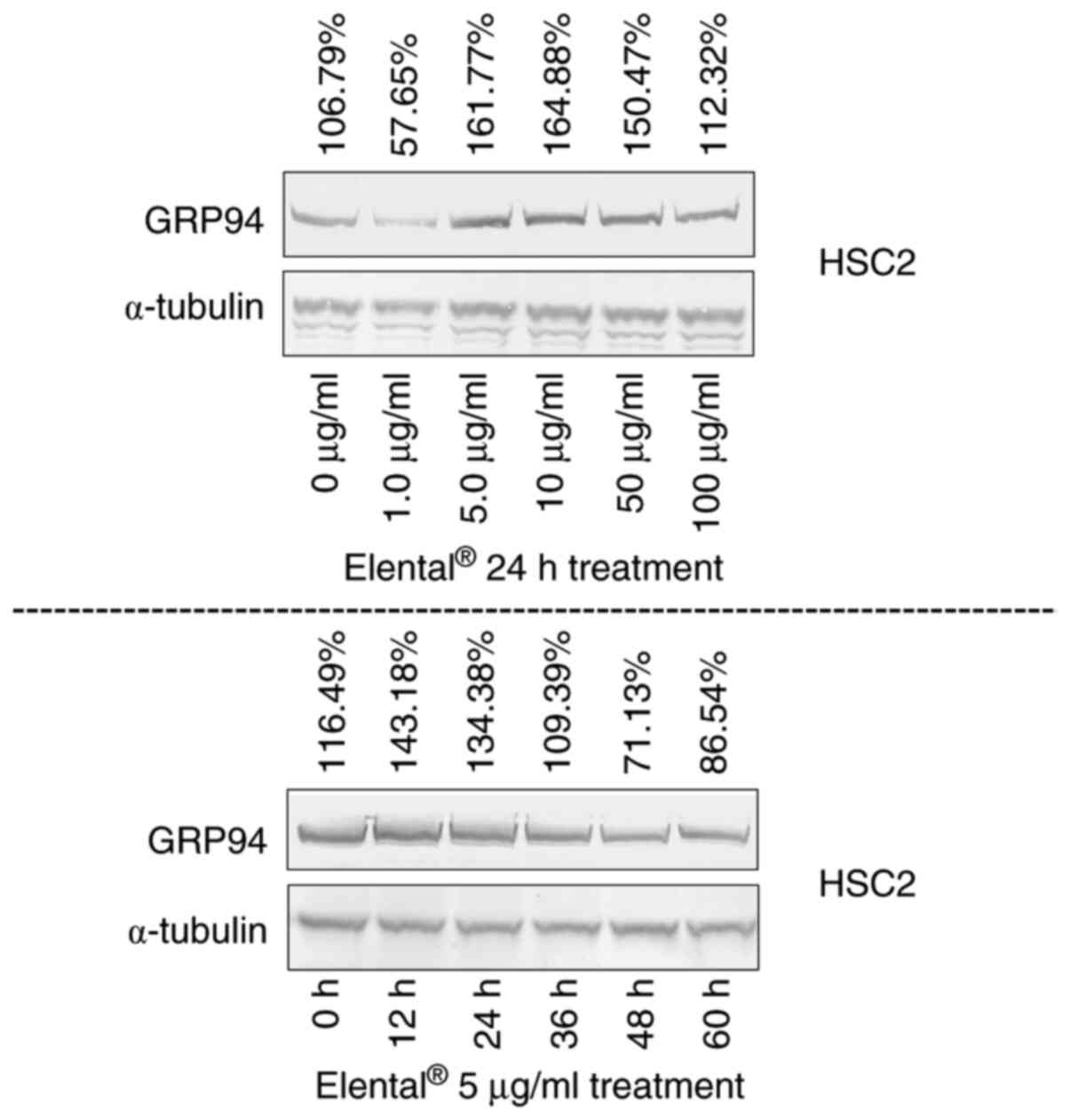
On the other hand, 24 h treatment of
Elental® (5.0-100 µg/ml) increased the expression of
GRP94 in HSC2 cells than in untreated cells; whereas 12-24 h of
treatment with Elental® (5.0 µg/ml) enhanced the
expression of GRP94 in treated cells. Briefly, 12-24 h of
Elental® treatment could induce GRP94 expression in HSC2
cells; whereas treatment >24 h could not (Fig. 7).
Sequential treatment effects of
Elental® and anticancer agents on OSCC cell
proliferation and apoptosis in vitro
We aimed to determine whether Elental®
pre-treatment can enhance the growth-limiting and
apoptosis-inducing ability of widely used anticancer agents or not.
The growth inhibitory effect of Elental® sequential
treatment with 5-FU or DOC on HSC2, HSC3 and HSC4 cells was
analyzed by MTT assay. Fig. 8A
summarizes the experimental methodology used for the MTT assay. As
shown in Fig. 8B-D,
Elental® (5.0 µg/ml) pre-treatment for 24 h followed by
5-FU (Elental®→ 5-FU) or DOC (Elental®→ DOC)
for 24 h slightly inhibited cell growth in all three cells than in
5-FU (No trt.→ 5-FU) or DOC (No trt.→ DOC) alone; however, we could
not find any significance. Elental® pre-treatment did
not significantly increase the growth inhibitory effect of 5-FU or
DOC in HSC2, HSC3 and HSC4 cells.
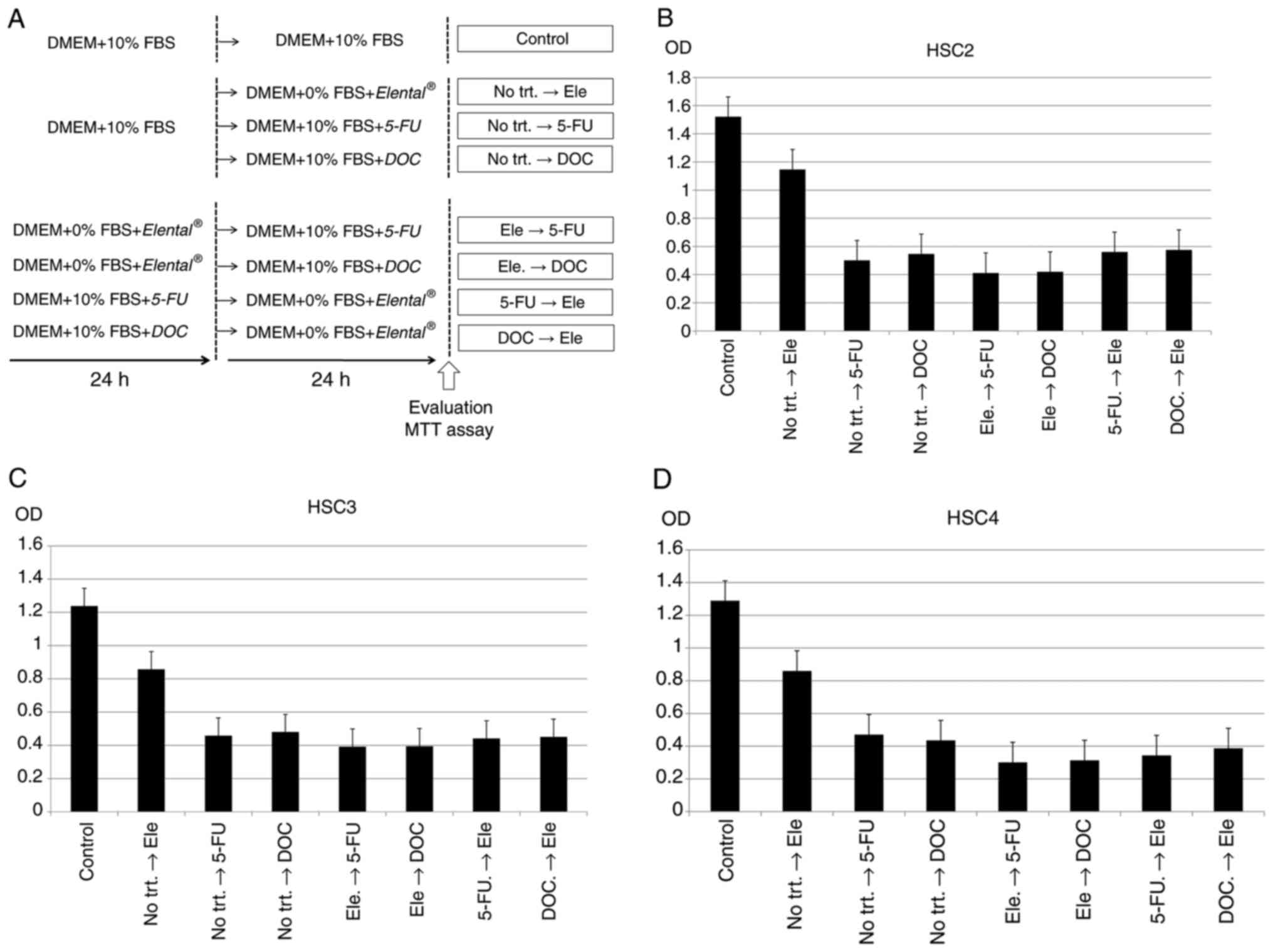 | Figure 8.Sequential treatment effects of
Elental® and anticancer agents on OSCC cell
proliferation in vitro. Inhibition of cell growth was
evaluated by MTT assay. (A) Experimental methodology of the MTT
assay. Twenty-four hours after cell seeding, the cells were
subjected to single or sequential treatments with
Elental® (5.0 µg/ml), 5-FU (1.0 µg/ml) and/or DOC
(docetaxel; 1.0 ng/ml). Untreated control cells were cultured for
48 h without any treatment. In case of single treatments, the cells
were without any treatment for 24 h followed by Elental®
(No trt.→ Ele), or 5-FU (No trt.→ 5-FU), or DOC (No trt.→ DOC)
treatment for 24 h. In case of sequential treatments, the cells
were treated with Elental® for 24 h followed by 5-FU for
24 h (Ele → 5-FU) or DOC for 24 h (Ele → DOC), 5-FU for 24 h
followed by Elental® for 24 h (5-FU → Ele), or DOC for
24 h followed by Elental® for 24 h (DOC → Ele). (B-D)
Elental® pre-treatment for 24 h followed by 5-FU
(Elental® → 5-FU) or DOC (Elental® → DOC) for
24 h could slightly inhibit cell growth in OSCC cell lines compared
to 5-FU (No trt.→ 5-FU) or DOC (No trt.→ DOC) alone; however
Elental® pre-treatment did not significantly increase
the growth inhibitory effect of these anticancer agents against (B)
HSC2, (C) HSC3 and (D) HSC4. Ele, Elental®; 5-FU,
5-fluorouracil; DOC, docetaxel; No trt., no treatment. |
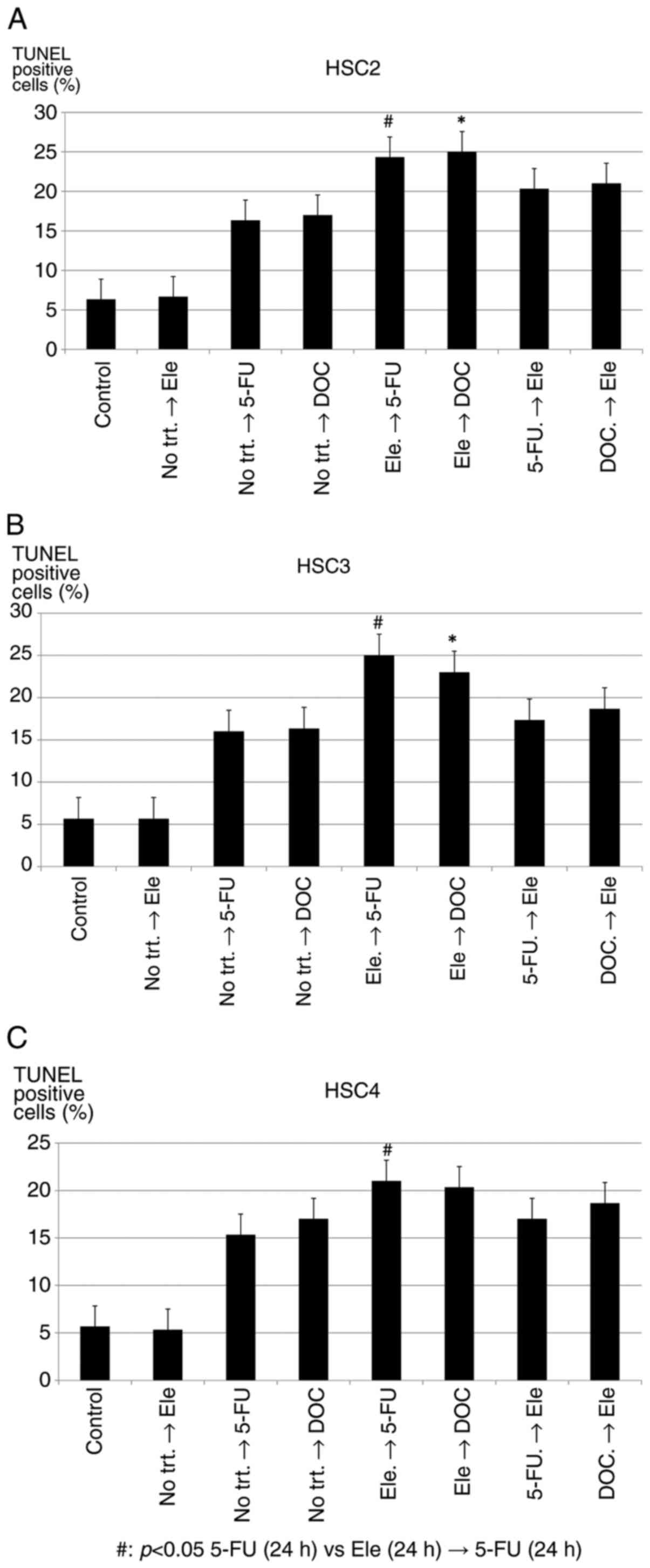
Therefore, we assumed that sequential or
pre-treatment of Elental® may increase
apoptosis-inducing ability of anticancer agents, and we performed
TUNEL assay to detect DNA fragmentation and chromatin condensation
in treated cells using the same experimental protocol described in
Fig. 8A. As shown in Fig. 9A and B, Elental®
pre-treatment significantly enhanced the apoptosis-inducing ability
of 5-FU (Elental®→ 5-FU) or DOC (Elental®→
DOC) in HSC2 and HSC3 cells. In the case of HSC4 cells (Fig. 9C), Elental® pre-treatment
could significantly increase apoptosis-inducing ability of 5-FU
(Elental®→ 5-FU), but not DOC (Elental®→
DOC). The highest number of apoptotic cell were observed in the
case of Elental® (5.0 µg/ml) sequential treatment for 24
h followed by 5-FU (1.0 µg/ml) for 24 h (Elental®→ 5-FU)
on the three types of cells. Briefly, pre-treatment of
Elental® may prompt apoptosis by anticancer
agents.
Discussion
Elental® is used in Japan as a treatment
for patients who are malnourished, or who have inflammatory bowel
disease. Elental® has been shown to be useful in the
management of chemotherapy-induced mucositis in patients with
various types of cancer (7–9,23–29,33).
We have also reported that Elental® reduced
chemotherapy-induced oral mucositis and dermatitis in patients with
OSCC (25,26). Therefore, we should consider more
extensive use of Elental® for patients with cancer in
the future. However, although, to the best of our knowledge, there
have been no reports about the possible harmful effects of
nutritional supplements or ED intake in cancer patients, we cannot
exclude the possibility that an ED could act as an extra source of
nutrition for cancer cells, which might facilitate cancer
progression. Therefore, it is important to weigh the beneficial
effects of Elental® against any possible harmful
effects.
In the present study, we aimed to examine the
efficacy of Elental® administration during treatment for
oral cancer by comparing the action of Elental® in HOK
and HSC2 cells. The aim also was to clarify whether
Elental® works differently on healthy oral cells
compared with its action on oral cancer cells. We observed that
Elental® promoted growth and migration of malnourished
and 5-FU-treated damaged HOK but did not significantly affect the
proliferation of 5-FU-treated damaged HSC2 cells. On the other
hand, a high dosage of Elental® (10 µg/ml) could
increase the growth rate of 5-FU-treated HSC3 and HSC4 even in some
poor nutritional condition (1% FBS); however, the proliferation
rate was still low compared to HOK cells in poor nutritional
condition (0 or 1% FBS). HOK and HSC2 were used for whole
transcriptome analysis and IPA analysis, as Elental®
affects the proliferation rate of the two cells differently.
Extensive gene analysis using whole transcriptome analysis followed
by IPA resulted in noteworthy data (Fig. 4A-D). Briefly, Elental®
may play a role in the growth and survival of HOK through the
integrin-mediated activation of ERK and may induce heat
shock protein via endoplasmic reticulum stress in HSC2 cells.
Crucially, under certain conditions Elental® may stress
cancer cells and stimulate the growth of healthy cells. This
finding could be important for patients with cancer, as it
indicates that Elental® may not exert any harmful
effects on healthy cells. We showed that Elental®
enhanced the expression of p-ERK in HOK but not so in HSC2 cells,
the expression level of p-ERK/ERK in HSC2 was low compared with its
expression level in HOK (Fig. 5).
Western blot analysis data also revealed that Elental®
enhanced the expression of BiP in HSC2 cells compared with its
expression in untreated cells (Fig.
6A). Almost identical results were observed in the case of HSC3
and HSC4 (Fig. 6B and C). From our
findings, we can conclude that Elental® may accelerate
wound healing and reduce oral mucositis via the integrin-mediated
activation of ERK, without promoting cancer progression.
GRP94 expression was analyzed in HSC2 because it is
another stress-related protein and is also involved in cellular
protein metabolic process. We observed that, Elental®
increased the expression of GRP94 in HSC2 cells; whereas 12-24 h of
treatment with Elental® (5.0 µg/ml) showed the best
results (Fig. 7). Moreover,
Elental® pre-treatment could enhance the
apoptosis-inducing ability of 5-FU against OSCC cell lines,
although they could not exert significant growth inhibitory effects
on OSCC cell lines compared to 5-FU alone (Figs. 8 and 9). Our results indicate that
Elental® may add stress to HSC2 and other OSCC cells,
but could provide growth stimulation to HOK.
It has been reported that the administration of
additional nutrition during perioperative periods in patients with
cancer could help with the maintenance of general health and
enhance wound healing (33).
According to the guidelines of the American Society for Parenteral
and Enteral Nutrition (ASPEN), early enteral feeding is strongly
recommended (advisability A) following surgery for digestive organ
cancer (34). However, until now
there has been no useful and decisive nutrition administration
strategy available for patients with cancer. Currently, we have to
develop nutritional supplement administration strategies based on
the state of the disease in individual cancer patients, after
carefully evaluating any potential beneficial or harmful effects on
cancer treatment. Our findings suggest a solution to this problem
through the use of an effective nutritional supplement that has no
adverse side effects for patients with cancer. Amino acid-based
dietary formulations such as Elental® may be able to
exert an incredible healing effect on oral mucositis and dermatitis
in patients with oral cancer, although this effect may vary
depending on the amino-acid formulation of each particular ED.
Further investigations into Elental® and other
amino-acid formulations may be necessary to clarify their
usefulness in the treatment of oral cancer. Moreover, in this
study, the cells were directly exposed to different concentrations
of Elental®. However, whether there is any positive
correlation between in vivo absorption levels of ingredients
and in vitro dissolution level of Elental®
remains to be determined and further investigation to clarify this
point in the future should be conducted.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported in part by a Grant-in-Aid
from the Japanese Ministry of Education, Science and Culture, grant
no. 15K11292.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
KH and YM designed the study. KH, TF, and KW
performed the experiments, analyzed the data, and wrote and revised
the manuscript. YM and KM revised the manuscript and provided
valuable suggestions during the study. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest and are
fully responsible for the content of this paper. All authors
reviewed and approved the paper.
References
|
1
|
Duncan M and Grant G: Oral and intestinal
mucositis-causes and possible treatments. Aliment Pharmacol Ther.
18:853–874. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burdelya LG, Gleiberman AS, Toshkov I,
Aygun-Sunar S, Bapardekar M, Manderscheid-Kern P, Bellnier D,
Krivokrysenko VI, Feinstein E and Gudkov AV: Toll-like receptor 5
agonist protects mice from dermatitis and oral mucositis caused by
local radiation: Implications for head-and-neck cancer
radiotherapy. Int J Radiat Oncol Biol Phys. 83:228–234. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sonis ST: Oral mucositis. Anticancer
Drugs. 22:607–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCarthy GM, Awde JD, Ghandi H, Vincent M
and Kocha WI: Risk factors associated with mucositis in cancer
patients receiving 5-fluorouracil. Oral Oncol. 34:484–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyano K, Ueno T, Yatsuoka W and Uezono Y:
Treatment for cancer patients with oral mucositis: Assessment based
on the mucositis study group of the multinational association of
supportive care in cancer in international society of oral oncology
(MASCC/ISOO) in 2013 and proposal of possible novel treatment with
a Japanese herbal medicine. Curr Pharm Des. 22:2270–2278. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lalla RV, Sonis ST and Peterson DE:
Management of oral mucositis in patients who have cancer. Dent Clin
North Am. 52:61–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skubitz KM: Glutamine as a potential
treatment for the prevention of chemotherapy induced mucositis. J
Infusional Chemotherapy. 4:64–67. 1994.
|
|
8
|
Kyllo RL and Anadkat MJ: Dermatologic
adverse events to chemotherapeutic agents, part 1: Cytotoxics,
epidermal growth factor receptors, multikinase inhibitors, and
proteasome inhibitors. Semin Cutan Med Surg. 33:28–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shou J, Lieberman MD, Hofmann K, Leon P,
Redmond HP, Davies H and Daly JM: Dietary manipulation of
methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr.
15:307–312. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campos MI, Campos CN, Aarestrup FM and
Aarestrup BJ: Oral mucositis in cancer treatment: Natural history,
prevention and treatment. Mol Clin Oncol. 2:337–340. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keefe DM, Schubert MM, Elting LS, Sonis
ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB,
Hutchins RD, Peterson DE, et al: Updated clinical practice
guidelines for the prevention and treatment of mucositis. Cancer.
109:820–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peterson DE, Bensadoun RJ and Roila F;
ESMO Guidelines Working Group, : Management of oral and
gastrointestinal mucositis: ESMO clinical recommendations. Ann
Oncol. 20 (Suppl 4):S174–S177. 2009. View Article : Google Scholar
|
|
13
|
Quinn B, Potting CM, Stone R, Blijlevens
NM, Fliedner M, Margulies A and Sharp L: Guidelines for the
assessment of oral mucositis in adult chemotherapy, radiotherapy
and haematopoietic stem cell transplant patients. Eur J Cancer.
44:61–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henke M, Alfonsi M, Foa P, Giralt J,
Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG
and Berger D: Palifermin decreases severe oral mucositis of
patients undergoing postoperative radiochemotherapy for head and
neck cancer: A randomized, placebo-controlled trial. J Clin Oncol.
29:2815–2820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bensinger W, Schubert M, Ang KK, Brizel D,
Brown E, Eilers JG, Elting L, Mittal BB, Schattner MA, Spielberger
R, et al: NCCN Task force report. Prevention and management of
mucositis in cancer care. J Natl Compr Canc Netw. 6 (Suppl
1):S1–S21; quiz S22-S24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Svanberg A, Ohrn K and Birgegard G: Oral
cryotherapy reduces mucositis and improves nutrition-a randomised
controlled trial. J Clin Nurs. 19:2146–2151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scully C, Epstein J and Sonis S: Oral
mucositis: A challenging complication of radiotherapy,
chemotherapy, and radiochemotherapy. Part 2: Diagnosis and
management of mucositis. Head Neck. 26:77–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cowen D, Tardieu C, Schubert M, Peterson
D, Resbeut M, Faucher C and Franquin JC: Low energy Helium-Neon
laser in the prevention of oral mucositis in patients undergoing
bone marrow transplant: Results of a double blind randomized trial.
Int J Radiat Oncol Biol Phys. 38:697–703. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Online EA Pharma Co., Ltd. Products
Information, Elental®, . simplehttp://www.eapharma.co.jp/medicalexpert/product/elental/elental_information.htmlWebpage
in Japanese. July 25–2019
|
|
20
|
Yamamoto T, Nakahigashi M, Umegae S,
Kitagawa T and Matsumoto K: Impact of elemental diet on mucosal
inflammation in patients with active Crohn's disease: Cytokine
production and endoscopic and histological findings. Inflamm Bowel
Dis. 11:580–588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johtatsu T, Andoh A, Kurihara M, Iwakawa
H, Tsujikawa T, Kashiwagi A, Fujiyama Y and Sasaki M: Serum
concentrations of trace elements in patients with Crohn's disease
receiving enteral nutrition. J Clin Biochem Nutr. 4:197–201. 2007.
View Article : Google Scholar
|
|
22
|
Yamamoto T, Nakahigashi M, Saniabadi AR,
Iwata T, Maruyama Y, Umegae S and Matsumoto K: Impacts of long-term
enteral nutrition on clinical and endoscopic disease activities and
mucosal cytokines during remission in patients with Crohn's
disease: A prospective study. Inflamm Bowel Dis. 13:1493–1501.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanai H, Iida T, Takeuchi K, Arai H, Arai
O, Abe J, Tanaka T, Maruyama Y, Ikeya K, Sugimoto K, et al:
Nutritional therapy versus 6-mercaptopurine as maintenance therapy
in patients with Crohn's disease. Dig Liver Dis. 44:649–654. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto T, Nakahigashi M, Umegae S,
Kitagawa T and Matsumoto K: Impact of long-term enteral nutrition
on clinical and endoscopic recurrence after resection for Crohn's
disease: A prospective, non-randomized, parallel, controlled study.
Aliment Pharmacol Ther. 25:67–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukui T, Itoh Y, Orihara M, Yoshizawa K,
Takeda H, Kawada S and Yoshioka T: Elental prevented and reduced
oral mucositis during chemotherapy in patients esophageal cancer.
Gan To Kagaku Ryoho. 38:2597–2601. 2011.(In Japanese). PubMed/NCBI
|
|
26
|
Ogata Y, Takeuchi M, Ishibashi N, Kibe S,
Takahashi K, Uchida S, Murakami N, Yahara T and Shirouzu K:
Efficacy of Elental on prevention for chemotherapy-induced oral
mucositis in colorectal cancer patients. Gan To Kagaku Ryoho.
39:583–587. 2012.(In Japanese). PubMed/NCBI
|
|
27
|
Harada K, Minami H, Ferdous T, Kato Y,
Umeda H, Horinaga D, Uchida K, Park SC, Hanazawa H, Takahashi S, et
al: The Elental® elemental diet for
chemoradiotherapy-induced oral mucositis: A prospective study in
patients with oral squamous cell carcinoma. Mol Clin Oncol.
10:159–167. 2019.PubMed/NCBI
|
|
28
|
Harada K, Ferdous T, Horinaga D, Uchida K,
Mano T, Mishima K, Park S, Hanazawa H, Takahashi S, Okita A, et al:
Efficacy of elemental diet on prevention for
chemoradiotherapy-induced oral mucositis in patients with oral
squamous cell carcinoma. Support Care Cancer. 24:953–959. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harada K, Ferdous T, Kobayashi H and
Ueyama Y: Elemental diet accelerates the recovery from oral
mucositis and dermatitis induced by 5-Fluorouracil through the
induction of fibroblast growth factor 2. Integr Cancer Ther.
17:423–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harada K, Ferdous T, Mizukami Y and
Mishima K: Elemental diet inhibits pro-inflammatory cytokine
production in keratinocytes through the suppression of NF-κB
activation. Oncol Rep. 40:361–368. 2018.PubMed/NCBI
|
|
31
|
Luo M, Brooks M and Wicha MS: Asparagine
and Glutamine: Co-conspirators fueling metastasis. Cell Metab.
27:947–949. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knott SRV, Wagenblast E, Khan S, Kim SY,
Soto M, Wagner M, Turgeon MO, Fish L, Erard N, Gable AL, et al:
Asparagine bioavailability governs metastasis in a model of breast
cancer. Nature. 554:378–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Ling W, Shen ZY, Jin X and Cao H:
Clinical application of immune-enhanced enteral nutrition in
patients with advanced gastric cancer after total gastrectomy. J
Dig Dis. 13:401–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
ASPEN Board of Directors and the Clinical
Guidelines Task Force, . Guidelines for the use of parenteral and
enteral nutrition in adult and pediatric patients. JPEN J Parenter
Enteral Nutr. 26 (Suppl 1):1SA–138SA. 2002. View Article : Google Scholar : PubMed/NCBI
|