Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
prevalent clinicopathological subtype of lymphoma in adults,
accounting for 30-40% of non-Hodgkin's lymphoma (1). Standard immunotherapy and chemotherapy
treatment regimens, such as R-CHOP (rituximab plus
cyclophosphamide, doxorubicin, vincristine and prednisone) have led
to significant improvements in the clinical outcome of DLBCL, with
the 5-year overall survival (OS) rate of DLBCL in the USA reaching
60% (2,3). However, 1/3 of DLBCL patients still
experience primary refractoriness or relapse after achieving
complete remission, comprising a major challenge in the mortality
and morbidity of DLBCL. DLBCL is generally considered a
heterogeneous entity in terms of genetics, morphology and
biological behavior. According to gene expression profiling, DLBCL
can further be classified into three subtypes, germinal center B
cell-like (GCB), activated B cell-like (ABC) and an unclassified
subtype; however, the mechanism underlying the pathogenesis of
DLBCL remains largely elusive (4).
MicroRNAs (miRNAs) are a class of short,
single-stranded, non-coding RNAs usually 18-22 nucleotides in
length, which regulate target genes post-transcriptionally and
result in either direct mRNA degradation or protein translation
inhibition. Compelling evidence has demonstrated that aberrant
miRNA expression plays an essential role in the pathogenesis of
hematological malignancies, including lymphoma (5,6).
Costinean et al generated Eµ-miR155 transgenic mice
overexpressing miR-155, which were shown to develop a
lymphoproliferative disease resembling high-grade lymphoma
(7). A study by Mihailovich et
al found that the miR-17-92 cluster regulates c-myc to ensure
optimal B-cell lymphoma growth in an established lymphoma model
(8). Several miRNAs have been
reported to discriminate between DLBCL subtypes and be correlated
with chemosensitivity, while some miRNAs can predict the outcomes
of DLBCL patients treated with the R-CHOP regime (9,10).
Given the pivotal role of aberrant miRNA expression in the
pathogenesis of DLBCL, the correction of abnormal miRNA expression
has emerged as a promising therapeutic strategy (11).
Transforming growth factor β1 (TGF-β1) is a
versatile cytokine that plays an important role in the pathogenesis
of cancer as a tumor suppressor or promoter, depending on the
context. Findings of previous studies have shown that TGF-β1 is
involved in the development and progression of malignant tumors by
regulating the expression of various miRNAs (12,13).
From a miRNA microarray profile following the knockdown of TGF-β1
in colorectal cancer cell lines, our group identified a set of
TGF-β1-associated differentially expressed miRNAs. RT-qPCR was used
to validate the differential expression of these miRNAs in DLBCL
cells, and the miR-196a-3p expression was found to decrease in two
DLBCL cell lines. The influence of overexpressed miR-196a-3p on the
survival of DLBCL cells was then evaluated, and it was
experimentally validated that miR-196a-3p directly inhibited the
translation of ADP ribosylation factor 4 (ARF4) in vitro.
The present findings suggested that miR-196a-3p was involved in the
tumorigenesis of DLBCL through the downregulation of ARF4.
Materials and methods
Patients and human tissue samples
A total of 68 patients with a confirmed diagnosis of
de novo DLBCL by biopsy in the First Affiliated Hospital of
Soochow University (Suzhou, China) were enrolled in the present
study. All 68 DLBCL patients had available formalin-fixed
paraffin-embedded (FFPE) tissues (3 µm in thickness) for RNA
extraction and had not received radiotherapy or chemotherapy.
Patients with DLBCL secondary to other B-cell hematological
malignancies, primary CNS B-cell lymphoma and mediastinal large
B-cell lymphoma were excluded from the present study. Ten reactive
lymph node hyperplasia (RLH) specimens from patients in the First
Affiliated Hospital of Soochow University were included as the
normal control. According to the Hans algorithm, all DLBCL cases
were classified into GCB and non-GCB subtypes, based on
immunohistochemical (IHC) markers.
All the patients provided written informed consent
and were followed in the outpatient clinic or by telephone every 3
months. This study was approved by the Institutional Review Board
of the First Affiliated Hospital of Soochow University.
Cell lines and cell culture
The human DLBCL cell lines used in this study
included Farage and OCI-LY3, and were obtained from the American
Type Culture Collection. Farage and OCI-LY3 cells were cultured in
RPMI-1640 medium (GE Healthcare Life Sciences) in a humidified
atmosphere with 5% CO2 at 37°C. The culture media was
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.), 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin. Following ethics approval from the Institutional
Review Board, normal B lymphocytes were isolated from the
peripheral blood of 6 healthy volunteers who signed written
informed consent (age range 24-39 years, 3 males and 3 females)
using the Human B cell Enrichment kit (10954; Stemcell
Technologies, Inc.). Volunteers who had a positive pregnancy test
or signs of active infection were excluded from the present
study.
RNA extraction from cell lines or FFPE
tissues, and RT-qPCR
Total RNA was extracted from DLBCL cells using
TRIzol reagent (204205; Thermo Fisher Scientific, Inc.). A MagMax™
FFPE DNA/RNA Ultra kit (A31881; Thermo Fisher Scientific, Inc.) was
used to extract total RNA from FFPE tissue samples, according to
the manufacturer's instructions. After measuring the concentration
with a NanoDrop 2000 spectrophotometer (NP80; IMPLEN), all RNA was
reverse-transcribed and then stored at −80°C until the time of
assay.
RT-qPCR was used to detect the miRNA expression
using the stem-loop method. The cDNA was used as the template for
the amplification of the RNA, and RT-qPCR was performed on
reverse-transcribed miRNAs and mRNA using Power SYBR™ Green PCR
Master Mix (4367659; Thermo Fisher Scientific, Inc.) for
quantification following amplification. GAPDH and small nuclear RNA
U6 were used as internal controls for mRNA and miRNA RT-qPCR
detection, respectively. RT-qPCR was carried out using a previously
described procedure (14). The
primer sequences of miRNA and candidate target genes were listed in
Tables SI and SII. RT-qPCR conditions for miRNA listed
as below: Activation 10 min at 95°C; denaturation for 15 sec at
95°C; extension 60 sec at 60°C. RT-qPCR thermocycling conditions
for mRNA: Activation 10 min at 95°C; denaturation 15 sec at 95°C;
extension 60 sec at 60°C. Relative expression changes were
calculated by the 2−ΔΔCq method (14).
Oligonucleotide synthesis and
transfection
miR-196a-3p mimic (mimic sequence,
5′-CGGCAACAAGAAACUGCCUGAG-3′) and control scrambled oligonucleotide
(scrambled sequence, 5′-UUUGUACUACACAAAAGUACUG-3′) were chemically
synthesized by Guangzhou RiboBio Co., Ltd. ARF4 gene-specific small
interfering RNA (siRNA) was designed using siCATCH™ siRNA Designer
(Guangzhou RiboBio Co., Ltd.). Following confirmation by RT-qPCR
(data not shown), optimal ARF4 siRNA (5′-AGACAACCATTCTGTATAA-3′)
and scrambled siRNA (5′-UUUTGATCAUTGATGAAA-3′) sequences were
selected for ARF4 RNA interference and synthesized by Guangzhou
RiboBio Co., Ltd (Fig. S3). For
miR-196a-3p mimic or ARF4 siRNA transfection, 5 µl Lipofectamine
3000 transfection reagent (Thermo Fisher Scientific, Inc.) in 200
µl serum-free medium was mixed with 100 pmol oligonucleotides
dissolved in 200 µl Opti-MEM (Thermo Fisher Scientific, Inc.)
Scrambled oligonucleotides were transfected to the negative
control, and Lipofectamine 3000 without oligonucleotides was added
to the mock control.
Cell counting kit-8 (CCK-8) cell
proliferation assay
Cell proliferation was analyzed using a CCK-8 assay
kit (Guangzhou RiboBio Co., Ltd.). Farage and OCI-LY3 cells
(2×104 cells/ml) were seeded in a 96-well cell culture
plate in a volume of 200 µl/well. Prior to detection, 20 µl CCK-8
solution was added to each well and incubated for 4 h in a 37°C
incubator. Cell proliferation was measured at 24, 48 and 72 h after
transfection, and 5 replicate test wells were used. The optical
density value at an absorbance of 450 nm was determined using a
microplate reader (Thermo Fisher Scientific, Inc.).
Flow cytometry for the detection of
cell cycle, viability and apoptosis
Farage and OCI-LY3 cells were treated with the same
transfection conditions described above. EdU
(5-ethynyl-2′-deoxyuridine) staining assays (C10310-1; Guangzhou
RiboBio Co., Ltd.) were performed to investigate the influence of
transfection on DLBCL cell viability. Cells were stained with EdU
reagent 24 h post-transfection and analyzed by flow cytometry.
Propidium iodide (PI) staining and flow cytometry were used to
analyze the cell cycle. For cell apoptosis analysis by cytometry,
the cells were stained with Annexin V and PI for 10 min at room
temperature in the dark using the Annexin V-FITC Apoptosis
Detection Kit (C1062M; Beyotime Biotechnology Co., Ltd.). All the
reactions were analyzed in triplicate by flow cytometry (BD
FACSCalibur; BD Biosciences).
Bioinformatics search
Potential miR-196a-3p target genes were predicted
through algorithms from four canonical online databases: Targetscan
(http://www.targetscan.org), miRDB
(http://mirdb.org), microRNA (http://www.microrna.org/microrna) and DIANA-microT
(http://diana.imis.athena-innovation.gr/DianaTools/).
Overlapping results underwent further functional analysis using the
miR-ontology database to screen candidate target genes.
Western blot analysis
Following transfection, DLBCL cells were collected
and lysed in RIPA buffer (b100020; ProteinTech Group, Inc.) and
total protein was extracted using bicinchoninic acid (BCA) method.
Equal aliquots of extracts were electrophoresed on
SDS-polyacrylamide gels and electrotransferred onto PVDF membranes.
In this experiment, 25 µg proteins were loaded per lane and 8-12%
gels were used. Membranes were blocked with TBS containing 5%
non-fat dry milk for at least 1 h at room temperature The blots
were then incubated with one of the following primary antibodies,
against ARF4 (11673-1-AP; dilution 1:500; ProteinTech Group, Inc.),
ZNF280 (dilution 1:500; DF9950, Affinity Bioscience), coronin-1C
(CORO1C) (14749-1-AP; dilution 1:500; ProteinTech Group, Inc.) or
neuropilin-2 (NRP2) (dilution 1:500; DF7032, Affinity Bioscience).
The blots were incubated with a peroxidase-conjugated secondary
antibody (BA1056; dilution 1:2,000; Boster Biological Technology)
at room temperature for 1 h and visualized using Immobilon Western
Chemiluminescent HRP Substrate (WBKLS0100; EMD Millipore). β-actin
was detected at the same time as the control, and the protein
expression was quantified using SkanIt™ Software (Thermo Fisher
Scientific).
Dual-luciferase reporter assays
The 3′-untranslated region (3′-UTR) sequence that
contained either a wild-type or mutant version of the ARF4 binding
sequence was inserted into a psi-Check2 luciferase reporter plasmid
(Promega Corporation), and the procedure was performed according to
the manufacturer's instructions. 293T cells were seeded in 24-well
plates (3.0×105 cells/well) and the cells were
transiently transfected by Lipofectamine 3000 with pGL3-X-baI
(Promega Corporation) and 100 ng/well psi-Check2 luciferase
reporter (Promega Corporation), and co-transfected with the
miR-196a-3p mimic or scrambled sequence. Renilla luciferase
activity was detected using the Luciferase Reporter System (Promega
Corporation) 48 h after transfection.
Immunostaining
The FFPE tissues of DLBCL and RLH were sectioned for
immunohistochemistry to determine the protein expression of the 4
candidate target genes. Following endogenous peroxidase blocking
and immunostaining with antibodies, the sections were incubated
with the following biotinylated anti-rabbit primary antibodies:
ARF4, ZNF280B, CORO1C or NRP2 (dilution 1:200; ProteinTech Group,
Inc.). DAB color-substrate solution was added following washing and
the slides were counterstained with hematoxylin. The total
immunoreactive score (IRS) algorithm was used to score the antibody
expression by immunohistochemistry (IHC) as follows: 0, no positive
cells; 1, ≤25% positive cells; 2, >25 and ≤50% positive cells;
3, >50% positive cells. Staining intensity was categorized as
follows: 0, negative; 1, weak; 2, moderate; 3, strong. IRS values
of 0-2 and 3-6 were defined as a low and high expression of ARF4
proteins, respectively. Slides were viewed using a Nikon Eclipse
E100 microscope (Nikon Corporation) at a magnification of ×100 and
×400. The immunostaining was reviewed by two independent
pathologists.
Statistical analysis
All data are presented as the mean ± SD. The
statistical software SPSS version 21.0 for Windows (IBM Corp) was
used for data analysis. Independent sample t-tests, ANOVA with
Tukey's post hoc test and chi-square tests were used to compare the
results between different groups. Survival curves were plotted
using the Kaplan-Meier method, and compared using the log-rank
test. Results are the mean ± SD of three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Decreased miR-196a-3p expression is
associated with poor survival in DLBCL
Previous findings identified the following 5
differentially expressed miRNAs by knocking down TGF-β1 expression
in colorectal cancer cell lines: miRNA-3691-3p, miR-4313,
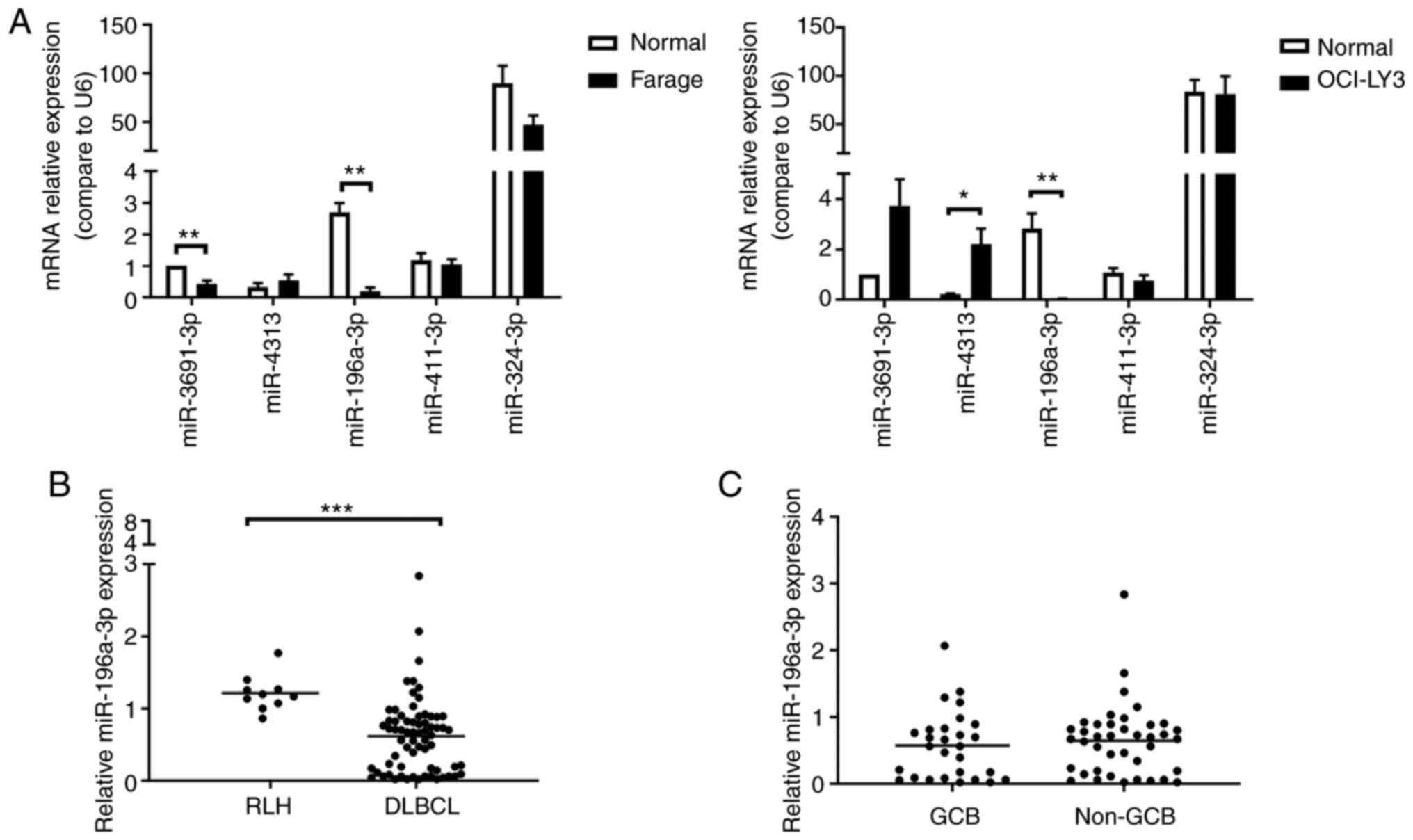
miR-196a-3p, miR-411-3p and miR-324-3p (GSE53338) (15). To investigate whether the 5 miRNAs
were also differentially expressed between DLBCL cell lines and
normal B cells, RT-qPCR was performed to compare the expression
level of these miRNAs in DLBCL cell lines and normal B cells. Two
DLBCL cell lines were analyzed in these experiments: Farage
(GCB-like DLBCL) and OCI-LY3 (ABC-like DLBCL), as described in the
Materials and methods section. Normal CD19+ B cells were
isolated from healthy individuals, with their consent, by
magnetic-activated cell sorting (Fig.
S1). Among the 5 miRNAs, only the expression of miR-196a-3p
significantly decreased in both Farage and OCI-LY3 cells, as
compared with normal CD19+ B cells (Fig. 1A).
To further determine whether miR-196a-3p was also
differentially expressed in clinical DLBCL specimens, the
expression of miR-196a-3p in 68 DLBCL FFPE samples was analyzed by
RT-qPCR. A total of 10 RLH tissues were analyzed as the control. It
was found that the miR-196a-3p expression was reduced by 50% in
DLBCL, as compared with RLH (P<0.001; Fig. 1B). However, there was no difference
in miR-196a-3p expression between the GCB and non-GCB DLBCL groups
(Fig. 1C).
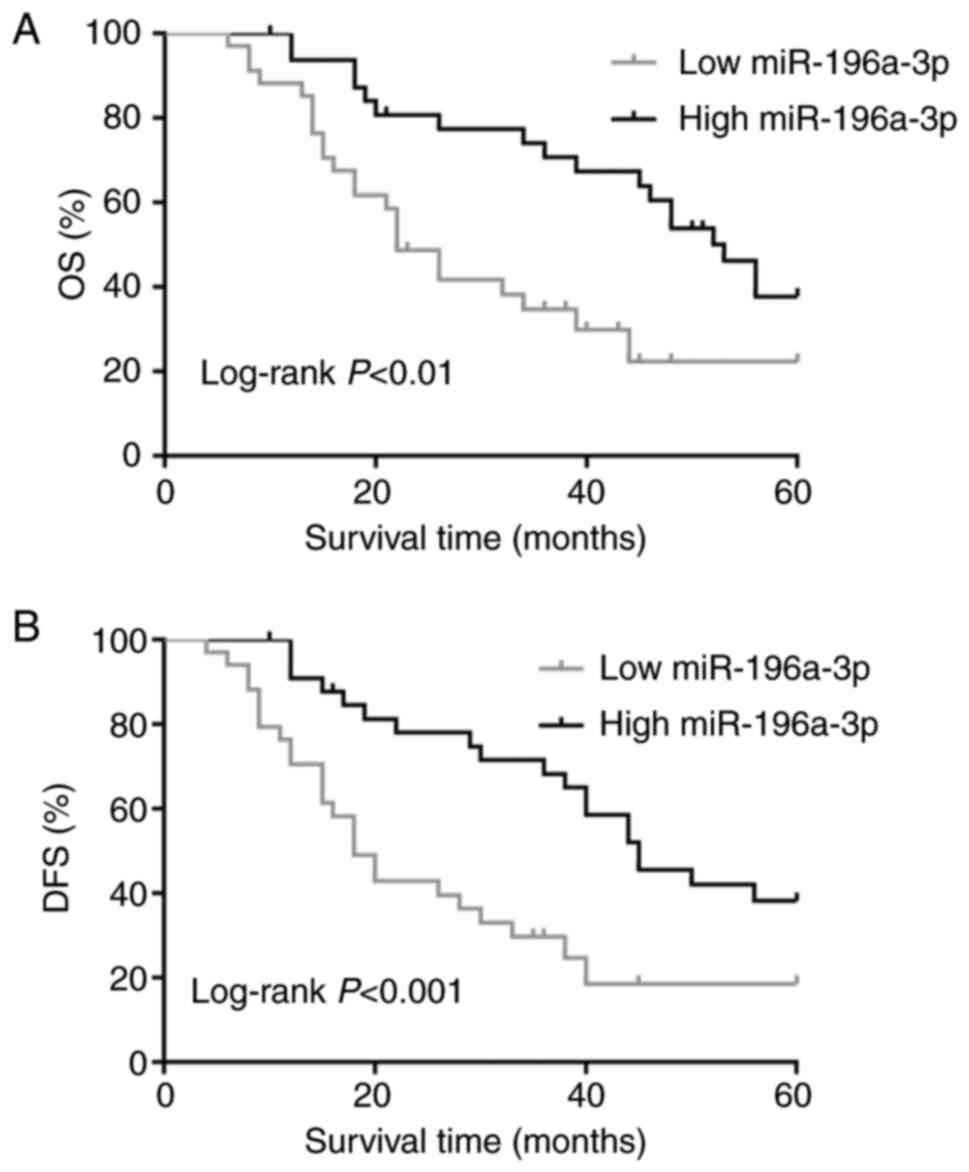
To investigate the clinical relevance of the
miR-196a-3p expression in DLBCL, 68 DLBCL patients were divided
into two groups according to the median level of the miR-196a-3p
expression (median=0.66-fold change). Table I summarizes the association between
the miR-196a-3p expression and clinicopathological variables of
these 68 DLBCL patients. Statistical analysis revealed that a low
miR-196a-3p expression level was correlated with Ann Arbor stage
(P=0.001), international prognosis index (P=0.049), bone marrow
involvement (P=0.017) and number of extranodal sites (P=0.027).
However, no correlations were identified between the miR-196a-3p
level and age, sex, B symptom, primary site, Eastern Cooperative
Oncology Group (ECOG) score, lactate dehydrogenase (LDH) level and
Hans classification. The Kaplan-Meier method and log-rank test were
then used to compare survival between the two groups with a
different miR-196a-3p expression (Fig.
2). The low miR-196a-3p expression group had a significantly
shorter OS and disease-free survival (DFS) than did the high
miR-196a-3p expression group (P<0.01 and P<0.001,
respectively).
 | Table I.Correlation of expression of
miR-196a-3p with clinicopathologic features of DLBCL patients. |
Table I.
Correlation of expression of
miR-196a-3p with clinicopathologic features of DLBCL patients.
|
| miR-196a-3p
expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameter | High (34) | Low (34) | P-value |
|---|
| Age (years) |
|
<60 | 25 | 21 | 0.300 |
|
≥60 | 9 | 13 |
|
| Sex |
|
Male | 19 | 17 | 0.627 |
|
Female | 15 | 17 |
|
| Ann Arbor
stage |
| I and
II | 18 | 5 | 0.001b |
| III and
IV | 16 | 29 |
|
| B symptom |
| No | 23 | 20 | 0.451 |
|
Yes | 11 | 14 |
|
| Primary site |
|
Nodal | 20 | 21 | 0.804 |
|
Extranodal | 14 | 13 |
|
| Performance status
(ECOG) |
|
0-1 | 27 | 22 | 0.177 |
| ≥2 | 7 | 12 |
|
| LDH |
|
Normal | 23 | 19 | 0.318 |
|
Elevated | 11 | 15 |
|
| BM involvement |
|
Absent | 31 | 22 | 0.017a |
|
Present | 3 | 12 |
|
| IPI group |
|
|
|
| Low
(0–2) | 24 | 16 | 0.049a |
| High
(3–5) | 10 | 18 |
|
| Hans
classification |
|
GCB | 11 | 17 | 0.139 |
|
Non-GCB | 23 | 17 |
|
| No. of extranodal
sites |
|
0-1 | 19 | 10 | 0.027a |
| ≥2 | 15 | 24 |
|
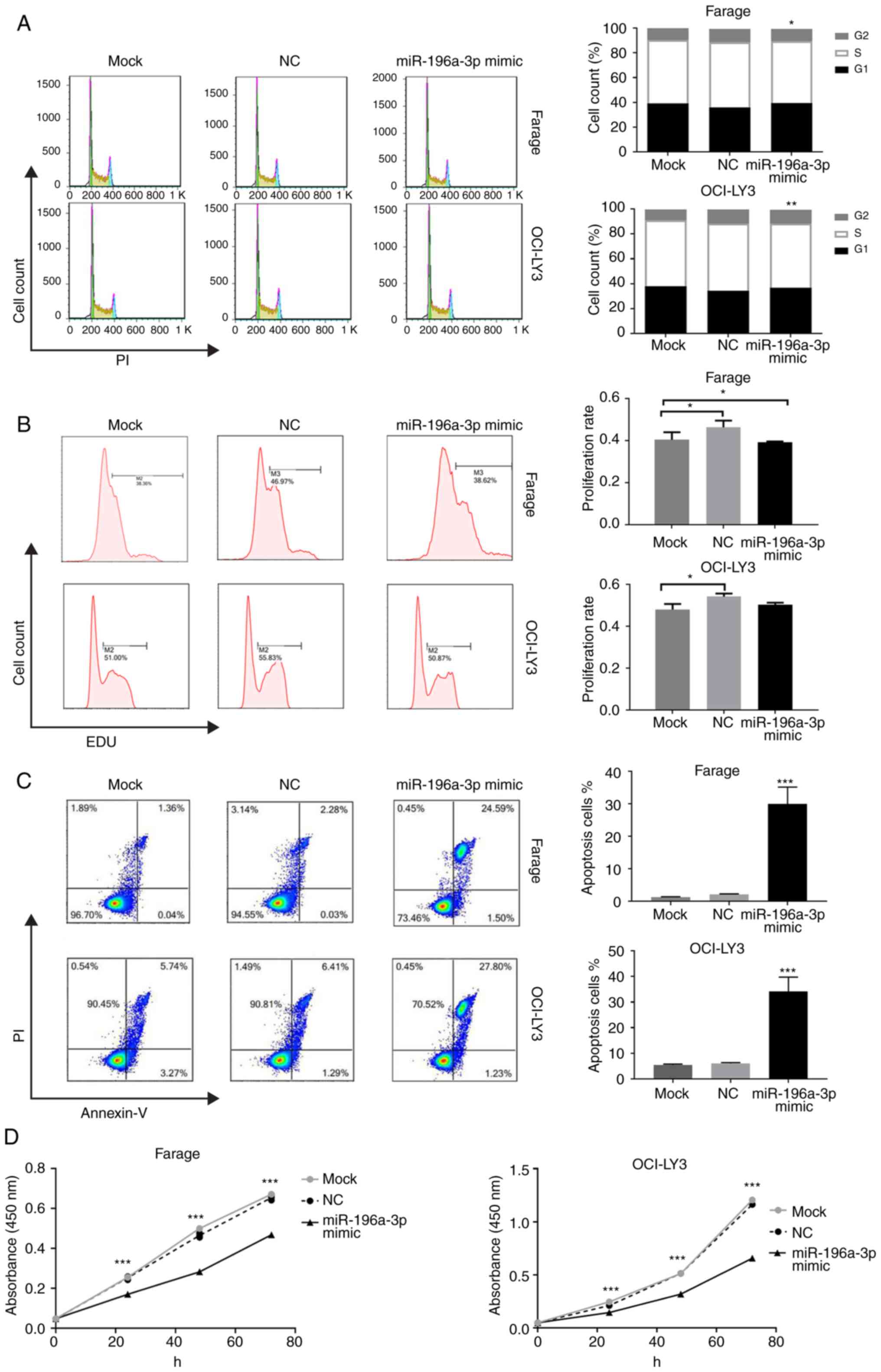
Ectopic overexpression of miR-196a-3p
induces cell cycle arrest and apoptosis and reduces cell
proliferation in DLBCL
In the previous experiment, a notably decreased
expression of miR-196a-3p was confirmed in both DLBCL cell lines
and pathological specimens, as compared with normal controls. To
further evaluate the biological role of miR-196a-3p in the
development and progression of DLBCL, and whether
miR-196a-3p-overexpression influenced DLBCL cell cycle, apoptosis
and proliferation were next investigated. Synthesized miR-196a-3p
mimic and scrambled mimic oligonucleotide as negative controls were
transiently transfected into Farage and OCI-LY3 cells, and the
transfection efficiency of the miR-196a-3p mimic was validated by
RT-qPCR (data not shown, Fig. S2).
Next, multiple functional assays, including cell cycle analysis,
EdU staining, cell apoptosis and CCK-8 proliferation assays were
performed to evaluate cell survival dysfunction of DLBCL in
vitro. As shown in Fig. 3A,
cell cycle analysis revealed a reduced percentage of S-phase cells
in Farage and OCI-LY3 cells following miR-196a-3p mimic
transfection (P<0.05 and P<0.01, respectively), and G1-phase
cells increased in OCI-LY3 cells (P<0.01). The EdU-positive
percentage of Farage and OCI-LY3 cells in the mimic-transfected
group was significantly lower than that of the control group
(Fig. 3B). In the apoptosis assays,
the overexpression of miR-196a-3p led to a markedly increased cell
apoptosis by 14.3-fold in Farage cells and 5.6-fold in OCI-LY3
cells (P<0.001; Fig. 3C).
Finally, a CCK-8 assay was performed to evaluate the collective
effect of miR-196a-3p on the growth and survival of DLBCL cells.
Proliferation rates 48 and 72 h after mimic transfection of both
Farage and OCI-LY3 cells decreased significantly, as compared with
the negative control group (P<0.01). Furthermore, the 72-h
proliferation rate was reduced to 56.5 and 71.5% in Farage and
OCI-LY3 cells, respectively (P<0.001; Fig. 3D).
ARF4 is the downstream target gene of
miR-196a-3p in DLBCL
To investigate the molecular mechanisms underlying
the influence of miR-196a-3p on the DLBCL cell phenotype, a
bioinformatics approach was used to explore the putative targets of
miR-196a-3p. From online prediction algorithms of TargetScan,
miRDB, microRNA and DIANA-microT, 8 candidate genes were identified
based on intersecting search results from these 4 bioinformatics
databases. After a functional analysis by the miR-Ontology
database, ARF4, Zinc finger protein (ZNF280B), CORO1C and NRP2 were
identified as 4 potential target genes (Fig. 4A).
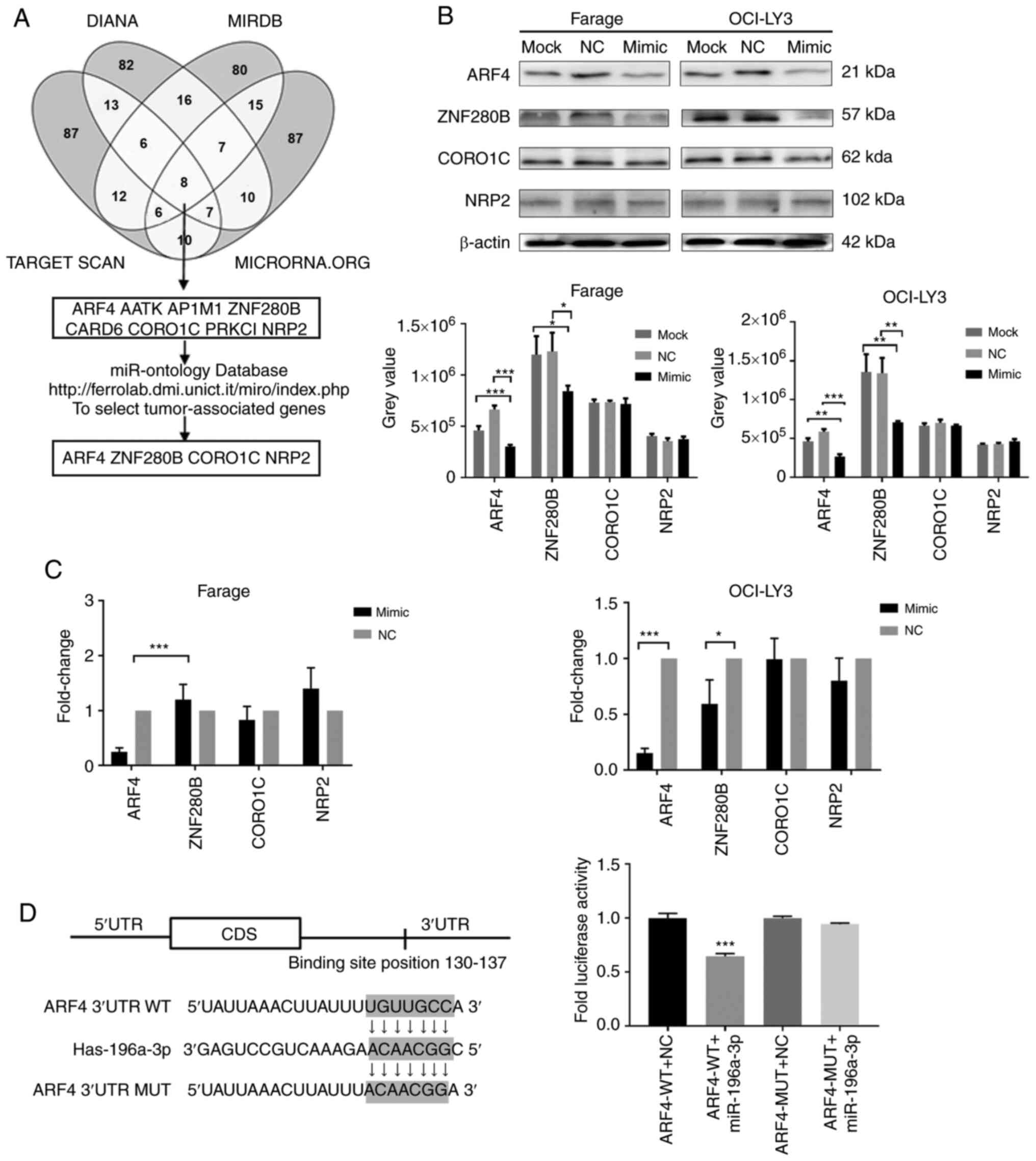 | Figure 4.Prediction and identification of
miR-196a-3p target genes in DLBCL cells. (A) miRNA targets were
predicted using TargetScan, microRNA, miRDB and DIANA-microT. (B)
The protein levels of 4 miR-196a-3p candidate target genes were
analyzed by western blot analysis. Up, protein bands; down, grey
value. (C) mRNA levels of 4 miR-196a-3p candidate target genes were
analyzed by RT-qPCR. (D) 293T cells were transiently transfected
with Renilla luciferase reporter vectors containing either
wild-type or mutant ARF4 3′-UTR seed sequence, and luciferase
activity was measured following co-transfection with miR-196a-3p or
NC. *P<0.05, **P<0.01 and ***P<0.001. DLBCL, diffuse large
B-cell lymphoma; miRNA, microRNA; ARF4, ADP ribosylation factor 4;
3′-UTR, 3′-untranslated region; NC, negative control. |
Our next aim was to further confirm the effect of
miRNA-196a-3 on these targets in Farage and OCI-LY3 cells. The
protein expression levels of the 4 target genes in both DLBCL cell
lines were examined by western blot analysis following miR-196a-3p
mimic transfection, as compared with the negative control.
Significantly decreased protein expression levels of AFR4 and
ZNF280B were observed in mimic-transfected Farage and OCI-LY3
cells, as compared with negative controls (Fig. 4B). RT-qPCR was used to detect the
level of target gene mRNA, which revealed that only the ARF4 mRNA
level was significantly decreased in both DLBCL cell lines, as
compared with control cells (Fig.
4C). Based on the above results, ARF4 was selected as our
target gene for further experimental verification.
Dual-luciferase gene reporter assays were then used
to determine whether miR-196a-3p regulated ARF4 expression directly
through specific base pairing in vitro. ARF4 3′-UTR
harboring the potential miR-196a-3p-binding site or a mutant
sequence were cloned and inserted into a luciferase reporter
vector. The relative luciferase activity in cells containing the
wild-type ARF4 3′-UTR was reduced by 35.3%, as compared with the
negative control, while no change was observed in cells containing
the mutated reporters (Fig. 4D).
These findings suggested that miR-196a-3p directly binds to the
3′-UTR of ARF4 mRNA to suppress ARF4 protein translation.
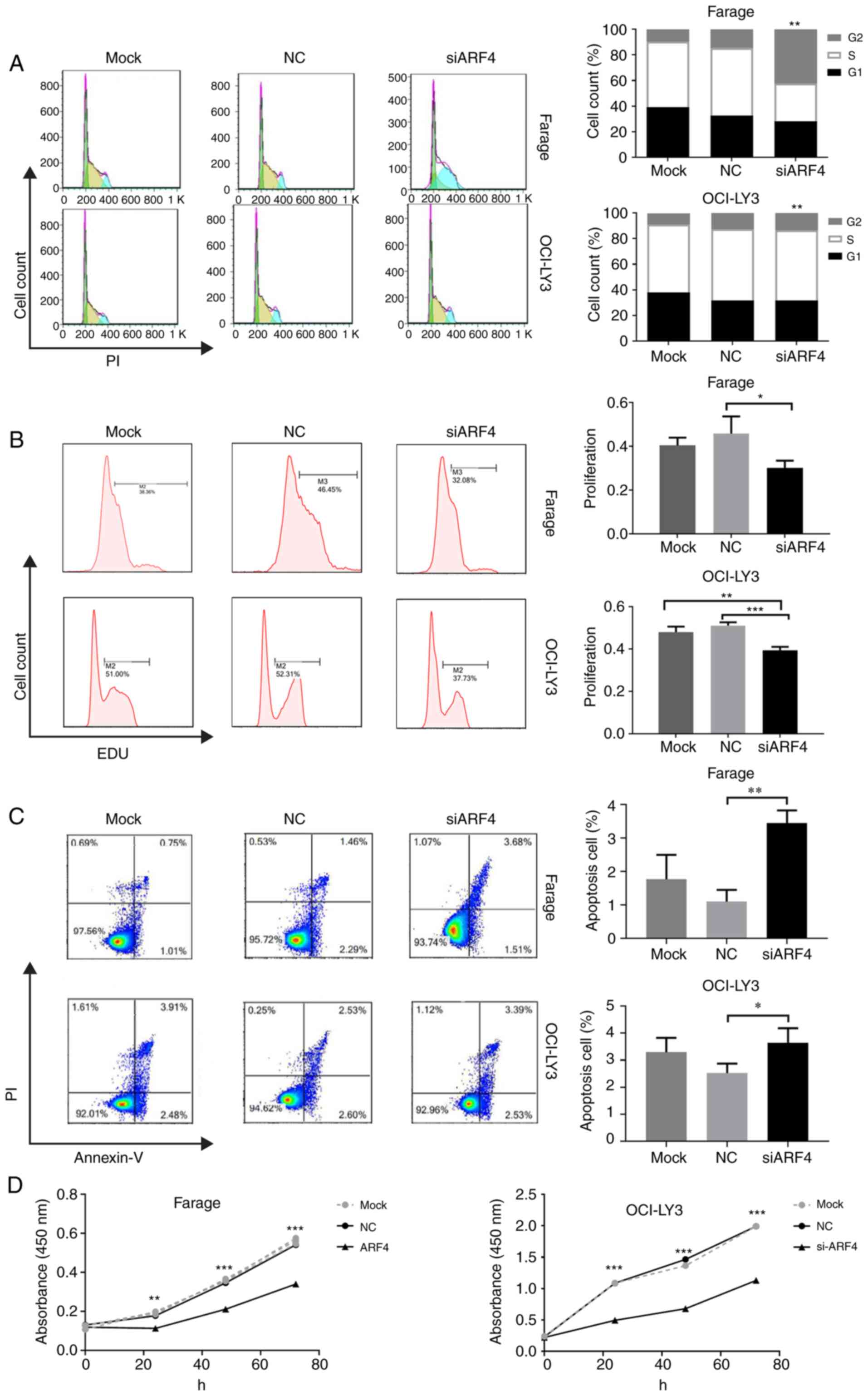
ARF4 induces cell proliferation and
inhibits apoptosis in DLBCL
To explore whether the downregulation of ARF4
produced a similar effect to that of the miR-196a-3p mimic on the
phenotype of DLBCL cells, siRNA technology was used to silence the
expression of ARF4 in vitro. Following ARF4 siRNA
transfection, functional assays evaluating DLBCL cell growth and
survival were performed individually.
Transfection of Farage and OCI-LY3 cells with ARF4
siRNA resulted in an increased accumulation of G1-phase cells, as
compared with control transfections (P<0.01), while no
difference in the number of S-phase cells was observed between the
two groups (Fig. 5A). EdU staining
and cytometric analysis indicated that the percentage of
EdU-positive cells significantly decreased, as compared with the
control (Fig. 5B). Similarly, by
annexin-PI double staining cytometric analysis, ARF4 siRNA
transfection induced an increase in the percentage of apoptosis in
both Farage and OCI-LY3 cells, as compared with nonsense control
transfection (P<0.01 and P<0.05, respectively; Fig. 5C). As determined by the CCK-8 assay,
the two DLBCL cell lines exhibited a significantly reduced cell
proliferation 24, 48 and 72 h after ARF4 siRNA inhibition (Fig. 5D). The 72-h proliferation inhibition
percentage following ARF4 knockdown was 62.8 and 56.7% in Farage
and OCI-LY3 cells, respectively (P<0.001). From these results,
it was concluded that ARF4 contributes to cell growth and survival
of DLBCL as an oncogene, which can be downregulated by
miR-196a-3p.
Upregulation of ARF4 is correlated
with clinicopathologic variables in DLBCL
By gain and loss of function tests and luciferase
reporter assays in vitro, it was validated that ARF4
contributes to DLBCL progression under the direct regulation of
miR-196a-3p. The protein expression of the 4 candidate target genes
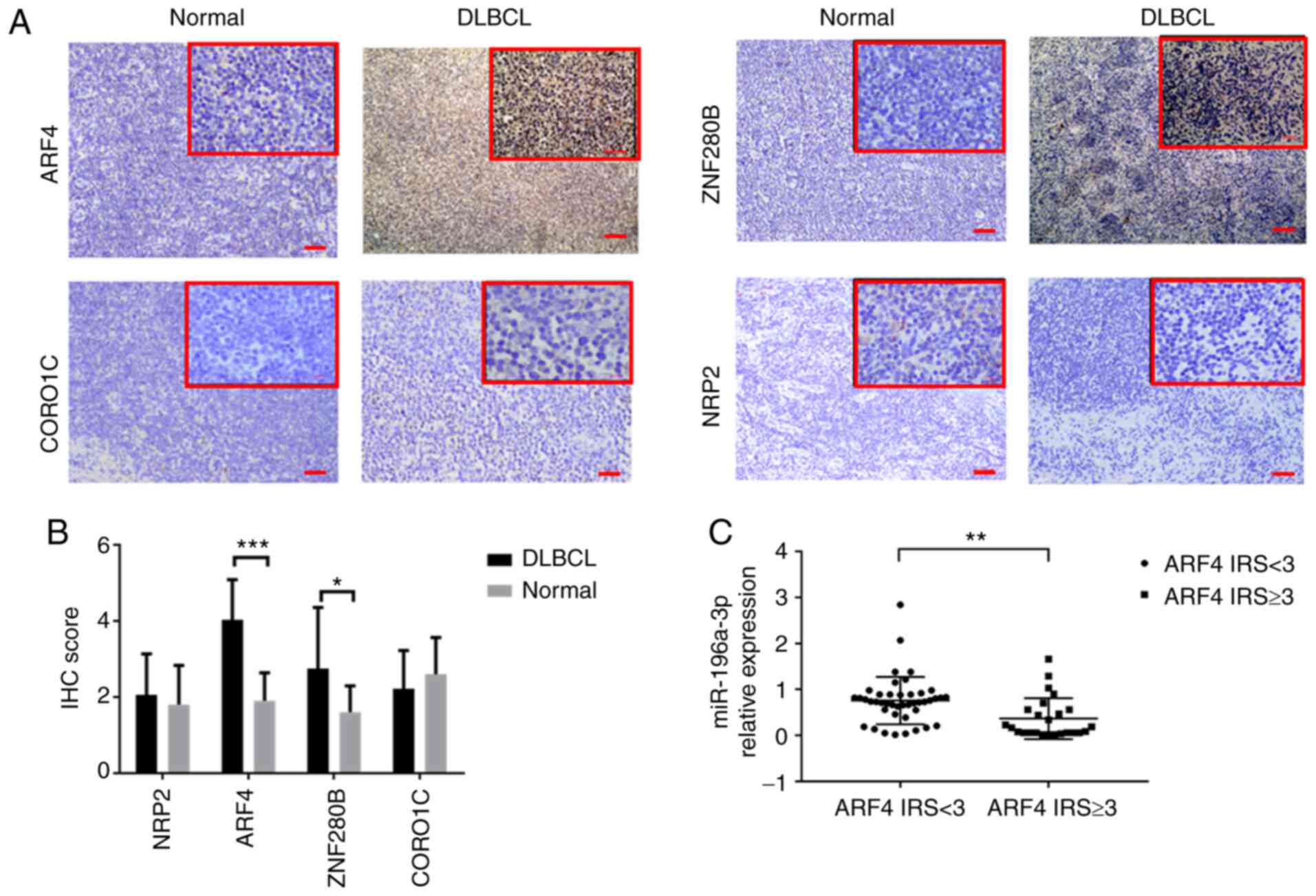
was measured in 32 DLBCL FFPE tissues by immunohistochemical
staining, as compared with 10 RLH FFPE tissues (Fig. 6A). The IRS score was used to compare
IHC results of target proteins. Compared with RLH, the ARF4 protein
expression levels were significantly higher in the DLBCL tissues
(P<0.001), while the ZNF280B protein expression was slightly
higher (P<0.05; Fig. 6B).
Furthermore, the high ARF4 proteinexpression group had a lower
miR-196a-3p expression level than the low ARF4 expression group in
the DLBCL specimens. (P<0.01; Fig.
6C).
To further elucidate the clinical significance of
ARF4 expression, the association between the ARF4 protein
expression level and clinicopathologic variables in 68 DLBCL
patients was analyzed. The ARF4 IHC staining was performed on the
FFPE slides of the DLBCL patients, and all patients were divided
into two groups, according to the ARF4 expression level determined
by the IRS score (≥3 and <3). High ARF4 expression levels were
significantly associated with Ann Arbor stage (P=0.040), LDH
(P=0.022) and the number of extranodal sites (P=0.013). However,
there was no correlation between the ARF4 level and age, sex, B
symptom, primary site, ECOG score, IPI, bone marrow involvement and
Hans classification (Table
II).
 | Table II.Association of expression of ARF4 IRS
with clinicopathological characteristics of DLBCL patients. |
Table II.
Association of expression of ARF4 IRS
with clinicopathological characteristics of DLBCL patients.
| Parameter | High ARF4 (IRS ≥3)
43 patients | Low ARF4 (IRS
<3) 25 patients | P-value |
|---|
| Age (years) |
|
<60 | 28 | 18 | 0.559 |
|
≥60 | 15 | 7 |
|
| Sex |
|
Male | 22 | 14 | 0.700 |
|
Female | 21 | 11 |
|
| Ann Arbor
stage |
| I and
II | 20 | 3 | 0.04a |
| III and
IV | 23 | 22 |
|
| B symptom |
| No | 30 | 13 | 0.143 |
|
Yes | 13 | 12 |
|
| Primary site |
|
Nodal | 29 | 21 | 0.804 |
|
Extranodal | 14 | 14 |
|
| Performance status
(ECOG) |
|
0-1 | 29 | 12 | 0.114 |
| ≥2 | 14 | 13 |
|
| LDH |
|
Normal | 31 | 11 | 0.022a |
|
Elevated | 12 | 14 |
|
| BM involvement |
|
Absent | 37 | 16 | 0.066 |
|
Present | 6 | 9 |
|
| IPI group |
| Low
(0–2) | 31 | 10 | 0.09 |
| High
(3–5) | 12 | 15 |
|
| Hans
classification |
|
GCB | 16 | 12 | 0.383 |
|
Non-GCB | 27 | 13 |
|
| No. of extranodal
sites |
|
0-1 | 6 | 24 | 0.013a |
| ≥2 | 19 | 19 |
|
Discussion
The miRNA-196 family, located in the homeobox gene
clusters, consisted of 3 miR-196 genes: miR-196a-1, miR-196a-2 and
miR-196b. It has been extensively reported that miR-196
participates in various fundamental biological processes including
development, inflammation, immunity, and cancer pathogenesis
(16,17). Various miRNA profiling studies
reported overexpressed miR-196 levels in malignant tumors (18–20).
miRNA-196a-5p levels were found to be inversely correlated with
survival in pancreatic duct adenocarcinoma patients (21). Lu et al found that miR-196
mediated an invasive phenotype in oral cancer through the JNK
signaling pathway (22). One recent
study from our group validated the decreased expression of
miR-196a-3p in breast cancer, and the downregulation of miR-196a-3p
was found to promote the metastasis of breast cancer by targeting
NRP2 (23). Several miRNAs are
involved in the pathogenesis of DLBCL by regulating downstream
target genes. However, the biological role of miR-196, particularly
miR-196a-3p, in the development and progression of DLBCL remains to
be elucidated.
As in the case of other malignant tumors, the
dysfunction of cell survival is a hallmark of DLBCL pathogenesis.
Several mechanisms have been attributed to survival dysfunction,
including intrinsic signaling aberrations, tumor environmental
dysfunction and viral factors (24). The dysfunction of multiple cellular
pathways, including the BCR signaling, BCL2 and p53 pathways, was
found to contribute to aberrant DLBCL cell survival (25). Various novel drugs targeting
abnormal cell survival pathways have been developed for DLBCL. The
BTK inhibitor ibrutinib, a novel drug targeting the BCR pathway,
has shown promising efficiency in relapsed and refractory DLBCL
patients (25). In recent years,
several miRNAs have been reported to play essential roles in cell
growth and DLBCL progression. A report by Zhu et al found
that the downregulation of miR-155 inhibited DLBCL cell growth by
inducing cell cycle arrest and promoting cell apoptosis, and
luciferase reporter assays demonstrated where TGFBR2 is a target of
miR-155 in lymphoma cell lines (26). Another study by Fan et al
found that miR-10a can suppress the proliferation and promote the
apoptosis of DLBCL cells by targeting BCL6 (27). Jia et al found that miR-27b
suppressed DLBCL cell viability and proliferation by targeting the
MET/PI3K/AKT pathway, which is regulated by HDAC6. Moreover, the
decreased expression of miR-27b was associated with poor survival
in DLBCL (28).
Findings of the present study showed that the
expression level of miR-196a-3p was reduced in DLBCL, and that the
downregulation of miR-196a-3p plays an important role in cell
survival dysfunction in DLBCL. Subsequent survival analysis
revealed that the decreased expression of miR-196a-3p was
associated with Ann Arbor stage, BM involvement, IPI, number of
extranodal sites and poor survival in DLBCL patients. To explore
the influence of miR-196a-3p on the DLBCL phenotype and the
underlying mechanism, we first transfected miR-196a-3p mimic and
target gene siRNA in gain and loss of function tests. Next, the
growth and survival of DLBCL cells were evaluated via 4 different
functional assays, including cell cycle, EdU staining, cell
apoptosis and CCK-8 assays. In addition to testing the effect of
miR-196a-3p on target gene expression, ARF4 was verified as the
target gene of miR-196a-3p by a bioinformatics approach and
luciferase reporter assay. It is well established that one single
miRNA can regulate multiple genes and multiple miRNAs can target
one gene. miR-196a-3p may be involved in DLBCL tumorigenesis
through a network of various target genes. However, as with
miR-196a-3p mimic restoration in DLBCL cells, silencing of the ARF4
expression induced cell cycle arrest and apoptosis, and inhibited
cell proliferation. Furthermore, using IHC staining analysis, it
was confirmed that the ARF4 protein was overexpressed in DLBCL
samples and inversely associated with the miR-196a-3p level. These
findings consistently suggested that the targeting of ARF4 is an
important mechanism through which miR-196a-3p plays its
tumor-suppressor role in DLBCL.
ARF4 is a member of the Ras superfamily of small
guanine nucleotide-binding proteins. ARFs, which consist of 6 Arf
isoforms (Arf1-Arf6), participate in several cellular processes,
including vesicular trafficking, cytokinesis, cell adhesion and
tumor cell invasion (29). Several
reports have suggested that ARF4 plays a pivotal role in
tumorigenesis. Woo et al found that ARF4 could function as
an anti-apoptotic protein and reduce the generation of reactive
oxygen species in response to either B-cell lymphoma 2-associated X
protein or N-(4-hydroxyphenyl) retinamide (30). In another study, miR-221-3p was
shown to inhibit the proliferation and migration of epithelial
ovarian cancer cells by targeting ARF4 (31). The present study revealed that the
ARF4 expression was increased in DLBCL, and that this increase was
associated with an advanced Ann Arbor stage (III, IV), elevated LDH
and increased number of extranodal sites (>2). Moreover, the
knockdown of ARF4 in vitro exhibited a pro-apoptotic and
anti-proliferative effect on DLBCL cells. These results indicated
that ARF4 may act as an oncogene mediating aberrant DLBCL cell
apoptosis and proliferation.
Two main limitations of the present study should be
addressed. First, this is a single-center retrospective study and
only 68 DLBCL patients were enrolled, and a prospective research
including multiple centers will further prove the value of
miR-196a-3p and ARF4. Second, we did not perform the ARF4 rescue
experiment which probably would alleviate the effect of
overexpressed miR-196a-3p on DLBCL cells.
In conclusion, the present study found a significant
decrease of miR-196a-3p in DLBCL, which was associated with poor
clinical prognosis. It was demonstrated that the downregulation of
the ARF4 expression by miR-196a-3p resulted in the accumulation of
cells in the G1 phase cell cycle, accompanied by an inhibited cell
proliferation and elevated apoptosis in DLBCL. The present findings
delineated a novel molecular regulatory network of miR-196a-3p and
ARF4 in DLBCL cell proliferation and apoptosis, which may have a
potential therapeutic or prognostic value for the management of
DLBCL in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key R&D
Program of China (grant nos. 2016YFC0902800, 2017YFA0104502 and
2017ZX09304021), Innovation Capability Development Project of
Jiangsu Province (grant no. BM2015004) and Jiangsu Provincial Key
Medical Center (grant no. YXZXA2016002).
Availability of data and materials
The dataset supporting the conclusions of this
article is included within this article.
Authors' contributions
JF and XL designed and performed the experiments,
statistical analysis and writing of the manuscript. DW and SWan
participated in experiment design and direction, and reviewed the
manuscript. ZC, XZ and MZ participated in the collection of
clinical specimens and patient follow-up. SWang and LG were
involved in the conception of the study and revising the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
All patients and volunteers recruited have signed
informed consent and this research was approved by the
Institutional Review Board of the First Affiliated Hospital of
Soochow University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lymphoma. Pathology. 50:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapuy B, Stewart C, Dunford AJ, Kim J,
Kamburov A, Redd RA, Lawrence MS, Roemer MS, Li AJ, Ziepert M, et
al: Molecular subtypes of diffuse large B cell lymphoma are
associated with distinct pathogenic mechanisms and outcomes. Nat
Med. 24:679–690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Lisio L, Martinez N, Montes-Moreno S,
Piris-Villaespesa M, Sanchez-Beato M and Piris MA: The role of
miRNAs in the pathogenesis and diagnosis of B-cell lymphomas.
Blood. 120:1782–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng B, Xi Z, Liu R, Yin W, Sui Z, Ren B,
Miller H, Gong Q and Liu C: The Function of MicroRNAs in B-cell
development, lymphoma, and their potential in clinical practice.
Front Immunol. 9:9362018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costinean S, Zanesi N, Pekarsky Y, Tili E,
Volinia S, Heerema N and Croce CM: Pre-B cell proliferation and
lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155
transgenic mice. Proc Natl Acad Sci USA. 103:7024–7029. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mihailovich M, Bremang M, Spadotto V,
Musiani D, Vitale E, Varano G, Zambelli F, Mancuso FM, Cairns DA,
Pavesi G, et al: miR-17-92 fine-tunes MYC expression and function
to ensure optimal B cell lymphoma growth. Nat Commun. 6:87252015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song G, Gu L, Li J, Tang Z, Liu H, Chen B,
Sun X, He B, Pan Y, Wang S and Cho WC: Serum microRNA expression
profiling predict response to R-CHOP treatment in diffuse large B
cell lymphoma patients. Ann Hematol. 93:1735–1743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Troppan K, Wenzl K, Pichler M, Pursche B,
Schwarzenbacher D, Feichtinger J, Thallinger GG, Beham-Schmid C,
Neumeister P and Deutsch A: miR-199a and miR-497 are associated
with better overall survival due to increased chemosensitivity in
diffuse Large B-cell lymphoma patients. Int J Mol Sci.
16:18077–18095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Babar IA, Cheng CJ, Booth CJ, Liang X,
Weidhaas JB, Saltzman WM and Slack FJ: Nanoparticle-based therapy
in an in vivo microRNA-155 (miR-155)-dependent mouse model of
lymphoma. Proc Natl Acad Sci USA. 109:E1695–E1704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun MM, Li JF, Guo LL, Xiao HT, Dong L,
Wang F, Huang FB, Cao D, Qin T, Yin XH, et al: TGF-β1 suppression
of microRNA-450b-5p expression: A novel mechanism for blocking
myogenic differentiation of rhabdomyosarcoma. Oncogene.
33:2075–2086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun M, Huang F, Yu D, Zhang Y, Xu H, Zhang
L, Li L, Dong L, Guo L and Wang S: Autoregulatory loop between
TGF-β1/miR-411-5p/SPRY4 and MAPK pathway in rhabdomyosarcoma
modulates proliferation and differentiation. Cell Death Dis.
6:e18592015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo L, Fu J, Sun S, Zhu M, Zhang L, Niu H,
Chen Z, Zhang Y, Guo L and Wang S: MicroRNA-143-3p inhibits
colorectal cancer metastases by targeting ITGA6 and ASAP3. Cancer
Sci. 110:805–816. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu YC, Chang JT, Chan EC, Chao YK, Yeh TS,
Chen JS and Cheng AJ: miR-196, an emerging cancer biomarker for
digestive tract cancers. J Cancer. 7:650–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Zhang Y, Zhang L, Weakley SM and
Yao Q: MicroRNA-196: Critical roles and clinical applications in
development and cancer. J Cell Mol Med. 15:14–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suh YE, Raulf N, Gaken J, Lawler K, Urbano
TG, Bullenkamp J, Gobeil S, Huot J, Odell E and Tavassoli M:
MicroRNA-196a promotes an oncogenic effect in head and neck cancer
cells by suppressing annexin A1 and enhancing radioresistance. Int
J Cancer. 137:1021–1034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang F, Tang J, Zhuang X, Zhuang Y, Cheng
W, Chen W, Yao H and Zhang S: MiR-196a promotes pancreatic cancer
progression by targeting nuclear factor kappa-B-inhibitor alpha.
PLoS One. 9:e878972014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Popovic R, Riesbeck LE, Velu CS, Chaubey
A, Zhang J, Achille NJ, Erfurth FE, Eaton K, Lu J, Grimes HL, et
al: Regulation of mir-196b by MLL and its overexpression by MLL
fusions contributes to immortalization. Blood. 113:3314–3322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong X, Du Y, Wang G, Gao J, Gong Y, Li L,
Zhang Z, Zhu J, Jing Q, Qin Y and Li Z: Detection of differentially
expressed microRNAs in serum of pancreatic ductal adenocarcinoma
patients: miR-196a could be a potential marker for poor prognosis.
Dig Dis Sci. 56:602–609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu YC, Chang JT, Liao CT, Kang CJ, Huang
SF, Chen IH, Huang CC, Huang YC, Chen WH, Tsai CY, et al:
OncomiR-196 promotes an invasive phenotype in oral cancer through
the NME4-JNK-TIMP1-MMP signaling pathway. Mol Cancer. 13:2182014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Huang S, Wu B, Fang J, Zhu M, Sun
L, Zhang L, Zhang Y, Sun M, Guo L and Wang S: Transforming growth
factor-β1 promotes breast cancer metastasis by downregulating
miR-196a-3p expression. Oncotarget. 8:49110–49122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao Y, Medeiros LJ, Xu-Monette ZY, Li J
and Young KH: Dysregulation of cell survival in diffuse large B
cell lymphoma: Mechanisms and therapeutic targets. Front Oncol.
9:1072019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson WH, Young RM, Schmitz R, Yang Y,
Pittaluga S, Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano
J, et al: Targeting B cell receptor signaling with ibrutinib in
diffuse large B cell lymphoma. Nat Med. 21:922–926. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu FQ, Zeng L, Tang N, Tang YP, Zhou BP,
Li FF, Wu WG, Zeng XB and Peng SS: MicroRNA-155 downregulation
promotes cell cycle arrest and apoptosis in diffuse large B-cell
lymphoma. Oncol Res. 24:415–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan Q, Meng X, Liang H, Zhang H, Liu X, Li
L, Li W, Sun W, Zhang H, Zen K, et al: miR-10a inhibits cell
proliferation and promotes cell apoptosis by targeting BCL6 in
diffuse large B-cell lymphoma. Protein Cell. 7:899–912. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia YJ, Liu ZB, Wang WG, Sun CB, Wei P,
Yang YL, You MJ, Yu BH, Li XQ and Zhou XY: HDAC6 regulates
microRNA-27b that suppresses proliferation, promotes apoptosis and
target MET in diffuse large B-cell lymphoma. Leukemia. 32:703–711.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donaldson JG and Jackson CL: ARF family G
proteins and their regulators: Roles in membrane transport,
development and disease. Nat Rev Mol Cell Biol. 12:362–375. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo IS, Eun SY, Jang HS, Kang ES, Kim GH,
Kim HJ, Lee JH, Chang KC, Kim JH, Han CW and Seo HG: Identification
of ADP-ribosylation factor 4 as a suppressor of N-(4-hydroxyphenyl)
retinamide-induced cell death. Cancer Lett. 276:53–60. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Q, Ren X, Zhang Y, Fu X, Li Y, Peng Y,
Xiao Q, Li T, Ouyang C, Hu Y, et al: MiR-221-3p targets ARF4 and
inhibits the proliferation and migration of epithelial ovarian
cancer cells. Biochem Biophys Res Commun. 497:1162–1170. 2018.
View Article : Google Scholar : PubMed/NCBI
|