Introduction
Worldwide, pancreatic cancer is the seventh major
cause of cancer-related deaths in both males and females (1). Economically developed countries
experience a higher incidence of pancreatic cancer cases and higher
mortality rates than developing countries (2). In the United States, it was estimated
that by the end of 2019, there would be 56,770 new pancreatic
cancer cases (males: 29,940; females: 26,830), and 45,750
pancreatic cancer-related deaths (males: 23,800; females: 21,950)
(2). Despite the developments in
screening methods and advances in cancer drug discovery approaches,
new pancreatic cancer cases are predicted to rise within the next
decade (3). Based on histological
and molecular characteristics, pancreatic cancer can be classified
into two major subtypes, namely, exocrine (pancreatic
adenocarcinoma) and endocrine tumors (3). Although molecular and histological
subtyping helps to determine treatment plans in other human
cancers, treatment plans for pancreatic cancer do not necessarily
depend on the pancreatic cancer subtype (4). Radiotherapy, chemotherapy, and surgery
are currently used to extend the survival of pancreatic cancer
patients (3,5). However, radiotherapy and chemotherapy
treatments are ineffective for advanced-stage pancreatic cancer.
Gemcitabine is a commonly used chemotherapy for all stages of
pancreatic cancer (6,7). Development of resistance to
gemcitabine has been identified as a major clinical barrier in
pancreatic cancer treatment, thereby limiting the clinical efficacy
of this drug. Surgical removal of the pancreas or surrounding
metastatic tumors has been reported to cause some digestive issues
and functional loss in pancreatic cancer patients (8). Therefore, there is an urgent necessity
to explore new anticancer therapeutics, which can effectively
target pancreatic cancer cells without causing adverse side
effects.
Various plant extracts and plant-derived secondary
metabolites have been used to treat a number of diseases and have
been reported to exert a range of pharmacological effects (9). In recent anticancer drug discovery
approaches, great effort has been invested in attempts to identify
plant-derived secondary metabolites as novel anticancer
therapeutics as they exert favorable therapeutic efficacies over
synthetic anticancer drugs (10).
Catechol (pyrocatechol) is a secondary metabolite found in some
fruits and vegetables (11,12). Catechol is chemically synthesized in
large quantities to be used as a precursor in the pharmaceutical
industry. Some phytochemicals with a catechol moiety, such as
fisetin, luteolin, procyanidin B2, quercetin, and
7,3′,4′-trihydroxyisoflavone have been revealed to exert anticancer
effects in several in vitro models (12). The anticancer efficacy of pure
catechol has been reported in lung cancer and breast cancer stem
cells (11,12). However, to the best of our
knowledge, in vitro anticancer effects of pure catechol in
pancreatic cancer cells have not been explored yet. Therefore, the
present study aimed to explore the possible anti-carcinogenic and
radio-sensitizing effects of catechol in pancreatic cancer cells
in-vitro.
Materials and methods
Materials
Cell culture reagents used in this investigation
were purchased from Invitrogen; Thermo Fisher Scientific, Inc. All
other chemicals used were purchased from Sigma-Aldrich; Merck KGaA
unless otherwise stated. Primary antibodies [Bax (product no.
2774), Bcl-2 (product no. 2872), cleaved caspase-3 (product no.
9664), cleaved poly(ADP-ribose) polymerase (PARP) (cat. no. 5625S),
GAPDH (product no. 5174S), phosphorylated-signal transducer and
activator of transcription 3 (p-STAT3) (product no. 9145S), STAT-3
(product no. 9139S), matrix metalloproteinase-2 (MMP2) (product no.
13132S), Snail (product no. 3879S), vimentin (product no. 5741S),
p-AMP-activated protein kinase (p-AMPKα) (product no. 2535S), AMPKα
(product no. 2532S), Yes-associated protein (YAP) (product no.
4912S), cysteine-rich angiogenic inducer 61 (Cyr61) (product no.
14479S), connective tissue growth factor (CTGF) (product no.
10095S), p-ataxia telangiectasia mutated kinase (p-ATM) (product
no. 13050S), and p-checkpoint kinase 2 (p-Chk2) (product no. 2661S)
were purchased from Cell Signaling Technology. The primary antibody
MMP9 was purchased from EMD Millipore (product no. AB19016).
Secondary antibodies [goat anti-rabbit (cat. no. PI-1000-1) and
horse anti-mouse immunoglobin (Ig)G (cat. no. PI-2000-1)] were
purchased from Vector Laboratories, Inc. The BS ECL Plus kit (cat.
no. W6002) was purchased from Biosesang, Inc.
Cell culture
Four human pancreatic cancer cell lines, Aspc-1,
MiaPaCa2, Panc-1, and SNU-213, were obtained from the laboratory of
Professor Jae-Hoon Kim at the Faculty of Biotechnology, Jeju
National University, Republic of Korea. Cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum and an antibiotic cocktail (100 µg/ml
streptomycin and 100 U/ml penicillin) and incubated at 37°C under
5% CO2. Dermal fibroblasts (13) were cultured in DMEM under similar
culture conditions. Cells were sub-cultured upon 80%
confluency.
Cell viability assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT)
assay was used as the cell viability assay in the present
investigation. Prior to the MTT assay, four types of pancreatic
cancer cells (Aspc-1, MiaPaCa2, Panc-1 and SNU-213) were cultured
in 96-well culture plates (5×103 cells/well) and
incubated for 48 h at 37°C. Following incubation, the cells were
exposed to different doses (12.5–200 µM) of catechol and incubated
again for 48 h at 37°C. In chemosensitivity evaluation experiments
of catechol, Panc-1 cells were co-treated with catechol (50 or 100
µM) and gemcitabine (0.25–1 µM) for 48 h. The MTT assay was then
conducted to assess cell viability. Dimethyl sulfoxide (DMSO) was
used to dissolve purple formazan and the absorbance was recorded at
540 nm using a micro-plate reader (Sunrise; Tecan Group, Ltd.). The
percentage of cell viability was determined using the formula:
[(AT-AB)/(AC-AB)] ×100,
where AT denotes the absorbance value of the treated
group, AB denotes the absorbance value of the blank, and
AC denotes the absorbance value of the untreated
control. GraphPad Prism 7.00 (GraphPad Software, Inc.) was used to
determine the half maximum inhibitory concentration
(IC50) of catechol in each pancreatic cancer cell
line.
Colony formation assay
Prior to the colony formation assay, Panc-1 cells
(200 cells/dish) were seeded in cell culture dishes and exposed to
catechol alone, or to a combination of catechol (50 µM) and
ionizing radiation (2 or 4 Gy). After 10 days, the cells were fixed
with 4% formaldehyde at room temperature for 30 min, stained with
crystal violet (0.5% crystal violet), incubated at room temperature
for 30 min and washed. NIST's Integrated Colony Enumerator software
(https://www.nist.gov/services-resources/software/nists-integrated-colony-enumerator-nice)
was used to count the number of colonies in the culture dishes.
Irradiation was carried out at the Applied Radiological Science
Institute at Jeju National University using a 60CO
Theratron-780 teletherapy unit.
Wound healing assay
Panc-1 cells (1×105/well) were cultured
in 6-well plates and incubated until the cells reached confluence.
A uniform scratch was then made through the cell monolayer using a
sterilized 200-µl pipette tip. After washing with
phosphate-buffered saline (PBS), different doses (25 or 50 µM) of
catechol were added to the wells with DMEM containing 5% FBS
(14), and the cells were incubated
for 24 h. Following incubation, the width of the wound was measured
under a phase-contrast microscope (×100).
Invasion assay
Prior to the invasion assay, cells were exposed to
different concentrations (12.5, 25, or 50 µM) of catechol and
incubated for 24 h. For this assay, a Transwell system (24-well
plate; 0.2 µm pore; Corning, Inc.) was used. The upper chambers
were coated with 1% Matrigel by incubating at 37°C for 1 h. The
upper chambers received 200 µl of DMEM and 1×105 cells
(without FBS) supplemented with or without catechol (12.5, 25 and
50 µM), while the lower chambers received DMEM supplemented with
10% FBS. Following 24 h of incubation at 37°C, invading Panc-1
cells were first fixed with methanol and then 4% paraformaldehyde
at room temperature for 30 min, stained with 2% crystal violet for
30 min at room temperature, washed with distilled water and
observed under a phase contrast microscope (×100).
Hoechst staining
Panc-1 cells (5×104/well) were cultured
on coverslips and incubated for 24 h. Following incubation cells
were exposed to catechol for 48 h. Cells were then fixed with 4%
formaldehyde for 30 min at room temperature and stained with
Hoechst 33342 solution (0.01 mg/ml) for 30 min at room temperature.
Stained Panc-1 cells were observed and images were captured under a
fluorescence microscope (×100) (IX73; Olympus Corporation).
Flow cytometry
The effects of catechol on the cell cycle and
apoptosis were investigated using flow cytometry at the Bio-Health
Materials Core-Facility at Jeju National University. Flow
cytometric analysis was performed with BD FACSDiva™ Software (BD
Biosciences). Prior to the analysis, the cells (3×104
cells) were washed twice with PBS and fixed in 70% ethanol for 30
min at 37°C. Following fixation, the cells were treated with RNase
A (25 ng/ml) and staining with propidium iodide (40 µg/ml) and
subjected to cell cycle analysis. The Annexin V-FITC Apoptosis
Detection Kit (BD Biosciences) was used according to the
manufacturer's instructions for apoptosis analysis.
Western blot analysis
Following treatments, radioimmunoprecipitation assay
buffer (RIPA; Thermo Fisher Scientific, Inc.) was used to extract
cell lysates. The protein concentrations were determined using a
BCA assay. Upon quantification of proteins, 40 µg of proteins were
electrophoresed (10-12.5% gels) and transferred to PVDF membranes.
After blocking with 5% dried non-fat milk overnight at 4°C,
membranes were exposed to different primary antibodies overnight at
4°C. Most of the primary antibodies and the horseradish
peroxidase-conjugated (HRP) goat anti-rabbit IgG secondary
antibodies were diluted prior to the experiments (primary
antibodies to 1:1,000 and secondary antibodies to 1:5,000). GAPDH
or β-actin (reference protein) was diluted to 1:10,000 prior to the
experiments. Protein bands were detected using the BS ECL-Plus kit
(Biosesang, Inc.), and the ImageJ software (version 1.48; National
Institutes of Health) was used to quantify bands.
Statistical analysis. Group comparisons were
performed using the paired Student's t-test and one-way analysis of
variance (with Duncan's post hoc test) with SPSS software (ver.
18.0; IBM Corp.). P-values <0.05 were considered to indicate a
statistically significant difference.
Results
Catechol inhibits proliferation and
induces apoptosis in Panc-1 cells
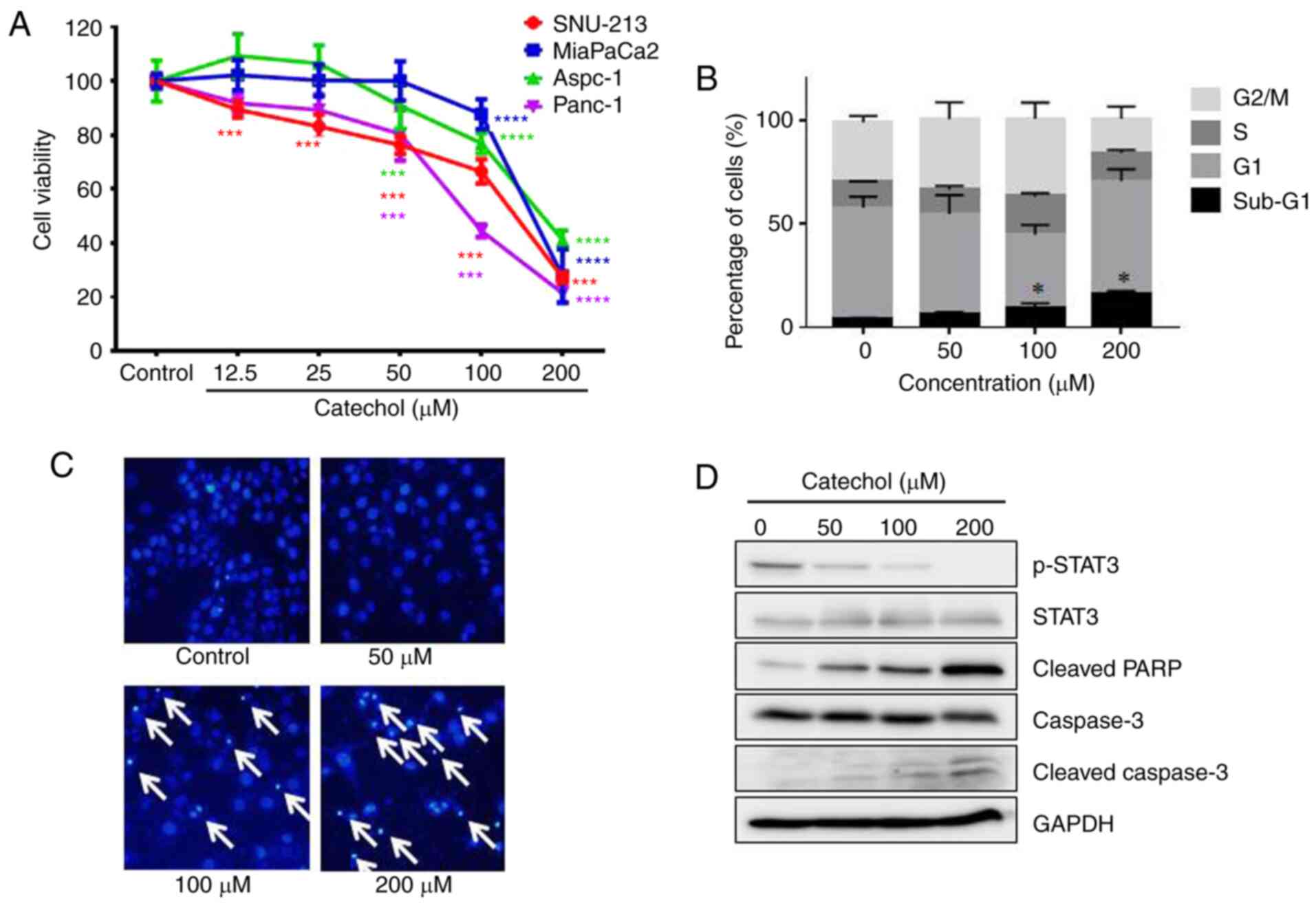
The MTT assay was used to evaluate the effects of
catechol on pancreatic cancer cell proliferation. As revealed in
Fig. 1A, catechol inhibited the
proliferation of pancreatic cancer cell lines (Aspc-1, MiaPaCa-2,
Panc-1 and SNU-213) in a dose-dependent manner at 48 h
post-incubation. Catechol had the greatest anti-proliferative
effect (IC50: 91.71±5.14 µM) on Panc-1 cells among the
four pancreatic cancer cell lines tested. As catechol exerted the
strongest anti-proliferative effect on Panc-1 cells, this cell line
was selected for other experiments in the present study. Moreover,
it has been demonstrated that Panc-1 cells are resistant to
gemcitabine, a commonly used chemotherapeutic drug in pancreatic
cancer treatments, compared to Aspc-1 cells (15). Catechol displayed an IC50
value of 162.6±15.6 µM in dermal fibroblasts following 48 h of
exposure (Fig. S1). It was then
assessed whether the anti-proliferative effects of catechol were
associated with Panc-1 cell cycle arrest. As revealed in Fig. 1B, catechol treatment caused the
sub-G1 population to increase, from 4.27±0.31% (0 µM) to
16.33±1.13% (200 µM). Moreover, as revealed in Fig. 1C, morphological changes associated
with apoptosis such as condensed and fragmented chromatin and
apoptotic bodies (arrows) were evident in catechol-treated (50, 100
and 200 µM) Panc-1 cells. Next, we assessed the effects of catechol
on the expression of apoptosis-associated proteins (cleaved PARP,
caspase-3, and cleaved caspase-3) and STAT3 via western blot
experiments. The data revealed that catechol increased cleaved PARP
and cleaved caspase-3 protein levels, confirming the
apoptosis-inducing effects of catechol on Panc-1 cells (Fig. 1D). Catechol also decreased the
phosphorylation of STAT3 in a dose-dependent manner (Fig. 1D). The transcription factor STAT3
has been reported to play a key role in tumorigenesis (11). This observation is also consistent
with findings from a previous study, which reported that catechol
inhibited STAT3 activation in breast cancer stem cells (11). Collectively, the results
demonstrated that catechol inhibited the proliferation of Panc-1
cells and promoted apoptosis through the inactivation of STAT3, one
of the known targets of apoptosis (16).
Catechol suppresses EMT
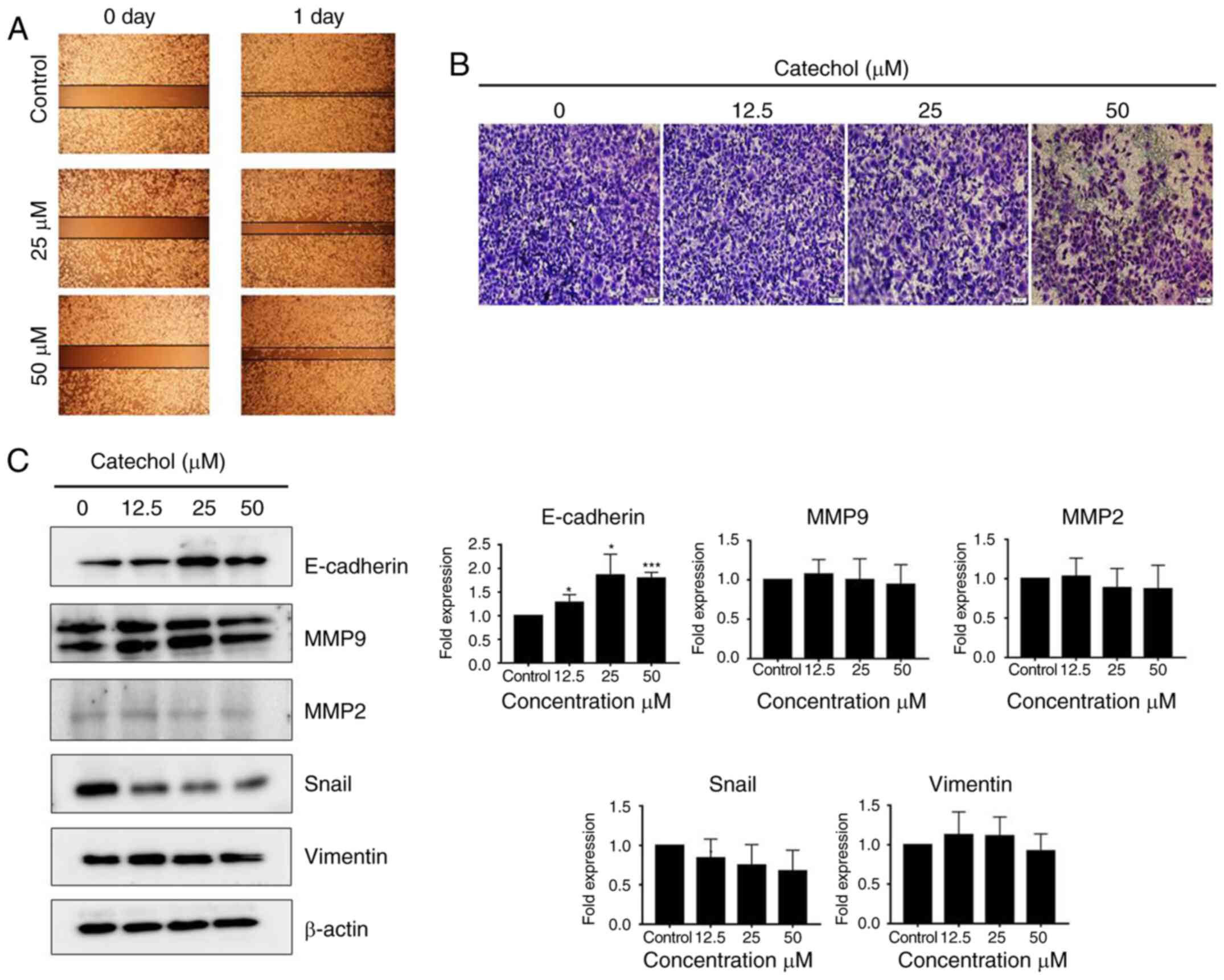
Next, two marginally cytotoxic doses (12.5 and 50
µM) of catechol were selected for migration and invasion assays to
examine whether catechol could suppress EMT in Panc-1 cells. The
results of the migration and invasion assays revealed that catechol
reduced cell migration (Fig. 2A)
and invasion (Fig. 2B) in Panc-1
cells at 24 h post-incubation. Furthermore, catechol treatment
caused an increase in the expression levels of E-cadherin protein
along with a gradual decrease in the expression of two EMT markers,
Snail and vimentin, in Panc-1 cells (Fig. 2C). MMP2 expression was also
decreased following catechol exposure in Panc-1 cells (Fig. 2C). The expression of MMP9 was
slightly affected by catechol exposure (Fig. 2C). These data indicated that
catechol suppressed Panc-1 cell migration and invasion by reducing
the expression of EMT-related proteins.
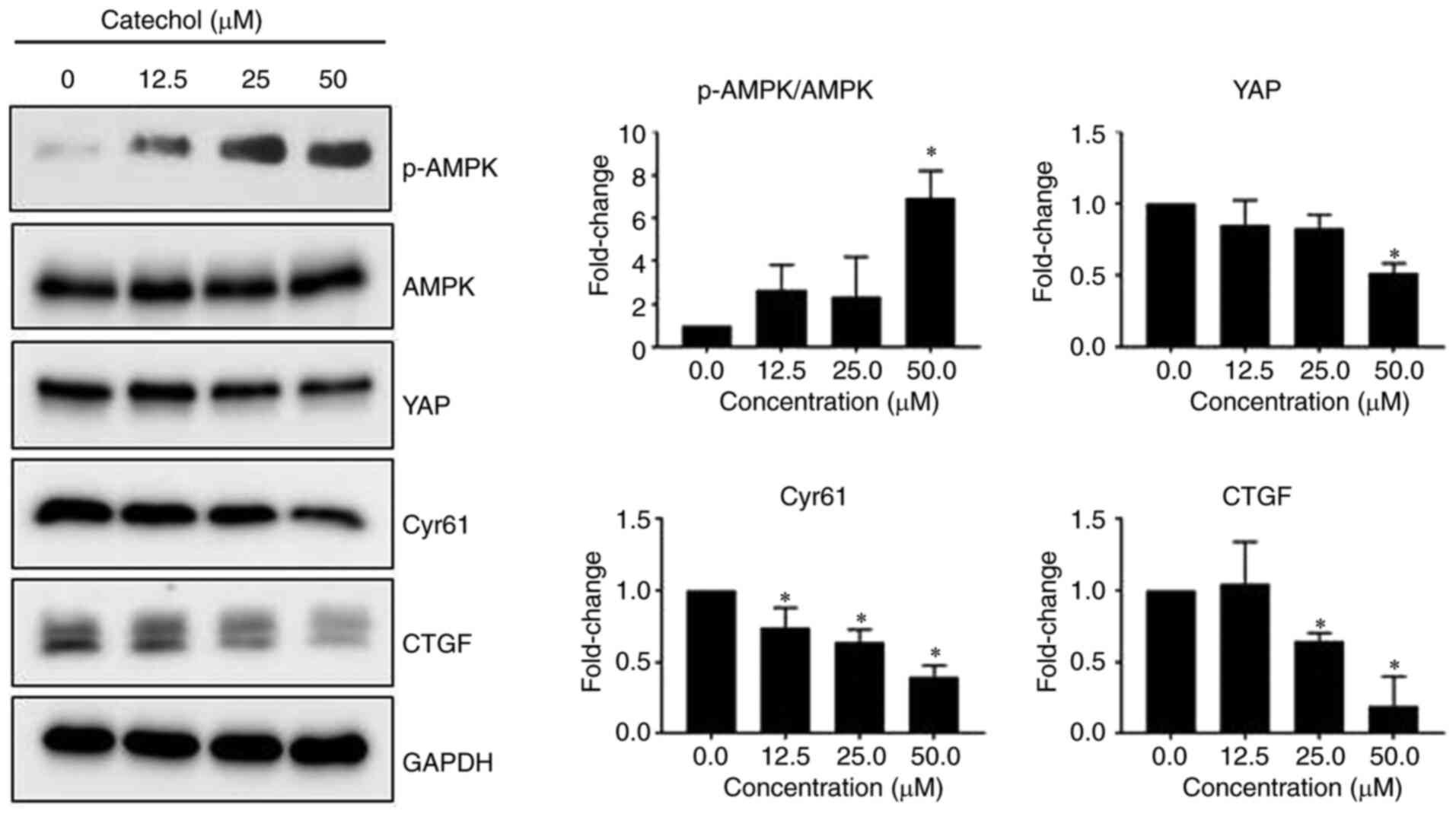
Catechol inhibits the AMP-activated
protein kinase (AMPK)/Hippo signaling pathway
The Hippo signaling pathway has been reported to
drive tumorigenesis and play a role in developing chemo- and
radio-resistance in a range of human cancers (17). YAP is a transcription co-activator
in the Hippo signaling pathway (17), and both CYR61 and CTGF are major
downstream targets of YAP (18). In
addition, activated AMPK has been reported to be associated with
YAP inhibition (19). Therefore,
the regulatory effects of catechol on the AMPK/Hippo signaling
pathway in Panc-1 cells were investigated. It was observed that
catechol induced the phosphorylation of AMPK in a dose-dependent
manner with a concomitant decrease in YAP, CYR61, and CTGF protein
levels (Fig. 3), indicating that
catechol could effectively target oncogenic Hippo signaling in
Panc-1 cells.
Catechol enhances the chemosensitivity
of Panc-1 cells to gemcitabine
To assess whether catechol enhances the in
vitro cytotoxic efficacy of gemcitabine. Cell viability was
then analyzed using an MTT assay. Combining catechol and
gemcitabine resulted in the reduction of Panc-1 cell viability
compared to the gemcitabine treatment alone (Fig. 4A). To investigate whether catechol
and gemcitabine had synergistic cytotoxic effects in Panc-1 cells,
combination index (CI) values were calculated. The CI values for
drug combinations in Panc-1 cells ranged from 0.43 to 0.68.
Combined treatment comprising 50 µM of catechol and 0.25 µM of
gemcitabine exerted the highest synergistic inhibitory effects with
a CI value of 0.43 (Fig. 4B). In
addition, as revealed in Fig. 4C,
condensed and fragmented chromatin and apoptotic bodies (arrows)
were prominent in the combined-treatment groups. As the combined
treatment (50 µM of catechol and 0.25 µM of gemcitabine)
demonstrated the highest synergistic inhibitory effects, this
combination was used in the following experiments to explore the
mechanisms by which catechol synergizes with gemcitabine in Panc-1
cells. Cell cycle analysis revealed that sub-G1 populations of
Panc-1 cells exposed to catechol and gemcitabine increased in a
dose-dependent manner (Fig. 4D).
The largest sub-G1 population (10.05±4.40%) of Panc-1 cells was
observed in the treatment combining 50 µM of catechol and 0.25 µM
of gemcitabine (Fig. 4D).
Furthermore, the combined treatment increased p-AMPK expression,
cleavage of PARP, and the Bax/Bcl-2 ratio, as well as decreased
YAP, CYR61, and p-STAT3 levels (Fig.
4E). Based on these observations, it was concluded that 50 µM
of catechol can enhance the in vitro cytotoxic efficacy of
gemcitabine and promote gemcitabine-induced apoptosis in Panc-1
cells.
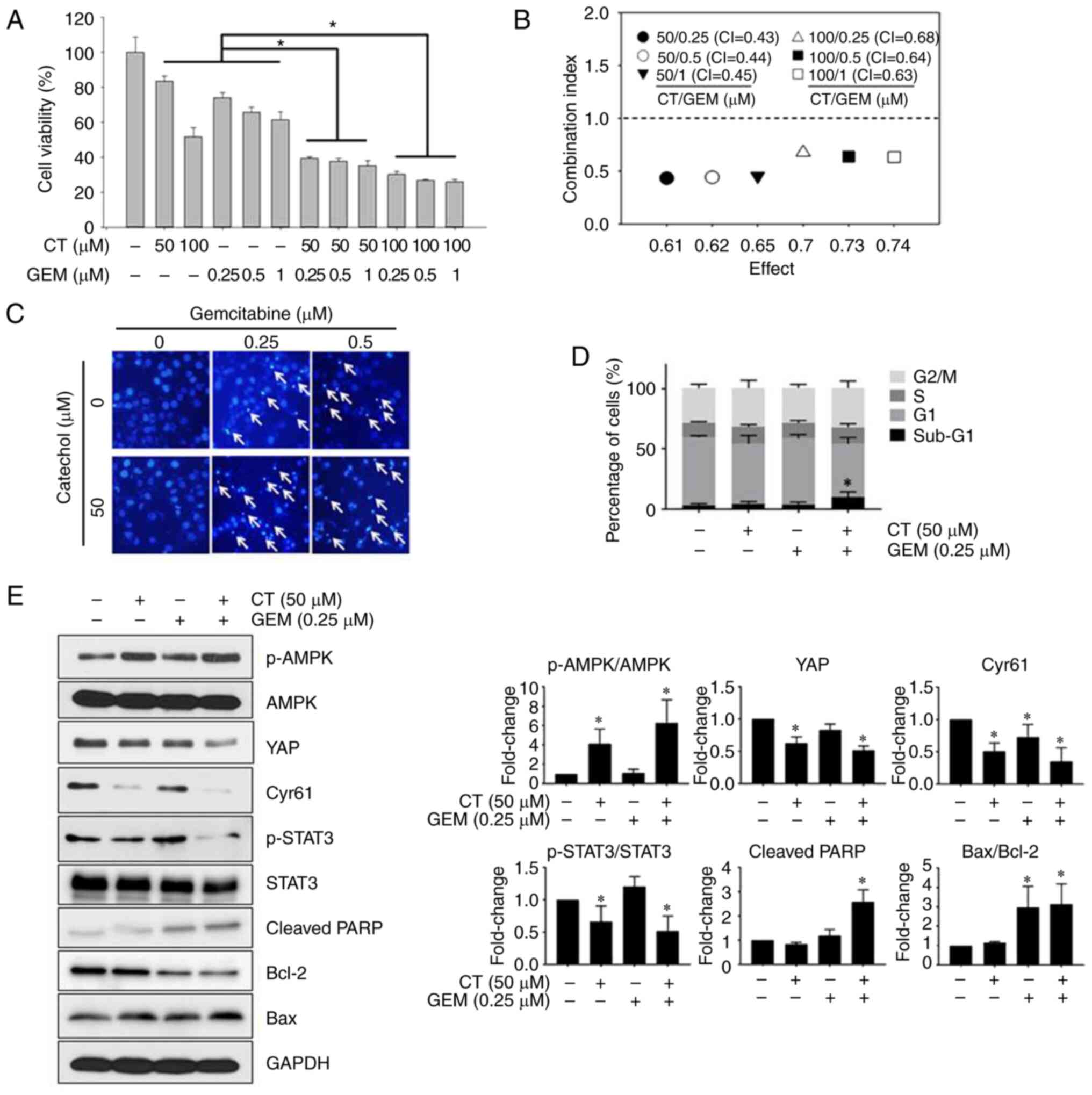 | Figure 4.Catechol enhances the
chemosensitivity of Panc-1 cells to gemcitabine. (A) Synergistic
anti-proliferative effects of catechol and gemcitabine in Panc-1
cells as measured by the MTT assay. (B) CI values calculated for
treatments exposing Panc-1 cells to combinations of various
concentrations of catechol and gemcitabine. (C and D) Following
exposure to 50 µM of catechol, 0.25 µM of gemcitabine, 0.5 µM of
gemcitabine, or catechol plus gemcitabine for 48 h, Hoechst 33342
staining and cell cycle analysis were carried out. (E) Protein
levels were examined using western blot analysis after exposing
Panc-1 cells to catechol. GAPDH was used as the loading control.
Band intensities were measured using ImageJ software (version
1.48). Values represent the means ± SDs (n=3). *P<0.05 vs. the
control. CT, catechol; GEM, gemcitabine; CI, combination index;
SDs, standard deviations; AMPK, AMP-activated protein kinase; p-,
phosphorylated; YAP, yes-associated protein; CYR61, cysteine-rich
angiogenic inducer 61; CTGF, connective tissue growth factor; PARP,
poly(ADP-ribose) polymerase. |
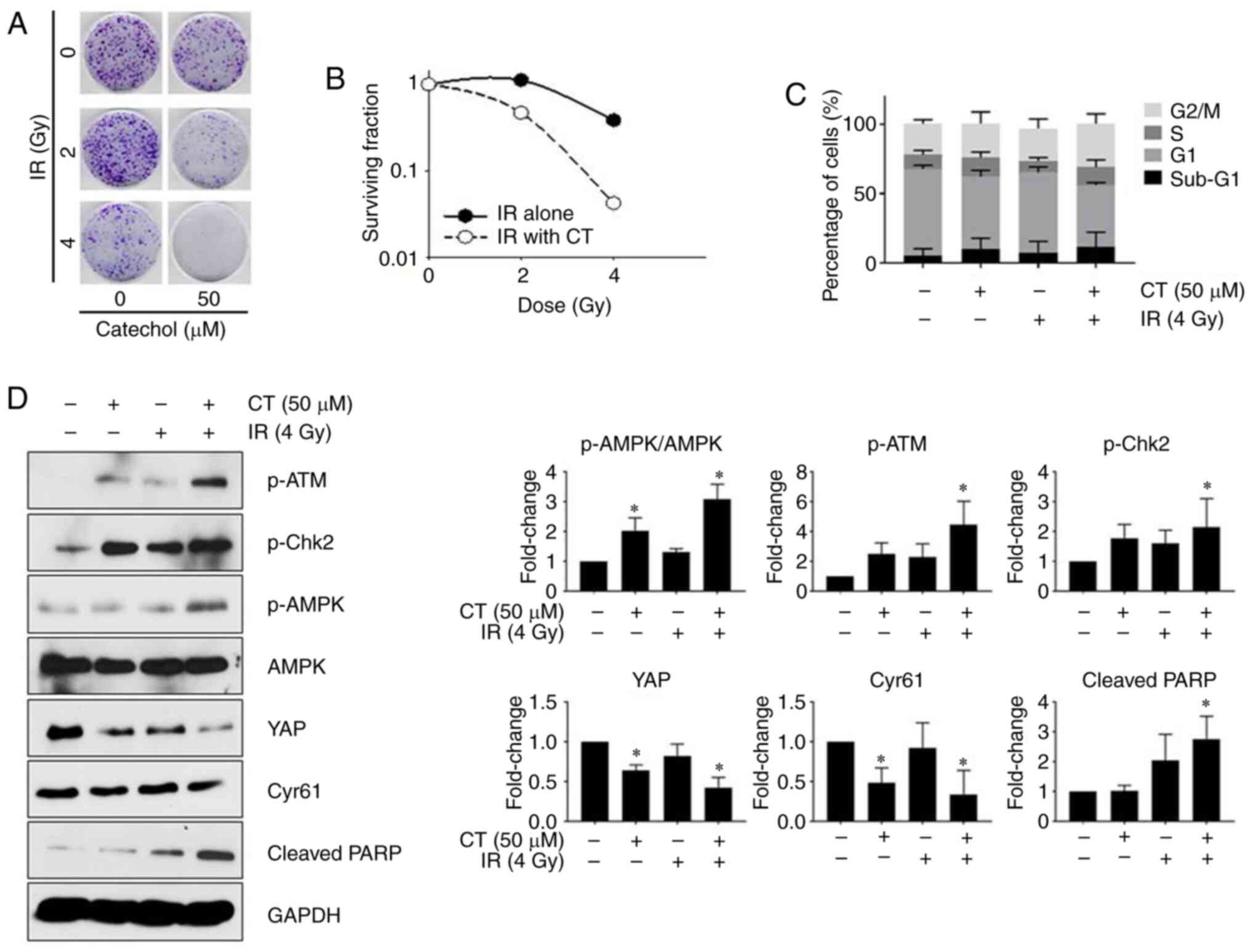
Catechol enhances the radiosensitivity
of Panc-1 cells
The response of Panc-1 cells to radiation treatment
in the presence or absence of catechol was examined by performing a
colony formation assay. Results of the colony formation assay
revealed that Panc-1 cell survival rates markedly decreased after
exposure to catechol and radiation treatments (Fig. 5A). The linear-quadratic model was
applied to generate radiosensitivity curves for each experimental
group to represent the results of the colony formation assay
(Fig. 5B). Catechol-treated cells
were revealed to be more sensitive to radiation compared to control
cells (Fig. 5B). Cell cycle
analysis (Fig. 5C) revealed that
combined catechol-exposure (50 µM) and radiation treatment (4 Gy)
increased the sub-G1 population of Panc-1 cells compared to the
control (radiation only). Furthermore, western blotting revealed
that the co-treatment group (50 µM catechol and 4 Gy radiation
dose) had higher expression levels of p-ATM, p-Chk2, and cleaved
PARP, known markers for radiation-induced DNA damage (20), compared to the untreated control
(Fig. 5D). Increased AMPK levels
have been reported to be associated with enhanced radiosensitivity
(21). In the present results,
increased p-AMPK levels in the combined treatment group were
observed, indicating that catechol could promote radiosensitivity
by activating p-AMPK. Moreover, the expression of YAP and CYR61 was
reduced in the combined treatment group (Fig. 5D). These results indicated that
catechol enhanced the effects of radiation treatments in Panc-1
cells.
Discussion
Pancreatic cancer is expected to become the third
major cause of cancer-related deaths, exceeding deaths due to
breast cancer, in most developed countries (22). Although chemotherapy, radiotherapy,
and surgery can control pancreatic cancer metastasis to some
extent, there is still no permanent cure. Clinically, resistance to
gemcitabine after treatment initiation has been identified as a
major therapeutic drawback in pancreatic cancer treatments
(6,7). In several pancreatic cancer clinical
studies, the combination of gemcitabine with other
chemotherapeutics has been revealed to improve the overall efficacy
of gemcitabine vs. using the drug on its own (23,24).
Radiation therapy is often used in adjuvant or neoadjuvant settings
in pancreatic cancer treatment. As in chemotherapy, adverse side
effects and development of radiotherapy resistance have been
identified as major clinical issues (3,5).
Numerous herbal remedies have been used to treat
various diseases and conditions for centuries. Some studies have
revealed that numerous cancer patients use herbal medicine as
complementary and alternative therapies (25). Various plant-derived herbal drugs or
compounds have been reported to improve the efficacy of
chemotherapeutics and decrease adverse side effects in cancer
patients (25). Catechol
(pyrocatechol) is a natural compound found in some fruits and
vegetables. Lim et al (12)
reported that catechol could target the ERKs/c-Myc signaling
pathway in lung cancer in vitro and in vivo. Another
study by Choi et al (11)
reported that catechol targets breast cancer stem cells by
dysregulating the Stat3/IL-6 survival pathway. In the present
study, it was revealed that catechol was most effective at
inhibiting the proliferation of Panc-1 cells among the four
pancreatic cancer cell lines tested. Moreover, it was determined
that catechol induced Panc-1 cell cycle arrest at the sub-G1 phase
in a dose-dependent manner. In addition, western blot experiments
confirmed that catechol could induce apoptosis in Panc-1 cells. EMT
plays a key role in tumorigenesis and the development of chemo- and
radio-therapy resistance (26). It
was revealed that catechol reduced cell migration and invasion of
Panc-1 cells and suppressed the expression of EMT-related markers
such as Snail, vimentin, and MMP2, indicating the ability of
catechol to inhibit Panc-1 cell migration and invasion by reducing
the expression of EMT-associated proteins. The Hippo signaling
pathway plays a regulatory role in tumorigenesis (27). The transcription co-activator YAP in
Hippo signaling is often overexpressed in a range of human cancers,
making it an attractive pharmacological target 27). Activated
AMPK-mediated YAP inhibition has been reported in previous
investigations (19,28). Moreover, studies have revealed that
phytochemicals such as curcumin and resveratrol can target YAP in
breast and bladder cancer cells (29,30).
In the present investigation, catechol was revealed to increase the
expression of p-AMPK with a concomitant decrease in the levels of
CTGF and CYR61, two main downstream targets of YAP in Hippo
signaling, suggesting that catechol can function as a new drug lead
that targets Hippo signaling in Panc-1 cells.
As resistance to gemcitabine is frequently observed
in pancreatic cancer patients, new approaches to improve the
clinical efficacy of gemcitabine in pancreatic cancer treatments
should be explored. Treatment using chemotherapeutics combined with
natural drugs has been reported to enhance the overall efficacy of
chemotherapy treatments as well as minimize adverse side effects
(31). In the present study, it was
demonstrated that catechol enhanced both the chemosensitivity of
Panc-1 cells to gemcitabine and gemcitabine-induced apoptosis,
which were confirmed by cell viability assays, flow cytometry, and
western blot experiments. A decreased sensitivity of pancreatic
tumors to radiation therapy significantly influences the life
expectancy of pancreatic cancer patients (6,7).
Several natural radio-sensitizers have been revealed to enhance the
overall efficacy of radiation treatment in a range of human cancers
(32). Notably, it was observed
that catechol improved radiosensitivity in Panc-1 cells. Moreover,
the results of western blot experiments confirmed that radiation
therapy along with catechol increased the expression of
radiation-induced DNA-damage markers, including p-ATM, p-Chk2,
p-AMPK, and cleaved PARP, in Panc-1 cells, indicating the potential
use of catechol/radiation combined therapy for pancreatic cancer
patients. Considering the importance of ATM serine/threonine kinase
(ATM) in DNA double strand breaks (DSBs) in response to IR, the
effects of catechol on the expression of ATM-associated pathway
proteins were assessed. Activation of ATM occurs by
auto-phosphorylation at Ser1981 upon DNA damage induced by IR.
Following DNA damage, ATM can phosphorylate Chk2 at Thr68 (33). Hence, considering ATM and Chk2 as
markers for DNA damage in response to IR, the activated forms
(phosphorylated forms) of ATM and Chk2 were only used (33).
In conclusion, catechol induced apoptosis and cell
cycle arrest and suppressed the expression of EMT-related markers
in Panc-1 cells. Above all, catechol enhanced the chemosensitivity
of Panc-1 cells to gemcitabine and induced radiation sensitization
in Panc-1 cells, indicating that catechol can play a prominent role
in overcoming chemo- and radio-resistance, a major clinical
challenge in pancreatic cancer. Additionally, catechol was revealed
to modulate the AMPK/Hippo signaling pathway in pancreatic cancer
cells. Results of the present study provide a strong rationale for
exploring the clinical efficacy of catechol in the treatment of
pancreatic cancer. We are currently investigating the in
vivo efficacy of catechol in nude mice xenografts and the
results will be published in future. Moreover, we are currently
planning to initiate new experiments to investigate the effects of
catechol to assess the loss- and gain-of-function of STAT-3.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present research was supported by the 2020
Scientific Promotion Program funded by Jeju National University,
the National Research Foundation of Korea (NRF) grant no.
2020R1A2C1004349, and by the Basic Science Research Program through
the NRF funded by the Ministry of Education (grant no.
2016R1A6A1A03012862).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JYM and SKC designed the study. JYM, MKE, JYR and
HYK performed experiments. JYM and MKE analyzed the data and wrote
the manuscript. SKC supervised the study and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMPK
|
AMP-activated protein kinase
|
|
CI
|
combination index
|
|
CTGF
|
connective tissue growth factor
|
|
CYR61
|
cysteine-rich angiogenic inducer
61
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
PI
|
propidium iodide
|
|
YAP
|
yes-associated protein
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collisson EA, Bailey P, Chang DK and
Biankin AV: Molecular subtypes of pancreatic cancer. Nat Rev
Gastroenterol Hepatol. 16:207–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amrutkar M and Gladhaug IP: Pancreatic
cancer chemoresistance to gemcitabine. Cancers (Basel). 9:1572017.
View Article : Google Scholar
|
|
7
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strobel O, Neoptolemos J, Jaeger D and
Buechler MW: Optimizing the outcomes of pancreatic cancer surgery.
Nat Rev Clin Oncol. 16:11–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: In vitro assays and techniques utilized in anticancer drug
discovery. J Appl Toxicol. 39:38–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi HS, Kim JH, Kim SL, Deng HY, Lee D,
Kim CS, Yun BS and Lee DS: Catechol derived from aronia juice
through lactic acid bacteria fermentation inhibits breast cancer
stem cell formation via modulation Stat3/IL-6 signaling pathway.
Mol Carcinog. 57:1467–1479. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim DY, Shin SH, Lee MH, Malakhova M,
Kurinov I, Wu Q, Xu J, Jiang Y, Dong Z, Liu K, et al: A natural
small molecule, catechol, induces c-Myc degradation by directly
targeting ERK2 in lung cancer. Oncotarget. 7:35001–35014. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tran TA, Ho MT, Song YW, Cho M and Cho SK:
Camphor induces proliferative and anti-senescence activities in
human primary dermal fibroblasts and inhibits UV-induced wrinkle
formation in mouse skin. Phytother Res. 12:1917–1925. 2015.
View Article : Google Scholar
|
|
14
|
Wang T, Luo R, Li W, Yan H, Xie S, Xiao W,
Wang Y, Chen B, Bai P and Xing J: Dihydroartemisinin suppresses
bladder cancer cell invasion and migration by regulating KDM3A and
p21. J Cancer. 11:11152020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tadros S, Shukla SK, King RJ, Gunda V,
Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, et al:
De novo lipid synthesis facilitates gemcitabine resistance through
endoplasmic reticulum stress in pancreatic cancer. Cancer Res.
77:5503–5517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao B, Lei QY and Guan KL: The Hippo-YAP
pathway: New connections between regulation of organ size and
cancer. Curr Opin Cell Biol. 20:638–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mo JS, Yu FX, Gong R, Brown JH and Guan
KL: Regulation of the Hippo-YAP pathway by protease-activated
receptors (PARs). Genes Dev. 26:2138–2143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mo JS, Meng Z, Kim YC, Park HW, Hansen CG,
Kim S, Lim DS and Guan KL: Cellular energy stress induces
AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell
Biol. 17:500–510. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu PC, Gopinath RK, Hsueh YA and Shieh
SY: CHK2-mediated regulation of PARP1 in oxidative DNA damage
response. Oncogene. 38:1166–1182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adeberg S, Bernhardt D, Harrabi SB,
Nicolay NH, Hörner-Rieber J, König L, Repka M, Mohr A, Abdollahi A,
Weber KJ, et al: Metformin enhanced in vitro radiosensitivity
associates with G2/M cell cycle arrest and elevated
adenosine-5′-monophosphate-activated protein kinase levels in
glioblastoma. Radiol Oncol. 51:431–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferlay J, Partensky C and Bray F: More
deaths from pancreatic cancer than breast cancer in the EU by 2017.
Acta Oncol. 55:1158–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Infante JR, Somer BG, Park JO, Li CP,
Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K and
Le N: A randomised, double-blind, placebo-controlled trial of
trametinib, an oral MEK inhibitor, in combination with gemcitabine
for patients with untreated metastatic adenocarcinoma of the
pancreas. Eur J Cancer. 50:2072–2081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Le Chevalier T, Scagliotti G, Natale R,
Danson S, Rosell R, Stahel R, Thomas P, Rudd RM, Vansteenkiste J,
Thatcher N, et al: Efficacy of gemcitabine plus platinum
chemotherapy compared with other platinum containing regimens in
advanced non-small-cell lung cancer: A meta-analysis of survival
outcomes. Lung Cancer. 47:69–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cassileth BR and Deng G: Complementary and
alternative therapies for cancer. Oncologist. 9:80–89. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeRan M, Yang J, Shen CH, Peters EC,
Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al:
Energy stress regulates hippo-YAP signaling involving AMPK-mediated
regulation of angiomotin-like 1 protein. Cell Rep. 9:495–503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K,
Wang K, Wang X, Chang LS, He D and Guo P: Curcumin promotes KLF5
proteasome degradation through downregulating YAP/TAZ in bladder
cancer cells. Int J Mol Sci. 15:15173–15187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YN, Choe SR, Cho KH, Kang J, Park CG
and Lee HY: Resveratrol suppresses breast cancer cell invasion by
inactivating a RhoA/YAP signaling axis. Exp Mol Med. 49:e296. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malik A, Sultana M, Qazi A, Qazi MH,
Parveen G, Waquar S, Ashraf AB and Rasool M: Role of natural
radiosensitizers and cancer cell radioresistance: An update. Anal
Cell Pathol (Amst). 2016:61465952016.PubMed/NCBI
|
|
33
|
Chen L, Gilkes DM, Pan Y, Lane WS and Chen
J: ATM and Chk2-dependent phosphorylation of MDMX contribute to p53
activation after DNA damage. EMBO J. 24:3411–3422. 2005. View Article : Google Scholar : PubMed/NCBI
|