Introduction
The loss of epithelial features in tumors, known as
epithelial-mesenchymal transition (EMT), is significantly involved
in the malignant transformation of cancers, such as tumor
initiation, migration, and metastasis (1,2). Previous
findings have identified several molecules associated with the
maintenance of the epithelial features of cells (3). Epithelial cell adhesion molecule (EpCAM)
is a cell adhesion transmembrane molecule, which is overexpressed
in tumors. EpCAM is also known as trophoblast cell surface antigen
1 (TROP1) and it is encoded by the tumor-associated calcium signal
transducer 1 (TACSTD1) gene (3). Trophoblast cell surface antigen 2
(TROP2), another molecule of the TACSTD gene family,
was identified as a cell surface marker for invasive trophoblast
cells (4). TROP2 is a promising
therapeutic target (5), and its
expression is associated with cancer malignancy in various solid
tumors including breast cancers (6).
TROP2 is a 46-kDa type I transmembrane glycoprotein
(323 amino acids), which consists of a large extracellular domain
(274 amino acids) with four N-glycosylation sites, a
transmembrane domain (23 amino acids), and a short intracellular
domain (26 amino acids). TROP2 possesses 49% identity and 67%
similarity with EpCAM (4,5), and is expressed in normal tissues, such
as skin, kidney, liver, breast, ureteric bud, and renal tubules.
TROP2 is highly expressed during the development of mammalian
embryos and fetus (4,7,8).
TROP2 has been reported to be overexpressed in
cancers, and is involved in cell proliferation, invasion,
metastasis, and poor prognosis in many cancer types (9–12). It has
been reported that membrane-localized TROP2 becomes an unfavorable
target of prognosis, while the intracellular retention of TROP2 is
associated with less frequent tumor relapse and better survival in
breast cancer patients (13). A high
expression of TROP2 and low expression of E-cadherin are associated
with lymph node status, metastasis, tumor/node/metastasis (TNM)
stage, and ER/PR/HER2 expression, indicating that TROP2 is
considered to have a potential role in the promotion of EMT
(14). Furthermore, TROP2 has been
reported to be involved in the chemotherapeutic resistance against
lung cancer (15).
Previously, we developed a highly sensitive
anti-TROP2 monoclonal antibody (mAb; clone TrMab-6; mouse
IgG2b, kappa) (16) using
a Cell-Based Immunization and Screening (CBIS) method (17). TrMab-6 was useful for investigations
using flow cytometry, western blot, and immunohistochemistry
(16). The aim of this study was to
investigate whether TrMab-6 possesses in vitro
antibody-dependent cellular cytotoxicity (ADCC) or
complement-dependent cytotoxicity (CDC) activities and in
vivo antitumor activities using breast cancer models.
Materials and methods
Cell lines
CHO-K1 and the breast cancer cell lines, MDA-MB-231
and MDA-MB-468 were obtained from the American Type Culture
Collection. The breast cancer cell line MCF7 was obtained from the
Cell Resource Center for Biomedical Research Institute of
Development, Aging and Cancer, at Tohoku University, Japan.
C-terminal PA-tagged TROP2-overexpressed CHO-K1 (CHO/TROP2) was
previously established by transfection of pCAG/TROP2-PA to CHO-K1
cells using Lipofectamine LTX Reagent (Thermo Fisher Scientific,
Inc.) (16). The TROP2
gene-knockout cell line, MCF7/TROP2-KO (BINDS-29), was previously
generated by transfection of CRISPR/Cas9 plasmids targeting TROP2
(http://www.med-tohoku-antibody.com/topics/001_paper_cell.htm),
using the Neon Transfection System (Thermo Fisher Scientific,
Inc.). Stable transfectants were established by cell sorting using
SH800 (Sony Biotechnology Corp.) (16). CHO-K1, CHO/TROP2, MCF7, and BINDS-29
were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(Nacalai Tesque, Inc.). MDA-MB-231 and MDA-MB-468 were cultured in
Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Inc.).
RPMI-1640 and DMEM were supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 U/ml
of penicillin (Nacalai Tesque, Inc.), 100 µg/ml streptomycin
(Nacalai Tesque, Inc.), and 0.25 µg/ml amphotericin B (Nacalai
Tesque, Inc.), and incubated at 37°C in a humidified atmosphere
containing 5% CO2.
Primary antibodies
Purified mouse IgG (cat. no. I8765) and mouse
IgG2b (cat. no. M1395) were purchased from
Sigma-Aldrich; Merck KGaA. An anti-TROP2 mAb was purified using
Protein G-Sepharose (GE Healthcare Biosciences).
Western blot analysis
Cell pellets were resuspended in phosphate-buffered
saline (PBS; Nacalai Tesque, Inc.) with 1% Triton X-100 (cat. no.
168-11805; FUJIFILM Wako Pure Chemical Corporation) and 50 µg/ml
aprotinin (product no. 03346-84; Nacalai Tesque, Inc.). Cell debris
was removed by centrifugation at 21,880 × g for 10 min at 4°C.
Protein concentration was determined by BCA method. Cell lysates
were boiled in sodium dodecyl sulfate sample buffer with a reducing
reagent (Nacalai Tesque, Inc.). These proteins (10 µg) were
electrophoresed on 5–20% polyacrylamide gels (FUJIFILM Wako Pure
Chemical Corporation) and transferred onto polyvinylidene
difluoride (PVDF) membranes (Merck KGaA). After blocking with 4%
skim milk (Nacalai Tesque, Inc.) at room temperature for 30 min,
the membranes were incubated with primary antibodies, such as 1
µg/ml of TrMab-6 or anti-β-actin for control (clone AC-15;
Sigma-Aldrich; Merck KGaA) at room temperature for 30 min, followed
by incubation with secondary peroxidase-conjugated anti-mouse
immunoglobulins (1:1,000; cat. no. P044701-2; Agilent Technologies
Inc.) at room temperature for 30 min. Finally, the proteins were
visualized with ImmunoStar LD (cat. no. 290-69904; FUJIFILM Wako
Pure Chemical Corporation) or Pierce™ ECL Plus Western Blotting
Substrate (cat. no. 32132; Thermo Fisher Scientific, Inc.), and
were detected using the Sayaca-Imager (DRC Co. Ltd.). Qcapture Pro
software (DRC Co. Ltd) was used for the densitometry.
Flow cytometry
Cells (2×105 cells/ml) were harvested
after brief exposure to 0.25% trypsin in 1 mM
ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). After
washing with 0.1% bovine serum albumin (BSA, Nacalai Tesque, Inc.)
in PBS the cells were treated with 1 µg/ml of an anti-TROP2 mAb or
control (1% BSA in PBS; blocking buffer) for 30 min at 4°C, and
then with Alexa Fluor 488-conjugated anti-mouse IgG (1:1,000;
product no. 4408; Cell Signaling Technology, Inc.). Fluorescence
data were collected using flow cytometer: SA3800 Cell Analyzer
(Sony Biotechnology Corp.).
ADCC
ADCC stimulation by an anti-TROP2 mAb was assayed as
follows. Five female five-week-old BALB/c nude mice (mean weight,
15±3 g) were purchased from Charles River Laboratories, Inc. Mice
were kept under specific pathogen-free conditions on an 11-h
light/13-h dark cycle at a temperature of 23±2°C and 55±5% humidity
with food and water supplied ad libitum during the
experimental periods. After euthanasia by cervical dislocation,
spleens were removed aseptically, and single-cell suspensions were
obtained by forcing spleen tissues through a sterile cell strainer
(product no. 352360; Corning, Inc.) with a syringe. Erythrocytes
were lysed with 10-sec exposure to ice-cold distilled water. The
splenocytes were then washed with DMEM and resuspended in DMEM with
10% FBS; this preparation was designated as effector cells. The
target tumor cells were labeled with 10 µg/ml Calcein-AM (Thermo
Fisher Scientific, Inc.) and resuspended in the same medium. The
target cells were transferred to 96-well plates, at
2×104 cells/well, and mixed with effector cells at an
effector-to-target ratio of 100:1, along with 100 µg/ml of an
anti-TROP2 mAb or control mouse IgG2b. After a 5-h
incubation at 37°C, the Calcein-AM release into the supernatant was
measured for each well. Fluorescence intensity was assessed using a
microplate reader (Power Scan HT; BioTek Instruments, Inc.) with an
excitation wavelength of 485 nm and an emission wavelength of 538
nm. Cytotoxicity (as % lysis) was measured using the formula:
Percentage of lysis (%)=(E-S)/(M-S) ×100, where E is the
fluorescence released in combined cultures of target cells and
effector cells, S is the spontaneous fluorescence released in
cultures of only target cells, and M is the maximum fluorescence
measured after lysis of all cells with buffer containing 0.5%
Triton X-100, 10 mM Tris-HCl (pH 7.4), and 10 mM EDTA. Animal
studies for ADCC and the antitumor activity were approved by the
Institutional Committee for experiments of the Institute of
Microbial Chemistry (permit no. 2020-015).
CDC
CDC stimulation by an anti-TROP2 mAb was assayed as
follows. Target cells were labeled with 10 µg/ml Calcein-AM (Thermo
Fisher Scientific, Inc.) and resuspended in medium. Target cells
were plated in 96-well plates, at 2×104 cells/well, and
10% rabbit complement (Low-Tox-M rabbit complement; Cedarlane
Laboratories) and 100 μg/ml of an anti-TROP2 mAb or control IgG
(mouse IgG2b) were added to each well. After 5 h of
incubation at 37°C, the Calcein-AM release into the supernatant was
measured for each well. Fluorescence intensity was calculated as
described in the ADCC section above.
Antitumor activity of an anti-TROP2
mAb in a mouse xenograft model
Sixty-four five-week-old female BALB/c nude mice
(mean weight, 15±3 g) were purchased from Charles River
Laboratories, Inc., and were divided into the following four groups
(n=16 in each group): i) CHO/TROP2-bearing mice, ii) CHO-K1-bearing
mice, iii) MCF7-bearing mice, and iv) BINDS-29-bearing mice. On day
7, each group was subdivided into 2 groups (n=8 in each group) with
equal mean tumor volume: A control mouse IgG-treated group or an
anti-TROP2 mAb-treated group. All animal experiments were performed
in accordance with institutional guidelines and regulations to
minimize animal suffering and distress in the laboratory. The
Institutional Committee for experiments of the Institute of
Microbial Chemistry (permit no. 2020-015) approved the animal
studies for antitumor activity.
Mice were maintained in a pathogen-free environment,
on an 11-h light/13-h dark cycle at a temperature of 23±2°C and
55±5% humidity, with food and water supplied ad libitum
throughout the experiments. Mice were monitored for health and
weight every three or four days. Experiments on mice were conducted
in four weeks. Weight loss >25% or tumor volume >3,000
mm3 was identified as humane endpoints for euthanasia.
At humane and experimental endpoints, mice were euthanized by
cervical dislocation, and death was verified by validating
respiratory and cardiac arrest.
After an acclimation period of one week, these mice
were used in experiments at six weeks of age (mean weight, 16±2 g).
Cells (0.3 ml of 1.33×108 cells/ml in DMEM) were mixed
with 0.5 ml BD Matrigel Matrix Growth Factor Reduced (BD
Biosciences). A total of 100 µl of this suspension
(5×106 cells) was injected subcutaneously into the left
flank of each animal. On day 7 post-inoculation, 100 µg of an
anti-TROP2 mAb or control mouse IgG in 100 µl PBS was injected
intraperitoneally (i.p.). Additional antibody inoculations were
performed on days 14 and 21. Twenty-four days after cell
implantation, all mice were euthanized by cervical dislocation, and
tumor diameters and volumes were measured and recorded.
Statistical analysis
Data are expressed as mean ± standard error of the
mean (SEM). Statistical analysis was conducted with Welch's t-test
for ADCC and CDC, ANOVA and Sidak's multiple comparisons tests for
tumor volume and mouse weight, and Welch's t-test for tumor weight.
All calculations were performed using GraphPad Prism 7 (GraphPad
Software, Inc.). P<0.05 was considered statistically
significant.
Results
Western blot analysis
We performed western blot analysis using TrMab-6.
TrMab-6 detected TROP2 with a 40-kDa band in CHO/TROP2 (16), MCF7, MDA-MB-231, and MDA-MB-468 cells;
however, it did not detect any proteins in CHO-K1 and BINDS-29
cells (Fig. 1A), indicating that
TrMab-6 is specific for TROP2. As TROP2 is over-expresed in
CHO/TROP2, the band in the CHO/TROP2 cell was broader than that of
the other cells, such as MCF7, MDA-MB-231, and MDA-MB-468. We used
β-actin as an internal control.
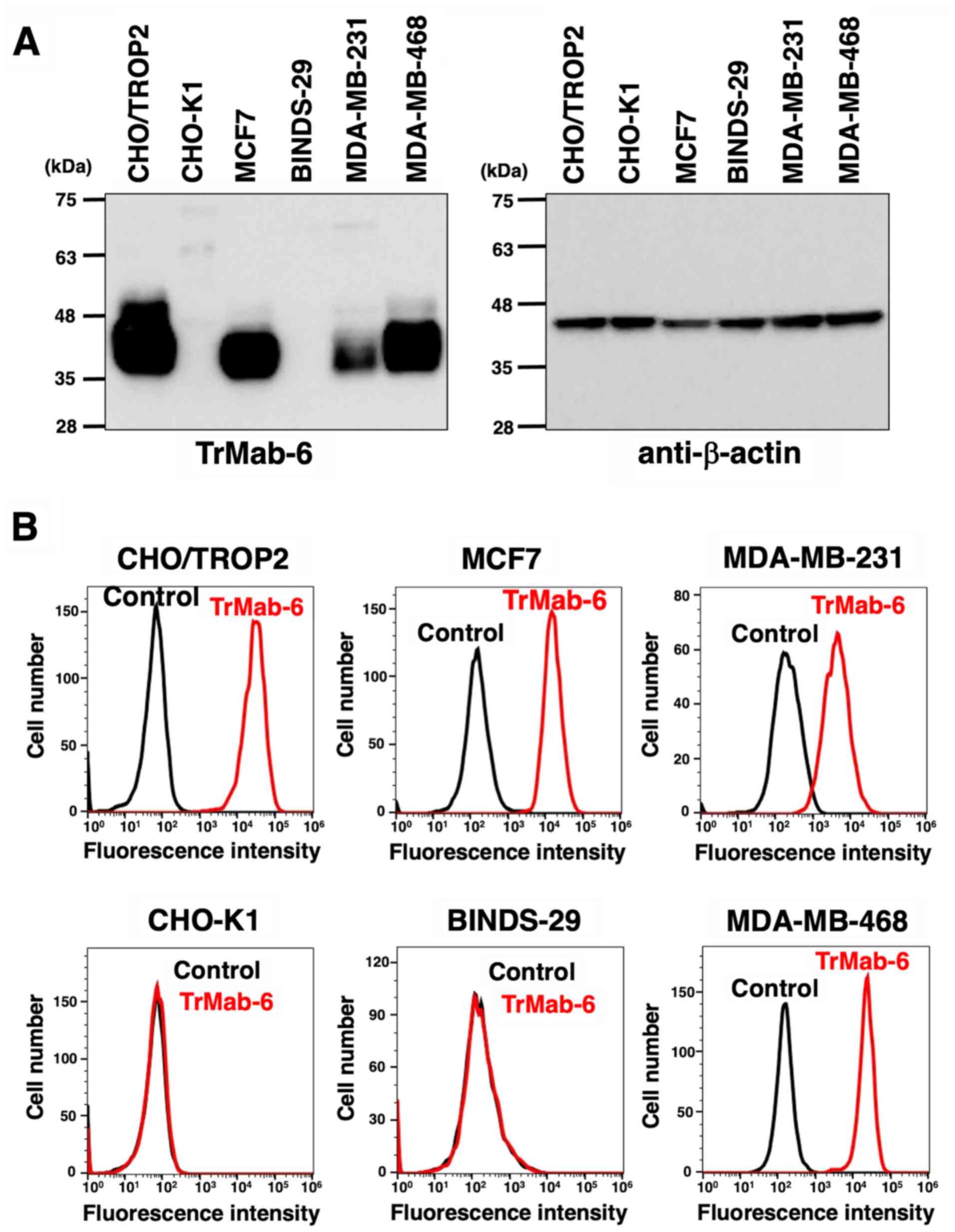 | Figure 1.(A) Detection of TROP2 by TrMab-6 by
western blot analysis. Cell lysates of CHO/TROP2, CHO-K1, MCF7,
BINDS-29, MDA-MB-231, and MDA-MB-468 cells were electrophoresed and
transferred onto PVDF membranes. These membranes were treated with
TrMab-6 (left panel) or anti-β-actin (right panel), followed by
incubation with peroxidase-conjugated anti-mouse immunoglobulin.
(B) Flow cytometry using TrMab-6. CHO/TROP2, CHO-K1, MCF7,
BINDS-29, MDA-MB-231, and MDA-MB-468 cells were treated with 1
µg/ml of TrMab-6, followed by a treatment with Alexa Fluor
488-conjugated anti-mouse IgG. Black line, negative control
(blocking buffer). |
Flow cytometry
We investigated whether TrMab-6 can react with
CHO-K1, CHO/TROP2 (16), MCF7,
BINDS-29 (MCF7/TROP2-KO), MDA-MB-231, and MDA-MB-468 by flow
cytometry. We used a blocking buffer as negative control. TrMab-6
recognized the CHO/TROP2 cells, but not the parental CHO-K1 cells
(Fig. 1B). TrMab-6 also recognized
the endogenous TROP2 in MCF7 breast cancer cells (Fig. 1B). By contrast, the reaction of
TrMab-6 to BINDS-29 was lost after the knockout of TROP2 in MCF7
cells (Fig. 1B), indicating that
TrMab-6 is specific for TROP2. TrMab-6 also detected TROP2 of
MDA-MB-231 and MDA-MB-468 (Fig.
1B).
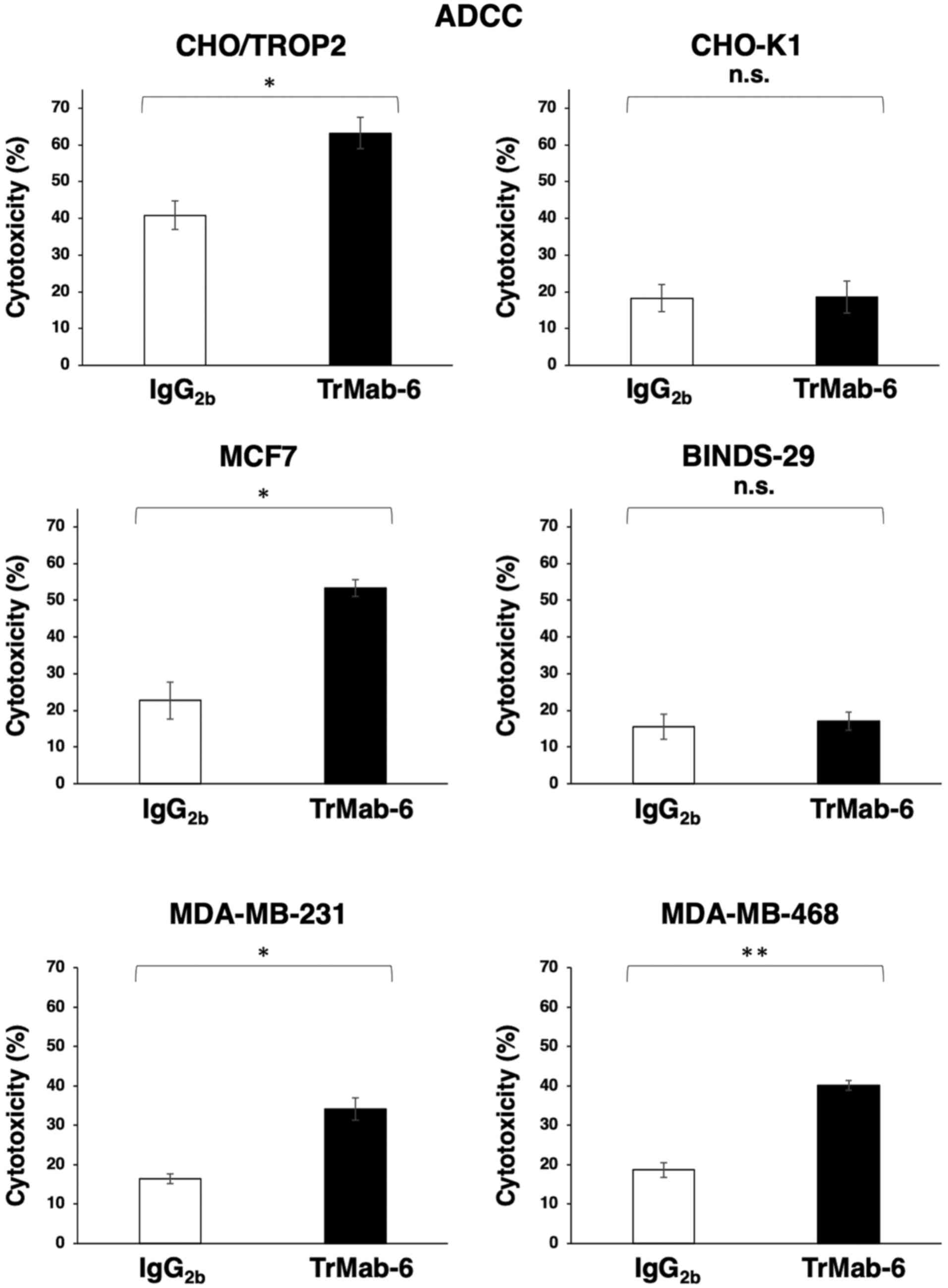
ADCC and CDC activities of TrMab-6 in
TROP2-expressing cell lines
The effect of TrMab-6 (mouse IgG2b) in
the ADCC and CDC activity in TROP2-expressing cells, such as
CHO/TROP2 (16) or MCF7, MDA-MB-231,
and MDA-MB-468 breast cancer cell lines, was analyzed. First,
TrMab-6 exhibited higher ADCC (63.2% cytotoxicity) in CHO/TROP2
cells than that of the control mouse IgG2b (40.9%
cytotoxicity; P<0.05) (Fig. 2). By
contrast, TrMab-6 did not show any ADCC activity in CHO-K1 cells
compared with the respective control (Fig. 2). Additionally, TrMab-6 exhibited
higher ADCC (53.3% cytotoxicity) in MCF7 cells that in the control
mouse IgG2b (22.7% cytotoxicity; P<0.05); however, no
ADCC activity was observed in BINDS-29 cells (Fig. 2). TrMab-6 also exhibited higher ADCC
(34.2% cytotoxicity) in MDA-MB-231 cells than that of the control
mouse IgG2b (16.4% cytotoxicity; P<0.05) (Fig. 2). Furthermore, TrMab-6 exhibited
higher ADCC (40.2% cytotoxicity) in MDA-MB-468 cells than that of
the control mouse IgG2b (18.7% cytotoxicity; P<0.01)
(Fig. 2).
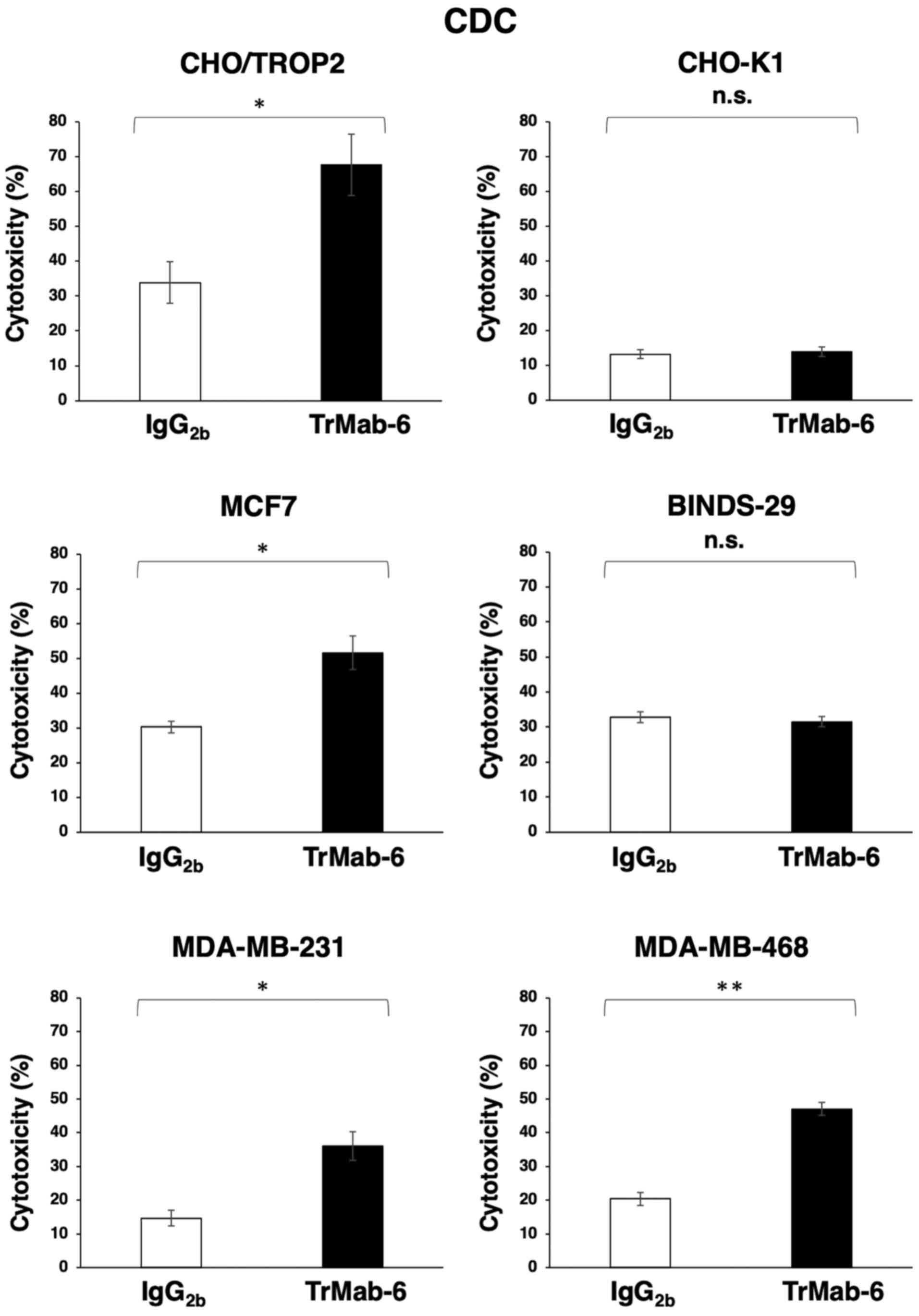
TrMab-6 was also associated with more robust CDC
activity (67.7% cytotoxicity) in CHO/TROP2 cells compared to
control mouse IgG2b (33.9% cytotoxicity; P<0.05), in
contrast to its CDC activity in CHO-K1 cells (Fig. 3). Furthermore, while TrMab-6 exhibited
higher CDC (51.6% cytotoxicity) in MCF7 cells compared to the
control (30.2% cytotoxicity; P<0.05), this was not evident in
BINDS-29 cells (Fig. 3). TrMab-6 also
exhibited higher CDC (36.0% cytotoxicity) in MDA-MB-231 cells than
that of the control mouse IgG2b (14.7% cytotoxicity;
P<0.05) (Fig. 3). Furthermore,
TrMab-6 exhibited higher CDC (47.0% cytotoxicity) in MDA-MB-468
cells than that of the control mouse IgG2b (20.4%
cytotoxicity; P<0.01) (Fig.
3).
These favorable ADCC/CDC activities indicated that
TrMab-6 may induce strong antitumor activity against breast cancer
cells in vivo.
Antitumor effect of TrMab-6 in mouse
xenografts of TROP2-expressed CHO/TROP2 cells
CHO/TROP2 was developed in our previous study
(16). Tumor formation of 16
CHO/TROP2-bearing mice was observed on day 7. Then, these 16
CHO/TROP2-bearing mice were divided into a TrMab-6-treated group
and a control group. On days 7, 14 and 21 after CHO/TROP2 cell
injections into the mice, TrMab-6 (100 µg) or control mouse IgG
(100 µg) were injected i.p. to the mice. Tumor volume was measured
on days 7, 10, 14, 17, 21 and 24 after CHO/TROP2 cell injection.
TrMab-6-treated mice exhibited significantly less tumor growth on
days 14 (P<0.05), 17 (P<0.01), 21 (P<0.01), and 24
(P<0.01) compared with IgG-treated control mice (Fig. 4A, upper panel). On day 24, there was a
reduction of the tumor volume of 61.9% in TrMab-6-treated mice
(Fig. 4A, upper panel). Tumors from
TrMab-6-treated mice weighed significantly less than tumors from
IgG-treated control mice on day 24 (52.9% reduction, P<0.05;
Fig. 4A, middle panels). These
results indicated that TrMab-6 reduced the growth of CHO/TROP2
×enografts, but without full elimination. Total body weights did
not significantly differ between the treatment and control groups
(Fig. 4A, lower panel).
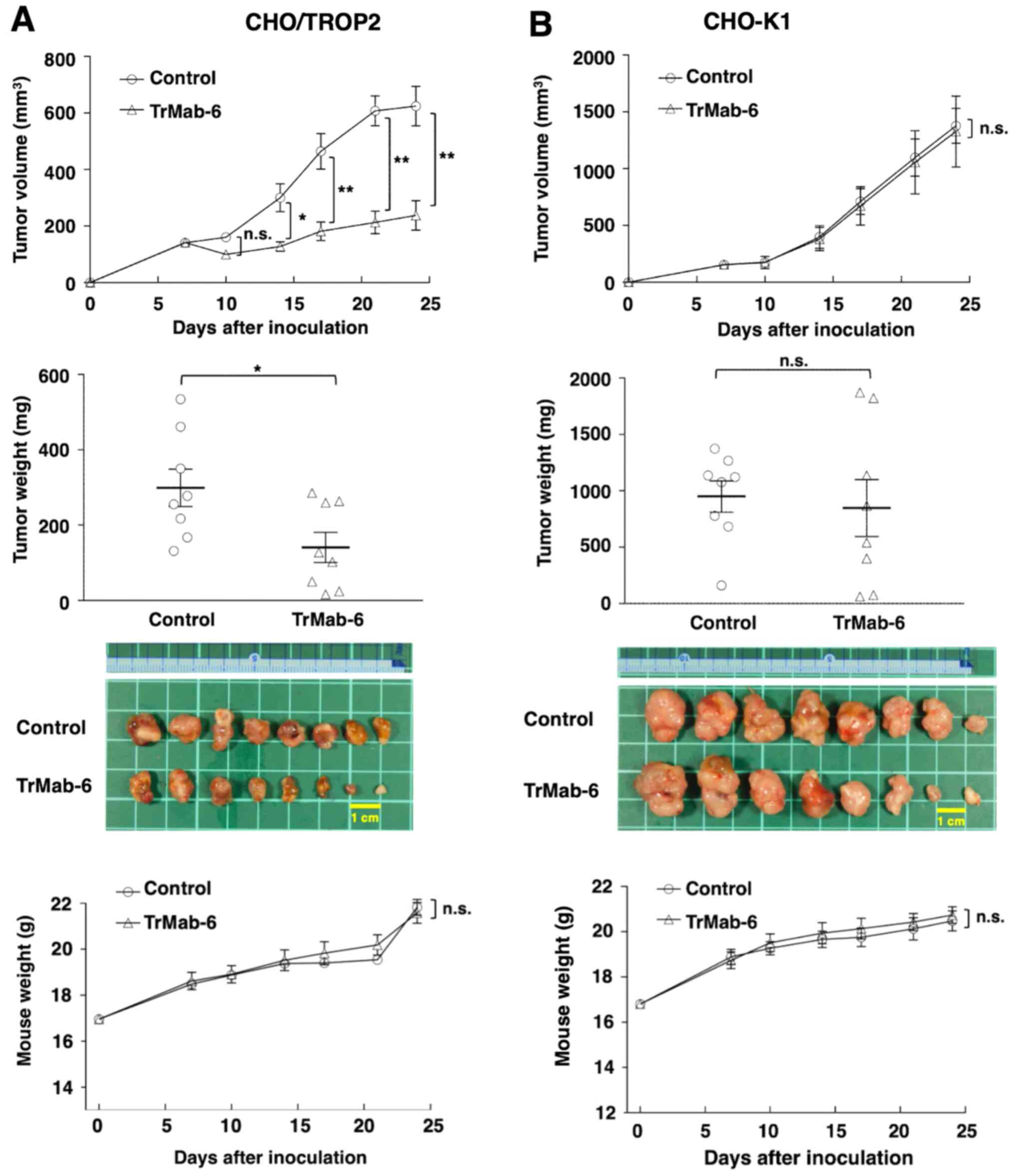 | Figure 4.Evaluation of antitumor activity of
TrMab-6 in CHO/TROP2 or CHO-K1 ×enografts. (A, upper panel)
CHO/TROP2 cells (5×106 cells) were injected
subcutaneously into the left flank. After day 7, 100 µg of TrMab-6
and control mouse IgG in 100 µl PBS were injected i.p. into treated
and control mice, respectively. Additional antibodies were then
injected on days 14 and 21. The tumor volume was measured on days
7, 10, 14, 17, 21 and 24. Values are mean ± SEM. Asterisk indicates
statistical significance (**P<0.01, *P<0.05, n.s., not
significant, ANOVA and Sidak's multiple comparisons test). (A,
middle panels) Tumors of CHO/TROP2 ×enografts were resected from
TrMab-6 and control mouse IgG groups. Tumor weight on day 24 was
measured from excised xenografts. Values are mean ± SEM. Asterisk
indicates statistical significance (*P<0.05, Welch's t-test).
Resected tumors of CHO/TROP2 ×enografts from control mouse IgG and
TrMab-6 groups on day 24. Scale bar, 1 cm. (A, lower panel) Body
weights of the mice implanted with CHO/TROP2 ×enografts were
recorded on days 7, 10, 14, 17, 21 and 24 (n.s., not significant).
(B, upper panel) CHO-K1 cells (5×106 cells) were
injected subcutaneously into the left flank. After day 7, 100 µg of
TrMab-6 and control mouse IgG in 100 µl PBS were injected i.p. into
treated and control mice, respectively. Additional antibodies were
then injected on days 14 and 21. Tumor volume was measured on days
7, 10, 14, 17, 21 and 24. Values are mean ± SEM. n.s., not
significant. (B, middle panels) Tumors of CHO-K1 ×enografts were
resected from TrMab-6 and control mouse IgG groups. Tumor weight on
day 24 was measured from excised xenografts. Values are mean ± SEM.
n.s., not significant. Resected tumors of CHO-K1 ×enografts from
control mouse IgG and TrMab-6 groups on day 24. Scale bar, 1 cm.
(B, lower panel) Body weights of the mice implanted with CHO-K1
×enografts were recorded on days 7, 10, 14, 17, 21 and 24 (n.s.,
not significant). |
Similarly, tumor formation of 16 CHO-K1-bearing mice
was observed on day 7, before they were divided into a
TrMab-6-treated group and a control group. On days 7, 14 and 21
after CHO-K1 cell injections, TrMab-6 (100 µg) or control mouse IgG
(100 µg) were injected i.p. into the mice. Tumor volume was
measured on days 7, 10, 14, 17, 21 and 24 after CHO-K1 cell
injection. Both TrMab-6-treated and control groups exhibited
similar tumor growth on all days (Fig.
4B, upper panel) and no difference in the tumor weight was
observed between the two groups on day 24 (Fig. 4B, middle panels). These results
indicated that TrMab-6 did not reduce the growth of TROP2-negative
CHO-K1 ×enografts. Additionally, the total body weights did not
significantly differ between the two study groups (Fig. 4B, lower panel).
Antitumor effect of TrMab-6 in mouse
xenografts of TROP2-expressing MCF7 breast cancer cell lines
The tumor formation of 16 MCF7-bearing mice was
observed on day 7 before mice were divided into a TrMab-6-treated
group and a control group. On days 7, 14 and 21 after MCF7 cell
injections into the mice, either TrMab-6 (100 µg) or control mouse
IgG (100 µg) was injected i.p. into the mice. The tumor volume was
measured on days 7, 10, 14, 17, 21 and 24 after MCF7 cell
injection. TrMab-6-treated mice exhibited significantly less tumor
growth on days 10 (P<0.01), 14 (P<0.01), 17 (P<0.01), 21
(P<0.01), and 24 (P<0.01) compared with IgG-treated control
mice (Fig. 5A, upper panel). On day
24, a reduction of the tumor volume of 46.6% was seen in
TrMab-6-treated mice (Fig. 5A, upper
panel). Tumors from TrMab-6-treated mice weighed significantly less
than tumors from IgG-treated control mice on day 24 (37.8%
reduction, P<0.05; Fig. 5A, middle
panels). These results indicated that TrMab-6 reduced the growth of
MCF7 ×enografts, but did not contribute towards their total
elimination. Total body weights did not significantly differ
between the treatment and control groups (Fig. 5A, lower panel).
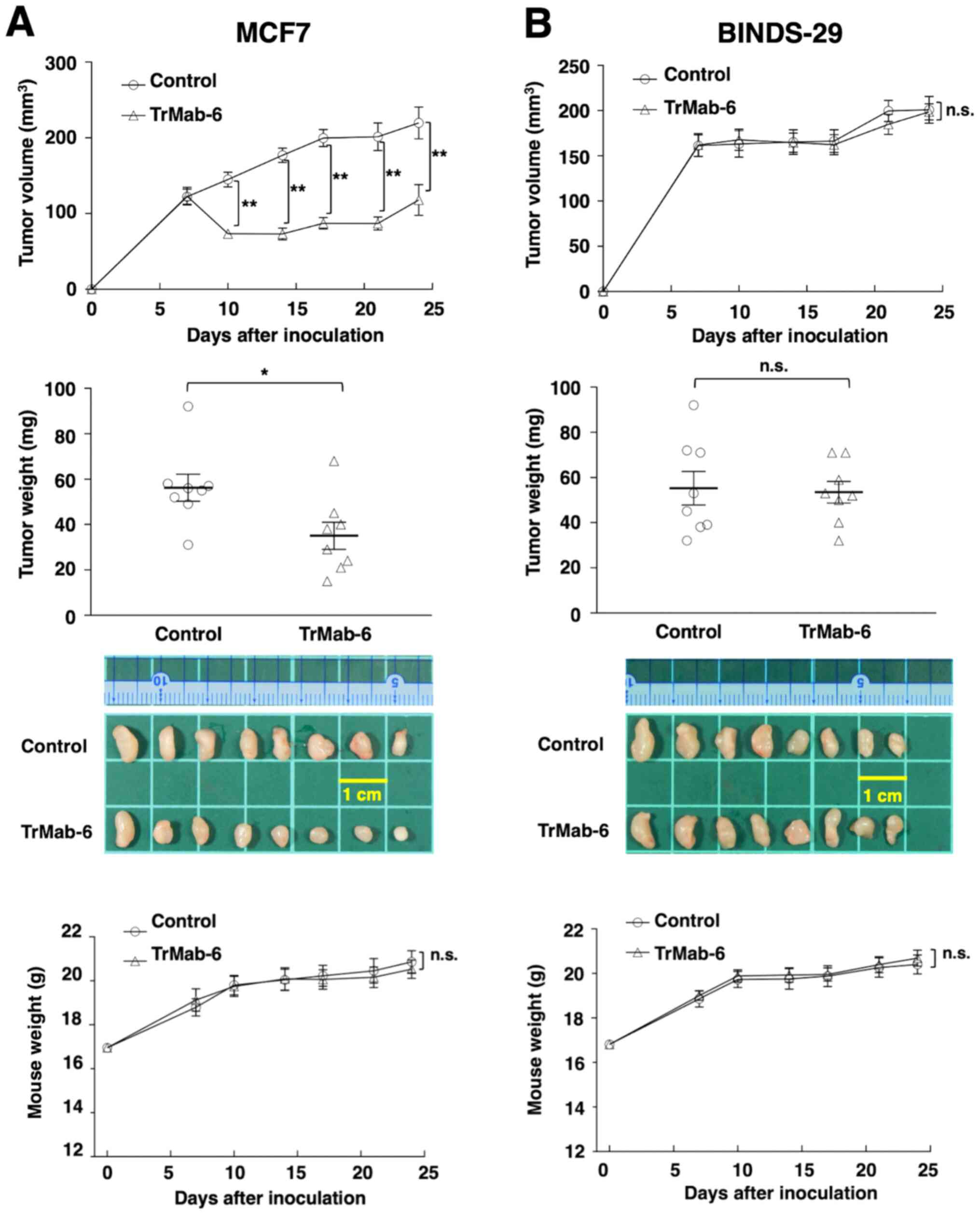 | Figure 5.Evaluation of antitumor activity of
TrMab-6 in MCF7 or BINDS-29 ×enografts. (A, upper panel) MCF7 cells
(5×106 cells) were injected subcutaneously into the left
flank. After day 7, 100 µg of TrMab-6 and control mouse IgG in 100
µl PBS were injected i.p. into treated and control mice,
respectively. Additional antibodies were then injected on days 14
and 21. Tumor volume was measured on days 7, 10, 14, 17, 21 and 24.
Values are mean ± SEM. Asterisk indicates statistical significance
(**P<0.01, ANOVA and Sidak's multiple comparisons test). (A,
middle panels) Tumors of MCF7 ×enografts were resected from TrMab-6
and control mouse IgG groups. Tumor weight on day 24 was measured
from excised xenografts. Values are mean ± SEM. Asterisk indicates
statistical significance (*P<0.05, Welch's t-test). Resected
tumors of MCF7 ×enografts from control mouse IgG and TrMab-6 groups
on day 24. Scale bar, 1 cm. (A, lower panel) Body weights of the
mice implanted with MCF7 ×enografts were recorded on days 7, 10,
14, 17, 21 and 24 (n.s., not significant). (B, upper panel)
BINDS-29 cells (5×106 cells) were injected
subcutaneously into the left flank. After day 7, 100 µg of TrMab-6
and control mouse IgG in 100 µl PBS were injected i.p. into treated
and control mice, respectively. Additional antibodies were then
injected on days 14 and 21. Tumor volume was measured on days 7,
10, 14, 17, 21 and 24. Values are mean ± SEM. n.s., not
significant. (B, middle panels) Tumors of BINDS-29 ×enografts were
resected from TrMab-6 and control mouse IgG groups. Tumor weight on
day 24 was measured from excised xenografts. Values are mean ± SEM.
n.s., not significant. Resected tumors of BINDS-29 ×enografts from
control mouse IgG and TrMab-6 groups on day 24. Scale bar, 1 cm.
(B, lower panel) Body weights of the mice implanted with BINDS-29
×enografts were recorded on days 7, 10, 14, 17, 21 and 24 (n.s.,
not significant). |
Similarly, the tumor formation of 16
BINDS-29-bearing mice was observed on day 7, before the 16
BINDS-29-bearing mice were divided into a TrMab-6-treated group and
a control group. On days 7, 14 and 21 after BINDS-29 cell
injections into the mice, TrMab-6 (100 µg) or control mouse IgG
(100 µg) was injected i.p. into the mice. The tumor volume was
measured on days 7, 10, 14, 17, 21 and 24 after BINDS-29 cell
injection. The TrMab-6-treated and control groups exhibited similar
tumor growth on all days (not significant; Fig. 5B, upper panel) and no difference in
the tumor weight was observed between the two groups, on day 24
(Fig. 5B, middle panels). These
results indicated that TrMab-6 did not reduce the growth of
TROP2-negative BINDS-29 ×enografts. Total body weights did not
significantly differ between the treatment and control groups
(Fig. 5B, lower panel).
Discussion
TROP2 has been demonstrated to be overexpressed in a
variety of tumors (18). A gene
expression pattern analysis comparing gastric tumors and their
normal counterparts revealed that TROP2 was not overexpressed in
normal tissues (19). In addition, in
a meta-analysis that included 16 studies involving 2,569
participants, TROP2 overexpression was found to be associated with
poor overall and disease-free survival across several types of
solid tumors (20). Furthermore, the
knockdown of TROP2 decreased cell proliferation and migration
(21). Altogether, these results
suggest that TROP2 is a potential target for antitumor
treatments.
Antibody-based therapy is a rapidly emerging field
for treatment of several diseases, including cancer. The
development of antibody drugs for TROP2 has been accelerated in
recent years due to identification of the extracellular domain of
TROP2 as a potential prominent target for TROP2-positive cancers
(5,6).
Among them, antibody-drug conjugates (ADCs) are the main modality
of antibody drugs (22). Recently,
the first anti-TROP2 ADC, sacituzumab govitecan, which is a
humanized IgG1 conjugated to irinotecan metabolite
(SN-38), has been approved by the US Food and Drug Administration
against metastatic triple-negative breast cancers (23). ADCs have also been developed against
hormone receptor-positive breast cancers and HER2-negative
metastatic breast cancers (23).
Preliminary findings have demonstrated that datopotamab deruxtecan
(DS-1062) is active in patients with advanced or metastatic
non-small cell lung cancer (24). In
phase I trials of datopotamab deruxtecan, this drug induced
responses in almost 25% of the patients trialed and had manageable
side effects (24). The combination
of these ADCs with immune checkpoint inhibitors is also expected to
be effective (5,22,23,25).
The adaptive or acquired resistance to targeted
antibody cancer therapies is of importance for clinical outcomes
(26). Development of new antibodies
and improvement of antibody-based drugs are required to overcome
therapeutic resistance and reduce the possibility of identifying
suitable candidates for clinical application (27). In this study, we demonstrated the
efficacy of a new anti-TROP2 antibody, TrMab-6. The development of
anti-TROP2 ADC is a potential therapeutic option for cancer
patients with therapy-resistant solid tumors by itself or in
combination with other anticancer drugs. Furthermore, TrMab-6 can
be used in flow cytometry, immunohistochemistry, and western blot
analyses (16). In histopathology,
immunohistochemistry is used for clinical diagnosis for biopsies
and resected specimens. TrMab-6 may be used to ascertain patients
who should receive the anti-TROP2-targeted therapy.
The CBIS method, which uses antigen-expressing cell
lines for both immunization and screening, can help to effectively
develop mAbs which may be useful as antitumor agents. We have
recently succeeded in developing numerous mAbs that target membrane
proteins, including CD19 (28), CD20
(29), CD44 (30), CD133 (17), EpCAM (31), and TROP2 (16). Of these, CMab-43 (mouse
IgG2a) for CD133 showed significant ADCC/CDC activities
against colon cancer cells and antitumor activity against colon
cancer xenograft models (32).
EpMab-16 (mouse IgG2a) for EpCAM also demonstrated
significant antitumor activity against colon cancer xenograft
models (31), and oral squamous cell
carcinomas (33). Furthermore,
5-mG2a-f (a defucosylated mouse IgG2a-type of
clone C44Mab-5) for CD44 exerted antitumor effects in
mouse xenograft models of oral squamous cell carcinomas (34).
In the present study, we investigated whether
TrMab-6 (16), developed using the
CBIS method, could exhibit ADCC/CDC activities in vitro and
antitumor activity in vivo against breast cancers. In
vitro experiments revealed strong ADCC/CDC inducement against
CHO/TROP2, MCF7, MDA-MB-231, and MDA-MB-468 cells by TrMab-6
(Figs. 2 and 3). In vivo experiments on CHO/TROP2
(Fig. 4) and MCF7 (Fig. 5) xenografts revealed that the TrMab-6
treatment significantly reduced tumor growth, compared with the
control mouse IgG. By contrast, TrMab-6 did not demonstrate
ADCC/CDC in vitro (Figs. 2 and
3) and antitumor activity in
vivo against TROP2-negative CHO-K1 (Fig. 4) and BINDS-29 (Fig. 5), demonstrating that the toxicity of
TrMab-6 is specific for TROP2. These data indicated that TrMab-6 is
a promising treatment option for TROP2-expressing breast cancers.
For future studies, several modalities, such as ADC or chimeric
antigen receptor (CAR)-T of TrMab-6, should be developed to
strengthen the antitumor activity against breast cancers.
Acknowledgments
We would like to thank Mr. Takuro Nakamura, Ms.
Miyuki Yanaka, Ms. Saori Handa, and Mr. Yu Komatsu (Department of
Antibody Drug Development, Tohoku University Graduate School of
Medicine) for technical assistance of in vitro experiments,
and Ms. Akiko Harakawa and Mr. Shun-ichi Ohba [Institute of
Microbial Chemistry (BIKAKEN), Numazu, Microbial Chemistry Research
Foundation] for technical assistance of animal experiments.
Funding
This research was supported in part by the Japan
Agency for Medical Research and Development (AMED) under grant nos.
JP21am0401013 (to YK) and JP21am0101078 (to YK), and by the Japan
Society for the Promotion of Science (JSPS) Grants-in-Aid for
Scientific Research (KAKENHI) grant nos. 21K15523 (to TA), 21K07168
(to MKK), 19K07705 (to YK) and 20K16322 (to MS).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TT, TO, TA, RN, HHo, and MS performed the
experiments. JT and MKK analyzed the experimental data. HHa, MK,
and YK designed the present study. TT, TO, and YK wrote the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal studies for ADCC and the antitumor activity
were approved by the Institutional Committee for experiments of the
Institute of Microbial Chemistry (permit no. 2020-015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADC
|
antibody-drug conjugate
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
BSA
|
bovine serum albumin
|
|
CBIS
|
Cell-Based Immunization and
Screening
|
|
CDC
|
complement-dependent cytotoxicity
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
FBS
|
fetal bovine serum
|
|
mAb
|
monoclonal antibody
|
|
PBS
|
phosphate-buffered saline
|
|
PVDF
|
polyvinylidene difluoride
|
|
SEM
|
standard error of the mean
|
References
|
1
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fagotto F and Aslemarz A: EpCAM cellular
functions in adhesion and migration, and potential impact on
invasion: A critical review. Biochim Biophys Acta Rev Cancer.
1874:1884362020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McDougall AR, Tolcos M, Hooper SB, Cole TJ
and Wallace MJ: Trop2: From development to disease. Dev Dyn.
244:99–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaman S, Jadid H, Denson AC and Gray JE:
Targeting Trop-2 in solid tumors: Future prospects. Onco Targets
Ther. 12:1781–1790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldenberg DM, Stein R and Sharkey RM: The
emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel
cancer target. Oncotarget. 9:28989–29006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stepan LP, Trueblood ES, Hale K, Babcook
J, Borges L and Sutherland CL: Expression of Trop2 cell surface
glycoprotein in normal and tumor tissues: Potential implications as
a cancer therapeutic target. J Histochem Cytochem. 59:701–710.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDougall AR, Hooper SB, Zahra VA, Cole
TJ, Lo CY, Doran T and Wallace MJ: Trop2 regulates motility and
lamellipodia formation in cultured fetal lung fibroblasts. Am J
Physiol Lung Cell Mol Physiol. 305:L508–L521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akarken İ and Dere Y: Could trop-2
overexpression indicate tumor aggressiveness among prostatic
adenocarcinomas? Ann Diagn Pathol. 50:1516802021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Pang B, Liang Y, Xu SC, Xin T, Fan
HT, Yu YB and Pang Q: Overexpression of EpCAM and Trop2 in
pituitary adenomas. Int J Clin Exp Pathol. 7:7907–7914.
2014.PubMed/NCBI
|
|
11
|
Li Z, Jiang X and Zhang W: TROP2
overexpression promotes proliferation and invasion of lung
adenocarcinoma cells. Biochem Biophys Res Commun. 470:197–204.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu N, Zhang Z, Zhu J, Xu L, Li Y, Duan L,
Mao Y and Li H: Overexpression of trophoblast cell surface antigen
2 as an independent marker for a poor prognosis and as a potential
therapeutic target in epithelial ovarian carcinoma. Int J Exp
Pathol. 97:150–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambrogi F, Fornili M, Boracchi P,
Trerotola M, Relli V, Simeone P, La Sorda R, Lattanzio R, Querzoli
P, Pedriali M, et al: Trop-2 is a determinant of breast cancer
survival. PLoS One. 9:e969932014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao W, Kuai X, Zhou X, Jia L, Wang J,
Yang X, Tian Z, Wang X, Lv Q, Wang B, et al: Trop2 is a potential
biomarker for the promotion of EMT in human breast cancer. Oncol
Rep. 40:759–766. 2018.PubMed/NCBI
|
|
15
|
Wang X, Long M, Dong K, Lin F, Weng Y,
Ouyang Y, Liu L, Wei J, Chen X, He T and Zhang HZ: Chemotherapy
agents-induced immunoresistance in lung cancer cells could be
reversed by trop-2 inhibition in vitro and in vivo by interaction
with MAPK signaling pathway. Cancer Biol Ther. 14:1123–1132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sayama Y, Kaneko MK and Kato Y:
Development and characterization of TrMab-6, a novel anti-TROP2
monoclonal antibody for antigen detection in breast cancer. Mol Med
Rep. 23:922021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Itai S, Fujii Y, Nakamura T, Chang YW,
Yanaka M, Saidoh N, Handa S, Suzuki H, Harada H, Yamada S, et al:
Establishment of CMab-43, a sensitive and specific Anti-CD133
monoclonal antibody, for immunohistochemistry. Monoclon Antib
Immunodiagn Immunother. 36:231–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shvartsur A and Bonavida B: Trop2 and its
overexpression in cancers: Regulation and clinical/therapeutic
implications. Genes Cancer. 6:84–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Zhou W, Velculescu VE, Kern SE,
Hruban RH, Hamilton SR, Vogelstein B and Kinzler KW: Gene
expression profiles in normal and cancer cells. Science.
276:1268–1272. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng P, Chen MB, Zhou LN, Tang M, Liu CY
and Lu PH: Impact of TROP2 expression on prognosis in solid tumors:
A systematic review and meta-analysis. Sci Rep. 6:336582016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu QZ, Nijiati A, Gao X, Tao KL, Li CD,
Fan XP and Tian Z: TROP2 promotes cell proliferation and migration
in osteosarcoma through PI3K/AKT signaling. Mol Med Rep.
18:1782–1788. 2018.PubMed/NCBI
|
|
22
|
Shaffer C: Trop2 deal heats up
antibody-drug conjugate space in cancer. Nat Biotechnol.
39:128–130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seligson JM, Patron AM, Berger MJ, Harvey
RD and Seligson ND: Sacituzumab Govitecan-hziy: An antibody-drug
conjugate for the treatment of refractory, metastatic,
triple-negative breast cancer. Ann Pharmacother. Oct 17–2020.(Epub
ahead of print). doi: 10.1177/1060028020966548. PubMed/NCBI
|
|
24
|
Authors not listed. TROP2 ADC Intrigues in
NSCLC. Cancer Discov. 11:OF52021. View Article : Google Scholar
|
|
25
|
Son S, Shin S, Rao NV, Um W, Jeon J, Ko H,
Deepagan VG, Kwon S, Lee JY and Park JH: Anti-Trop2
antibody-conjugated bioreducible nanoparticles for targeted triple
negative breast cancer therapy. Int J Biol Macromol. 110:406–415.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crystal AS, Shaw AT, Sequist LV, Friboulet
L, Niederst MJ, Lockerman EL, Frias RL, Gainor JF, Amzallag A,
Greninger P, et al: Patient-derived models of acquired resistance
can identify effective drug combinations for cancer. Science.
346:1480–1486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beck A, Goetsch L, Dumontet C and Corvaia
N: Strategies and challenges for the next generation of
antibody-drug conjugates. Nat Rev Drug Discov. 16:315–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamada S, Kaneko MK, Sayama Y, Asano T,
Sano M, Yanaka M, Nakamura T, Okamoto S, Handa S, Komatsu Y, et al:
Development of novel mouse monoclonal antibodies against human
CD19. Monoclon Antib Immunodiagn Immunother. 39:45–50. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Furusawa Y, Kaneko MK and Kato Y:
Establishment of C20Mab-11, a novel anti-CD20 monoclonal
antibody, for the detection of B cells. Oncol Lett. 20:1961–1967.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamada S, Itai S, Nakamura T, Yanaka M,
Kaneko MK and Kato Y: Detection of high CD44 expression in oral
cancers using the novel monoclonal antibody, C44Mab-5.
Biochem Biophys Rep. 14:64–68. 2018.PubMed/NCBI
|
|
31
|
Hosono H, Ohishi T, Takei J, Asano T,
Sayama Y, Kawada M, Kaneko MK and Kato Y: The anti-epithelial cell
adhesion molecule (EpCAM) monoclonal antibody EpMab-16 exerts
antitumor activity in a mouse model of colorectal adenocarcinoma.
Oncol Lett. 20:3832020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato Y, Ohishi T, Yamada S, Itai S,
Furusawa Y, Sano M, Nakamura T, Kawada M and Kaneko MK: Anti-CD133
monoclonal antibody CMab-43 exerts antitumor activity in a mouse
xenograft model of colon cancer. Monoclon Antib Immunodiagn
Immunother. 38:75–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaneko MK, Ohishi T, Takei J, Sano M,
Nakamura T, Hosono H, Yanaka M, Asano T, Sayama Y, Harada H, et al:
Anti-EpCAM monoclonal antibody exerts antitumor activity against
oral squamous cell carcinomas. Oncol Rep. 44:2517–2526. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takei J, Kaneko MK, Ohishi T, Hosono H,
Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, et al: A
defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts
antitumor effects in mouse xenograft models of oral squamous cell
carcinoma. Oncol Rep. 44:1949–1960. 2020.PubMed/NCBI
|