Introduction
Circular RNAs (circRNAs/circs) are a type of
noncoding RNA lacking 5′-3′ ends and poly(A) tails, with a
closed-loop structure that is more stable than that of linear RNA
(1). circRNAs were first revealed to
exist via electron microscopy in 1976 (2). In 1991, the first spliced circRNAs were
discovered through analyses of a human candidate tumor suppressor
gene (3). Despite extensive research,
no evidence of the translation of circRNAs has been found, and
researchers typically considered circRNAs to be the result of
splicing errors (3). However, in
early 2012, researchers were surprised to find that circRNA was a
transcriptional product of thousands of human and mouse genes
(4).
circRNAs can be roughly divided into three
categories based on their genomic origin. Exonic circRNAs, the main
type of circRNA, are formed via exon skipping or head-to-end
connection. Intronic circRNAs are composed of lariat introns
(5). Exon-intron circRNAs, which
comprise the third category, consist of both exons and introns
(5). Exonic circRNAs indicate the
development of disease, and can be used as noninvasive biomarkers
for diagnosis and prognosis in a number of diseases (6).
In the last few decades, the development of improved
chemotherapy regimens with multiple chemotherapeutics, such as
5-fluorouracil (5-FU), and molecular targeting drugs, such as
erlotinib and gemcitabine, have provided various treatments and
significantly extended the survival time of patients with cancer
(7). However, the acquisition of
resistance to chemotherapeutics is an intractable problem in
clinical chemotherapy for cancer, reducing the effectiveness of
treatment. To escape the attack of multiple chemotherapeutics,
cancer cells have evolved multiple strategies, including promoting
drug transport, altering drug targets, elevating DNA repair
capacity, evading apoptosis and autophagy, and promoting adaptive
responses (8). Nevertheless, the
exact mechanisms of chemotherapy resistance remain to be fully
elucidated.
With increasing in-depth research, circRNAs have
been found to serve important roles in cancer development,
including cancer proliferation, metastasis, chemoresistance and
radioresistance, suggesting the potential of circRNA as a new tool
for overcoming tumor resistance. The present study focused on the
signaling pathways via which circRNAs promote the development of
tumor chemoresistance, with the aim of finding general molecular
mechanisms that stimulate further exploration of the pathway(s)
through which circRNAs are involved in chemoresistance and
therapeutic targets associated with these pathways.
Function of circRNAs
Multiple functions of circRNAs have been revealed;
circRNAs can not only act as microRNA (miRNA/miR) sponges, protein
sponges, and transcriptional and translational regulators, but also
interact with proteins and translated peptides, and compete with
pre-mRNA splicing (9). These
functions may provide insight into the roles of circRNAs in the
chemoresistance pathway.
miRNA sponges
Endogenous circRNAs can act as sponges of miRNAs and
modulate the effects of miRNAs on target genes (10). miRNA dysregulation has been confirmed
to play important roles in cancer growth via several mechanisms,
including changes in genomic miRNA copy numbers and gene locations
(11,12). The discovery of the circRNA-miRNA code
has increased understanding of the dysregulation of miRNAs. For
example, circ-DOCK1 can regulate baculoviral IAP repeat-containing
protein 3 by sponging miR-196a-5p and thereby participating in the
regulation of oral squamous cell carcinoma (OSCC) (13). However, the effects of circRNAs on
their potential sponge miRNAs in chemoresistance remain largely
undiscovered (14). Furthermore,
concerns regarding the quantity of circRNAs required to achieve a
measurable effect have been proposed due to the low levels of
circRNAs and limited binding sites with miRNAs in cells (15).
Protein sponges and interactions with
proteins
In addition to interacting with miRNA, circRNAs are
processed cotranscriptionally to combine with various proteins as
protein sponges or protein scaffolds (14). An example of circRNAs serving as
protein scaffolds is the feedback loop between mannose-binding
lectin (MBL) and circ-MBL (16).
Excess levels of MBL promote the expression of circ-MBL, which
absorbs the excess MBL, thereby decreasing its own mRNA expression
and maintaining a balance in MBL production (14). Furthermore, circRNAs have been
reported to engage with proteins and sequester proteins. For
example, it was reported that the CDK2 and p21 can bind to
circ-FoxO3 and form a circ-FoxO3-p21-CDK complex; the formation of
this circ-FoxO3-p21-CDK2 complex can inhibit the function of CDK2
and induce cell cycle disorders (17). Furthermore, circ-FoxO3 can also
interact with E2F1, ID-1, hypoxia-inducible factor 1α and focal
adhesion kinase as an upstream signaling molecule (9). Thus, circRNAs can participate in and
regulate a variety of cellular behaviors through proteins.
Transcriptional and translational
regulators
To regulate gene transcription, the sequence of the
circRNA itself is duplicated with the DNA sequence of the host
gene. When circRNA stays in the nucleus for a certain period of
time, it will form an RNA:DNA hybrid strand with the maternal DNA
double strand. Under these circumstances, the maternal DNA
transcription of other transcripts will be halted, which is
referred to as negative feedback loop regulation (18). In addition, circRNAs have the
potential to be pseudogenes; some circRNAs can be inserted into the
genome to alter the genetic information of the genome via
retrotransposition, regulating gene expression (19). A computational pipeline, CIRCpseudo,
was developed to identify potential circRNA-derived pseudogenes in
the mouse reference genome, and it was found that circ-SATB1 from
mouse can be inserted into the CTCF gene sequence as a pseudogene
(19).
Although circRNAs have been found to be
predominantly located in the cytoplasm, circRNAs in the nucleus are
involved in the regulation of transcription, alternative splicing
and chromatin looping (20). circRNAs
formed by processed intron lariats (ciRNAs) or by back-splicing
with retained introns (EIciRNAs) are limited to the nucleus in
human cells (14). RNA polymerase II
(Pol II) consists of U1 small nuclear ribonucleoprotein (snRNP) and
other proteins, and EIciRNA-U1 snRNP complexes can combine with RNA
Pol II at the promoters of their parent genes to enhance gene
expression (14). Additionally, a
ciRNA called ci-ankrd52 was revealed to modulate RNA Pol II
transcription by accumulating at its own sites of transcription
(21).
Furthermore, circRNAs have been found to play an
essential regulatory role in translation. circ-polyadenylate
binding protein 1 (PABPN1) has been shown to sequester HuR to
regulate the translation rate of the PABPN1 gene and reduce PABPN1
translation (22). The ribosome is
the main organelle involved in translation; it was reported that
the circ-ANRIL can modulate ribosomal RNA maturation to control
ribosome biogenesis and nucleolar stress (23), which highlights the association
between circRNA and translation regulation.
circRNAs can be translated
Most endogenous circRNAs cannot be translated, as
they lack a 5′ 7-methylguanosine triphosphate cap and a 3′-end
poly(A) tail (20). However, studies
have shown that certain endogenous circRNAs can be translated into
proteins or peptides (24). The
majority of circRNAs spliced from coding genes contain open reading
frames (ORFs) and thus have protein coding potential (25). Although thousands of circRNAs are
predicted to contain putative ORF and upstream internal ribosome
entry sites (IRESs), to date only a few endogenous circRNAs, such
as circ-PINTexon2, circ-F-box/WB repeat-containing protein 7,
circ-Mb1, circ-zinc finger protein 609 (ZNF609) and circ-SHPRH,
have been shown to be useful protein templates (26). Recently, it was found that ~50% of
male germ cell circRNAs exhibited protein-coding potential,
containing large ORFs and m6A-modified start codons in junction
sequences (25). Additionally, a ~10
kDa protein encoded by circ-MBL3 has been detected via mass
spectrometry (24). circ-ZNF609
contains an ORF and is translated into a protein in a
splicing-dependent/cap-independent manner (26). Furthermore, circRNAs can promote the
direct binding of translatable circRNAs to initiation factors or
ribosomes, acting as IRESs (14).
Furthermore, as circRNA-derived peptides are typically truncated
forms of standard proteins lacking essential functional domains
(26), they may serve as
dominant-negative protein variants, decoys or modulators of
alternative protein complexes.
Mechanisms of drug resistance in cancer
Chemotherapy is one of the main methods for treating
malignancy, and multidrug resistance (MDR) is the main problem
limiting the success of chemotherapy. The resistance of cancer
cells can be categorized as primary resistance or acquired
resistance. The former is resistance that existed in the tumor
cells before the use of antitumor drugs, and is unassociated with
their use. The latter is resistance induced by drug administration;
that is, the cells were sensitive to the drug(s) before drug
administration and became resistant afterwards. Acquired resistance
may limit the application of chemotherapeutics and involves the
gradual loss of the initial promising effect of chemotherapy.
Numerous mechanisms have been described to explain MDR, including
mechanisms involving drug transport and metabolism, alterations of
drug targets, DNA damage repair, downstream resistance mechanisms,
adaptive responses and the tumor microenvironment (Fig. 1) (8).
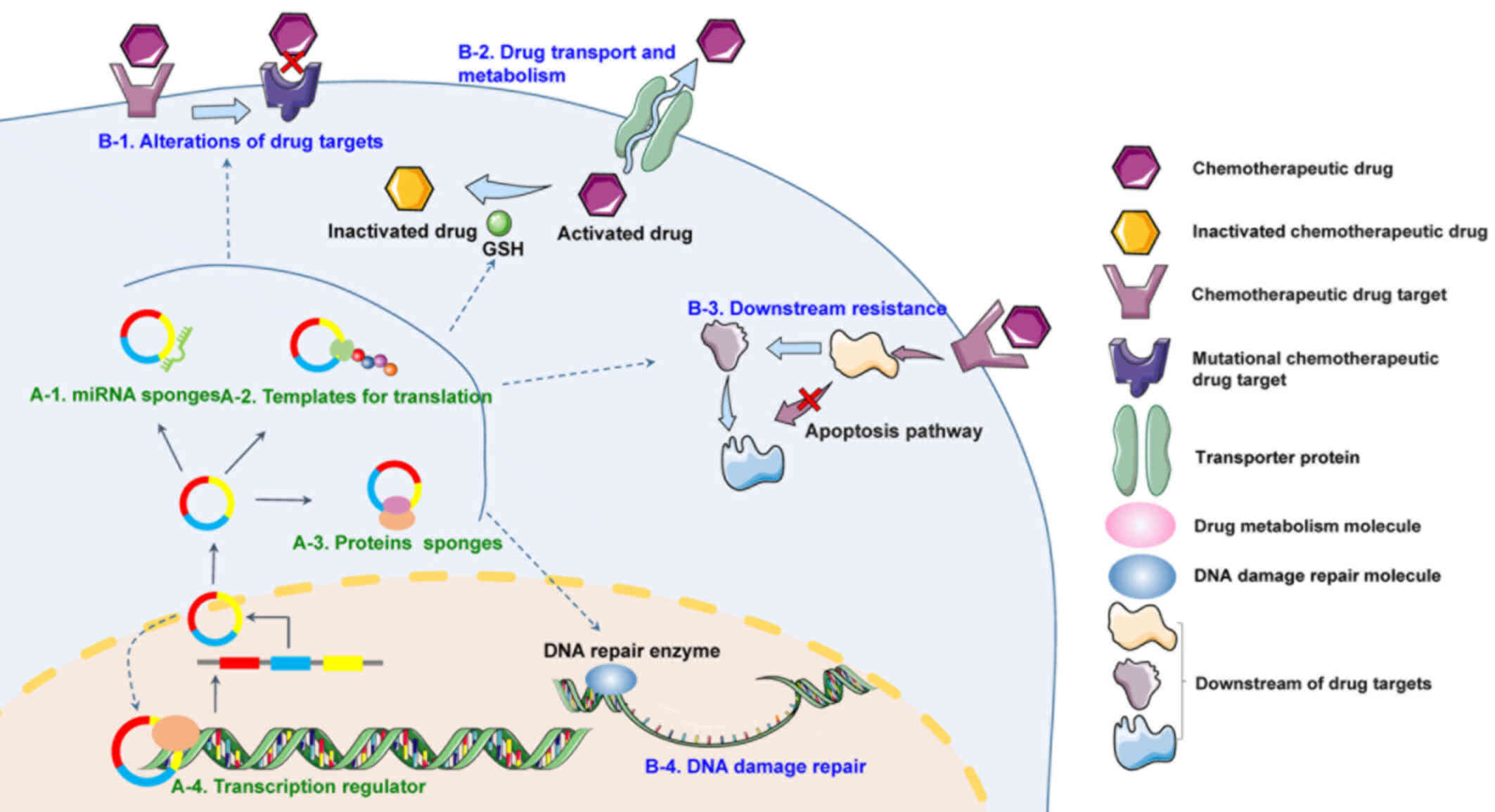 | Figure 1.Mechanisms of circRNA involvement in
chemotherapy resistance. Typical circRNAs are formed by precursor
mRNA back-splicing or exon-skipping events in eukaryotes. (A)
General mechanisms of circRNA functions in chemotherapy resistance.
(1) circRNAs can sponge miRNAs,
resulting in enhanced levels of ribosome binding and translation of
target RNAs. (2) circRNAs with
internal ribosome entry site elements and AUG sites may be
translated and generate unique peptides. (3) Certain circRNAs can bind proteins to form
circRNA-ribonucleoproteins, such as circ-FoxO3-p21-CDK, thus
regulating their functions. (4)
circRNAs can act as transcriptional regulators by recruiting
specific proteins to certain loci or subcellular compartments. (B)
Mechanisms via which circRNAs participate in chemotherapy
resistance. (1) Alterations of drug
targets. Drug inactivation resulting from alterations of drug
targets is an important means of drug resistance. For example,
increased expression of AR in prostate cancer has been found to
reduce the efficacy of AR antagonists such as bicalutamide.
(2) Drug transport and metabolism.
Tumor cells can resist drug actions by promoting drug efflux via
several cell membrane transporter proteins, such as members of the
ATP-binding cassette transporter family, or drug inactivation; for
instance, platinum drugs can be inactivated by the thiol GSH.
(3) Downstream resistance. After
drugs have inhibited their cellular target, various innate adaptive
responses can be triggered to promote the survival of cancer cells,
such as dysregulation of apoptosis pathways. (4) DNA damage repair. Tumor cells can avoid
cell death and cell cycle arrest by promoting the repair of DNA
damage induced by agents such as cisplatin. circRNA/circ, circular
RNA; AR, androgen receptor; GSH, glutathione. |
Drug transport and metabolism
Reducing the intracellular concentration of the
drugs is an effective method for cancer cells to avoid the damage
induced by drugs. A group of membrane proteins have been found to
contribute to the drug resistance of commonly used antitumor drugs
by promoting drug efflux. For example, multi-drug resistance
(MDR)1, a member of the ATP-binding cassette (ABC) transporter
family, has been reported to regulate the absorption, distribution
and excretion of various anticancer drugs and inhibit the efficacy
of chemotherapy in numerous cancers (27). Other ABC family transporters
associated with MDR include multidrug resistance-associated protein
2 and breast cancer resistance protein (28). These three ABC transporters are
commonly co-expressed in cancer and exhibit a wide range of
substrate specificity overlap involving drugs such as doxorubicin,
epirubicin, etoposide, irinotecan and mitoxantrone (28). Drug metabolism is another important
means to induce drug resistance, particularly when a
chemotherapeutic drug is combined with a specific targeting
molecule. For example, metallothionein and glutathione can bind
cisplatin (CDDP), leading to drug inactivation (29).
Alterations to drug targets
Alterations to drug targets, such as mutations or
changes in expression levels, may affect drug response and
resistance. For example, altered expression of thymidylate synthase
and ribonucleotide reductase reduces the effectiveness of
inhibitors of these targets (30).
Furthermore, cases of alterations to genes, including EGFR and
anaplastic lymphoma kinase (ALK), leading to drug resistance have
been observed. For example, a common drug resistance-related
mutation, EGFR T790M, is associated with the acquisition of drug
resistance (31). Additionally, in
non-small cell lung cancer (NSCLC), gefitinib and erlotinib can
activate mutations in the EGFR tyrosine kinase domain (32). In ALK-positive patients with NSCLC
treated with tyrosine kinase inhibitor (TKI), ALK tyrosine kinase
domain mutations or ALK fusion gene amplifications may occur
(33).
DNA damage repair
A number of drugs, such as platinum and
topoisomerase inhibitors, can lead to cell cycle arrest by inducing
DNA damage, which will result in cancer cell death (34). However, some tumor cells can escape
drug-induced damage by means of DNA damage repair, thus achieving
drug resistance. The level of ERCC1-XPF has been found to be
elevated in CDDP-treated testis tumor cells and result in increased
DNA repair (35). It has been
established that p53 is an important tumor suppressor protein for
various types of human tumor; when platinum drugs cause damage to
the DNA of tumor cells, p53 can initiate DNA damage repair and cell
cycle arrest, and its mutation is frequently associated with drug
resistance (36). To block the
activation of DNA damage repair mechanisms, molecular targeting
drugs have been developed, such as inhibitors of the
single-strand-break DNA repair enzyme poly(ADP-ribose) polymerase
1; these drugs have proved effective against breast and ovarian
tumors that involve mutations in the BRCA1 or BRCA2 genes (37). Therefore, targeting or blocking DNA
repair processes is an effective treatment strategy.
Downstream resistance mechanisms
Even if enough active drug molecules accumulate on a
cellular target, numerous intrinsic adaptive responses can be
triggered that promote cancer cell survival (8). Under such conditions, cancer cells can
evade drug-induced cell death in two ways: Apoptotic evasion and
autophagy (8). Apoptosis is triggered
when cells are in an adverse environment, such as the environment
observed during anticancer therapy. Tumor cells have evolved
multiple strategies to limit or evade apoptosis. One common way to
block apoptosis is via the loss of the damage sensor TP53 (38). Autophagy is the process of
phagocytosis and decomposition of cytoplasmic proteins or
organelles by lysosomes; this process allows the resulting
catabolites to be recycled to maintain cellular biosynthesis and
viability (39). Paradoxically,
nutritional starvation, radiation therapy and certain cytotoxic
drugs can lead to elevated levels of autophagy, which instead of
promoting the anticancer effects of chemotherapeutic drugs, has a
protective effect on cancer cells (40). Numerous anticancer treatments can
activate the autophagy pathway; conversely, hydroxychloroquine, an
autophagy inhibitor, has been developed, and it can cause human
cancer cells to become sensitive to chemotherapy (41).
Promotion of adaptive responses
The promotion of adaptive responses consists of
three parts: Activation of prosurvival signaling, oncogenic bypass
and pathway redundancy, and epithelial-mesenchymal transition
(8). The addition of EGFR-targeted
therapies to irinotecan-based chemotherapy in KRAS-wild-type
colorectal cancer has shown beneficial effects. However,
KRAS-mutant colorectal cancer is unresponsive to EGFR inhibitors as
oncogenic KRAS is not dependent upon upstream activation by EGFR;
this is an example of both activation of prosurvival signaling and
oncogenic bypass resistance to EGFR inhibitors that was observed in
cell lines undergoing epithelial-mesenchymal transition (42,43).
EGFR-targeted drugs are promising for drug resistance
treatment.
Tumor microenvironment
In both solid tumors and hematological malignancies,
the complex tumor microenvironment provides shelter for cancer
cells, protecting them from chemotherapeutic drugs and facilitating
disease relapse. In addition, communication between cancer cells
may mediate the development of chemoresistance (43). For example, increased expression of
integrins can promote drug resistance (44). Furthermore, cytokines and growth
factors are associated with resistance. Wilson et al
(45) found that hepatocyte growth
factor in the tissue microenvironment can induce drug resistance by
reactivating either or both of the PI3K-AKT and MEK-ERK pathways.
Moreover, exosomes play roles in the regulation of drug resistance.
It has been shown that CDDP-resistant ovarian cancer cells release
more protein and export higher levels of CDDP through exosomes than
CDDP-sensitive cells (46). Exosomes
containing miR-21 from CDDP-resistant OSCC cells were found to
promote chemoresistance by targeting PTEN and programmed cell death
protein 4 (PDCD4) in recipient OSCC cells (47).
Extrachromosomal circular DNA
(ecDNAs)
ecDNA, a type of circular DNA structure found
outside of the normal chromosome structure, has begun to receive
increasing attention. A previous study reported a novel targeted
drug resistance mechanism mediated by ecDNA in glioblastoma: Tumor
cells could show resistance to EGFR TKI by eliminating mutant EGFR
in ecDNA (48). It is hypothesized
that ecDNA is related to resistance mechanisms; however, there
remains a lack of research in this area.
The mechanisms of drug resistance for different
anticancer drugs during the process of drug resistance in different
tumors do not exist independently. Multifaceted drug resistance
pathways may complement each other during the development of drug
resistance. An example is EGFR, which has been extensively studied;
EGFR is involved in multiple mechanisms, including alterations of
drug targets, dysregulation of apoptosis, activation of prosurvival
signaling and epithelial-mesenchymal transition (8).
Mechanisms of circRNAs in cancer drug
resistance
The mechanisms via which circRNAs promote drug
resistance were divided into four categories: circRNA-miRNA
patterns; fusion circRNAs; circRNA in exosomes; and mechanisms
mediating chemoresistance potentially related to circRNAs in
cancers. This classification emphasizes the roles of circRNAs in
various drug-resistant pathways, providing a theoretical basis for
future research aimed at overcoming tumor drug resistance with
circRNAs as the targets.
circRNA-miRNA patterns
miRNAs serve important roles in cell development,
cell differentiation, chemoresistance and the immune system, and
function as oncogenes and tumor suppressors (49). For example, the FOXC1/miR-31-5p/large
tumor suppressor kinase 2 pathway can modulate chemoresistance in
colorectal cancer (50).
Additionally, miR-375 can promote colorectal cancer cell
sensitivity to 5-FU by directly targeting yes-associated protein 1
and SP1 (51). As sponges of miRNAs,
circRNAs can modulate cancer cell chemoresistance by absorbing and
degrading miRNAs (52). Identified
circRNA-miRNA pathways involved in the development of cancer drug
resistance are summarized in Table I.
Most of the contributing studies aimed to elucidate the regulatory
effects of circRNA-miRNA interactions on target proteins involved
in chemoresistance. Several proteins listed in Table I, such as STAT3, EGFR and p53, are
associated with the dysregulation of apoptosis, the activation of
prosurvival signaling and DNA damage repair, respectively.
Interactions between circRNAs and miRNAs can promote drug
resistance by regulating protein expression (Fig. 2).
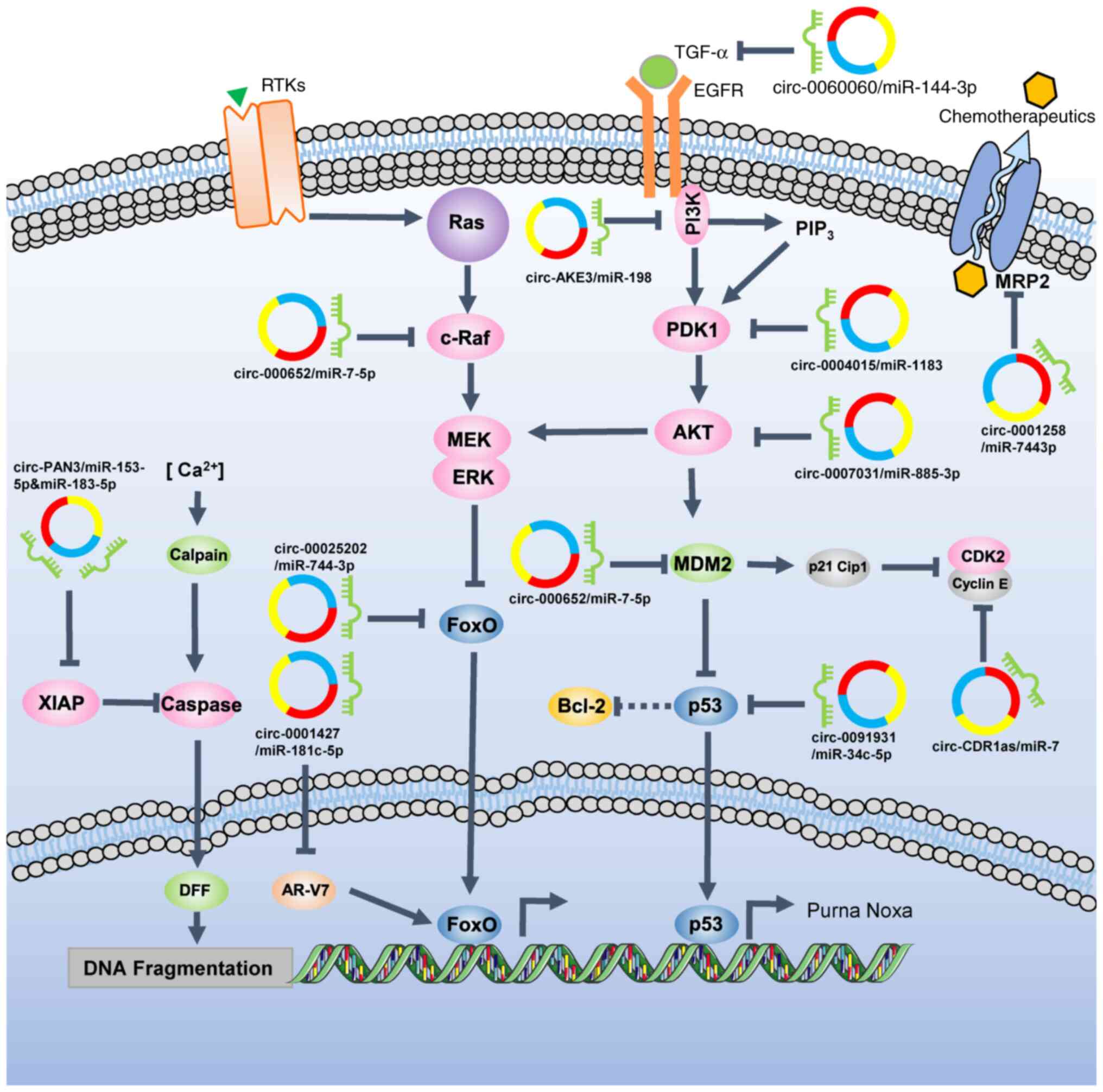 | Figure 2.Network regulation of chemotherapy
resistance via circRNA-miRNA interactions. In the apoptosis
pathway, circ-PAN3 elevate the expression of XIAP by sponging
miR-153-5p and miR-183-5p, thereby promoting DNA damage repair.
circ-0001427 sponges miR-181c-5p, which targets AR-V7 and increases
its expression, resulting in the reduced efficacy of AR
antagonists. In the AKT-ERK regulatory network,
circ-000652/miR-7-5p/c-Raf, circ-00025202/miR-744-3p/FoxO,
circ-0060060/miR-144-3p/IGF, circ-0004015/miR-1183/PDK1,
circ-0007031/miR-885-3p/AKT, circ-000652/miR-7-5p/MDM2,
circ-0091931/miR-34c-5p/p53 and circ-CDR1as/miR-7/CDK2-cyclin E
axes may induce proliferation signal transmission, leading to
evasion of drug-induced cell death. By sponging miR-744-3p,
circ-0001258 could increase the levels of MRP2, which can transfer
chemotherapeutics out of cancer cells. circRNA/circ, circular RNA;
miRNA/miR, microRNA; AR-V7, androgen receptor V7; DFF, DNA
fragmentation factor; MDM2, mouse double minute homolog 2; MRP2,
multidrug resistance-associated protein 2; PDK1,
phosphoinositide-dependent protein kinase 1; RTK, receptor tyrosine
kinase; XIAP, X-linked inhibitor of apoptosis protein. |
 | Table I.circRNAs-miRNAs and drug
resistance. |
Table I.
circRNAs-miRNAs and drug
resistance.
| First author,
year | Cancer type | circRNA |
Expressiona | Validated/putative
targets/pathways | Drug
resistance-related effects | (Refs.) |
|---|
| Yan et al,
2019 | Renal clear cell
carcinoma | circ-0035483 | Up | miR-335 | Promotes
gemcitabine resistance | (53) |
| Yang et al,
2019 | Breast cancer | circ-CDR1as | Up | miR-7/CCNE1 | Promotes docetaxel
resistance | (79) |
| Huang et al,
2019 | Gastric cancer | circ-AKT3 | Up | miR-198/PIK3R1 | Promotes CDDP
resistance | (52) |
| Yu et al,
2019 | Lung
adenocarcinoma | circ-0003998 | Up | miR-326 | Promotes
doxorubicin resistance | (80) |
| Shang et al,
2019 | Acute myeloid
leukemia | circ-PAN3 | Up | miR-153-5p and
miR-183-5p-XIAP | Promotes
doxorubicin resistance | (81) |
| Kun-Peng et
al, 2018 | Osteosarcoma | circ-0004674 | Up | miR-490-3p/ABCC2
and miR-1254/EGFR | Promotes
doxorubicin/cisplatin/methotrexate resistance | (82) |
| Liu et al,
2018 | Thyroid cancer | circ-0060060 | Up |
miR-144-3p/TGF-α | Promotes CDDP
resistance | (83) |
| Gao et al,
2019 | Breast cancer | circ-000652 | Up | miR-7-5p/Raf1 | Promotes adriamycin
resistance | (84) |
| Zhou et al,
2019 | NSCLC | circ-0004015 | Up | miR-1183/PDPK1 | Promotes gefitinib
resistance | (85) |
| Xu et al,
2018 | NSCLC | circ-0000567 | Up | miR-141 | Promotes taxol
resistance | (5) |
| Xu et al,
2018 | NSCLC | circ-0091931 | Down | miR-34c-5p/p53 | Promotes taxol
resistance | (5) |
| Hua et al,
2019 | NSCLC | circ-0000567 | Up | miR-124 | Promotes pemetrexed
resistance | (86) |
| Xiong et al,
2017 | Colorectal
cancer | circ-0000504 | Up | miR-485-5p on
STAT3 | Promotes 5-FU
resistance | (87) |
| Xiong et al,
2017 | Colorectal
cancer | circ-0007031 | Up |
miR-885-3p/AKT3/BCL2 | Promotes 5-FU
resistance | (87) |
| Xiong et al,
2017 | Colorectal
cancer | circ-0048234 | Down |
miR-671-5p/EGFR | Promotes 5-FU
resistance | (87) |
| Sang et al,
2019 | Breast cancer | circ-0025202 | Down |
miR-182-5p/FoxO3a | Suppresses
tamoxifen resistance | (76) |
| Zhu et al,
2019 | Osteosarcoma | circ-0001258 | Down |
miR-744-3p/GSTM2 | Suppresses drug
resistance | (88) |
| Chi et al,
2019 | Bladder cancer | circ-0000285 | Down | miR-124 or
miR-558 | Suppresses CDDP
resistance | (89) |
| Wu et al,
2019 | Prostate
cancer | circ-0001427 | Down |
miR-181c-5p/ARv7 | Suppresses
enzalutamide resistance | (90) |
Research into circRNA-miRNA-protein three-stage
regulatory networks has added an extra layer of complexity to
understanding of cancer drug resistance; however, it also provides
numerous potential targets to reverse chemoresistance that cover
proteins and RNAs at same time. A recent study suggested that
circRNA can promote gemcitabine resistance via autophagy regulation
(53). Furthermore, numerous studies
have found that circRNAs can influence tumor cell resistance to
chemotherapy drugs via miRNA-mRNA axes (54–56).
However, certain studies lack detailed resistance mechanisms;
studies into the regulation of drug resistance via circRNA-protein
pathways may neglect the involvement of miRNAs. The detailed
mechanisms through which circRNAs are involved in tumor resistance
remain unclear. At present, most research into modulation of cancer
cell chemoresistance by circRNAs has focused on circRNA-miRNA
pathways, which may overshadow circRNAs that regulates cancer drug
resistance via other mechanisms.
Fusion circRNAs (f-circRNAs)
As genes are misallocated due to abnormal chromosome
translocations and chromosomal rearrangements, complementary
repeating intron sequences such as the Alu-sequence may be
introduced close enough to facilitate novel reverse splicing events
during RNA maturation, leading to the production of abnormal
circRNAs (57). Therefore, the
juxtaposition of complementary sequences in the upstream and
downstream introns of the translocation breakpoint region may form
new circRNAs, called f-circRNAs, which are formed from the fusion
of two translocation genes (57).
Roles for this new type of circRNA in chemotherapy resistance have
been identified. For example, in acute promyelocytic leukemia,
general translocation occurs between the promyelocytic leukemia
protein (PML) and retinoic acid receptor (RAR) genes, which then
form f-circRNAs (58). F-circ-M9_1
and f-circ-M9_2 are two f-circRNAs formed via MLL/AF9 translocation
in acute myeloid leukemia; it was reported that f-circM9 can
promote chemotherapy resistance in acute myeloid leukemia (57). Furthermore, an f-circRNA from the
BCR-ABL1 fusion gene, circ-BA9.3, was found to be associated with
resistance to TKIs by increasing the production of C-ABL1 or
BCR-ABL1 protein in leukemic cells (59).
circRNA in exosomes
Exosomes, containing a variety of proteins, DNA,
mRNA, miRNA and other molecules, serve important roles in
intercellular communication and the triggering of physiological
responses (60). It has been reported
that exosomes released from CDDP-resistant OSCC cells transmit
miR-21, which targets PTEN and PDCD4 to decrease the drug
resistance of OSCC cells (47).
circ-CDR1as, which suppresses CDDP resistance in ovarian cancer,
has been reported to be downregulated in serum exosomes from
CDDP-resistant patients (61).
However, the mechanism via which exosomes regulate drug resistance
in ovarian cancer cells is unclear. Additionally, exosomal circ-Myc
in the serum is associated with recurrence and bortezomib
resistance in multiple myeloma (62).
Recently, it was observed that exosomal circ-nuclear factor 1
X-type (NFIX) was upregulated in the serum of temozolomide
(TMZ)-resistant patients and exosomal circ-NFIX from TMZ-resistant
cells conferred TMZ resistance to recipient sensitive cells in
glioma (63). This newly identified
resistance mechanism may provide novel resistance targets.
Mechanisms mediating chemoresistance
potentially related to circRNAs in cancers
circRNAs participate in the regulation of cancer
cell chemoresistance not only by interacting with miRNAs, but also
by affecting certain signaling pathways. Tumor resistance involves
the mutual regulation of signaling networks. For example, BRCA1
serves an important role in the homology-directed repair of DNA
double-strand breaks, which modulates chemotherapy resistance
(64). AKT activation regulates
resistance to CDDP-induced apoptosis by inhibiting
apoptosis-inducing factor-related pathways (65). Furthermore, circ-PAN3 facilitates drug
resistance in acute myeloid lymphoma cells via the AMPK/mTOR
pathway (66). circ-mitochondrial
tRNA translation optimization 1 reverses monastrol resistance by
regulating the TNF receptor associated factor 4/Eg5 axis (67). In addition, circ-plasmacytoma variant
translocation 1 facilitates the expression of ABCB1 to enhance the
doxorubicin and CDDP resistance of osteosarcoma cells (67). It has been reported that lung
adenocarcinoma can activate autophagy via the AMPK/mTOR signaling
pathway and thus induce CDDP resistance (68). Additionally, ABCB1 is involved in drug
transport and metabolism (69).
However, there are few studies concerning the direct binding of
circRNAs to proteins to regulate drug resistance.
circRNAs are involved in multiple drug resistance
pathways and can form complex drug-resistance networks. The roles
of circRNAs in resistance, including circRNA-miRNA interactions,
circRNA-protein interactions and F-circRNAs, have been reviewed.
However, some circRNAs have been identified to be associated with
cancer drug resistance, but information concerning their mechanisms
is lacking. For example, circ-0004350 and circ-0092857 are involved
in the drug sensitivity of lung cancer, and circ-elongator complex
protein 3 contributes to CDDP resistance (70,71).
Additionally, circ-100053 can promote imatinib resistance in
chronic myeloid leukemia (72), and
circ-coiled-coil domain containing 66 can increase the EGFR
resistance of lung adenocarcinoma cells (73). Nevertheless, the mechanisms via which
these circRNAs alter drug resistance remain unclear. This topic
merits further investigation, and future research may lead to the
identification of potential protein targets to overcome
chemotherapy resistance.
Potential applications of circRNAs in cancer
drug resistance treatment
With developments in molecular oncology, targeted
drugs that regulate drug resistance genes have become an important
tool for reversing drug resistance. A previous study found that RNA
interference can significantly reduce the expression of STAT3,
allowing the resensitization of resistant cancer cells (74). Findings concerning the multiple
mechanisms of circRNA in cancer drug resistance have indicated the
potential of circRNAs to serve as a new treatment tool.
One important mechanism of tumor resistance entails
modulation of the regulatory effects of miRNA. In vitro
research has suggested that certain miRNA mimics or antagomirs can
enhance the treatment effect of anticancer drugs by regulating
target protein expression (75). In
the case of imbalanced circRNAs in drug-resistant cancer, circRNA
levels could be adjusted by cloning the circRNA sequence and its
regulatory flanking regions or by using small interfering RNA
(siRNA). In various animal experiments, it has been shown that
circRNA can be used as a target to achieve substantial therapeutic
efficacy (52,76). Additionally, it has been reported that
packaging siRNA in extracellular vesicles via a pre-microRNA
backbone can allow a reduced therapeutic dose of siRNA (77). It is hypothesized that circRNA may
serve as a novel type of miRNA vector with multiple miRNA
adsorption target sites that promote chemoresistance in order to
block drug resistance, utilizing the function of circRNA as a miRNA
sponge.
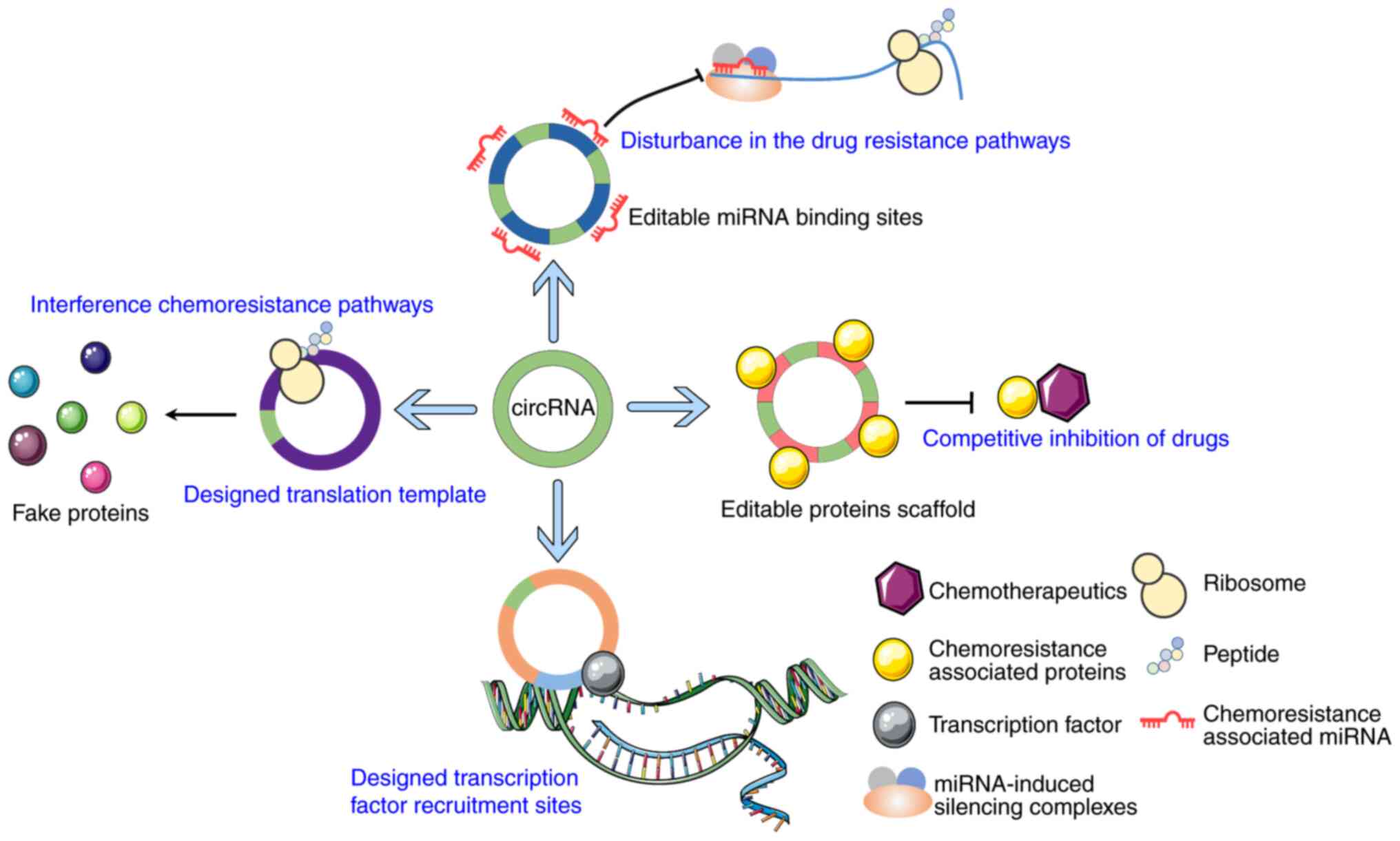
It is proposed that circRNAs could be designed to
sponge miRNAs and proteins that promote chemoresistance; due to the
size of circRNAs, multiple sponge sites could be designed and
incorporated to enhance their function. Additionally, as circRNAs
can function as transcriptional and translational regulators, it
may be possible to design artificial circRNAs to regulate the
transcription and translation of essential genes involved in the
pathway of drug resistance by interacting with their promoters.
However, a lack of information concerning the mechanisms via which
circRNA can act as a transcriptional and translational regulator is
an obstacle to realizing this possibility. It may also be possible
to design certain circRNAs similar to plasmids that can carry a
target gene sequence and transmit it into cancer cells to express
specific proteins and inhibit drug resistance (Fig. 3). Furthermore, it may be possible to
design circRNAs with several of the functions mentioned above to
enhance their ability to regulate drug resistance.
circRNAs are increasingly recognized as important
factors in maintaining cellular homeostasis. circRNAs are closely
associated with chemoresistance and may be used as potential
therapeutic targets and prognostic markers in solid tumors or
hematological malignancies. Furthermore, circRNAs may be used as an
early marker of tumor drug resistance, as they can readily enter
the circulatory system by the exosome pathway (52,63,78).
Conclusions and perspectives
The present review provided a novel perspective on
the roles of circRNAs in chemotherapy resistance. By consulting
studies into the various resistance pathways, it was found that
most research concerning the modulation of chemoresistance in
cancer cells by circRNAs has focused on circRNA-miRNA pathways.
Furthermore, a novel form of circRNA that has been discovered,
f-circRNA, may play important roles in chemoresistance. circRNA
research will increase present understanding of the mechanisms
underlying tumor resistance and identify therapeutic targets to
combat drug resistance. The detailed mechanisms via which circRNAs
affect drug resistance remain to be elucidated.
Although not all drug resistance-associated circRNAs
are included in this review, the studies summarized above
demonstrate that circRNAs serve important regulatory roles in
chemotherapy. circRNAs are involved in multiple drug resistance
pathways and can form complex drug resistance networks. The roles
of circRNA in resistance were reviewed, addressing circRNA-miRNA
and circRNA-protein interactions, as well as f-circRNAs. However,
some circRNAs have been identified to be associated with cancer
drug resistance, but information on the mechanisms is lacking.
Therefore, an in-depth understanding of the molecular mechanisms
via which circRNAs participate in cancer resistance is
required.
There is great promise concerning circRNAs that
could serve as important biomarkers for diagnosis and prognosis in
clinical settings. For example, Kaplan-Meier survival analysis
revealed that recurrent patients with glioma in a low circ-NFIX
expression group exhibited improved survival compared with those in
the high circ-NFIX expression group (63). Additionally, a previous study reported
that low levels of circ-AKT3 in patients with gastric cancer
receiving CDDP therapy were associated with poorer 5-year
disease-free survival (52).
Furthermore, decreased expression of circ-KDM4C in breast cancer
was associated with poorer overall survival (78). Accumulating evidence indicates that
circRNAs may serve an important role in the diagnosis and prognosis
of various tumors.
miRNAs and proteins that may be associated with drug
resistance should be investigated for their relationships with
circRNAs. There is great clinical potential for findings from
research into circRNAs; however, due to the low intracellular
concentrations of circRNAs, their adsorption and biological
functions are limited. Additionally, the large number of
resistance-related circRNAs may lead to failure in the treatment of
drug resistance. Nevertheless, circRNAs exhibit great potential for
overcoming drug resistance, especially for tumors that can readily
develop chemoresistance.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81872211, 81672675 and
81771081), the 111 Project of MOE China (grant no. B14038), the
Sichuan University Innovation and Entrepreneurship Training Program
for Undergraduates (grant no. C2019106455) and the Graduate
Student's Research and Innovation Fund of Sichuan University (grant
no. 2018YJSY018).
Availability of data and materials
Not applicable.
Authors' contributions
CX, FH, JL and JKW wrote the manuscript and designed
the figures. JL and JKW provided guidance and revised the
manuscript. QC revised this manuscript. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bach DH, Lee SK and Sood AK: Circular RNAs
in cancer. Mol Ther Nucleic Acids. 16:118–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nigro JM, Cho KR, Fearon ER, Kern SE,
Ruppert JM, Oliner JD, Kinzler KW and Vogelstein B: Scrambled
exons. Cell. 64:607–613. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrett SP and Salzman J: Circular RNAs:
Analysis, expression and potential functions. Development.
143:1838–1847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu N, Chen S, Liu Y, Li W, Liu Z, Bian X,
Ling C and Jiang M: Profiles and bioinformatics analysis of
differentially expressed circrnas in taxol-resistant non-small cell
lung cancer cells. Cell Physiol Biochem. 48:2046–2060. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou R, Chen KK, Zhang J, Xiao B, Huang Z,
Ju C, Sun J, Zhang F, Lv XB and Huang G: The decade of exosomal
long RNA species: An emerging cancer antagonist. Mol Cancer.
17:752018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solassol I, Pinguet F and Quantin X: FDA-
and EMA-Approved tyrosine kinase inhibitors in advanced
EGFR-Mutated non-small cell lung cancer: Safety, tolerability,
plasma concentration monitoring, and management. Biomolecules.
9:6682019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei K, Bai H, Wei Z, Xie C, Wang J, Li J
and Chen Q: The mechanism and function of circular RNAs in human
diseases. Exp Cell Res. 368:147–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong DD, Dang YW, Lin P, Wen DY, He RQ,
Luo DZ, Feng ZB and Chen G: A circRNA-miRNA-mRNA network
identification for exploring underlying pathogenesis and therapy
strategy of hepatocellular carcinoma. J Transl Med. 16:2202018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayani J, Kuzmanov U, Saraon P, Fung WA,
Soosaipillai A, Squire JA and Diamandis EP: Copy number and
expression alterations of miRNAs in the ovarian cancer cell line
OVCAR-3: Impact on kallikrein 6 protein expression. Clin Chem.
59:296–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Wei Y, Yan Y, Wang H, Yang J,
Zheng Z, Zha J, Bo P, Tang Y, Guo X, et al: CircDOCK1 suppresses
cell apoptosis via inhibition of miR196a5p by targeting BIRC3 in
OSCC. Oncol Rep. 39:951–966. 2018.PubMed/NCBI
|
|
14
|
Li X, Yang L and Chen LL: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kristensen LS, Hansen TB, Venø MT and
Kjems J: Circular RNAs in cancer: Opportunities and challenges in
the field. Oncogene. 37:555–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conn VM, Hugouvieux V, Nayak A, Conos SA,
Capovilla G, Cildir G, Jourdain A, Tergaonkar V, Schmid M, Zubieta
C and Conn SJ: A circRNA from SEPALLATA3 regulates splicing of its
cognate mRNA through R-loop formation. Nat Plants. 3:170532017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong R, Zhang XO, Zhang Y, Ma XK, Chen LL
and Yang L: CircRNA-derived pseudogenes. Cell Res. 26:747–750.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdelmohsen K, Panda AC, Munk R,
Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM,
Martindale JL and Gorospe M: Identification of HuR target circular
RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA
Biol. 14:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holdt LM, Stahringer A, Sass K, Pichler G,
Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou
A, et al: Circular non-coding RNA ANRIL modulates ribosomal RNA
maturation and atherosclerosis in humans. Nat Commun. 7:124292016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang C, Xie Y, Yu T, Liu N, Wang Z,
Woolsey RJ, Tang Y, Zhang X, Qin W, Zhang Y, et al:
m6A-dependent biogenesis of circular RNAs in male germ
cells. Cell Res. 30:211–228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 Is a Circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: An update on the clinical strategy
of inhibiting p-glycoprotein. Cancer Control. 10:159–165. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shapira A, Livney YD, Broxterman HJ and
Assaraf YG: Nanomedicine for targeted cancer therapy: Towards the
overcoming of drug resistance. Drug Resist Updat. 14:150–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashida S, Soh J, Toyooka S, Furukawa M,
Shien K, Yamamoto H, Asano H, Tsukuda K, Hagiwara K and Miyoshi S:
Presence of the minor EGFR T790M mutation is associated with
drug-sensitive EGFR mutations in lung adenocarcinoma patients.
Oncol Rep. 32:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sequist LV, Martins RG, Spigel D, Grunberg
SM, Spira A, Jänne PA, Joshi VA, McCollum D, Evans TL, Muzikansky
A, et al: First-line gefitinib in patients with advanced
non-small-cell lung cancer harboring somatic EGFR mutations. J Clin
Oncol. 26:2442–2449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shaw AT, Yeap BY, Solomon BJ, Riely GJ,
Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, et
al: Effect of crizotinib on overall survival in patients with
advanced non-small-cell lung cancer harbouring ALK gene
rearrangement: A retrospective analysis. Lancet Oncol.
12:1004–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zakharenko A, Dyrkheeva N and Lavrik O:
Dual DNA topoisomerase 1 and tyrosyl-DNA phosphodiesterase 1
inhibition for improved anticancer activity. Med Res Rev.
39:1427–1441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Usanova S, Piée-Staffa A, Sied U, Thomale
J, Schneider A, Kaina B and Köberle B: Cisplatin sensitivity of
testis tumour cells is due to deficiency in interstrand-crosslink
repair and low ERCC1-XPF expression. Mol Cancer. 9:2482010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan S, el-Deiry WS, Bae I, Freeman J,
Jondle D, Bhatia K, Fornace AJ Jr, Magrath I, Kohn KW and O'Connor
PM: p53 gene mutations are associated with decreased sensitivity of
human lymphoma cells to DNA damaging agents. Cancer Res.
54:5824–5830. 1994.PubMed/NCBI
|
|
37
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
White E, Mehnert JM and Chan CS:
Autophagy, metabolism, and cancer. Clin Cancer Res. 21:5037–5046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasaki K, Tsuno NH, Sunami E, Tsurita G,
Kawai K, Okaji Y, Nishikawa T, Shuno Y, Hongo K, Hiyoshi M, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arnold D, Lueza B, Douillard JY, Peeters
M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP,
Tabernero J, et al: Prognostic and predictive value of primary
tumour side in patients with RAS wild-type metastatic colorectal
cancer treated with chemotherapy and EGFR directed antibodies in
six randomized trials. Ann Oncol. 28:1713–1729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fuchs BC, Fujii T, Dorfman JD, Goodwin JM,
Zhu AX, Lanuti M and Tanabe KK: Epithelial-to-mesenchymal
transition and integrin-linked kinase mediate sensitivity to
epidermal growth factor receptor inhibition in human hepatoma
cells. Cancer Res. 68:2391–2399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoyt K, Castaneda B, Zhang M, Nigwekar P,
di Sant'agnese PA, Joseph JV, Strang J, Rubens DJ and Parker KJ:
Tissue elasticity properties as biomarkers for prostate cancer.
Cancer Biomark. 4:213–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilson TR, Fridlyand J, Yan Y, Penuel E,
Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al:
Widespread potential for growth-factor-driven resistance to
anticancer kinase inhibitors. Nature. 487:505–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Safaei R, Larson BJ, Cheng TC, Gibson MA,
Otani S, Naerdemann W and Howell SB: Abnormal lysosomal trafficking
and enhanced exosomal export of cisplatin in drug-resistant human
ovarian carcinoma cells. Mol Cancer Ther. 4:1595–1604. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu T, Chen G, Sun D, Lei M, Li Y, Zhou C,
Li X, Xue W, Wang H, Liu C and Xu J: Exosomes containing miR-21
transfer the characteristic of cisplatin resistance by targeting
PTEN and PDCD4 in oral squamous cell carcinoma. Acta Biochim
Biophys Sin (Shanghai). 49:808–816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nathanson DA, Gini B, Mottahedeh J,
Visnyei K, Koga T, Gomez G, Eskin A, Hwang K, Wang J, Masui K, et
al: Targeted therapy resistance mediated by dynamic regulation of
extrachromosomal mutant EGFR DNA. Science. 343:72–76. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hsu HH, Kuo WW, Shih HN, Cheng SF, Yang
CK, Chen MC, Tu CC, Viswanadha VP, Liao PH and Huang CY: FOXC1
regulation of miR-31-5p confers oxaliplatin resistance by targeting
LATS2 in colorectal cancer. Cancers (Basel). 11:15762019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu X, Chen X, Xu M, Liu X, Pan B, Qin J,
Xu T, Zeng K, Pan Y, He B, et al: miR-375-3p suppresses
tumorigenesis and partially reverses chemoresistance by targeting
YAP1 and SP1 in colorectal cancer cells. Aging (Albany NY).
11:7357–7385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang X, Li Z, Zhang Q, Wang W, Li B, Wang
L, Xu Z, Zeng A, Zhang X, Zhang X, et al: Circular RNA AKT3
upregulates PIK3R1 to enhance cisplatin resistance in gastric
cancer via miR-198 suppression. Mol Cancer. 18:712019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yan L, Liu G, Cao H, Zhang H and Shao F:
Hsa_circ_0035483 sponges hsa-miR-335 to promote the
gemcitabine-resistance of human renal cancer cells by autophagy
regulation. Biochem Biophys Res Commun. 519:172–178. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lapa RML, Barros-Filho MC, Marchi FA,
Domingues MAC, de Carvalho GB, Drigo SA, Kowalski LP and Rogatto
SR: Integrated miRNA and mRNA expression analysis uncovers drug
targets in laryngeal squamous cell carcinoma patients. Oral Oncol.
93:76–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen D, Bao C, Zhao F, Yu H, Zhong G, Xu L
and Yan S: Exploring specific miRNA-mRNA axes with relationship to
taxanes-resistance in breast cancer. Front Oncol. 10:13972020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kong F, He S, Shen X, Li L, Fang J and
Lian M: Integrated analysis of different mRNA and miRNA profiles in
human hypopharyngeal squamous cell carcinoma sensitive and
resistant to chemotherapy. Neoplasma. 67:473–483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guarnerio J, Bezzi M, Jeong JC, Paffenholz
SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH and Pandolfi PP:
Oncogenic role of Fusion-circRNAs Derived from cancer-associated
chromosomal translocations. Cell. 165:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dos Santos GA, Kats L and Pandolfi PP:
Synergy against PML-RARa: Targeting transcription, proteolysis,
differentiation, and self-renewal in acute promyelocytic leukemia.
J Exp Med. 210:2793–2802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pan Y, Lou J, Wang H, An N, Chen H, Zhang
Q and Du X: CircBA9.3 supports the survival of leukaemic cells by
up-regulating c-ABL1 or BCR-ABL1 protein levels. Blood Cells Mol
Dis. 73:38–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shi Q, Huo N, Wang X, Yang S, Wang J and
Zhang T: Exosomes from oral tissue stem cells: Biological effects
and applications. Cell Biosci. 10:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao Z, Ji M, Wang Q, He N and Li Y:
Circular RNA Cdr1as Upregulates SCAI to suppress cisplatin
resistance in ovarian cancer via miR-1270 suppression. Mol Ther
Nucleic Acids. 18:24–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luo Y and Gui R: Circulating Exosomal
circMYC is associated with the recurrence and bortezomib resistance
in patients with multiple myeloma. Turk J Haematol. 37:248–262.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ding C, Yi X, Wu X, Bu X, Wang D, Wu Z,
Zhang G, Gu J and Kang D: Exosome-mediated transfer of circRNA
CircNFIX enhances temozolomide resistance in glioma. Cancer Lett.
479:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Anantha RW, Simhadri S, Foo TK, Miao S,
Liu J, Shen Z, Ganesan S and Xia B: Functional and mutational
landscapes of BRCA1 for homology-directed repair and therapy
resistance. Elife. 6:e213502017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang X, Fraser M, Abedini MR, Bai T and
Tsang BK: Regulation of apoptosis-inducing factor-mediated,
cisplatin-induced apoptosis by Akt. Br J Cancer. 98:803–808. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shang J, Chen WM, Liu S, Wang ZH, Wei TN,
Chen ZZ and Wu WB: CircPAN3 contributes to drug resistance in acute
myeloid leukemia through regulation of autophagy. Leuk Res.
85:1061982019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Y, Dong Y, Zhao L, Su L and Luo J:
Circular RNA-MTO1 suppresses breast cancer cell viability and
reverses monastrol resistance through regulating the TRAF4/Eg5
axis. Int J Oncol. 53:1752–1762. 2018.PubMed/NCBI
|
|
68
|
Wu T, Wang MC, Jing L, Liu ZY, Guo H, Liu
Y, Bai YY, Cheng YZ, Nan KJ and Liang X: Autophagy facilitates lung
adenocarcinoma resistance to cisplatin treatment by activation of
AMPK/mTOR signaling pathway. Drug Des Devel Ther. 9:6421–6431.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jiang B, Yan LJ and Wu Q: ABCB1 (C1236T)
polymorphism affects P-glycoprotein-mediated transport of
methotrexate, doxorubicin, actinomycin D, and etoposide. DNA Cell
Biol. 38:485–490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang MS, Yuan FQ, Gao Y, Liu JY, Chen YX,
Wang CJ, He BM, Zhou HH and Liu ZQ: Circular RNA screening from
EIF3a in lung cancer. Cancer Med. 8:4159–4168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Su Y, Yang W, Jiang N, Shi J, Chen L,
Zhong G, Bi J, Dong W, Wang Q, Wang C and Lin T: Hypoxia-elevated
circELP3 contributes to bladder cancer progression and cisplatin
resistance. Int J Biol Sci. 15:441–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ping L, Jian-Jun C, Chu-Shu L, Guang-Hua L
and Ming Z: High circ_100053 predicts a poor outcome for chronic
myeloid leukemia and is involved in imatinib resistance. Oncol Res.
Feb 14–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Joseph NA, Chiou SH, Lung Z, Yang CL, Lin
TY, Chang HW, Sun HS, Gupta SK, Yen L, Wang SD and Chow KC: The
role of HGF-MET pathway and CCDC66 cirRNA expression in EGFR
resistance and epithelial-to-mesenchymal transition of lung
adenocarcinoma cells. J Hematol Oncol. 11:742018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kotmakçı M, Çetintaş VB and Kantarcı AG:
Preparation and characterization of lipid nanoparticle/pDNA
complexes for STAT3 downregulation and overcoming chemotherapy
resistance in lung cancer cells. Int J Pharm. 525:101–111. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Garofalo M and Croce CM: MicroRNAs as
therapeutic targets in chemoresistance. Drug Resist Updat.
16:47–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sang Y, Chen B, Song X, Li Y, Liang Y, Han
D, Zhang N, Zhang H, Liu Y, Chen T, et al: circRNA_0025202
regulates tamoxifen sensitivity and tumor progression via
regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther.
27:1638–1652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Reshke R, Taylor JA, Savard A, Guo H, Rhym
LH, Kowalski PS, Trung MT, Campbell C, Little W, Anderson DG and
Gibbings D: Reduction of the therapeutic dose of silencing RNA by
packaging it in extracellular vesicles via a pre-microRNA backbone.
Nat Biomed Eng. 4:52–68. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liang Y, Song X, Li Y, Su P, Han D, Ma T,
Guo R, Chen B, Zhao W, Sang Y, et al: circKDM4C suppresses tumor
progression and attenuates doxorubicin resistance by regulating
miR-548p/PBLD axis in breast cancer. Oncogene. 38:6850–6866. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang W, Gu J, Wang X, Wang Y, Feng M, Zhou
D, Guo J and Zhou M: Inhibition of circular RNA CDR1as increases
chemosensitivity of 5-FU-resistant BC cells through up-regulating
miR-7. J Cell Mol Med. 23:3166–3177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yu W, Peng W, Sha H and Li J:
Hsa_circ_0003998 promotes chemoresistance via modulation of miR-326
in lung adenocarcinoma cells. Oncol Res. 27:623–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shang J, Chen WM, Wang ZH, Wei TN, Chen ZZ
and Wu WB: CircPAN3 mediates drug resistance in acute myeloid
leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp Hematol.
70:42–54.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kun-Peng Z, Xiao-Long M, Lei Z, Chun-Lin
Z, Jian-Ping H and Tai-Cheng Z: Screening circular RNA related to
chemotherapeutic resistance in osteosarcoma by RNA sequencing.
Epigenomics. 10:1327–1346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu F, Zhang J, Qin L, Yang Z, Xiong J,
Zhang Y, Li R, Li S, Wang H, Yu B, et al: Circular RNA EIF6
(hsa_circ_0060060) sponges miR-144-3p to promote the
cisplatin-resistance of human thyroid carcinoma cells by autophagy
regulation. Aging (Albany NY). 10:3806–3820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gao D, Qi X, Zhang X, Fang K, Guo Z and Li
L: hsa_circRNA_0006528 as a competing endogenous RNA promotes human
breast cancer progression by sponging miR-7-5p and activating the
MAPK/ERK signaling pathway. Mol Carcinog. 58:554–564. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou Y, Zheng X, Xu B, Chen L, Wang Q,
Deng H and Jiang J: Circular RNA hsa_circ_0004015 regulates the
proliferation, invasion, and TKI drug resistance of non-small cell
lung cancer by miR-1183/PDPK1 signaling pathway. Biochem Biophys
Res Commun. 508:527–535. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hua X, Sun Y, Chen J, Wu Y, Sha J, Han S
and Zhu X: Circular RNAs in drug resistant tumors. Biomed
Pharmacother. 118:1092332019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xiong W, Ai YQ and Li YF, Ye Q, Chen ZT,
Qin JY, Liu QY, Wang H, Ju YH, Li WH and Li YF: Microarray analysis
of circular RNA expression profile associated with
5-fluorouracil-based chemoradiation resistance in colorectal cancer
cells. Biomed Res Int. 2017:84216142017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T and
Zhang L: Analyzing the interactions of mRNAs and ncRNAs to predict
competing endogenous RNA Networks in osteosarcoma Chemo-Resistance.
Mol Ther. 27:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chi BJ, Zhao DM, Liu L, Yin XZ, Wang FF,
Bi S, Gui SL, Zhou SB, Qin WB, Wu DM and Wang SQ: Downregulation of
hsa_circ_0000285 serves as a prognostic biomarker for bladder
cancer and is involved in cisplatin resistance. Neoplasma.
66:197–202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu G, Sun Y, Xiang Z, Wang K, Liu B, Xiao
G, Niu Y, Wu D and Chang C: Preclinical study using circular RNA 17
and micro RNA 181c-5p to suppress the enzalutamide-resistant
prostate cancer progression. Cell Death Dis. 10:372019. View Article : Google Scholar : PubMed/NCBI
|