Introduction
In the female population, breast cancer exhibits the
highest cancer incidence and is a leading cause of death worldwide
(1,2). It was estimated in 2018 that more
than 2.1 million women were newly diagnosed with breast cancer with
600,000 deaths (3) and 2.3 million
new cases are estimated by 2030 (4). Although breast conservation surgery
combined with neoadjuvant therapy can reduce the mortality rate,
some breast cancer patients have potential drug-resistance genetic
profiles leading to the unsatisfactory outcome of treatments
(5).
Breast cancer is categorized into 4 major subtypes
based on the presence or absence of molecular markers by
immunohistochemical staining for the estrogen receptor (ER),
progesterone receptor (PR) and human epidermal receptor 2 (HER2):
luminal subtypes, A and B: ER-positive and/or
PR-positive/HER2-negative (luminal A) or -positive (luminal B)
accounted for 70% of breast cancer cases; HER2-positive subtype:
ER- and/or PR-negative/HER2-positive with an estimation of 15–20%,
and triple-negative (TN) or basal subtype: lacking all ER/PR/HER2
with around 15% of the total cases (6). Luminal and HER2-positive subtypes
respond well to standard treatment; however, some of them have
treatment failures (5). The TN
subtype is of great interest to explore since having no ER/PR/HER2,
these patients with advanced stage have no targeted drugs
available.
Several breast cancer cell lines have been widely
used in research to unravel the mechanisms of cancer progression
and drug resistance driven by certain genes (7). There are 27 TN breast cancer cell
lines used in breast cancer research filed of which MDA-MB-231 is
the most popular cell line with high proliferative, invasive, and
metastatic properties (8). The
trastuzumab-resistant HER2-positive MDA-MB-453 cell line has an
abnormal gene expression profile, in particular, the upregulation
of transforming growth factor (TGF)-β1 and epidermal growth factor
(EGF), and insulin-liked growth factor binding protein-3 (IGFBP3)
(9). Although, novel breast cancer
cell lines have been established (10–12),
no recent cell models of TN and HER2-positive subtypes have been
reported for mechanistic investigation of metastasis and drug
resistance.
In the present study, two novel breast cancer cell
lines designated as PC-B-142CA and PC-B-148CA were established from
fresh breast cancer tissues. The epithelial markers and chromosome
aberrations were investigated to confirm epithelial-derived cancer
cells. The 2-dimensional (2-D) and 3-D tumor spheroids and 3-D
organoids were used to demonstrate the tumorigenic phenotypes
including cell proliferation, cell growth, cell migration, cancer
stemness (CSCs) and doxorubicin (DOX)/paclitaxel (PTX) resistance.
The DNA sequences of drug-targeted genes were investigated and
discussed for the chemotherapeutic and target drugs response of
these cells. Programmed death-ligand 1 (PD-L1) was checked to
propose its use for the sensitivity of cancer cells by T cell
killing. The obtained findings revealed that PC-B-148CA is a good
TN breast cancer model for investigating migration and cancer
stemness properties, while PC-B-142CA is a new HER2-positive breast
cancer model for drug resistance.
Materials and methods
Cancer cell isolation and culture
Breast cancer tissues were obtained from two
patients who underwent surgery at Siriraj Hospital, Bangkok,
Thailand, designated as PC-B-142CA and PC-B-148CA. PC-B-142CA was
derived from a 58-year-old female patient diagnosed with stage IV
HER-2 positive breast cancer, while PC-B-148CA was isolated from a
50-year-old female patient diagnosed with stage II TN breast
cancer.
The tissue collection protocol was approved by the
Siriraj Institutional Review Board (COA no. Si 329/2017). Single
cell suspensions from tumor tissues were prepared using the
GentleMACS single cell isolation machine (Miltenyi Biotec GmbH)
according to the manufacturer's instructions. Briefly, the tissues
were minced into 1–2 mm3 pieces and incubated for 30 min
at 37°C with the enzyme cocktail mix (Miltenyi Biotec GmbH). The
digested cells were harvested and filtered over a 70 µm nylon
filter (SPL Life Sciences). The cell suspensions were washed by
centrifugation and the cell pellets were resuspended in DMEM F/12
media (Gibco BRL) supplemented with 10 ng/ml of epidermal growth
factor (EGF, PeproTech, Inc.), 5 µg/ml insulin (Sigma-Aldrich;
Merck KGaA), 0.32 µg/ml hydrocortisone (Sigma-Aldrich; Merck KGaA)
and 10 µM ROCK inhibitor (Y27632, StemMACS, Miltenyi Biotec GmbH).
The contaminated fibroblasts were isolated using a tumor cell
isolation kit (Miltenyi Biotec GmbH). The primary breast cancer
cells were cultured in DMEM F/12 medium supplemented with 10% fetal
bovine serum (FBS) (Thermo Fisher Scientific Inc.) and 10X
antibiotic mixture containing 1 U/ml penicillin G sodium and 1
mg/ml streptomycin (Thermo Fisher Scientific, Inc.). Cells were
subcultured, periodically checked for negative mycoplasma and kept
in liquid nitrogen for storage.
Commercial human breast cancer cell lines,
MDA-MB-231 and MCF-7 (purchased from American Type Culture
Collection, ATCC) were cultured at 37°C in a humidified atmosphere
of 5% CO2 in Dulbecco's modified Eagle's medium (DMEM,
Gibco, Thermo Fisher Scientific Inc.) supplemented with 10% FBS
(v/v) (Gibco, Thermo Fisher Scientific Inc.), 100 U/ml of
penicillin and 100 µg/ml of streptomycin (both from Sigma-Aldrich;
Merck KGaA).
Detection of epithelial markers by
immunocytochemistry and immunofluorescence staining
Cell pellets were fixed in 10% formalin and
subjected for staining on sterile glass coverslips by antibodies
against estrogen receptor (rabbit anti-ER monoclinal antibody,
790-4325, ready-to-use; clone SP1, Ventana Laboratories),
progesterone receptor (rabbit anti-PR monoclonal antibody,
790-4296, ready-to-use; clone 1E2, Ventana Laboratories) and human
epidermal growth factor receptor 2 (rabbit anti-HER2 monoclonal
antibody, 790-2991, ready-to-use; clone 4B5, Ventana Laboratories).
This process was performed by the routine service at the Department
of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol
University. Ki-67 (1:300 dilution, M7240, rabbit anti-Ki-67
monoclonal antibody, clone MIB-1, Dako Laboratories; Agilent
Technologies, Inc.) percentages at cut-point <14% were defined
as luminal A subtype (13,14).
The epithelial cytokeratin (CK) including CK-4, −5,
−6, −8, −10, −13, −18 and −19 were investigated. α-SMA and FAP,
specific markers for the stromal fibroblast, were used as a quality
control of cancer cell purity. The presence of PD-L1 was evaluated
for the ability of the obtained cancer cells to resist T cell
killing. Cells at 1×104 were plated on sterile glass
coverslips for 24 h, fixed in ice-cold absolute methanol,
permeabilized with 0.2% Triton-1X PBS, and then incubated overnight
at 4°C in a humidified chamber with the indicated primary antibody
as follows: mouse anti-human panCK antibody (dilution 1:200,
sc-8018, Santa Cruz Biotechnology, Inc.), mouse anti-human CK-19
antibody (dilution 1:200, sc-6278, Santa Cruz Biotechnology, Inc.),
mouse anti-human α-SMA antibody (dilution 1:500, A5228,
Sigma-Aldrich; Merck KGaA), rabbit anti-human fibroblast activation
protein (FAP) antibody (dilution 1:500 ab53066, Abcam), and rabbit
anti-human PD-L1 antibody (dilution 1:500, ab205921, Abcam). The
goat anti-mouse IgG-Cy3 antibody (dilution 1:2,000, #115-166-071,
Jackson ImmunoResearch Laboratories Inc.) or the donkey anti-rabbit
IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 488
(dilution 1:2,000, 21206, Thermo Fisher Scientific, Inc.) was
applied for 3 h at room temperature. The nuclei were stained with
1:2,000 Hoechst 33342 (Invitrogen, Thermo Fisher Scientific, Inc.).
Fluorescence was captured with a ZEISS LSM 800 confocal laser
fluorescence scanning microscope (Axio Observer7, LSM 800, Zeiss
GmbH).
Three-dimensional (3-D) spheroid and
3-D organoid formation
Spheroids were created by 1×103 breast
cancer cells supplemented with 2.5% cold Matrigel™ (BD Biosciences)
in 200 µl of complete DMEM F/12 medium and seeded into individual
wells of pre-cooled 96-well ultra-low attachment multiple well
plates (CLS7007, Costar/Corning, Inc.). Centrifugation at 4°C at
300 × g for 3 min was performed and the cells were maintained at
37°C in a humidified 5% CO2 atmosphere for 5 days to
form spheroids. Medium was renewed twice weekly and the
proliferation rate of spheroids was monitored for up to 10 days.
For the organotypic cultures, 1×104 of PC-B-142 and
PC-B-148CA cells were generated in 24-well clear flat bottom
ultra-low attachment multiple well plates (CLS3473, Costar/Corning,
Inc.) at the concentration of 4% cold Matrigel™ (BD Biosciences) in
300 µl of complete DMEM F/12 medium. The plate was placed in the
incubator with 5% CO2 at 37°C. The organoid culture
medium was refreshed with 300 µl complete DMEM F/12 medium every
2–3 days. The organoid culture was ended on day 14.
Cell proliferation and colony
formation assays
Growth curves of PC-B-142CA and PC-B-148CA cells,
compared to commercial cell lines, MDA-MB-231 and MCF-7, were
determined by using the MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium)
(G3581, Promega.) assay following the manufacturer's instructions.
Briefly, 5,000 cells were seeded in each well of 96-well plates in
complete DMEM F/12 medium and cultured overnight in a 5%
CO2 atmosphere at 37°C. At time intervals, 20 µl of MTS
reagent was added to each well, followed by incubation in a
humidified, 5% CO2 atmosphere for a minimum of 2 h.
Absorbance at 490 nm was recorded at 24, 48, 72 and 96 h. For the
colony formation assay, 2,000 cancer cells were seeded in 6-well
plates in complete DMEM F/12 medium and cultured overnight. The
medium was refreshed every 3 days. At day 10, the adherent cells
were washed with 1X PBS, fixed with cold methanol, and stained with
a 0.5% crystal violet solution. The colony numbers were counted by
photometric measurements using CellCounter software version 0.2.1
(Nghia, Ho) and a 1–5 µm diameter colony was determined as one
colony. Three independent experiments were performed for each assay
condition.
Drug cytotoxicity assay
The 2-D culture was treated for 0, 24, 48 and 72 h
with 0, 0.01, 0.1, 1 and 10 µM DOX (Selleckchem) or PTX
(Selleckchem) diluted in 10% FBS of DMEM/F12 medium. The selected
concentrations at 0.1, 1 and 10 µM of DOX or PTX were tested in 3-D
spheroids for 72 h. The 2-D killing was measured by the MTS assay
and cell viability of 3-D killing was analyzed by the calculation
of the volume (µm3) with the formula 4/3πr3
in a spheroid with or without drug treatment.
Cell migration assay
For the wound healing assay, 5×104 cells
were adhered in a 24-well plate and cultured until they reached
>90% confluency. Scratch wounds were made with a sterile yellow
tip pipette. The cells were incubated in complete 10% FBS DMEM F/12
and the wound area was recorded and digitally photographed at 30
min, 18 and 24 h by an inverted microscope (IX71 Olympus). The
closing of the wound gap was calculated using ImageJ software
version 1.48v (NIH, Bethesda, MD, USA). Quantification of cell
migration was determined with the formula: Migration area=(Area of
original wound-Area of wound after healing)/Area of original wound.
Cell migration was performed in 8.0-µm Transwell Boyden chambers
(Corning, Inc.). A total of 5×104 cells in serum-free
medium were seeded in the upper chamber insert. Subsequently, 500
µl of DMEM F/12 medium containing 10% FBS was added to the lower
chamber. Then at 24 h, the cells which passed through the membrane
were fixed with absolute methanol, stained with 0.5% crystal
violet, and quantitated with ImageJ software version 1.48v.
Western blot analysis
Cells were lysed with RIPA buffer containing 0.5 M
NaF, 0.2 M NaVO4, 1 M Tris-HCl pH 7.5, 0.5 M EDTA, 2.5 M
NaCl, 10% (v/v) NP-40, 10% (w/v) SDS, Triton X-100, and protease
inhibitor cocktails (Cell Signaling Technology, Inc.). The proteins
were quantitated by Bradford kits (Bio-Rad Laboratories Srl.).
Sixty micrograms of protein lysates were electrophoresed in 10%
SDS-polyacrylamide gel and blotted onto a polyvinylidene fluoride
membrane (Bio-Rad Laboratories Srl.). The proteins were blocked in
5% non-fat dried milk diluted in 1X TBS/0.1% Tween-20. Primary
antibodies against E-cadherin (1:1,000 dilution, mouse anti-human
E-cadherin antibody, 13-1700, Invitrogen; Thermo Fisher Scientific,
Inc.), MMP-9 (1:200 dilution, mouse anti-human MMP-9 antibody, 2C3,
sc-21733, Santa Cruz Biotechnology, Inc.), MMP-13 (1:100 dilution,
rabbit anti-human MMP-13 antibody, H-230, sc-30073, Santa Cruz
Biotechnology, Inc.), BAX (mouse anti-human BAX antibody, 1:1,000
dilution, 610983, Becton Dickinson Holdings Pte. Ltd.), BCL-2
(rabbit anti-human BCL-2 antibody, 1:2,000 dilution, ab196495,
Abcam) and β-actin (1:10,000 dilution, sc-47778, Santa Cruz
Biotechnology, Inc.) were used. The immunoreactive signals were
visualized by ECL (Thermo Fisher Scientific, Inc.) under Gel
Document Syngene (Syngene). The bands were quantified by ImageJ
version 1.48v. β-actin was used as the loading control protein to
verify the amount of total loading protein.
Targeted next-generation
sequencing
The genomic DNA isolation of PC-B-142CA, PC-B-148CA,
MDA-MB-231 and MCF-7 was performed using Cobas® DNA
sample preparation kit (05985536190, Hoffman-La Roche) according to
manufacturer's instructions. The quantity of the extracted DNA
samples was determined using the Qubit dsDNA HS Assay Kit (Q32854,
Thermo Fisher Scientific, Inc.) and Qubit 2.0 fluorometer (Thermo
Fisher Scientific, Inc.). One-hundred nanograms of DNA was used as
a template to generate libraries by the GeneRead QIAact AIT DNA UMI
Kit (181911, Qiagen) and the GeneRead QIAact BRCA Advanced DNA UMI
panel (181925, Qiagen). Sequencing was performed by the GeneReader
NGS System (Qiagen). GeneRead UMI Advanced Sequencing Q Kit
(185251, Qiagen), GeneRead UMI Advanced Sequencing Q Wash Buffers
(185905, Qiagen) were used according to the manufacturer
instructions. Single-end sequencing was performed on the GeneReader
NGS System (Qiagen). The single-end sequencing was performed. The
average read lengths were as follows: MDA-MB-231 [AIT panel: 121.90
base pairs (bps)], MCF-7 (AIT panel: 111.38 bps), PC-B-142CA (AIT
panel: 132.57 and BRCA panel: 126.73 bps), and PC-B-148CA (AIT
panel: 119.04 and BRCA panel: 99.17 bps). Quality control and
variant data reviews were performed in Qiagen Clinical Insight
Analyze. Qiagen Clinical Insight Interpret software was used for
variant interpretation and reporting. The sequences were submitted
to GenBank database (https://www.ncbi.nlm.nih.gov/) under the accession
number: PRJNA762209 (https://www.ncbi.nlm.nih.gov/sra/PRJNA762209). The
BioSample accession numbers were: SAMN21380367, SAMN21380368,
SAMN21380369, SAMN21380370, SAMN21380371 and SAMN21380372,
respectively.
Cancer stem cell analysis
PC-B-142CA and PC-B-148CA cells were harvested and
incubated with Allophycocyanin (APC)-labeled anti-CD44 (21270446,
ImmunoTools GmbH, Friesoythe Germany, 1:10 dilution) and
FITC-labeled anti-CD24 (21270443, ImmunoTools GmbH, 1:10 dilution)
antibodies in 1X PBS/2% FBS for 30 min at 4°C. Mouse IgG1 control
FITC-conjugated (21275513, ImmunoTools GmbH) and mouse IgG1 control
APC-conjugated (21275516, ImmunoTools GmbH) were used as the
isotype controls. The CytoFLEX flow cytometer (Beckman Coulter,
Inc.) was used for flow cytometric and data analysis using
CytExpert software version 2.1 (Beckman Coulter, Inc.).
Data collection and statistical
analysis
The values are represented as mean ± standard
deviation (SD) from three independent assays. All statistical
calculations were performed with the SPSS version 17.0 (SPSS Inc.).
The data from two groups were analyzed by paired Student's t-tests
and from multiple groups by one-way repeated-measure analysis of
variance (ANOVA) followed by Tukey's post-hoc test using GraphPad
Prism software version 7.04 (GraphPad Software, Inc.) or SigmaPlot
16.0v (Systat Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
Characterizations of PC-B-142CA and
PC-B-148CA
PC-B-142CA cells were isolated from stage IV
HER2-positive breast cancer tissues with pathological features of
negative ER and PR, positive HER2 (>90%) and Ki-67 (47%).
PC-B-148CA was derived from a patient diagnosed with stage II TN
breast cancer with negative expression of ER, PR and HER2, but
positive Ki-67 (84%) (Table I).
Fingerprint results confirmed different origins of these 2 cell
lines (Table SI). The PC-B-148CA
fingerprint was identical to that of the white blood cells of the
patient whose tissue was used to establish PC-B-148CA (data not
shown), while that of PC-B-142CA could not be checked due to the
unavailability of the sample. The immunocytochemical staining
results confirmed PC-B-142CA as a HER2-positive breast cancer cell
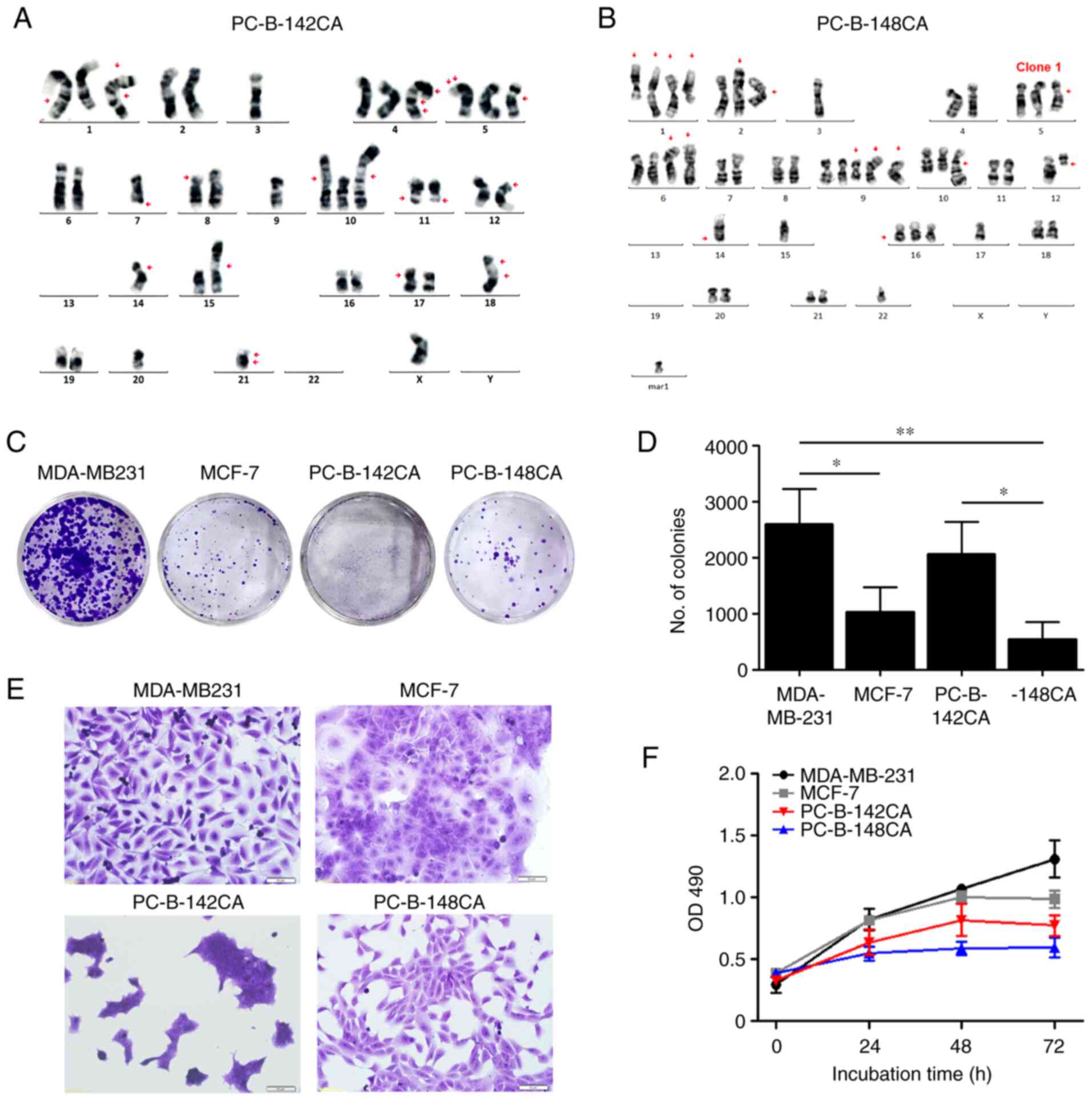
line, and PC-B-148CA as a TN breast cancer cell line (Table I). PC-B-142CA revealed a great
karyotypic heterogeneity with 38 chromosomes with a monosomy X
chromosome (Fig. 1A) whereas
PC-B-148CA had around 47–58 chromosomes with loss of sex
chromosomes and several numerical and structural rearrangements and
unidentifiable aberrations (Figs.
1B and S1).
 | Table I.Demographic data of the patients and
the characteristics of the established cell lines. |
Table I.
Demographic data of the patients and
the characteristics of the established cell lines.
|
Characteristics | PC-B-142CA | PC-B-148CA |
|---|
| Patients |
|
|
|
Origin | Breast, metastasis
to axilla skin | Breast, right |
| Age
(years) | 58 | 50 |
|
Sex | Female | Female |
| Tumor
size (cm3) | 3.9×3.6×3.0 | 3.5×3.0×3.0 |
| Gross
pathology | Angiolymphatic
invasion | Angiolymphatic
invasion |
|
Clinical stage | IV | II |
| ER | Negative | Negative |
| PR | Negative | Negative |
|
HER2 | Positive | Negative |
|
Ki-67 | Positive | Positive |
| Cell lines |
|
|
| Growth
pattern | Adherent | Adherent |
|
Doubling time (h) | 45.0±3.0 | 155.7±5.2 |
| CK | Positive | Positive |
|
α-SMA | Negative | Negative |
|
FAP | Negative | Negative |
|
PD-L1 | Positive | Positive |
| ER | Negative | Negative |
| PR | Negative | Negative |
|
HER2 | Positive | Negative |
The colony formation assay exhibited that TN
PC-B-148CA had a significantly slower growth rate than the
commercial TN MDA-MB-231 (Fig. 1C and
D). PC-B-142CA formed a colony faster than PC-B-148CA, but
slower that MDA-MB-231. PC-B-142CA had a very small size and grew
in cluster of cells, whereas PC-B-148CA showed a polygonal shape
(Fig. 1E). The growth rates of
PC-B-142CA and PC-B-148CA were lower than those of MDA-MB-231 and
MCF-7 (Fig. 1F). PC-B-148CA had
the slowest growth rate among these four cells with a doubling time
of 155.7±5.2 h, while that of PC-B-142CA was 45.0±3.0 h (Table I).
Morphology and cellular markers of
PC-B-142CA and PC-B-148CA
PC-B-142CA cells adhered to form a big tight colony
with blurred cell borders (Fig.
2A, bright field), while PC-B-148CA exhibited characteristic of
spindle shape with multiple processes and seldom multinucleated
cells (Fig. 2C, bright field).
PC-B-148CA had a bigger cell size than PC-B-142CA. Both cells were
positive for all CKs but no α-SMA and FAP fibroblast markers were
detected (Fig. 2A and C). PD-L1
was found in both PC-B-142CA and PC-B-148CA. The
immunocytochemistry staining confirmed positive expression of HER2
in PC-B-142CA, whereas negative ER, PR and HER2 were detected in
PC-B-148CA (Fig. 2A and C). The
3-D spheroid of PC-B-142CA was larger than that of PC-B-148CA
(Fig. 2B and D). The 3-D mean
diameter continuously increased with time from 0.048±0.04
µm3 at day 0 to 0.118±0.21 µm3 at day 5 for
PC-B-142CA and from 0.021±0.04 µm3 at day 0 to
0.095±0.058 µm3 at day 5 for PC-B-148CA. The aggregation
and compaction of 3-D organotypic modeling was observed at day 14
of culture. The 3-D organoids of PC-B-142CA exhibited a round shape
with smooth surface, whereas those of PC-B-148CA had invadopodia
representing the specialized adhesive structures capable to invade
surrounding tumor microenvironment. This similar feature was also
observed in the PC-B-148CA spheroid.
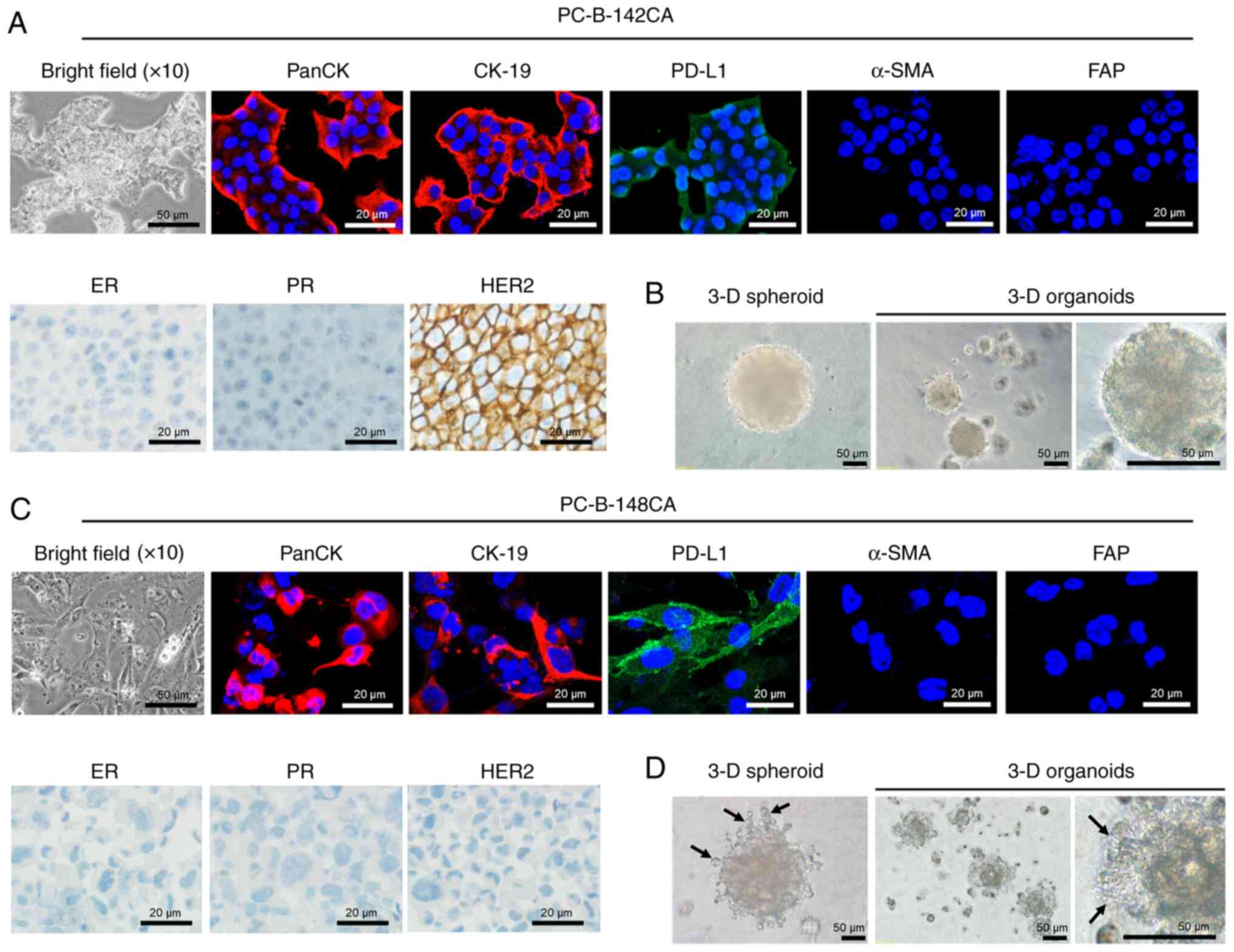 | Figure 2.Morphology of the in-house breast
cancer cells. (A) PC-B-142CA and (C) PC-B-148CA cell lines. Typical
morphology of stable culture cells under a phase contrast light
microscopy (×200 magnification; scale bars, 50 µm). (Top panels)
Expression of biological markers of epithelial cells by
immunofluorescence staining of PanCK (red fluorescence), CK-19 (red
fluorescence), PD-L1 (green fluorescence), α-SMA (red fluorescence)
and FAP (green fluorescence); images captured at ×400
magnification; scale bars, 20 µm. Staining with Hoechst33342 (blue
fluorescence) was conducted to visualize chromatin. (Lower left
panels) Phase-contrast micrographs showing the immunohistochemistry
staining of ER, PR and HER2; scale bars, 20 µm. (B and D) The 3-D
formation ability is shown by 3-D spheroids at day 5 and 3-D
organoids at day 14. Invadopodia were observed in only 3-D
PC-B-148CA cells (black arrow). CK, cytokeratin; PD-L1, programmed
death-ligand 1; α-SMA, α-smooth muscle actin; FAP,
fibroblast-activation protein; ER, estrogen receptor; PR,
progesterone receptor; HER2, human epidermal growth factor receptor
2. |
Migration and cancer stem cell
properties of PC-B-142CA and PC-B-148CA
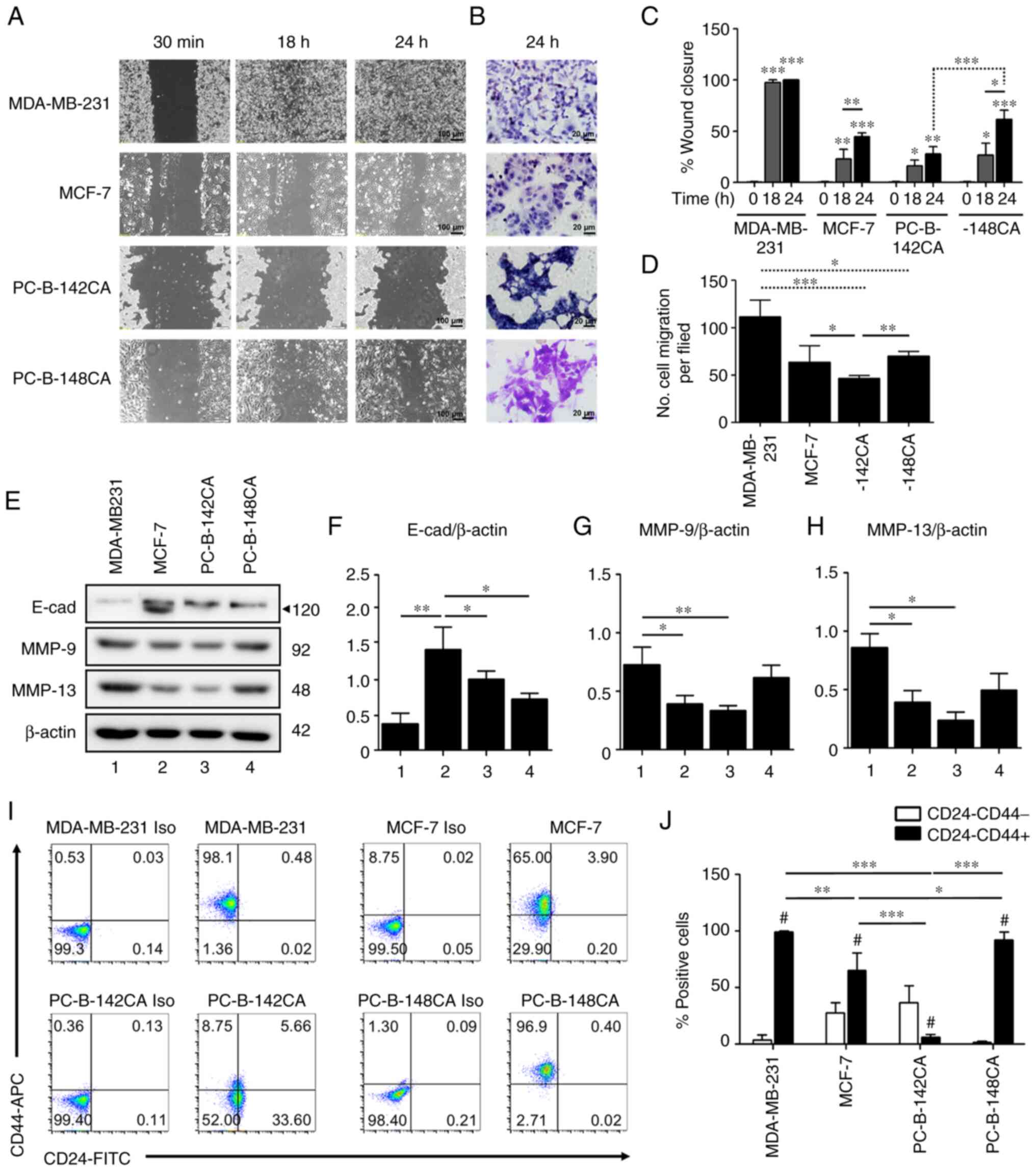
MBA-MB-231 TN breast cancer cells confirmed their
most rapid migration by wound healing and Transwell migration
assays in 10% FBS media (Fig. 3A and
D). The results showed that PC-B-148CA closed a 70% wound gap
at 24 h (Fig. 3A and C), while at
this time point, PC-B-142CA had only 30% wound closure and needed
more than 96 h to completely heal the wound gap (data not shown).
We tried to culture it in serum free, unluckily, the cells did not
grow confluently and we could not do wound scratching. The primary
cells did not tolerate serum starving as well as the established
cell lines, hence wound healing assay in our experiment was
performed in the presence of serum. The doubling times of
PC-B-142CA and PC-B-148CA were approximately 45±3.0 and 155.7±5.2
h, respectively, implying that within 24 h of this assay, cells did
not proliferate. The Transwell migration assay essentially
confirmed the migration capability observations from high to low
as: MBD-MB-231>PC-B-148CA>MCF-7>PC-B-142CA (Fig. 3D). The western blot analysis of the
proteins involved in cancer cell migration exhibited that
E-cadherin was basally expressed in all 4 cell lines in this study
and showed the highest level in MCF-7, especially, the 120-kDa
mature isoform whereas MMP-9 and MMP-13 were markedly higher in
MDA-MD-231 and PC-B-148CA cells (Fig.
3E-H).
CD24−/CD44+ representing
cancer stem cells (CSCs) of breast cancer, were detected in
PC-B-142CA and PC-B-148CA as 8.75 and 96.9% (Fig. 3I and J). MDA-MB-231 had around
98.1% CSCs, while that of MCF-7 was 65.0%.
DOX and PTX resistance of PC-B-142CA
and PC-B-148CA
To determine the relevant toxic concentration of DOX
and PTX, the breast cancer cells were exposed to the increasing
concentrations of DOX and PTX (0.01, 0.1, 1 and 10 µM) and cell
viability was assayed at 24, 48, 72 and 96 h. In 2-D culture, 0.01
µM of DOX (Fig. 4C) or 1 µM of PTX
(Fig. 4D) was the minimum
concentration which elicited the highest toxic effect in PC-B-148CA
cells, whereas no cytotoxic effect was found in the PC-B-142CA cell
line (Fig. 4A and B).
Interestingly, 3-D spheroid formation showed that after exposure to
1 µM DOX and 10 µM PTX, the size of tumor spheroids was
significantly reduced from the control without drug treatment in
PC-B-142CA and PC-B-148CA cells at 72 h (Fig. 4E and F). Moreover, DOX and
PTX-treated PC-B-142CA and PC-B-148CA increased the pro-apoptotic
BAX protein, while decreased the expression of the anti-apoptotic
BCL-2 protein (Fig. 4G and H).
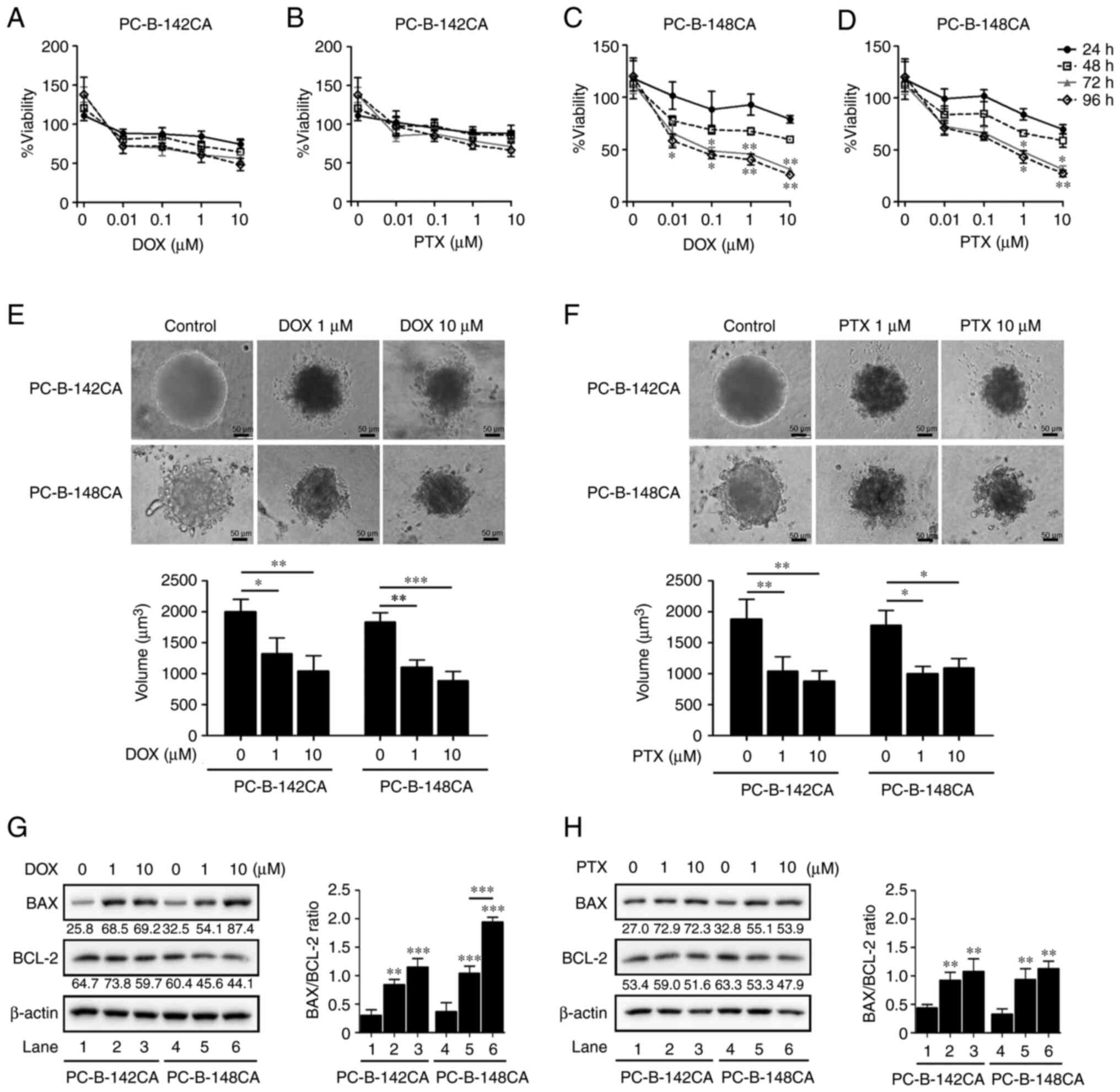 | Figure 4.DOX and PTX induce cell death in
PC-B-142CA and PC-B-148CA cells. (A) PC-B-142CA and (C) PC-B-148CA
cells were plated and exposed to 0, 0.01, 0.1, 1 and 10 µM of DOX
and (B) PC-B-142CA and (D) PC-B-148CA cells were treated with 0,
0.01, 0.1, 1 and 10 µM of PTX for 24, 48, 72 and 96 h (0 h was used
as the normalization). Quantitative results of MTS staining were
performed in triplicate. (E and F) Phase-contrast micrographs
showing the morphology of 3-D sphere-formation of two breast cancer
cell lines tested with 0, 1 and 10 µM of DOX (E) and of PTX (F)
were tested for 72 h (day 7 of culture). Images were captured at
×200 magnification; scale bar, 50 µm. (G and H) Expression of BAX
and BCL-2 in PC-B-142CA and PC-B-148CA treated or not with 0, 1 and
10 µM of DOX (G) and of PTX (H) for 72 h. β-actin was used as
protein loading control. Densitometry analysis of the relative band
intensity of the western blotting. *P<0.05; **P<0.01 and
***P<0.001 compared with the untreated control. DOX,
doxorubicin; PTX, paclitaxel. |
Mutation of the drug-targeted
genes
Drug-targeted gene mutations were checked in the
PC-B-142CA and PC-B-148CA cells in comparison to those in the
MDA-MB-231 and MCF-7 cells (Table
II). The pathogenic mutations represented the variants well
established as disease causing including KIT (E839K, 50.0%),
PIK3CA (C420R, 68.0%), SMAD4 (Q224*, 100.0%, *
represented stop codon), and TP53 loss-of-function (I202T,
98.0%) were detected in PC-B-142CA, whereas BRCA2
(T3033fs*11, 16.0%) and TP53 (R196*, 100%) loss-of-function,
and NRAS gain-of-function (G12C, 35.0%) were found in
PC-B-148CA. ERBB2 amplification and PIK3CA mutation
at exon 8 (c.1258T>C, 68%) were found in PC-B-142CA.
 | Table II.The drug-targeted gene alterations in
the breast cancer cell lines. |
Table II.
The drug-targeted gene alterations in
the breast cancer cell lines.
|
|
|
|
| % Mutation
(Pathogenica) |
|---|
|
|
|
|
|
|
|---|
| Gene | Exon | Nucleotide
alteration | Amino acid
variant | MCF-7 | MDA-MB-231 | PC-B-142CA | PC-B-148CA |
|---|
| ALK | 29 | c.4587C>G | p.D1529E | WT | 62.49% | 33% | 31% |
| ALK | 29 | c.4472A>G | p.K1491R | WT | 64.03% | 33% | 32% |
| ALK | 29 | c.4381A>G | p.I1461V | 99% | 99.03% | 99% | 99% |
| ALK | 23 | c.3600G>C | p.A1200A | WT | WT | WT | 34% |
| ALK | 18 | c.3036G>A | p.T1012T | WT | WT | 65% | WT |
| ALK | 15 | c.2535T>C | p.G845G | 100% | WT | 65% | WT |
| ALK | 2 | c.702T>A | p.P234P | 100% | 66.88% | 68% | 100% |
| ALK | 1 | c.27C>G | p.L9L | 100% | 100% | 100% | 100% |
| PIK3CA | 1 |
c.-77+8483C>T | – | WT | 28.28% | WT | WT |
| PIK3CA | 1 |
c.-76-23509A>G | – | WT | 30.66% | WT | WT |
| PIK3CA | 10 | c.1633G>A | p.E545K | 58%a | WT | WT | WT |
| PIK3CA | 8 | c.1258T>C | p.C420R | WT | WT | 68%a | WT |
| FGFR3 | 14 | c.1953G>A | p.T651T | 100% | 99.15% | 99% | 100% |
| PDGFRA | 7 | c.939T>G | p.G313G | WT | 35.17% | 48% | WT |
| PDGFRA | 10 | c.1432T>C | p.S478P | WT | 30.59% | WT | WT |
| PDGFRA | 12 | c.1701A>G | p.P567P | 99% | 99.44% | 100% | 100% |
| PDGFRA | 13 | c.1809G>A | p.A603A | WT | 35.37% | WT | WT |
| PDGFRA | 18 | c.2472C>T | p.V824V | WT | 34.80% | WT | WT |
| KIT | 16 |
c.2362-77G>A | – | WT | 36.76% | WT | WT |
| KIT | 18 | c.2586G>C | p.L862L | WT | 34.51% | WT | WT |
| KIT | 18 | c.2515G>A | p.E839K | WT | WT | 50%a | WT |
| EGFR | 4 | c.474C>T | p.N158N | 100% | 25% | WT | 100% |
| EGFR | 13 | c.1562G>A | p.R521K | WT | WT | 100% | WT |
| EGFR | 16 | c.1968C>T | p.H656H | 83% | WT | WT | 49% |
| EGFR | 18 |
c.2184+19G>A | – | 78% | WT | WT | 48% |
| EGFR | 20 | c.2361G>A | p.Q787Q | 99% | 98.77% | WT | 48% |
| EGFR | 25 | c.2982C>T | p.D994D | 83% | WT | 34% | 100% |
| BRAF | 15 | c.1805C>G | p.S602C | WT | WT | 45.00% | WT |
| BRAF | 16 | c.1929A>G | p.G643G | WT | 99.36% | 100% | WT |
| BRAF | 11 | c.1391G>T | p.G464V | WT | 96.08%a | WT | WT |
| KRAS | 5 | c.*2505T>G | – | 31% | WT | WT | WT |
| KRAS | 2 | c.38G>A | p.G13D | WT | 98.83%a | WT | WT |
| ERBB2 | – | – | – | WT | WT | Amplificationa | WT |
| ERBB2 | 27 | c.3508C>G | p.P1170A | 99% | 98.51% | 3.77% | WT |
| ERBB2 | 17 | c.1960A>G | p.I654V | 100% | WT | WT | WT |
| ERBB2 | 17 | c.1963A>G | p.I655V | 100% | WT | WT | WT |
| ERBB2 | 27 | c.3631C>G | p.P1211A | WT | WT | 3.67% | WT |
| ERBB2 | 27 | c.3651C>T | p.F1217F | WT | WT | 4.21% | WT |
| ERBB3 | 27 | c.3355A>T | p.S1119C | WT | WT | WT | 99% |
| ESR1 | 10 | c.1782G>A | p.T594T | 65% | WT | WT | 56% |
| ESR1 | 3 | c.30T>C | p.S10S | 23% | WT | 100% | WT |
| MAP2K2 | 2 | c.192C>T | p.V64V | WT | 53.04% | WT | WT |
| MET | 20 | c.3912C>T | p.D1304D | 49% | WT | 99% | 67% |
| NOTCH1 | 27 | c.5094C>T | p.D1698D | 43% | WT | 99% | 99% |
| SMAD4 | 6 | c.670C>T | p.Q224* | WT | WT | 100%a | WT |
| BRCA1 | 10 | c.2612C>T | p.P871L | ND | ND | 93% | WT |
| BRCA2 | 10 | c.1274C>G | p.S425C | ND | ND | 99% | WT |
| BRCA2 | 10 | c.1114A>C | p.N372H | ND | ND | WT | 99% |
| BRCA2 | 11 | c.4563A>G | p.L1521L | ND | ND | WT | 100% |
| BRCA2 | 11 | c.6513G>C | p.V2171V | ND | ND | WT | 99% |
| BRCA2 | 14 | c.7397T>C | p.V2466A | ND | ND | WT | 100% |
| BRCA2 | 17 |
c.7806-14T>C | – | ND | ND | 100% | WT |
| BRCA2 | 23 | c.9097dupA | p.T3033fs*11 | ND | ND | WT | 16%a |
| TP53 | 6 | c.586C>T | p.R196* | ND | ND | WT | 100%a |
| TP53 | 8 | c.839G>A | p.R280K | ND | ND | WT | WT |
| TP53 | 4 | c.215C>G | p.P72R | ND | ND | WT | WT |
| TP53 | 7 | c.695T>C | p.1232T | ND | ND | 98%a | WT |
Discussion
The incidence of breast cancer in females is
increasing worldwide (3). Luminal
breast cancer is the most common subtype whereas HER2-positive is
the second most common (6). High
local recurrence and bone metastasis in patients with luminal
breast cancer is commonly detected during a 2- to 5-year period
(15). The median overall survival
for metastatic TN breast cancer is approximately 1 year compared to
approximately 5 years for the other 2 subtypes (6). Moreover, drug resistance is common in
all breast cancer types despite the different treatment modalities
applied (16). Several breast
cancer cell lines are existing and widely used in research fields.
In the present work, two novel cell lines from tumor tissues of two
breast cancer patients diagnosed with HER2-positive and
triple-negative (TN) breast cancer were established and designated
as PC-B-142CA and PC-B-148CA. The characterization for their
biological, molecular, and genetic properties confirmed that
PC-B-148CA had high aggressive properties including migration,
doxorubicin (DOX)/paclitaxel (PTX) resistance and stemness
properties, whereas HER2-positive PC-B-142CA had pathogenic gene
mutations related to the resistance to several chemotherapeutic
drugs.
Both PC-B-142CA and PC-B-148CA cell lines grew as
adherent monolayer cells with morphology of epithelial cells. The
PC-B-142CA proliferation rate doubling time compared to PC-B-148CA
was around 45 vs. 155 h. The presence of fibroblast-activation
protein (FAP) and α-smooth muscle actin (α-SMA) confirmed the
characteristic of cancer-associated fibroblasts (17), whereas the presence of cytokeratin
(CK) represented cancer cells (18). Thus, having negative α-SMA and FAP,
with positive CK ensures the purity of PC-B-142CA and PC-B-148CA
without fibroblast contamination. The expression of programmed
death-ligand 1 (PD-L1) in both PC-B-142CA and PC-B-148CA cell lines
implies the ability of these cells to resist T cell killing as
PD-L1 is a checkpoint molecule acting as a break to inhibit T cell
function (19,20). Atezolizumab, an anti-PD-L1
antibody, has just been approved by the US Food and Drug
Administration (US FDA) for use in combination with chemotherapy
for the treatment of patients with PD-L1-positive, non-operable,
locally advanced/metastatic TN breast cancer (21). Hence, PD-L1-expressing PC-B-142CA
and PC-B-148CA cells may be a valuable aid in the search to
overcome immune checkpoint-mediated T cell dysfunction in breast
cancer.
It is widely acknowledged that cancer stem cells
(CSCs) serve as an important part in the occurrence and development
of tumors on account of their ability for self-renewal,
differentiation, proliferation and induction of tumor growth
(22,23). Surface CD44, overexpressed in
several types of cells, is a cell-surface glycoprotein involved in
tumorigenesis, metastasis and recurrence (24,25).
PC-B-148CA showed the highest stemness property. TN breast cancer
cell lines including MDA-MB-231, MDA-MB-436, Hs578T, SUM1315 and
HBL-100 with a higher percentage of
CD44+/CD24− cells (>30%) express higher
levels of pro-invasive genes and are highly invasive (26). PC-B-148CA cells showed marked
migration activity when compared to the PC-B-142CA cells. This
property was found to be correlated with the histological data of
the tissue of origin of PC-B-148CA cells including invasive lobular
carcinoma and angiolymphatic invasion. Serum starving is the most
common non-pharmaceutical method for minimizing proliferation in
wound healing assays, but the degree of serum starving has to be
calculated for each cell type under investigation (27). Please note that primary cells do
not tolerate serum starving as well as established cell lines.
PC-B-142CA and PC-B-148CA cells were tested for their migration
capability in the presence of serum. Hence, the proliferation
parameter cannot be ruled out from this assay results.
Metastatic cancer cells invade surrounding tissues
and blood vessels by forming F-actin-rich protrusions known as
invadopodia, which degrade the extracellular matrix and enable
invasion of tumor cells (28). The
proportion of CD44+/CD24− in breast cancer
cell populations has been reported to enrich mammosphere formation
(29) and tumorigenesis in mice
(30) and have been well studied
(31). Invadopodia were observed
in PC-B-148CA cells forming as 3-D structures, both spheroid and
organoid. A key step in tumor progression is the transition of
stationary epithelial cells to become motile by the loss of
cell-cell adhesion and matrix degradation. The process of
epithelial-mesenchymal transition (EMT) exhibits molecular hallmark
by downregulation of E-cadherin (26,32).
The phenotype of cell migration was consistent with the western
blot analysis which revealed low expression of E-cadherin in the
PC-B-148CA cells. All of these characteristics support the high
invasive property of PC-B-148CA cells. However, MCF-7 cells have a
high E-cadherin, yet a high migration rate was detected. This may
be explained by the fact that not only the E-cadherin level
reflects the migration ability, but also other proteins such as
matrix metalloproteinases (MMPs) and N-cadherin were previously
found with high levels in MCF-7 cells (33,34).
In breast cancer, overexpression of several MMPs has been reported
which is generally associated with breast tumor progression. MMP-9
is a potential biomarker which is widely found to play a role in
tumor invasion, metastasis and angiogenesis and to mediate tumor
microenvironment (35). MMP-9
protein expression in MDA-MB-231 and MCF-7 cells was found to be
significantly higher than in normal breast cells (36). Since MMP-13 is expressed in a broad
range of breast cancer cells, it has emerged as a novel metastatic
biomarker. MDA-MB-231 breast cancer cells that secrete higher
levels of MMP-13 are less aggressive than MCF7 cells (37). Consistent with our results, MMP-9
and MMP-13 were highly expressed in MDA-MB-231, MCF-7 and
PC-B-148CA cells when compared with these levels in PC-B-142CA
cells. The lack of N-cadherin expression assessment is a limitation
of this study.
The 3-D spheroid and 3-D organoid models enable
mimicking of the in vivo tumor condition in patients
(38). Both PC-B-142CA and
PC-B-148CA cell lines showed the capability of producing 3-D
multicellular tumor spheroids and 3-D tumor organoids.
Additionally, cells within tumor spheroids may have activities
similar as that in a patient's body, promotion of migration and
invasion; such features are absent in 2D culture. The different
sensitivity to DOX or PTX was detected in these two cell lines. DOX
and PTX had a strong impact on the spheroid size of both PC-B-142CA
and PC-B-148CA cell lines, with small size and outer loose layer of
sphere cells in a dose-dependent manner.
In the mutation analysis, ERBB2 (a gene
encoding the HER2 protein) amplification found in PC-B-142CA cells
was consistent with the presence of HER2 by an immunocytochemistry
result and confirmed that it had originated from HER2-positive
breast cancer tissue. Five mutations of drug-targeted genes
including KIT, PIK3CA, SMAD4 and TP53 found in
PC-B-142CA together with the ERBB2 amplification are related
to the resistance of several targeted drugs. Amplification of
HER2 together with PIK3CA may aggravate the
resistance to HER2-targeted drugs and suggest the combination of
treatment with PI3K inhibitors (16). Multiple advances in the treatment
of HER2-positive breast cancer due to multiple mutated genes were
found leading to the benefit of the combination of HER2 and
targeted inhibitors. PI3KCA mutant HER2-positive bearing
mice demonstrated tumor regression after combined lapatinib and
trastuzumab treatment (39).
Further studies using PC-B-142CA as a model of drug resistance may
be valuable for identification of better targeted molecules in
HER2-positive breast cancer.
As to the review of literature, the well-known
HER2-positive breast cancer cell lines are the cells with
HER2 gene overexpression and more aggressive phenotypes. In
comparison to these existing cell lines, the newly established
PC-B-142CA cell line exhibited BRCA1 and BRCA2
mutations. BRCA1 mutation at exon 10 (c.2612C>T) was
found in PC-B-142CA (93%) cells and the BRCA2 mutation
showed in exon 10 (c.12740C>G, 99%) and exon 17
(c.7806-14T>C, 100%). Commercial HER2-positive cell lines
including AU565, HCC1569, HCC1954, HCC202, KPL-4, OCUB-F, SKBR3,
SKBR5, SUM190PT, SUM225CWN and UACC893 cells have wild-type
BRCA1 (12,40,41).
The breast cancer susceptibility genes, BRCA1 and BRCA2, are
critically involved in the repair of DNA double-strand breaks
(42) and drug resistance in
cancer treatment (43). The
BRCA1 and BRCA2 mutations showed an association with
the development of breast cancer (44), ovarian cancer (45), prostate cancer (46) and pancreatic cancer (47). The BRCA1/2-mutated PC-B-142CA cells
can be used in the area of breast cancer research for insight into
the effect of BRCA aberration in breast cancer progression.
Moreover, ERBB2 amplification was found in
HER2-positive PC-B-142CA cells which may be related to drug
resistance of anastrozole, anthracycline, capecitabine,
docetaxel/trastuzumab, exemestane, fulvestrant, lapatinib,
lapatinib/letrozole, lapatinib/trastuzumab, letrozole, neratinib,
pertuzumab, pertuzumab/trastuzumab, tamoxifen and
trastuzumab/emtansine; whereas PIK3CA mutation at exon 8
(c.1258T>C) is a pathogenic mutation correlated with alpelisib
and combined alpelisib/fulvestrant resistance.
In the PC-B-148CA cell line, the gain-of-function
mutation of NRAS (G12C) leads to NRAS activation involving the
RAS/RAF/MARK/PI3K pathway resulting in drug resistance (48). In addition, the loss-of-function
mutations of BRCA2 (16%) and TP53 (100%) were found
in PC-B-148CA cells, which is common in tumorigenesis (49,50).
Loss of TP53 is common in advanced cancers; TP53 exon 6
single nucleotide variant mutant displays a relationship in
promoting cancer cell proliferation, survival and EMT features
(51,52). In comparison to TP53
mutations in MDA-MB-231 cells, over 90% of the mutations in
TP53 in MDA-MB-231 were found at exons 8 (codon 280:
Arg>Lys (R280K) (53), that
indicates the impact of mutant TP53 upon the tumorigenic properties
of MDA-MB-231 cell by loss of cytoplasmic pro-apoptotic activity
(54). There are no mutations of
TP53 in MCF-7 cells (53,55).
In conclusion, this study established two breast
cancer cell lines from HER2-positive and TN breast cancer cell
lines and characterized their tumorigenic phenotypes including cell
growth, migration and DOX/PTX responses and targeted drug-related
gene aberrations. PC-B-142CA cells can serve as a novel
HER2-positive cell line for drug resistance and unravelling the
effect of BRCA aberration in breast cancer progression, while
PC-B-148CA is a novel TN cell line suitable for invasive and
stemness-related properties. Further in vivo study on these
two breast cancer cells, such as tumor biology, cellular and
molecular carcinogenesis and drug response, are needed.
Importantly, these novel breast cancer cell lines represent
valuable tools in breast cancer research.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor James A.
Will, University of Wisconsin-Madison, USA for the English
edition.
Funding
This study was funded by the National Research
Council of Thailand (NRCT), Ministry of Higher Education, Science,
Research and Innovation (grant no. RSA6280091) Thailand and
Research Grant, Faculty of Medicine Siriraj Hospital, Mahidol
University (R016033015) to CT.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. Other related data can be
available upon request to the authors.
Authors' contributions
ST, PT, PTY, NP and CT designed the experiments; ST
performed the main research work. PJ, JP and NS prepared the
samples for the experiments. MW, DSN and POC acquired the patient
breast cancer tissues and analyzed the results. ST analyzed and
interpreted all the data, prepared figures/tables and wrote the
manuscript, and finally submitted the manuscript. CT performed the
research grant application, wrote and improved the scientific
quality of the manuscript, and finally submitted the manuscript.
All authors read and approved the manuscript and agreed to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work.
Ethics approval and consent to
participate
All experimental procedures performed in the present
study were approved by Siriraj Institutional Review Board (COA no.
Si 329/2017) and written informed consent was provided by the
subjects. All patients recruited in this study were accepted for
information of the study by written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
α-SMA
|
α-smooth muscle actin
|
|
BRCA1/2
|
breast cancer 1/2
|
|
CK
|
cytokeratin
|
|
DOX
|
doxorubicin
|
|
ER
|
estrogen receptor
|
|
ERBB2
|
erb-b2 receptor tyrosine kinase 2
|
|
FAP
|
fibroblast-activation protein
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
KIT
|
tyrosine-protein kinase
|
|
PD-L1
|
programmed death-ligand 1
|
|
PIK3CA
|
phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α
|
|
PR
|
progesterone receptor
|
|
PTX
|
paclitaxel
|
|
SMAD4
|
mothers against decapentaplegic
homolog 4
|
|
TP53
|
tumor protein p53
|
References
|
1
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alanazi IO and Khan Z: Understanding EGFR
signaling in breast cancer and breast cancer stem cells:
Overexpression and therapeutic implications. Asian Pac J Cancer
Prev. 17:445–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang Y, Wang Y, Kiani MF and Wang B:
Classification, treatment strategy, and associated drug resistance
in breast cancer. Clin Breast Cancer. 16:335–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elstrodt F, Hollestelle A, Nagel JH, Gorin
M, Wasielewski M, van den Ouweland A, Merajver SD, Ethier SP and
Schutte M: BRCA1 mutation analysis of 41 human breast cancer cell
lines reveals three new deleterious mutants. Cancer Res. 66:41–45.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharieh EA, Awidi AS, Ahram M and Zihlif
MA: Alteration of gene expression in MDA-MB-453 breast cancer cell
line in response to continuous exposure to Trastuzumab. Gene.
575:415–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hámori L, Kudlik G, Szebényi K, Kucsma N,
Szeder B, Póti Á, Uher F, Várady G, Szüts D, Tóvári J, et al:
Establishment and characterization of a brca1−/−,
p53−/− mouse mammary tumor cell line. Int J Mol Sci.
21:11852020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han Y, Nakayama J, Hayashi Y, Jeong S,
Futakuchi M, Ito E, Watanabe S and Semba K: Establishment and
characterization of highly osteolytic luminal breast cancer cell
lines by intracaudal arterial injection. Genes Cells. 25:111–123.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai X, Cheng H, Bai Z and Li J: Breast
cancer cell line classification and its relevance with breast tumor
subtyping. J Cancer. 8:3131–3141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prat A, Cheang MC, Martín M, Parker JS,
Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen
TO and Perou CM: Prognostic significance of progesterone
receptor-positive tumor cells within immunohistochemically defined
luminal A breast cancer. J Clin Oncol. 31:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li ZH, Hu PH, Tu JH and Yu NS: Luminal B
breast cancer: Patterns of recurrence and clinical outcome.
Oncotarget. 7:65024–65033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chun KH, Park JH and Fan S: Predicting and
overcoming chemotherapeutic resistance in breast cancer. Adv Exp
Med Biol. 1026:59–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shiga K, Hara M, Nagasaki T, Sato T,
Takahashi H and Takeyama H: Cancer-associated fibroblasts: Their
characteristics and their roles in tumor growth. Cancers (Basel).
7:2443–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barak V, Goike H, Panaretakis KW and
Einarsson R: Clinical utility of cytokeratins as tumor markers.
Clin Biochem. 37:529–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu-Monette ZY, Zhang M, Li J and Young KH:
PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor
immune response? Front Immunol. 8:15972017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia L, Zhang Q and Zhang R: PD-1/PD-L1
pathway blockade works as an effective and practical therapy for
cancer immunotherapy. Cancer Biol Med. 15:116–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narayan P, Wahby S, Gao JJ,
Amiri-Kordestani L, Ibrahim A, Bloomquist E, Tang S, Xu Y, Liu J,
Fu W, et al: FDA approval summary: Atezolizumab plus paclitaxel
protein-bound for the treatment of patients with advanced or
metastatic TNBC whose tumors express PD-L1. Clin Cancer Res.
26:2284–2289. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capp JP: Cancer stem cells: From
historical roots to a new perspective. J Oncol. 2019:51892322019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Najafi M, Farhood B and Mortezaee K:
Cancer stem cells (CSCs) in cancer progression and therapy. J Cell
Physiol. 234:8381–8395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lathia JD and Liu H: Overview of cancer
stem cells and stemness for community oncologists. Target Oncol.
12:387–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S and
Nakshatri H: CD44+/CD24− breast cancer cells
exhibit enhanced invasive properties: An early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jonkman JE, Cathcart JA, Xu F, Bartolini
ME, Amon JE, Stevens KM and Colarusso P: An introduction to the
wound healing assay using live-cell microscopy. Cell Adh Migr.
8:440–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meirson T and Gil-Henn H: Targeting
invadopodia for blocking breast cancer metastasis. Drug Resist
Updat. 39:1–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu W, Prasadam I, Yu M, Zhang F, Ling P,
Xiao Y and Yu C: Gamma tocotrienol targets tyrosine phosphatase
SHP2 in mammospheres resulting in cell death through RAS/ERK
pathway. BMC Cancer. 15:6092015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF
and Wicha MS: Stem cells in normal breast development and breast
cancer. Cell Prolif. 36 (Suppl 1):S59–S72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bailey PC, Lee RM, Vitolo MI, Pratt SJP,
Ory E, Chakrabarti K, Lee CJ, Thompson KN and Martin SS:
Single-cell tracking of breast cancer cells enables prediction of
sphere formation from early cell divisions. iScience. 8:29–39.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bruner HC and Derksen PWB: Loss of
E-cadherin-dependent cell-cell adhesion and the development and
progression of cancer. Cold Spring Harb Perspect Biol.
10:a0293302018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ziegler E, Hansen MT, Haase M, Emons G and
Gründker C: Generation of MCF-7 cells with aggressive metastatic
potential in vitro and in vivo. Breast Cancer Res Treat.
148:269–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:32492018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
37
|
Pivetta E, Scapolan M, Pecolo M,
Wassermann B, Abu-Rumeileh I, Balestreri L, Borsatti E, Tripodo C,
Colombatti A and Spessotto P: MMP-13 stimulates osteoclast
differentiation and activation in tumour breast bone metastases.
Breast Cancer Res. 13:R1052011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rexer BN, Chanthaphaychith S, Dahlman KB
and Arteaga CL: Direct inhibition of PI3K in combination with dual
HER2 inhibitors is required for optimal antitumor activity in HER2+
breast cancer cells. Breast Cancer Res. 16:R92014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riaz M, van Jaarsveld MT, Hollestelle A,
Prager-van der Smissen WJ, Heine AA, Boersma AW, Liu J, Helmijr J,
Ozturk B, Smid M, et al: miRNA expression profiling of 51 human
breast cancer cell lines reveals subtype and driver
mutation-specific miRNAs. Breast Cancer Res. 15:R332013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hollestelle A, Nagel JH, Smid M, Lam S,
Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal
MJ, et al: Distinct gene mutation profiles among luminal-type and
basal-type breast cancer cell lines. Breast Cancer Res Treat.
121:53–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen CC, Feng W, Lim PX, Kass EM and Jasin
M: Homology-directed repair and the role of BRCA1, BRCA2, and
related proteins in genome integrity and cancer. Ann Rev Cancer
Biol. 2:313–336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lord CJ and Ashworth A: Mechanisms of
resistance to therapies targeting BRCA-mutant cancers. Nat Med.
19:1381–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moran A, O'hara C, Khan S, Shack L,
Woodward E, Maher ER, Lalloo F and Evans DG: Risk of cancer other
than breast or ovarian in individuals with BRCA1 and BRCA2
mutations. Fam Cancer. 11:235–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ledermann JA, Drew Y and Kristeleit RS:
Homologous recombination deficiency and ovarian cancer. Eur J
Cancer. 60:49–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gallagher DJ, Gaudet MM, Pal P, Kirchhoff
T, Balistreri L, Vora K, Bhatia J, Stadler Z, Fine SW, Reuter V, et
al: Germline BRCA mutations denote a clinicopathologic subset of
prostate cancer. Clin Cancer Res. 16:2115–2121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iqbal J, Ragone A, Lubinski J, Lynch HT,
Moller P, Ghadirian P, Foulkes WD, Armel S, Eisen A, Neuhausen SL,
et al: The incidence of pancreatic cancer in BRCA1 and BRCA2
mutation carriers. Br J Cancer. 107:2005–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Corcoran RB, Dias-Santagata D, Bergethon
K, Iafrate AJ, Settleman J and Engelman JA: BRAF gene amplification
can promote acquired resistance to MEK inhibitors in cancer cells
harboring the BRAF V600E mutation. Sci Signal. 3:ra842010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kolinjivadi AM, Sannino V, de Antoni A,
Técher H, Baldi G and Costanzo V: Moonlighting at replication
forks-a new life for homologous recombination proteins BRCA1, BRCA2
and RAD51. FEBS Lett. 591:1083–1100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Holloman WK: Unraveling the mechanism of
BRCA2 in homologous recombination. Nat Struct Mol Biol. 18:748–754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shirole NH, Pal D, Kastenhuber ER, Senturk
S, Boroda J, Pisterzi P, Miller M, Munoz G, Anderluh M, Ladanyi M,
et al: TP53 exon-6 truncating mutations produce separation of
function isoforms with pro-tumorigenic functions. Elife.
5:e179292016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shirole NH, Pal D, Kastenhuber ER, Senturk
S, Boroda J, Pisterzi P, Miller M, Munoz G, Anderluh M, Ladanyi M,
et al: TP53 exon-6 truncating mutations produce separation of
function isoforms with pro-tumorigenic functions. Elife.
6:e255322017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huovinen M, Loikkanen J, Myllynen P and
Vähäkangas KH: Characterization of human breast cancer cell lines
for the studies on p53 in chemical carcinogenesis. Toxicol In
Vitro. 25:1007–1017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hui L, Zheng Y, Yan Y, Bargonetti J and
Foster D: Mutant p53 in MDA-MB-231 breast cancer cells is
stabilized by elevated phospholipase D activity and contributes to
survival signals generated by phospholipase D. Oncogene.
25:7305–7310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lu X, Errington J, Curtin NJ, Lunec J and
Newell DR: The impact of p53 status on cellular sensitivity to
antifolate drugs. Clin Cancer Res. 7:2114–2123. 2001.PubMed/NCBI
|