Introduction
Naltrexone is an opiate receptor antagonist
preventing opiate stimulation; it was licensed in 1984 as a
treatment for addiction to opiates as it prevented the euphoria
induced by recreational use of morphine and heroin. It was
observed, however, that naltrexone action also had an
immune-modulatory element, which was therapeutically beneficial to
certain patients (1). This effect
appeared to be observed only at doses much lower than given to
treat addiction, and its profile of action was distinct from that
seen with the higher dose (2).
Since then, naltrexone used at this lower dose was informally
referred to as low-dose naltrexone (LDN), to distinguish it from
naltrexone used at the conventional higher dose.
Mechanistically, naltrexone interferes physically
with the interaction between opiate and the receptor, and repeated
and chronic stimulation/blockade by naltrexone could lead to
unintentional changes in the expression and distribution of these
receptors as well as others. Specifically, complex and varied
dimerisation of the receptor with other G-protein coupled receptors
(GPCR) means that its blockade can often impact the distribution
and functioning of other members of the receptor superfamily
(3). Ultimately, binding to these
receptors can feed into and affect the action of central signalling
pathways such as the PI3 kinase and MAPK pathways, which together
influence cell fate (4). There are
many drugs that exert a therapeutic effect through these pathways;
this is not a surprise considering conservative estimates put the
value of cancers involving or driven by malfunctions in these
cascades at 90% (5).
Another drug that our laboratory has actively
researched is cannabidiol (CBD), which is a cannabinoid commonly
extracted from the cannabis plant. Surprisingly, it shares many
features with LDN. It possesses biological activity and has
recently been approved for use by the EMA and FDA as a treatment
for a rare type of childhood epilepsy (6). Evidence from the earlier 1970s
suggests that, in general, phytocannabinoids possess anticancer
activity, and it took until the mid-2000s before CBD was
specifically mentioned as having anticancer action (7). The principal mechanism of action for
CBD is slightly unclear, but it was first thought to involve
binding of the canonical cannabinoid receptors; however, binding
studies have shown its affinity for the receptor was low (8). A number of possible receptors were
suggested as being important in determining the function, with
agonism and antagonism of related GPCRs being mooted as central to
the MOA (9). Modulation of these
receptors gives CBD the ability to interact with intracellular
signalling cascades, and as with LDN, tapping into these allows CBD
to fundamentally manipulate key processes such as cell growth and
survival (7).
The effects that LDN and CBD have on these
signalling pathways in cancer cells often does not lead to an
increase in cytotoxicity. Instead, cell proliferation is reduced
resulting in smaller numbers of cells. Another effect that changes
to these pathways, particularly MAPK, can produce are alterations
to the proteins that regulate and determine apoptosis (10). This effect is important as this can
lead to a way that LDN and CBD can influence further cell fate.
Apoptosis is a process that is strictly regulated by the BCL-2
family of proteins. The balance of these members, which are made up
of those that can either oppose or promote apoptosis, is crucial in
determining whether a cell commits to apoptosis (11). These proteins therefore act as an
apoptosis switch, and when correctly engaged, apoptosis can proceed
fully when the cytotoxic signal/stimulus is received (12).
Some cancer cells have aberrant signalling within
the BCL-2 family, that results in disruption to apoptotic
capability; cancer cells may overexpress the family members that
oppose apoptosis, making it more difficult for the cell to initiate
apoptosis. The inverse scenario can also exist, namely when the
proteins that support apoptosis are mutated or absent (13). Overall, this means that cancer
cells are unable to activate apoptosis even though there is a
‘kill’ signal, and are effectively resistant to certain treatments.
Approaches, such as BCL-2 inhibitors and/or mimetics have been
developed to correct the errors in the expression levels of these
proteins, and in doing so, restore the ability to undergo cell
death (14). It is by altering the
balance of these apoptosis proteins that LDN and CBD are able to
restore a level of cell killing in cancer cells (2,15).
In the present study, a part of our ongoing
investigations into the anticancer actions of LDN and CBD is
described. Specifically, combination studies were performed to
understand how these two related agents can be combined in a way to
prime cancer cells to conventional chemotherapy. The best
combinations identified by in vitro experiments were then
forwarded on to further examinations in a murine model.
Materials and methods
Cell culture and drugs
All cell lines were purchased from the European
Collection of Cell Cultures (Salisbury, UK), and maintained and
grown in the culture medium specified by the depositor. For A549
(human lung cancer) and HCT116 (human colorectal cancer) cells this
was DMEM (Sigma-Aldrich; Merck KGaA), while for MCF7 (human breast
cancer) cells the medium was RPMI-1640 (Sigma-Aldrich; Merck KGaA).
Media were supplemented with 5% foetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.) and 2 mM L-glutamine (Thermo Fisher
Scientific, Inc.). All cells were grown in a humidified atmosphere
with 5% CO2 in air at 37°C, and discarded after 8 weeks.
Authentication of the cell lines was performed by the service
providers using the AmpFISTR Identifier Plus PCR amplification kit
looking for the presence of <10 known loci for each cell
line.
Naltrexone hydrochloride (naltrexone; Sigma-Aldrich;
Merck KGaA), cannabidiol (CBD; THC Pharm GmbH, Germany);
gemcitabine (GEM; Sigma-Aldrich; Merck KGaA) and oxaliplatin (OXP;
Sigma-Aldrich; Merck KGaA) were dissolved in DMSO, with the final
DMSO concentration in individual tests being <0.05%.
Viability assays
Our previous research has shown that the effects of
naltrexone are highly dependent on dose; at lower concentrations
<50 nM, the cellular and molecular effects are mainly anticancer
in nature, while these are lost at conventionally higher doses that
are typically >10 µM (2). To
study the effects of each agent on cell growth, exponentially
growing cells were seeded onto 6-well plates at a density of
1×105 cells per well and left to adhere overnight. Cells
were then treated with the agents. Naltrexone was used at 10 µM
(designated NTX) and at a lower 10 nM concentration. This lower
concentration was the Cmax as determined by
pharmacokinetic studies in volunteers administered approximately
4.5 mg of naltrexone, which is the low dose of naltrexone (LDN)
that is used clinically. CBD was used at 1 µM, which is a
concentration seen in the sera of patients given 400 mg i.v.
(intravenously). GEM and OXP were used at ~IC20 as
established in a previous study (16). Cell number and viability were
assessed after 48 h by using trypan blue staining to discriminate
live from dead cells. Aliquots were also harvested for flow
cytometric analysis of the cell cycle using propidium iodide (PI)
and RNAse staining as western blot analysis, both as previously
described (16).
Combination studies
The effect of combining the drugs was assessed by
culturing the cells according to a regimen that was made up of two
phases of treatment, with each lasting 48 h. The total duration was
therefore 96 h. In between the phases, exhausted medium containing
any drugs was removed, and the cells were gently washed and
replenished with fresh culture medium containing the next phase of
treatment. Methodologically, cells were seeded onto 6-well plates
at a density of 1×105 cells per well and left to adhere
overnight. After this time, the drugs were added to the cells as
part of the first phase of the treatment at concentrations detailed
in the ‘Viability assays’ section, and left for 48 h. The second
phase of treatment followed directly after the exhausted medium was
removed from the cells, and fresh medium added containing the
appropriate concentration of the test drug. Cells were left for a
further 48 h before the cell number and viability were assessed by
cell counting and processing for western blot analysis.
Immunoblot analysis
Following individual treatments, the cells were then
harvested by scraping into lysis buffer (New England Biolabs, UK),
and standard western blot protocols were followed as described
previously (2). Primary probing
was with specific antibodies generated against phosphorylated
(p)AKT (cat#: 9271S), AKT (cat#: 9272S), pERK (cat#: 9101S), ERK
(cat#: 9102S), cannabinoid receptor (CBR) 1 (cat#: 93851S) and 2
(cat#: PA1-744) (all New England Biolabs), used at a dilution of
1:1,000. GAPDH (1:5,000; New England Biolabs; cat#: 2118S) was the
loading control, and secondary probing was performed using the
species-appropriate HRP conjugated antibody (New England Biolabs;
cat#: 7074S) at a dilution of 1:1,000. Bands were visualised using
the SuperSignal chemiluminescent detection system (Thermo Fisher
Scientific, Inc., UK). Densitometry of band intensity was
determined using Adobe Photoshop CS3, v10.0 (Maidenhead, UK), and
normalised to GAPDH.
In vivo tumour model
The present study assessed the effect that differing
treatment-schedules had on the growth of HCT116 cells in athymic
nu/nu BALB/c mice. A total of 60 female mice (Charles River
Laboratories, Harlow, UK), aged 6–8 weeks, were separated into
groups each containing at least 4-mice, and treatments began after
one week of acclimatisation. The average starting weight for each
animal was ~17.2 g. The schedules involved two phases of treatment
that each lasted one week. Exponentially growing HCT116 cells, with
viabilities >90% were resuspended in phosphate-buffered saline
(PBS) at a concentration of 5×107 cells/ml. Tumour
suspension (100 µl) was injected subcutaneously in the
dorso-lateral flanks of the mice and allowed to grow until the
masses were palpable. This was often after about 7 days, and for
ease of recording, this day was designated day 1. Drugs were then
administered on days 1 and 4 for the first phase of treatment, and
on days 8 and 11 for the second phase. The drugs used were LDN (1.2
µg/mouse), CBD (35 µg/mouse) or GEM (9 µg/mouse), and administered
intraperitoneally (100 µl). Mice were housed under standard
conditions appropriate for nu/nu mice, in rooms with filtered air.
Tumour growth was checked daily, and final tumour volume was
determined on day 14 by taking measurements of the tumour in two
dimensions [width (W) and length (L)], and using the equation V=0.5
× W × L2. Mice were sacrificed on day 14 by using a
schedule 1 method according to the UK Home Office and involved
cervical dislocation and confirmation by snipping the femoral
artery. Tumours were excised for further analysis by western
blotting.
Statistical analyses
All statistical analyses were carried out using
Graph-Pad Prism 7 (GraphPad Software, Inc.) or Microsoft Excel
(v1808). Datasets were tested for normality by the Shapiro-Wilk
test, and differences between variable and control groups were
determined by the appropriate one-way ANOVA with multiple
comparisons at a level to P<0.01. Paired tests were then
performed to further determine any differences following
Bonferroni's testing. Unless where otherwise stated, data where a
statistical difference was noted are indicated in the text and/or
in figures. All sets consisted of data from at least three separate
experiments, and data are presented as the mean and SD.
Results
CBD, LDN and NTX have minimal effects
on cell growth and viability
Our earlier studies showed that both LDN and CBD had
minimal effects on cell viability (2,17),
and the results of the present study recapitulated this. The two
agents, however, were capable of significantly altering the
expression of intracellular signalling proteins such as AKT and
ERK, which underpinned key cell functions such as cell survival and
apoptosis. We also showed that affecting these proteins caused a
‘priming’ effect, rendering cancer cells more susceptible to the
cytotoxic effects of certain chemotherapy drugs. A principle
component of the present work was to understand the effects of
using LDN and CBD together on intracellular signalling in cancer
cells, and whether or not they could exert similar priming effects
seen when used individually. We therefore combined LDN or
naltrexone at a more conventional concentration (10 µM; designated
NTX) with CBD, and assessed the effect it may have on cancer
cells.
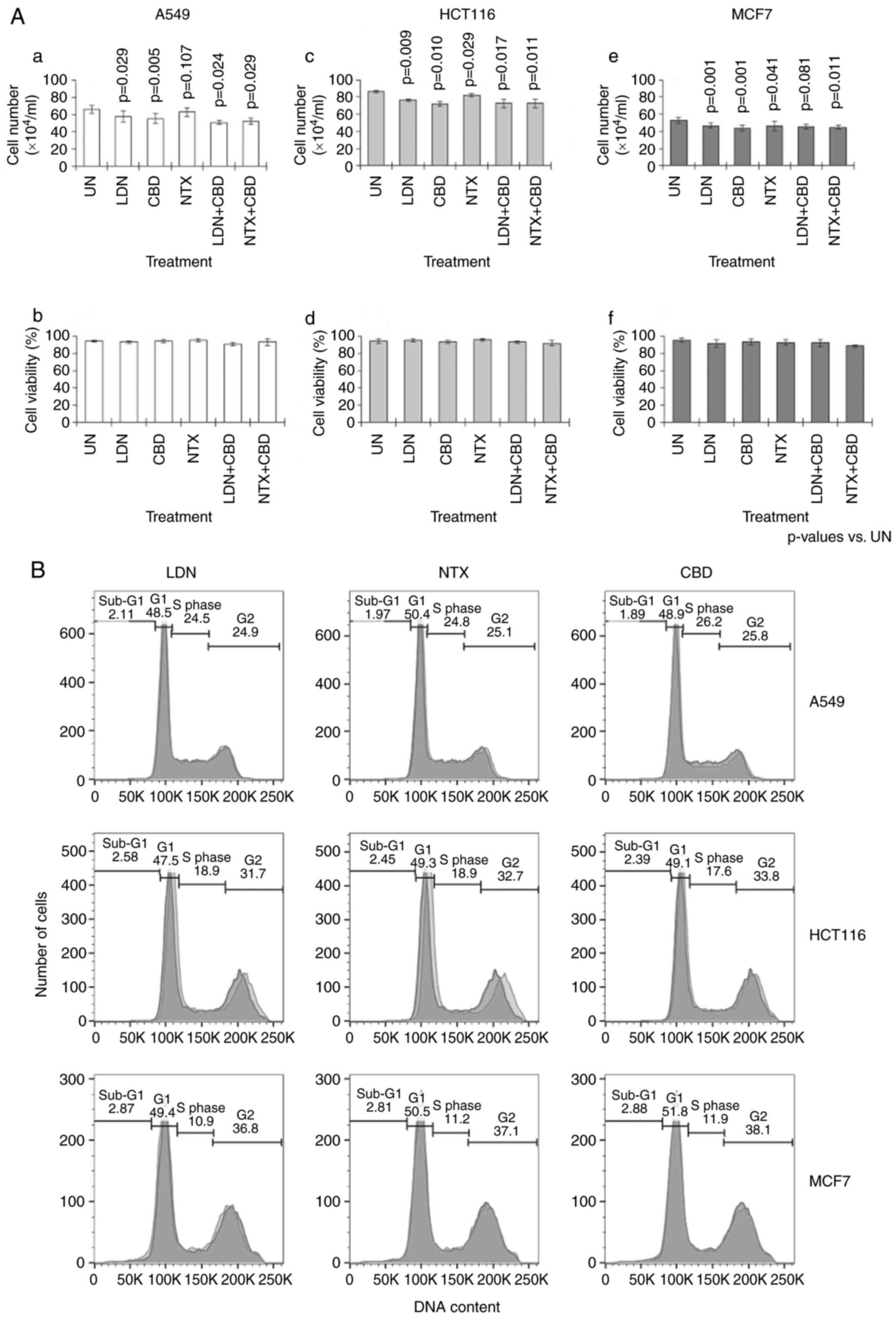
The results showed that single-agent CBD, LDN and
NTX had no significant effect on cell viability (Fig. 1A-b, d and f). NTX alone also had no
effect on cell numbers; however, CBD alone resulted in small but
significant reductions in cell number when compared to the
untreated (UN) cells (P<0.01) (Fig.
1A-a, c and e). Similarly, LDN alone also significantly reduced
cell numbers, but only in HCT116 and MCF7 cells (P<0.01)
(Fig. 1A-c and e). Combining
either LDN or NTX with CBD generally had no significant effect on
cell parameters (Fig. 1A).
Although in A549 cultures, there appeared to be fewer cells in the
combination groups; comparing cell numbers actually observed in
these groups against predicted numbers showed no significant
differences (Fig. 1A-a). For
example, the number of cells predicted to have remained after LDN
and CBD were administered together was 47±8.3×104 cells,
which was not significantly different to 51±2.1×104
cells that was actually observed (P=0.508). Thus overall, the
effect of combining LDN with CBD did not affect the number of cells
predicted to remain after the combination treatment.
Parenthetically, predicted cell numbers were calculated by
subtracting the number of cells reduced by individual treatments
with LDN and CBD alone from the number of cells seen in untreated
control cultures. There were no significant changes in the
distribution of the cells in the different phases of the cell cycle
(Fig. 1B).
The sequence of drugs influences
overall activity
Guided by our previous studies that showed that the
actions of LDN and CBD were influenced by the sequence of
administration, we next examined the effect using one drug before
the other may have on cell number and viability. We did not have a
positive control in this section as LDN and CBD alone have little
cytotoxic activity on its own. This is an important consideration
when assessing the current dataset. This was tested by comparing
cell numbers and viabilities after treatment schedules where one
drug was used before the other and vice versa. ANOVA showed
statistical differences within some of the groups (P<0.01),
which were then analysed further using paired t-tests. The results
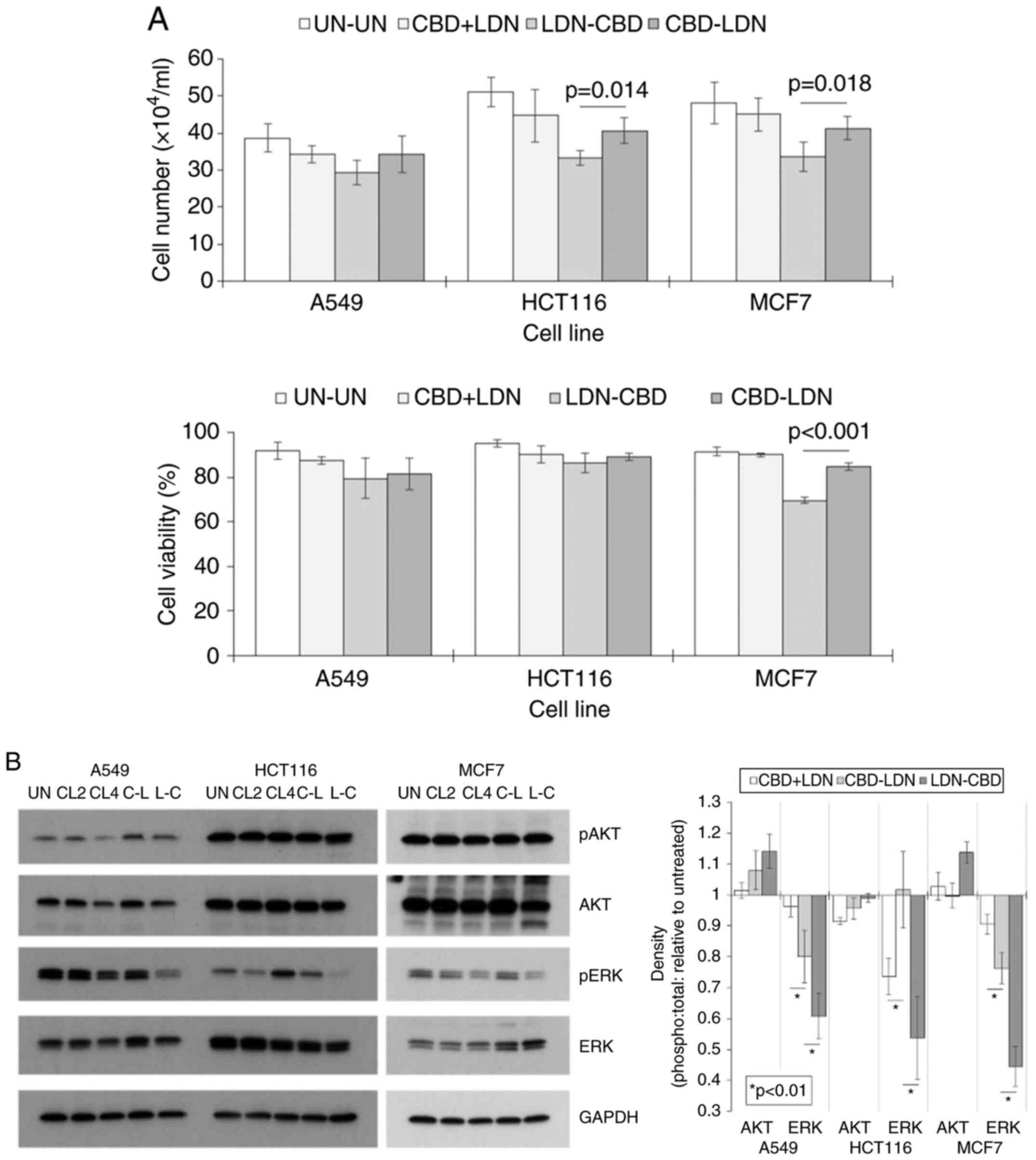
showed that the sequential administration of LDN and CBD
irrespective of order (LDN-CBD or CBD-LDN), appeared to be more
active than a treatment-regimen involving the concomitant
administration of the drugs (CBN + LDN) (Fig. 2A). More importantly, cell number
and viability were generally reduced more when LDN was used before
CBD (LDN-CBD) compared to the reverse treatment order (Fig. 2A). In some instances, the
differences were significant; for example, in HCT116 cells, the
cell number was 40±3.5×104 after treatment with the
sequence CBD-LDN vs. 33±2.1×104 using the LDN-CBD
sequence (P=0.014).
Cell number and viability are modulated in part by
intracellular signalling pathways. Thus, whether the two sequences
of CBD and LDN exerted differing effects on AKT and ERK was
ascertained. These parameters were then compared to untreated (UN)
controls and to cells treated with CBD and LDN simultaneously for
either 2 or 4 days (CL2 or CL4). The results showed that pAKT
levels were generally unchanged in cells treated with LDN and CBD
in any of the treatment schedules, and that also the sequence by
which the drugs were applied had no bearing on this (Fig. 2B). Conversely, pERK levels were
generally reduced following treatments. Crucially, the reduction in
expression was greater when the sequence LDN followed by CBD was
used (P<0.01). For example, in HCT116 cells, the ratio of the
densities of the pERK:tERK bands relative to untreated bands were
+2% after treatment with CBD-LDN vs. −46% after treatment with
LDN-CBD (Fig. 2B).
CBD and LDN can sensitise cells to the
effects of chemotherapy
ERK signalling regulates cell proliferation and
survival, so consequently, drugs that alter this may sensitise
cancer cells to the cytotoxic effects of chemotherapy drugs.
Considering the effect that LDN and CBD combinations had on pERK
expression, we explored the possibility that pre-treating cancer
cells with LDN and CBD could influence their sensitivity to the
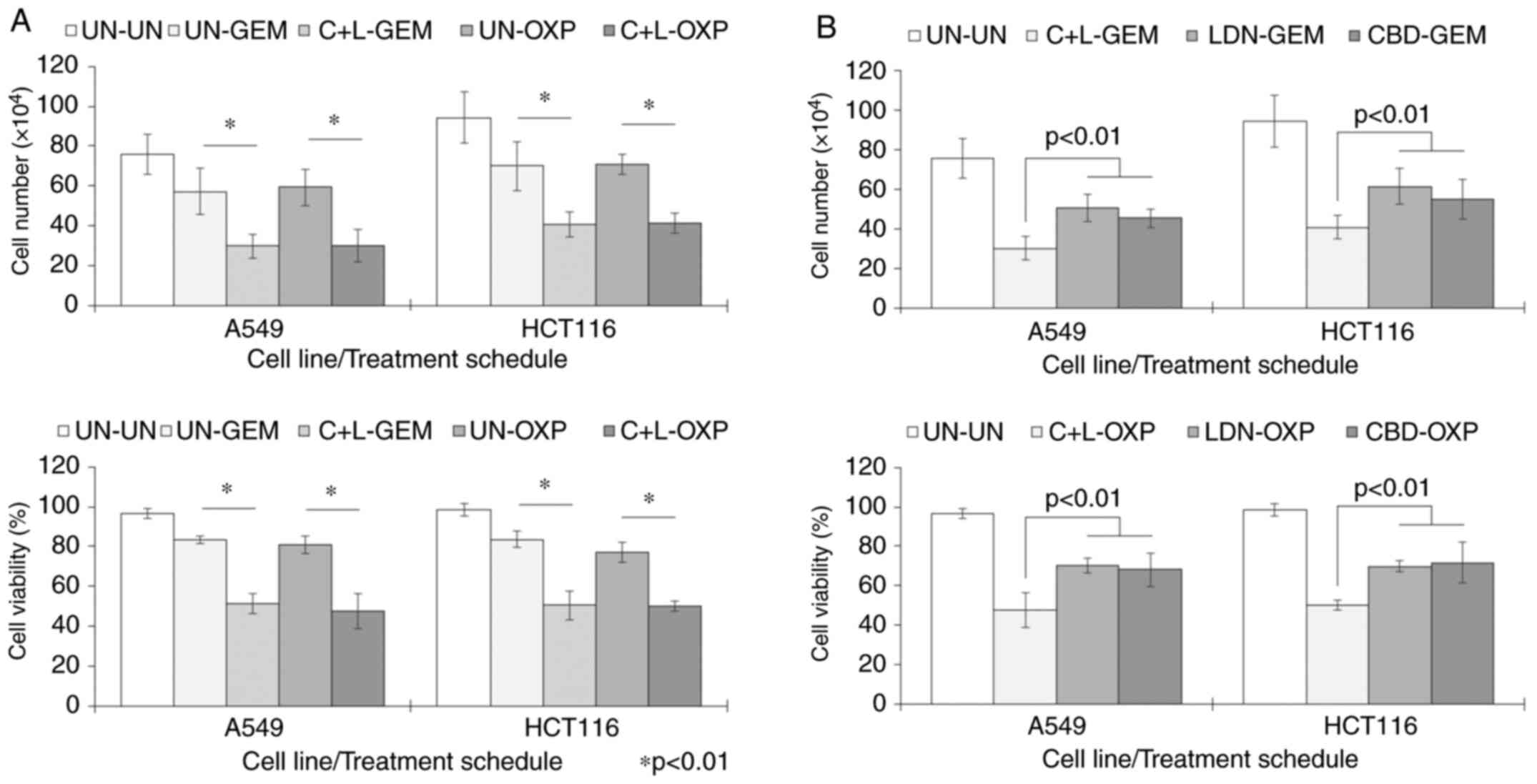
common drugs GEM and OXP. Cells were pre-treated for 2 days with
CBD and LDN at a molar ratio of 100:1, before treatment with
chemotherapy used at suboptimal cytotoxic concentrations. The
results showed that cell numbers and cell viability were
significantly reduced compared to the untreated (UN) controls
(Fig. 3A). For example, in HCT116
cells pre-treated with CBD + LDN (C + L), OXP significantly reduced
cell number to 41±5.0×104 vs. 94±13×104 cells
in the untreated cells (P<0.001), and cell viability to 50±2.7%
from 98±3.0% seen in the untreated cells (P<0.001).
There was also an element of a ‘priming’ effect of C
+ L, as both number and viability of cells pre-treated with these
prior to chemotherapy were significantly lower than cultures when
chemotherapy was used without the C + L pre-treatment (Fig. 3A). For example, cell number and
viability of HCT116 cells exposed to OXP without the C+L
pre-treatment was 71±5.0×104 cells and 77±4.8%,
respectively. However, with the C + L pre-treatment, these values
were reduced significantly further to 41±5.0×104 cells
and 50±2.7% (P<0.01). As both CBD and LDN were not cytotoxic on
their own, the increase in death suggested cells were primed to the
killing effects of OXP.
This priming effect also appeared to be most
effective when CBD and LDN were used in combination, as results
also showed that priming separately with just CBD or LDN did not
enhance the cytotoxic effect of GEM or OXP as much as if both
components were used together (P<0.01 in all cases) (Fig. 3B).
GEM is tolerated better when used with
CBD and LDN
The efficacy of treatments using CBD and/or LDN were
also assessed in a xenograft murine model. The growth of human
HCT116 cells implanted into the flanks of nude mice was tracked for
about 25 days, and the effects of CBD ± LDN with or without GEM on
final tumour volumes were examined. The treatment regimens were
composed of two stages of treatment that each lasted 7 days. Those
involving just LDN and/or CBD were well tolerated, and there were
no marked changes in body weights compared to the untreated groups
(data not shown). Mice that were treated with just a single agent,
viz. either LDN or CBD only for both stages of the regimen,
exhibited tumours that were not significantly different to those
observed in the untreated mice (Fig.
4A-a). However, the sequential administration of LDN and CBD,
regardless of their particular order generally resulted in tumour
volumes that appeared to be smaller than those seen in the control
(UN) group; however, this was not statistically significant
(Fig. 4A-b).
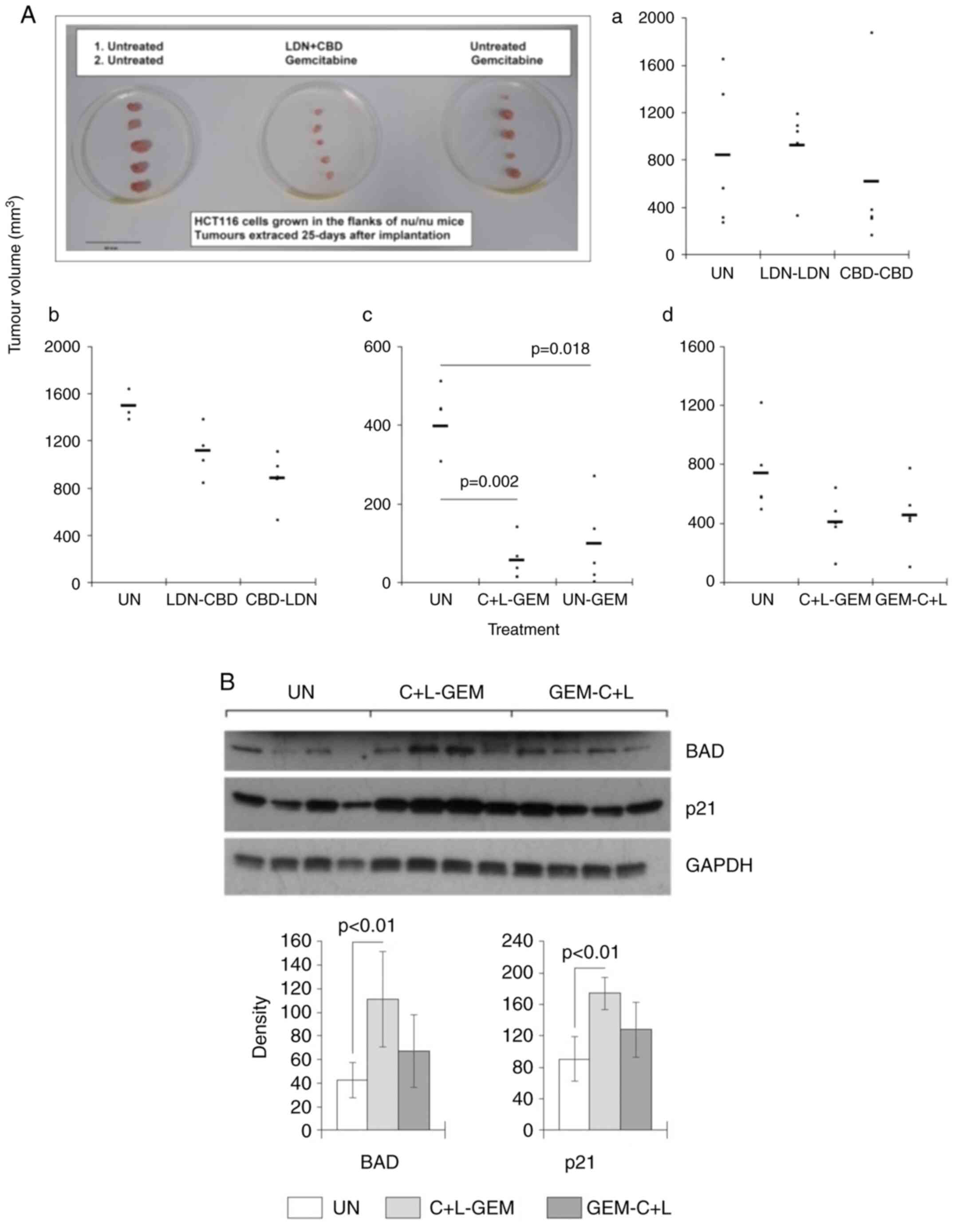 | Figure 4.Effect of CBD, LDN and GEM on tumour
cells in vivo. HCT116 cells were implanted subcutaneously
into the flanks of immune-compromised mice, and when palpable,
tumours were treated with CBD, LDN and/or GEM according to a
treatment schedule consisting of two stages of treatment that each
lasted one week. For example, LDN could be used in the first period
followed by CBD in the second week (designated LDN-CBD). In some
situations, CBD and LDN were used together (C + L). (Aa-d) Tumour
sizes were assessed at the end of the treatment (end of week 2).
The tumours extracted from the mice in experiment c were
photographed and included in the figure. (B) Tumours were
disaggregated at the end of week 2, and subjected to western blot
analysis for expression of BAD and p21. Black bars represent the
mean of each mouse represented by the circles. The blots are of
four mice, and the columns represent the mean of all the mice in
each group. CBN, cannabidiol; LDN, low-dose naltrexone; GEM,
gemcitabine. |
Next, mice were either given C + L or PBS in the
first phase of treatment before being treated with GEM. Tumour
volumes were then measured and compared at the end of treatment.
The results showed that tumour volumes when compared with the
control group were smaller in the groups where GEM was used after
PBS (P=0.018) or with C + L (P=0.002) (Fig. 4A-c). There was no difference in
tumour volumes between C + L primed and un-primed animals; however,
GEM was better tolerated by mice in the group pre-treated with C +
L compared to those without. Specifically, the change [median
(IQR)] in animal body-weight after treatment with C + L then GEM
was 1.5 g/mouse (1.0-1.8) vs. −0.25 g/mouse (−0.55-0.3) in the PBS
then GEM group (data not shown).
The order in which C + L and GEM was administered
was also examined to see whether this influenced overall efficacy.
The results revealed there was no significant difference in the
final tumour volumes from animals in the C + L-GEM group vs. the
GEM-C + L group (Fig. 4A-d).
However, there was a difference in how the treatments were
tolerated as the change [median (IQR)] in animal body-weight was
1.3 (1.2-2.3) in the C + L-GEM group vs. −0.60 (−1.1-0) in the
GEM-C + L group (data not shown). The effect of these two
treatments on standard markers of cell growth and survival were
also measured. The results showed the treatment using C + L
followed by GEM resulted in a significant increase in the
expression of BAD and p21 compared to the untreated group
(P<0.01) (Fig. 4B).
Discussion
The present study was a continuation of our earlier
ones, which focused on understanding further the anticancer effects
of low-dose naltrexone (LDN) and cannabidiol (CBD). Our previous
studies showed that both agents when used in isolation were able to
enhance the activities of common chemotherapy agents.
Mechanistically, this was because both LDN and CBD were able to
enhance the level of certain proteins within cell signalling
cascades such as BAD, BAX and p21 that influence cell death and
survival. Both agents were found to share a similar MOA, which is
that they are able to prime cancer cells to the cytotoxic effects
of standard cytotoxic agents (2,17,18).
The potential anticancer effects of these two agents
have been known for some time and are supported by a range of in
vitro studies and animal modelling. Many of the key studies
have been examined and collated into review articles (19,20),
yet there have been scant clinical trials to confirm the efficacy
and benefit; especially as single-use agents. This has not,
however, dissuaded the public from using these sorts of drugs in
the hope that they will work ‘better’ than conventional treatments.
Indeed, in more recent times, LDN and CBD have actually been used
together in the belief they are complementary irrespective of the
fact that there are no data suggesting a benefit to using them
together as anticancer agents. The only study we could find that
reported the use of LDN and CBD was a murine study reporting a
benefit to using the two as a way of reducing the motivation of
mice to self-administer ethanol (21). The benefit was only observed when
the two drugs were used concomitantly as CBD appeared to
support/promote the action of LDN. Although the MOA was not
defined, the need for signalling cascades common to both agents
appeared important. Thus, as an attempt to shed more light in this
field, we examined the effect that combining LDN with CBD may have
on the growth and survival characteristics of cell lines in
vitro. We also assessed the effect of these novel combinations
on the cytotoxic effects of certain chemotherapy drugs. Finally, we
examined the best drug-combinations utilizing murine studies to
establish the optimum tumour-reducing treatment schedule.
LDN and CBD display actions and features that are
extremely similar to each other. They both interact loosely with
receptors that result in intracellular modifications, which lead to
the desired response. The receptors involved are not the same for
both drugs, but are all part of the G-protein coupled receptor
(GPCR) superfamily (22).
Activation of these receptors can cause changes to similar
downstream signalling cascades, which although overlapping in
places, lead to effects specific to each drug individually.
Anticancer actions have been loosely attributed to and associated
with two routes of action. Firstly, both CBD and LDN can directly
interfere with cell signalling systems within cancer cells that can
result in an arrest of cell proliferation. In some circumstances,
this arrest in cell cycling can lead to apoptosis (23,24).
Second, both are capable of modifying the host immune system, which
can re-educate and stimulate and direct an immune-based cytotoxic
response against cancer cells (1,25).
Given these actions, it appears that both CBD and LDN reduce cell
numbers by inducing a cytostatic effect rather than killing cells
directly.
The cannabinoid receptor and opioid receptor systems
are known to interact with each other (26), which suggests that the use of LDN
with CBD together could lead to an enhanced effect overall. In
vitro and murine models have indeed shown that the binding to
cannabinoid receptor 1 can influence activity of the δ-opioid
receptor (27). The cross-play
between receptor systems is not uncommon and can involve a
compensatory increase in expression of a receptor to make up for
the loss or antagonism of another (28). In fact, our previous study
examining the effect of LDN on the gene expression of a range of
receptors within this family, including the adrenergic, GABA,
glutamate and serotonin, revealed 15% were upregulated and 17% were
downregulated following culture with LDN (2). Crucially, as the incoming
‘substitute’ receptor may interact differently with intracellular
signalling, the ultimate effect achieved may actually differ from
that originally initiated. This introduces the possibility that a
ligand with classical actions can engage other processes distinct
from what would be expected, and two drugs that work on receptors
may work in unison. Taken together, these introduce the overarching
concept that using methods to modulate the activity of one receptor
may be exploited as a mechanism by which the activity of another
can be altered. We therefore utilised LDN and CBD together to see
if their interplay could influence further cancer cell fate.
Using LDN and CBD at the same time had no effect on
cell number and cell viability. Using the two together was no
better than using the agents separately, and in some situations
negated the reduction in cell number seen when the drugs were used
separately. This was not a complete surprise as GPCR signalling is
not a uniform process but instead one that is spatial and temporal
in nature (29). Receptor
activation and signalling via secondary messengers are timed and
orchestrated, with one event having to occur before another can. We
thus examined the effects on cell number and viability when LDN and
CBD were used sequentially and showed that the order in which they
were used was crucial. Using CBD before LDN was no different to
using the two drugs at the same time; however, cell number and
viability were significantly reduced when LDN was used before CBD.
The levels of pERK expression in cells were also tracked, and were
reduced after treatment with CBD and LDN, with the largest
reduction seen in the schedule where LDN was used before CBD. There
are other readouts such as migration and invasion that may have
been affected by these treatments, which is something that should
be explored further in the future.
Signalling through pERK plays a central role in
determining the fate of cancer cells, as their activation generally
increases cell numbers by promoting cell proliferation and survival
(30). It plays an important
enough role that targeting its action as well as other members of
the cascade has been the aim of several therapeutic approaches
(31). The anticancer actions of
both LDN and CBD alone are associated in part with changes to pERK
signalling (2,32), and our results showed that
combining the two both simultaneously or sequentially could still
reduce its expression. There was no market increase in cell killing
by using the two, but this was not surprising as both LDN and CBD
are actually not cytotoxic agents, and even though pERK was
reduced, the absence of an active ‘kill’ signal would mean death is
unlikely to happen. Indeed, studies have shown that pERK is
involved in the balancing of anti- and pro-apoptotic proteins, and
as such its modification alone does not necessarily result in cell
death, but instead modulates the likelihood of apoptosis occurring
(33). Taken together, this
suggests partnering a cytotoxic chemotherapy agent with LDN or CBD
would be a way of increasing cytotoxicity, which is precisely
something we and others have shown. Specifically, there are data
showing CBD is effective with chemotherapy in a wide range of
cancer types, with the MOAs underpinning synergy involving the
modification to the balance of BAX:BCL2-related proteins downstream
of pERK (34–36). Similarly, studies involving LDN and
chemotherapy combinations report a similar MOA involving these
apoptosis-determining proteins (2,37).
The results of the present study showed that using
CBD and LDN together could also sensitise cells to the cytotoxic
effects of two common chemotherapy agents in vitro. For
example, the cytotoxic effect of GEM in HCT116 cells was enhanced
by 40% when CBD and LDN were used up-front as a priming agent (%
cell death: 49±7.3 vs. 16±4.0% in GEM-treated cells without
priming; P<0.01). Notably, using the two together was superior
to using just one of the drugs individually. The priming effect was
not clear cut in the studies with mice bearing a human tumour, and
an expected divergence of tumour sizes in the primed and un-primed
groups was not observed. Post experimental analysis of the data
indicated that GEM generated an effect that was much higher than
expected. For logistical reasons, an equivalent dosage for the
mouse experiment was extrapolated from standard guides (38), based on previously published
studies, and in accordance with our in vitro work targeted
to be in the region of an IC20. The actual dose turned
out to be more effective than expected, and any benefit of the
priming effect was swamped by GEM working at the more efficacious
dose. Nevertheless, the mouse studies indicated that priming with
CBD and LDN made GEM at this concentration more tolerable to mice.
Specifically, the loss of body weight with GEM treatment was
negated and in fact reversed when mice were pre-treated with CBD
and LDN. This suggested that another possible benefit of using CBD
and LDN especially in vivo would be an improvement in the
capacity of patients to tolerate cytotoxic treatments (39).
In conclusion, these data reinforce the idea that
CBD and LDN are drugs that have the capacity to enhance the action
of other treatments. Although they have a minimal effect on their
own on cell growth and death, their benefits lie in the way that
they can enhance the activity of chemotherapy drugs. This effect is
not only seen when LDN and CBD are used individually, but when used
together following a treatment schedule that involves a
sequenced-administration of the drugs. Our in vivo
examinations recapitulated the laboratory studies and also showed
that adding CBD and LDN as a priming agent resulted in animals
bearing the chemotherapy much better. Finally, all our data
reported here were based on cancer cell lines and did not take into
consideration the tumour microenvironment and the inflammatory
immune responses. We previously demonstrated that naltrexone blocks
Toll-like receptors (TLR)-7, −8 and −9 that reduce the production
of interleukin (IL)-6, which is a major determinant of cancer
progression (40). Hence the
anticancer effect may be even more important in the human clinical
situation where significant responses have been reported with LDN
alone (41). Overall, these
studies provide evidence to support the role for LDN/CBD and
chemotherapy in clinical trials, especially in those cancer that
have an issue of high toxicity with standard chemotherapy
regimens.
Acknowledgements
The authors wish to thank Mr Robert Bond, Miss Emma
Mustafa and the rest of the staff at the Biological Research
Facility at St George's University of London for assistance with
the animal experiments.
Funding
This work was funded by a research grant awarded to WML from LDN
Pharma Ltd., London UK, who had no input into the study concept and
study design. AGD receives funding from the Institute for Cancer
Vaccines and Immunotherapy, St George's University of London.
Availability of data and materials
The datasets used in the present study are available
from the corresponding author upon reasonable request.
Authors' contributions
The study was conceived and written by WML and AGD.
The experiments were performed by WML, NKH and HSYL. The data were
analysed, examined, and assessed by WML. Interpretation of the data
and studies and confirmation of the integrity of all data were
performed by WML, FLH and AGD. All authors read and approved the
final manuscript for publication.
Ethics approval and consent to
participate
All animal experiments were performed according to
the strict guidelines in accordance with the UK Animals (Scientific
Procedures) Act and associated guidelines. All work was approved by
the Ethics Committee of St George's University of London (SGUL),
and performed at the Biological Research Facility of SGUL under
project licence PP1419291.
Patient consent for publication
Not applicable.
Competing interests
WML and AGD are named inventors on patents (some
that are pending) related to the use of LDN and/or cannabinoids as
a potential cancer therapy. FLH is contracted by LDN Pharma Ltd.
(London, UK)
References
|
1
|
Li Z, You Y, Griffin N, Feng J and Shan F:
Low-dose naltrexone (LDN): A promising treatment in immune-related
diseases and cancer therapy. Int Immunopharmacol. 61:178–184. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu WM, Scott KA, Dennis JL, Kaminska E,
Levett AJ and Dalgleish AG: Naltrexone at low doses upregulates a
unique gene expression not seen with normal doses: Implications for
its use in cancer therapy. Int J Oncol. 49:793–802. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milligan G: G protein-coupled receptor
hetero-dimerization: Contribution to pharmacology and function. Br
J Pharmacol. 158:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto Gutierrez A and McDonald PH: GPCRs:
Emerging anti-cancer drug targets. Cell Signal. 41:65–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al: Oncogenic signaling pathways in the cancer genome atlas.
Cell. 173:321–337.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arzimanoglou A, Brandl U, Cross JH,
Gil-Nagel A, Lagae L, Landmark CJ, Specchio N, Nabbout R, Thiele
EA, Gubbay O, et al: Epilepsy and cannabidiol: A guide to
treatment. Epileptic Disord. 22:1–14. 2020.PubMed/NCBI
|
|
7
|
Massi P, Solinas M, Cinquina V and
Parolaro D: Cannabidiol as potential anticancer drug. Br J Clin
Pharmacol. 75:303–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McPartland JM, Glass M and Pertwee RG:
Meta-analysis of cannabinoid ligand binding affinity and receptor
distribution: Interspecies differences. Br J Pharmacol.
152:583–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shahbazi F, Grandi V, Banerjee A and Trant
JF: Cannabinoids and cannabinoid receptors: The story so far.
iScience. 23:1013012020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb
Perspect Biol. 5:a0087142013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5:a0087222013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Lu Z and Zhao X: Targeting Bcl-2
for cancer therapy. Biochim Biophys Acta Rev Cancer.
1876:1885692021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shrivastava A, Kuzontkoski PM, Groopman JE
and Prasad A: Cannabidiol induces programmed cell death in breast
cancer cells by coordinating the cross-talk between apoptosis and
autophagy. Mol Cancer Ther. 10:1161–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu WM, Fowler DW, Smith P and Dalgleish
AG: Pre-treatment with chemotherapy can enhance the antigenicity
and immunogenicity of tumours by promoting adaptive immune
responses. Br J Cancer. 102:115–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scott KA, Dalgleish AG and Liu WM:
Anticancer effects of phytocannabinoids used with chemotherapy in
leukaemia cells can be improved by altering the sequence of their
administration. Int J Oncol. 51:369–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scott KA, Shah S, Dalgleish AG and Liu WM:
Enhancing the activity of cannabidiol and other cannabinoids in
vitro through modifications to drug combinations and treatment
schedules. Anticancer Res. 33:4373–4380. 2013.PubMed/NCBI
|
|
19
|
Liubchenko K, Kordbacheh K, Khajehdehi N,
Visnjevac T, Ma F, Khan JS, Storey M, Abd-Elsayed A and Visnjevac
O: Naltrexone's impact on cancer progression and mortality: A
systematic review of studies in humans, animal models, and cell
cultures. Adv Ther. 38:904–924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Cancer Institute, .
PDQ® integrative, alternative and complementary
therapies editorial board. Cannabis and cannabinoids
(PDQ®): Health professional version. 2021 Jun 3. PDQ
Cancer Information Summaries. National Cancer Institute (US);
Bethesda, MD: 2002, https://www.cancer.gov/about-cancer/treatment/cam/hp/cannabis-pdqOctober
15–2021PubMed/NCBI
|
|
21
|
Viudez-Martínez A, García-Gutiérrez MS,
Fraguas-Sánchez AI, Torres-Suárez AI and Manzanares J: Effects of
cannabidiol plus naltrexone on motivation and ethanol consumption.
Br J Pharmacol. 175:3369–3378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rios C, Gomes I and Devi LA: mu Opioid and
CB1 cannabinoid receptor interactions: Reciprocal inhibition of
receptor signaling and neuritogenesis. Br J Pharmacol. 148:387–395.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kovalchuk O and Kovalchuk I: Cannabinoids
as anticancer therapeutic agents. Cell Cycle. 19:961–989. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu N, Meng Y, Handley MK, Wang C and Shan
F: Preclinical and clinical studies into the bioactivity of
low-dose naltrexone (LDN) for oncotherapy. Int Immunopharmacol.
96:1077142021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu WM, Fowler DW and Dalgleish AG:
Cannabis-derived substances in cancer therapy-an emerging
anti-inflammatory role for the cannabinoids. Curr Clin Pharmacol.
5:281–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manzanares J, Ortiz S, Oliva JM,
Pérez-Rial S and Palomo T: Interactions between cannabinoid and
opioid receptor systems in the mediation of ethanol effects.
Alcohol Alcohol. 40:25–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bushlin I, Gupta A, Stockton SD Jr, Miller
LK and Devi LA: Dimerization with cannabinoid receptors
allosterically modulates delta opioid receptor activity during
neuropathic pain. PLoS One. 7:e497892012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allouche S, Noble F and Marie N: Opioid
receptor desensitization: Mechanisms and its link to tolerance.
Front Pharmacol. 5:2802014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lohse MJ and Hofmann KP: Spatial and
temporal aspects of signaling by G-protein-coupled receptors. Mol
Pharmacol. 88:572–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sever R and Brugge JS: Signal transduction
in cancer. Cold Spring Harb Perspect Med. 5:a0060982015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Yang X, Geng M and Huang M:
Targeting ERK, an achilles' heel of the MAPK pathway, in cancer
therapy. Acta Pharm Sin B. 8:552–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Howlett AC: Cannabinoid receptor
signaling. Handb Exp Pharmacol. 168:53–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Go YY, Kim SR, Kim DY, Chae SW and Song
JJ: Cannabidiol enhances cytotoxicity of anti-cancer drugs in human
head and neck squamous cell carcinoma. Sci Rep. 10:206222020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seltzer ES, Watters AK, MacKenzie D Jr,
Granat LM and Zhang D: Cannabidiol (CBD) as a promising anti-cancer
drug. Cancers (Basel). 12:32032020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pagano C, Navarra G, Coppola L, Bifulco M
and Laezza C: Molecular mechanism of cannabinoids in cancer
progression. Int J Mol Sci. 22:36802021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma M, Wang X, Liu N, Shan F and Feng Y:
Low-dose naltrexone inhibits colorectal cancer progression and
promotes apoptosis by increasing M1-type macrophages and activating
the Bax/Bcl-2/caspase-3/PARP pathway. Int Immunopharmacol.
83:1063882020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma V and McNeill JH: To scale or not
to scale: The principles of dose extrapolation. Br J Pharmacol.
157:907–921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kleckner AS, Kleckner IR, Kamen CS, Tejani
MA, Janelsins MC, Morrow GR and Peppone LJ: Opportunities for
cannabis in supportive care in cancer. Ther Adv Med Oncol.
11:17588359198663622019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cant R, Dalgleish AG and Allen RL:
Naltrexone inhibits IL-6 and TNFα production in human immune cell
subsets following stimulation with ligands for intracellular
toll-like receptors. Front Immunol. 8:8092017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dalgleish A and Liu W: Chapter 9-cancer.
The LDN book: How a little-known generic drug-Low Dose
Naltrexone-could revolutionize treatment for autoimmune diseases,
cancer, autism, depression and more. Elsegood L: Chelsea Green
Publishing; London: pp. 143–151. 2016
|