Introduction
Angiosarcomas in humans are rare, highly aggressive,
malignant, endothelial cell tumors of vascular or lymphatic origin.
Treatment is challenging in a number of cases, and the prognosis is
poor (1). Canine hemangiosarcoma
(HSA) is also an aggressive malignant neoplasm with a poor
prognosis. Surgery and chemotherapy have had limited success in
prolonging survival and increasing the quality of life of canines
with HAS (2). HSA tissues
overexpress vascular endothelial growth factor (VEGF), fibroblast
growth factor (FGF) and their receptors (1,3).
These growth factors generally induce tyrosine kinase activation of
receptors and activate downstream signaling pathways, including the
MAPK/ERK and phosphatidyl-inositol-3 kinase/Akt/mammalian target of
rapamycin (PI3K/Akt/mTOR) pathways, which are involved in tumor
progression (4–6). MAPK/ERK pathways in endothelial cells
are activated in tumors (7–9). The
PI3K/Akt/mTOR pathway also participates in the pathogenesis of
endothelial cells (10,11). Phosphatase and tensin homolog
(PTEN) mutation, which is an antagonist of PI3K, has been detected
in human and canine HSA cases (12,13),
and the hyperactivation of the Akt/mTOR pathway has been reported
in sporadic human HSA cases (14).
However, the role of the PI3K/Akt/mTOR pathway in canine HSA has
not yet been well investigated (15).
HSA occurs in ~50,000 canines per year in the United
States (16), and its frequency is
higher than that in humans, rendering this a useful model for the
study of human intractable disease that requires early detection
and effective therapeutic strategy. Developments in genome-wide
approaches have enabled the comprehensive analysis of
disease-related gene mutations in humans and animals (17,18).
Mutated canine genes related to HSA have been searched using exome
sequencing, and the 1047th histidine residue (H1047) of p110α
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha (PIK3CA) was found to be highly mutated (19). PIK3CA acts downstream of the EGFR
pathway. In human tumor cases, mutations of the 1047th histidine
(e.g., H1047R and H1047L) of PIK3CA have been shown to induce the
hyperactivation of EGFR signaling. Akt phosphorylation and tumor
cell proliferation are enhanced by this mutation (20). The importance of the PIK3CA
mutation in canine HSA is well recognized; however, the open
reading frame of canine PIK3CA has not yet been cloned, at least to
the best of our knowledge. The present study thus cloned canine
PIK3CA and produced mutants by substituting the H1047 residue and
investigated functional alterations in EGFR signaling via Akt
phosphorylation. The anti-proliferative effects of alpelisib, which
suppresses the hyperactivation of EGFR signaling by the PIK3CA
mutation (21), on canine HSA cell
lines were also investigated.
Materials and methods
cDNA cloning, sequencing and
mutagenesis of canine PIK3CA
cPIK3CA was amplified by polymerase chain reaction
(PCR) using the following oligonucleotide primers: cPIK3CA-F
(5′-CTGGGACCCGATGTGGTTAGAG-3′) and cPIK3CA-R
(5′-GAAATGAACTAGTTTAGTGCTC-3′). These primers were designed based
on canine-predicted canine PIK3CA (cPIK3CA) sequences (GenBank
accession no. XM_022414352.1). RNA was obtained from canine total
kidney RNA (Zymo Research Corp.). The total RNA (4 µg) was
denatured at 70°C for 10 min, cooled immediately, and reverse
transcribed, using 200 units of SuperScript III (Thermo Fisher
Scientific Inc.), 25 pmol of random primer, and 10 nmol dNTPs in
total volume of 20 µl at 37°C for 50 min. PCR amplification was
performed using PrimeSTAR (Takara Bio, Inc.). PCR was conducted for
30 cycles, each consisting of denaturation at 96°C for 30 sec,
annealing at 55°C and extension at 72°C for 3 min. Sequence data
were determined for at least five independent clones using an ABI
3730 platform (Applied Biosystems; Thermo Fisher Scientific Inc.).
For sequence analysis, human PIK3CA (GenBank accession no.
NP_006209.2) and cPIK3CA (GenBank accession no. LC625864.1) were
compared using Genetyx software ver. 15 (Genetyx Corporation)
(Fig. S1). dATP was added to the
PCR products using a 10X A-attachment kit (Toyobo Life Science) and
cloned into pGEM-T Easy (Promega Corporation). To construct the
H1047R and H1047L mutants, nucleotide substitutions were performed
by PCR mutagenesis using a pGEM-T Easy vector carrying the
wild-type (WT) sequence as the template and the following
oligonucleotide primers: cPIK3CA-H1047R-F
(5′-GAATGATGCACGTCATGGTGGCTG-3′), cPIK3CA-H1047R-R
(5′-CAGCCACCATGACGTGCATCATTC-3′), cPIK3CA-H1047L-F
(5′-GAATGATGCACTTCATGGTGGCTG-3′) and cPIK3CA-H1047L-R
(5′-CAGCCACCATGAAGTGCATCATTC-3′). These clones were subcloned into
the Halo-tagged expression vector pFN21A (Promega Corporation)
containing SgfI/PmeI sites.
Canine cell lines, formalin-fixed and
paraffin-embedded (FFPE) tissue sample preparation and
sequencing
Genomic DNA of canine cell lines or FFPE tissues
from paraffin scrolls (Table SI)
was extracted from canine tumor samples using the
ReliaPrepTM gDNA Tissue Miniprep System (for cell lines:
Promega Corporation) or QIAamp DNA FFPE Tissue kit (for FFPE
samples: Qiagen GmbH) following the manufacturer's instructions,
respectively. PCR amplification was performed using PrimeSTAR
(Takara Bio, Inc.). PCR was conducted for 30 cycles, each
consisting of denaturation at 96°C for 30 sec, annealing at 55°C
and extension at 72°C for 1 min. The primer pairs used for
amplifying and sequencing canine cPIK3CA exon 21 were
5′-CTCAATGATGCTTGGCTCTGG-3′ and 5′-CTAATGCTGTTCATGGATTGTG-3′.
Histological analysis
With permission from the University Ethics
Committee, we obtained tissue samples from the Department of
Veterinary Pathology, School of Veterinary Science, Nippon
Veterinary and Life Science University (approval no. 11–50, May 27,
2018). All samples were classified by veterinary pathologists in
accordance with the World Health Organization classification
(22) (Table SI). FFPE cancer tissues were
sliced at a thickness of 4 µm and the sections were placed on
slides, following which hematoxylin and eosin (H&E) staining
was performed. Tissues resected were fixed in 10% neutral buffered
formalin for 24 h at 25°C, processed routinely and embedded in
paraffin wax. FFPE cancer tissues were sliced at a thickness of 4
µm and the sections were placed on slides, following which
hematoxylin and eosin (H&E; Muto pure chemicals, Tokyo, Japan)
staining was performed. H&E sections were examined using a
light microscope (BX53, Olympus Corporation).
Cells and cell culture
HeLa cells were purchased from the American Type
Culture Collection (ATCC). Canine mammary gland tumor (CMT) and HSA
cell lines were established and characterized in previous studies
(15,23). The HeLa cells and CMT cell lines
were maintained in Dulbecco's modified Eagle's medium (FUJIFILM
Wako Pure Chemical Corporation), and HSA cell lines were maintained
in RPMI-1640 medium (FUJIFILM Wako Pure Chemical Corporation),
supplemented with 10% fetal bovine serum, penicillin and
streptomycin (FUJIFILM Wako Pure Chemical Corporation) and
incubated at 37°C in a 5% CO2 atmosphere.
Transfections
HeLa cells (2×105/well in 6-well plates,
500 µl medium/well) were transfected with 1 µg pFN21A vector
(Promega Corporation) containing cPIK3CA WT or H1047R-L mutant
plasmids using FuGENE HD Transfection Reagent (Promega
Corporation). An empty pFN21A vector was transfected as a control.
At 48 h (37°C incubation) after the cells were transfected, they
were used for western blot analysis.
Stimulation with anticancer agents and
EGF stimulation for Akt phosphorylation
Following a 24 h-incubation at 37°C, the cells were
arranged in 6-well plates at a concentration of 2×105
cells/well. The medium was replaced with alpelisib (LC
Laboratories) or doxorubicin (FUJIFILM Wako Pure Chemical
Corporation) at suitable concentrations (alpelisib: 0, 1, 2, 5, 10,
20, 50 and 100 µM; doxorubicin: 0, 0.1, 0.5, 1, 5, 10 and 50 µM)
followed by incubation for 24 h at 37°C, following which the cells
were treated with EGF (PeproTech, Inc., 100 ng/ml) for 30 min.
Western blot analysis
Cells that were treated with EGF and/or drugs were
lysed with mammalian lysis buffer (Promega Corporation)
supplemented with a protease inhibitor cocktail (Promega
Corporation). Protein concentrations were determined using a BCA
protein assay kit (Nacalai Tesque). The protein extract from the
cells (10 µg) was mixed with 2X loading buffer and separated on
5–20% gradient SDS-PAGE [Dream Realization and Commucation (DRC);
https://www.drc2002.com/index.html].
The separated proteins were transferred to polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked with
EzBlock Chemi reagent (ATTO Corporation) for 1 h at 25°C. Western
blot analysis was performed using the following primary antibodies
for 16 h at 4°C: Rabbit polyclonal anti-Halo (1:1,000; cat. no.
G9281, Promega Corporation), rabbit monoclonal anti-Akt (Pan;
1:1,000; cat. no. 4691), p-Akt (Ser473; 1:1,000; cat. no. 4060),
Akt1 (1:1,000; cat. no. 2938, CST), Akt2 (1:1,000; cat. no. 3063),
p-Akt1 (1:1,000; cat. no. 9018) p-Akt2 (1:1,000; cat. no. 8599)
(all from Cell Signaling Technology, Inc.) and mouse monoclonal
anti-α-tubulin (1:2,000; cat. no. 013-25033, FUJIFILM Wako Pure
Chemical Corporation). Horseradish peroxidase-conjugated secondary
antibodies for 1 h at 25°C and EzWestLumi plus (ATTO Corporation)
were used to detect antibody-bound proteins, and band intensities
were quantified by densitometry using ImageJ ver. 1.53e software
(National Institutes of Health).
MTT assay
The cytotoxic effects of alpelisib and doxorubicin
were evaluated using MTT assay. The cells were plated in 96-well
plates at a density of 5×103 cells/well. After 24 h, the
medium was replaced with 75 µl medium containing alpelisib or
doxorubicin at various concentrations (0, 0.1, 0.5, 1, 5, 10 and 50
µM) and incubated for 24, 48 or 72 h at 37°C. At the end of the
treatment period, 7.5 µl MTT (5 mg/ml PBS) was added to each well.
The cells were then incubated for 4 h at 37°C in an incubator
(WAKENYAKU Co., Ltd.). The colored crystals of the produced
formazan crystals were dissolved in 150 µl DMSO. The purple blue
formazan formed was measured using a MULTISKAN FC (Thermo Fisher
Scientific, Inc.) at 570 nm. The optical density of each sample was
compared with the control optical density, and graphs were plotted
using SkanItTM Software ver. 4.1 (Thermo Fisher
Scientific, Inc.). IC50 values were obtained from the
generated inhibition curve plots.
Tumor cell migration assay
The inhibition of HSA cell migration with alpelisib
was assessed using a wound healing assay (24). HSA cells were seeded in RPMI-1640
medium (FUJIFILM Wako Pure Chemical Corporation), supplemented with
10% fetal bovine serum, penicillin and streptomycin (FUJIFILM Wako
Pure Chemical Corporation) and grown in confluent monolayers in
6-well plates at a density of 4×105 cells/well for 24 h.
A single scratch wound was created using a sterile micropipette
tip. Subsequently, the cell debris was removed by washing the
plates twice with PBS, and the cell culture medium was refreshed
with or without 20 µM alpelisib. The cells were cultivated for up
to 8 h. Three independent experiments were performed, and the area
of cell migration was examined using an inverted microscope (DM IL
LED, Leica Microsystems) and quantified using ImageJ ver. 1.53e
software (National Institutes of Health).
Caspase-3/7 activity assay
The HSA cell lines were seeded in 96-well plates at
a concentration of 1×104 cells/well and treated with
DMSO as a vehicle for alpelisib at 5 or 20 µM and incubated for 24
h at 37°C. Caspase-3/7 activity was measured using the Caspase-Glo
3/7 Assay (Promega Corporation) according to the manufacturer's
instructions. Luminescence was measured using a GloMax 96
microplate luminometer (Promega Corporation). Caspase-3/7
activities following alpelisib treatment are shown as relative
values to those following treatment with the vehicle (DMSO).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Analysis of variance with Tukey's post hoc test were used when
multiple comparisons were required. A value of P<0.01 was
considered to indicate a statistically significant difference.
Results
Isolation of the H1047R and H1047L
mutations from canine HSA
Genomic DNA was isolated from tumor FFPE samples
from 19 canine HSA samples. PCR amplification of exon 21 of cPIK3CA
containing the 3140th nucleotide coding H1047 revealed two genetic
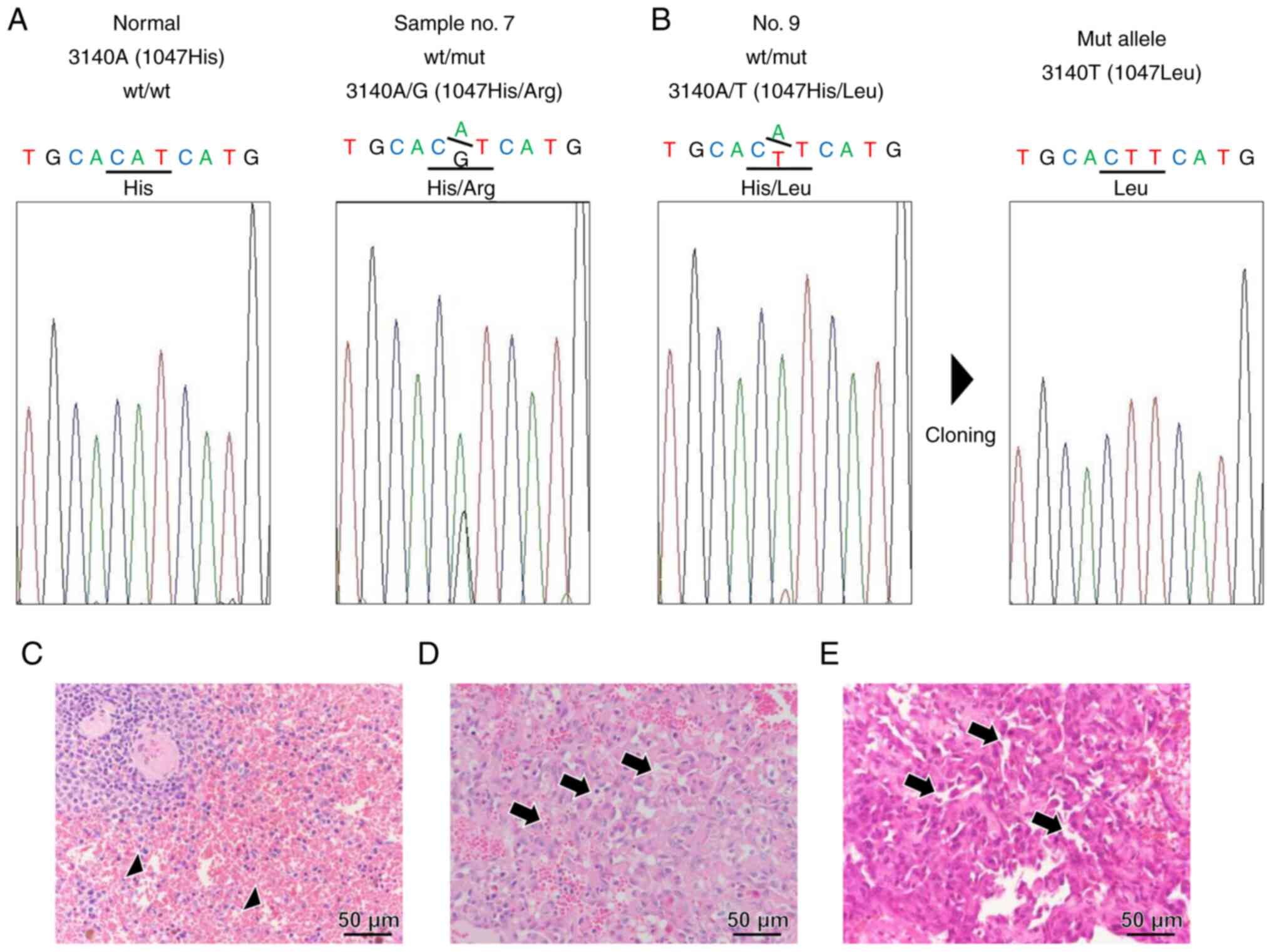
alternations in samples 7 and 9. Sample 7 exhibited a definite
heterozygous 3140 A/G peak, wherein histidine was replaced with
arginine at residue 1407 (H1407R) (Fig. 1A). On the other hand, sample 9
exhibited a minimal 3140 A/T peak (Fig. 1B). When this PCR product was cloned
and sequenced, the 3140T clone was confirmed, in which histidine
was replaced with leucine at residue 1407 (H1407L) (Fig. 1B). No other somatic mutations in
H1047 of cPIK3CA were found in these samples. Representative
H&E-stained normal (Fig. 1C)
and HSA samples (Fig. 1D and E)
with H1047R (case no. 7 in Table
SI) or H1047L (case no. 9) are shown. Microscopically, the
neoplasm was composed of neoplastic endothelial cells with large
round-to-oval nuclei that form irregular vascular channels compared
to normal vascular channels.
The enforced expression of PIK3CA
mutants induces the hyperphosphorylation of Akt in HeLa cells
The Halo-tagged (WT: 1047H) and mutant (1047R or
1047 L) of cPIK3CA were forcibly expressed into HeLa cells. Both
1047R and 1047L mutants induced the hyperphosphorylation of Akt
with/without EGF stimulation compared to the untransfected cells
and WT-transfected cells (Fig. 2).
In particular, Akt2 was phosphorylated by cPIK3CA mutant
transfection.
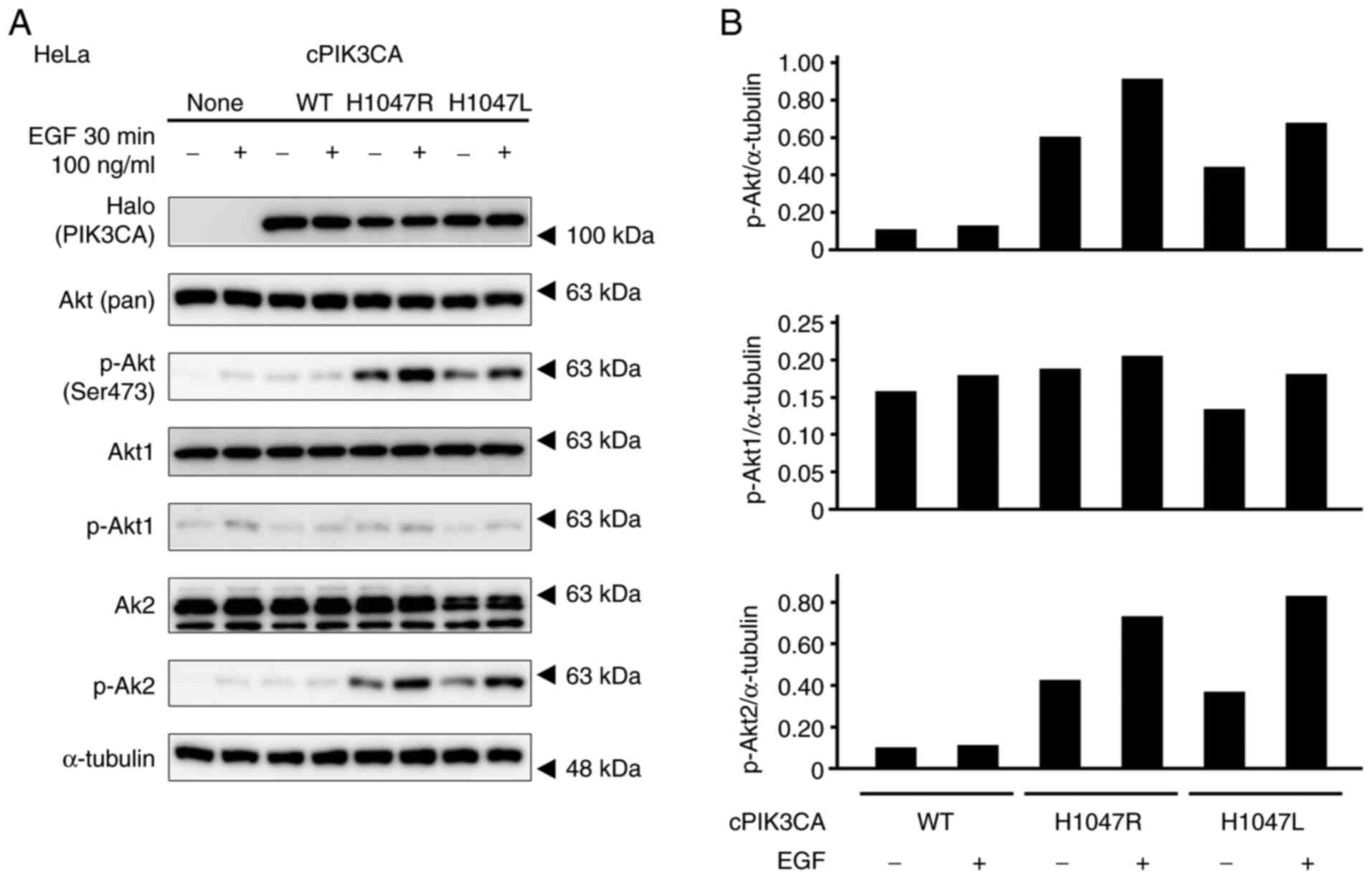 | Figure 2.Phosphorylation profiles of Akt of
HeLa cells with the enforced expression of PIK3CA mutants, which
were stimulated with 100 µM EGF for 30 min. (A) Expression levels
of Halo-tagged PIK3CA, Akt (pan), p-Akt (Ser473), Akt1, p-Akt1,
Akt2, and p-Akt2 were determined by western blotting. α-tubulin was
used as a loading control. (B) Graphs depict densitometric analysis
of the western blots. The intensities of the p-Akt, p-Akt1, and
p-Akt2 bands were quantified by densitometry and normalized to
those of α-tubulin. PIK3CA, p110α
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha. |
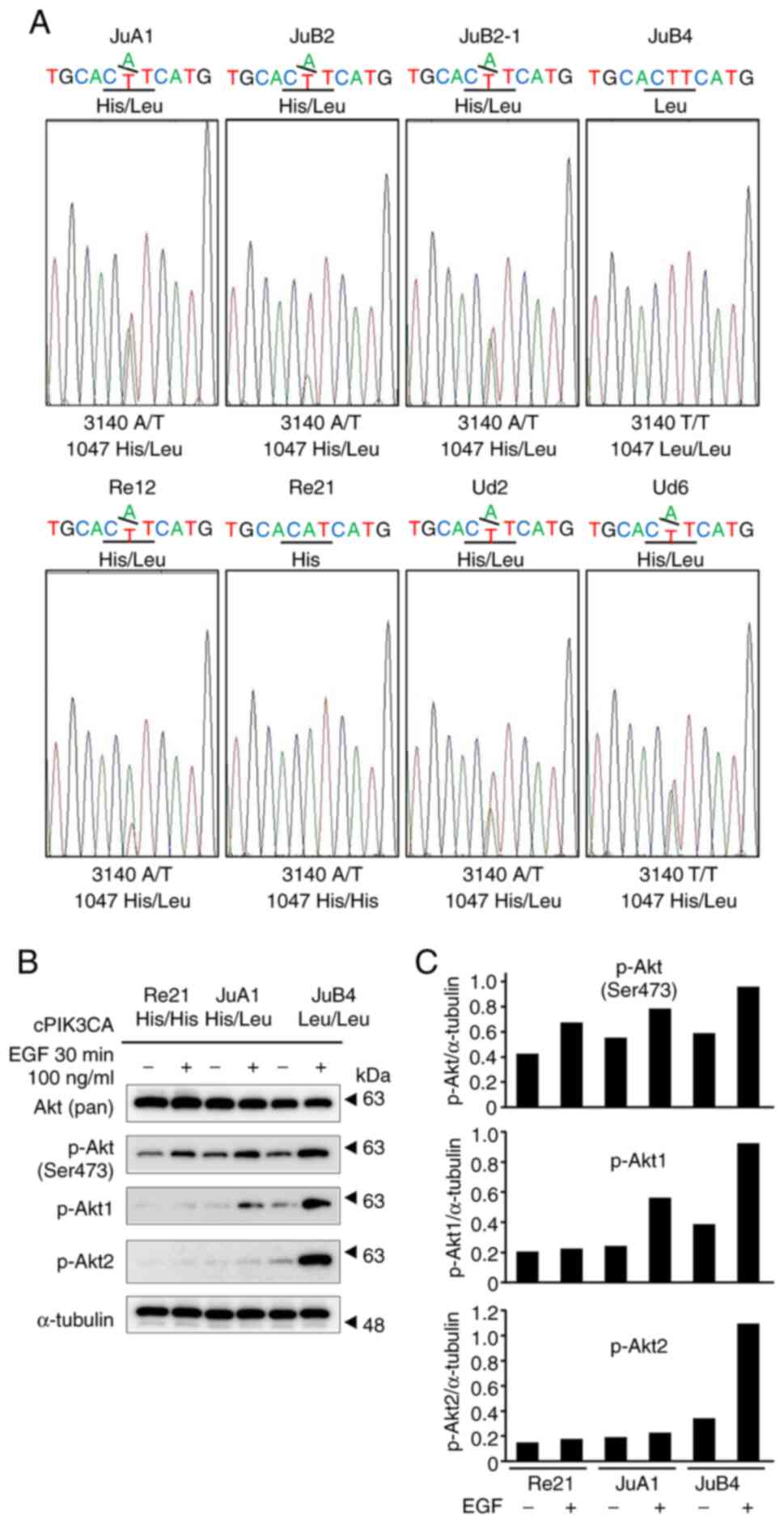
Seven of eight of canine HSA cell
lines have mutated alleles that code 1047L
Exon 21 of PIK3CA from eight canine cell lines
(JuA1, JuB2, JuB2-1, JuB4, Re12, Re21, Ud2 and Ud6), which were
established from canine spontaneous HSA cases, were PCR-amplified
and directly sequenced (15).
Apart from Re21, the JuA1 cells had a 1047L heterozygous mutation
and the JuB4 cells had a homozygous 1047L mutation (Fig. 3A). Following stimulation with 100
ng/µl EGF in three different representative cell lines [Re21
(1047H/H WT), JuA1 (1047H/L heterozygous) and JuB4 (1047 L/L
homozygous)] for 30 min, all three cell lines exhibited the
hyperphosphorylation of Akt at Ser473 (Fig. 3B and C). The JuB4 cells exhibited
the phosphorylation of both Akt1 and Akt2 upon EGF stimulation. By
contrast, the JuA1 cells exhibited only Akt1 phosphorylation upon
EGF stimulation (Fig. 3B and
C).
Inhibitory effects of alpelisib on the
proliferation of canine HSA cell lines
Canine HSA cell lines were treated with increasing
concentrations of alpelisib (0, 1, 2, 5, 10, 20, 50 and 100 µM) for
24, 48 and 72 h. The PIK3CA-mutant cell lines (JuA1 and JuB4) were
more sensitive to alpelisib, with a decrease in cell proliferation
observed in a concentration-dependent manner, compared with the
PIK3CA WT cell line (Re21) at 48 and 72 h following alpelisib
treatment, but not at 24 h (Fig.
4A-C). The IC50 values of the PIK3CA-mutant cell
lines were 11.26 µM (48 h) and 7.39 µM (72 h) for the JuA1 cells,
and 19.62 µM (48 h) and 18.23 µM (72 h) for the JuB4 cells. The
PIK3CA WT cell line exhibited higher IC50 values for
alpelisib treatment (IC50 values: 52.85 µM at 48 h,
26.63 µM at 72 h). By contrast, the Re21 cell line was inhibited
more effectively by doxorubicin treatment than the JuA1 and JuB4
cells (Fig. 4D). Akt
phosphorylation at Ser473 was markedly inhibited by alpelisib
treatment in the JuA1 cells and moderately inhibited in the JuB4
cells (Fig. 4E and F). On the
other hand, the Re21 cells exhibited Akt Ser473 phosphorylation
following alpelisib treatment. The phosphorylation analysis of
p-Akt1 and p-Akt2 revealed that the JuA1 cells exhibited decreased
p-Akt1 and p-Akt2 levels.
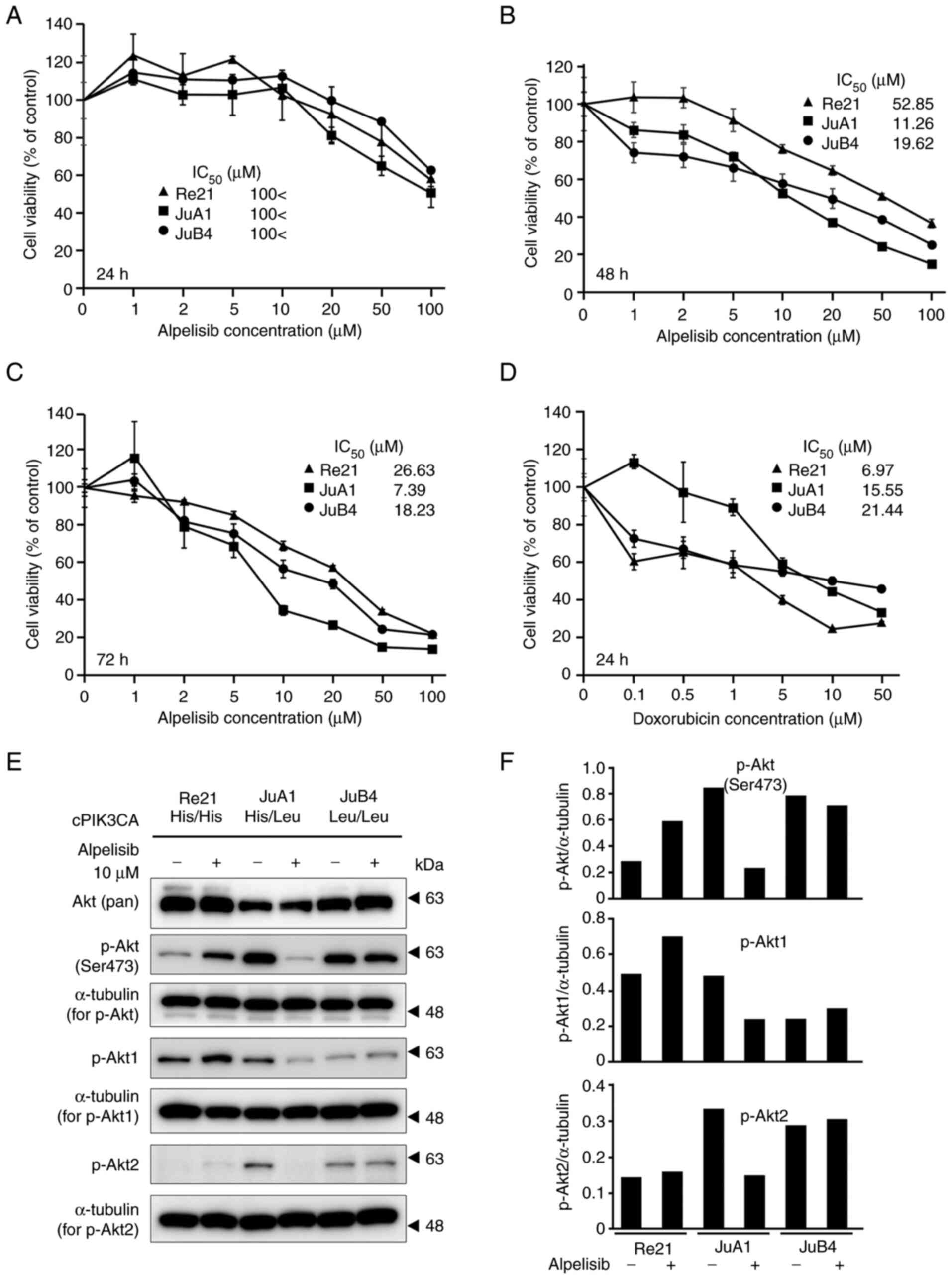 | Figure 4.Anti-proliferative activity of
alpelisib in canine HSA cell lines. PIK3CA wild-type or
heterozygous/homozygous mutant cell lines were treated with
increasing concentrations of alpelisib (0, 1, 2, 5, 10, 20, 50 and
100 µM) for (A) 24, (B) 48 and (C) 72 h. Cells were also treated
with doxorubicin (0, 0.1, 0.5, 1, 5, 10 and 50 µM) for 24 h (D).
The IC50 values and cell viability using an MTT assay
were determined by measuring the absorbance at 560 nm on a
microplate reader. The values of no treatment were 100%, and the
values are shown from four independent experiments as the mean ±
SD. (E) Western blot analysis of Akt phosphorylation in canine HSA
cell lines with 10 µM alpelisib treatment for 6 h. (F) Graphs
depicting densitometric analysis of the western blots compared with
the loading controls. The intensities of the p-Akt, p-Akt1, and
p-Akt2 bands were quantified through densitometry and normalized to
the intensity of α-tubulin. PIK3CA, p110α
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha; HSA, hemangiosarcoma. |
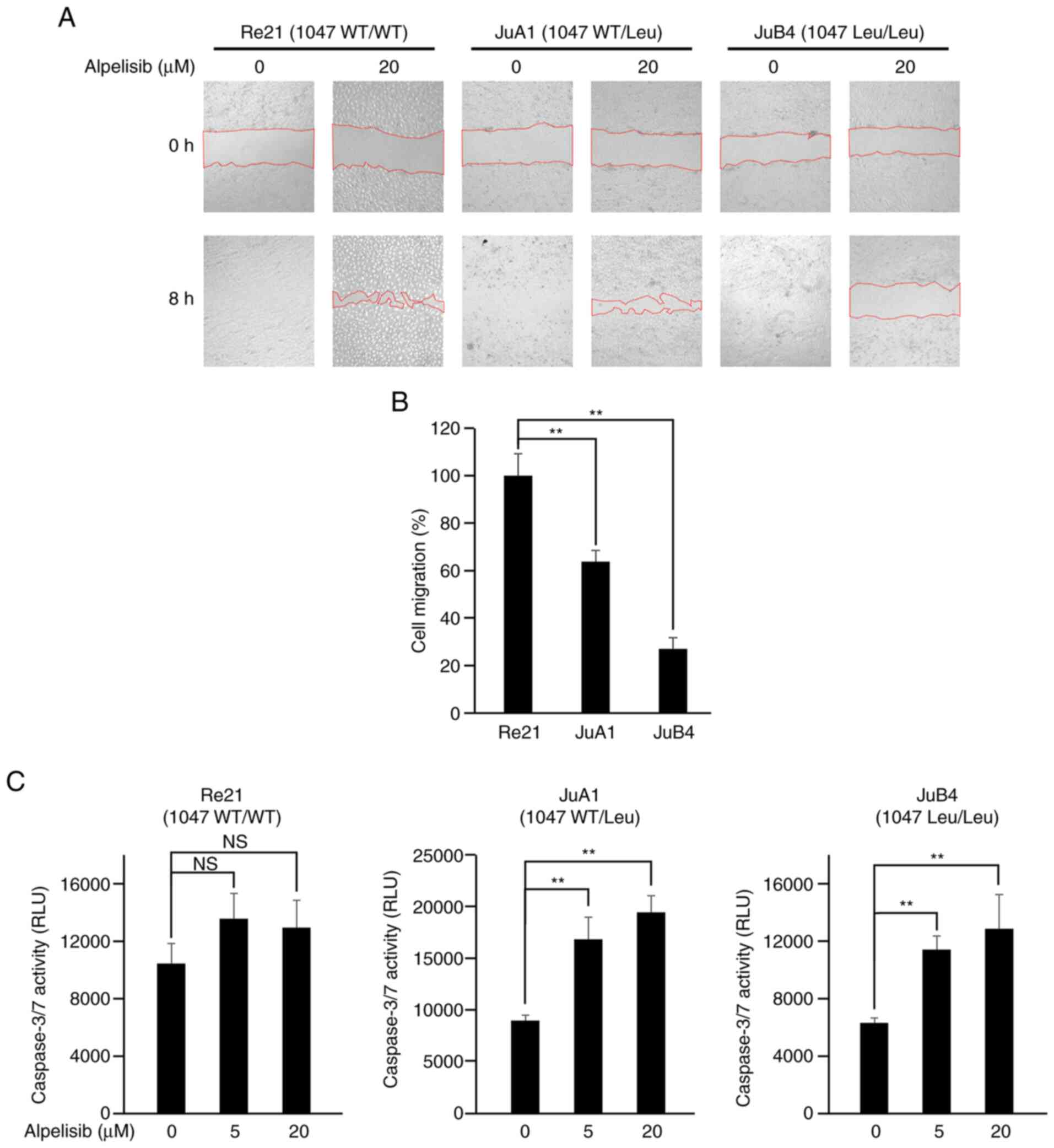
Alpelisib inhibits cell migration
At 8 h after scratching without alpelisib treatment,
all cell lines recovered completely (Fig. 5A, lower left panels of cell lines,
respectively). However, with 20 µM alpelisib treatment, only the
Re21 cells recovered almost completely from the wound after 8 h;
the JuA1 and JuB4 cells exhibited a significantly delayed wound
healing ability (Fig. 5B).
Alpelisib induces caspase-3/7
activities in PIK3CA mutant cells
The cells were exposed to alpelisib at a range of
concentrations (0, 5 or 20 µM) for 24 h; the Re21 cells did not
exhibit an increase in caspase-3/7 activity, while the PIK3CA
mutant JuA1 and JuB4 cell lines exhibited a significant increase in
caspase-3/7 activity following treatment with 5 and 20 µM alpelisib
(Fig. 5C).
Discussion
The present study demonstrates that there are
mutations in PIK3CA H1047 in both canine HSA cases and cell lines.
In humans, the PIK3CA H1047th residue is mutated in a number of
types of tumors (25). Although
canine PIK3CA is also mutated in mammary gland tumor and
hemangiosarcoma cases (19,26),
the cloning and molecular characterization of canine PIK3CA have
not yet been performed, at least to the best of our knowledge.
Therefore, the present study cloned and characterized canine
PIK3CA. The novel cloned canine PIK3CA (GenBank accession no.
LC625864) exhibited a high homology with human PIK3CA
(NP_006209.2), and functional domains were well conserved (Fig. S1). The H1047 residue was conserved
in canine PIK3CA in the same position. These structural properties
suggest that canine PIK3CA possesses EGFR signaling activities.
In the mutation analysis of 19 cases of canine
PIK3CA in HSA FFPE samples, either a H1047R or H1047L heterozygous
mutation was detected. Although the 3140A>G (case no. 7: H1047R)
mutant sequence waveform was absent, a small waveform of 3140A>T
(case no. 9: H1047L) was detected. This mutant could be cloned and
confirmed; however, there may be other invisible mutants in other
cases. It has been previously reported that the detection limit of
rare alleles by Sanger sequencing is ~10% (27). High-resolution methods (e.g.,
pyrosequencing and next-generation sequencing) may be able to
detect mutations in the 1047th residue of canine PIK3CA, which was
isolated from tumor tissues (28,29).
A definite pathological difference between the presence or absence
of PIK3CA 1047th residue mutation was not detected by microscopic
observation.
The analysis of Akt phosphorylation with the
enforced expression of canine PIK3CA WT or H1047 mutants in HeLa
cells revealed that H1047R- and H1047L-transfected cells exhibited
the hyperphosphorylation of Akt; in particular, Akt2 but not Akt1
was phosphorylated with or without EGF stimulation. In the human
mammary tumor cell line, MCF10A, the enforced expression of H1047R
of human PIK3CA mutant was previously shown to specifically induce
Akt2 phosphorylation (30,31). The Akt isoforms Akt1 and Akt2 play
differential roles in tumor metastasis (32). Akt1 has been demonstrated to
suppress, while Akt2 promotes breast cancer cell migration and
invasion in vitro (33).
This result suggests that the canine PIK3CA H1047 mutation induces
the upregulation of EGFR signaling and may promote tumor growth and
invasion via Akt2 phosphorylation in HeLa cells.
Canine cell lines established from HSA or mammary
gland tumor tissue were highly mutated in the H1047th residue of
PIK3CA (Fig. S2). Herein, the
overexpression of the canine PIK3CA H1047 mutation induced the
phosphorylation of Akt2, but not Akt1 in HeLa cells. Alternatively,
EGF stimulation induced the phosphorylation of endogenous canine
Akt at Ser473 in the canine HSA cell lines, Re21, JuA1, and JuB4.
JuA1 and JuB4, which were heterozygous/homozygous for the PIK3CA
H1047L mutation, and induced the phosphorylation of Akt1 upon EGF
stimulation, but not in the PIK3CA normal cell line, Re21. Given
that only JuB4 exhibited Akt2 phosphorylation, a homozygous H1047L
mutation may induce a stronger gain-of-function in PIK3CA. In
canine HSA cell lines, the H1047L mutation of PIK3CA induced the
phosphorylation of Akt1 and/or Akt2 with EGF stimulation, which may
result in a severe pathogenesis (34).
Alpelisib (BYL719) is a targeted compound against
mutated PIK3CA and is ~50-fold stronger than other isoforms
(35). In the present study,
alpelisib inhibited cell proliferation by suppressing Akt
phosphorylation and inducing apoptosis via the activation of
caspase-3/7 pathways, particularly in PIK3CA-mutant canine HSA cell
lines. In MTT assays of canine HSA cell lines exposed to alpelisib,
the JuA1 cell line, which is heterozygous for the H1047L mutation,
was the most sensitive, although the JuB4 cell line, which is
homozygous for the H1047L mutation, exhibited moderate sensitivity
to alpelisib. JuA1 cells exhibited higher viability than JuB4 cells
in a previous study (15). Thus,
alpelisib may exert a potent antitumor effect on PIK3CA mutant
tumor cells, which have a higher proliferative capacity, by
suppressing Akt phosphorylation. On the other hand, although
alpelisib also suppressed Akt phosphorylation in PIK3CA mutant cell
lines, which were derived from canine mammary gland tumors
(23), there was no marked
difference in the tumor suppressive effect between normal and
mutant PIK3CA cells (Fig. S2).
Additionally, 20 µM alpelisib also significantly inhibited cell
migration in PIK3CA mutant cell lines compared with PIK3CA WT cell
lines. It has been reported that canine HSA cells promote migration
by interacting with CXCR4 and CXCL12 (36–38),
and the overexpression of CXCR4 promotes the invasion and migration
of non-small cell lung cancer via EGFR (39). These phenomena suggest that the
suppression of abnormal EGFR signaling, induced by PIK3CA mutation,
by alpelisib may be able to control canine HSA progression.
Furthermore, alpelisib induced significant apoptotic cell death
specifically in PIK3CA mutant canine HSA cell lines via caspase-3/7
activation; thus, alpelisib can be used as an agent for the
treatment of canine HSA. In addition to the tumor-suppressive
effect on canine PIK3CA mutant HSA in vivo, alpelisib has
been confirmed to be safe for use in dogs based on safety
examinations during drug development processes (e.g., http://www.ema.europa.eu/documents/assessment-report/piqray-epar-public-assessment-report_en.pdf).
Therefore, the authors aim to investigate its direct clinical
effects on canine HSA cases in future studies.
In conclusion, the present study detected a PIK3CA
H1047 mutation in canine HSA tissues and cell lines derived from
canine HSA cases. The H1047R and H1047L mutations in canine PIK3CA
induced EGFR signaling via Akt hyperphosphorylation. Alpelisib
suppressed Akt phosphorylation, cell viability and migration, and
induced apoptosis (determined by caspase-3/7 activation) in PIK3CA
mutated canine HSA cell lines. These data suggest that the H1047
mutation of PIK3CA is a crucial and useful marker of canine HSA,
and alpelisib may prove to be an effective agent against
PIK3CA-mutant canine HSA.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by KAKENHI scientific research
grants from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan (nos. 18H02334 and 19K06390).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO, MMi, HS, KI, MW and YT designed the study. MMa,
MMo, NK, MS, DA, MY, EO and TS performed the laboratory
experiments. KO was responsible for the statistical analysis and
wrote the manuscript. MW, YT, KI and KO supervised the study. KO,
MMi and YT confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
With permission from the University Ethics Committee
of Nippon Veterinary and Life Science University, tissue samples
were obtained from the Department of Veterinary Pathology, School
of Veterinary Science, Nippon Veterinary and Life Science
University (approval no. 11–50, May 27, 2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Itakura E, Yamamoto H, Oda Y and
Tsuneyoshi M: Detection and characterization of vascular
endothelial growth factors and their receptors in a series of
angiosarcomas. J Surg Oncol. 97:74–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clifford CA, Mackin AJ and Henry CJ:
Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Intern
Med. 14:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yonemaru K, Sakai H, Murakami M, Yanai T
and Masegi T: Expression of vascular endothelial growth factor,
basic fibroblast growth factor, and their receptors (flt-1, flk-1,
and flg-1) in canine vascular tumors. Vet Pathol. 43:971–980. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia F, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
di Blasio L, Puliafito A, Gagliardi PA,
Comunanza V, Somale D, Chiaverina G, Bussolino F and Primo L:
PI3K/mTOR inhibition promotes the regression of experimental
vascular malformations driven by PIK3CA-activating mutations. Cell
Death Dis. 9:452018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumura I, Mizuki M and Kanakura Y:
Roles for deregulated receptor tyrosine kinases and their
downstream signaling molecules in hematologic malignancies. Cancer
Sci. 99:479–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arbiser JL, Weiss SW, Arbiser ZK, Bravo F,
Govindajaran B, Caceres-Rios H, Cotsonis G, Recavarren S, Swerlick
RA and Cohen C: Differential expression of active mitogen-activated
protein kinase in cutaneous endothelial neoplasms: Implications for
biologic behavior and response to therapy. J Am Acad Dermatol.
44:193–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kroll J and Waltenberger J: The vascular
endothelial growth factor receptor KDR activates multiple signal
transduction pathways in porcine aortic endothelial cells. J Biol
Chem. 272:32521–32527. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang X, Wang J, Deng X, Xiong F, Zhang S,
Gong Z, Li X, Cao K, Deng H, He Y, et al: The role of
microenvironment in tumor angiogenesis. J Exp Clin Cancer Res.
39:2042020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blasio A, Wang J, Wang D, Varodayan FP,
Pomrenze MB, Miller J, Lee AM, McMahon T, Gyawali S, Wang HY, et
al: Novel small-molecule inhibitors of protein kinase C epsilon
reduce ethanol consumption in mice. Biol Psychiatry. 84:193–201.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Cras TD, Goines J, Lakes N, Pastura P,
Hammill AM, Adams DM and Boscolo E: Constitutively active PIK3CA
mutations are expressed by lymphatic and vascular endothelial cells
in capillary lymphatic venous malformation. Angiogenesis.
23:425–442. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dickerson EB, Thomas R, Fosmire SP,
Lamerato-Kozicki AR, Bianco SR, Wojcieszyn JW, Breen M, Helfand SC
and Modiano JF: Mutations of phosphatase and tensin homolog deleted
from chromosome 10 in canine hemangiosarcoma. Vet Pathol.
42:618–632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tate G, Suzuki T and Mitsuya T: Mutation
of the PTEN gene in a human hepatic angiosarcoma. Cancer Genet
Cytogenet. 178:160–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou
C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, et al:
Angiosarcoma: Clinical and molecular insights. Ann Surg.
251:1098–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murai A, Asa SA, Kodama A, Hirata A, Yanai
T and Sakai H: Constitutive phosphorylation of the
mTORC2/Akt/4E-BP1 pathway in newly derived canine hemangiosarcoma
cell lines. BMC Vet Res. 8:1282012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tamburini BA, Trapp S, Phang TL, Schappa
JT, Hunter LE and Modiano JF: Gene expression profiles of sporadic
canine hemangiosarcoma are uniquely associated with breed. PLoS
One. 4:e55492009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lequarre AS, Andersson L, Andre C,
Fredholm M, Hitte C, Leeb T, Lohi H, Lindblad-Toh K and Georges M:
LUPA: A European initiative taking advantage of the canine genome
architecture for unravelling complex disorders in both human and
dogs. Vet J. 189:155–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Espejo-Freire AP, Elliott A, Rosenberg A,
Costa PA, Barreto-Coelho P, Jonczak E, D'amato G, Subhawong T,
Arshad J, Diaz-Perez JA, et al: Genomic landscape of angiosarcoma:
A targeted and immunotherapy biomarker analysis. Cancers.
13:48162021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Wu M, Maloneyhuss MA, Wojcik J,
Durham AC, Masson NJ and Royh DB: Actionable mutations in canine
hemangiosarcoma. PLoS One. 12:e01886672017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arafeh R and Samuels Y: PIK3CA in cancer:
The past 30 years. Semin Cancer Biol. 59:36–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
André F, Ciruelos E, Rubovszky G, Campone
M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufmanet K, et
al: Alpelisib for PIK3CA-mutated, hormone receptor-positive
advanced breast cancer. N Engl J Med. 380:1929–1940. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Misdorp W; Armed Forces Institute of
Pathology (U.S.); American Registry of Pathology; WHO Collaborating
Center for Worldwide Reference on Comparative Oncology, :
Histological classification of mammary tumors of the dog and the
cat. International Histological Classification of Tumors of
Domestic Animals. Vol 7. 2nd series. Armed Forces Institute of
Pathology in cooperation with the American Registry of Pathology
and the World Health Organization Collaborating Center for
Worldwide Reference on Comparative Oncology; Washington, DC:
1999
|
|
23
|
Uyama R, Nakagawa T, Hong SH, Mochizuki M,
Nishimura R and Sasaki N: Establishment of four pairs of canine
mammary tumour cell lines derived from primary and metastatic
origin and their E-cadherin expression. Vet Comp Oncol. 4:104–113.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghazanchaei A, Mansoori B, Mohammadi A,
Biglari A and Baradaran B: Restoration of miR-152 expression
suppresses cell proliferation, survival, and migration through
inhibition of AKT-ERK pathway in colorectal cancer. J Cell Physiol.
234:769–776. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madsen RR, Vanhaesebroeck B and Semple RK:
Cancer-associated PIK3CA mutations in overgrowth disorders. Trends
Mol Med. 24:856–870. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JH: PIK3CA mutations matter for cancer
in dogs. Res Vet Sci. 133:39–41. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ney JT, Froehner S, Roesler A, Buettner R
and Merkelbach-Bruse S: High-resolution melting analysis as a
sensitive prescreening diagnostic tool to detect KRAS, BRAF,
PIK3CA, and AKT1 mutations in formalin-fixed, paraffin-embedded
tissues. Arch Pathol Lab Med. 136:983–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beca F, Krings G, Chen YY, Hosfield EM,
Vohra P, Sibley RK, Troxell ML, West RB, Allison KH and Bean GR:
Primary mammary angiosarcomas harbor frequent mutations in KDR and
PIK3CA and show evidence of distinct pathogenesis. Mod Pathol.
33:1518–1526. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang G, Wu M, Durham AC, Mason NJ and Roth
DB: Canine oncopanel: A capture-based, NGS platform for evaluating
the mutational landscape and detecting putative driver mutations in
canine cancers. Vet Comp Oncol. 20:91–101. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Zhao W, Guo H, Fang Y, Stockman
SE, Bai S, Ng PK, Li Y, Yu Q, Lu Y, et al: AKT isoform-specific
expression and activation across cancer lineages. BMC Cancer.
18:7422018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo H, Gao M, Lu Y, Liang J, Lorenzi PL,
Bai S, Hawke DH, Li J, Dogruluk T, Scott KL, et al: Coordinate
phosphorylation of multiple residues on single AKT1 and AKT2
molecules. Oncogene. 33:3463–3472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dillon RL, Marcotte R, Hennessy BT,
Woodgett JR, Mills GB and Muller WJ: Akt1 and akt2 play distinct
roles in the initiation and metastatic phases of mammary tumor
progression. Cancer Res. 69:5057–5064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ihle MA, Fassunke J, König K, Grunewald I,
Schlaak M, Kreuzberg N, Tietze L, Schildhaus HU, Büttner R and
Merkelbach-Bruseet S: Comparison of high resolution melting
analysis, pyrosequencing, next generation sequencing and
immunohistochemistry to conventional Sanger sequencing for the
detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer.
14:132014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rascio F, Spadaccino F, Rocchetti MT,
Castellano G, Stallone G, Netti GS and Ranieri E: The pathogenic
role of PI3K/AKT pathway in cancer onset and drug resistance: An
updated review. Cancers. 13:39492021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fritsch C, Huang A, Chatenay-Rivauday C,
Schnell C, Reddy A, Liu M, Kauffmann A, Guthy D, Erdmann D, De
Pover A, et al: Characterization of the novel and specific PI3Kα
inhibitor NVP-BYL719 and development of the patient stratification
strategy for clinical trials. Mol Cancer Ther. 13:1117–1129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Im KS, Graef AJ, Breen M, Lindblad-Toh K,
Modiano JF and Kim JH: Interactions between CXCR4 and CXCL12
promote cell migration and invasion of canine hemangiosarcoma. Vet
Comp Oncol. 15:315–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH, Graef AJ, Dickerson EB and Modiano
JF: Pathobiology of hemangiosarcoma in dogs: Research advances and
future perspectives. Vet Sci. 2:388–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim KH, Chung WS, Kim Y, Kim KS, Lee IS,
Park JY, Jeong HS, Na YC, Lee CH and Jang HJ: Transcriptomic
analysis reveals wound healing of morus alba root extract by
up-regulating keratin filament and CXCL12/CXCR4 signaling.
Phytother Res. 29:1251–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuo J, Wen M, Li S, Lv X, Wang L, Ai X and
Lei M: Overexpression of CXCR4 promotes invasion and migration of
non-small cell lung cancer via EGFR and MMP-9. Oncol Lett.
14:7513–7521. 2017.PubMed/NCBI
|