Introduction
Fluoropyrimidines (FP), namely 5-fluorouracil (5-FU)
and capecitabine, are widely used to treat solid malignancies,
including those arising from gastrointestinal tract. In addition,
FP are frequently used concurrently with radiotherapy, due to their
radio-sensitizing properties. 5-FU and capecitabine are generally
well tolerated, being myelosuppression, gastrointestinal, and skin
toxicity (hand-foot syndrome) the most common adverse events, which
vary according to dose and schedule (1).
Cardiotoxicity is a feared, uncommon toxicity of FP;
although the most common manifestation of FP-induced cardiotoxicity
(FIC) is unstable angina (2),
other severe adverse cardiac events, including cardiomyopathy,
myocarditis, and sudden cardiac death, have been reported (3). FIC typically occurs during the first
few treatment cycles, which usually results in the discontinuation
of treatment (4). As a
consequence, FIC represents an important issue particularly in
colorectal cancer (CRC) treatment, due to the central role of FP in
the management of this disease, both in adjuvant and palliative
settings. Patients with CRC that experienced FIC have a
considerable reduction in their therapeutic options with a negative
influence on prognosis (5).
Proposed mechanisms that underlie FIC include
coronary artery vasospasms, vascular endothelial dysfunction,
direct toxicity on the myocardium, and thrombogenicity due to
altered blood rheology (6).
Coronary artery vasospasms are considered to be the
main pathogenic mechanism (7), and
it has been suggested that N-terminal (NT)-pro hormone BNP (proBNP)
may act as a biomarker of FIC (8).
The compound that may be responsible for these effects is
fluoroacetate. 5-FU and capecitabine are catabolised by
dehydro-pyrimidine dehydrogenase (DPYD) and in several steps
degraded into α-fluoro-β-alanine, and then into fluoroacetate, a
highly cardiotoxic and neurotoxic compound (9).
The reported incidence of cardiac symptoms and
events varies greatly in the existing literature, with a range from
0–34.6% of patients (10). Serious
cardiac events, including myocardial infarction, cardiogenic shock
and cardiac arrest, occurred in 0–2% (11). No significant differences were
observed in the incidence of cardiac events between capecitabine
and 5-FU (11).
The identification of patients at risk for adverse
cardiac events remains the major challenge, either due to the
limited data on baseline cardiac risk factors or the lack of
predictive biomarkers (10).
Indeed, the correlation between cardiovascular (CV) risk factors,
CV comorbidities and FIC remains unclear. Circulating biomarkers,
such as proBNP and the cardiac structural proteins troponin I and T
(TnI and TnT), useful for the diagnosis and prognosis of patients
suffering from heart disease, have a limited role in the
identification of FIC (10).
Other suitable and reliable plasma biomarkers could
be represented by microRNAs (miRNA), small molecules of 20–22
nucleotides of non-coding RNA with a post-transcriptional
regulation function. Different miRNAs are involved in CV diseases,
including myocardial infarction, heart failure and cardiac
hypertrophy. Certain of them, including miR-1, miR-133, miR-145,
miR-208 and miR-499, abundantly expressed in the myocardium, have a
diagnostic and prognostic role in cardiac damage (12,13).
Consistent results were obtained for doxorubicin-treated patients
with breast cancer: in particular, miR-1 is significantly
overexpressed in the case of cardiotoxicity and has higher
sensitivity compared with the classical TnI dosage (14). However, the ability of circulating
miRNAs expression to predict FIC has not been explored.
In the present study, it was aimed to prospectively
evaluate incidence, clinical manifestations, risk factors and
predictive biomarkers of FIC in FP-treated patients with CRC.
Materials and methods
A monocentric prospective study linked to a
real-world intervention in patients with either localized or
advanced stage CRC who were eligible for treatment with FP-based
therapy (CHECKPOINT trial, NCT02665312), was conducted. Patients
were enrolled at Candiolo Cancer Institute, Italy, between January
2016 and February 2020. An accrual of 200 patients was originally
planned, however, due to the COVID-19 pandemic and the restrictions
applied to reduce not necessary accesses, the enrolment was
suspended and the study was prematurely closed. The study was
approved (approval no. 251/2015; October, 22nd 2015) by the local
review board and by the institutional Ethical Committee of Candiolo
Cancer Institute (Turin, Italy).
Inclusion criteria for the study were: lack of any
prior treatment with FP, age ≥18 years; histologically confirmed
diagnosis of localized or metastatic CRC; a clinical indication to
receive a 5-FU or capecitabine-based chemotherapy regimen,
according to the current literature and national guidelines
(15,16). A personal history of cardiac
diseases was not an exclusion criterion for the study. Written
informed consents were obtained from all patients before their
participation. None of these patients were examined for DPYD
variant. Although accumulating data now suggest a correlation
between DPYD polymorphisms and FP toxicities, at the time of the
present study, such preliminary analyses were not routinely
recommended by regulatory authorities (15).
5-FU and capecitabine treatments
The choice of the chemotherapy regimen was not
dictated by the study protocol, but was left to clinical judgement
according to the treatment setting. 5-FU-based chemotherapy was
administered according to the mFOLFOX6, FOLFOX6, FOLFIRI, FOLFOXIRI
and DeGramont regimens. Specifically, the doses were given in
2-week cycles. Capecitabine-based chemotherapy (capecitabine
monotherapy or CAPOX) was given in 21-day cycles as a
2-week-on/1-week-off regimen (chemotherapy regimens are described
in detail at Appendix S1).
Study procedures and FIC
assessment
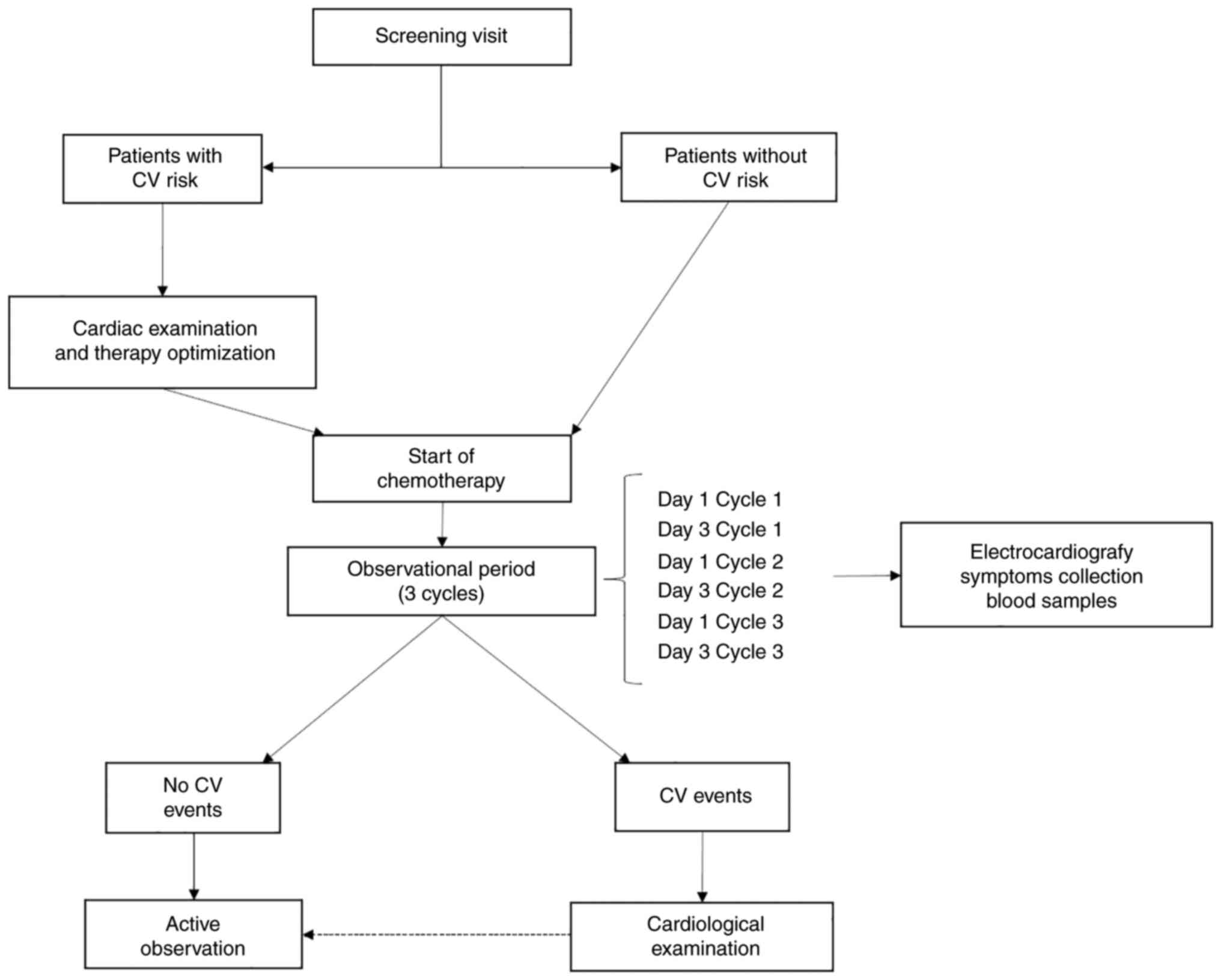
All enrolled patients were evaluated for potential
CV risk factors (including physical measurements and collection of
physiological, family and past medical history) at the screening
visit and divided into two groups, low and high CV risk. Patients
were considered at high CV risk in case of i) history of coronary
artery disease; ii) uncontrolled hypertension; iii) cerebrovascular
accident/stroke within six months prior to the enrolment; iv)
chronic heart failure of NYHA Grade II or higher; v) cardiac
arrhythmia; vi) significant electrocardiogram (ECG) abnormalities;
vii) symptoms considered potentially related to relevant
cardiovascular diseases. Patients without any of these conditions
were considered at low CV risk. All patients deemed with high CV
risk at the screening visit received a cardiological evaluation and
cardiac ultrasonography. In this evaluation, the cardiologist could
optimize therapy before the start of chemotherapy, if
necessary.
In the present study, patients were actively
monitored from day 1 (D1) of the first cycle (C1) to day 3 (D3) of
the third cycle (C3). During this period, patients underwent
complete physical examination (D1, C1-3), blood samples collection
for cell blood count and serum biochemical analysis, proBNP dosage,
(D1, C1-3), ECG and blood samples for high-sensitivity cardiac TnI
levels and miRNAs expression analysis (D1, C1-3 + D3, C1-3), and
they filled the patient-reported outcomes (PROs) with a 5-items
simplified questionnaire on symptoms and concomitant medications
(D1, C1-3 + D3, C1-3). After the first three cycles, patients
continued active observation with PROs collection.
If patients reported symptoms potentially related to
cardiotoxicity on other days than D1 or D3, or during the
observation period, they were referred for an unscheduled visit
with the same aforementioned procedures, including biochemical
analyses, ECG and cardiac examination, if needed. The scheme of the
trial is presented in Fig. 1.
Cardiovascular toxicities
CV toxicity was defined as the occurrence of any
signs, symptoms or ECG changes potentially related to impaired
cardiac function. Cardiotoxicity was recorded and graded according
to the Common Toxicity Criteria version 4.0, using recorded signs,
PROs questionnaires and ECG changes at predefined points (D1, C1-3
+ D3, C1-3 and during follow-up). CV events were defined as:
significant ECG changes (ST deviation, T inversion/variation,
ventricular arrhythmia, new-onset bundle branch block) with or
without signs or symptoms; typical chest pain, with or without ECG
changes; syncope, with or without ECG changes; dyspnoea or
palpitations (of new and sudden onset), with or without ECG
changes.
Patients developing CV events were referred within
24 h to cardiological examination, cardiac ultrasonography, blood
samples and further investigations and treatments, if considered
appropriate by the cardiologist (Fig.
1). Patients developing CV events were treated by the
cardiologist and treatment with FP was discontinued or resumed upon
clinical judgement.
Cardiac biomarker evaluation
Plasma proBNP and TnI levels were determined at
predefined points (D1C1-3 + D3C1-3 and during follow-up). Levels of
BNP were determined with the Triage BNP test (Beckman Coulter,
Inc.) using the Beckman Access DxI 800 platform. The upper limit of
normal was 100 pg/ml. Levels of high-sensitivity TnI were
determined by the Access AccuTnI immunometric assay (Beckman
Coulter, Inc.) using the Beckman DxI 800 platform. The upper limit
of normal was 0.05 ng/ml. Determinations were carried out according
to the manufacturer's instructions.
Collection of samples for circulating
biomarkers
Two tubes of 7 ml blood samples were drawn at D1 and
D3 of the first three cycles of therapy to collect serum (in
heparin) and plasma (in EDTA). Briefly, both tubes were centrifuged
at room temperature for 10 min at 1,800 × g without brake. Serum
was directly collected, while supernatant of EDTA tubes was
collected and transferred in a 15 ml tube and centrifuged for
further 10 min at 2,200 × g with brake. Both serum and plasma
samples were aliquoted in 1.5 ml collection tubes and stored at
−80°C.
miRNA profiling
Total circulating RNA was extracted from 200 µl of
plasma using the Mirneasy serum/plasma advanced kit (Qiagen GmbH)
following the manufacturer's instructions. To improve RNA
extraction, MS2 carrier (Merck Life Science S.r.l.) was added to
each sample before lysis step. RNA elution was performed in 30 µl
of nuclease-free water.
For miRNA profiling, 4 µl of total RNA of each
sample were used and individually processed with the miRNA Complete
Labeling and Hybridization kit (Agilent Technologies S.p.A.) and
hybridized on 8×60 K oligonucleotides arrays miRbase V21 (Agilent
Technologies Italia S.p.A.). After hybridization, washing and
scanning, by Agilent G2505C scanner, images were analyzed using
Feature Extraction software v10.7 (Agilent Technologies S.p.A). Raw
data were processed by means of the limma R library, applying
background correction (normexp) and quantile normalization, and
averaging miRNA levels when they are represented with the same
probe on the array.
Statistical analysis
The primary objective of the present study was to
assess the incidence of FIC during FP-based chemotherapy. Secondary
objectives were: i) to assess the relationship between FIC and CV
risk factors, ii) to investigate the role of known circulating
biomarkers of cardiac damage (including TnI, proBNP), iii) to
explore the possible role of circulating miRNAs as predictive
biomarkers of a CV event.
Two class comparison was then applied to detect
differentially expressed miRNAs between patients with and without a
CV event, between high and low CV risk patients, and between
patients with CV event and low CV risk patients without an event,
using the limma R package version 3.52.4 (https://bioconductor.org/packages/release/bioc/html/limma.html).
Associations between CV events and patient characteristics were
studied by using the Chi-Square test (dichotomous variables).
Two-tailed paired t-test was applied to evaluate proBNP changes
between C1 and C3 of the first three cycles in both CV event and no
CV event patients and according to the chemotherapy administered
(5-FU-based vs. capecitabine-based). Two-tailed unpaired t-test was
applied to evaluate proBNP changes between CV event and no CV event
patients within the same time point. P<0.05 was considered to
indicate a statistically significant difference. Chi-square and
t-tests were performed using the GraphPad Prism software, version
9.0 (GraphPad Software, Inc.).
Univariate logistic regression was used to predict
the correlation with the occurrence of a CV event, [95% confidence
intervals (CI) for odds ratios, P<0.05 considered as
statistically significant]. Since none of the single variables
provided a significant prediction value, a logistic stepwise
analysis was used to identify the variables to retain in a
multivariable analysis. These analyses were carried out using the
glmnet R package version 4.1-4 (https://cran.r-project.org/web/packages/glmnet/index.html).
Results
Study population
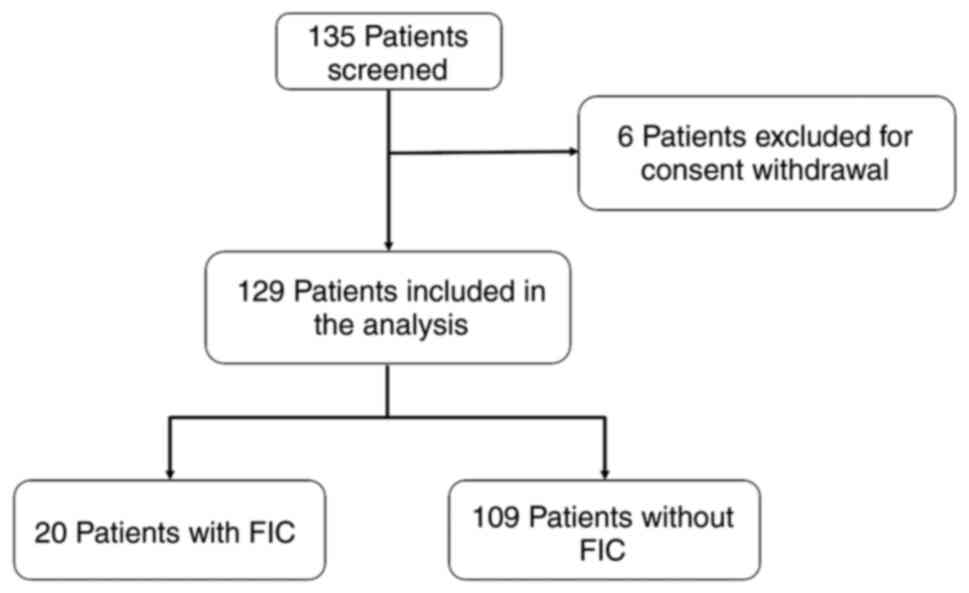
A total of 135 patients with CRC were enrolled in
the study after the informed consent was signed. A total of 6
patients were excluded from this analysis owing to subsequent
consent withdrawal.
A total of 129 consecutive patients with CRC were
thus eligible for analysis (Fig.
2). Patient characteristics are listed in Tables I and SI. The median age was 69 years (range
27–83 years), 121 patients (93.8%) initially received 100% dose,
while 8 patients (6.2%) were treated with 75% dose. A total of 62
patients (48%) resulted at high CV risk prior to treatment
initiation and were referred to the cardiologist for a baseline
assessment. Of these, 7 (5.4%) had a history of acute myocardial
infarction, while 21 (16.3%) had other types of cardiac disease,
including coronary artery disease (n=3), atrial fibrillation (n=5),
other cardiac arrhythmias or previous syncopal episodes (n=7),
heart failure history (n=2), and heart valve disease (n=1).
 | Table I.Patient characteristics and
pre-existing disease of patients at baseline. |
Table I.
Patient characteristics and
pre-existing disease of patients at baseline.
| Patients | Number | Percentage of
patients (%) |
|---|
| Sex |
|
|
|
Female | 51 | 39.5 |
|
Male | 78 | 60.5 |
| Primary site |
|
|
|
Colon | 106 | 82.2 |
|
Rectum | 23 | 17.8 |
| Age |
|
|
| <70
years | 66 | 51.2 |
| ≥70
years | 63 | 48.8 |
| FP |
|
|
|
Capecitabine | 60 | 46.5 |
|
5-fluoropyrimidine | 69 | 53.5 |
| Setting |
|
|
|
Adjuvant | 68 | 52.7 |
|
Metastatic | 61 | 47.3 |
| Cardiological
risk |
|
|
|
Low | 67 | 51.9 |
|
High | 62 | 48.1 |
| Body mass
index |
|
|
|
<25 | 55 | 42.6 |
|
≥25 | 74 | 57.4 |
| Smoke |
|
|
| Current
or former | 68 | 52.7 |
|
Never | 61 | 47.3 |
| Alcohol intake |
|
|
| (< or ≥10 g per
day) |
|
|
|
Yes | 72 | 55.8 |
|
Never | 57 | 44.2 |
| Sedentary (< or
≥30 min aerobic activity/3 times per week) |
|
|
| No | 53 | 41.1 |
|
Yes | 75 | 58.1 |
| NA | 1 | 0.8 |
| Hypertension |
|
|
|
Yes | 71 | 55 |
| No | 58 | 45 |
| Diabetes |
|
|
|
Yes | 24 | 18.6 |
| No | 105 | 81.4 |
| Dyslipidaemia |
|
|
|
Yes | 39 | 30.2 |
| No | 88 | 68.2 |
| NA | 2 | 1.6 |
| Myocardial
infarction |
|
|
|
Yes | 7 | 5.4 |
| No | 121 | 93.8 |
| NA | 1 | 0.8 |
| Stroke |
|
|
|
Yes | 4 | 3.1 |
| No | 124 | 96.1 |
| NA | 1 | 0.8 |
| Heart disease |
|
|
|
Yes | 21 | 16.3 |
| No | 107 | 82.9 |
| NA | 1 | 0.8 |
Cardiotoxicity
A total of 20 out of 129 patients (15.5%)
experienced one or more symptoms of FIC. The most common symptoms
were dyspnoea (12 of 20 patients, 60%), chest pain (8 patients,
40%) and palpitations (8 patients, 40%) (Table SII). A total of 3 patients (15%)
had clinically relevant ECG changes. One patient had new-onset
supraventricular paroxysmal tachycardia, one had a new-onset left
bundle branch block, and one had an ST deviation. The latter case
showed also elevated TnI levels and was diagnosed as acute
myocardial infarction. No patient had a lethal outcome. The first
occurrence of FIC was at the first cycle for 7 of 20 patients
(35%), at the second for 4 patients (20%) and at the third for 7
patients (25%). A total of 3 other patients (15%) experienced FIC
after the third cycle.
A cardiological treatment was initiated in 6 of
these patients. FP-based chemotherapy was resumed in 18 cases: 14
patients were retreated with the same FP dose without initiation of
cardiac therapy, and 4 were retreated with the same FP dose but
received concomitant cardiac therapy with angiotensin-converting
enzyme inhibitors, antiarrhythmics or beta-blockers. In these
patients, neither further interventions nor dose reductions were
needed.
Only one of the 14 patients treated with full dose
FP-based chemotherapy and who had not received new cardiological
therapy had recurrent symptoms at retreatment. This patient had
chest pain, palpitation and dyspnoea during C2 of adjuvant
mFOLFOX6. A complete cardiological assessment was performed,
including coronary angiography, without evidence of cardiac
disease; thus, FP treatment was resumed under strict cardiac
monitoring. Nevertheless, during C6, he presented a symptomatic
recurrence with chest pain, dyspnoea, elevated cardiac enzymes and
ST deviation that required hospitalization. She had a complete
recovery from this acute event, but FP treatment was
discontinued.
The patient with new onset of left branch block had
his FP-based chemotherapy discontinued with the resolution of the
aberrant ECG pattern. The patient with supraventricular paroxysmal
tachycardia was treated with antiarrhythmics with a symptomatic
resolution, but FP treatment was discontinued by cardiologist's
decision. Nine events of FIC occurred in the subgroup of patients
with cardiac comorbidity (n=62) (14.5%), a similar rate (P=0.77) to
the one observed among patients without overt cardiac comorbidities
(n=67) (11 events, 16.4%).
Correlation between CV event and
clinical pathological characteristics
Firstly, we investigated a potential association of
the occurrence of a CV event with CV risk factors, tumor type, and
FP-based chemotherapy regimen. As shown in Table II, only sex was minimally
associated with CV events, with female patients displaying a higher
risk (30%, vs. male 11.6%; P=0.05). A trend was observed for higher
body mass index (BMI) and capecitabine-based regimens (P=0.07 for
both).
 | Table II.Contingency table (2×2) analyzed by
χ-square test (P<0.05 as statistically significant). |
Table II.
Contingency table (2×2) analyzed by
χ-square test (P<0.05 as statistically significant).
|
| FIC | No FIC | P-value (Chi square
test) |
|---|
| Primary site |
|
| 0.32 |
|
Colon | 18 | 88 |
|
|
Rectum | 2 | 21 |
|
| Tumor stage |
|
| 0.09 |
|
Non-metastatic | 14 | 54 |
|
|
Metastatic | 6 | 55 |
|
| Sex |
|
| 0.05 |
|
Male | 8 | 69 |
|
|
Female | 12 | 40 |
|
| Age at
diagnosis |
|
| 0.4 |
| ≤70
years | 13 | 60 |
|
| >70
years | 7 | 49 |
|
| Fluoropyrimidine
based regimen |
|
| 0.07 |
|
5-FU-based | 7 | 62 |
|
|
Capecitabine-based | 13 | 47 |
|
| Cardiovascular risk
at screening |
|
| 0.76 |
|
Low | 11 | 56 |
|
|
High | 9 | 53 |
|
| Smoke |
|
| 0.45 |
|
Yes | 9 | 59 |
|
| No | 11 | 50 |
|
| Alcohol |
|
| 0.16 |
|
Yes | 14 | 58 |
|
| No | 6 | 51 |
|
| Hypertension |
|
| 0.62 |
|
Yes | 10 | 61 |
|
| No | 10 | 48 |
|
| Concomitant heart
disease |
|
| 0.26 |
|
Yes | 5 | 16 |
|
| No | 15 | 92 |
|
| Diabetes |
|
| 0.28 |
|
Yes | 2 | 22 |
|
| No | 18 | 86 |
|
| Body mass
index |
|
| 0.07 |
| ≤25
kg/m2 | 15 | 58 |
|
| >25
kg/m2 | 5 | 51 |
|
Univariate logistic regression was also applied to
examine any association of CV events with age, single risk factors
(smoke, alcohol intake, BMI, diabetes, hypertension, sedentary
habits, and cholesterol), type of chemotherapy (5-FU/capecitabine),
or inflammatory indexes (i.e. neutrophils/lymphocytes ratio or
platelets). None of the variables tested was able to predict the
occurrence of a CV event. Low infusion volume displayed the lowest
P-value (0.06), showing a modest protective effect (Table III).
 | Table III.Association between
clinical-pathological characteristics and occurrence of CV event
analyzed by univariate logistic regression. |
Table III.
Association between
clinical-pathological characteristics and occurrence of CV event
analyzed by univariate logistic regression.
| Univariate
analysis |
|---|
|
|---|
| Characteristics of
patients | Odds ratio (95%
confidence interval) | P-value |
|---|
| Age
(≤70 vs. >70) | 0.971
(0.934–1.012) | 0.15 |
| Sex
(female vs. male) | 2.18
(0.805–5.654) | 0.13 |
| Therapy
(5-FU vs. capecitabine) | 0.620
(0.232–1.618) | 0.33 |
| Type of
infusion (low vs. high volume) | 0.377
(0.126–1.014) | 0.06 |
| Risk factor |
|
|
| Body
mass index (≤25 vs. >25 kg/m2) | 0.949
(0.859–1.045) | 0.29 |
| Smoke
(yes vs. no) | 0.544
(0.199–1.421) | 0.22 |
| Alcohol
(yes vs. no) | 2.052
(0.762–6.155) | 0.17 |
|
Hypertension (yes vs. no) | 0.786
(0.299–2.066) | 0.62 |
|
Diabetes mellitus (yes vs.
no) | 0.439
(0.067–1.682) | 0.29 |
|
Sedentary (yes vs. no) | 1.071
(0.409–2.935) | 0.89 |
|
Previous Heart diseases (yes
vs. no) | 1.917
(0.562–5.766) | 0.26 |
| Basal
CV risk (high vs. low) | 0.679
(0.248–1.772) | 0.43 |
| Hematochemical
indexes |
|
|
| Lactate
dehydrogenase (≤ vs. >300 U/l) | 0.100
(0.996–1.001) | 0.5 |
|
Haemoglobin (≤10 vs. >10
g/dl) | 0.935
(0.714–1.122) | 0.58 |
|
Platelets (≤400 vs. >400
103/mm3) | 0.100
(0.993–1.004) | 0.78 |
|
Neutrophil/Lymphocyte ratio
(≤5 vs. >5) | 1.063
(0.825–1.310) | 0.59 |
Stepwise logistic regression selected sex and
alcohol intake (defined as < or ≥10 g per day) as variables to
perform multiple logistic regression. As revealed in Table IV, the chance of having a CV event
in males who drank less than 10 g of alcohol per day was very low
[exp(b0)=0.04]. The chance of CV among females who drank
less than 10 g per day is exp(b1) times the chance of
the reference group and among males who drink more than 10 g per
day is exp(b2) times the chance of the reference group,
which gives 0.17 for both groups and remains very low. However,
among women who drank more than 10 g per day, the chance of a CV
event increased to 0.7 [exp(b1+b2) times
exp(b0)].
 | Table IV.Association between sex, alcohol
intake and occurrence of cardiovascular event, analyzed by
multivariable logistic regression. |
Table IV.
Association between sex, alcohol
intake and occurrence of cardiovascular event, analyzed by
multivariable logistic regression.
|
| Coefficients | SE | 95% CI | Odds ratio (95%
CI) | P-value |
|---|
| Intercept |
b0=−3,239 | 0,6674 | −4,649; −2,029 |
|
|
| Sex (female vs.
male) |
b1=1,438 | 0,5855 | 0,3108; 2,626 | 4.12
(1.364–13.81) | 0.01 |
| Alcohol (yes vs.
no) |
b2=1,441 | 0,6211 | 0,2741; 2,731 | 4.225
(1.315–15.35) | 0.02 |
Circulating biomarkers of FIC
Plasma TnI increased to 0.07 g/l in the patient
experiencing acute myocardial infarction during 5-FU treatment,
while remaining below the lower limit of detection in all other
patients. For this reason, the statistical analysis for this
variable was not performed. The influence of FP-based chemotherapy
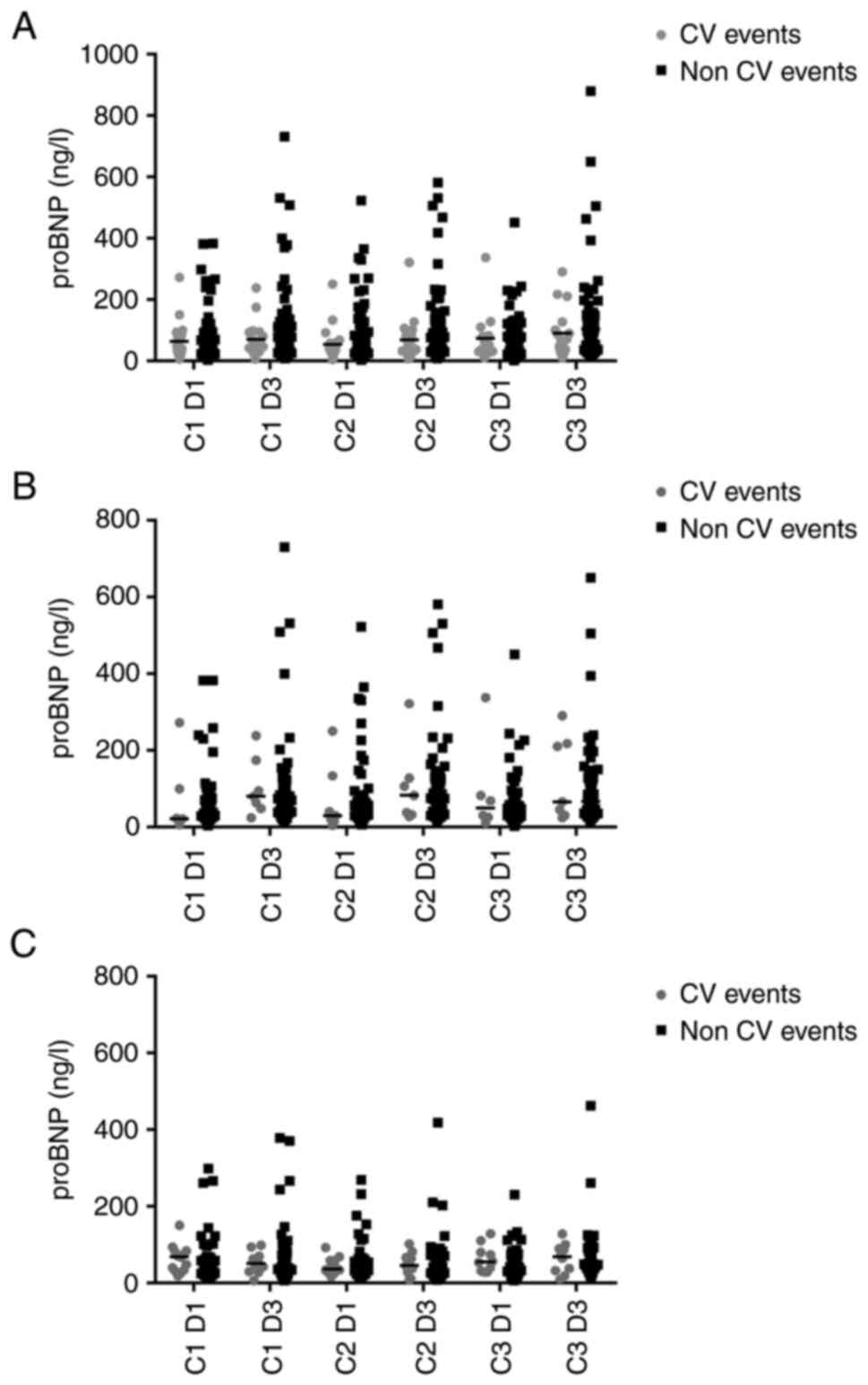
on proBNP levels is demonstrated in Fig. 3A; proBNP values changes considering
5-FU and capecitabine separately are presented in Fig. 3B and C.
No significant differences were observed in proBNP
levels, measured at the same time point, between patients who
experienced FIC and those who did not (Table SIII).
The proBNP levels registered in the observational
period increased after drug administration in patients without FIC,
but they did not significantly change in patients with FIC
(Table SIV). In particular, in
patients without FIC, there was an increase of proBNP in patients
treated with 5-FU.
Baseline circulating miRNA profiling in the plasma
of 8 patients who experienced a CV event was compared with that of
16 patients without CV event. In Table SV, clinical and pathological
characteristics of this subset of patients are reported.
No significant differences in terms of miRNA
expression were found comparing patients with and without a CV
event. Similarly, a lack of difference was observed when comparing
high CV risk with low CV risk patients. Since the number of CV
events was slightly higher in low CV risk patients, miRNA profiling
of patients experiencing CV events was compared with control
patients, selecting among them only those at low CV risk. In this
analysis, three downregulated miRNAs were identified in CV-events
patients: hsa-miR-6089, which regulates the expression of genes
involved in inflammation processes and is already described as a
biomarker of stroke and vascular damage (17); hsa-miR-4459, which stimulates
endothelial cells, promotes autophagy and regulates atherosclerosis
through a miRNA-long non coding RNA loop (18) and has already been described as a
putative biomarker of cardiovascular risk; hsa-miR-4505, which did
not have any known role in CV risk or disease.
Discussion
In this prospective observational study, incidence
and clinical manifestations of FIC were evaluated in patients with
CRC. The combination of female sex and high alcohol intake was
identified as risk factor for FIC development.
The observed incidence of FIC, 15.5%, is in stark
contrast with the general notion that this is an infrequent
complication. The existing literature displays a moderate-high
variability in the delineation of this value, to which different
factors can contribute: retrospective nature, selection bias,
differences in CV risk profiles of the analysed samples, and,
importantly, the lack of a shared and widely accepted standard for
a practical definition of FIC.
In the present study, a collection of PROs together
with an objective assessment of the cardiac function was proposed.
This led to the individuation and inclusion in our cohort of
asymptomatic and mildly symptomatic patients with cardiovascular
disease, potentially excluded from other prospective or
retrospective analyses. Suspected FIC cases were subject to a
cardiological verification.
In the present trial, 40% of patients with FIC
reported chest pain, but only 15% presented an ECG alteration. Most
of the patients with chest pain and normal ECG had also normal
coronary enzymes, whereas severe events, such as acute myocardial
infarction, were rare. This pattern is in accordance with other
studies. Both the sudden onset of chest pain and the rarity of the
occurrence of life-threatening complications support the
pathogenetic hypothesis of a FP-induced transient vasospastic
mechanism. In our experience, this was further corroborated by the
subsequent angiographic finding of normal coronary arteries in the
patient who had experienced FP-related acute myocardial
infarction.
In line with this hypothesis, only a minority of
patients displayed objective signs (e.g. ECG changes) of
cardiotoxicity, and FIC was initially suspected on the clinical
relevance of subjective symptoms. In this context, the physician's
assessment of the patient's symptoms takes on a relevant role,
passing from a passive registration of symptomatology to active
discrimination of cardiac events.
Compared with other case series, the symptoms
reported were prospectively and actively analyzed according to a
global patient evaluation. This led to the discrimination between
non-specific symptoms and clinically relevant cardiological events,
showing the actual FIC incidence in the real-world CRC population.
As an example, patient 13 was correctly identified at C2 as a CV
event and a consequent cardiological assessment was performed.
Nevertheless, due to the completely negative cardiological work-up,
retreatment with FP was performed with strict monitoring and
patient experienced an acute CV event at C6.
Except for this single event, retreatment with FP
after the occurrence of cardiotoxicity was attempted in all other
patients without any FIC recurrence. Overall, out of 18 patients
experiencing FIC, 4 started a cardiological protective therapy
without recurrence, whilst among 14 patients who were not
prescribed any further cardiological therapy, only one experienced
a FIC recurrence. The real value of cardiological therapy and FP
dose reduction is questionable. A retrospective study on 668
patients and a prospective study on 644 patients treated with 5-FU
or capecitabine reported a benefit from initiation of a
prophylactic cardiological therapy that prevented symptoms at
retreatment in 9 out of 12 patients and 12 out of 15 patients,
respectively (19,20). By contrast, a small and
non-randomised study could not demonstrate a prophylactic effect of
calcium channel blockers on the occurrence of cardiotoxicity
(21). With the limitation of the
small numbers, the complex of these data suggested that resumption
of FP-based chemotherapy is a generally feasible strategy with the
following caveats: i) however carefully conducted, selection of
patients to be subject to retreatment is rendered difficult by the
proteiform manifestations of FIC; ii) rather than pharmacological
secondary prevention, close cardiac monitoring is crucial; iii)
retreatment requires considerable precaution given the
unpredictable time-cardiotoxicity correlation. As more general
considerations, the added value offered in this context by the
collaboration between the oncologist and the
cardiologist/cardioncologist should be noted, as well as the need
for a frank discussion with the patient, in which duty of the
oncologist is to carefully review the risk/benefit balance of
resuming the treatment.
The multivariate logistic regression, applied to sex
and alcohol consumption, revealed that both males and females are
more protected from CV events if they do not consume alcohol; the
subpopulation of females drinking more than 10 g of alcohol per day
are the most at risk to FIC development and thus deserve particular
attention.
Pursuing the objective of a real-world study, 44.6%
of patients with high CV risk factors (such as myocardial
infarction history) were included in the present cohort.
Nevertheless, a predictive role for FIC was not demonstrated for
pre-existing cardiac disease. High-risk subgroups cannot be
identified on these bases, and, since FIC can be lethal in patients
without a history of cardiac disease, attention must be paid in all
FP-treated patients indiscriminately.
Although previous studies suggested that
capecitabine is more likely to induce cardiotoxicity compared with
5-FU chemotherapy (20,22), results from the literature are
contradictory (11,19). In our cohort, a comparable
incidence of FIC between the oral pro-drug and the infusion
regimens was observed.
Based on these results, careful monitoring of
possible FIC throughout in all patients is recommended regardless
of CV comorbidities, FP chemotherapy regimen, through all treatment
cycles and, particularly, upon the occurrence of mildly symptomatic
FIC. An effective work-up should be applied (e.g. ECG monitoring or
use of cardiac therapy) but, in relation to its unpredictable
nature, strict monitoring must be performed to avoid lethal events,
particularly in the adjuvant setting, in which FP have a curative
role and the risk-benefits should be carefully considered.
Albeit routinely used in the clinical management of
cardiac disease, TnI and proBNP cannot be efficiently used for FIC
prediction. Particularly, neither significantly changed their
expression in CV-event patients at any cycle of treatment, except
for patient 17 in which a TnI elevation was observed at the
recurrence. No variation in proBNP expression between FIC and no
FIC patients was found. A statistically significant increase of
proBNP levels between D3 and D1 of all the first three cycles was
observed only in patients treated with 5-FU; a potential
explanation could be the cardiac parietal stress exerted by the
transient mild hypervolemia due to the fluids infused during the
5-FU-based chemotherapy administration.
The exploratory analysis of miRNA profiling
conducted on a small subset of patients who experienced FIC (n=8)
and 16 control patients, at baseline, revealed no significant
differences in terms of miRNA expression. A comparison between
patients who experienced FIC and low risk controls revealed three
differentially expressed microRNAs, two of which had been
previously associated with cardiovascular diseases. Exploration of
miRNA expression, either as a baseline regulators of enzymes
activities or as modulators induced by treatment with chemotherapy
and targeted therapy, is a promising field (23–25).
Nevertheless, this was not pursued as the present study aimed to
identify potential predictive biomarkers in FP-naïve patients; for
this reason, plasma samples collected after the completion of FP
treatment were not analyzed.
Certain limitations to the present study need to be
discussed. Due to the early closure of accrual, a smaller sample
size than planned was obtained, resulting in a low number of events
(20 CV events) leading to wide CIs, low statistical power and
increased risk of type II statistical errors. This, moreover,
reduces the number of risk factors which could be could confidently
examined in our multivariate model. The low number of events and
multiple testing (increasing the risk of false-positive results)
are weaknesses of most studies analysing risk factors and render
the conclusions that can be drawn from these studies less valid and
may conceal clinically significant differences between events and
no event.
Lastly, the DPYD status was not available. While
DPYD polymorphisms exert a well-known influence on enzymatic
activity, FP catabolism and toxicity, the impact of the enzyme
deficiency on cardiotoxicity remains unclear but appears to be
limited.
In a large prospective study, 487 oncologic patients
treated with a 5-FU-based chemotherapy regimen were characterized
for single nucleotide polymorphisms (SNPs) of DPYD. Although 187
patients had SNPs, only 4 of these patients (2%) developed severe
(grade 3 or 4) cardiotoxicity (26).
Nevertheless, DPYD polymorphisms are not the only
factors that could affect DPDYD enzyme activities. The miR-27 level
expression has been linked to the function of the DPD enzyme in the
liver cells, and p53 status plays an important role in controlling
pyrimidine catabolism through repression of DPYD expression
(25,27). In addition, even abnormalities of
thymidylate synthase, the main target of FP actions, appear to
predict the risk of severe toxicity and the combination of DPYD and
TYMS genotyping could identify ≥50% of patients at the greatest
risk of adverse events (28).
However, differently from most other large studies
on this topic, the present study is a monocentric, rigorous,
prospective trial. This may result in a minor inter-variability in
symptomatology assessment and cardiological evaluation, but it may
not reflect the characteristics of the general population and
practice patterns.
In conclusion, cardiotoxicity can be a
life-threatening, unexpected complication of FP therapy that limits
the appropriate treatment of a highly morbid and aggressive cancer.
Despite females with an alcohol consumption habit being more prone
to experience FIC at our institution, FIC was not clearly
associated with classical CV comorbidities. Consequently, optimal
information about the risk and close monitoring represent the best
option to prevent serious events and lethal outcome in all patient
subgroups, regardless of common risk factors.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Raw and processed data or miRNA analysis are
available on Gene Expression Omnibus (GEO) database (accession no.
GSE217768).
Authors' contributions
ID, AB and FL conceived and designed the present
study. PA, GA, AB, ID, EF, RF, VQ and MM collected the data. PA,
GA, CPN, MB, GCa, LG, CC, MA, PO and GCh analyzed and interpreted
the results. PL, GA, CPN, RF and FL prepared the draft of the
manuscript. All authors reviewed the results, read and approved the
final version of the manuscript. CPN and GCh confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
251/2015; October, 22nd 2015) by the Ethics Committee of Candiolo
Cancer Institute (Turin, Italy). Written informed consent was
provided by all patients before the start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoff PM, Ansari R, Batist G, Cox J, Kocha
W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, et al:
Comparison of oral capecitabine versus intravenous fluorouracil
plus leucovorin as first-line treatment in 605 patients with
metastatic colorectal cancer: Results of a randomized phase III
study. J Clin Oncol. 19:2282–2292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiga T and Hiraide M: Cardiotoxicities of
5-fluorouracil and other fluoropyrimidines. Curr Treat Options
Oncol. 21:272020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polk A, Vaage-Nilsen M, Vistisen K and
Nielsen DL: Cardiotoxicity in cancer patients treated with
5-fluorouracil or capecitabine: A systematic review of incidence,
manifestations and predisposing factors. Cancer Treat Rev.
39:974–984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Becker K, Erckenbrecht JF, Häussinger D
and Frieling T: Cardiotoxicity of the antiproliferative compound
fluorouracil. Drugs. 57:475–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deboever G, Hiltrop N, Cool M and
Lambrecht G: Alternative treatment options in colorectal cancer
patients with 5-fluorouracil- or capecitabine-induced
cardiotoxicity. Clin Colorectal Cancer. 12:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polk A, Vistisen K, Vaage-Nilsen M and
Nielsen DL: A systematic review of the pathophysiology of
5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol.
15:472014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Südhoff T, Enderle MD, Pahlke M, Petz C,
Teschendorf C, Graeven U and Schmiegel W: 5-Fluorouracil induces
arterial vasocontractions. Ann Oncol. 15:661–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen SA, Hasbak P, Mortensen J and
Sørensen JB: Fluorouracil induces myocardial ischemia with
increases of plasma brain natriuretic peptide and lactic acid but
without dysfunction of left ventricle. J Clin Oncol. 28:5280–5286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arellano M, Malet-Martino M, Martino R and
Gires P: The anti-cancer drug 5-fluorouracil is metabolized by the
isolated perfused rat liver and in rats into highly toxic
fluoroacetate. Br J Cancer. 77:79–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Depetris I, Marino D, Bonzano A, Cagnazzo
C, Filippi R, Aglietta M and Leone F: Fluoropyrimidine-induced
cardiotoxicity. Crit Rev Oncol Hematol. 124:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Cutsem E, Hoff PM, Blum JL, Abt M and
Osterwalder B: Incidence of cardiotoxicity with the oral
fluoropyrimidine capecitabine is typical of that reported with
5-fluorouracil. Ann Oncol. 13:484–485. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao RY, Li Q, Miao Y, Zhang Y, Yuan W, Fan
L, Liu G, Mi Q and Yang J: The emerging role of MicroRNA-155 in
cardiovascular diseases. Biomed Res Int. 2016:98692082016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Navickas R, Gal D, Laucevičius A,
Taparauskaitė A, Zdanytė M and Holvoet P: Identifying circulating
microRNAs as biomarkers of cardiovascular disease: A systematic
review. Cardiovasc Res. 111:322–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rigaud VO, Ferreira LR, Ayub-Ferreira SM,
Ávila MS, Brandão SM, Cruz FD, Santos MH, Cruz CB, Alves MS, Issa
VS, et al: Circulating miR-1 as a potential biomarker of
doxorubicin-induced cardiotoxicity in breast cancer patients.
Oncotarget. 8:6994–7002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Argilés G, Tabernero J, Labianca R,
Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P,
Yoshino T, Taieb J, et al: Localised colon cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 31:1291–1305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sonoda T, Matsuzaki J, Yamamoto Y, Sakurai
T, Aoki Y, Takizawa S, Niida S and Ochiya T: Serum MicroRNA-based
risk prediction for stroke. Stroke. 50:1510–1518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zhang L, Wang Y, Ding H, Xue S,
Qi H and Li P: MicroRNAs or long noncoding RNAs in diagnosis and
prognosis of coronary artery disease. Aging Dis. 10:353–366. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jensen SA and Sørensen JB: Risk factors
and prevention of cardiotoxicity induced by 5-fluorouracil or
capecitabine. Cancer Chemother Pharmacol. 58:487–493. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kosmas C, Kallistratos MS, Kopterides P,
Syrios J, Skopelitis H, Mylonakis N, Karabelis A and Tsavaris N:
Cardiotoxicity of fluoropyrimidines in different schedules of
administration: A prospective study. J Cancer Res Clin Oncol.
134:75–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eskilsson J and Albertsson M: Failure of
preventing 5-fluorouracil cardiotoxicity by prophylactic treatment
with verapamil. Acta Oncol. 29:1001–1003. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng J, Dong C, Wang C, Li W, Yu H, Zhang
M, Zhao Q, Zhu B, Zhang J, Li W, et al: Cardiotoxicity of
5-fluorouracil and capecitabine in Chinese patients: A prospective
study. Cancer Commun (Lond). 38:222018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leger KJ, Leonard D, Nielson D, de Lemos
JA, Mammen PP and Winick NJ: Circulating microRNAs: Potential
markers of cardiotoxicity in children and young adults treated with
anthracycline chemotherapy. J Am Heart Assoc. 6:e0046532017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindholm EM, Ragle Aure M, Haugen MH,
Kleivi Sahlberg K, Kristensen VN, Nebdal D, Børresen-Dale AL,
Lingjaerde OC and Engebraaten O: miRNA expression changes during
the course of neoadjuvant bevacizumab and chemotherapy treatment in
breast cancer. Mol Oncol. 13:2278–2296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Offer SM, Butterfield GL, Jerde CR, Fossum
CC, Wegner NJ and Diasio RB: microRNAs miR-27a and miR-27b directly
regulate liver dihydropyrimidine dehydrogenase expression through
two conserved binding sites. Mol Cancer Ther. 13:742–751. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morel A, Boisdron-Celle M, Fey L, Soulie
P, Craipeau MC, Traore S and Gamelin E: Clinical relevance of
different dihydropyrimidine dehydrogenase gene single nucleotide
polymorphisms on 5-fluorouracil tolerance. Mol Cancer Ther.
5:2895–2904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gokare P, Finnberg NK, Abbosh PH, Dai J,
Murphy ME and El-Deiry WS: P53 represses pyrimidine catabolic gene
dihydropyrimidine dehydrogenase (DPYD) expression in response to
thymidylate synthase (TS) targeting. Sci Rep. 7:97112017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saif MW, Hachem H, Purvey S, Hamal R,
Zhang L, Siddiqui NS, Godara A and Diasio RB: Pharmacogenetic
variants in the DPYD and TYMS genes are clinically significant
predictors of fluoropyrimidine toxicity: Are we ready for use in
our clinical practice. Arch Pharmacol Ther. 2:6–8. 2020.PubMed/NCBI
|