Introduction
Breast cancer accounts for the highest mortality
among female cancer patients in India (1). It is also the most prevalent cancer
type among Indian females with an estimated age-adjusted rate of
25.8 per 100,000 women and a mortality rate of 12.7 per 100,000
women (2). India has a worse
survival outcome for patients with breast cancer compared with
western countries (3).
Specifically, 90,408 mortalities are reported in India due to
breast cancer as per the Globocan data 2020 (3). Advances in novel therapies remain
untranslated to improved outcomes for patients with
therapy-resistant cancer, which remains incurable (4).
An existing challenge in pre-clinical research and
drug discovery is the lack of accurate in vitro and in
vivo models that mimic the patients' phenotypes of resistance.
Immortalized cell lines and xenograft models derived from such cell
lines are markedly different from patients' tumors in terms of
molecular and genetic profiles (5).
Traditional cancer cell lines are usually grown clonally in plastic
flasks with uniform morphology of undifferentiated phenotypes.
These cell line models acquire irreversible genetic and behavioral
changes upon serial passaging (6,7). Such
pre-clinical models also lack stromal elements that are unable to
mirror the architecture and heterogeneity of human tumors (7–9). Guo
et al (10) have reported
poor cancer type specificity in cancer cell lines, suggesting that
they deviate both histologically, pathologically and molecularly
from patient tumors. Their data demonstrate a high degree of
similarity for molecular profiles of human tumors with subsequently
established patient-derived xenografts (PDXs) from these tumors as
compared to cell line-based xenografts or cell lines (10).
PDXs, also termed patient avatars, retain several of
the molecular and functional features of patients' native tumors.
PDXs are reliable models for investigating tumor heterogeneity and
identifying drug targets for personalized medicine. Over the years,
several groups have developed and reported the molecular
characterization of PDX models for preclinical drug screening
programs worldwide (11–13). A recent study has reported that a
novel claudin-low triple-negative breast cancer PDX model maintains
a histopathological phenotype throughout subsequent passages in
mice, which holds promise for preclinical drug testing (12). Ramani et al (13) have highlighted the usage of PDX
models for assessing the impact and outcome of different anticancer
therapeutics on circulating tumor cell shedding and metastasis in
breast cancer. However, a limited number of preclinical PDX models
have been established from hormone receptor-positive,
HER2/Neu-negative patients with breast cancer who have progressed
on endocrine hormone therapy.
The present study aimed to establish a preclinical
patient-derived orthotopic xenograft (PDOX) model from a breast
cancer patient with the aforementioned phenotype for preclinical
drug screening. The development and serial passage of this PDOX
model were successful. In addition, multiple generations of
PDOX-derived breast tumor tissues were compared along with the
patient's tissues, both histopathologically and at the molecular
level. The expression levels of specific biomarkers for luminal,
epithelial and mesenchymal phenotypes were analyzed by
immunofluorescence and western blot analyses. The data indicated
that the newly developed PDOX was a suitable model system for
preclinical drug screening, biomarker development and personalized
treatment for this hormone therapy-resistant patient.
Materials and methods
Patient recruitment and
biobanking
A hormone receptor-positive, HER2/Neu-negative
patient who progressed on endocrine hormone therapy participated in
the present study. The study was approved by Institutional Ethics
Committee III of the Advanced Centre for Treatment, Research and
Education in Cancer (ACTREC), and registered with the clinical
trial registry of India (CTRI; registration no.
CTRI/2017/11/010553; registered on 17/11/2017). The patient's
sample was deposited in a biobank at the time of biopsy in a
magnetic-activated cell sorting tissue storage solution (Miltenyi
Biotec, Inc.). Half of the tissues were used for the development of
PDOX and the other half was used for the establishment of primary
cultures from patients' tumors. These dissected tissues were fixed
in formalin solution.
Development of PDOX model and in vivo
passaging
The animal procedures were approved by and performed
in compliance with the guidelines of the Institutional Animal Care
and Use Committee of the National Centre for Cell Science (Pune,
India). The breast tumor tissues were minced, collected in 1X
Dulbecco's PBS and centrifuged at 123 × g for 10 min at room
temperature. Non-dissociated tissue fragments (5–10 pieces of <1
mm in size) were administered orthotopically along with 1:1
Matrigel® (Corning, Inc.) into the right inguinal
mammary fat pad of a single female NOD/SCID mouse (age, 8 weeks;
body weight, 19 g), which was obtained from the institutional
animal facility, National Centre for Cell Science (Pune, India).
The mouse was maintained at the in-house pathogen-free facility
with ad libitum access to sterile food and chlorinated
sterile water at 22±1°C temperature with 55±2% humidity and a 12-h
light/dark cycle, and the tumor gradually developed. Following the
generation of palpable tumors (approximately within 180 days from
date of implantation), the tumor volume was measured using a
digital caliper twice a week. The mice were sacrificed when the
tumors attained a volume of >2,000 mm3 and a diameter
of >20 mm. The mice were sacrificed using CO2
asphyxiation by maintaining the CO2 flow rate at 30–70%
of the cage volume per minute to minimize sudden distress and pain.
Death of the mice was verified by observing the cessation of breath
and heartbeat, as well as areflexia as per the guidelines of the
Institutional Animal Care and Use Committee of the National Centre
for Cell Science, India. The tumors were excised and the weights
were measured. A part of the tumor tissue was again implanted
orthotopically into the right mammary fat pad of the next set of
female NOD/SCID mice (n=1-3) for the development of subsequent
generations of PDOXs.
Hematoxylin and eosin (H&E)
staining
Formalin-fixed and paraffin-embedded tissue sections
with 5-µm thickness were stained with H&E. Patients' and PDOX
tumor sections were deparaffinized using 3 rounds of fixation in
xylene and rehydration in descending grades of ethanol (100, 95 and
75%), followed by distilled water at room temperature. The tissue
sections were incubated with a hematoxylin solution for 8 min and
washed with water. The sections were soaked in 70% ethanol solution
containing 1% HCl and washed again. Subsequently, they were stained
with eosin for 5 min and rinsed with absolute alcohol and xylene
for 5 min each at room temperature. The images were captured using
a bright-field microscope and analyzed by an expert
histopathologist.
Western blot analysis
The tumor tissues from various generations were
lysed using RIPA lysis buffer. Following centrifugation, the
cleared supernatant was used to measure the total proteins using
Bradford's method. The lysates containing an equal amount of total
protein (30 µg) were resolved by 10 and 12.5% SDS-PAGE. Protein was
transferred onto PVDF membrane (Bio-Rad Laboratories, Inc.) and
further analyzed by western blot analysis. The expression levels of
ERα, PR, HER2, E-cadherin, N-cadherin and vimentin in tumor tissues
derived from different generations of PDOX models were analyzed
using their respective primary antibodies, namely anti-ERα (cat.
no. ab32063; 1:1,000 dilution; Abcam), anti-PR (cat. no. 8757;
1:1,000 dilution; Cell Signaling Technology, Inc.), anti-HER2 (cat.
no. ab2428; 1:1,000 dilution; Abcam), anti-E-cadherin (cat. no.
ab1416; 1:1,000 dilution; Abcam), anti-N-cadherin (cat. no.
ab18203; 1:1,000 dilution; Abcam) and anti-vimentin (cat. no.
sc-7558; Santa Cruz Biotechnology, Inc.; 1:1,000 dilution),
followed by incubation for 1 h at room temperature with specific
secondary antibodies, including goat anti-rabbit IgG HRP (cat no.
114038001A; Genei Laboratories Pvt. Ltd.), rabbit anti-mouse IgG
HRP (cat no. 114058001A; Genei Laboratories Pvt. Ltd.) and rabbit
anti-goat IgG HRP (cat no. 114048001A; Genei Laboratories Pvt.
Ltd.) at 1:2,000 dilution. All the blots were visualized using
Clarity Western ECL (Bio-Rad Laboratories, Inc.) reagent.
Establishment of primary culture
The primary cultures were established as previously
described with minor modifications (14). The clinical specimens were
aseptically transferred into DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 4% penicillin and streptomycin solution.
The tissue specimens were washed twice with 1X PBS containing an
antibiotic solution. The tissue specimens were cut into fine pieces
and incubated in 0.15% collagenase II and collagenase IV (Gibco;
Thermo Fisher Scientific, Inc.) solution in a water bath at 37°C
for 2 h for proper enzymatic digestion. Following incubation, the
tissue specimens were centrifuged at 123 × g for 10 min at room
temperature. The pellets were resuspended in DMEM supplemented with
20% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 25 µl hydrocortisone (Calbiochem), 10 µl insulin (Gibco;
Thermo Fisher Scientific, Inc.), 5 µl epidermal growth factor
(Peprotech) and a solution containing 2% penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The cells
were grown in a 95% humidified incubator with 5% CO2 at
37°C. When the cultures were established, the amount of FBS was
reduced to 10% to maintain the selective growth of cancer
epithelial cells.
Characterization of primary
cultures
Primary cultures were characterized by
immunofluorescence as previously described (15). The primary culture cells were grown
on coverslips. When the cells were confluent, they were fixed in 2%
paraformaldehyde for 20 min, quenched with 100 mM glycine for 10
min, permeabilized with 0.1% Triton-X100 for 10 min and incubated
with 10% FBS in PBS to block non-specific binding for 1 h at room
temperature. The cells were incubated overnight at 4°C with primary
antibodies to the following proteins: estrogen receptor (ER)-α
(cat. no. ab32063; Abcam), progesterone receptor (PR) (cat. no.
ab8757; Cell Signaling Technology, Inc.), HER2 (cat. no. ab2428;
Abcam), E-cadherin (cat. no. sc-7870; Santa Cruz Biotechnology,
Inc.), N-cadherin (ab18203; Abcam), vimentin (sc-7558; Santa Cruz
Biotechnology, Inc.), Ki-67 (sc-23900; Santa Cruz Biotechnology,
Inc.), α-smooth muscle actin (SMA; ab5694; Abcam) and
pan-cytokeratin (cat. no. C5992; Sigma-Aldrich; Merck Millipore) at
1:100 dilution. Fluorescent-labeled secondary antibodies such as
goat anti-mouse (cat. no. AP124C), donkey anti-rabbit (cat. no.
AP182C) and donkey anti-goat (cat. no. AP180C) Cy3 antibodies were
added to cells at 1:200 dilution and incubated for 1 h at room
temperature. The cells were visualized using a confocal microscope
(Leica Microsystems).
Statistical analysis
The data were analyzed and graphs were prepared
using SigmaPlot version 10.0 (Systat software).
Results
Patient and clinical phenotype
A 51-year-old post-menopausal female with no
comorbidities was referred to the Tata Memorial Hospital (Mumbai,
India) in October 2013 with a 5-cm lump in the left breast and a
2-cm lymph node (left axilla). The case had been determined to be
positive for the presence of a tumor based on a computerized
tomography scan. The patient had previously received four cycles of
cyclophosphamide, adriamycin and 5-fluorouracil prior to
registering at our center. A bone scan revealed the presence of
asymptomatic oligometastatic disease with suspected osteoblastic
lesions in the ribs. The patient provided consent for further
clinical treatment options and agreed to the treatment with radical
curative intent by simple mastectomy. Subsequently, the patient
received localized radiation therapy (RT) at the site of excision,
taxane-based chemotherapy and aromatase inhibitors, which were
completed in April 2014. The patient was disease-free for the next
two years and presented with a recurring disease in the liver in
October 2016. Therapy with tamoxifen and paclitaxel was initiated,
along with zoledronic acid. The patients also received palliative
RT to the D4-D6 region to alleviate symptomatic pain. The patient
remained stable on tamoxifen in the absence of disease progression
for 1 year. In 2017, following the appearance of new metastatic
nodules in the liver, the patient received therapy containing
exemestane. The patient remained stable on exemestane for another
year prior to the appearance of new nodules in the lung, which were
consistent with the presence of lung metastatic disease. The
patient provided consent to being recruited for the present study
in 2018. A biopsy was performed in 2–3 tumor cores from the
patient's liver using ultrasound guidance. The tissues were added
to a biobank for subsequent processing. The patient was
subsequently treated with capecitabine therapy along with
concurrent palliative RT. The patient progressed within two months
and received therapy based on a protocol of gemcitabine and
carboplatin. The patient responded to therapy for six months and
had stable disease. Subsequently, disease progression was observed
in early 2019. The patient received letrozole and zoledronic acid
followed by palliative RT. The patient continued receiving therapy
containing letrozole and zoledronic acid in 2019, exhibited stable
disease and was alive at the last follow-up in April 2022.
Histopathological analysis of the
patient's tumor
The histopathological examination of the tumor
resected in 2013 identified it as an infiltrating duct carcinoma of
the cribriform type with a high nuclear grade (grade III) and
necrosis. The tumor exhibited a focal micropapillary pattern and
extracellular mucin with a modified Bloom Richardson Score of
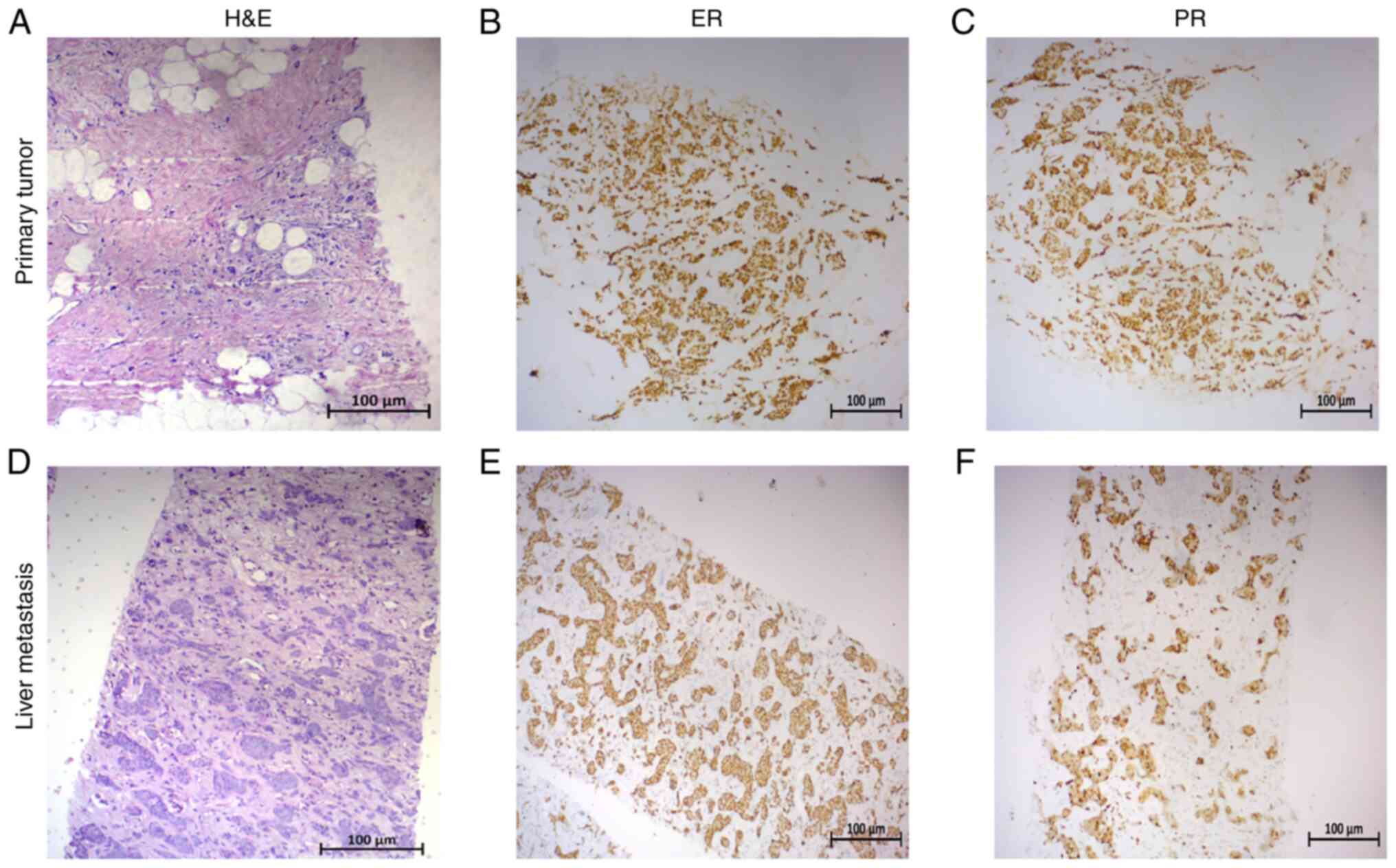
3+3+2=8 in the absence of Paget's disease (Fig. 1A). The tumor exhibited positive
staining for ER (all-red score, 8) and PR (all-red score, 8), as
determined by immunohistochemistry (IHC) (Fig. 1A-C) and negative for HER2 expression
on the Ventana system (score +1; data not shown). Histopathological
assessment of lymph nodes assessed by axillary clearance during
surgery revealed the presence of cancer cells in 10/20 lymph nodes
assessed. Histopathological re-examination of the tumor biopsied
from the patient's liver at progression in January 2018 revealed
consistency with the resected tumor prior to five years. The tumor
also demonstrated positive staining for ER (all-red score, 8) and
PR (all-red score, 8; Fig. 1D-F)
and negative for HER2 expression based on the Ventana system (score
+1; data not shown).
Generation and serial passaging of
breast cancer PDOX
Subcutaneous tumor PDX models are standard
pre-clinical models that are widely used to study treatment
response. However, they also exhibit low clinical relevance and
biological features to patients' tumors as compared to orthotopic
PDXs. Furthermore, other major limitations of the subcutaneous
models include a lower potential to metastasize and failure to
accurately mimic the tumor microenvironment and imitate a patient's
clinical treatment response. In contrast to these observations,
PDOXs may accurately and precisely epitomize the clinical behavior
and treatment response of a patient's unique cancer (11).
In the present study, a breast PDOX model was
developed using hormone-resistant-breast cancer by orthotopically
implanting tumor fragments into the mammary fat pad of female
NOD/SCID mice. The tissues were mixed with Matrigel to mimic the
organ microenvironment prior to implantation. The rate of
successful engraftment from the patient tumors to the NOD/SCID mice
was estimated to be 20% (3 PDOXs were established out of 15 samples
implanted; data not shown), which was consistent with previously
reported studies (16,17). However, the in vivo passaging
rate was considerably higher (~90%) following the establishment of
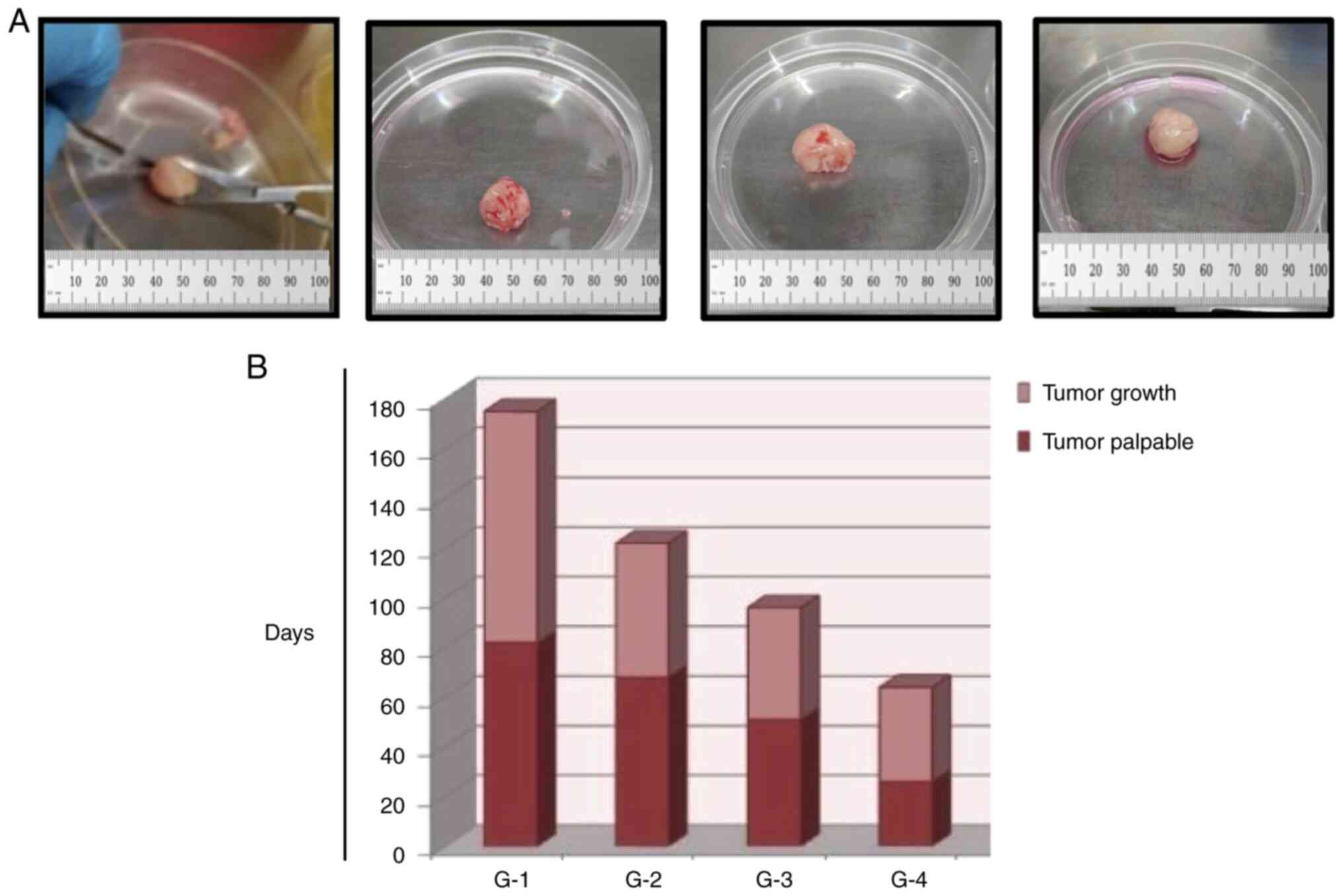
PDOXs. Following 70 days of implantation, the tumor was grown to a
palpable size (Generation 1; G1). The tumor was grown to the
endpoint volume, (~2,000 mm3) within 90 days after the
observation of the palpable tumors. Serial passaging of established
PDOXs was performed in the next cohort of immunocompromised mice to
establish G2 PDOXs. PDOXs exhibited an unstable growth pattern. The
time required to attain palpable tumors (tumor take) for G2 was 60
days, whereas that for G3 was ~40 days. The time required to reach
the tumor burden from the stage of palpable tumors for G2 was 60
days, while that for G3 (to reach the same volume as G1) was 50
days (Fig. 2A and B). The time
periods required to develop palpable tumors and to reach the tumor
burden for G4 were considerably decreased compared with those of
the G1-G3 generations of PDOXs. This is likely due to the
corresponding patient having undergone chemotherapy prior to
surgery. Therefore, PDOXs exhibited signs of chemotherapy-induced
differentiation and slow tumor-cell proliferation in the lower
passage and aggressive growth in advanced cohorts (Fig. 2A and B).
PDOXs reflect the histological traits
of primary tumors
The tissue sections derived from primary tumors and
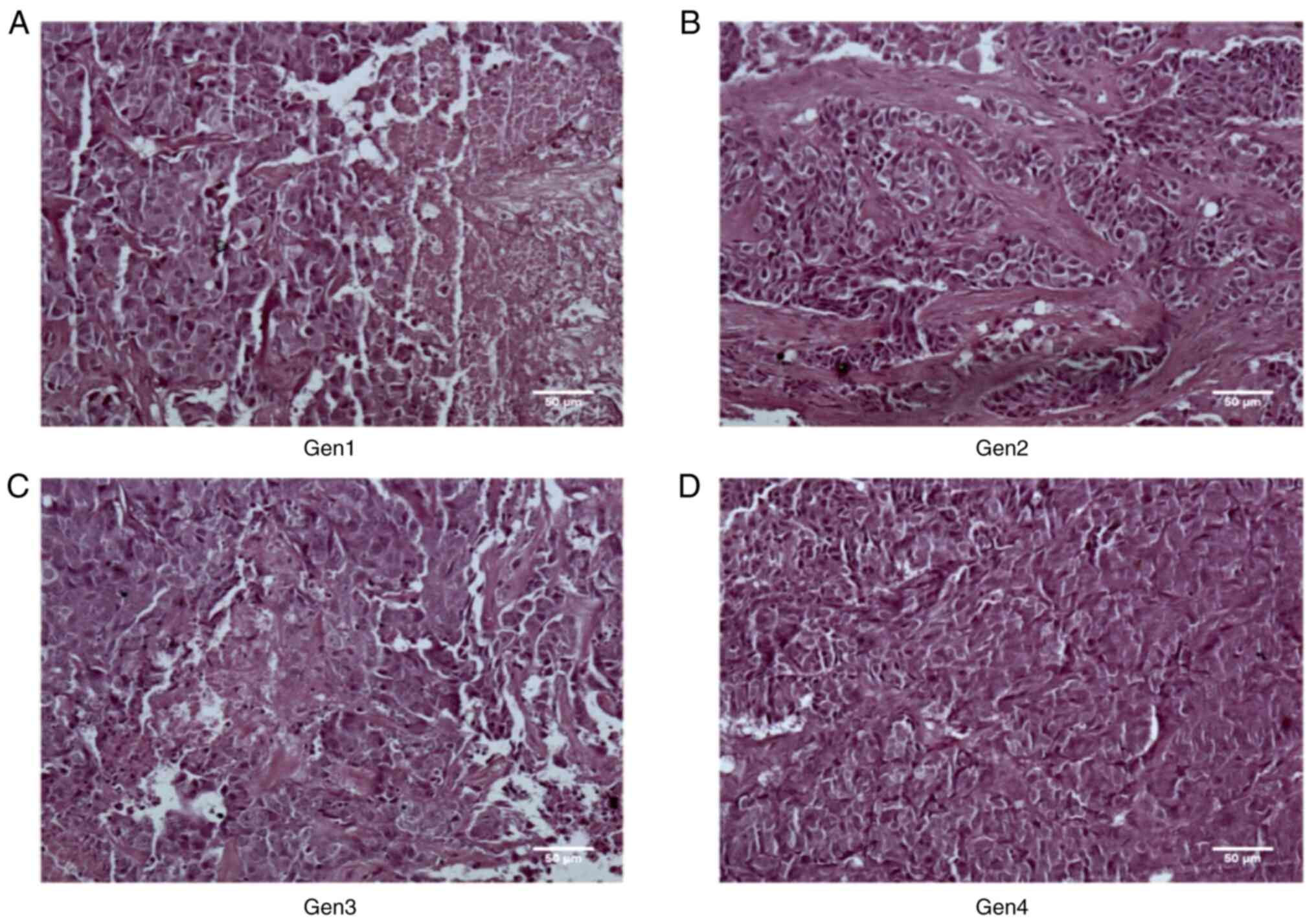
PDOXs were stained with H&E to assess their histological
features and determine growth stability and tissue morphology. The
histological reports of primary tumors and PDOXs revealed similar
grade histology represented by minimal to maximal nuclear
polymorphism and vessel formation, numerous mitotic activities with
occasional abnormal mitoses including atypical mitotic figures and
areas of necrosis (Figs. 1A and
3A-D, Table I). Mitotic activity and necrosis
were significantly increased from G1 to G4 (Fig. 3A-D, Table I). The increasing trend was in
parallel with the unstable growth patterns observed in the PDOXs
(Fig. 2B, Table I). The tumor tissues of different
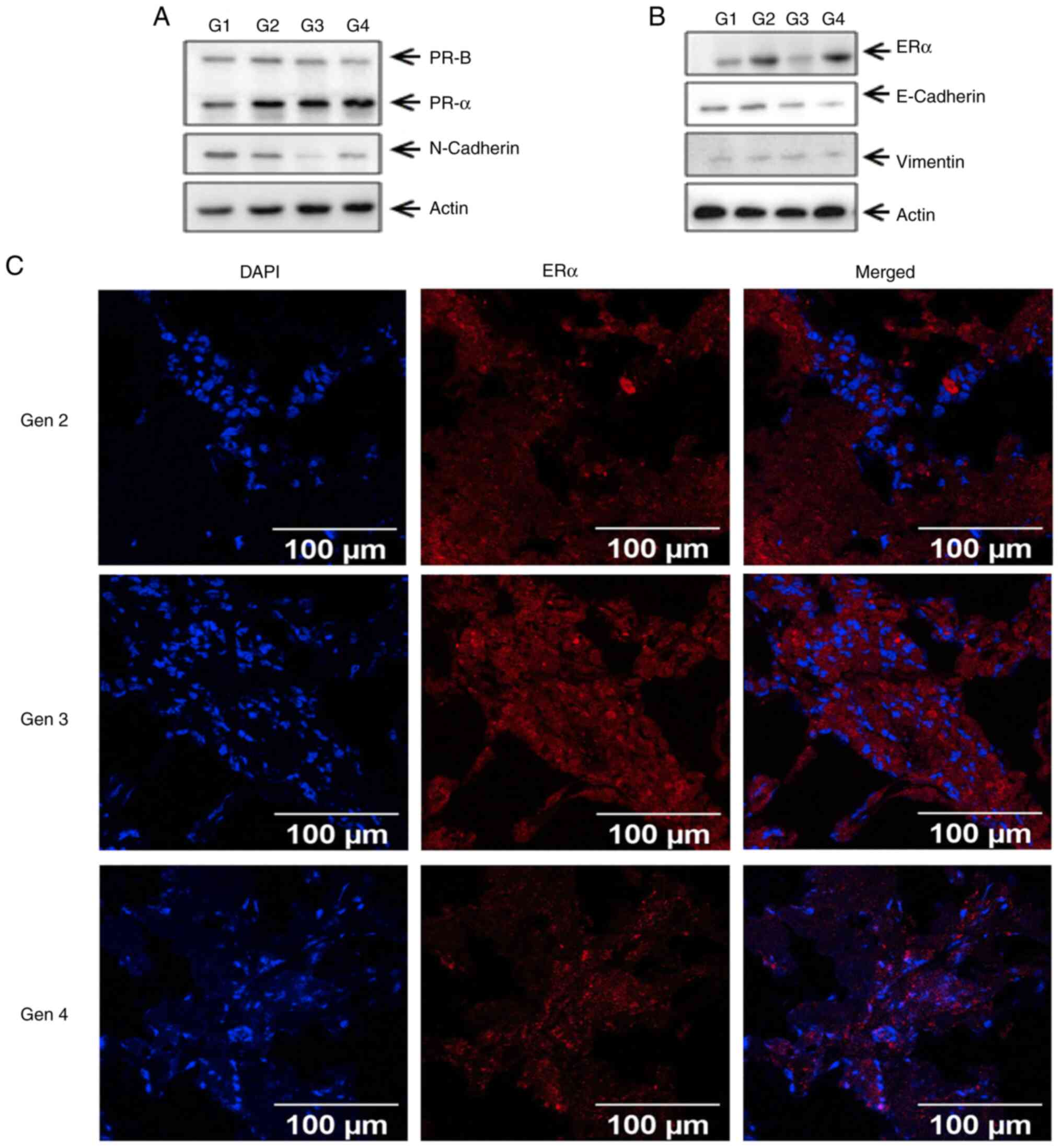
passages of PDOXs were subjected to immunofluorescence and western
blot analyses to further characterize the expression of subtype-
and epithelial-to-mesenchymal transition (EMT)-specific markers.
The results indicated that the expression levels of breast
cancer-specific markers, such as ERα and PR, as well as those of
the EMT markers, such as E-cadherin, N-cadherin and vimentin, were
similar in the different passages of PDOXs (Fig. 4A-C). To further characterize their
subtype, the primary cultures were established and
immunofluorescence analysis was performed to examine the expression
levels of ERα, PR and HER2, and those of the epithelial- and
mesenchymal-specific markers, such as E-cadherin, N-cadherin,
vimentin and α-SMA. The expression levels of subtype-specific
markers, such as ERα, PR and Ki-67, were observed in the primary
culture derived from G1 PDOXs and were mostly retained in primary
culture of G4 PDOXs. The epithelial and mesenchymal markers, such
as E-cadherin, N-cadherin, vimentin and pan-cytokeratin, were also
similar between primary cultures of G1 and G4 PDOXs (Fig. 5A and B).
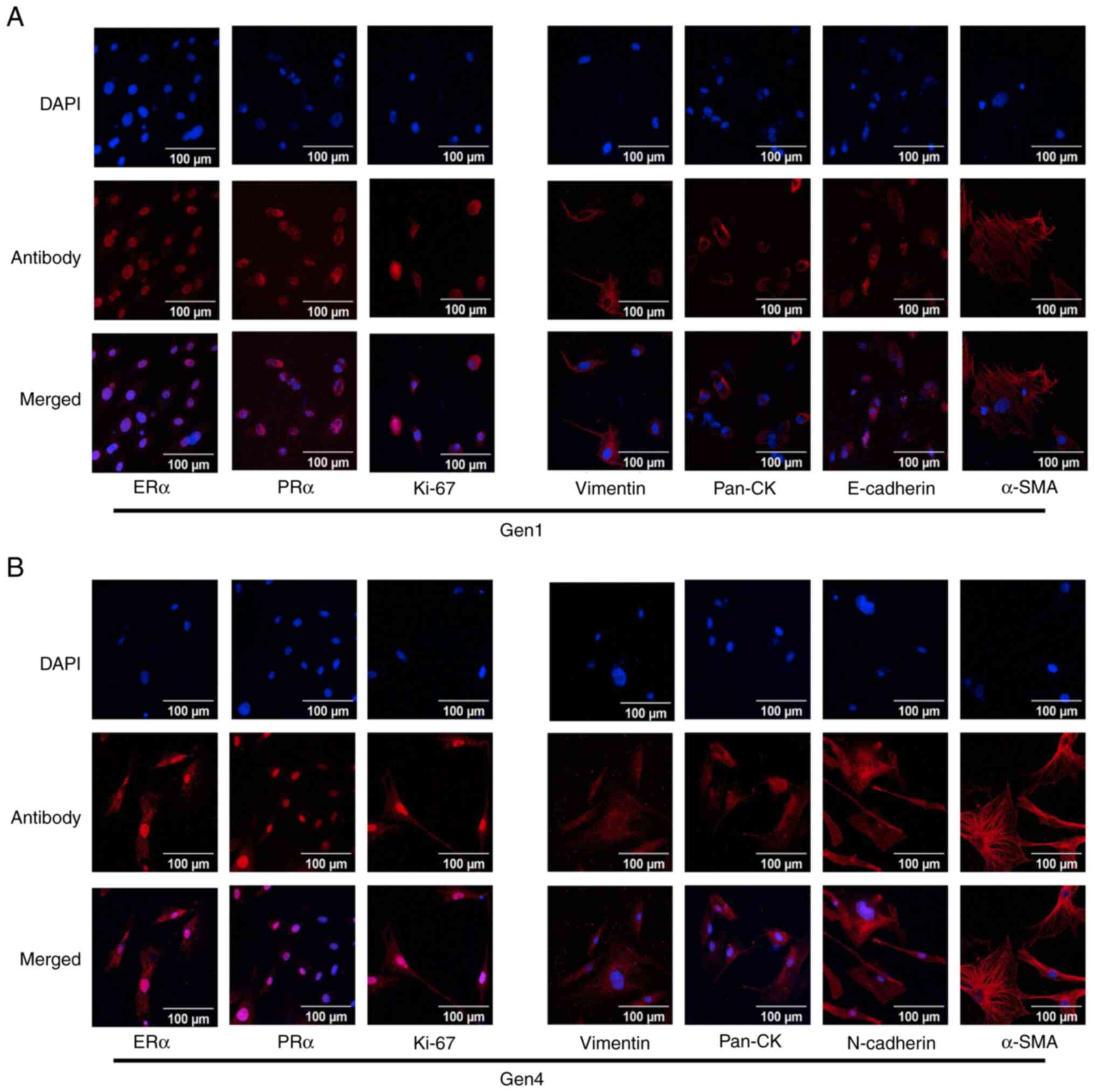 | Figure 5.Immunofluorescence analysis of primary
culture derived from PDOXs. (A and B) Established primary cultures
were seeded on coverslips and fixed. (A) Primary cultures of G1 of
PDOXs were stained with breast cancer subtype-specific markers,
such as ERα, PRα and Ki-67 and epithelial and mesenchymal markers
such as pan-CK, E-cadherin, vimentin and α-SMA. (B) Primary
cultures of G4 of PDOXs were stained with breast cancer
subtype-specific markers, ERα, PRα and Ki-67 and epithelial and
mesenchymal markers such as Pan-CK N-Cadherin, Vimentin and α-SMA.
PDOX, patient-derived orthotopic xenograft; SMA, smooth muscle
actin; ER, estrogen receptor; PR, progesterone receptor; CK,
cytokeratin. |
 | Table I.Histopathological analysis of
patient-derived orthotopic xenografts. |
Table I.
Histopathological analysis of
patient-derived orthotopic xenografts.
| Generation of
P014R | Cell infiltration in
tumor | Angiogenesis | Mitotic
activity/hpf | Abnormal mitosis | Tumor giant
cells | Nuclear
polymorphism | Tumor necrosis |
|---|
| 1 | L+; M focal | Focal | 1-2 | Occasional | Absent | ++ | ++ |
| 2 | L ++; M++ | Focal | 2-3 | Occasional | Absent | ++ | ++ |
| 3 | L++; N+ | + | 5-6 | Occasional | Absent | +++ | +++ |
| 4 | L+; N++ | ++ | 8-10 | Occasional | Absent | +++ | ++++ |
From these observations, it was suggested that the
histological and molecular features of PDOXs were mostly retained
in different passages of PDOXs. All these data demonstrated the
successful development of the hormone therapy-resistant breast
cancer PDOX models from an Indian patient. These models were
extended to four generations and their luminol-positive features
were characterized compared with those of the patient's tissues.
These models will be used for drug screening and the development of
therapeutically relevant biomarkers using proteomic and
genomic-based approaches. All of these works are in progress and
will be published soon in a separate article.
Discussion
Patients who progress following multiple rounds of
therapy constitute a major proportion of patients with cancer who
attain mortality. Such cancers are difficult to treat and lead to
rapid degeneration of patient health (18–20).
In the majority of these cases, all potential therapeutic
interventions are exhausted in the treatment of these patients.
Therefore, the development of specific models for such diseases may
allow for personalized treatment and to identify novel molecules
that may counter the disease course and improve the outcomes.
Hormone receptor-positive, HER2-Neu-negative metastatic breast
cancer is one such disease. Currently, there is a lack of
preclinical models derived from patients with the aforementioned
phenotype.
In the present study, a novel breast PDOX model was
successfully developed from a patient with hormone
receptor-positive, HER2-Neu-negative metastatic breast cancer who
progressed on multiple rounds of therapy. The model was established
by directly implanting tumor fragments into the mammary fat pad of
an immunocompromised mouse followed by serial passages. An
engraftment rate of 20% was noted for generating G1 PDOXs from
primary tumors. Previous studies have also reported an engraftment
rate of ~20% for the generation of PDXs in NOD/SCID mice (16,17),
which may be due to residual activities of natural killer cells in
these mice (17,21,22).
Different approaches were used to eliminate or suppress these cells
and increase the engraftment of the patient's tumors, including the
usage of anti-IL2 receptor antibodies or crossbreeding with
β-macroglobulin/perforin-deficient mice (17,21).
In addition, several studies reported improvements in the
engraftment rate by using additional types of immunocompromised
mice, such as NOD/Shi-SCID/IL-2Rγnull/NOD SCID gamma
(NSG) mouse, SCID/beige and BALB/c background (21,23–25).
Histopathological analysis of the patient's tumors
and the PDOXs revealed that these cellular entities have similar
morphology and histopathology grade. This is consistent with
previous reports that indicate the ability of PDXs to mirror the
histopathological features of the patient's tumor (11). However, it was observed that the
mitotic activity and necrosis were enhanced following the increased
passage number of PDOXs. This is likely due to the patient having
received multiple rounds of therapy. Decreased time required for
formation of palpable tumors and attaining tumor burden in higher
passages of PDOXs explain the increased mitotic activity as noted
in these tissues. Chen et al (26) reported that the site-specificity of
primary tumors is retained in PDOX models. To examine whether the
information of the primary tumor is highly preserved in PDOXs, the
gene expression profiles of the primary tumor and PDOXs were
analyzed using immunofluorescence analysis in primary cultures and
western blot analysis of the tumor tissue sections. The data
indicated that primary tumors and PDOXs (G1-G4) exhibited similar
expression profiles of breast-subtype-specific epithelial and
mesenchymal markers. This is consistent with the previous reports
indicating that PDOXs retain histological and molecular features of
the primary tumor. These results suggested that this model is
highly reliable for preclinical drug screening and biomarker
development. While the present study successfully developed a PDOX
model for hormone therapy-resistant breast cancer, the sample
number was limited and it was not possible to provide any
statistical data for the development of PDOX. Due to the limited
sample size of the primary tumor, the present study was confined to
histology and IHC and no other comparison studies with PDOX were
possible. In the case of the present study, liver metastasis of the
primary tumor was confirmed using histology and IHC. However, the
lack of metastatic experiments in mice is a limitation of the
present study as patient of the present study exhibited liver
metastasis. It was also not evaluated whether PDOXs reflect gene
expression patterns of primary tumors. Further studies are in
progress to determine whether the xenografts recapitulate the
mutational burden and gene expression patterns of the primary tumor
using next-generation sequencing.
In conclusion, in the present study, a PDOX model
for a hormone receptor-positive, HER2/Neu-negative metastatic
breast cancer case was successfully developed. The data indicated
that certain histological/molecular features were retained among
PDOXs as demonstrated by immunohistochemical, immunofluorescence
and western blot analyses. The data also suggest that the developed
PDOXs are a reliable pre-clinical model for the further development
of novel therapeutics.
Acknowledgements
The authors acknowledge the help of Dr Khushboo
Gandhi (Clinician Scientist Laboratory, ACTREC) in acquiring
microscopy images for patient histology. The authors also thank Dr
Tandrima Mitra and Ms Diksha Malhotra, School of Biotechnology,
KIIT University (Bhubaneswar, India) for editing the entire
manuscript.
Funding
This study was supported by a Department of Biotechnology,
Government of India-funded VNCI program (grant no.
BT/MED/30/VNCI-Hr-BRCA/2015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RB, PK, GB and SSM designed and performed most of
the experiments. RC, RK, PP and SG devised the patient recruitment
strategy, wrote the study protocol and obtained IEC approval. RB,
RC, SG and GCK drafted the manuscript. SA, RC, PP, RK, TS, AD and
SG acquired clinical samples. SA, RC, PP, RK, TS, AD and SG
followed-up patients and acquired the clinical data. RB, PK, GB and
SSM developed different generations of PDOX models and established
and characterized the primary cultures. TS characterized patient
tumor histology and performed IHC on primary patient tumor tissue
for ER and PR in the clinical setting. SG and TS analyzed the raw
data (H&E staining, ER, PR and HER2 staining) from clinical
samples of primary patient tumor at surgical resection and relapse
and confirmed the integrity of these data. RB, RC, PP, SG and GCK
revised the manuscript draft. GCK conceptualized, designed,
supervised the entire work, drafted and corrected the manuscript.
AD, SG and GCK checked and confirmed the authenticity of the raw
data. All authors have read and approved the manuscript and agreed
to be accountable for all aspects of the research. All authors are
responsible for the accuracy or integrity of any part of this
study.
Ethics approval and consent to
participate
The patient was recruited after informed consent
under IEC Study 239 (2017) on a protocol approved by Institutional
Ethics Committee III of the ACTREC. This study has been registered
in the CTRI under the registration no. CTRI/2017/11/010553. All the
animal experiments were approved by the Institutional Animal Care
and Use Committee of the National Centre for Cell Science (Pune,
India; ethics approval no. B-372).
Patient consent for publication
The patient consented to the publication of
information about her case presentation/disease course.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mathur P, Sathishkumar K, Chaturvedi M,
Das P, Sudarshan KL, Santhappan S, Nallasamy V, John A, Narasimhan
S and Roselind FS: ICMR-NCDIR-NCRP Investigator Group: Cancer
statistics, 2020: Report from national cancer registry programme,
India. JCO Glob Oncol. 6:1063–1075. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malvia S, Bagadi SA, Dubey US and Saxena
S: Epidemiology of breast cancer in Indian women. Asia Pac J Clin
Oncol. 13:289–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehrotra R and Yadav K: Breast cancer in
India: Present scenario and the challenges ahead. World J Clin
Oncol. 13:209–218. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chakraborty S and Rahman T: The
difficulties in cancer treatment. Ecancermedicalscience.
6:ed162012.PubMed/NCBI
|
|
5
|
Tentler JJ, Tan AC, Weekes CD, Jimeno A,
Leong S, Pitts TM, Arcaroli JJ, Messersmith WA and Eckhardt SG:
Patient-derived tumour xenografts as models for oncology drug
development. Nat Rev Clin Oncol. 9:338–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillet JP, Calcagno AM, Varma S, Marino M,
Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, et al:
Redefining the relevance of established cancer cell lines to the
study of mechanisms of clinical anti-cancer drug resistance. Proc
Natl Acad Sci USA. 108:18708–18713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burdall SE, Hanby AM, Lansdown MR and
Speirs V: Breast cancer cell lines: Friend or foe? Breast Cancer
Res. 5:89–95. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cifani P, Kirik U, Waldemarson S and James
P: Molecular portrait of breast-cancer-derived cell lines reveals
poor similarity with tumors. J Proteome Res. 14:2819–2827. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manning HC, Buck JR and Cook RS: Mouse
models of breast cancer: Platforms for discovering precision
imaging diagnostics and future cancer medicine. J Nucl Med. 57
(Suppl 1):60S–68S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo S, Qian W, Cai J, Zhang L, Wery JP and
Li QX: Molecular pathology of patient tumors, patient-derived
xenografts, and cancer cell lines. Cancer Res. 76:4619–4626. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Braekeveldt N, von Stedingk K, Fransson S,
Martinez-Monleon A, Lindgren D, Axelson H, Levander F, Willforss J,
Hansson K, Øra I, et al: Patient-derived xenograft models reveal
intratumor heterogeneity and temporal stability in neuroblastoma.
Cancer Res. 78:5958–5969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matossian MD, Burks HE, Bowles AC, Elliott
S, Hoang VT, Sabol RA, Pashos NC, O'Donnell B, Miller KS, Wahba BM,
et al: A novel patient-derived xenograft model for claudin-low
triple-negative breast cancer. Breast Cancer Res Treat.
169:381–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramani VC, Lemaire CA, Triboulet M, Casey
KM, Heirich K, Renier C, Vilches-Moure JG, Gupta R, Razmara AM,
Zhang H, et al: Investigating circulating tumor cells and distant
metastases in patient-derived orthotopic xenograft models of
triple-negative breast cancer. Breast Cancer Res. 21:982019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pandrangi SL, Raju Bagadi SA, Sinha NK,
Kumar M, Dada R, Lakhanpal M, Soni A, Malvia S, Simon S, Chintamani
C, et al: Establishment and characterization of two primary breast
cancer cell lines from young Indian breast cancer patients:
Mutation analysis. Cancer Cell Int. 14:142014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chakraborty G, Jain S and Kundu GC:
Osteopontin promotes vascular endothelial growth factor-dependent
breast tumor growth and angiogenesis via autocrine and paracrine
mechanisms. Cancer Res. 68:152–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhimani J, Ball K and Stebbing J:
Patient-derived xenograft models-the future of personalised cancer
treatment. Br J Cancer. 122:601–602. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okada S, Vaeteewoottacharn K and Kariya R:
Application of highly immunocompromised mice for the establishment
of patient-derived xenograft (PDX) models. Cells. 8:8892019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kessous R, Wissing MD, Laskov I, Abitbol
J, Bitharas J, Agnihotram VR, Yasmeen A, Salvador S, Lau S and
Gotlieb WH: Multiple lines of chemotherapy for patients with
high-grade ovarian cancer: Predictors for response and effect on
survival. Int J Cancer. 148:2304–2312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanker LC, Loibl S, Burchardi N, Pfisterer
J, Meier W, Pujade-Lauraine E, Ray-Coquard I, Sehouli J, Harter P
and du Bois A: AGO and GINECO study group: The impact of second to
sixth line therapy on survival of relapsed ovarian cancer after
primary taxane/platinum-based therapy. Ann Oncol. 23:2605–2612.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park IH, Lee KS and Ro J: Effects of
second and subsequent lines of chemotherapy for metastatic breast
cancer. Clin Breast Cancer. 15:e55–e62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shultz LD, Schweitzer PA, Christianson SW,
Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV,
Greiner DL, et al: Multiple defects in innate and adaptive
immunologic function in NOD/LtSz-scid mice. J Immunol. 154:180–191.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chijiwa T, Kawai K, Noguchi A, Sato H,
Hayashi A, Cho H, Shiozawa M, Kishida T, Morinaga S, Yokose T, et
al: Establishment of patient-derived cancer xenografts in
immunodeficient NOG mice. Int J Oncol. 47:61–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shultz LD, Goodwin N, Ishikawa F, Hosur V,
Lyons BL and Greiner DL: Human cancer growth and therapy in
immunodeficient mouse models. Cold Spring Harb Protoc.
2014:694–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown KM, Xue A, Mittal A, Samra JS, Smith
R and Hugh TJ: Patient-derived xenograft models of colorectal
cancer in pre-clinical research: A systematic review. Oncotarget.
7:66212–66225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mosier DE, Stell KL, Gulizia RJ, Torbett
BE and Gilmore GL: Homozygous scid/scid; beige/beige mice have low
levels of spontaneous or neonatal T cell-induced B cell generation.
J Exp Med. 177:191–194. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Pan X, Zhang YH, Hu X, Feng K,
Huang T and Cai YD: Primary tumor site specificity is preserved in
patient-derived tumor xenograft models. Front Genet. 10:7382019.
View Article : Google Scholar : PubMed/NCBI
|