Culture of circulating tumor cells using a microfilter device
- Authors:
- Published online on: March 31, 2023 https://doi.org/10.3892/or.2023.8538
- Article Number: 101
-
Copyright: © Furukawa et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Circulating tumor cells (CTCs) are malignant cells, which may metastasize from a primary site to a secondary site (1,2). Their number may serve as a prognostic marker in cancer patients, since increases in CTCs after treatment indicate therapy failure (3–6). Of note, to achieve the goal of personalized therapy, CTC phenotype details are more important than total counts (7–9). For this reason, the analysis of CTCs in liquid biopsy enables us to improve the management of therapies for patients with cancer and elucidate the mechanisms of cancer metastasis.
Conventional technologies mainly rely on antibody-based methods for detecting CTCs with an epithelial phenotype, since epithelial-mesenchymal transition (EMT) has a core role in promoting metastasis by facilitating high mobility and invasiveness (10,11). Since CTCs in the bloodstream gain a mesenchymal phenotype, current antibody-based methods (including the CellSearch system, which detects CTCs based on epithelial characteristics), have decreased detection sensitivity in these cases. Therefore, sized-based CTC isolation methods were developed to overcome antibody-based limitations and enable EMT-independent detection of CTC (12–14).
For such blood-based cell analyses to work, in vitro expansion is required, as the analysis of physiological functions, such as drug sensitivity, requires a large number of cells and whole blood may only contain a small number of total cells in circulation. Several studies have attempted to cultivate CTCs isolated from whole blood; however, the periods of such reported in vitro CTC cultures are short (15–17). The establishment of long-term CTC cultures therefore remains an urgent issue. The purpose of the present study was thus to establish a novel approach to culture captured CTCs using a microfilter device. The primary aim was to reduce damage to cultured CTCs captured on the filter and investigate the effects of the filter material and a culture medium exchange method on long-term culture viability. It was hypothesized that the less invasive peripheral collection and microfiltering are able to rapidly isolate and culture CTCs (including CTC clusters), which may be subjected to functional and genome analyses to better predict treatment responses and design antitumor strategies.
Materials and methods
Study design
The present study was a prospective study performed in patients with pancreatic cancer at the University of Tsukuba Hospital (Tsukuba, Japan). A total of 15 whole-blood specimens from patients with pancreatic cancer were collected between October 2021 and April 2022. The study protocols were approved by the Institutional Review Board of the University of Tsukuba Hospital (Tsukuba, Japan; approval no. H30-150) and all patients provided written informed consent.
Single and cluster CTC isolation
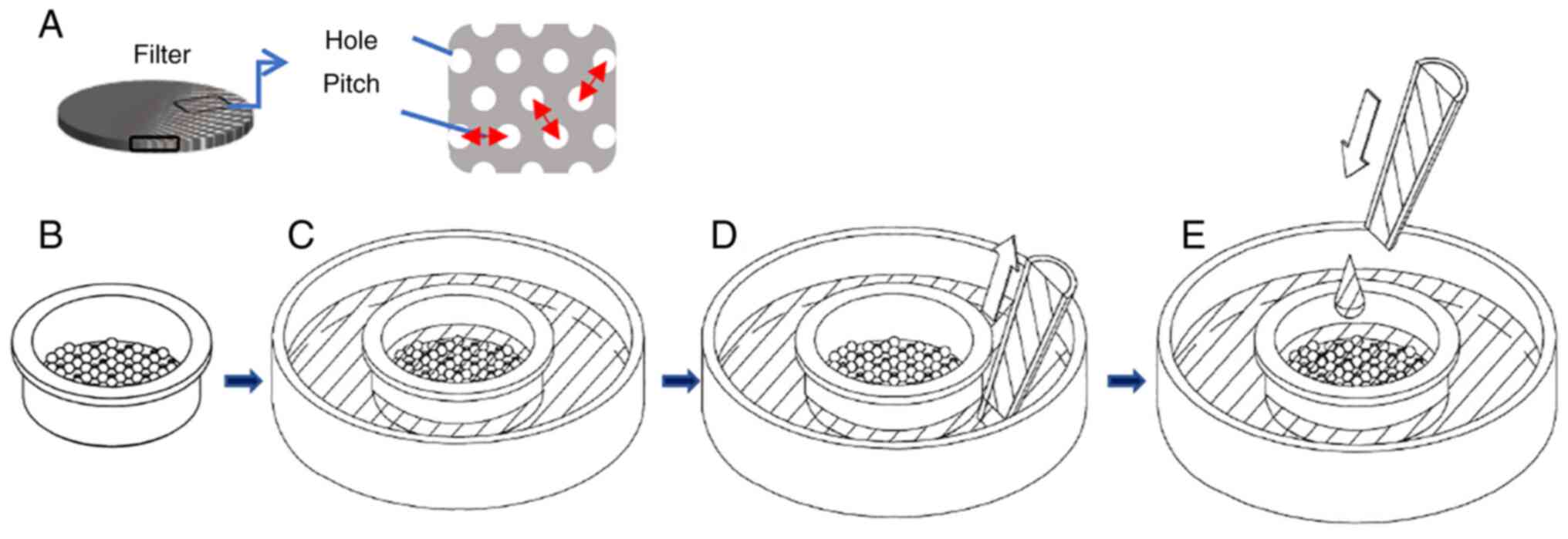
From each patient, 5 ml whole blood was collected into an EDTA blood collection tube (Venoject II vacuum blood collection tube; Terumo) and aspirated with a 10-ml syringe (SS-10SZ; Terumo), and the syringe was attached to an automatic pump (KDS100; KD Scientific, Inc.). The following operations were all performed at a flow rate of 50 ml/h. A 13-mm diameter 15±0.5-µm pore filter (φ15-RM-P30d-t10; Optnics Precision) and a 8±0.5-µm pore filter (φ8-RM-P17d-t10; Optnics Precision) were respectively mounted on the filter holders (CSS-00352; Optnics Precision), and these two filter devices were connected in tandem to the syringe containing the sample. Blood samples were passed through the 15-µm pore filter and 8-µm pore filter, and cells were collected by each filter. Subsequently, the filter was washed by flushing with 10 ml PBS/2 mM EDTA. In cases 1 to 6, 2 ml of staining solution [PE-conjugated mouse anti-human epithelial cellular adhesion molecule (EpCAM; 1:200 dilution; IgG2b clone 9C4; cat. no. 324206; BioLegend) and Hoechst 33342 (1:2,000 dilution; cat. no. H3570; Invitrogen; Thermo Fisher Scientific, Inc.) in PBS], was added and incubated at room temperature for 30 min. Subsequently, the filter was washed by flushing with 10 ml PBS. Finally, the filter device was removed from the syringe and placed on the 35-mm dish (MS-10350; Sumitomo Bakelite Co., Ltd.) in all cases. The cells on the filter were cultured at 37°C in a humidified atmosphere containing 5% CO2 in Iscove's Modified Dulbecco's Media (IMDM; cat. no. 098-06465; Fujifilm) 1:1 with Nutrient Mixture F-12 Ham (cat. no. N6658; Sigma-Aldrich; Merck KGaA), 20% FBS (cat. no. 35-079-CV; Corning, Inc.), Matrigel® (cat. no. 354234; Corning, Inc.), recombinant mouse EGF (cat. no. 053-07751) and recombinant human basic fibroblast growth factor (FGF; cat. no. 064-04541; both from Fujifilm) and B27 (cat. no. 17504-044; Gibco; Thermo Fisher Scientific, Inc.). The culture medium was made to flow between the inside and outside of the filter device. The culture medium was exchanged by aspirating from the outside of the filter device and then adding the medium to the inside of the filter device (Fig. 1). After being cultured, the cells were washed 3 times with 200 µl PBS and 200 µl staining solution (PE-conjugated mouse anti-human EpCAM and Hoechst 33342, as specified above) was added and the mixture was incubated at room temperature for 30 min. For cases 14 and 15, 200 µl of staining solution (Calcein-AM; 1:2,000 dilution; cat. no. 341-07901; Dojindo; and PE-conjugated mouse anti-human EpCAM-PE; 1:200 dilution; cat. no. 324206; clone 9C4; Biolegend; in PBS) was added and cells were incubated at room temperature for 30 min. After the above-mentioned staining, the cells were washed 3 times with 200 µl PBS.
Enumeration of CTCs, CTC clusters and CTC colonies
The collected filter was placed on a 35-mm dish. For observation, an automatic fluorescence microscope (MSZ25; Nikon Corporation) was used and an image of the entire area of the filter was acquired with a ×10 objective lens (excitation wavelengths of 395, 472 and 545 nm and emission wavelengths of 460, 520 and 615 nm), before automatically generating a single image file (software: Nikon NIS-Elements BR v.5.21; Nikon Corp.).
Results
Patient and tumor characteristics
The clinicopathological characteristics of the patients are presented in Table I. The age range of the 15 cases included was 44–87 years with a median age of 74 years. The cohort comprised 6 males and 9 females. The histologic type was invasive ductal adenocarcinoma in 13 cases and intraductal papillary mucinous carcinoma in 2 cases. The blood was collected from all patients just before the surgery. Among the 15 patients, 4 patients with borderline resectable or unresectable locally advanced pancreatic cancer received pre-operative treatment, 2 patients received systemic chemotherapy and 14 underwent a course of gemcitabine/nab-paclitaxel and 2 cycles of gemcitabine (1,000 mg/m2) with S1 oral intake prior to surgery. A total of 2 patients received chemoradiotherapy, including 2 cycles of gemcitabine infusion with concurrent proton beam radiation (67.5 Gy) (Table I).
Enumeration of single CTCs, CTC clusters and CTC colonies
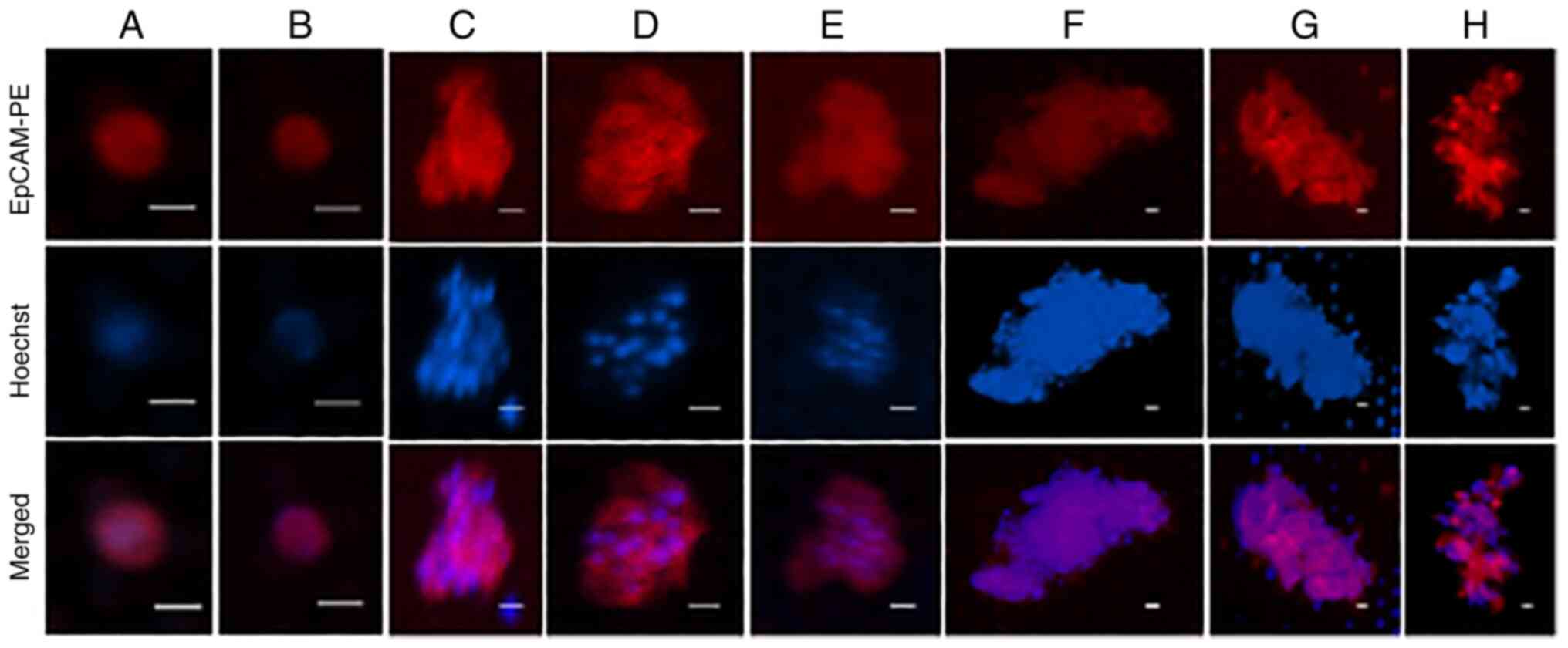
CTCs isolated on the precision microfiltration membrane with 8- and 15-µm pore size were cultured, and enumeration and evaluation of the state of the cancer cells on the filter was performed by fluorescent labeling in the real-time (approximate culture time: 0, 25, 45 and 100 days). CTCs captured on the filter were cultured using IMDM-F12 medium containing 20% FBS, −2% Matrigel, EGF, bFGF and B27. A CTC was defined as a single, intact round oval cell with a visible nucleus (Hoechst 33342-positive) that stained positive with anti-EpCAM (Fig. 2A and B). Clusters were defined as aggregates of two or more CTCs and <50 µm in the long diameter (Fig. 2C-E), and colonies were defined as aggregates of >50 µm in the long diameter (Fig. 2F-H). The numbers of single CTCs, clusters and colonies for cases 1–6, which were observed at three sequential time-points, are provided in Table II. In several cases, there were no CTCs immediately after filtration, but single cells, clusters and colonies were detected after cultivation in each case. The numbers of single CTCs, clusters and colonies for cases 7–13, after culturing for different durations, at one time-point, are presented in Table III.
Table II.Enumeration of single CTCs, CTC clusters and CTC colonies for cases 1–6 at three sequential points. |
Table III.Enumeration of single CTCs, CTC clusters and CTC colonies for cases 7–13 at one point of measurement only. |
Activity of cultured CTCs
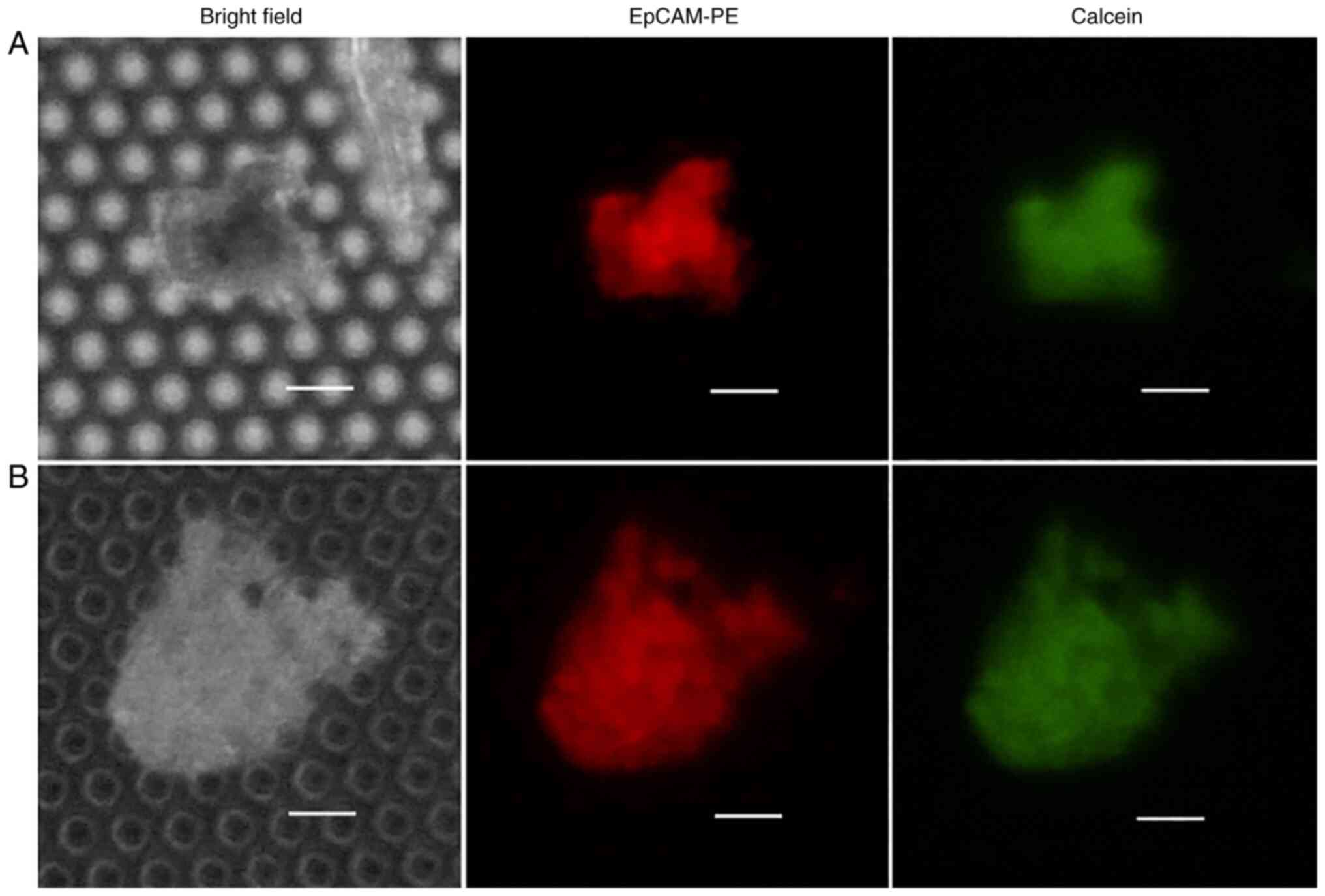
The number and state of cancer cells on the filter were evaluated by staining with Calcein AM to confirm cultured CTC activity. The cells were observed after exposing them to Calcein AM in cases 14 and 15. The EpCAM-positive cells cultured on the filter stained with Calcein AM were considered viable CTCs (Table IV, Fig. 3).
Discussion
In the present study, a culture system was constructed using a microfilter device to isolate and culture both CTC and CTC clusters simultaneously from peripheral blood samples of patients with pancreatic cancer. In addition, staining was performed to confirm cell viability in clinical practice.
Long-term in vitro CTC culturing poses multiple challenges, particularly with regard to cellular viability after filtering. Yusa et al (18) reported that filters electroformed from pure Pd or a Pd alloy (Pd/Nickel, 4:1) had lower toxicity to cultured cells during three days of culture than a pure nickel filter. However, while an electroformed Ni-Pd alloy does not elute toxic nickel, the cells on the filter may be damaged when the medium is exchanged. Therefore, in the present study, a method for exchange, in which the CTCs would not detach from the filter, was devised. In this system, the medium was aspirated from the outside of the filter device with a pipette, followed by the addition of medium directly into the filter device for replacement of the medium. In this way, the cells remained adsorbed on the filter and were able to be viable over a long culturing period.
CTCs reduce or eliminate the expression of EpCAM through EMT to acquire invasive potential. Our group previously developed a simple and inexpensive system for accurate detection, in which CTCs are concentrated by using a microfilter and 5-aminolevulinic acid (12). Kitz et al (13) have additionally developed EMT-independent CTC enumeration and harvesting protocols using the Parsortix®. In the present study, CTCs were not detected immediately after filtering the blood, presumably due to attenuated EpCAM expression in certain cases, but were able to be eventually detected after recovering the epithelial phenotype in the process of culturing on the filter. In CTCs undergoing EMT, the transition may be reversed by attachment on the filter. Furthermore, this result indicates that size-based CTC isolation methods may capture EMT-independent CTC.
To confirm whether cultured cancer cells on the filter retain cell viability, Calcein AM staining was used, indicating a positive result. This means that cultured cells proliferating from cancer cells may be analyzed for morphological and genetic markers of cancer. Long-term culture of filtered CTCs may result in a more specific analysis of the physiological characteristics of these cells, which, in turn, will translate into individualized pancreatic cancer treatments.
The present data are limited by the small sample size and focus on only pancreatic cancer. It has been reported that the size of CTCs varies depending on the cancer site (19); therefore, it is necessary to examine the filter pore size for each cancer type. Future studies require to be prospectively considered in larger populations.
In conclusion, a non-invasive, size-based filtering modality was developed in the present study, which is able to effectively isolate and culture CTCs and CTC clusters from clinical patients with cancer to characterize these peripheral cells for therapy response predictions. Future validation studies will serve to facilitate functional and genomic analyses, such as in patient-specific anticancer drug susceptibility testing.
Acknowledgements
The authors would like to thank Dr Seishin Kinuta, Dr Yoshiyuki Ichinomiya and Dr Masashi Kobayashi (Optnics Precision Co., Ltd.) for useful experimental suggestions. Preliminary data of this paper (abstract no. e16230) were presented at the 2022 American Society of Clinical Oncology annual meeting (June 3–6, 2022, Chicago, USA). The authors would like to thank Dr Bryan J. Mathis of the University of Tsukuba Hospital International Medical Center (Tsukuba, Japan) for language revision.
Funding
This work was supported by JSPS KAKENHI (grant no. 21H02997) and a research grant from Optnics Precision Co., Ltd.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AF: Methodology (equal); formal analysis (equal); investigation (lead); data curation (lead); writing-original draft (lead); visualization (equal). TM: Methodology (equal); investigation (equal); resources (equal); writing-original draft (equal). OS: Investigation (equal); writing-review & editing (equal). KA: Investigation (equal); writing-review & editing (equal). TO: Investigation (equal); writing-review & editing (equal). SM: Conceptualization (equal); methodology (equal); resources (equal); writing-review & editing (equal); supervision (lead); project administration (equal); funding acquisition (equal).
Ethics approval and consent to participate
The study protocols were approved by the Institutional Review Board of the University of Tsukuba Hospital (Tsukuba, Japan; approval no. H30-150) and all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
SM received a research grant and free filters and filter devices provided for from Optnics Precision Co., Ltd. The other authors have no competing interests to disclose.
Glossary
Abbreviations
Abbreviations:
|
IPD |
invasive ductal carcinoma |
|
IPMC |
intraductal papillary mucinous carcinoma |
|
PDAC |
pancreatic ductal adenocarcinoma |
|
DP |
distal pancreatectomy |
|
TP |
total pancreatectomy |
|
SSpPD |
subtotal stomach-preserving pancreatoduodenectomy |
|
Lap-DP |
laparoscopic distal pancreatectomy |
References
|
Paoletti C and Hayes DF: Circulating tumor cells. Adv Exp Med Biol. 882:235–258. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Pantel K and Brakenhoff RH: Dissecting the metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW and Hayes DF: Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Cohen SJ, Plunt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al: Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 31:3213–3221. 2008. View Article : Google Scholar | |
|
Matsusaka S, Suenaga M, Mishima Y, Kuniyoshi R, Takagi K, Terui Y, Mizunuma N and Hatake K: Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci. 102:1188–1192. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Matsusaka S, Chìn K, Ogura M, Suenaga M, Shinozaki E, Mishima Y, Terui Y, Mizunuma N and Hatake K: Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 101:1067–1071. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Mishima Y, Matsusaka S, Chin K, Mikuniya M, Minowa S, Takayama T, Shibata H, Kuniyoshi R, Ogura M, Terui Y, et al: Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target Oncol. 12:341–351. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Matsusaka S, Hanna DL, Ning Y, Yang D, Cao S, Berger MD, Miyamoto Y, Suenaga M, Dan S, Mashima T, et al: Epidermal growth factor receptor mRNA expression: A potential molecular escape mechanism from regorafenib. Cancer Sci. 111:441–450. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kozuka M, Battaglin F, Jayachandran P, Wang J, Arai H, Soni S, Zhang W, Hirai M, Matsusaka S and Lenz HJ: Clinical significance of circulating tumor cell induced epithelial-mesenchymal transition in patients with metastatic colorectal cancer by single-cell RNA-sequencing. Cancers (Basel). 13:48622021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al: Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 339:580–584. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Nieto MA: Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 342:12348502013. View Article : Google Scholar : PubMed/NCBI | |
|
Matsusaka S, Kozuka M, Takagi H, Ito H, Minowa S, Hirai M and Hatake K: A novel detection strategy for living circulating tumor cells using 5-aminolevulinic acid. Cancer Lett. 355:113–120. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kitz J, Goodale D, Postenka C, Lowes LE and Allan AL: EMT-independent detection of circulating tumor cells in human blood samples and pre-clinical mouse models of metastasis. Clin Exp Metastasis. 38:97–108. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sonoda T, Yanagitani N, Suga K, Yoshizawa T, Nishikawa S, Kitazono S, Horiike A, Shiba K, Ishizuka T, Nishio M and Matsusaka S: A novel system to detect circulating tumor cells using two different size-selective microfilters. Anticancer Res. 40:5577–5582. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z, et al: Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 345:216–220. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Brungs D, Minaei E, Pipper AK, Perry J, Splitt A, Carolan M, Ryan S, Wu XJ, Corde S, Tehei M, et al: Establishment of novel long-term cultures from EpCAM positive and negative circulating tumour cells from patients with metastatic gastroesophageal cancer. Sci Rep. 10:5392020. View Article : Google Scholar : PubMed/NCBI | |
|
Carmona-Ule N, González-Conde M, Abuín C, Cueva JF, Palacios P, López-López R, Costa C and Dávila-Ibáñez AB: Short-term ex vivo culture of CTCs from advance breast cancer patients: Clinical implications. Cancers (Basel). 13:26682021. View Article : Google Scholar : PubMed/NCBI | |
|
Yusa A, Toneri M, Masuda T, Ito S, Yamamoto S, Okochi M, Kondo N, Iwata H, Yatabe Y, Ichinosawa Y, et al: Development of a new rapid isolation device for circulating tumor cells (CTCs) using 3D palladium filter and its application for genetic analysis. PLoS One. 9:e888212014. View Article : Google Scholar : PubMed/NCBI | |
|
Mendelaar PAJ, Kraan J, Van M, Zeune LL, Terstappen LWMM, Oomen-de Hoop E, Martens JWM and Sleijfer S: Defining the dimensions of circulating tumor cells in a large series of breast, prostate, colon, and bladder cancer patients. Mol Oncol. 15:116–125. 2021. View Article : Google Scholar : PubMed/NCBI |