Introduction
Liver cancer is the fifth most prevalent cancer and
the second most common cause of tumor-related deaths worldwide
(1,2). China accounts for approximately 50% of
the world's new cases and deaths regarding this disease (3). The main etiological factors of liver
cancer include hepatitis B virus, hepatitis C virus, aflatoxin
contamination and alcoholic liver disease. The current clinical
treatment of liver cancer is lacking due to the low curative ratio
and high recurrence rate. Despite their importance as a treatment
method, chemotherapeutic drugs have numerous serious side effects;
therefore, developing natural agents with improved therapeutic
efficacy and low toxicity, to combat liver cancer, is
essential.
Saikosaponin (SS) is the main active component of
Radix Bupleuri, accounting for ~7% of the total dry weight of the
roots of Bupleurum chinense DC. It possesses many important
pharmacological activities, including immune regulation (4), liver protection (5), liver fibrosis inhibition as well as
anti-inflammatory (6), antiviral
(7) and antitumor (8) activities. SS's antitumor activity
regulates fundamental cellular processes, such as S-phase DNA
synthesis, protein metabolism, proliferation and apoptosis. Several
monomers have been identified in SS, including SSa, SSb1, SSb2,
SSc, SSd and SSe, based on their different chemical structures.
Numerous studies have demonstrated that SSa and SSd have potent
antitumor effects (8). However,
little is known about SSb2′s effect on liver protection and cancer
prevention. We previously demonstrated that SSb2 significantly
mitigated LPS/GalN-induced acute liver injury in mice. This effect
may be attributable to decreased NF-κB and increased Sirt-6 protein
expression levels, both of which improve inflammatory injury and
energy metabolism (9). Further
study on SSb2′s role in liver cancer is crucial for the development
of safe and effective new anticancer agents.
Liver cancer is a highly vascularized solid tumor in
which the growth of new blood vessels continuously supplies oxygen
and nutrients to tumor cells (10).
As a prerequisite for continued tumor growth, angiogenesis
significantly contributes to liver cancer development. Recent
cancer research studies have concentrated on anti-angiogenesis as a
novel approach for the treatment of cancers with poor prognoses
(11,12). Antiangiogenic therapy has become an
important adjunct to conventional chemotherapy in the treatment of
many solid tumors (13,14). The vascular endothelial growth
factor (VEGF) is a key regulator of angiogenesis and its expression
is closely associated with liver cancer (15). In most experimental systems,
hypoxia-inducible factor-1α (HIF-1α) regulates VEGF transcription
(10,16). HIF-1α is closely associated with
important aspects of tumor biology, such as angiogenesis, invasion,
glucose metabolism and cell survival, and it is overexpressed in
many human cancers (17). Notably,
VEGF/HIF-1α is the target of many anti-liver cancer drugs, which
are considered a promising treatment strategy for liver cancer
(18–20). The present study evaluated the role
of SSb2 in liver cancer to determine whether SSb2 suppresses liver
cancer development through regulation of the expression of proteins
involved in angiogenic pathways.
Materials and methods
Materials and reagents
SSb2 was purchased from Chengdu Must Bio-Technology
Co., Ltd. Doxorubicin (DOX) was purchased from Haizheng
Pharmaceutical Co., Ltd. Dulbecco's modified eagle medium (DMEM)
was purchased from Gibco (Thermo Fisher Scientific, Inc.). Trypase
and methylthiazolyl tetrazolium bromide (MTT) were purchased from
MilliporeSigma. The BCA Protein Assay Kit and Super Signal West
Pico Chemiluminescent Substrate were purchased from Pierce (Thermo
Fisher Scientific, Inc.). Radioimmunoprecipitation assay (RIPA)
Lysis Buffer and 10× poly-L-lysine were purchased from Beijing
Solarbio Science & Technology Co., Ltd. Antibodies against CD34
(1:10,000, cat. no. 60180-1-Ig), VEGF (1:500, cat. no. 19003-1-AP),
MMP9 (1:500, cat. no. 10375-2-AP) and β-actin (1:1,000, cat. no.
CL594-66009) were purchased from Wuhan Sanying Biotechnology;
antibodies against MMP2 (1:500, cat. no. TA806846) and ERK1/2
(1:500, cat. no. TA325139) were purchased from Origene
Technologies, Inc.; and antibodies against p-ERK1/2 (1:500, cat.
no. sc-7383), mouse anti-rabbit IgG-HRP (1:1,000, cat. no. sc-2357)
and goat anti-mouse IgG-HRP (1:1,000, cat. no. sc-2005) were
purchased from Santa Cruz Biotechnology, Inc.
Animals and ethics statement
A total of 50 Male Balb/c mice weighing 18–22 g were
purchased from the Experimental Animal Center of The Medical
College of Henan University of Science and Technology. The
Experimental Animal Ethics Committee of Henan University of Science
and Technology approved the experimental protocols involving
animals and fertilized chicken eggs (approval no. 20200519). All
animal experiments were performed according to The National Act on
the Use of Experimental Animals (China). Appropriate measures were
taken to minimize the use and suffering of animals.
Cell culture
The human liver cancer cell line HepG2 was purchased
from the Shanghai Institutes for Biological Sciences (cat. no.
SNL-083). Human umbilical vein endothelial cells (HUVECs) were
purchased from Procell Life Science & Technology Co., Ltd.(cat.
no. CL-0675). H22 liver cancer cells were purchased from the Henan
Institute of Medical Sciences (cat. no. CL-0341). The identify of
each cell line was confirmed using short tandem repeat profiling
analysis. HepG2 and HUVECs were cultured in high-glucose DMEM
(Gibco; Thermo Fisher Scientific, Inc.) and H22 cells were cultured
in Roswell Park Memorial Institute 1640 medium (Gibco; Thermo
Fisher Scientific, Inc) both supplemented with 10% fetal bovine
serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.). All cells were
incubated at 37°C in a 5% CO2, humidified
atmosphere.
Cell viability assay
HepG2 liver cancer cells and HUVECs were seeded at a
density of 2.5×104 cells/well in 96-well plates for 24 h
at 37°C in a 5% CO2 incubator before treatment with
different SSb2 concentrations at 37°C. After 20 h of culturing with
SSb2, 20 µl MTT (5 mg/ml) was added to each well, followed by
incubation for 4 h at 37°C. Then, 200 µl dimethyl sulfoxide was
added to each well after removing the supernatant. The final
absorbance was measured at a wavelength of 490 nm using a
microplate reader.
H22 liver cancer transplanted tumor
model
Mice were housed in a sterile environment at 22±1°C,
a relative humidity of 40–70% and a 12 h light/dark cycle. The mice
had free access to rodent chow and drinkable water. The H22 model
was established through subcutaneous injection, as described
previously (21,22). The suspension of H22 liver cancer
cells was adjusted to a density of 1×106/ml, and 0.2 ml
of H22 liver cancer cell suspensions were subcutaneously inoculated
into the right armpit region of each mouse. After 24 h, the mice
were randomly divided into 5 groups (n=10) as follows: The control
group [normal saline, intraperitoneal (i.p.) injection, once a
day], SSb2-low, middle and high dose groups (5, 10 and 20
mg/kg/day, respectively, i.p. injection, once a day) and the
positive control group (DOX, 2 mg/kg/day, i.p. injection, once
every two days). All animals were treated for 10 days. The mice's
health and behavior were monitored and recorded daily. On the 10th
day, blood samples were collected to perform a white blood cell
count using a cell counting plate. Isoflurane was administered via
inhalation as anesthesia with an induction concentration of 4–5%
and a maintenance concentration of 2–3%. At the study endpoint all
mice were euthanized by cervical dislocation under anesthesia.
Humane endpoints were identified as xenograft tumor diameter >20
mm, the xenograft tumor weight >10% of the animal's body weight,
body weight loss due to tumor growth >20% of the animal's body
weight, or an animal was deemed to be in poor health and did not
eat. Observations of pupil dilation and cessation of heartbeat and
breath were used to confirm the animal's death. Following
euthanasia, the tumors were carefully isolated and processed for
further analysis. Tumor tissues, the thymus and the spleen were
collected and weighed in order to calculate the tumor inhibitory
rate and organ index, as follows: Tumor growth inhibition rate
(%)=1-(tumor weight in SSb2 or DOX group/tumor weight in control
group) ×100; and organ index=organ weight (mg)/body weight (g).
Hematoxylin and eosin (H&E)
staining, CD34 immunostaining and microvessel density (MVD)
counting
The tumor tissues were fixed using a 10%
formaldehyde solution for 12 h at room temperature, followed by
rinsing in running water for 12 h. The fixed tumor tissues were
then dehydrated using increasing ethanol concentrations, embedded
in paraffin and sectioned to a 4 µm thickness. The tumor sections
were stained using H&E for histologic examination. In this
process, the paraffin embedded sections were placed into xylene two
successively times for dewaxing, for 15 min each at room
temperature. Then the sections were successively placed in 100,
100, 95, 80 and 70% ethanol, and double distilled water, 5 min each
at room temperature. The tissue sections were stained with
hematoxylin for 2 min and eosin for 1.5 min, both at room
temperature. After that, the sections were dehydrated in 70, 80 and
95% ethanol, followed by immersion in absolute ethanol twice and
xylene twice for treatment, 3 min each at room temperature.
Finally, the sections were sealed using neutral resin. Microscopic
images were captured using a light microscope (Olympus
Corporation). For immunohistochemistry staining, tumor sections
were placed into a 60°C incubator for 60 min, and then the slides
were dewaxed twice in xylene (the first for 30 min and the second
for 15 min). Then, the slides were rehydrated using decreasing
ethanol concentrations. The slides were then incubated in trypsin
solution (trypsin solution:trypsin diluent, 1:3, MilliporeSigma)
for 30 min at 37°C, and then washed in phosphate-buffered saline
(PBS) three times. The slides were incubated with 3%
H2O2 solution for 15 min at room temperature,
and after washed three times in PBS, goat serum was used for
blocking at 37°C for 20 min. The primary antibody (anti-CD34
antibody, 1:50, cat. no. 60180–1-Ig, Proteintech Group, Inc.) was
added for incubation overnight at 4°C. After washing three times in
PBS, the slides were incubated with secondary antibody (bio-goat
anti-mouse IgG, 1:1,000, cat. no. sc-2005, Santa Cruz
Biotechnology, Inc) for 20 min at 37°C. The slides were incubated
with streptavidin-peroxidase for 20 min at room temperature after 3
washes with PBS. Finally, diaminobenzidine was used for 4 min at
room temperature followed by washing with PBS, and hematoxylin was
used for staining for 2 min at room temperature. At last, the
slides were dehydrated with ethanol and xylene, and then were
covered. The images were obtained using a light microscope (Olympus
Corporation). Microvessel density was evaluated using five randomly
selected fields of view from each section at 200× magnification.
Any single cell or discrete cluster, stained brown, that indicated
positive CD34 reactivity was identified as a single countable
vessel. The MVD for each case was determined as the mean count of
the five fields of view.
Western blotting
Total proteins were extracted from tumor tissues and
HepG2 liver cancer cells using RIPA lysis buffer, the concentration
of extracted protein was quantified using a bicinchoninic acid
(BCA) protein assay kit, and the protein lysates (70 µg/lane) were
subjected to 12% SDS-PAGE and transferred onto a nitrocellulose
membrane. The membranes were then incubated overnight at 4°C with
primary antibodies after being blocked with 5% non-fat dry milk at
37°C for 1 h. After three washes with PBST (0.05% Tween-20),
membranes were incubated with the aforementioned secondary
antibodies for 1 h at room temperature. Signals were assessed using
an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.)
and the gray values were measured by Gel-Pro analyzer 32 (Media
Cybernetics. Inc.).
Transwell migration and invasion
assay
For the Transwell migration assay, HUVECs were
seeded into the upper chamber of the Transwell plate at a density
of 3×105 cells/well into wells containing 100 µl
serum-free medium. A total of 600 µl HUVEC special medium (cat. no.
CM-0122, Procell Life Science & Technology Co., Ltd.) with 20%
FBS (Gibco; Thermo Fisher Scientific, Inc.) was added in the bottom
chamber as a chemoattractant. After SSb2 (25, 50, 100 µg/ml)
treatment, the cells were cultured in a 37°C incubator for 8 h.
HUVECs that migrated to the bottom chamber were fixed using 4%
paraformaldehyde at room temperature for 30 min and stained for 30
min using 0.1% crystal violet at room temperature. HUVEC migration
was observed and quantified using an inverted light microscope. For
the Transwell invasion assay, 50 µl of diluted Matrigel was
pre-applied to the upper chamber, then placed in a 37°C incubator
overnight. All other procedures were identical to those for the
Transwell migration experiments.
Wound-healing assay
HUVECs and HepG2 liver cancer cells were seeded in
6-well plates and cultured in high-glucose DMEM supplemented with
10% FBS at 37°C for 24 h (23,24)
until the cells were densely confluent (80–90%), after which the
monolayer cells were scratched using a sterile 200 µl pipette tip
and washed with PBS to remove nonadherent cells. Then the cells
were incubated for an additional 24 h (HUVECs) or 48 h (HepG2) in
serum-free medium with or without SSb2 (25, 50, 100 µg/ml)
treatment., Plates were imaged using an inverted light microscope.
The width of the cell-free gap was measured to calculate the
migration rate using Image J (version 1.53m, National Institutes of
Health).
Chicken embryo chorioallantoic
membrane (CAM) assay
For 10 days, fertilized chicken eggs were incubated
at 37°C and 65–70% relative humidity in an incubator. On day 11, a
window of approximately 1 cm2 was gently opened with a
tweezer in the chick embryo air sac. The eggs were selected at
random and divided into 5 groups (n=10). SSb2 (0.1, 0.2 0.4
µg/embryo) and DOX (0.2 µg/embryo) were injected into the chick
embryonic blood vessel branch, the control group were administered
the same volume of normal saline.
An additional, 60 chicken embryos were selected and
divided into 6 groups (n=10). Groups were treated as above, with an
additional group basic fibroblast growth factor (b-FGF) group (0.1
µg/embryo) introduced; the aforementioned SSb2 groups also
contained the growth factor b-FGF (0.1 µg/embryo) as an inducer.
The windows were then covered with sterile membranes, and the eggs
were returned for incubation for an additional 3 days.
Subsequently, the CAM microvessels were imaged using a light
microscope (Olympus Corporation) after fixation using 4%
formaldehyde for 8 h at room temperature. The number of vascular
branches were recorded in eight fields of view per embryo and a
mean calculated to quantify the effect of SSb2 on angiogenesis.
Statistical analysis
SPSS22.0 (IBM Corp.) was used for statistical
analysis. Data were presented as mean ± SD. Comparisons among
multiple groups were performed using a one-way analysis of variance
followed by Dunnett's or Tukey's post hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of SSb2 on tumor growth in H22
tumor-bearing mice
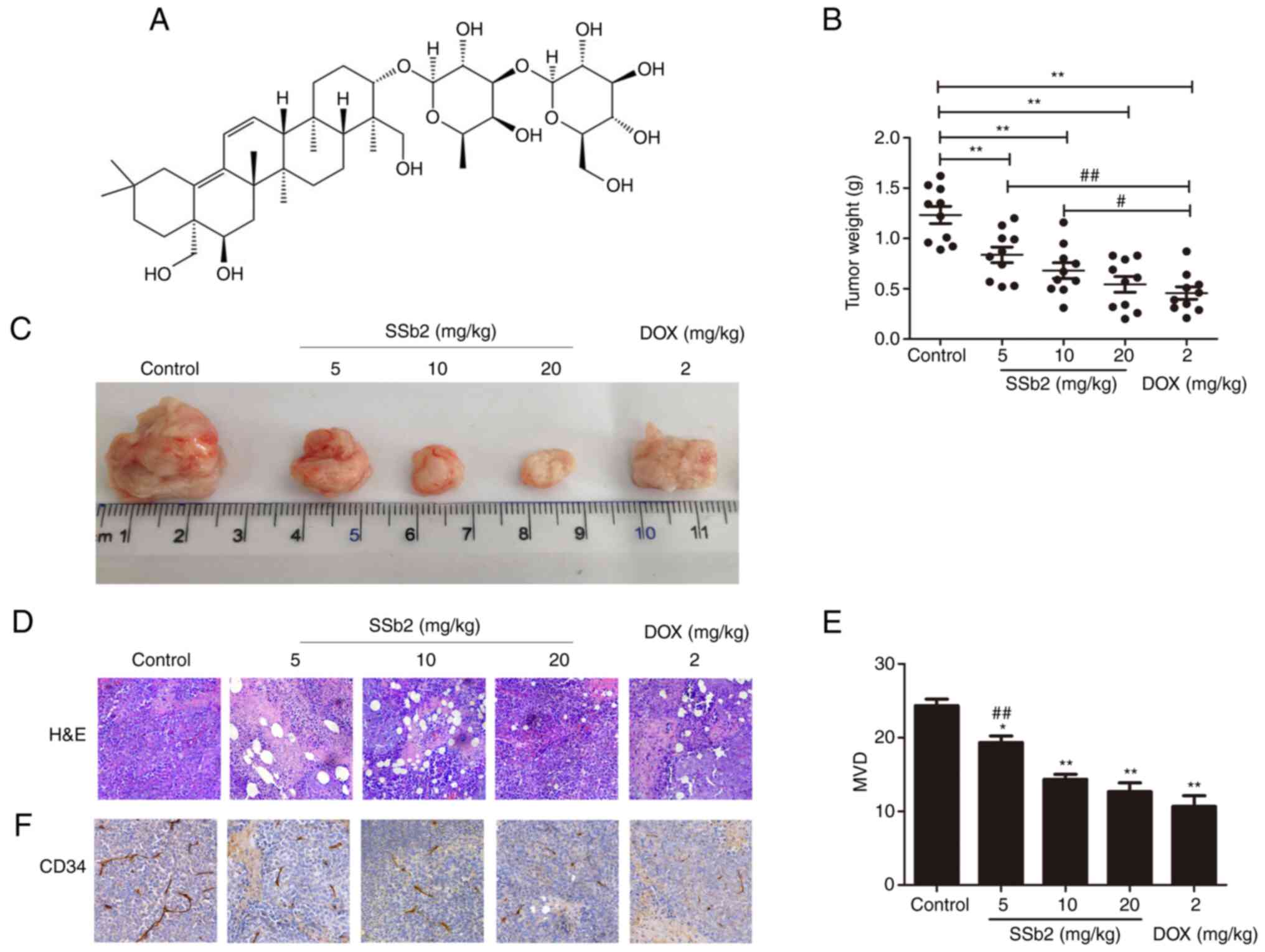
Fig. 1A presented
the molecular structure of SSb2. To evaluate its antitumor effect
on tumor growth in vivo, the tumor weights of H22
tumor-bearing mice treated with 5, 10 or 20 mg/kg SSb2 or DOX for
10 days were assessed. Fig. 1B
presented the inhibitory effect of SSb2 on tumor growth, the
average tumor weights of the SSb2- and DOX-treated groups were
significantly lower compared with those of the control group. The
inhibitory rates of tumor growth in the low-, medium- and high-dose
SSb2-treated groups and the DOX-treated group were 32.12, 44.85,
55.88 and 62.94%, respectively, which suggested that SSb2 had a
concentration-dependent antitumor effect on H22 tumor-bearing mice.
Furthermore, the tumor inhibitory rate of high-dose SSb2 did not
differ significantly from that of the DOX group.
H&E staining was performed to evaluate
pathological changes in the tumors. As presented in Fig. 1D, the control group's tumor cells
were heteromorphic and densely arranged, and many obvious nuclear
atypia could also be observed in the tumor tissue. However, in the
SSb2 and DOX groups, tumor cell growth was disrupted and
vacuolated, and the tumor cells displayed a disorganized
arrangement and a large, red-stained nucleolytic region. These
results demonstrated that SSb2 had a significant antitumor effect
on H22 tumor-bearing mice.
Effect of SSb2 on immune function in
H22 tumor-bearing mice
To evaluate whether SSb2 administration had any
adverse effects on the immune system, the white blood cell count,
thymus index and spleen index of the H22 tumor-bearing mice were
assessed. As presented in Table I,
the white blood cell counts in H22 tumor-bearing mice did not
differ significantly between the control, SSb2-treated and
DOX-treated groups; however, the thymus and spleen indices in the
SSb2-treated mice were significantly lower compared with those in
the control group. The thymus and spleen indices of the
SSb2-treated mice were, significantly higher compared with those of
the DOX group, which indicated that SSb2′s immunotoxicity was less
severe than that of DOX.
 | Table I.Effects of SSb2 on immune function in
H22 tumor-bearing mice. |
Table I.
Effects of SSb2 on immune function in
H22 tumor-bearing mice.
|
|
| Organ index
(×10−3) |
|---|
|
| White blood cell
count (×109/l) |
|
|---|
| Groups | Thymus | Spleen |
|---|
| Control | 7.3±3.01 | 1.80±0.35 | 6.93±0.62 |
| SSb2, mg/kg |
|
|
|
| 5 | 7.3±1.14 |
1.65±0.30a,c |
6.61±0.53d |
| 10 | 7.7±1.90 |
1.54±0.23a,c |
5.83±0.65a,c |
| 20 | 8.0±1.31 |
1.16±0.25b |
5.74±0.55a,c |
| DOX, 2 mg/kg | 6.1±2.30 |
0.93±0.28b |
4.49±0.57a |
Effect of SSb2 on MVD in H22
tumor-bearing mice
To evaluate whether SSb2 treatment affected
angiogenesis in tumor tissues, MVD was assessed using
immunohistochemistry with an anti-CD34 antibody. The micrographs in
Fig. 1F demonstrated the changes in
MVD, where the brown regions indicate angiogenesis. As presented in
Fig. 1E, SSb2 or DOX treatment
significantly decreased MVD in the tumor tissue. Moreover, SSb2
decreased MVD in a markedly concentration dependent manner, with no
significant difference demonstrated between the medium/high-dose
SSb2 and DOX groups. These results indicated that SSb2 inhibited
angiogenesis in H22 tumor-bearing mice.
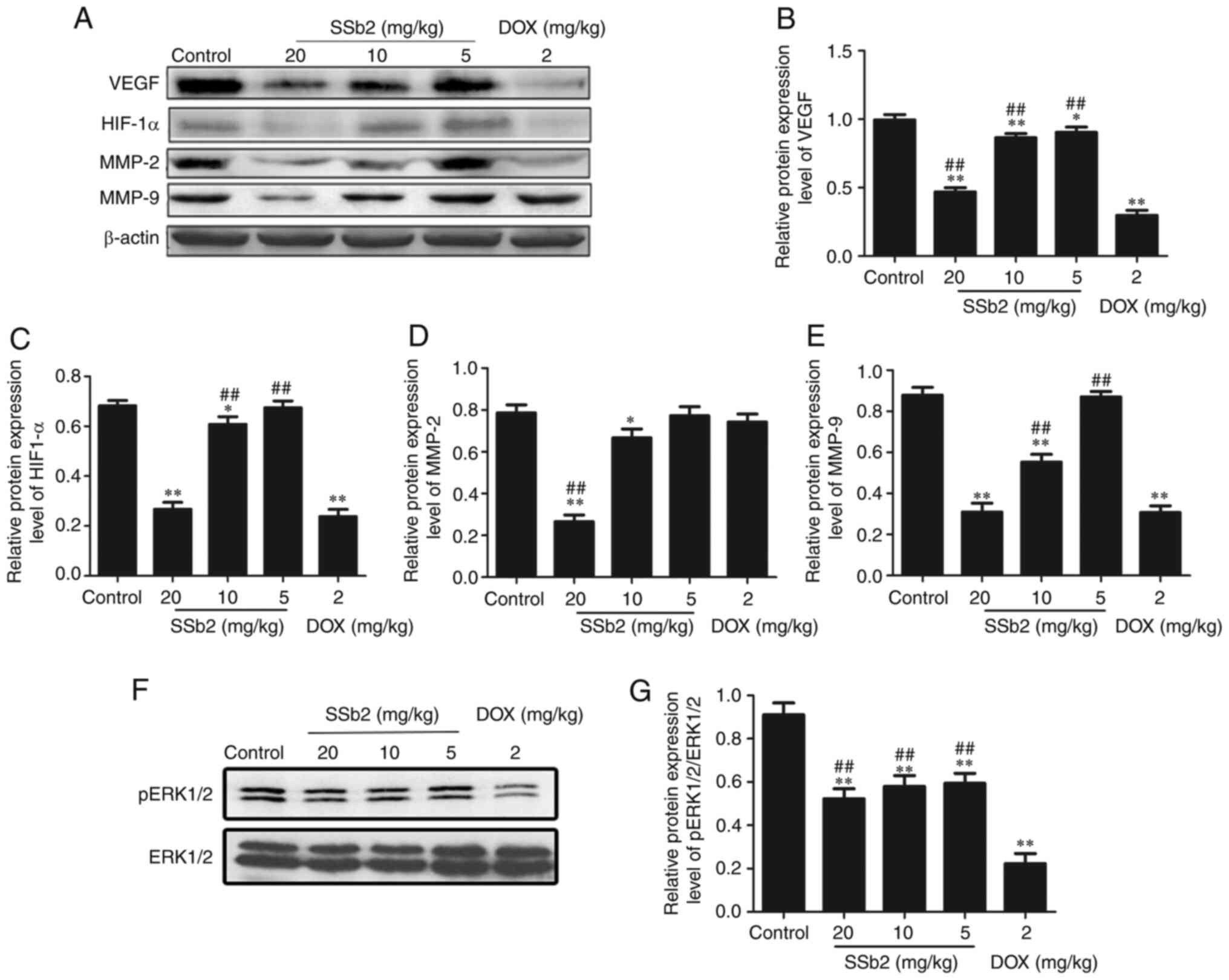
Effect of SSb2 on the expression of
angiogenesis-related proteins in H22 tumor-bearing mice
To evaluate the mechanisms underlying the
suppressive effects of SSb2 on tumor growth, western blotting was
used to assess the expression level of proteins involved in the
regulation of angiogenesis in tumor tissue. As presented in
Fig. 2A-E, the protein expression
levels of VEGF, HIF-1α, MMP-2 and MMP-9 markedly decreased in the
SSb2-treated groups as the SSb2 concentration increased. As
presented in Fig. 2F and G, the
concentration-dependent phosphorylation of ERK1/2 was markedly
decreased in response to SSb2 treatment; however, the total
expression level of ERK1/2 was unaffected. The protein expression
levels of VEGF, HIF-1α, MMP-9 and p-ERK1/2 were downregulated in
the DOX group. These results suggested that the antitumor mechanism
of SSb2 was associated with its antiangiogenic effect via
inhibition of the VEGF/ERK/HIF-1α signal pathway.
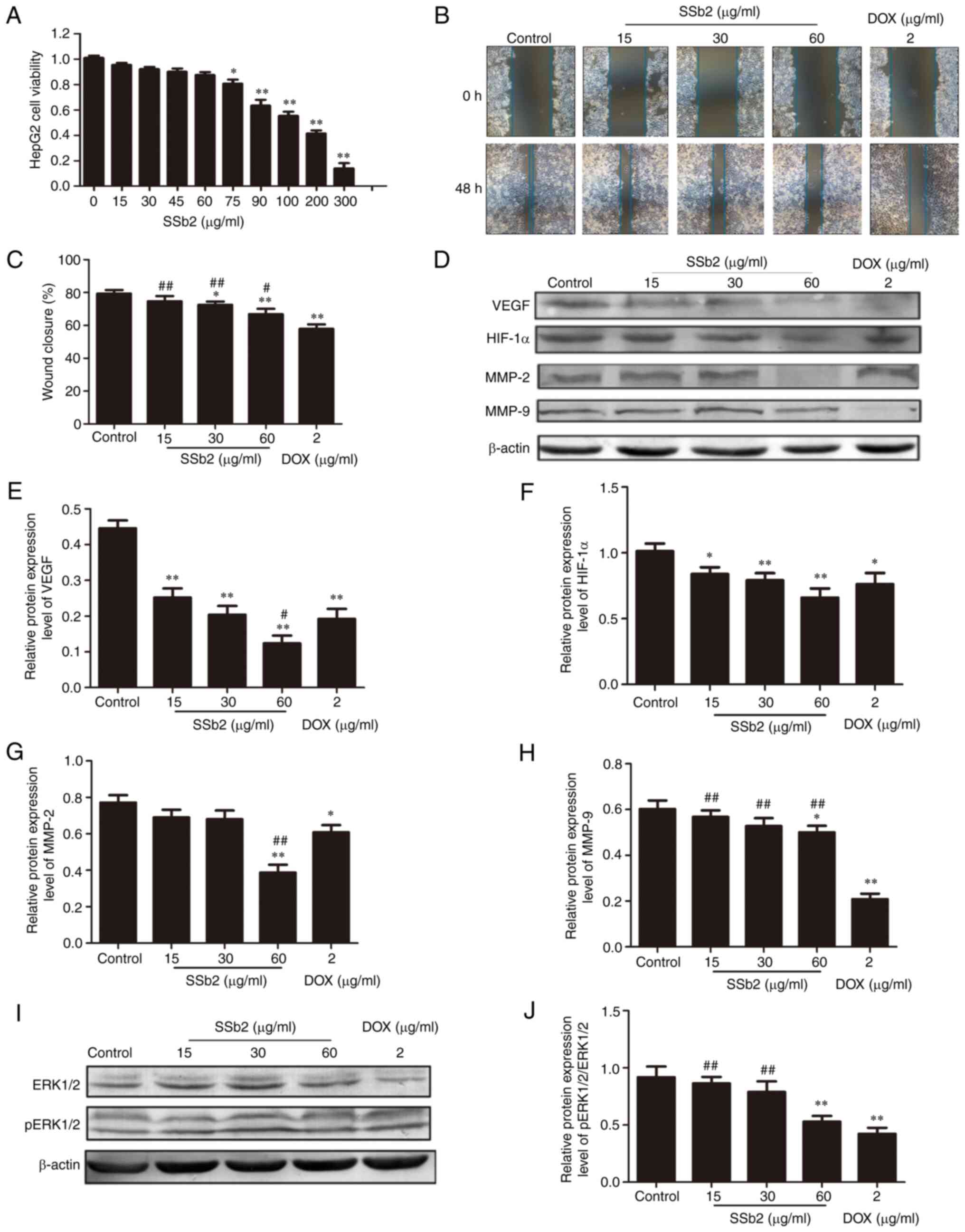
Effect of SSb2 on HepG2 liver cancer
cell viability, migration and expression of angiogenesis-related
proteins
HepG2 liver cancer cells were used to investigate
the antitumor effect of SSb2 in vitro. First, an MTT assay
was performed to assess the effect of SSb2 on HepG2 liver cancer
cell viability and to determine the nontoxic concentration of SSb2
in the cells. As presented in Fig.
3A, SSb2 significantly inhibited HepG2 liver cancer cell
proliferation, in a markedly concentration dependent manner. Based
on these results, 15, 30 and 60 µg/ml SSb2 were chosen for use in
the following scratch experiment. SSb2 significantly inhibited
HepG2 liver cancer cell migration compared with the control group
in the scratch wound experiments, as presented in Fig. 3B and C.
To further assess the mechanism underlying the
inhibitory effect of SSb2 on HepG2 liver cancer cell proliferation,
the protein expression levels of VEGF, HIF-1α, MMP-2 and MMP-9 were
analyzed. As presented in Fig.
3D-H, SSb2 at different concentrations significantly inhibited
VEGF and HIF-1αprotein expression levels compared with the control.
However, MMP2 and MMP9 expression levels were significantly reduced
compared with the control, in only the 60 µg/ml SSb2-treated group.
The effect of SSb2 on the phosphorylation of ERK1/2 was also
assessed; as presented in Fig. 3I and
J, protein expression levels of p-ERK1/2 were markedly reduced
in different concentrations of the SSb2-treated group, and were
significantly reduced compared with the control in the 60 µg/ml
SSb2-treated group. These results indicated that SSb2 inhibited the
proliferation and migration of HepG2 liver cancer cells, which may
be related to its inhibitory effect on the expression of
angiogenesis-related proteins in HepG2 liver cancer cells.
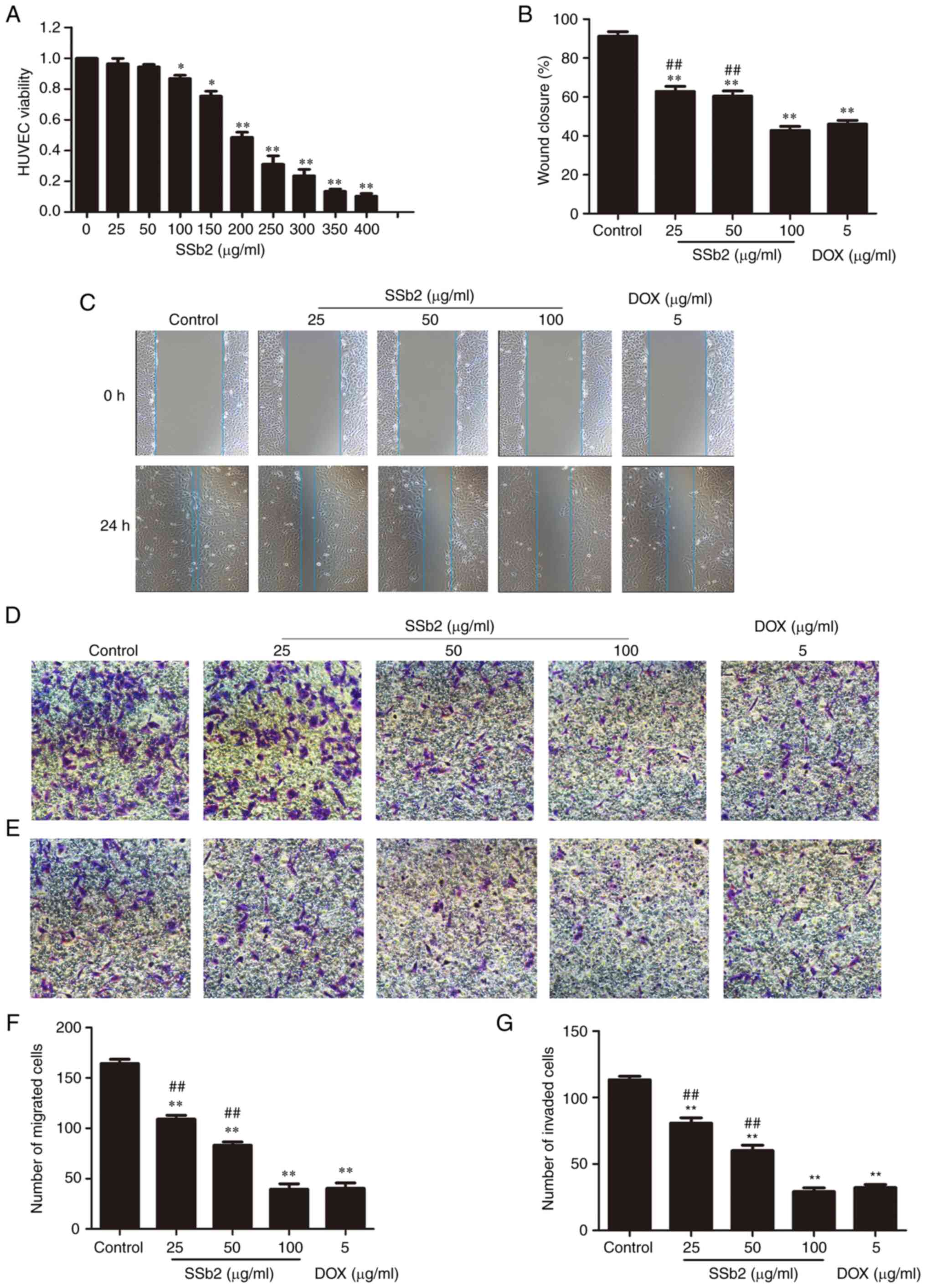
Effect of SSb2 on HUVEC viability,
migration and invasion
An MTT assay was used to determine the cell
viability of HUVECs treated with varying concentrations of SSb2. As
shown in Fig. 4A, HUVEC viability
significantly decreased compared with the control as the SSb2
concentration increased.
Because the migration of endothelial cells is a
critical step in angiogenesis, wound-healing and Transwell assays
were performed to evaluate the effect of SSb2 on HUVEC migration.
Based on the MTT assay results, 25, 50 and 100 µg/ml SSb2 were
selected for use in the subsequent cell migration and invasion
experiments. As presented in Fig. 4B
and C, after 48 h incubation, HUVECs in the control group had
migrated into most of the wound area. However, certain
concentrations of SSb2-treated HepG2 significantly decreased the
wound-healing capacity in HUVECs compared with the untreated cells.
SSb2 also significantly inhibited the migration activity of HUVECs
in a concentration-dependent manner, compared with the control, as
demonstrated by the Transwell migration assay, presented in
Fig. 4D and F, which was used to
evaluate the ability of cells to migrate vertically. As presented
in Fig. 4E and G, in the Transwell
invasion assay, HUVECs demonstrated high levels of invasive
activity in the control group. After exposure to SSb2 at 25, 50 or
100 µg/ml, or 5 µg/ml DOX, cell invasion was suppressed by 28.7,
47.1, 74.3, and 71.6%, respectively, which suggested that SSb2
effectively suppressed HUVEC migration and invasion. These results
demonstrated the effective in vitro antiangiogenic activity
of SSb2.
Effect of SSb2 on tube formation as
measured using the CAM assay in vivo
A CAM assay was performed to further elucidate the
potential function of SSb2 in angiogenesis. SSb2 was injected into
the chick embryonic blood vessel branch on incubation day 11 in the
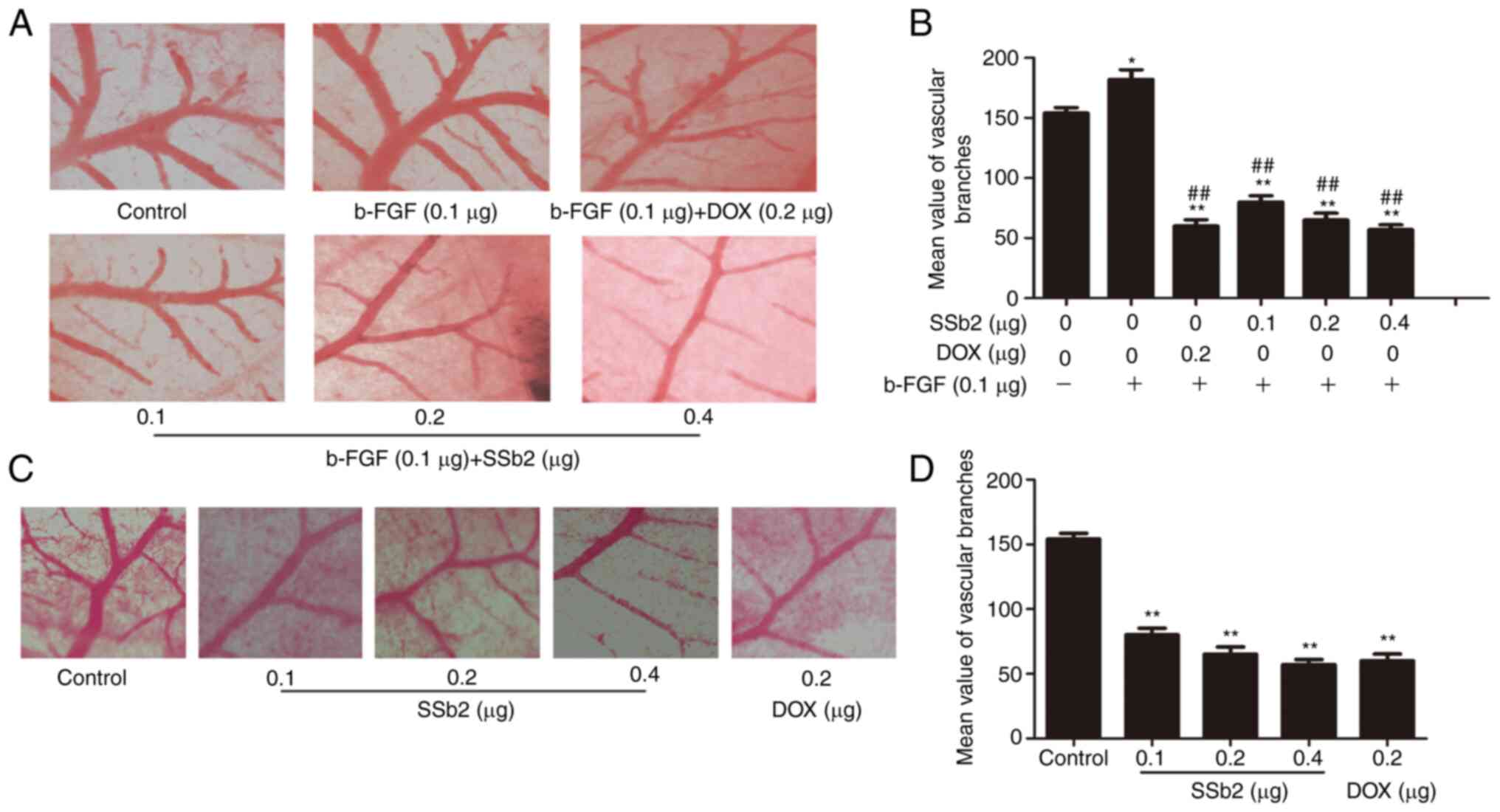
presence of b-FGF. As presented in Fig.
5A and B, b-FGF triggered a potent angiogenic response, and the
number of branches and vessel diameters of CAM induced by b-FGF
were significantly increased compared with the control. Moreover,
the 0.1 µg/embryo SSb2 significantly inhibited b-FGF-induced
angiogenesis, and SSb2 at concentrations of 0.2 µg/embryo and 0.4
µg/embryo demonstrated greater inhibitory effects on the formation
of tube-like vessels, which indicated that SSb2 caused a
concentration-dependent blockage of the capillary tubes.
Fig. 5C and D
presented the inhibitory effect of SSb2 on the CAM model without
any inducement. SSb2 and DOX significantly reduced angiogenesis
compared with the control, as demonstrated by the induction of
fewer vessel branches and a smaller vessel diameter; the effect was
amplified at higher SSb2concentrations. These findings suggested
that SSb2 was capable of inhibiting angiogenesis in
vivo.
Discussion
Angiogenesis serves a critical role in solid tumor
development by supplying nutrients and oxygen to sustain continuous
tumor growth, and numerous malignancies are characterized by
intense and rapid angiogenesis. Moreover, angiogenesis is a crucial
process in vascular remodeling, tissue damage, tumor migration and
invasion (25–27). Therefore, inhibition of tumor
angiogenesis is regarded as an effective cancer prevention and
treatment strategy. Numerous angiogenesis inhibitors, such as
bevacizumab, sunitinib and sorafenib, have been reported to date
(28,29). However, recent clinical studies have
reported that these antiangiogenic drugs do not have a sufficient
curative effect in preventing angiogenesis and tumor development.
Furthermore, numerous adverse effects, including hypertension,
cardiotoxicity, bleeding, gastrointestinal perforation and birth
defects, have been reported during treatment with these drugs
(30,31). Therefore, in order to alleviate the
suffering of patients and improve their quality of life, more
effective and safe angiogenesis drugs must be discovered and
developed.
In the present study, the in vivo antitumor
efficacy of SSb2 was evaluated by assessing the inhibition of tumor
growth in H22 tumor-bearing mice. These data showed that SSb2
exhibited remarkable antitumor activity in the H22 tumor-bearing
mice model, as a significant concentration-dependent reduction in
tumor weight was observed following SSb2 treatment. DOX is a
chemotherapeutic drug that is frequently used to treat liver
cancer. In the present study, DOX was used as a positive control.
These data demonstrated that the tumor-inhibiting efficacy of
high-dose SSb2 was only marginally inferior to that of DOX.
However, the spleen and thymus indices of all SSb2 groups did not
decrease as much as those of the DOX group, which indicated that
SSb2 induced less immune damage than DOX. Therefore, the antitumor
effects of SSb2 were worthy of further study.
MVD is a good indicator of tumor angiogenesis, and
the measurement of tumor MVD has become the primary method for
determining antiangiogenic drug efficacy (27,32).
According to tumor immunohistochemistry, SSb2 markedly decreased
the CD34 protein expression level and significantly decreased the
corresponding MVD value in tumor tissue, which indicated that SSb2
had antitumor and antiangiogenic activity. These findings were also
demonstrated in the CAM model. Due to its advantages of convenient
sampling, simple operation, and widespread use, the CAM assay is
regarded as a good experimental model for angiogenesis studies and
is widely used for screening and evaluating the antiangiogenic
activity of drugs (33). As
expected, an increase in vessel diameter and branches was observed
in the b-FGF control group. However, SSb2 treatment significantly
reduced the number of vessel branches and decreased vessel diameter
in the CAM model with or without b-FGF. Migration of endothelial
cells is an important step in angiogenesis (34). Therefore, HUVECs were used to assess
the antiangiogenic effect of SSb2. SSb2 was demonstrated to
significantly inhibit both HUVEC migration and invasion. These
results validated the antiangiogenic activity of SSb2 both in
vivo and in vitro, which suggested that SSb2 was a
potential therapeutic agent for the inhibition of cancer metastasis
and tumor angiogenesis. Supporting evidence may provide an
experimental basis for further study of SSb2 as a potent
angiogenesis inhibitor in clinical applications.
To further investigate the mechanism underlying
SSb2′s antiangiogenic properties, angiogenesis-related signaling
pathways were assessed. VEGF is overexpressed in liver cancer, and
its high expression is closely associated with tumor angiogenesis,
invasion and metastasis (35,36).
VEGF can promote endothelial cell proliferation, increase vascular
permeability, enable endothelial cell migration, induce tumor
angiogenesis and maintain continued tumor growth by binding and
activating VEGFR. As the most powerful angiogenic factor currently
known (37), VEGF is related to
numerous physiological and pathological processes, including liver
cancer development. Any agent inhibiting VEGF-related processes
could inhibit angiogenesis and thus restrain tumor growth and
metastasis (34). The present study
demonstrated that SSb2 inhibited VEGF expression in liver cancer,
which suggested that SSb2 suppressed angiogenesis by downregulating
VEGF expression, which may be one of the mechanisms by which SSb2
inhibits liver cancer.
The primary characteristic of malignant tumors is
their high metastatic potential, which is the primary cause of
patient mortality. According to a previous report, >80% of
patients with tumors die as a result of tumor metastasis (38). Tumor metastasis is a complex process
(39) because many proteolytic
enzymes are involved in the degradation of environmental barriers,
such as the basal membrane and extracellular matrix, among which
MMPs serve an important role in promoting the migration of cancer
cells to neighboring tissues by degrading the main components of
the basement membrane, such as collagen IV (40,41).
Furthermore, HIF-1α acting as a signaling hub can affect the
expression of proangiogenic factors, such as VEGF, IL-6 and TNFα
(42–45) and proangiogenic enzymes, such as
inducible nitric oxide synthase andMMP-9 (43,46,47).
VEGF and HIF-1α, which are involved in the regulation of
angiogenesis, are deemed the most promising therapeutic targets for
direct or indirect angiogenesis inhibitors.
Activation of the MAPK family, including ERK, P38
MAPK and PI3K/Akt, is essential for the promotion of angiogenesis.
Therefore, suppression of these pathways may induce
anti-angiogenesis and antitumor effects (48–50).
The results of the present study demonstrated that SSb2 markedly
downregulated the expression of p-ERK1/2, which indicated that SSb2
was capable of modulating ERK1/2 signaling. ERK1/2, a downstream
protein of various growth factors, such as VEGF, regulates cell
proliferation, differentiation and survival. HIF-1α is a substrate
for ERK phosphorylation, and Ser641 and Ser643 phosphorylation of
HIF-1α is required for nuclear location and transcriptional
activity (51). Zhang et al
(51) reported that angiogenesis
was inhibited by blocking VEGF or downstream molecules such as
ERK1/2 and HIF-1α. The present study demonstrated that SSb2
significantly decreased the protein expression levels of VEGF,
ERK1/2, HIF-1α, MMP2 and MMP9 compared with the control group.
Therefore, we hypothesized that VEGF protein expression levels
decreased after SSb2 administration and that VEGF downregulated the
expression of ERK1/2, which could inhibit its downstream molecules,
including HIF-1α, MMP2 and MMP9. However, HIF-1α can regulate the
expression of the VEGF gene, resulting in further VEGF reduction.
These effects led to the subsequent inhibition of tumor metastasis
and angiogenesis. The present study did not investigate any other
pathway activated by VEGF, such as AKT, which is a limitation of
the present study and requires study in future research.
Furthermore, a gene knockdown or knockout experiment is required to
assess the significance of VEGF signaling in SSb2′s anticancer
activity. Future research must continue to focus on this area.
Overall, the present study demonstrated that SSb2
possesses an antiangiogenic effect both in vivo and in
vitro and that the mechanism appears to involve the inhibition
of the VEGF/ERK/HIF-1α signaling pathway. The present study's
findings provide new insights into how SSb2 inhibits liver cancer
and suggest that SSb2 could be a potential natural product for
treating liver cancer. Further studies are needed in to other
aspects of SSb2 in the treatment of angiogenesis and cancers.
Acknowledgements
Not applicable.
Funding
This study was supported financially by the Henan Province Key
Science and Technology Project (grant no. 202102310486) and Luoyang
Science and Technology Medical and Health Project (grant no.
1603001A-3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and JF analyzed the data and wrote the
manuscript. JF, XL, LW and HW performed the experiments and data
collection. RL designed the study and provided the funding and
facilities. LW, HW and RL critically reviewed and edited the
manuscript. All authors read and approved the final manuscript. JF
and RL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Animal experiment protocols were approved by the
Experimental Animal Ethics Committee of Henan University of Science
and Technology (approval no. 20200519). All animal experiments were
performed in accordance with the National Act on the Use of
Experimental Animals (China). Appropriate measures were taken to
minimize the use of animals as well as their suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caines A, Selim R and Salgia R: The
changing global epidemiology of hepatocellular carcinoma. Clin
Liver Dis. 24:535–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L, Huang Z, Chen J, Wang J and Wang S:
Protein phosphatase 2A mediates JS-K-induced apoptosis by affecting
Bcl-2 family proteins in human hepatocellular carcinoma HepG2
cells. J Cell Biochem. 119:6633–6643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu C, Wu J and Chang Z: Trends and
age-period-cohort effects on the prevalence, incidence and
mortality of hepatocellular carcinoma from 2008 to 2017 in Tianjin,
China. Int J Environ Res Public Health. 18:60342021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi X, Fan M, Huang N, Zhang XY, Liu J, Li
XY and Sun R: Saikosaponin d contributed to cancer chemotherapy
induced neutropenia therapy by promoting neutrophil differentiation
via activation CBL-dependent ERK pathway. Pharmacol Res.
160:1051492020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang GR, Lin WL, Lin TC, Liao HJ and Lu
YW: The ameliorative effects of saikosaponin in
thioacetamide-induced liver injury and non-alcoholic fatty liver
disease in mice. Int J Mol Sci. 22:113832021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu M, Zhang GF, Naqvi S, Zhang F, Kang T,
Duan Q, Wang ZY, Xiao SX and Zheng Y: Cytotoxicity of Saikosaponin
A targets HEKa cell through apoptosis induction by ROS accumulation
and inflammation suppression via NF-κB pathway. Int
Immunopharmacol. 86:1067512020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang W, Yang YJ, Guo BL and Cen S:
Anti-influenza triterpenoidsaponins (saikosaponins) from the roots
of Bupleurum marginatum var. stenophyllum. Bioorganic Med Chem
Lett. 27:1654–1659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Li X, Huang N, Liu R and Sun R: A
comprehensive review and perspectives on pharmacology and
toxicology of saikosaponins. Phytomedicine. 50:73–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You M, Li RF, Gao ZH, Li YY, Liu WY, Wang
JG, Wang HW and Li SQ: Effects of saikosaponin b_2 on inflammation
and energy metabolism in mice with acute liver injury induced by
LPS/GalN. Zhongguo Zhong Yao Za Zhi. 44:2966–2971. 2019.(In
Chinese). PubMed/NCBI
|
|
10
|
Wen Y, Zhou X, Lu M, He M, Tian Y, Liu L,
Wang M, Tan W, Deng Y, Yang X, et al: Bclaf1 promotes angiogenesis
by regulating HIF-1α transcription in hepatocellular carcinoma.
Oncogene. 38:1845–1859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang R, Song X, Wang P, Zhou Y, Lu X,
Wang J, Xu M, Chen X, Utpatel K, Che L, et al: Cabozantinib-based
combination therapy for the treatment of hepatocellular carcinoma.
Gut. 70:1746–1757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao H, Liu N, Lin MC and Zheng J: Positive
feedback loop between cancer stem cells and angiogenesis in
hepatocellular carcinoma. Cancer Lett. 379:213–219. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tampellini M, Sonetto C and Scagliotti GV:
Novel anti-angiogenic therapeutic strategies in colorectal cancer.
Exp Opin Investig Drugs. 25:507–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi SB, Han HJ, Kim WB, Song TJ and Choi
SY: VEGF overexpression predicts poor survival in hepatocellular
carcinoma. Open Med (Wars). 12:430–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo D, Wang Z and Wu J, Jiang C and Wu J:
The role of hypoxia inducible factor-1 in hepatocellular carcinoma.
Biomed Res Int. 2014:4092722014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju C, Colgan SP and Eltzschig HK:
Hypoxia-inducible factors as molecular targets for liver diseases.
J Mol Med (Berl). 94:613–627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Wang N, Tan HY, Guo W, Chen F,
Zhong Z, Man K, Tsao SW, Lao L and Feng Y: Direct inhibition of the
TLR4/MyD88 pathway by geniposide suppresses HIF-1α-independent VEGF
expression and angiogenesis in hepatocellular carcinoma. Br J
Pharmacol. 177:3240–3257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu P, Atkinson SJ, Akbareian SE, Zhou ZG,
Munsterberg A, Robinson SD and Bao Y: Sulforaphane exerts
anti-angiogenesis effects against hepatocellular carcinoma through
inhibition of STAT3/HIF-1α/VEGF signalling. Sci Rep. 7:126512017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang HM, Sun CY, Liang JL, Xu LQ, Zhang
ZB, Luo DD, Chen HB, Huang YZ, Wang Q, Lee DYW, et al:
Supercritical-carbon dioxide fluid extract from chrysanthemum
indicum enhances anti-tumor effect and reduces toxicity of
bleomycin in tumor-bearing mice. Int J Mol Sci. 18:4652017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao W, Hu C, Wu L, Xu L and Jiang W:
Rosmarinic acid inhibits inflammation and angiogenesis of
hepatocellular carcinoma by suppression of NF-κB signaling in H22
tumor-bearing mice. J Pharmacol Sci. 132:131–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ting W, Feng C, Zhang M, Long F and Bai M:
Overexpression of microRNA-203 suppresses proliferation, invasion,
and migration while accelerating apoptosis of CSCC cell line SCL-1.
Mol Ther Nucleic Acids. 28:428–440. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferreira KCB, Valle ABCDS, Gualberto ACM,
Aleixo DT, Silva LM, Santos MM, Costa DS, Oliveira LL, Gameiro J,
Tavares GD, et al: Kaurenoic acid nanocarriers regulates cytokine
production and inhibit breast cancer cell migration. J Control
Release. 352:712–725. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Liu Y, Li W and Song Z: Growth
differentiation factor 15 promotes cell viability, invasion,
migration, and angiogenesis in human liver carcinoma cell line
HepG2. Clin Res Hepatol Gastroenterol. 41:408–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao
HL, Li H, Zhang SR, Xu JZ, Qi ZH, et al: Angiogenesis in pancreatic
cancer: Current research status and clinical implications.
Angiogenesis. 22:15–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Zhang XF, Lu X, Jia HL, Liang L,
Dong QZ, Ye QH and Qin LX: MicroRNA-26a suppresses angiogenesis in
human hepatocellular carcinoma by targeting hepatocyte growth
factor-cMet pathway. Hepatology. 59:1874–1885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung HJ and Kwon HJ: Exploring the role of
mitochondrial UQCRB in angiogenesis using small molecules. Mol
Biosyst. 9:930–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim H, Jang JP, Han JM, Jang JH, Ahn JS
and Jung HJ: Antiangiogenic potential of microbial metabolite
elaiophylin for targeting tumor angiogenesis. Molecules.
23:5632018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu M, Tian Y, Yue WM, Li L, Li SH, Qi L,
Hu WS, Gao C, Si LB and Tian H: GOLPH3, a good prognostic indicator
in early-stage NSCLC related to tumor angiogenesis. Asian Pac J
Cancer Prev. 15:5793–5798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lou C, Zhu Z, Xu X, Zhu R, Sheng Y and
Zhao H: Picroside II, an iridoid glycoside from Picrorhizakurroa,
suppresses tumor migration, invasion, and angiogenesis in vitro and
in vivo. Biomed Pharmacother. 120:1094942019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Varinská L, Fáber L, Kello M, Petrovová E,
Balážová L, Solár P, Coma M, Urdzík P, Mojžiš J, Švajdlenka E, et
al: β-Escin effectively modulates HUVECs proliferation and tube
formation. Molecules. 23:1972018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carbajo-Pescador S, Ordoñez R, Benet M,
Jover R, García-Palomo A, Mauriz JL and González-Gallego J:
Inhibition of VEGF expression through blockade of Hif1α and STAT3
signalling mediates the anti-angiogenic effect of melatonin in
HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tseng PL, Tai MH, Huang CC, Wang CC, Lin
JW, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, et al: Overexpression
of VEGF is associated with positive p53 immunostaining in
hepatocellular carcinoma (HCC) and adverse outcome of HCC patients.
J Surg Oncol. 98:349–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kämmerer PW, Koch FP, Schiegnitz E, Berres
M, Toyoshima T, AI-Nawas B and Brieger J: Associations between
single-nucleotide polymorphisms of the VEGF gene and long-term
prognosis of oral squamous cell carcinoma. J Oral Pathol Med.
42:374–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kobus-Bianchini K, Bourckhardt GF, Ammar
D, Nazari EM and Müller YMR: Homocysteine-induced changes in cell
proliferation and differentiation in the chick embryo spinal cord:
Implications for mechanisms of neural tube defects (NTD). Reprod
Toxicol. 69:167–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng G, Zhou F, Wu Z, Zhang F, Niu K, Kang
YJ, Liu XJ, Wang QJ, Wang Y and Wang Q: Inhibition of cancer cell
migration with simpleCuS@mSiO2-PEG
nanoparticles by repressing MMP-2/MMP-9 expression. Int J
Nanomedicine. 13:103–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zou Y, Xiong H, Xiong H, Lu T, Zhu F, Luo
Z, Yuan X and Wang Y: A polysaccharide from mushroom Huaier retards
human hepatocellular carcinoma growth, angiogenesis, and metastasis
in nude mice. Tumor Biol. 36:2929–2936. 2014. View Article : Google Scholar
|
|
42
|
Medrek C, Pontén F, Jirström K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Werno C, Menrad H, Weigert A, Dehne N,
Goerdt S, Schledzewski K, Kzhyshkowska J and Brune B: Knockout of
HIF-1α in tumor-associated macrophages enhances M2 polarization and
attenuates their pro-angiogenic responses. Carcinogenesis.
31:1863–1872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1α and VEGF in
laser-induced rat choroidal neovascularization. Invest Opthalmol
Visual Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Coffelt SB, Tal AO, Scholz A, Palma MD,
Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y and
Lewis CE: Angiopoietin-2 regulates gene expression in
TIE2-expressing monocytes and augments their inherent proangiogenic
functions. Cancer Res. 70:5270–5280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Du R, Lu KV, Petritsch C, Liu P, Ganss R,
Passegué E, Song H, VandenBerg S, Johnson RS and Werb Z: HIF1α
induces the recruitment of bone marrow-derived vascular modulatory
cells to regulate tumor angiogenesis and invasion. Cancer Cell.
13:206–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen WT, Hung WC, Kang WY, Huang YC, Su
YC, Yang CH and Chai CY: Overexpression of cyclooxygenase-2 in
urothelial carcinoma in conjunction with
tumor-associated-macrophage infiltration, hypoxia-inducible
factor-1α expression, and tumor angiogenesis. APMIS. 117:176–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Herrera-Vargas AK, García-Rodríguez E,
Olea-Flores M, Mendoza-Catalán MA, Flores-Alfaro E and Navarro-Tito
N: Pro-angiogenic activity and vasculogenic mimicry in the tumor
microenvironment by leptin in cancer. Cytokine Growth Factor Rev.
62:23–41. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Baek SH, Ko JH, Lee JH, Kim C, Lee H, Nam
D, Lee J, Lee SG, Yang WM, Um JY, et al: Ginkgolic acid inhibits
invasion and migration and TGF-β-induced EMT of lung cancer cells
through PI3K/Akt/mTOR inactivation. J Cell Physiol. 232:346–354.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Singh SS, Yap WN, Arfuso F, Kar S, Wang C,
Cai W, Dharmarajan AM, Sethi G and Kumar AP: Targeting the PI3K/Akt
signaling pathway in gastric carcinoma: A reality for personalized
medicine? World J Gastroenterol. 21:12261–12273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu
M, Bai O, Liu W, Xie X, Wang Z, et al: RKTG inhibits angiogenesis
by suppressing MAPK-mediated autocrine VEGF signaling and is
downregulated in clear-cell renal cell carcinoma. Oncogene.
29:5404–5415. 2010. View Article : Google Scholar : PubMed/NCBI
|