Introduction
Breast cancer (BC) is one of the most common types
of cancer affecting women (1–3) and
the number of patients diagnosed with BC remains high worldwide as
indicated in the WHO data up to 2016 (1). In Japan, BC is the most frequently
diagnosed cancer in women (4).
However, recent reports present that mortality rates of BC tend to
decrease especially in developed countries (1,2), which
could be attributed to the progress of diagnostic methods and
therapies for BC (1–5). Despite advances in BC research and
treatment, due to the lack of therapeutic targets, triple-negative
BC (TNBC) is regarded as an aggressive subtype with a poor
prognosis and its clinical outcome remain, unsatisfactory (1,6,7).
Fascin, an actin-bundling protein, serves a
significant role in the regulation of cell adhesion, migration, and
invasion (8–15). Fascin is strongly upregulated in
several types of human carcinoma and sarcoma (8–12,14).
Fascin overexpression is associated with higher grade of BC and its
expression commonly predicts an aggressive clinical course in
patients (7,10,13,16,17).
Filopodia, bundles of actin, are fibrous protrusions on the cell
membrane. They are also essential in processes of cell
proliferation, including adhesion, migration and the formation of
cell-cell contacts. Filopodia allow cells to migrate to the
surrounding tissue through the extracellular matrix by interacting
with various types of intercellular adhesive structure such as
tight junctions, adherens junctions containing cadherin and
desmosomes (18).
The present study reviewed clinical data from 100
patients diagnosed with BC in 2015. Fresh immunohistochemical
assessment of fascin in tissue samples was performed to examine the
association between BC malignancy with fascin expression and TNBC
subtype. The present study aimed to investigate the association
between fascin and BC invasion by morphological observation of
cytoplasm and the cell surface. Fascin knockdown (FKD) was induced
in MDA-MB-231, a TNBC cell line, to detect morphological effects of
fascin on TNBC cells.
Materials and methods
Clinical data of patients with BC
Clinical data were reviewed from 100 consecutive
patients who had been diagnosed with early-stage BC at Kochi
University Hospital, Nankoku, Japan, from January to December 2015.
The study was reviewed and approved by the Ethic Committee for
Clinical Research of the School of Medicine, Kochi University
(approval no. 2020-123; 4th December 2020). Written consent to
participate was obtained from all patients. All 100 patients had
undergone surgical treatment and completed follow-up for >5
years at the hospital. All tissues obtained during surgery had been
embedded in paraffin blocks following formalin fixation for
preservation. The immunohistochemical evaluation of estrogen
receptor (ER), progesterone receptor (PR) and human epidermal
growth factor receptor 2 (HER2) were also reviewed from clinical
pathology reports at the time of initial diagnoses.
Fascin immunohistochemical
evaluation
A total of 11 tissue samples from patients with
metastasis or recurrence during 5-year follow-up were cut into 4 µm
thick slices and heat-treated at 95°C for 30 min with ULTRA cell
conditioning 1 retrieval solution (Ventana Automated Systems).
Immunohistochemical examination was performed using a Ventana
automated system with anti-fascin-1 mouse monoclonal antibody
(1:100; cat. no. M3567; Dako, Agilent Technologies, Inc.). Another
set of 17 consecutive tissue samples from patients without
metastasis or recurrence underwent the same procedure as a control
group. To evaluate immunohistochemical expression of fascin, the
Allred scoring system was used (13,14).
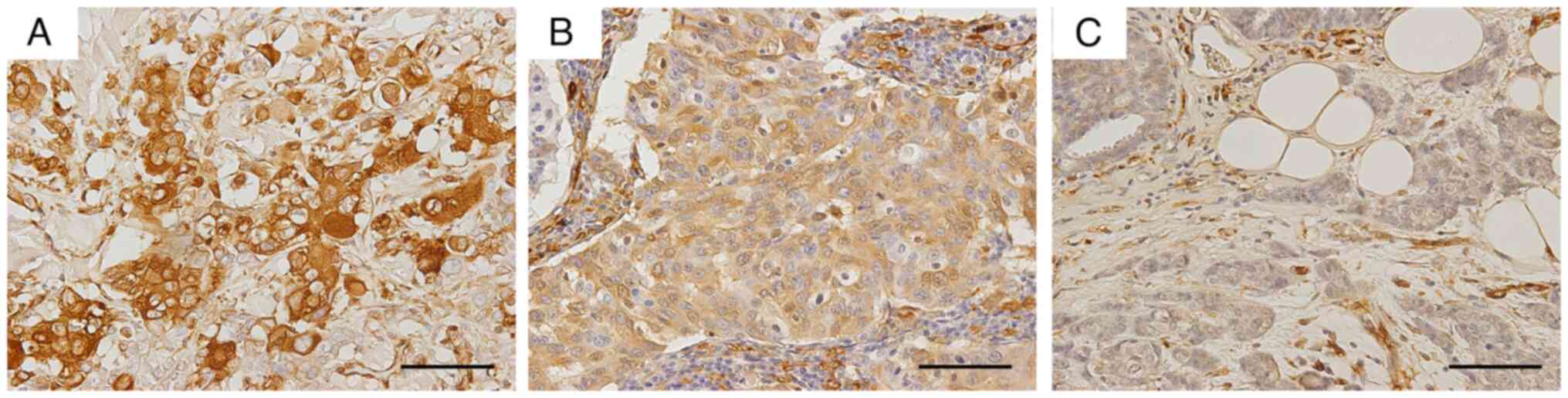
Briefly, the proportion of stained cells was categorized as
negative (0), <1 (1), 1–10
(2), 11–33 (3), 34–66 (4) and >66% (5) positive. The intensity of the most
predominantly stained area was categorized as no (0), weak
(1), intermediate (2), or strong (3) staining (Fig. 1). Allred score (0–8 points) was
calculated by adding the proportion and intensity values.
Independent evaluation of immunostaining was performed by two
expert pathologists (YH, IM).
FKD MDA-MB-231 cells
Human TNBC MDA-MB-231 cells (American Type Culture
Collection), were cultured at 37°C for 24 h with 5% CO2
in DMEM (Sigma-Akdrech, Merck KGaA) with 10% fetal bovine serum
(Biosera France SAS). Following recombination of the short hairpin
RNA (shRNA) against fascin, the pLKO.1-puro plasmid vector (1
µg/µl; Clone ID: NM_003088.2-1699s1c; Sigma-Aldrich, Merck KGaA),
containing the puromycin-resistance gene, was transfected into
MDA-MB-231 cells with FuGene®6 Transfection Reagent
(Roche Diagnostics) at 37°C for 24 h, according to the
manufacturer's instructions. Cells were incubated at 37°C with 2.2
µg/ml puromycin (Sigma-Akdrech, Merck KGaA) for 2 weeks.
Puromycin-resistant colonies (~20) were obtained and cultured with
the medium containing puromycin (2.2 µg/ml) in 100 mm-diameter
dishes. When the cell confluency reached 80%, dishes were provided
to carry out western blot analysis and to gain FKD MDA-MB-231
cells, respectively. Transfection of the pLKO.1-puro plasmid vector
without shRNA against fascin was used to generate non-FKD
MDA-MB-231 cells.
Immunocytochemical and phalloidin
staining of MDA-MB-231 cells
For immunocytochemical staining, non-FKD and FKD
MDA-MB-231 cells were incubated with anti-fascin-1 mouse monoclonal
antibody (1:100; cat. no. M3567; Dako, Agilent Technologies, Inc.)
at 4°C overnight reacted with fluorescein isothiocyanate
(FITC)-labeled anti-mouse IgG antibody (1:200; cat. no. F-2761;
Molecular Probes, Thermo Scientific, Inc.) at room temperature for
1 h and nuclei were stained with DAPI (Sigma-Akdrech, Merck KGaA).
For phalloidin staining, cells (2×104/well) were
cultured at 37°C for 24 h on a slide chamber (AGC Techno Co., Ltd),
fixed with 100% acetone at −20°C for 20 min and stained with
FITC-labeled phalloidin (Sigma-Akdrech, Merck KGaA) to bind actin
filaments, according to the manufacturer's instructions.
Fluorescence microscopy was performed using an Olympus BX53
(Olympus Corporation; magnification ×200) with cellSens standard
(ver.1.12, Olympus Corporation).
Western blot analysis
Concentration of samples lysed in RIPA Buffer
(Fujifilm Wako Pure Chemical Corp) was measured by Pierce BCA
Protein Assay Kit (cat. no. 23227; Thermo Fisher Scientific, Inc.).
20 µg/lane samples were prepared for SDS-PAGE separation (10%
SDS-PAGE Gel; Bio-Rad Laboratories, Inc.) and transferred to
polyvinylidene difluoride membranes using the Trans-Blot Turbo
Transfer System (Bio-Rad Laboratories, Inc.). Membranes were
blocked with Blocking One (cat. no. 03953-93; Nacalai Tesque, Inc.)
at room temperature for 1 h, and incubated at 4°C overnight with
following antibodies: anti-fascin-1 mouse monoclonal (1:500; cat.
no. M3567; Dako, Agilent Technologies, Inc.), E-cadherin mouse
monoclonal (1:500; cat. no. M3612; Dako, Agilent Technologies,
Inc.), Snail1 rabbit polyclonal antibody (1:200; cat. no. Ap205Aa;
Adgent, Inc.), and GAPDH mouse monoclonal antibody (1:2000; cat.
no. 20035; ProMab Biotechnologies, Inc.). Then, the membranes were
incubated at room temperature for 1 h with either of following
secondary antibodies: HRP-labeled anti-mouse polyclonal antibody
(1:2000; cat. no. P0447; Dako, Agilent Technologies, Inc.) or
anti-rabbit polyclonal antibody (1:2000; cat. no. P0399; Dako,
Agilent Technologies, Inc.). Bands were visualized by ECL Prime
Western Blotting Detection Reagents (Amersham, Cytiva) and observed
using LAS-4000 Lumino-Image Analyzer (FUJIFILM Wako Pure Chemical
Corporation). ImageJ (version no. 1.53; National Institutes of
Health) was used for analysis.
Wound healing assay
Trypsinized 2×104/ml parent, non-FKD and
FKD cells were counted with SKC, Inc. C-Chip™ Disposable
(Thermo Fisher Scientific), disposable hemocytometer, seeded onto a
35 mm-diameter dish and cultured at 37°C for 48 h. When the cell
confluence reached 90%, a scratch was made through the center of
the cell layer using a 20-µl pipette tip. The cells were incubated
using 10% FBS at 37°C for 20 h. Phase-contrast microscopy was
performed at 0, 12, 16 and 20 h on 50 spots, which were randomly
marked on 35-mm dishes containing each cell (Olympus Corporation,
CKX41, original magnification ×200). To investigate cell migration
ratio, the area of each scratch without cell migration was measured
in the same size dimension with the same magnification, using
ImageJ software, ver.1.53. The mean migration of 50 spots in each
cell sheet was examined. Then, the mean value at 0 h was defined as
1.0 and relative cell migration ratio was detected at each time of
each cell.
Correlative light and electron
microscopy (CLEM)
As aforementioned, a scratch was made using a 20-µl
pipette tip on a layer of non-FKD and FKD cells
(2×104/well each) seeded onto the slide chambers (AGC
Techno Co., Ltd). Immunocytochemical staining with anti-fascin-1
mouse monoclonal antibody and FITC-labeled anti-mouse IgG antibody
was performed as aforementioned. Following immunofluorescence
microscopy, cells were fixed with 2.5% glutaraldehyde and 1% osmic
acid at 4°C for 6 h and 1h, respectively. Then, cells were stained
with 1% phosphotungstic acid at room temperature for 10 min and
low-vacuum scanning electron microscopy (LV-SEM) was performed.
Finally, images of immunofluorescence and LV-SEM were superimposed
using ImageJ software, ver.1.53.
Hematoxylin-eosin (HE) and
immunohistochemical stain of non-FKD and FKD MDA-MB-231 cells
cultured in Cellmatrix®
A total of 1×104/ml non-FKD and FKD
MDA-MB-231 cells were counted as aforementioned and Cellmatrix
(Nitta Gelatin Inc.) was prepared according to the manufacturer's
instructions. Subsequently, each group of cells and 500 µl
Cellmatrix® were poured into a 35-mm-diameter dish
(coated with Cellmatrix at 37°C for 30 min), covered with 200 µl
DMEM and cultured at 37°C for 10 days. The gels were fixed in 20%
buffered formaldehyde at room temperature overnight and embedded in
paraffin. Following HE stain (Mayer's hematoxylin and 1% eosin
staining at room temperature for 10 and 5 min, respectively),
immunohistochemical staining for E-cadherin, Snail1 and vimentin
was performed as follows. 4 µm-thick tissue samples were immersed
in 0.01 M citrate buffer (pH 7.0) for antigen retrieval (98°C, 30
min). Sections were immersed in 0.3% hydrogen peroxide/methanol at
room temperature for 10 min to remove endogenous peroxidase. Then,
the sections were blocked using Blocking One (cat. no. 03953-93;
Nacalai Tesque, Inc.) at room temperature for 1 h and incubated at
4°C overnight with following antibodies: anti-E-cadherin mouse
monoclonal antibody (1:100; cat. no. M3612; Dako, Agilent
Technologies, Inc.), Snail1 rabbit polyclonal antibody (1:100; cat.
no. Ap2054a; Adbent, Inc.) and vimentin mouse monoclonal antibody
(1:200; cat. no. M725; Dako, Agilent Technologies, Inc.). After
washing with PBS, sections were incubated in N-Histofine Simple
Stain MAX PO (MULTI; cat. no. 424151; Nichirei Biosciences Inc.) at
room temperature for 1 h and washed again with PBS. Finally, the
sections were immersed in DAB substrate solution (tablet/15 ml
distilled water; SIGMAFAST 3,3′-Diaminobenzin tablets; D4418;
Sigma-Aldrich, Merck KGaA) and the nucleus was stained with Mayer
Hematoxylin at room temperature for 1 min. Optical microscope
images were performed using an Olympus BX53 with cellSens (Olympus
Corporation). An additional set of gels was fixed in 2.5%
glutaraldehyde at 4°C for 6 h for LV-SEM.
Spheroids of non-FKD and FKD
MDA-MB-231 cells
A total of 1×104/well parent, non-FKD and
FKD MDA-MB-231 cells were counted as aforementioned and seeded onto
PrimeSurface® (Sumitomo Bakelite Co., Ltd.), 96-well
plate with ultra-low adhesion round bottom dishes. Following
incubation at 37°C with 5% CO2 for 5 days, multicellular
spheroids were generated. As aforementioned, fixation of the
samples, HE and immunohistochemical staining of fascin and
E-cadherin for microscopy (Olympus BX53), and LV-SEM were
completed. A total of 10 spheroids was collected in a
35-mm-diameter dish (coated with Cellmatrix at 37°C for 30 min)
with 500 µl Cellmatrix, covered with 200 µl DMEM and cultured at
37°C for 3 days. After fixation in 20% buffered formaldehyde at
room temperature for 6 h, the samples were observed under
stereoscopic microscope MZ16FA (Leica Microsystems Tokyo, Japan,
original magnification ×200).
Immunohistochemistry of non-FKD and
FKD MDA-MB-231
Immunohistochemical and immunofluorescent analyses
were performed as previously described (9). The antibodies and chemical agents are
shown in Table I.
 | Table I.Reagents and suppliers. |
Table I.
Reagents and suppliers.
| Reagent | Supplier | Dilution |
|---|
| Anti-fascin-1 mouse
monoclonal antibody | Dako (Agilent
Technologies, Inc.) | 1:100 |
| Anti-vimentin mouse
monoclonal antibody | Dako (Agilent
Technologies, Inc.) | 1:200 |
| Anti-E-cadherin
mouse monoclonal antibody | Dako (Agilent
Technologies, Inc.) | 1:100 |
| Anti-Snail1 rabbit
polyclonal antibody | Abgent, Inc. | 1:100 |
| Anti-GAPDH mouse
monoclonal antibody | ProMab
Biotechnologies, Inc. | 1:2,000 |
| Biotinylated goat
anti-rabbit IgG antibody | Abcam | 1:200 |
| Biotinylated rabbit
anti-mouse IgG antibody | Dako (Agilent
Technologies, Inc.) | 1:200 |
| FITC-labeled
streptavidin | Dako (Agilent
Technologies, Inc.) | 1:200 |
| Texas Red-labeled
anti-rabbit IgG antibody | Molecular Probes
(Thermo Fisher Scientific, Inc.) | 1:200 |
| N-Histofine Simple
Stain MAX PO (MULTI) | Nichirei
Biosciences Inc. | Ready to use |
| DAB | Sigma Aldrich
(Merck KGaA) | Tablet/15 ml
distilled water |
LV-SEM
Samples containing cells or spheroids were fixed
using 2.5% glutaraldehyde in 0.1M phosphate buffer (PB, pH 7.4) at
4°C for 4 h and postfixed with 1% osmium tetroxide in PB at 4°C for
1 h. Then, each block was washed with distilled water for 30 min
and stained with 1% phosphotungstic acid solution at room
temperature for 10 min. Following a final wash with distilled water
for 30 min, the block was dried on an electrically conductive tape
(Nisshin EM Co., Ltd.) and observed using a Miniscope®
TM3030 (Hitachi Ltd.).
Statistical analysis
χ2 test was performed to detect the
association between a TNBC subtype and the incidence of metastasis
or recurrence. Cochran-Armitage test was performed to detect the
relationship between the Allred score and the incidence of
metastasis or recurrence. χ2 test was performed to
analyze the relationship between E-cadherin expression in FKD and
non-FKD cells, and Snail1 expression as well. Tukey-Kramer test was
used to compare the mean values of cell migration ratios in wound
healing assay. Two-side test was applied for all analyses except
Cochran-Armitage test. JMP (ver. 14.3.0, SAS Institute inc.) was
used for statistical analyses.
Results
Clinical data of patients with BC
patients
A total of 11 out of 100 consecutive patients with
BC developed metastasis or recurrence within five years (Table II). Fresh fascin immunostaining was
performed on tissue samples from these patients as well as 17
patients without metastasis or recurrence. χ2 test
result showed a significant association between TNBC subtype and
the incidence of metastasis or recurrence (P<0.05, Table II). Cochran-Armitage test showed a
significant association between the Allred score of fascin and the
incidence of metastasis or recurrence (P<0.05, Table II). However, Cases #2 and #6
developed poor prognosis regardless of negative or slightly
positive fascin expression (0 and 2, respectively). Cases #14 and
#22 did not develop metastasis or recurrence during follow-up
periods, although they showed high Allred scores (6 and 8,
respectively).
 | Table II.Clinical data of patients with breast
cancer. |
Table II.
Clinical data of patients with breast
cancer.
|
|
|
| Hormone receptor
status | Fascin
expression |
|---|
| Case | Age, years | Metastasis or
recurrence |
|
|
|---|
| ER | PgR | HER2 | Proportion | Intensity | Allred score |
|---|
| 1 | 71 | + | - | - | - | 5 | 3 | 8 |
| 2 | 57 | + | + | + | - | 0 | 0 | 0 |
| 3 | 63 | + | + | + | - | 2 | 1 | 3 |
| 4 | 42 | + | + | + | - | 5 | 1 | 6 |
| 5 | 65 | + | + | - | - | 2 | 1 | 3 |
| 6 | 51 | + | - | - | - | 1 | 1 | 2 |
| 7 | 65 | + | - | - | - | 3 | 3 | 6 |
| 8 | 74 | + | + | + | - | 3 | 1 | 4 |
| 9 | 53 | + | + | + | - | 2 | 1 | 3 |
| 10 | 74 | + | + | - | - | 2 | 2 | 4 |
| 11 | 77 | + | - | - | - | 3 | 1 | 4 |
| 12 | 60 | - | + | + | - | 0 | 0 | 0 |
| 13 | 64 | - | + | + | - | 0 | 0 | 0 |
| 14 | 59 | - | + | - | - | 4 | 2 | 6 |
| 15 | 40 | - | + | + | - | 2 | 1 | 3 |
| 16 | 58 | - | + | + | - | 0 | 0 | 0 |
| 17 | 71 | - | + | + | - | 0 | 0 | 0 |
| 18 | 59 | - | + | - | - | 0 | 0 | 0 |
| 19 | 69 | - | + | + | - | 0 | 0 | 0 |
| 20 | 85 | - | + | + | - | 0 | 0 | 0 |
| 21 | 57 | - | + | + | - | 2 | 1 | 3 |
| 22 | 71 | - | - | - | - | 5 | 3 | 8 |
| 23 | 60 | - | + | + | - | 0 | 0 | 0 |
| 24 | 69 | - | + | + | - | 2 | 2 | 4 |
| 25 | 65 | - | + | + | - | 0 | 0 | 0 |
| 26 | 64 | - | + | + | - | 0 | 0 | 0 |
| 27 | 61 | - | + | + | - | 0 | 0 | 0 |
| 28 | 40 | - | + | + | - | 2 | 2 | 4 |
Establishment of FKD MDA-MB-231
cells
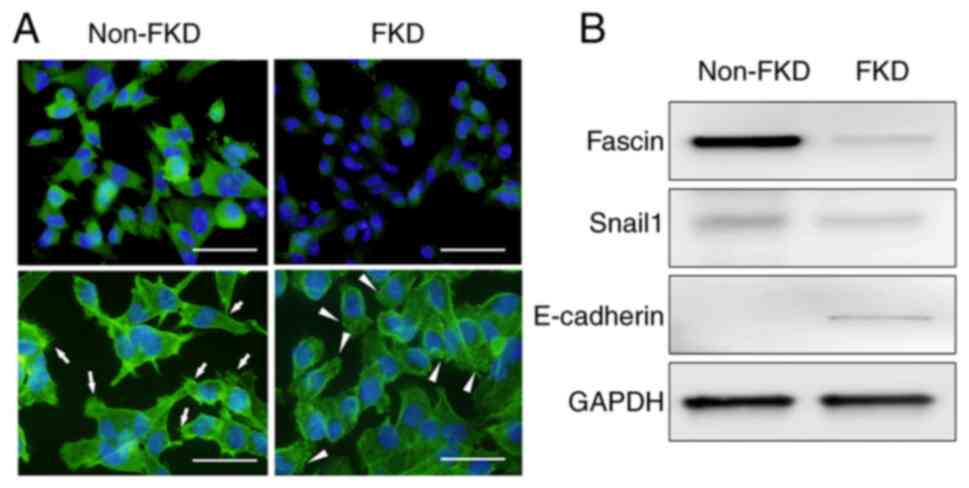
The expression of fascin (green) was strongly
positive in non-FKD cells and effectively suppressed in FKD cells
(Fig. 2A). Numerous filopodia,
including actin filaments (arrows), were observed on the membrane
of non-FKD cells, however, these filopodia were decreased and actin
positive granules (arrowheads) were observed on the membrane of FKD
cells. Fascin was strongly positive in non-FKD cells and was
suppressed in FKD cells (Fig. 2B).
Snail1 expression was also decreased in FKD cells. By contrast,
E-cadherin expression increased in FKD cells.
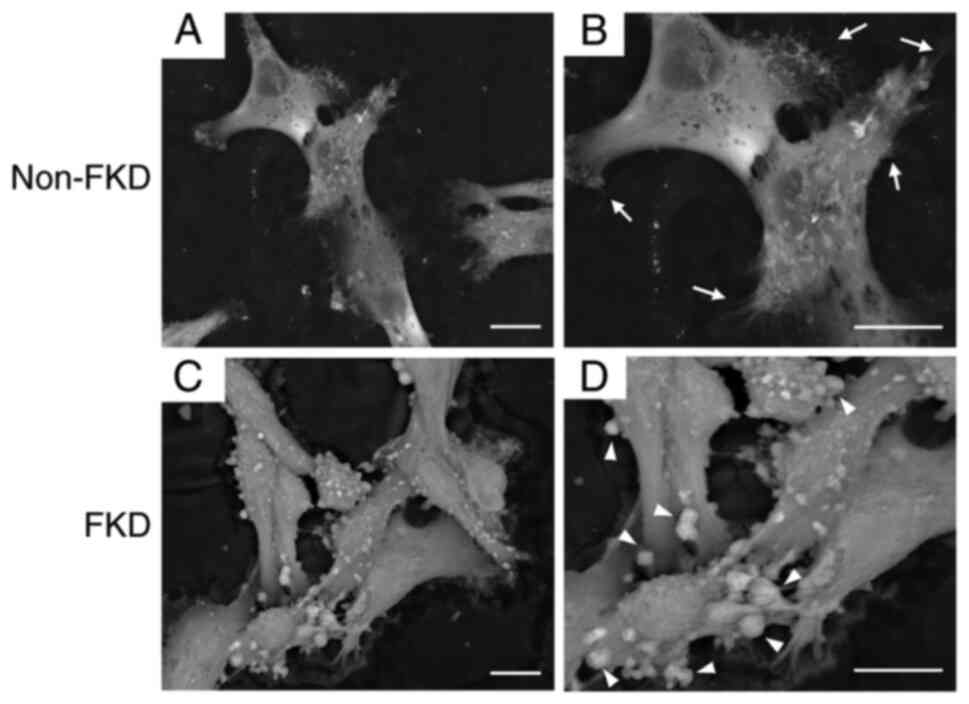
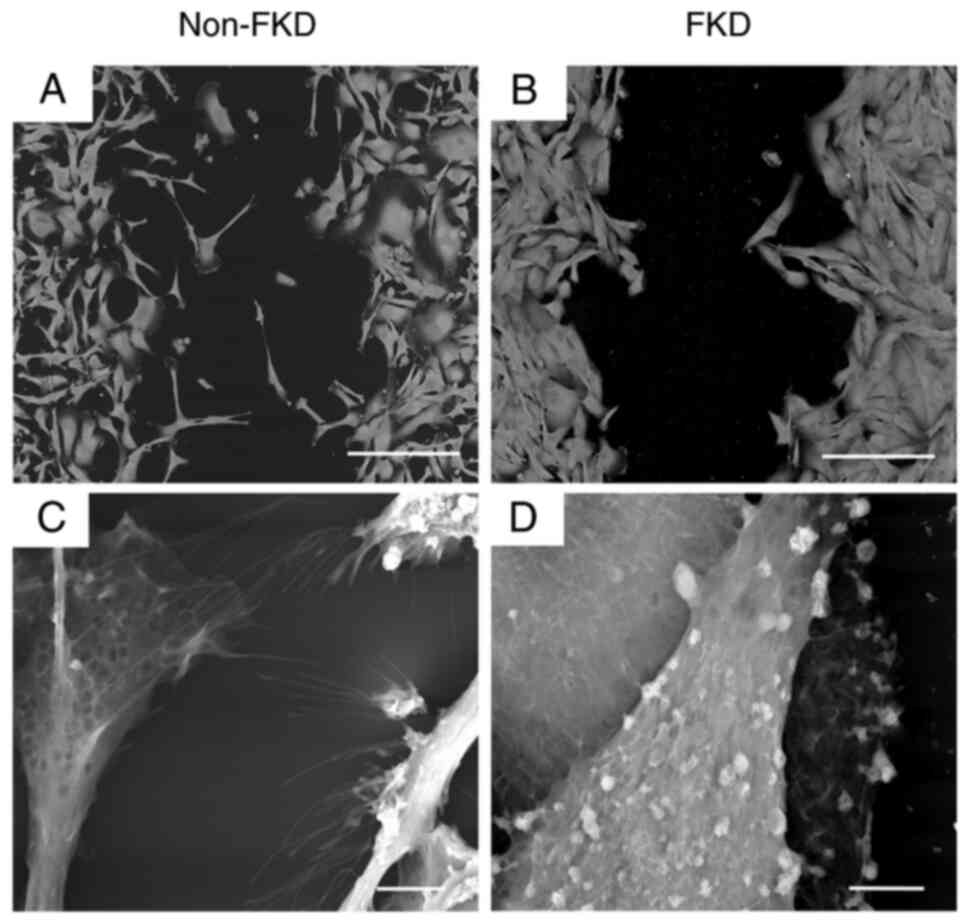
2D LV-SEM were performed following 3 day cultivation
of non-FKD and FKD MDA-MB-231 cells. Non-FKD cells exhibited loose
cell-cell connections (Fig. 3A),
however, bundles of extremely thin microfibrils on the cell surface
were observed (arrows; Fig. 3B).
FKD cells exhibited cell-cell adhesion (Fig. 3C) and granular nodules of various
sizes were observed on the cell surface (arrowheads; Fig. 3D).
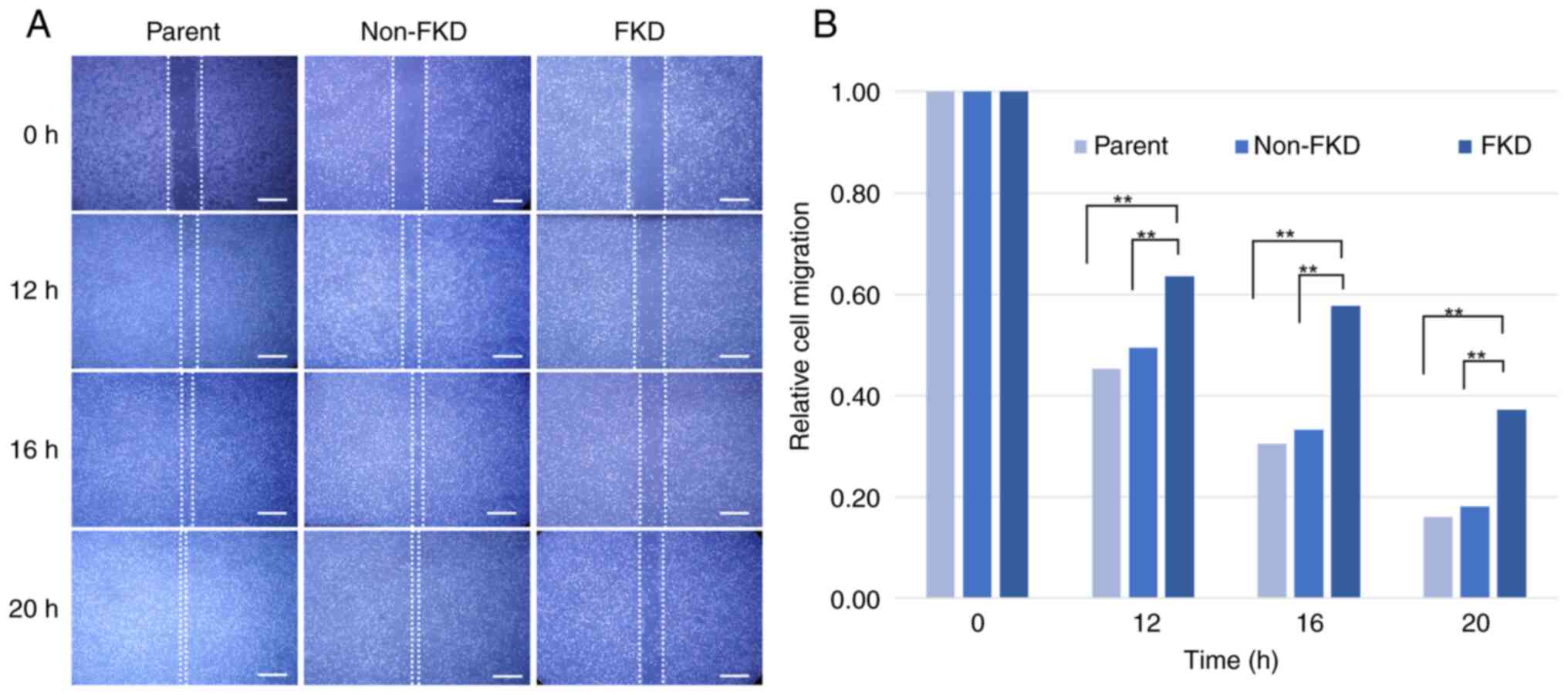
Wound healing assay
Following 20 h incubation, the mean scratch areas in
parent and non-FKD cell sheets decreased notably due to the cell
migration, however, the mean scratch area in FKD cell sheets
decreased only slightly (Fig. 4A and
B). FKD cell migration showed a statistical difference from
parent and non-FKD cells (Tukey-Kramer test, P<0.01).
In 2D LV-SEM observation, non-FKD cell sheet
exhibited loose cell-cell connections (Fig. 5A), whereas the cells of FKD cell
sheet were observed as groups with tight cell-cell connections
(Fig. 5B). Bundles of extremely
thin microfibrils (filopodia) existed on the surface of leading
migratory non-FKD cells (Fig. 5C).
By contrast, globular-shaped lamps of different sizes were observed
on the surface of FKD cells (Fig.
5D).
Correlative light and electron
microscopy (CLEM)
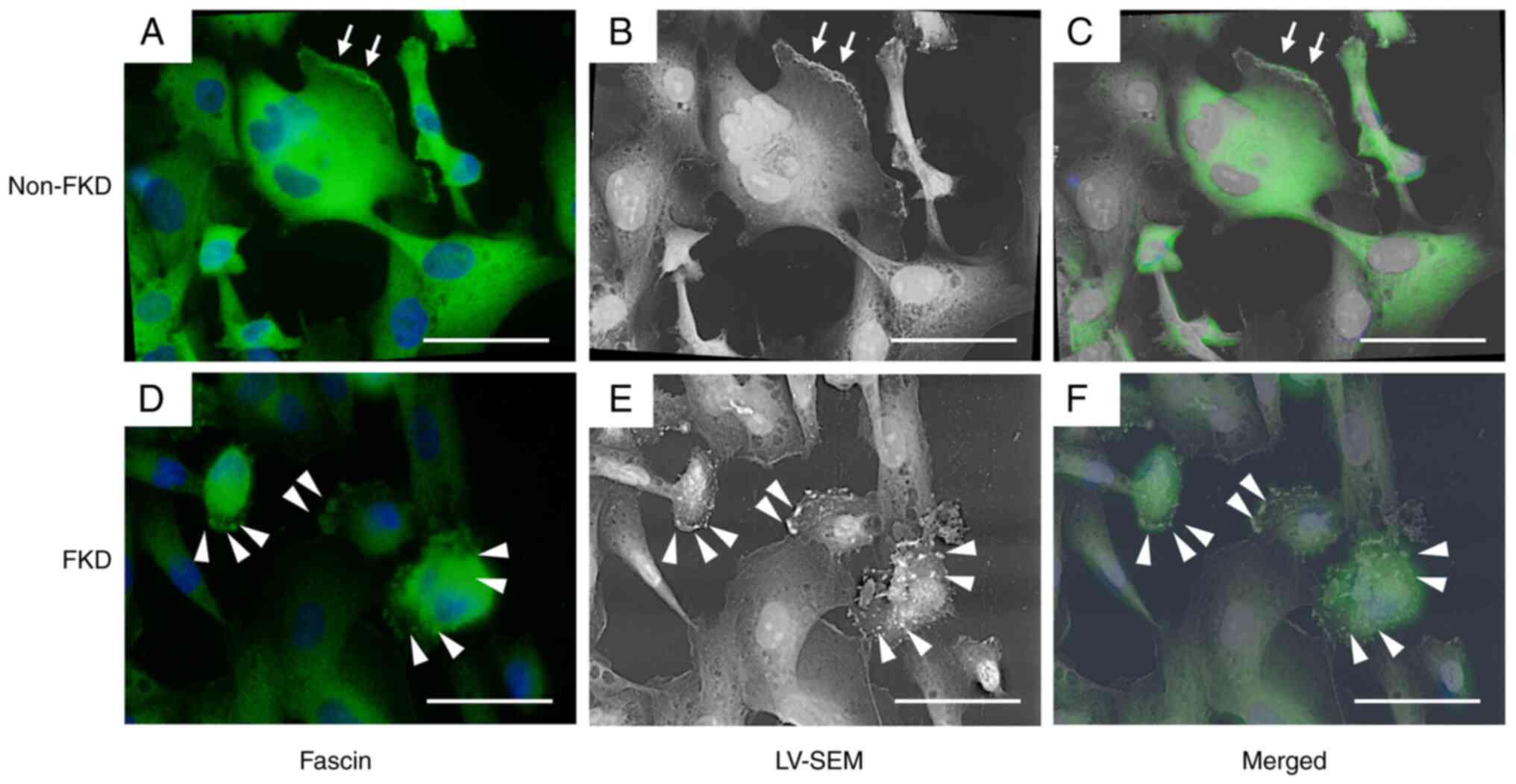
Fascin expression (green; Fig. 6) was strongly positive in non-FKD
cells, which exhibited thin filopodia on the cell membrane
(arrows). Meanwhile, fascin expression was effectively suppressed
in FKD cells, and globular-shaped lamps were observed on the cell
membrane (arrowheads). In LV-SEM, numerous lamps were recognized as
whitish spots on the surface of FKD cells (arrowheads). Thus,
fascin-positive spots observed by optical microscope were
demonstrated to locate at the whitish spots in the bulbous-shaped
protrusions on the FKD cell surface in LV-SEM.
HE and immunohistochemical study
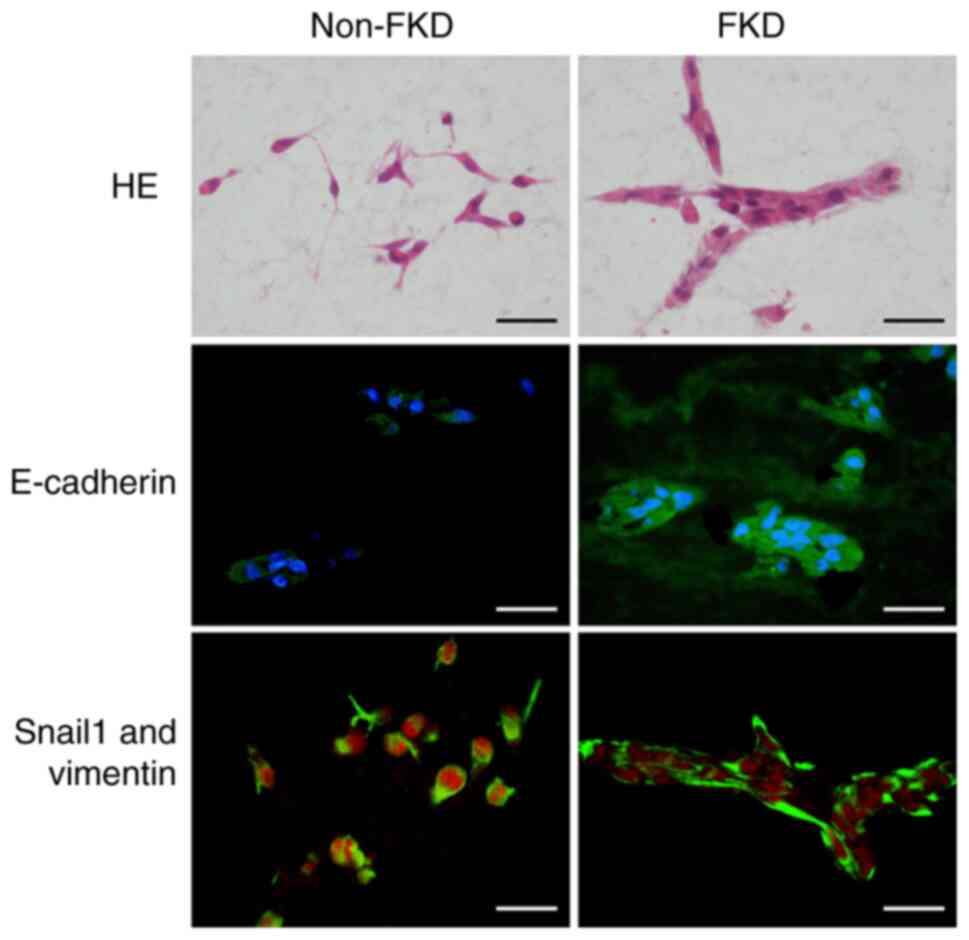
Non-FKD and FKD cells were cultured in
Cellmatrix® for 10 days. Following HE staining, non-FKD
cells were observed to have loose cell-cell adhesions, whereas
clusters with cell-cell connections were observed in FKD cells
(Fig. 7). E-cadherin
immunohistochemical staining was negative in non-FKD cells but
positive in FKD cells. Snail1 expression (red) was observed in the
nucleus of non-FKD cells but was decreased in FKD cells; however,
vimentin was strongly positive in both cell lines (green).
E-cadherin expression was significantly different between non-FKD
and FKD cells, and Snail1 expression as well (P<0.01).
3D LV-SEM observation
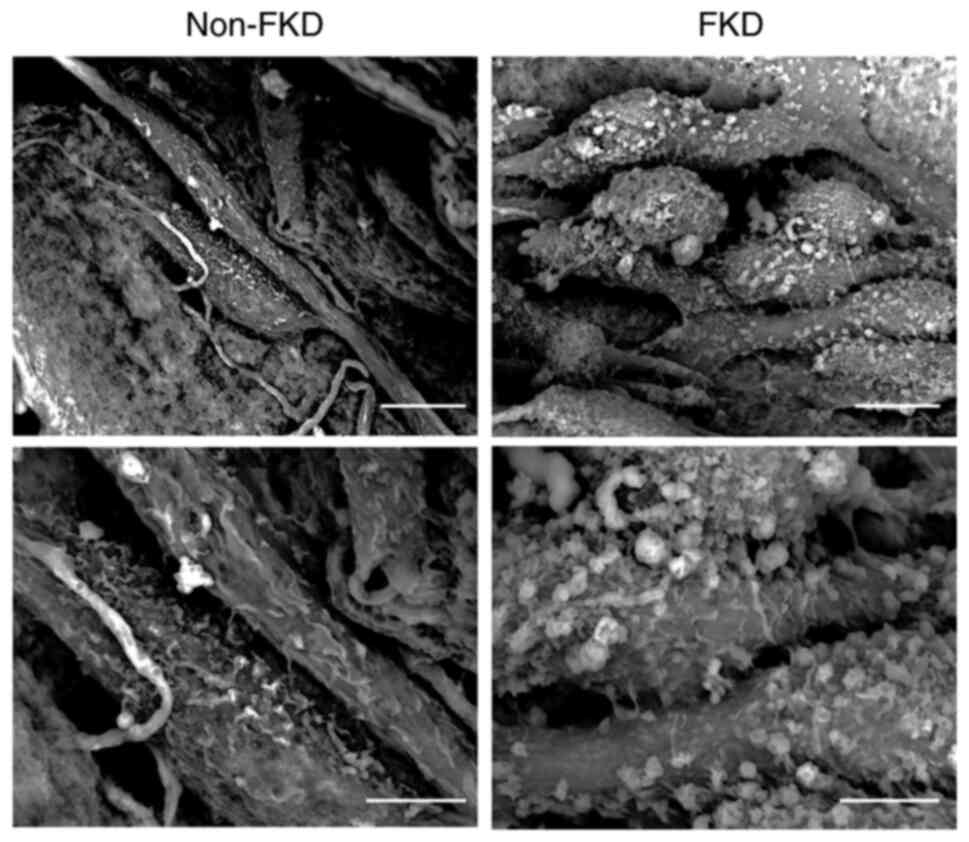
Numerous thin cell protrusions (filopodia) were
observed on the surface of non-FKD cells (Fig. 8). These cells exhibited loose
cell-cell adhesions. By contrast, bulbous-shaped protrusions of
varied sizes were detected on the surface of FKD cells, which
exhibited partial cell-cell adhesions.
Spheroid cell culture
Densely aggregated tumor cells with irregularly
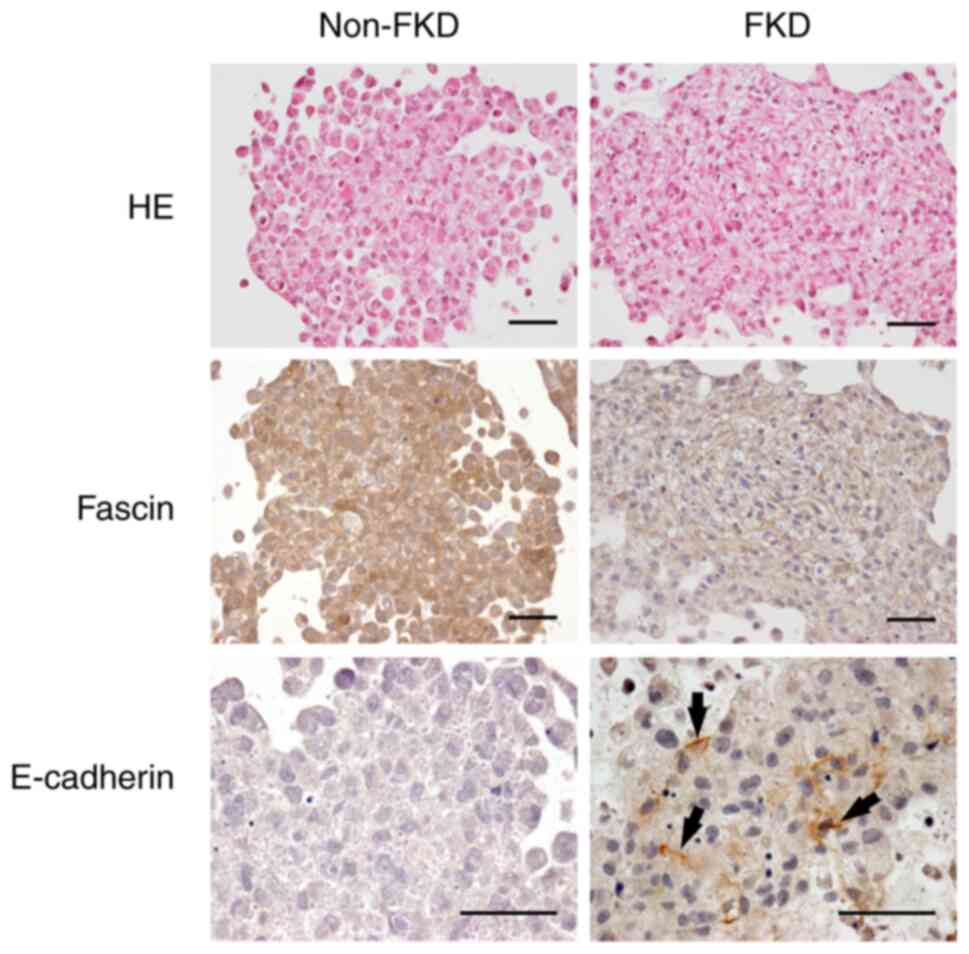
shaped nuclei were observed in both non-FKD and FKD cells (Fig. 9). Fascin expression was strongly
positive in non-FKD cells. Arrows indicate E-cadherin
positive-stained membranes observed in FKD cells.
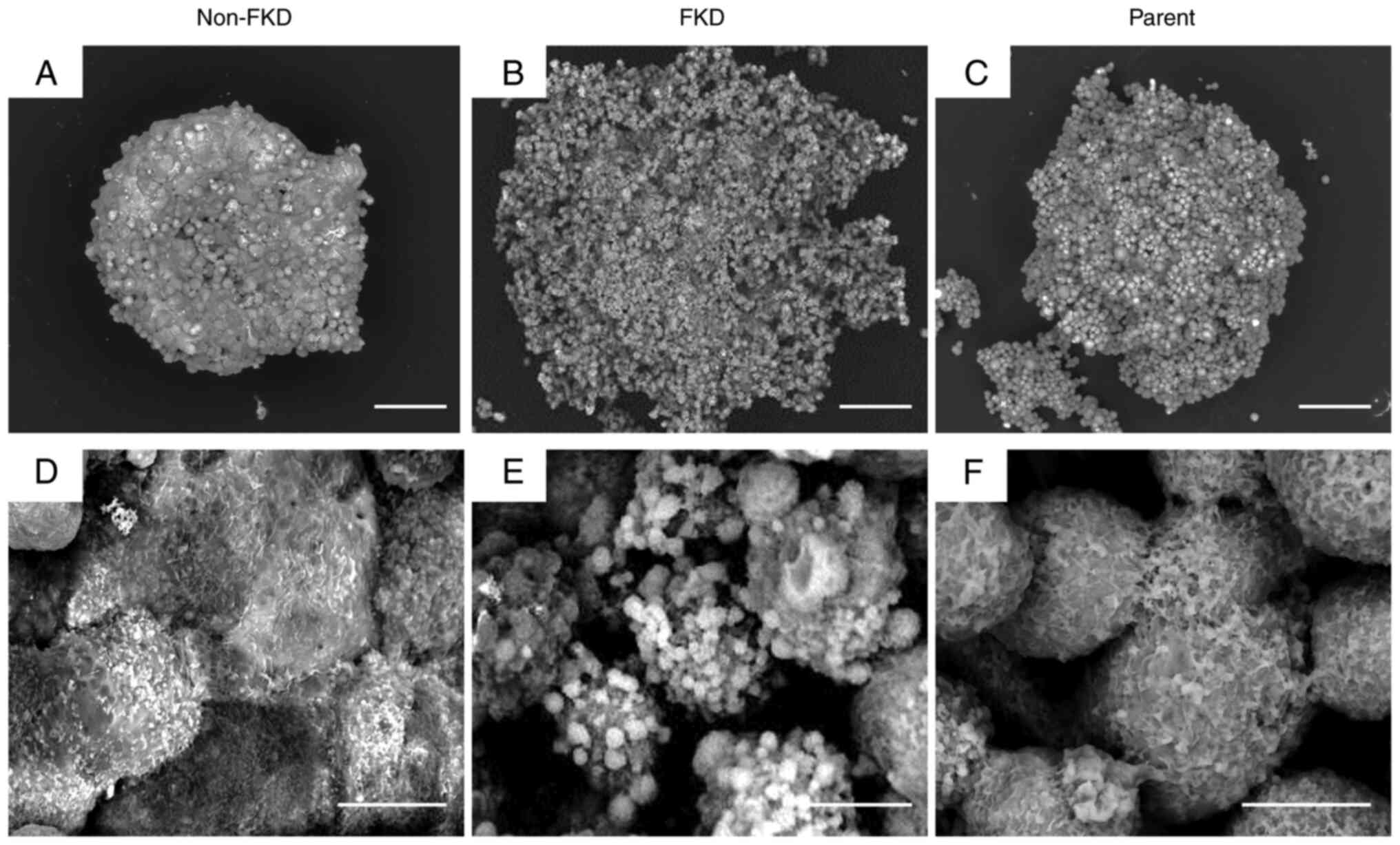
In 3D LV-SEM observation, non-FKD spheroid cells
gathered densely forming a spherical spheroid block, by contrast,
cells of the FKD spheroid gathered sporadically and eventually
formed an irregularly shaped spheroid block (Fig. 10). There were numerous cell
protrusions (filopodia) on the surface of non-FKD spheroid cells,
in contrast, globular-shaped protrusions of varied sizes were
observed on the surface of FKD spheroid cells. The morphological
appearances of parent cells were similar to non-FKD cells.
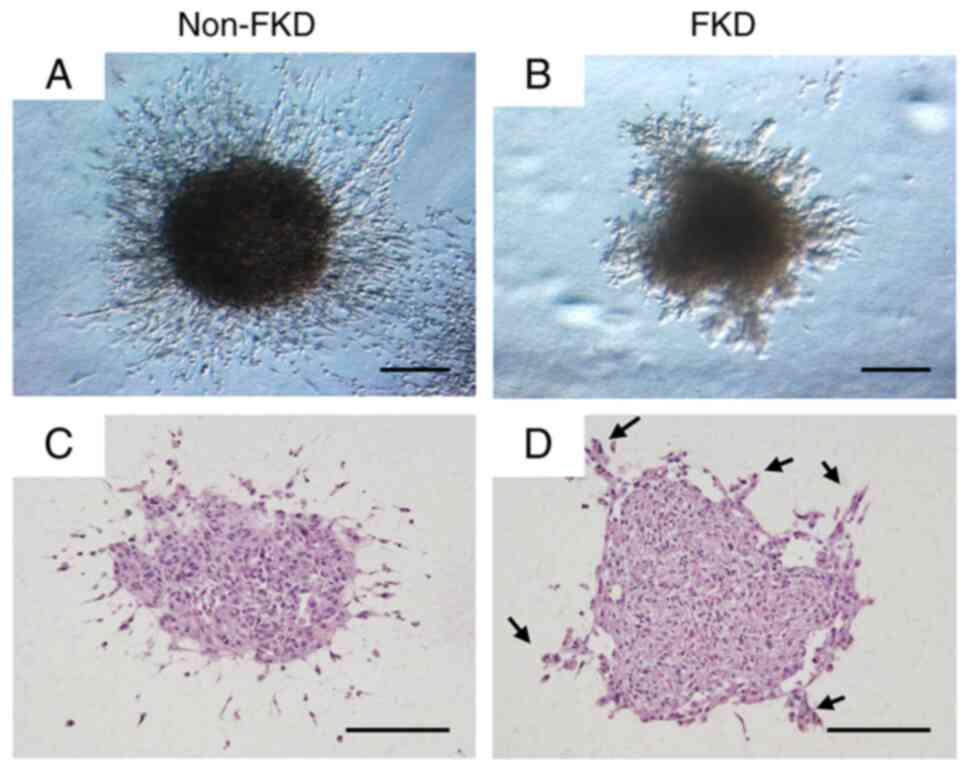
In stereoscopic microscope observation, homogeneous
filamentous cells were observed infiltrating into the surrounding
gel from the non-FKD spheroid (Fig.
11A), while irregular heterogeneous fascicular cell invasion
into the gel was observed from the FKD spheroid (Fig. 11B). HE staining revealed fibrous
spindle-shaped tumor cells invading the surrounding gel from the
non-FKD spheroid (Fig. 11C), while
clusters of tumor cells (arrows; Fig.
11D) originating from the FKD spheroid were observed invading
the gel.
Discussion
BC is the most frequently diagnosed cancer in female
patients. Treatment of BC has improved, however the mortality of BC
remains high among cancer-associated death in female patients
(1–3). In the present study, during 5-year
follow-ups, metastasis or recurrence occurred in 11 out of 100
consecutive Japanese patients diagnosed with early-stage BC at
Kochi University Hospital, Japan, in 2015. In the United States,
similarly, it is reported that nearly 12% of patients with BC
develop metastasis or recurrence (19). However, rising rates of BC incidence
and mortality in underdeveloped countries have been reported
(1,20). Different factors, such as diet,
alcohol, smoking, contraceptive pills and physical exercise may
affect prognosis of BC (2). When BC
is detected and treated at the earlier stages, more favorable
prognosis can be achieved, as with other types of cancer (3,21).
Fascin is an actin-bundling protein. Fascin composes
filopodia, slender bundled actin containing plasma membrane
protrusions, which serve an important role in cellular processes
such as cell adhesion, migration, wound healing, and the formation
of cell-cell contacts by stimulating migration at the leading edge
of cells (22,23). Recent studies have shown that fascin
also localizes to invadopodia, actin-rich protrusions of the plasma
membrane at the adherent cell surface, which facilitate
extracellular matrix invasion (24,25).
Lamb and Tootle (23) suggested
that fascin may serve multiple functions to control cell migration;
decreased fascin and prostaglandin expression induces delayed
migration of border cells and elongated cell clusters (26). Higher fascin expression is
associated with poorer prognosis in numerous types of cancer and
sarcoma. Thus, fascin is considered to be key for cancer
progression (7,10,13,16,17).
The present study investigated the association between fascin and
BC cell invasion via morphological observation of the cytoplasm and
the cell surface. Previous studies have investigated the mechanism
of how fascin increases cell migration and invasiveness to uncover
an effective treatment for malignant tumors, including BC, by
targeting fascin (17,27,28).
Accordingly, at the annual meeting of the American Society of
Clinical Oncology, 2021, Novita Pharmaceuticals presented Phase 1A
results of fascin inhibitor NP-G2-044 in patients with advanced and
metastatic solid tumor, which was shown to be safe and effective
(29).
Despite advances in research, diagnosis and
treatment of BC, TNBC is an aggressive subtype with a high rate of
metastasis or recurrence (1,6,7).
Esnakula et al (7) confirmed
a strong association between TNBC and fascin. Wang et al
(10) suggested that fascin may be
used as a novel diagnostic marker of TNBC. The present results
similarly suggested that fascin may serve as an index to evaluate
malignancy of early-stage BC and there was a significant
association between TNBC subtype and the incidence of metastasis or
recurrence. Patients who developed metastasis with negative or
slightly positive fascin expression were also included in the
present study. Hence, to examine how TNBC cells migrate following
downregulation of fascin, the present study established FKD and
non-FKD MDA-MB-231 cells and performed wound healing assay,
spheroid cell cultures, and 2D and 3D LV-SEM.
MDA-MB-231, a cell line isolated from a patient with
invasive ductal BC, is characterized by negative ER, PR and
E-cadherin expression and p53 mutation (30). The cells also lack growth factor
receptor HER2 and represent a good model of TNBC (30). Hoa et al (31) knocked down fascin in human U251
glioma cells and confirmed that these cells lost microvilli and
altered the glioma morphology. The present study successfully
established FKD and non-FKD TNBC cells using MDA-MB-231.
The wound healing assay may be performed under
various circumstances, such as mechanical, thermal or chemical
damage, however, it is a 2D approach and its utility is limited to
the observation of cells migrating as a collective epithelial sheet
(32). Therefore, the wound healing
assay does not simulate the natural environment where cells exist
with cell-cell and cell-extracellular matrix interactions. A more
ideal in vitro cell model that facilitates observation of
migration of malignant cells is 3D spheroid cell culture. Compared
with 2D monolayer culture, such as wound healing assay, spheroid
cell cultures more accurately reflect the microenvironment in
vivo (32).
LV-SEM in the present study demonstrated that FKD
morphologically modified the surface of TNBC cells. When fascin was
knocked down, cells lost filopodia, which are key for cell
migration into the surrounding microenvironment. FKD cells
developed granular lamps of various sizes that facilitated
cell-cell connection. The spheroid culture showed that following
FKD, tumor cells developed clusters and migrated into the
surrounding gel. Fox et al (26) demonstrated that fascin promotes
single cell migration; the present findings suggested that
suppression of fascin induced collective migration of TNBC cells.
The wound healing assay indicated that cell migration ability was
impaired due to FKD, however, 3D spheroid cell culture suggested
that modification of the surface of tumor cells facilitated
collective cell migration. These results indicated that TNBC cells
maintained the ability to migrate following FKD via collective cell
migration.
Cancer metastasis is a radical progression of
malignant cells that migrate into the surrounding microenvironment
and its mechanism is generally classified as single or collective
cell migration (15,33). Single cell migration has been
studied widely, primarily using 2D cell cultivation methods that do
not reflect the in vivo environment (33–36).
The theory of collective cell migration is relatively new. 3D
methods, such as a spheroid cell culture used in the present study,
facilitate analysis of collective cell migration, which is
considered to be a primary mechanism of cancer metastasis (33–36).
However, cell migration process does not occur only at the single
focal adhesion level, but it is also affected by the integration
with surrounding cells (36). Thus,
it is indispensable to observe the mechanism of different migration
patterns. The present study performed the classic 2D wound healing
assay and modern 3D spheroid cell culture, which indicated that
MDA-MB-231 cells exhibited collective migration following FKD.
Certain patients did not develop metastasis or
recurrence despite strong positive fascin expression. The Allred
scores of Cases #14 and #22 were 6 and 8, respectively. These
patients may have exhibited more favorable prognosis because the
cancer subtype was not TNBC. These patients had detected breast
tumors by self-exam, had an immediate consultation with a
specialist and underwent surgery within two months of self-exam.
Regular breast self-exam can detect cancer at an early stage,
allowing more effective and less invasive treatment and leading to
a more favorable prognosis (3,21).
The present study performed CLEM, one of the most
advanced methods to observe cells morphologically and understand
the dynamics of organelles. Recently, CLEM has achieved insights
into cell biology by making it possible to observe the same area as
an optical microscope with an electron microscope (37–39).
Here, CLEM demonstrated fascin-positive spots observed by optical
microscope located at the whitish spots in the bulbous-shaped
protrusions on the FKD cell surface in LV-SEM.
The present study demonstrated collective migration
of TNBC cells by 3D LV-SEM. TNBC cells may migrate into the
surrounding microenvironment through collective migration in FKD
cells that lack filopodia on the cell surface.
Acknowledgements
The authors would like to thank Dr Nobuaki Yamanaka,
Dr Kazuho Honda, and Mr. Takeshi Kamimura (The LVSEM Study Group of
Renal Biopsy, Tokyo, Japan) for technical assistance of the LVSEM
equipment.
Funding
The present study was supported by grant research No. 006 of the
LVSEM Study Group of Renal Biopsy from the LVSEM Study Group of
Renal Biopsy and Hitachi High-Tech.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and YH conceived the study and performed
experiments. YH and IM evaluated Allred score of fascin expression.
YY and HS performed statistical analysis. YY wrote the manuscript.
IM supervised the study. YY and YH confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethic Committee for Clinical Research of the School of Medicine,
Kochi University (approval no. 2020-123). Written consent to
participate was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fahad Ullah M: Breast cancer: Current
perspectives on the disease status. Adv Exp Med Biol. 1152:51–64.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coughlin SS: Epidemiology of breast cancer
in women. Adv Exp Med Biol. 1152:9–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Bray F, Ferlay J,
Lortet-Tieulent J, Anderson BO and Jemal A: International variation
in female breast cancer incidence and mortality rates. Cancer
Epidemiol Biomarkers Prev. 24:1495–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Statistics, . Cancer Information
Service, National Cancer Center, Japan (National Cancer Registry,
Ministry of Health, Labour and Welfare). https://ganjoho.jp/public/index.html
|
|
5
|
Katanoda K, Ito Y and Sobue T:
International comparison of trends in cancer mortality: Japan has
fallen behind in screening-related cancers. Jpn J Clin Oncol.
51:1680–1686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grayson M: Breast cancer. Nature.
485:S492012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esnakula AK, Ricks-Santi L, Kwagyan J,
Kanaan YM, DeWitty RL, Wilson LL, Gold B, Frederick WA and Naab TJ:
Strong association of fascin expression with triple negative breast
cancer and basal-like phenotype in African-American women. J Clin
Pathol. 67:153–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto Y, Hayashi Y, Sakaki H and
Murakami I: Fascin-1 is associated with recurrence in solitary
fibrous tumor/hemangiopericytoma. Mol Clin Oncol. 15:1992021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CQ, Tang CH, Chang HT, Li XN, Zhao
YM, Su CM, Hu GN, Zhang T, Sun XX, Zeng Y, et al: Fascin-1 as a
novel diagnostic marker of triple-negative breast cancer. Cancer
Med. 5:1983–1988. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arlt MJ, Kuzmanov A, Snedeker JG, Fuchs B,
Silvan U and Sabile AA: Fascin-1 enhances experimental osteosarcoma
tumor formation and metastasis and is related to poor patient
outcome. BMC Cancer. 19:832019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richmond AM, Blake EA, Torkko K, Smith EE,
Spillman MA and Post MD: Fascin is associated with aggressive
behavior and poor outcome in uterine carcinosarcoma. Int J Gynecol
Cancer. 27:1895–1903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
14
|
Yamamoto Y, Hayashi Y, Sakaki H and
Murakami I: Evaluation of clinical and immunohistochemical factors
relating to melanoma metastasis: Potential roles of nestin and
fascin in melanoma. Diagnostics (Basel). 12:2192022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamb MC, Kaluarachchi CP, Lansakara TI,
Mellentine SQ, Lan Y, Tivanski AV and Tootle TL: Fascin limits
Myosin activity within Drosophila border cells to control substrate
stiffness and promote migration. Elife. 10:e698362021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abbasi A, Noroozinia F, Anvar S, Abbasi M,
Hosseinzadeh S and Mokhtari S: Fascin overexpression is associated
with higher grades of breast cancer. Pol J Pathol. 70:264–268.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xing P, Li JG, Jin F, Zhao TT, Liu Q, Dong
HT and Wei XL: Fascin, an actin-bundling protein, promotes breast
cancer progression in vitro. Cell Biochem Funct. 29:303–310. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arjonen A, Kaukonen R and Ivaska J:
Filopodia and adhesion in cancer cell motility. Cell Adh Migr.
5:421–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peart O: Metastatic breast cancer. Radiol
Technol. 88:519M–539M. 2017.PubMed/NCBI
|
|
20
|
Iqbal J, Ginsburg O, Rochon PA, Sun P and
Narod SA: Differences in breast cancer stage at diagnosis and
cancer-specific survival by race and ethnicity in the United
States. JAMA. 313:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ginsburg O, Yip CH, Brooks A, Cabanes A,
Caleffi M, Dunstan Yataco JA, Gyawali B, McCormack V, McLaughlin de
Anderson M, Mehrotra R, et al: Breast cancer early detection: A
phased approach to implementation. Cancer. 126 (Suppl
10):S2379–S2393. 2020. View Article : Google Scholar
|
|
22
|
Mattila PK and Lappalainen P: Filopodia:
Molecular architecture and cellular functions. Nat Rev Mol Cell
Biol. 9:446–454. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamb MC and Tootle TL: Fascin in cell
migration: more than an actin bundling protein. Biology (Basel).
9:4032020.PubMed/NCBI
|
|
24
|
Lin S, Li Y, Wang D, Huang C, Marino D,
Bollt O, Wu C, Taylor MD, Li W, DeNicola GM, et al: Fascin promotes
lung cancer growth and metastasis by enhancing glycolysis and
PFKFB3 expression. Cancer Lett. 518:230–242. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Song M, Liu M, Zhang G, Zhang X,
Li MO, Ma X, Zhang JJ and Huang XY: Fascin inhibitor increases
intratumoral dendritic cell activation and anti-cancer immunity.
Cell Rep. 35:1089482021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fox EF, Lamb MC, Mellentine SQ and Tootle
TL: Prostaglandins regulate invasive, collective border cell
migration. Mol Biol Cell. 31:1584–1594. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin S, Taylor MD, Singh PK and Yang S: How
does fascin promote cancer metastasis? FEBS J. 288:1434–1446. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ristic B, Kopel J, Sherazi SAA, Gupta S,
Sachdeva S, Bansal P, Ali A, Perisetti A and Goyal H: Emerging role
of fascin-1 in the pathogenesis, diagnosis, and treatment of the
gastrointestinal cancers. Cancers (Basel). 13:25362021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung V, Jhaveri KL, Van Hoff DD Von,
Huang XY, Garmey EG, Zhang J and Tsai FYC: Phase 1A clinical trial
of the first-in-class fascin inhibitor NP-G2-044 evaluating safety
and anti-tumor activity in patients with advanced and metastatic
solid tumors. J Clin Oncol. 39 (15 Suppl):S25482021. View Article : Google Scholar
|
|
30
|
JoEllen W: Chapter 40 - Animal Models for
Studying Prevention and Treatment of Breast Cancer. Animal Models
for the Study of Human Disease. Elsevier; Amsterdam: pp. 997–1018.
2013
|
|
31
|
Hoa NT, Ge L, Erickson KL, Kruse CA,
Cornforth AN, Kuznetsov Y, McPherson A, Martini F and Jadus MR:
Fascin-1 knock-down of human glioma cells reduces their
microvilli/filopodia while improving their susceptibility to
lymphocyte-mediated cytotoxicity. Am J Trransl Rransl Res.
7:271–284. 2015.
|
|
32
|
Huang Z, Yu P and Tang J: Characterization
of triple-negative breast cancer MDA-MB-231 cell spheroid model.
Onco Targets Ther. 13:5395–5405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trepat X, Chen Z and Jacobson K: Cell
migration. Compr Physiol. 2:2369–2392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamb MC, Anliker KK and Tootle TL: Fascin
regulates protrusions and delamination to mediate invasive,
collective cell migration in vivo. Dev Dyn. 249:961–982. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lintz M, Muñoz A and Reinhart-King CA: The
mechanics of single cell and collective migration of tumor cells. J
Biomech Eng. 139:0210051–0210059. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Pascalis C and Etienne-Manneville S:
Single and collective cell migration: The mechanics of adhesions.
Mol Biol Cell. 28:1833–1846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanada T, Yamaguchi J, Furuta Y, Kakuta S,
Tanida I and Uchiyama Y: In-resin CLEM of Epon-embedded cells using
proximity labeling. Sci Rep. 12:111302022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng D, Li N, He W, Drasbek KR, Xu T,
Zhang M and Xu P: Improved fluorescent proteins for dual-colour
post-embedding CLEM. Cells. 11:10772022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Van den Dries K, Fransen J and Cambi A:
Fluorescence CLEM in biology: Historic developments and current
super-resolution applications. FEBP Lett. 596:2486–2496. 2022.
View Article : Google Scholar : PubMed/NCBI
|