Introduction
Pancreatic cancer (PaCa) is one of the most
intractable malignancies in the digestive system. The 5-year
overall survival (OS) rate for PaCa is ~11%, one of the lowest
among the various carcinomas (1).
One likely reason is the high local invasive and metastatic
potential in PaCa. PaCa can easily invade adjacent tissues (e.g.,
major blood vessels and nerves), and it is not rare for PaCa to be
unresectable or to have metastasized at the time of diagnosis.
Therefore, suppressing the migratory ability of cancer cells (which
is an important process in tissue invasion and metastasis), is
important in the treatment of PaCa. In addition, in a previous
study, it was reported that there is a correlation between the
metastatic potential and angiogenic potential of PaCa (2). However, the molecular biological
mechanisms involved in PaCa invasion and angiogenesis have not yet
been fully elucidated.
In the present study, Girdin was the protagonist,
which was identified in 2005, as a novel substrate of Akt,
serine/threonine kinase, a product of the proto-oncogene AKT1.
Girdin is activated by Akt and is involved in the formation of
lamellipodia, which are important for cell motility, through the
reorganization of actin fibers that form the cytoskeleton (3–5).
Girdin also has a wide range of other functions, including cell
proliferation and microangiogenesis (6–8).
Girdin is the product of the CCDC88A gene (Coiled-Coil
Domain Containing 88A). It is also called Akt phosphorylation
enhancer (APE) (9–11) or guanine nucleotide-binding protein
α subunit (Gα)-interacting vesicle-associated protein (GIV) and is
involved in a wide variety of functions. Previously, Girdin has
been reported to be involved in migration invasion and angiogenesis
in some carcinomas (12,13). It has been previously reported that
the expression of Girdin in esophageal carcinoma correlates with
the depth and prognosis of the tumor (14). In the present study, tumor migration
and angiogenesis in PaCa was the primary objective, which is more
aggressive than esophageal cancer.
To the best of our knowledge, there are only a few
studies on substances that inhibit Girdin. Scutellarin (SCU;
PubChem ID: 185617) is a flavonoid that inhibits signal transducer
and activator of transcription 3 (STAT3)/Girdin/Akt signaling in
hepatocellular carcinoma, and it suppresses tumor invasion and
metastasis (15). SCU is a natural
compound known for its antitumor, hepatoprotective and
neuroprotective effects, and has long been used in China for the
treatment of cerebrovascular diseases (16). There have been studies reporting its
tumor suppressive effects in other carcinomas (17–19).
However, there are no studies focusing on the antitumor effects of
SCU in PaCa.
In the present study, the function of Girdin in PaCa
was analyzed and the role of Girdin in tumor progression was
elucidated. The inhibitory effect of SCU on Girdin in PaCa and its
tumor suppressive effect was also investigated.
Materials and methods
Antibodies and chemicals
The primary antibodies polyclonal rabbit anti-Girdin
(cat. no. ab113890), for immunohistochemical (IHC) staining, and
anti-GIV [EPR18433] (cat. no. ab179481), for western blotting, were
purchased from Abcam. In addition, the polyclonal rabbit
anti-phosphate-Girdin (Tyr-1746) antibody (cat. no. GP5801) was
purchased from ECM Biosciences, monoclonal mouse anti-β-Actin
(8H10D10) antibody (cat. no. 3700) from Cell Signaling Technology,
Inc., and monoclonal mouse anti-GAPDH (0411) antibody (sc-47724)
from Santa Cruz Biotechnology, Inc. The secondary antibodies used
in the present study included polyclonal goat anti-rabbit
immunoglobulins/HRP (cat. no. P0448), polyclonal rabbit anti-mouse
immunoglobulins/HRP (P0161) (Dako A/S; Agilent Technologies, Inc.)
goat anti-rabbit IgG H&L (Cy3®) (cat. no. ab6939)
and goat anti-mouse IgG H&L (Alexa Fluor® 488; cat.
no. ab150113; Abcam). SCU analytical standard (cat. no. 73577) was
purchased from Sigma-Aldrich and recombinant human epidermal growth
factor (EGF) (236-EG) was purchased from R&D Systems, Inc.
Patients and tumor samples
The patients included in the present study underwent
surgery due to a diagnosis of PaCa at Nagoya City University
Hospital from June 2006 to August 2016. All patients were monitored
for at least 5 years after surgery, except for death and data
censoring. In addition, in the study of clinicopathological
characteristics, cases with no pathological findings described were
excluded. The present retrospective study was approved (approval
no. 60-18-0025) by the Institutional Review Board (Ethical Review
Committee for Medical Research, Nagoya City University Hospital,
Nagoya, Japan). Written informed consent was obtained from all
patients. Clinicopathological characteristics of patients are
listed in Table I.
 | Table I.Association of Girdin expression with
clinicopathological characteristics. The clinicopathological
characteristics of the two patient groups whose tumors stained
strongly and weakly in immunohistochemistry for Girdin.
Pathological findings were not available in two cases, and a total
of 88 cases were analyzed (Mean age: Mann-Whitney U test, Others:
Fisher's exact test). |
Table I.
Association of Girdin expression with
clinicopathological characteristics. The clinicopathological
characteristics of the two patient groups whose tumors stained
strongly and weakly in immunohistochemistry for Girdin.
Pathological findings were not available in two cases, and a total
of 88 cases were analyzed (Mean age: Mann-Whitney U test, Others:
Fisher's exact test).
|
| Expression level of
Girdin |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | Weakly stained
(n=28) | Strongly stained
(n=60) | P-value |
|---|
| Sex |
|
|
|
|
Male | 21 | 40 |
|
|
Female | 7 | 20 | 0.47 |
| Mean age,
years | 69 | 67 | 0.48 |
| IQR, years | 63-74 | 60-75 |
|
| TNM factors |
|
|
|
|
T1/2/3/4 | 4/3/17/4 | 4/3/29/24 |
|
|
T1-3 | 24 | 36 |
|
| T4 | 4 | 24 | 0.02 |
|
N0/1/2/3 | 14/11/2/1 | 22/25/10/3 |
|
| N- | 14 | 22 |
|
| N+ | 14 | 38 |
|
|
M0/1 | 28/0 | 56/4 | 0.3 |
| TNM stage |
|
|
|
|
1/2/3/4 | 4/2/15/7 | 4/2/21/33 |
|
|
1-3 | 21 | 27 |
|
| 4 | 7 | 33 | 0.01 |
| Venous
invasion |
|
|
|
|
v0/1/2/3 | 6/13/7/2 | 12/22/15/11 |
|
| v- | 6 | 12 |
|
| v+ | 22 | 48 | 1 |
| Lymphatic
invasion |
|
|
|
|
ly0/1/2/3 | 3/19/3/3 | 6/33/10/11 |
|
|
ly- | 3 | 6 |
|
|
ly+ | 25 | 54 | 1 |
| Neural
invasion |
|
|
|
|
ne0/1/2/3 | 3/13/9/3 | 15/16/11/18 |
|
|
ne- | 3 | 15 |
|
|
ne+ | 25 | 45 | 0.16 |
IHC staining
IHC staining using an anti-Girdin antibody was
performed in PaCa tissues obtained from surgically resected
specimens at Nagoya City University Graduate School of Medical
Sciences. The IHC staining procedure was performed as previously
described (20).
Survival Kaplan-Meier analysis
Kaplan-Meier plots were generated based on the
results of immunohistochemistry at our own institution and
validated for OS and RFS with logrank test. In addition, prognostic
predictions based on mRNA were also generated using the
Kaplan-Meier plotter online tool (https://kmplot.com/analysis/, accessed September 8,
2020).
Cell culture
Human PaCa cell lines [MIA PaCa-2 (cat. no.
CRL-1420™), SW 1990 (cat. no. CRL-2172™), AsPC-1 (cat. no.
CRL-1682™), BxPC-3 (cat. no. CRL-1687™), PANC-1 (cat. no.
CRL-1469™), Capan-2 (cat. no. HTB-80™) (21)] and the human pancreatic duct
epithelial cell line H6c7 (cat. no. PCS-600-010) were obtained from
the American Type Culture Collection (ATCC). The EA.hy926 cell line
(cat. no. CRL-2922™), used as immortalized human umbilical vein
endothelial cell (HUVECs) in the present study, was also purchased
from ATCC. The AsPC-1 and BxPC-3 cell lines were cultured in
RPMI-1640 medium, whereas the other PaCa cell lines and the
EA.hy926 cell line were cultured in DMEM (both media from
Sigma-Aldrich; Merck KGaA). Each medium was supplemented with 10%
FBS, 10 mg/ml streptomycin, 10,000 U/ml penicillin and 25 µg/ml
amphotericin B (all from Gibco; Thermo Fisher Scientific, Inc.).
The H6c7 cell line was cultured in keratinocyte SFM basal medium
with 2.5 µg/ml EGF and 25 mg/ml bovine pituitary extract
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 1X
antibiotic-antimycotic (Gibco; Thermo Fisher Scientific, Inc.). All
the cells were incubated at 37°C in a humidified incubator
containing 5% CO2.
Immunofluorescence staining
MIA PaCa-2, AsPC-1 and PANC-1 cell lines were seeded
on glass slides at 2×105 cells per slide and allowed to
adhere in optimal medium for 24 h. The cells were then incubated
for 2 h at 37°C in medium with or without reagents. Cells were
fixed and permeabilized by incubation with cold methanol on ice for
20 min and blocked with 3% BSA (Wako Chemicals USA, Inc.) for 1 h
at room temperature. Immunofluorescence staining was performed
using anti-β-actin mouse antibody and anti-Girdin (phospho Y1764)
rabbit antibody as primary antibodies for 1 h at room temperature.
Anti-mouse goat IgG (Alexa Fluor® 488; green) and
anti-rabbit goat IgG (Cy3; red) were used as secondary antibodies
for 1 h at room temperature under shading, and DAPI for nuclear
staining (blue) [ProLong® Gold Antifade Reagent with
DAPI (cat. no. 8961; Cell Signaling Technology, Inc.) was applied a
few drops per slide]. Fluorescence intensities were observed using
a fluorescence microscope BZ-X710 at ×100 field of view (Keyence
Co., Ltd.).
Small interfering RNA (siRNA)-mediated
knockdown of Girdin
Girdin was knocked down by RNA interference. Thermo
Fisher's Silencer® Select pre-Designed and Validated
siRNA was used. Cells were transfected with Girdin siRNA (assay ID:
s31296, cat. no. 4392420) or negative control siRNA (Select
Negative Control No.1, cat. no. 4390844) using
Lipofectamine® RNAiMAX (Both reagents were purchased
from Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The sequences of siRNAs were not
available from the supplier due to confidentiality issues.
Concretely, the transfection of siRNA into cells was performed as
follows: 1×106 cells were seeded per well using Falcon
six-well Clear Flat Bottom TC-treated Multiwell Cell Culture
Plates. A total of 106 cells were seeded and left
overnight to settle, then 25 mol siRNA and 7.5 µl Lipofectamine
RNAiMAX per well were mixed at room temperature (Opti-MEM medium
was used as solvent). After 5 min, the cells were added to each
well and incubated for 48 h before being used as a knockdown cell
line.
Reverse transcription-quantitative PCR
(RT-PCR) analysis
The total RNA extraction from cells, purification of
cDNA obtained by reverse transcription, and subsequent real-time
PCR procedures were performed as previously described (22). The primer probes used were from
TaqMan Gene Expression Assays and were purchased from Thermo Fisher
Scientific, Inc. (CCDC88A: H01554974_m1, GAPDH:
Hs9999999905_m1, VEGF-A: Hs00900055_m1).
Western blotting
Proteins were extracted from cultured cells (H6c7,
MIA PaCa-2, SW 1990, AsPC-1, BxPC-3, PANC-1 and Capan-2) using RIPA
buffer containing Protease Inhibitor Single Use Cocktail and
Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The protein
concentrations were measured using the Pierce BCA protein assay kit
(Thermo Fisher Scientific, Inc.). A total of 20 µg of each protein
extract was denatured at 90°C for 5 min and separated on 10%
Mini-PROTEAN TGX Precast gels (Bio-Rad Laboratories, Inc.). The
protein bands were transferred to nitrocellulose membranes and
blocked in iBind Flex Solution (iBind Flex Buffer, iBind Flex
Additive and distilled water; Thermo Fisher Scientific, Inc.) for
15 min at room temperature. The primary and secondary antibody
reactions were performed using the iBind Flex Western System
(Thermo Fisher Scientific, Inc.) for 3 h at room temperature
according to the manufacturer's instructions. The membranes were
incubated with anti-GIV (EPR18433; (1:1,000), polyclonal rabbit
anti-phosphate-Girdin (Tyr-1746; 1:500), or anti-GAPDH (0411)
(1:2,000) primary antibody, followed by polyclonal HRP-conjugated
goat anti-rabbit or rabbit anti-mouse secondary antibody (1:2,000).
The protein-antibody complexes were visualized using SuperSignal
West Pico Chemiluminescent Substrate, SuperSignal West Femto
Chemiluminescent Substrate, or Pierce ECL Western Blotting
Substrate (all from Thermo Fisher Scientific, Inc.). The
immunoreactive protein bands were detected using the Amersham
Imager 600 (Cytiva), and the densities of the detected bands were
calculated using ImageJ software 1.52v (National Institutes of
Health).
In vitro cell migration assays (Boyden
chamber assays) and wound healing assays
Transwell assays were performed by the Boyden double
chamber method using Falcon 24-well Clear Flat Bottom TC-treated
Multiwell Cell Culture Plates and Falcon Cell Culture Inserts (pore
size, 8.0-µm diameter). After knockdown of Girdin in MIA PaCa-2,
AsPC-1 and PANC-1, the cells were seeded into the upper chamber at
50,000 cells/insert. The bottom chamber was loaded with medium
containing 10% FBS. In the EGF-stimulated group, 0.1 ng/ml EGF was
added. After 24 h of incubation at 37°C, the cells on the upper
surface of the insert filter were removed, and the cells migrating
to the bottom side of the insert were fixed and stained using the
Diff-Quik staining kit (Dade Behring; Siemens Healthineers) (~3 min
total, at room temperature). The migration cells were quantified by
counting in nine randomly selected high-power (×200) fields of view
using a compound light microscope.
Wound healing assays were performed as follows: The
treated cells were seeded in Falcon 24-well plates at 100,000
cells/well so that the cells were relatively dense, and after 24 h,
the monolayer was scratched using a pipette tip. Following 48 h of
incubation at 37°C with medium containing 2% FBS and reagents, the
scratched gaps were measured in low-power (magnification, ×40)
fields of view with a phase contrast microscope (BZ-X710, Keyence
Co., Ltd.). For quantification, the company's optional Measurement
Module (BZ-H3M) was used.
Enzyme-linked immunosorbent assay
(ELISA)
The following procedure was used to prepare the
treated cell supernatant. Each PaCa cell line was seeded at 50,000
cells/well in six-well plates and incubated overnight at 37°C.
Then, the knockdown of Girdin was performed or SCU was
administered, and the cell supernatant was collected after 72 h of
incubation at 37°C. The supernatant was centrifuged to remove
particulate matter. ELISA was performed using the VEGF-A ELISA kit
(cat. no. DVE00; R&D Systems, Inc.) according to the
manufacturer's protocol.
Matrigel tube formation assay
To assess the angiogenic activity of vascular
endothelial cells in vitro, a tube formation assay using
Matrigel (Corning, Inc.) with reduced growth factors was used as
previously described (23–25). The EA.hy926 cell line (immortalized
vascular endothelial cells) was established by fusion of primary
human umbilical vein cells with a thioguanine-resistant clone of
A549 using polyethylene glycol. It has been screened for factor
VIII-related antigens and has been used to evaluate angiogenic
function in vitro (26). The
conditioned medium (CM) was prepared from the culture supernatants
of Girdin-knockdown PaCa cell lines (MIA PaCa-2, AsPC-1 and
PANC-1), and DMEM containing 2% FBS Matrigel was applied to 96-well
plates (50 µl/well). EA.hy926 cell lines were incubated in this CM.
The number of endotubes was then measured by phase contrast
microscopy. The assay was repeated three times for evaluation.
Cytotoxicity assay
The cytotoxicity of SCU against PaCa cell lines was
evaluated using the Premix WST-1 Cell Proliferation Assay System
(Takara Bio, Inc.) according to the manufacturer's protocol. MIA
PaCa-2 and PANC-1 cell lines were seeded in 96-well plates at
2×103 cells/100 µl/well and cultured for 1 day. Various
concentrations of SCU (1-200 µM) were then added to the cells;
after 72 h of incubation at 37°C, absorbance was measured at 450 nm
using a spectraMax 340 spectrophotometer (Molecular Devices,
LLC).
Statistical analysis
All experimental data are shown as the mean ± SEM.
Comparisons between two groups were assessed using unpaired
t-tests. One-way ANOVA was used to compare multiple groups,
followed by Dunnett's or Bonferroni's multiple comparison test for
individual comparisons. P<0.01 was considered to indicate a
statistically significant difference. Regarding clinical data, the
age was expressed as median and quartiles, and comparisons between
the groups were made using the Mann-Whitney U test. Other clinical
data were analyzed using Fisher's test. All statistical analyses
were performed with EZR (27),
which is based on R software (https://www.r-project.org/). More precisely, it is a
modified version of R commander designed to add statistical
functions frequently used in biostatistics. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of Girdin in human PaCa
tissues
IHC was used to evaluate the expression of Girdin in
surgical specimens from 90 PaCa patients in Nagoya City University
Hospital between 2006 to 2016. In all PaCa tissues, Girdin staining
consistent with the cytoplasm of cancer cells was observed. The
degree of staining was graded as follows: No staining was
designated as ‘-’; staining weaker than that in isles of Langerhans
was designated as ‘+’; staining stronger than that in islets of
Langerhans was designated as ‘+++’; and those other than those were
designated ‘++’. Staining intensity was determined by three
independent observers blinded to the disease stage and patient
outcome. The concordance rate was >90%. Differences in opinion
were resolved by consensus with a fourth evaluator (Fig. 1A).
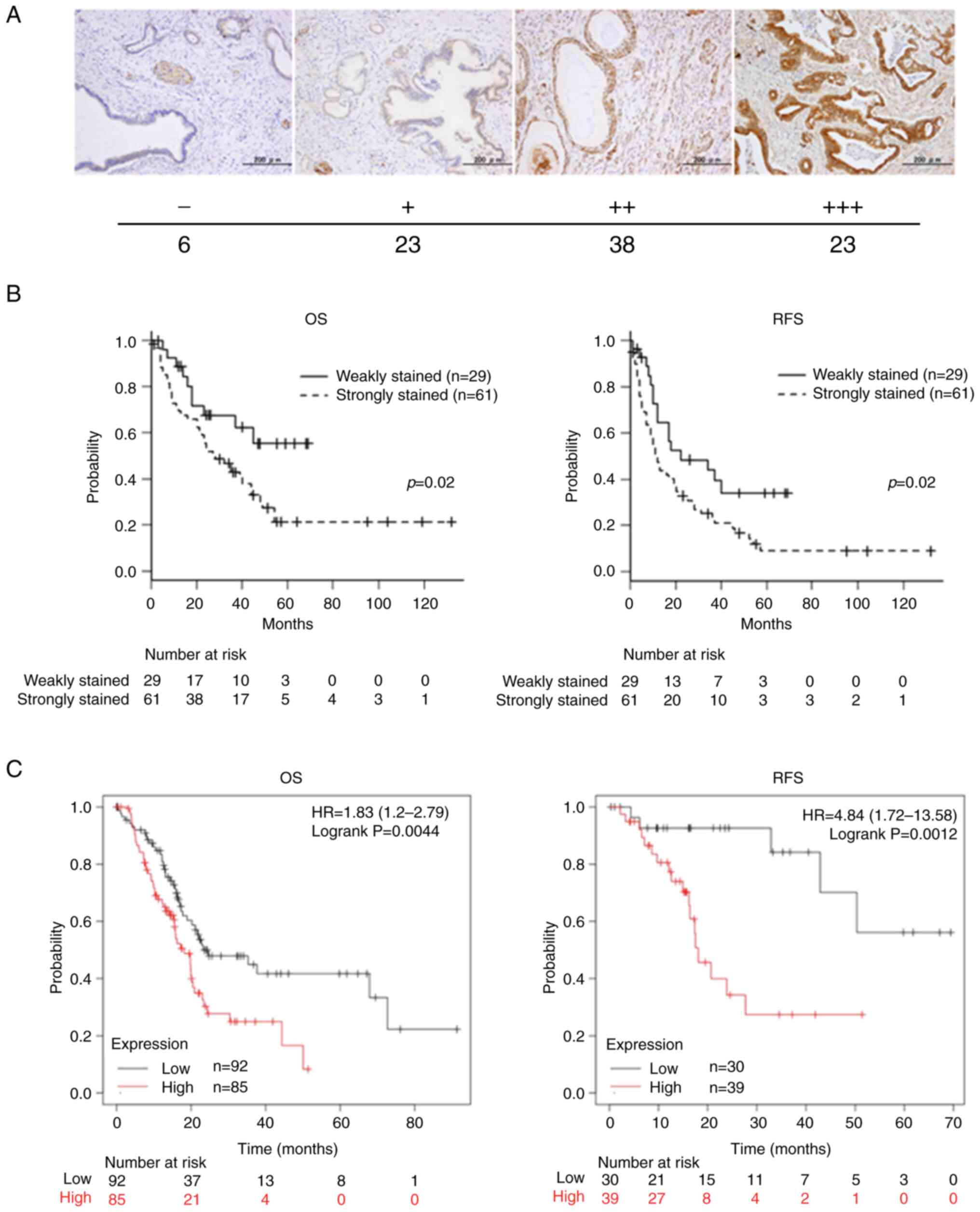 | Figure 1.Relationship between Girdin
expression in PaCa specimens and its prognosis. (A) IHC of tissues
resected from patients with PaCa at Nagoya City University Graduate
School of Medical Sciences. Paraffin-embedded PaCa tissues were
analyzed for Girdin expression by IHC using an anti-Girdin
antibody. In the PaCa tissues, there were differences in the
staining intensity for Girdin, and they were classified into four
types according to the intensity (−, +, ++ and +++). The number of
cases in each of the four staining intensity categories was
follows: -, 6 cases; +, 23 cases; ++, 38 cases; and +++, 23 cases.
(B) Kaplan-Meier curves showing OS RFS in 90 PaCa patients at
Nagoya City University Graduate School of Medical Sciences. The
tissues in both survival groups were classified into the weakly
stained (n=29) and the strongly stained (n=61) according to the
staining intensity. (i.e. ‘-’ and ‘+’ represent weak staining, ‘++’
and ‘+++’ represent strong staining). (C) Kaplan-Meier curves
showing OS and RFS of 177 and 69 PaCa patients, respectively, from
the database in the Kaplan-Meier plotter website (https://kmplot.com/analysis/). PaCa, pancreatic
cancer; IHC, Immunohistochemistry; OS, overall survival; RFS,
relapse-free survival. |
Association of Girdin expression with
OS and relapse-free survival (RFS)
The associations between the intensity of Girdin
expression and postoperative survival of PaCa patients was
investigated and the OS and RFS of patients in the weakly stained
(− and +) and the strongly stained (++ and +++) groups were
compared using the log-rank test. Both OS and RFS suggested a
significantly poorer prognosis in the group that stained strongly
for Girdin (both P=0.02) (Fig. 1B).
An online database (https://kmplot.com/analysis/) was utilized to confirm
the clinical significance of Girdin expression in PaCa. The gene
expression data and OS and RFS sources of the Kaplan-Meier plotter
website are based on NCBI Gene Expression Omnibus, The European
Genome-phenome Archive and The Cancer Genome Atlas (28). The gene expression of Girdin was
evaluated at the level of mRNA. As found in the present study, the
online databases also showed that strong expression of Girdin in
PaCa is a poor prognostic factor for both OS and RFS (OS and RFS
were examined in 177 and 69 patients, respectively) (Fig. 1C).
In PaCa, high Girdin expression is
associated with the invasion of cancer cells into the surrounding
tissues, including major blood vessels
Clinicopathological characteristics were analyzed
according to the intensity of Girdin staining in 88 PaCa patients
(two patients lacking histopathological findings were excluded). At
the time of surgery, there were no differences in sex or age ratios
between the two groups. Evaluation was performed based on the T4
classification, which indicates the invasion into major organs
adjacent to the pancreas (General Rules for the Study of PaCa in
Japan). Accordingly, the progression of the cancer from Stage 4 or
less than Stage 4 was also evaluated. As a result, the T factor was
significantly greater in the strongly stained group than in the
weakly stained group (T1-3 vs. T4, P=0.02). The TNM stage was also
significantly higher in the strongly stained group (Stages 1–3 vs.
Stage 4, P=0.01). However, there was no significant difference in
venous invasion, lymphatic invasion, or neural invasion (Table I).
Girdin expression and the knockdown of
Girdin in PaCa cells
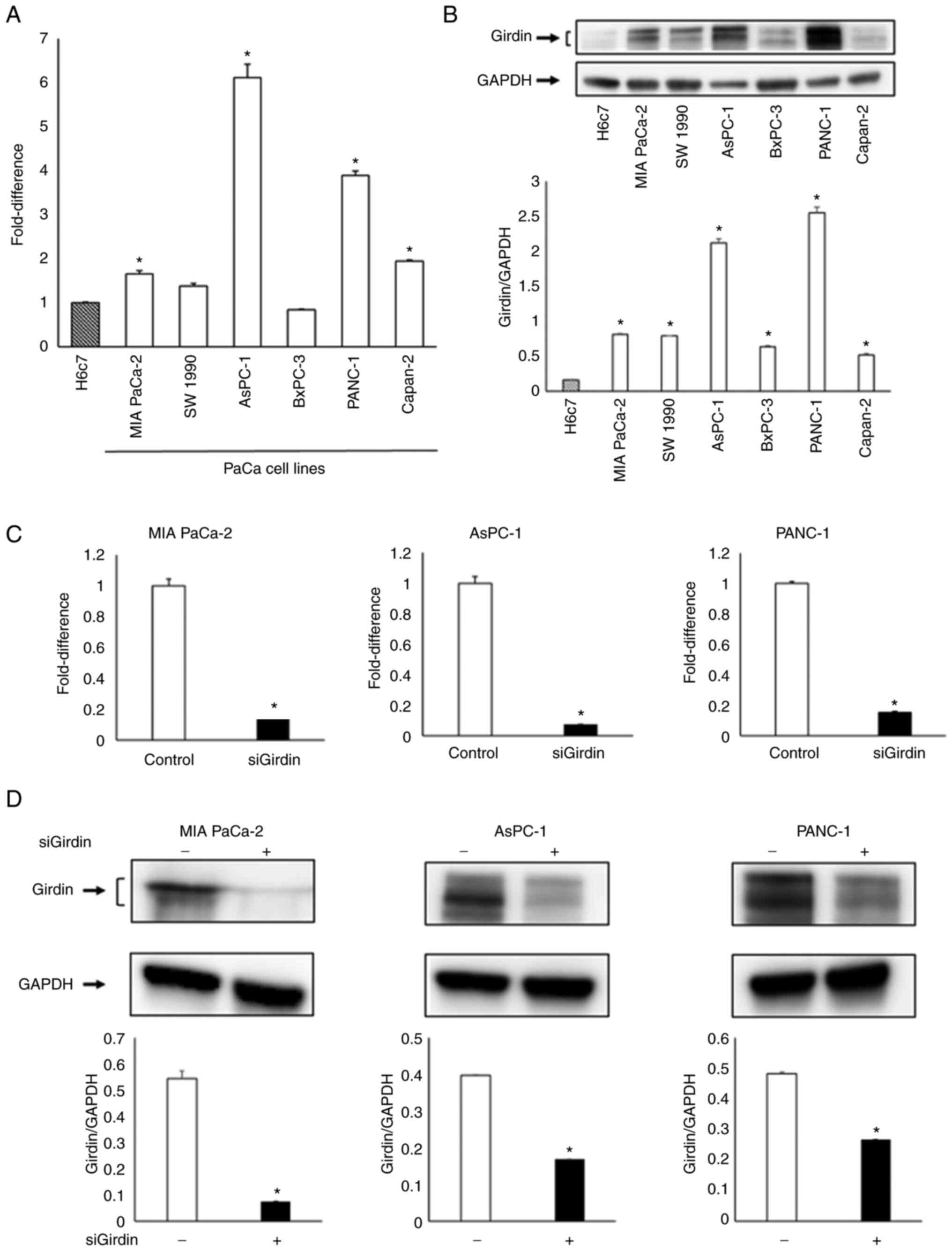
RT-qPCR and western blotting detected Girdin
expression in all cell lines, upregulation of Girdin was observed
in several PaCa cell lines, particularly in MIA PaCa-2, AsPC-1 and
PANC-1 cells, compared with the human pancreatic ductal epithelial
cell line H6c7 (Fig. 2A and B).
Although the Capan-2 cell line also had high Girdin expression, it
was not used in this experiment because its proliferation cycle is
longer than that of other cell lines, and this line was therefore
considered unsuitable for this experiment [(Capan-2, 96 h; MIA
PaCa-2, 26 h; AsPC-1, 38–40 h; PANC-1, 25.83 h) based on
Cellosaurus (https://www.cellosaurus.org/) data]. RT-qPCR and
western blotting confirmed that Girdin expression was suppressed
after Girdin knockdown in the aforementioned cell lines (Fig. 2C and D).
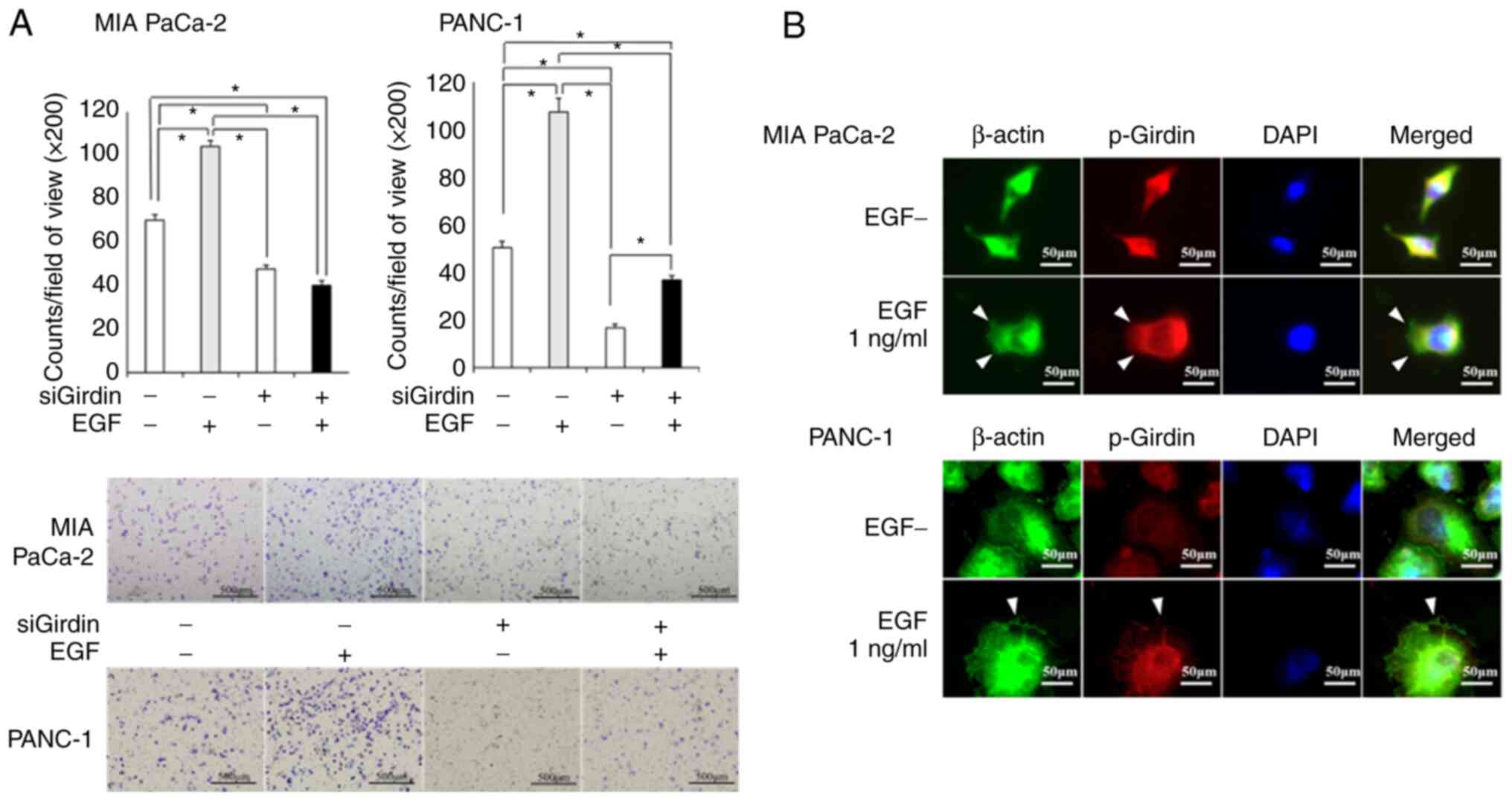
PaCa cell migration is enhanced by EGF
stimulation and decreased by suppression of Girdin activation
The Transwell assay revealed that EGF stimulation
increased the migratory potential of cells. Moreover, the knockdown
of Girdin inhibited the migration that was induced by EGF
stimulation in PaCa cells (Fig.
3A). PaCa cells form lamellipodia by restructuring actin fibers
that form the cytoskeleton upon EGF stimulation (white arrowheads).
It is suggested that the phosphorylation of Girdin, which binds to
actin fibers, is promoted by the EGF signaling system (Fig. 3B).
SCU, a flavonoid, suppresses the
migration ability of PaCa cells by inhibiting the phosphorylation
of Girdin
SCU is a chemical compound found in Scutellaria
barbata and Scutellaria lateriflora, which are medicinal
herbs, and it is a flavonoid glycoside with glucuronic acid
attached to the flavone skeleton (Fig.
4A). SCU did not inhibit the proliferation of MIA PaCa-2 or
PANC-1 cells at any concentration (Fig.
4B). SCU at 100 µM was used for further experiments.
Immunocytochemistry (ICC)/Immunofluorescence (IF) showed that
stimulation of PaCa cell lines with 1 ng/ml EGF resulted in the
formation of lamellipodia and the expression of phosphorylated
Girdin in the same regions; however, 100 µM SCU inhibited the
formation of lamellipodia and the phosphorylation of Girdin even
under EGF stimulation (Fig. 4C).
Western blotting also revealed that EGF stimulation increased,
whereas SCU treatment decreased, the level of phosphorylated Girdin
(Fig. 4D). The number of cells that
formed lamellipodia was evaluated using ICC/IF. The proportion of
cells that formed lamellipodia was significantly increased after
EGF stimulation, while it was suppressed after SCU treatment
(Fig. S1). In addition, the wound
healing assay suggested that migration ability was significantly
decreased in the 100 µM SCU group after EGF stimulation. However,
SCU alone did not significantly affect the migration ability of
PaCa cells (Fig. 4E).
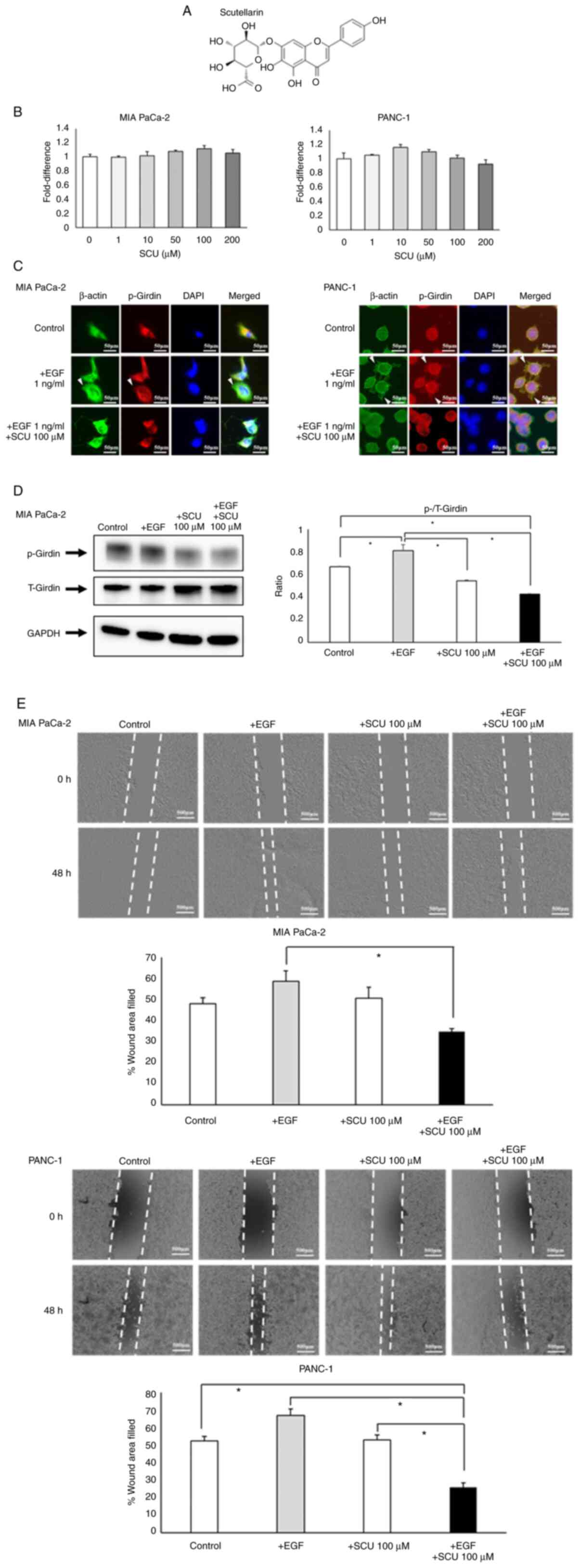 | Figure 4.SCU suppresses PaCa cell migration by
inhibiting Girdin phosphorylation. (A) The chemical structure of
SCU. SCU is a flavonoid with a molecular weight of 462.36 g/mol.
(B) The toxicological effects of SCU on PaCa cells were evaluated
using the WST-1 assay. SCU was administered to MIA PaCa-2 and
PANC-1 at various concentrations and incubated for 72 h, followed
by addition of WST-1 reagent and measurement of absorbance. The
values are expressed relatively to the control. (C)
Immunocytochemistry/Immunofluorescence was used to evaluate the
level of phosphorylated Girdin in MIA PaCa-2 and PANC-1 cells with
or without EGF and 100 µM SCU. The cells were incubated with each
reagent for 30 min and then stained. White arrows indicate the
location of the lamellipodia enhanced by EGF stimulation. In
addition, western blotting was used to quantify phosphorylated
Girdin (Y1764). (D) The changes in Girdin and p-Girdin expression
with SCU treatment were evaluated by western blotting. The ratio of
p-Girdin to total Girdin (T-Girdin) is shown in the graph. The
expression of p-Girdin was enhanced by EGF (1 ng/ml) stimulation,
whereas p-Girdin expression was significantly suppressed under SCU
treatment, even under EGF stimulation. (E) The effects of SCU on
the migration ability of PaCa cells was evaluated by wound healing
assay. MIA PaCa-2 and PANC-1 cells were seeded in 24-well plates at
100,000 cells per well, respectively. On the following day, after
cell settlement, scratches were made in a monolayer using a pipette
tip. Each reagent was added to medium containing 2% FBS, and the
cells were incubated for 48 h and then observed under a phase
contrast microscope. The length of the gaps at three random
locations were measured and averaged. The data are presented as the
mean ± SEM. All experiments were performed in triplicate and
repeated three times. *P<0.01 (B: One-way ANOVA with Dunnett's
multiple comparison test. D and E: One-way ANOVA with Bonferroni's
multiple comparison test, two-tailed). SCU, scutellarin; PaCa,
pancreatic cancer; p-, phosphorylated. |
Girdin regulates angiogenic activity
by upregulating the gene expression of VEGF-A, an angiogenic
factor
Both gene expression and secretion of the angiogenic
factor VEGF-A were suppressed in three PaCa cell lines (MIA PaCa-2,
AsPC-1 and PANC-1) with knockdown of Girdin (Fig. 5A and B). In the Matrigel tube
formation assay using EA.hy926 cells, tube formation was enhanced
in the CM with the culture supernatant of those PaCa cell lines,
whereas tube formation was significantly suppressed in the PaCa
cell line with knockdown of Girdin (Fig. 5C). As demonstrated in Fig. 4C, SCU inhibited the phosphorylation
of Girdin (tyrosine 1764). When the effect of SCU treatment on the
expression of VEGF-A was evaluated, no difference was observed in
the expression and secretion of VEGF-A treated with SCU with or
without EGF (Fig. 5D). These
results suggested that Girdin regulates the angiogenic factor
VEGF-A through a pathway different from that of tyrosine 1764
phosphorylation, which is considered to be at least related to
migration ability.
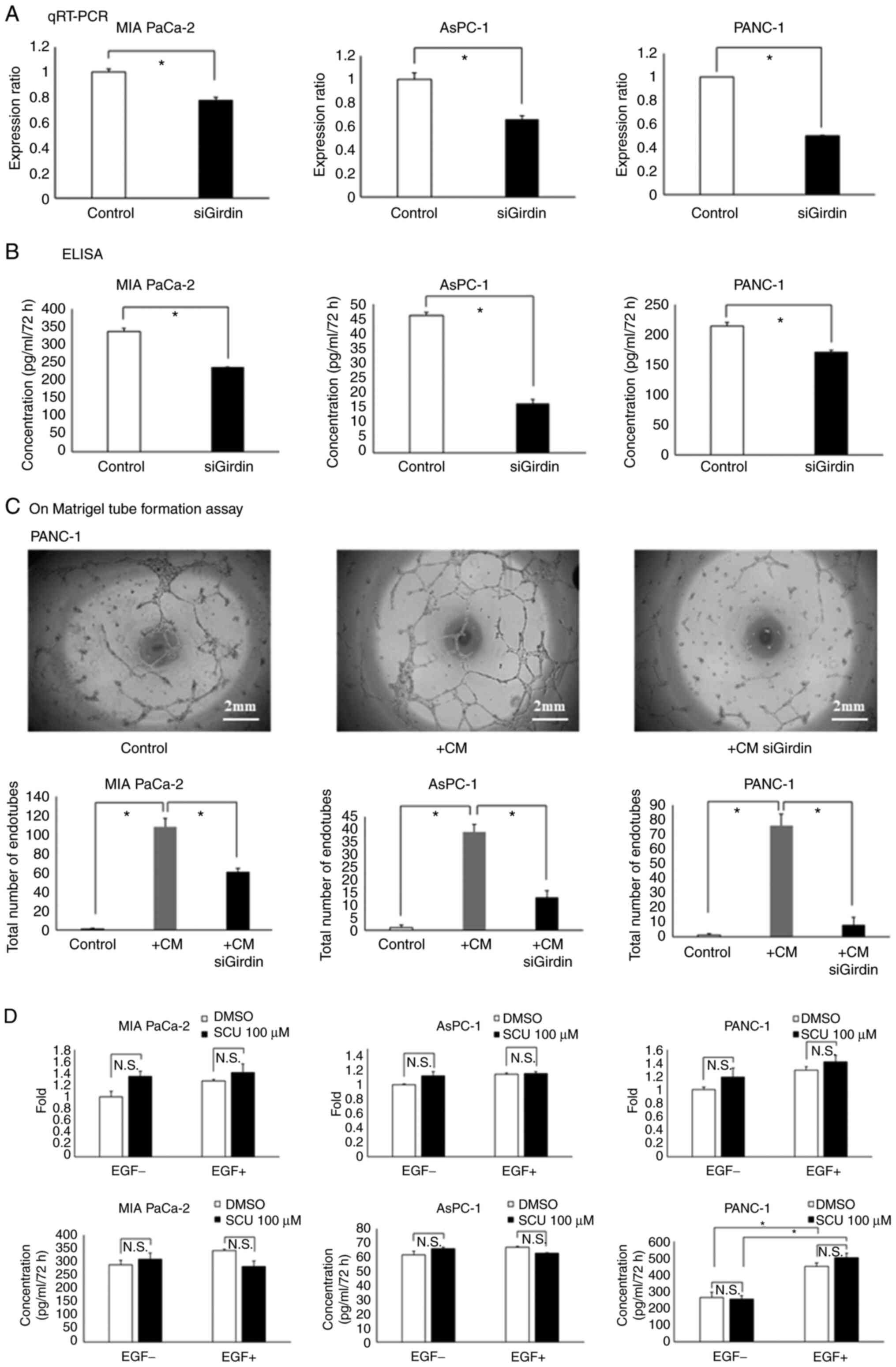 | Figure 5.Girdin regulates angiogenic activity
by upregulating VEGF-A gene expression. (A and B) Evaluation of
VEGF-A (A) expression and (B) secretion in PaCa cell lines (MIA
PaCa-2, AsPC-1 and PANC-1) with knockdown of Girdin. (A) RT-qPCR
experiments were calculated using a comparative quantitative method
with calibration curves. The vertical axis of the figure shows the
ratio of VEGF-A expression to that of the respective control group.
(B) After knockdown of Girdin in each cell line, the cells were
cultured in medium containing 2% FBS for 72 h, and the supernatant
was collected to measure VEGF-A by ELISA. The vertical axis of the
figure shows the absolute concentration of VEGF-A. (C) The HUVEC
tube formation assay was used to evaluate the angiogenic ability
in vitro. The PaCa cell lines after knockdown of Girdin were
cultured in 2% FBS-containing medium for 72 h, and then the cell
supernatant was mixed with the same volume of 2% FBS-containing
medium to make conditioned medium (CM). In the present study, the
EA.hy926 cell line was used as immortalized HUVECs. EA.hy926 cells
were cultured in the prepared conditioned medium for 16 h on
Matrigel, and the number of endotubes was counted by phase-contrast
microscopy. (D) Effect of SCU on the angiogenic ability of PaCa
cell lines. MIA PaCa-2, AsPC-1 and PANC-1 cells were treated with 1
ng/ml EGF and 100 µM SCU in 2% FBS-containing medium to verify the
alteration of VEGF-A expression and secretion. For RT-qPCR, cDNA
prepared 2 h after the addition of reagents was used; for ELISA,
the cell supernatant collected 72 h after the addition of reagents
was used. The data are presented as the mean ± SEM. All experiments
were performed in triplicate and repeated three times. *P<0.01
(A and B: Student's t-test. C and D: One-way ANOVA with
Bonferroni's multiple comparison test, two-tailed). PaCa,
pancreatic cancer; SCU, scutellarin; RT-qPCR, reverse
transcription-quantitative PCR; HUVEC, human umbilical vein
endothelial cell. |
Discussion
The malignant potential of cancer cells is likely to
be defined by their migratory, invasive and micro-angiogenic
potentials. Various intracellular and extracellular signaling
networks in the cancer microenvironment are involved. One of the
key processes in cancer cell invasion and metastasis is cell
motility. Three steps are important for cells to crawl between
tissues: Protrusion, adhesion, and traction (29). In protrusion, different cell types
generate various protruding structures such as filopodia,
lamellipodia and pseudopodia (30).
Epithelial cells, certain fibroblasts, and some neurons are known
to form lamellipodia, two-dimensional sheet-like structures
containing a cross-linking network of actin filaments (31).
Girdin, a novel substrate of Akt identified by
Enomoto et al (3), is a
protein bound to actin filaments that form the cytoskeleton. It is
activated by Akt, a serine/threonine kinase, and is involved in the
formation of lamellipodia, which are important for cytoskeletal
remodeling and cell migration. Girdin is also an APE (9–11),
G-alpha-interacting vesicle-associated protein (GIV) (32–34)
and Hook-related protein 1 (HkRP1) (35). For example, GIV is transcriptionally
induced by STAT3 and functions as a non-receptor guanine nucleotide
exchange factor for the Gai protein, promoting tumor invasion and
metastasis via STAT3 activation (32,36).
Thus, Girdin likely plays multiple physiological roles in
cells.
Girdin is highly expressed in various human
malignancies, including breast, colon, lung, thyroid and cervical
cancer (37–40). The Department of Gastroenterological
Surgery, Nagoya City University Graduate School of Medical Sciences
also reported that high Girdin expression was present in esophageal
cancer and it is correlated with invasive potential and prognosis
(14).
The purpose of the present study was to clarify the
role of Girdin in the migration and progression of PaCa. In the
present study of resected PaCa specimens, the expression of Girdin
varied in intensity. The results were divided into strong and weak
Girdin expression groups and it was found that the strong Girdin
expression group had a significantly poorer prognosis in OS and RFS
than the weak Girdin expression group, similar to previous studies
on other carcinomas. For confirmation of the data that were
provided, a web database (Kaplan Meier plotter) was addressed for
mRNA levels, and the results were similar. Evidently, expression of
Girdin in cancer cells is associated with greater invasiveness of
organs. On the other hand, there was no significant difference in
lymph node metastasis or distant metastasis, and the effect of the
T factor significantly correlated with the intensity of Girdin
expression at stage 4 and lower levels. However, no significant
difference was obtained for venous or lymphatic invasion. Neural
invasion was not significantly different but tended to be slightly
more common in the Girdin-expressing group. It was hypothesized
that this is because Girdin is involved in not only migration, but
also angiogenesis, as suggested in the present study, as well as in
proliferation and inhibition of apoptosis (10). Thus, Girdin expression could be a
biomarker for PaCa prognosis.
In vitro, Girdin was more highly expressed in
human pancreatic ductal cells than in numerous other PaCa cell
lines. MIA PaCa-2, AsPC-1 and PANC-1 also expressed Girdin at high
levels. It was found that the migration ability of these PaCa cell
lines was enhanced by EGF stimulation. By contrast, migration was
inhibited by EGF stimulation when Girdin was suppressed. In
addition, it was confirmed that EGF stimulation enhanced the
phosphorylation of Girdin, resulting in cell morphological changes
and the formation of lamellipodia. These results indicated that
Girdin is involved in cell migration via EGF signaling in PaCa. On
the other hand, the present ICC/IF results revealed that
phosphorylated Girdin was also expressed in the absence of EGF.
Enomoto et al (3) reported
that phosphorylated Girdin is also expressed in the absence of EGF.
This may indicate that phosphorylated Girdin is necessary but not
sufficient for lamellipodia formation. It was hypothesized that
phosphorylated Girdin has other functions besides lamellipodia
formation. Unfortunately, one of the limitations of the present
study was that it was not possible to search for phosphorylated
Girdin in all cell lines due to time constraints and the only
results that were obtained were from the MIA PaCa-2 cell line. It
is aspired to obtain similar results by conducting experiments on
other cell lines in the future.
There are a few substances that inhibit Girdin
signaling. In the present study the main focus was SCU, a type of
flavonoid, which it has long been used in Chinese medicine as a
treatment for cerebrovascular diseases, and is known for its
antitumor, hepatoprotective and neuroprotective effects. Natural
compounds have been used in China for the treatment of
cerebrovascular and other diseases (16). SCU was reported to inhibit cancer
invasion and metastasis by suppressing STAT3/Girdin/Akt signaling
in hepatocellular carcinoma (15).
SCU was selected for evaluation of its effects on Girdin
suppression in PaCa cells. As in the case of Girdin knockdown, the
PaCa migration enhanced by EGF was significantly reduced by SCU.
ICC/IF demonstrated that EGF-induced increase in lamellipodia
formation was suppressed by SCU treatment, and western blotting
indicated that this effect was mediated by phosphorylation of
Tyr-1764 in Girdin. On the other hand, knockdown of Girdin also
reduced cell migration ability. It was hypothesized that SCU
competitively interacts with the phosphorylation pathway of
Girdin.
Since Girdin also increases the activity of Akt and
STAT3, as aforementioned, the effect of Girdin was examined on
angiogenesis during tumor progression. In non-small cell lung
cancer, the production of VEGF was increased by IL-17, and it was
suppressed by knockdown of Girdin (41). It was also reported to be involved
in the level of mRNA expression for the angiogenic factor VEGF in
breast cancer (42). PaCa is
commonly referred to as an ischemic tumor based on
microscopic-level imaging findings, but there are numerous studies
showing an association between microvascular density and the
prognosis of PaCa (43). In fact,
certain studies have demonstrated the efficacy of anti-angiogenic
therapy for PaCa (44,45). Furthermore, with regard to novel
therapies targeting angiogenesis, it has been previously
demonstrated by the authors that inhibition of PaCa proliferation
at the in vivo level suppressed gene expression and the
production/secretion of the angiogenic factor VEGF-A. It was also
demonstrated that inhibition of Girdin expression suppressed the
angiogenic potential of VEGF-A in vitro. In the Matrigel
tube formation assay performed to evaluate angiogenic potential
in vitro, a significant decrease in tube formation was found
in the cell supernatants from Girdin-knockdown cell lines. No
obvious involvement of EGF stimulation in the expression and
production of these angiogenic factors and in angiogenesis could be
noted. On the other hand, the addition of SCU, which inhibits
Girdin phosphorylation, did not significantly alter the expression,
production or secretion of angiogenic factors. This suggests that
the regulatory mechanism of angiogenic potential of Girdin uses a
different pathway than the control of migratory activity without
EGF signaling. Further detailed investigation of these mechanisms
is warranted.
In the present study, experiments were performed to
suppress Girdin in order to analyze the function of Girdin. On the
other hand, it is necessary to examine the effects of strong Girdin
expression in order to further support the present findings, as it
was not possible to perform Girdin knock-in experiments due to
limited time, technology and facilities in the authors' laboratory.
In addition, a limitation to the study on phosphorylated Girdin is
that it was conducted only in MIA PaCa-2 cells and that the
multiple phosphorylation sites were not verified sufficiently.
These research limitations will be further developed and studied in
the future.
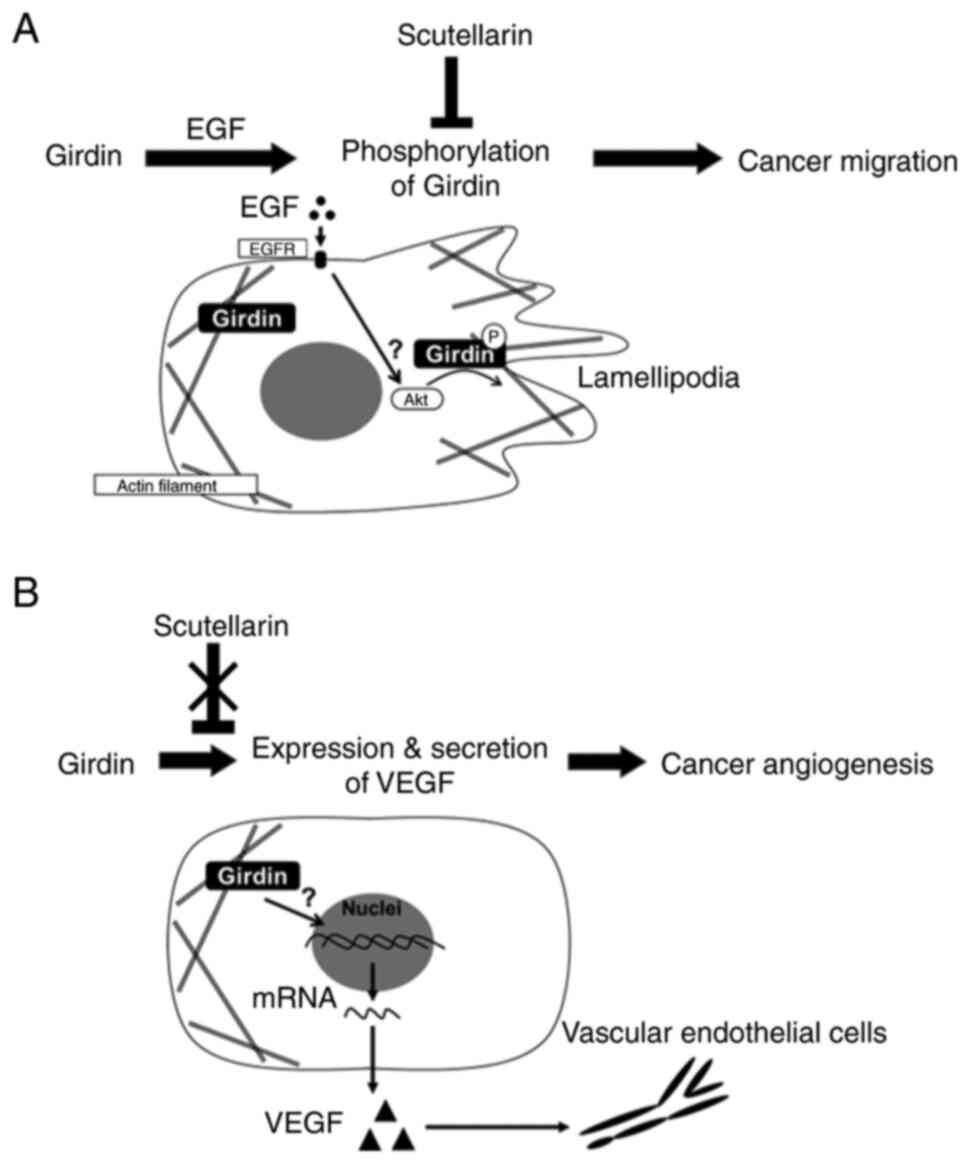
In conclusion, the results of the present study
suggested that Girdin is involved in EGF signal-mediated migration
in PaCa and that SCU may suppress cancer invasion by inhibiting it
(Fig. 6). Moreover, Girdin levels
associated with PaCa angiogenesis by regulating VEGF-A production
from PaCa. As the expression of Girdin is associated with PaCa
prognosis, Girdin may be a prognostic biomarker. SCU, a novel
targeting Girdin, may improve the prognosis of PaCa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
YH and YM contributed to the conception and design
of the study, analyzed and interpreted the data, and wrote and
reviewed the manuscript. YH, YM, GU, YA, TK, KO, HI, KS, MM, HT and
ST designed the study. YH, YD, KN, HM, GU, YA and TK performed the
experiments and acquired the data. YM, HI, KS, MM and HT provided
technical support in performing the experiments. YH, YM, AM and MK
confirm the authenticity of all the raw data. YM, RO, HT and ST
supervised the study. YH and YM planned all experiments, analyzed
and interpreted the data, and drafted the manuscript. All authors
read and approved the final version of the manuscript and are
equally responsible for all aspects of the study, ensuring that the
integrity or accuracy of all part of the study.
Ethics approval and consent to
participate
Ethics approval (approval no. 60-18-0025) for the
use of human tissue was granted by the Institutional Review Board
(Ethical Review Committee for Medical Research, Nagoya City
University Hospital, Nagoya, Japan). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APE
|
Akt phosphorylation enhancer
|
|
CM
|
conditioned medium
|
|
EGF
|
epidermal growth factor
|
|
GIV
|
guanine nucleotide-binding protein α
subunit (Gα)-interacting vesicle-associated protein
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
OS
|
overall survival
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
RFS
|
relapse-free survival
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
VEGF-A
|
vascular endothelial growth factor
A
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuo Y, Sawai H, Ochi N, Yasuda A,
Sakamoto M, Takahashi H, Funahashi H, Takeyama H and Guha S:
Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic
cancer by blocking NF-kappaB activity. Dig Dis Sci. 55:1167–1176.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enomoto A, Murakami H, Asai N, Morone N,
Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K and
Takahashi M: Akt/PKB regulates actin organization and cell motility
via Girdin/APE. Dev Cell. 9:389–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omori K, Asai M, Kuga D, Ushida K, Izuchi
T, Mii S, Enomoto A, Asai N, Nagino M and Takahashi M: Girdin is
phosphorylated on tyrosine 1798 when associated with structures
required for migration. Biochem Biophys Res Commun. 458:934–940.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamura Y, Asai N, Enomoto A, Kato T, Mii
S, Kondo Y, Ushida K, Niimi K, Tsunoda N, Nagino M, et al:
Akt-Girdin signaling in cancer-associated fibroblasts contributes
to tumor progression. Cancer Res. 75:813–823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitamura T, Asai N, Enomoto A, Maeda K,
Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, Kondo T, et al:
Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate
Girdin. Nat Cell Biol. 10:329–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito T, Komeima K, Yasuma T, Enomoto A,
Asai N, Asai M, Iwase S, Takahashi M and Terasaki H: Girdin and its
phosphorylation dynamically regulate neonatal vascular development
and pathological neovascularization in the retina. Am J Pathol.
182:586–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Enomoto A, Ping J and Takahashi M: Girdin,
a novel actin-binding protein, and its family of proteins possess
versatile functions in the Akt and Wnt signaling pathways. Ann N Y
Acad Sci. 1086:169–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anai M, Shojima N, Katagiri H, Ogihara T,
Sakoda H, Onishi Y, Ono H, Fujishiro M, Fukushima Y, Horike N, et
al: A novel protein kinase B (PKB)/AKT-binding protein enhances PKB
kinase activity and regulates DNA synthesis. J Biol Chem.
280:18525–18535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Lei Y, Cai Z, Ye X, Li L, Luo X
and Yu C: Girdin regulates the proliferation and apoptosis of
pancreatic cancer cells via the PI3K/Akt signalling pathway. Oncol
Rep. 40:599–608. 2018.PubMed/NCBI
|
|
11
|
Wang W, Chen H, Gao W, Wang S, Wu K, Lu C,
Luo X, Li L and Yu C: Girdin interaction with vimentin induces EMT
and promotes the growth and metastasis of pancreatic ductal
adenocarcinoma. Oncol Rep. 44:637–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weng L, Enomoto A, Ishida-Takagishi M,
Asai N and Takahashi M: Girding for migratory cues: Roles of the
Akt substrate Girdin in cancer progression and angiogenesis. Cancer
Sci. 101:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu F, Wang L, He J, Liu X, Zhang H, Li W,
Fu L and Ma Y: Girdin, an actin-binding protein, is critical for
migration, adhesion, and invasion of human glioblastoma cells. J
Neurochem. 131:457–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibata T, Matsuo Y, Shamoto T, Hirokawa
T, Tsuboi K, Takahashi H, Ishiguro H, Kimura M, Takeyama H and
Inagaki H: Girdin, a regulator of cell motility, is a potential
prognostic marker for esophageal squamous cell carcinoma. Oncol
Rep. 29:2127–2132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ke Y, Bao T, Wu X, Tang H, Wang Y, Ge J,
Fu B, Meng X, Chen L, Zhang C, et al: Scutellarin suppresses
migration and invasion of human hepatocellular carcinoma by
inhibiting the STAT3/Girdin/Akt activity. Biochem Biophys Res
Commun. 483:509–515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Q, Chen XY and Martin C: Scutellaria
baicalensis, the golden herb from the garden of Chinese medicinal
plants. Sci Bull (Beijing). 61:1391–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu PT, Mao M, Liu ZG, Tao L and Yan BC:
Scutellarin suppresses human colorectal cancer metastasis and
angiogenesis by targeting ephrinb2. Am J Transl Res. 9:5094–5104.
2017.PubMed/NCBI
|
|
18
|
Yang N, Zhao Y, Wang Z, Liu Y and Zhang Y:
Scutellarin suppresses growth and causes apoptosis of human
colorectal cancer cells by regulating the p53 pathway. Mol Med Rep.
15:929–935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou L, Chen L and Fang L: Scutellarin
inhibits proliferation, invasion, and tumorigenicity in human
breast cancer cells by regulating HIPPO-YAP signaling pathway. Med
Sci Monit. 23:5130–5138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato T, Matsuo Y, Ueda G, Murase H, Aoyama
Y, Omi K, Hayashi Y, Imafuji H, Saito K, Morimoto M, et al:
Enhanced CXCL12/CXCR4 signaling increases tumor progression in
radiation-resistant pancreatic cancer. Oncol Rep. 47:682022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Omi K, Matsuo Y, Ueda G, Aoyama Y, Kato T,
Hayashi Y, Imafuji H, Saito K, Tsuboi K, Morimoto M, et al: Escin
inhibits angiogenesis by suppressing interleukin-8 and vascular
endothelial growth factor production by blocking nuclear
factor-κB activation in pancreatic cancer cell lines. Oncol
Rep. 45:552021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao R and Guo X: Human microvascular
endothelial cells immortalized with human telomerase catalytic
protein: A model for the study of in vitro angiogenesis. Biochem
Biophys Res Commun. 321:788–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Francescone RA III, Faibish M and Shao R:
A matrigel-based tube formation assay to assess the vasculogenic
activity of tumor cells. J Vis Exp. 30402011.PubMed/NCBI
|
|
26
|
Bauer J, Margolis M, Schreiner C, Edgell
CJ, Azizkhan J, Lazarowski E and Juliano RL: In vitro model of
angiogenesis using a human endothelium-derived permanent cell line:
Contributions of induced gene expression, G-proteins, and
integrins. J Cell Physiol. 153:437–449. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lánczky A and Győrffy B: Web-based
survival analysis tool tailored for medical research (KMplot):
Development and implementation. J Med Internet Res. 23:e276332021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roy S, Miao F and Qi HJ: Cell crawling
assisted by contractile stress induced retraction. J Biomech Eng.
132:0610052010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rivas RJ and Hatten ME: Motility and
cytoskeletal organization of migrating cerebellar granule neurons.
J Neurosci. 15:981–989. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dunkel Y, Ong A, Notani D, Mittal Y, Lam
M, Mi X and Ghosh P: STAT3 protein up-regulates Gα-interacting
vesicle-associated protein (GIV)/Girdin expression, and GIV
enhances STAT3 activation in a positive feedback loop during wound
healing and tumor invasion/metastasis. J Biol Chem.
287:41667–41683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dunkel Y, Diao K, Aznar N, Swanson L, Liu
L, Zhu W, Mi XY and Ghosh P: Prognostic impact of total and
tyrosine phosphorylated GIV/Girdin in breast cancers. FASEB J.
30:3702–3713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leyme A, Marivin A and Garcia-Marcos M:
GIV/Girdin (Gα-interacting, vesicle-associated protein/Girdin)
creates a positive feedback loop that potentiates outside-in
integrin signaling in cancer cells. J Biol Chem. 291:8269–8282.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simpson F, Martin S, Evans TM, Kerr M,
James DE, Parton RG, Teasdale RD and Wicking C: A novel
hook-related protein family and the characterization of
hook-related protein 1. Traffic. 6:442–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta V, Bhandari D, Leyme A, Aznar N,
Midde KK, Lo IC, Ear J, Niesman I, López-Sánchez I, Blanco-Canosa
JB, et al: GIV/Girdin activates Gαi and inhibits Gαs via the same
motif. Proc Natl Acad Sci USA. 113:E5721–E5730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang P, Enomoto A, Jijiwa M, Kato T,
Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y and Takahashi M:
An actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghosh P, Tie J, Muranyi A, Singh S,
Brunhoeber P, Leith K, Bowermaster R, Liao Z, Zhu Y, LaFleur B, et
al: Girdin (GIV) expression as a prognostic marker of recurrence in
mismatch repair-proficient stage II colon cancer. Clin Cancer Res.
22:3488–3498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanouchi A, Taniuchi K, Furihata M,
Naganuma S, Dabanaka K, Kimura M, Watanabe R, Kohsaki T, Shimizu T,
Saito M, et al: CCDC88A, a prognostic factor for human pancreatic
cancers, promotes the motility and invasiveness of pancreatic
cancer cells. J Exp Clin Cancer Res. 35:1902016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu J, Zhang L, Zhou H, Du Z and Zhang G:
Silencing of Girdin suppresses the malignant behavior of colorectal
carcinoma cells. Oncol Rep. 40:887–894. 2018.PubMed/NCBI
|
|
41
|
Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin
S, Cao S, Che D, Liu F and Yu Y: Interleukin-17 promotes
angiogenesis by stimulating VEGF production of cancer cells via the
STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep.
5:160532015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Zhang J, Zhang M, Wei L, Chen H
and Li Z: A systematic study of Girdin on cell proliferation,
migration and angiogenesis in different breast cancer subtypes. Mol
Med Rep. 16:3351–3356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Benckert C, Thelen A, Cramer T, Weichert
W, Gaebelein G, Gessner R and Jonas S: Impact of microvessel
density on lymph node metastasis and survival after curative
resection of pancreatic cancer. Surg Today. 42:169–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Z, Ji S, Zhang B, Liu J, Qin Y, Xu J
and Yu X: Role of angiogenesis in pancreatic cancer biology and
therapy. Biomed Pharmacother. 108:1135–1140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Annese T, Tamma R, Ruggieri S and Ribatti
D: Angiogenesis in pancreatic cancer: Pre-clinical and clinical
studies. Cancers (Basel). 11:3812019. View Article : Google Scholar : PubMed/NCBI
|