Introduction
The global incidence and mortality rates of lung
cancer remain high and the five-year survival rate has been
reported to be ~20% (1–3). Lung adenocarcinoma (LUAD) is one of
the most common pathological subtypes of lung cancer, accounting
for ~40% of cases (4,5). Despite considerable advances in
anticancer therapy, the overall survival and prognosis of patients
with LUAD has not improved significantly (6,7).
Therefore, an improved understanding of the drivers of LUAD and the
molecular mechanisms could improve the diagnosis and treatment of
this deadly disease.
Heat shock proteins act as chaperones at the
molecular level and have been investigated in numerous diseases
associated with oxidative stress, including obesity (8,9). A
specific subfamily of heat-shock proteins are the HSPB family of
molecular chaperones, which comprises ten members (HSPB1-10, also
called small HSP) (10). HSPB7 is a
member of the HSPB family and has been shown to be ineffective in
suppressing the amorphous aggregation of model proteins, however,
it is very effective in preventing the aggregation of huntingtin
fragments enriched with Gln residues (11). HSPB7 is crucial for heart
development as it modulates actin filament assembly (12). HSPB7 interacts with dimerized
filamin C, and its absence results in progressive myopathy in
skeletal muscle (13).
Other genes in the HSPB family have been associated
with glycolysis. Increased glycolysis, oxidative phosphorylation,
and stem cell characteristics of esophageal cancer stem-like cells
depend on the Hsp27 (HSPB1)-AKT-HK2 pathway (14). The bidirectional gene
HspB2/αB-crystallin may be involved in the levels of reactive
oxygen species and glycolysis in MCF7 cells (15). However, the effect of HSPB7 on the
expression of glycolytic enzymes and level of glycolysis has not
been previously reported. It was hypothesized that HSPB7 may
indirectly affect the expression of glycolytic enzymes and the
level of glycolysis, but further studies are required to elucidate
the molecular mechanisms by which HSPB7 participates in
glycolysis.
Increasing evidence has indicated that HSPB7 acts as
a tumour suppressor in a variety of malignant tumours (16,17).
However, there is no study on the role of HSPB7 in LUAD. In the
present study, it was hypothesized that HSPB7 participates in LUAD
progression and its potential role and mechanism of action were
investigated. Preliminary analysis revealed that HSPB7 was
significantly downregulated in LUAD tumour tissues and cells,
suggesting that it may be a candidate tumour suppressor gene in
LUAD.
Materials and methods
Bioinformatics analysis
The expression profiles of myelodysplastic syndrome
1 (MDS1) and ecotropic viral integration site 1 (EVI1) complex
locus (MECOM) of patients with LUAD was analysed using the Gene
Expression Profiling Interactive Analysis (GEPIA) website
(http://gepia2.cancer-pku.cn/#index)
(18). In GEPIA website, it was not
possible to acquire clinical characteristics of 483 LUAD patients.
To show clinical characteristics, HSPB7 expression profiles and
clinical characteristics of patients with LUAD [515 cases,
International Classification of Diseases (ICD-10) (19), C34] were downloaded from TCGA
(https://portal.gdc.cancer.gov/), and
then analyzed via R software (version 3.6.1, http://www.r-project.org/). A survival curve for HSPB7
expression was obtained using GEPIA. The survival curve of MECOM
expression was obtained from the Kaplan Meier plot (http://kmplot.com/analysis/). Survival analysis was
performed using the Kaplan-Meier method, and the log-rank test was
used to compare survival times between the low and high expression
groups. Gene set enrichment analysis (GSEA) was used to identify
HSPB7 related signaling pathways in the LUAD dataset using the
following parameters: Number of permutations=1,000; permutation
type=gene_set; enrichment statistic=weighted; and metric for
ranking genes=Signal2Noise. The binding site of HSPB7 and MECOM was
predicted using the JASPAR website (http://jaspar.genereg.net/).
Tissue collection
Between September 2020 and December 2021, 23 tumour
tissues and paired normal tissues from patients with LUAD (13 males
and 10 females; age range: 28–75 years old; TNM stage: I–IV;
ICD-10: C34.1 and C34.3) were collected from Jinan Central Hospital
Affiliated to Shandong University (Jinan, China) and stored
immediately at −80°C for subsequent analysis. Pathologists
confirmed the correct identification of the tumour tissues and
paired normal tissues. The present study was approved (approval no.
W202203060147) by the Ethics Committee of Jinan Central Hospital
Affiliated to Shandong First Medical University (Jinan, China).
Written informed consent was obtained from all enrolled
patients.
Cell culture and transfection
Normal human lung epithelial cells (BEAS-2B; cat.
no. CRL-3588) and lung cancer cells [H1975 (cat. no. CRL-5908),
H1688 (cat. no. CCL-257), H1299 (cat. no. CRL-5803) and A549 (cat.
no. CRM-CCL-185)] were obtained from the American Type Culture
Collection. Cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich; Merck KGaA) at 37°C with 5%
CO2. Small interfering (si)RNAs si1-HSPB7 and si2-HSPB7,
which specifically targeted HSPB7 were synthesized and purified by
Guangzhou RiboBio Co., Ltd. The sequences were as follows:
si1-HSPB7 sense, 5′-GGUGCUGUGGGAGGACAAAGA-3′ and antisense,
5′-UUUGUCCUCCCACAGCACCUG-3′; si2-HSPB7 sense,
5′-GGAAGACUAUGUCACACUGCC-3′, and antisense,
5′-CAGUGUGACAUAGUCUUCCUG-3′; si1-MECOM sense,
5′-GGAUGAUGAAGAAGUUGAAGA-3′ and antisense,
5′-UUCAACUUCUUCAUCAUCCAG-3′; si2-MECOM sense,
5′-CCUGCUAGUUCUCCUGUUAAA-3′ and antisense,
5,-UAACAGGAGAACUAGCAGGUA-3′ and si-NC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5,′-ACGUGACACGUUCGGAGAATT-3′. HSPB7 and MECOM were cloned into the
pcDNA3.1 eukaryotic expression vector to allow their
overexpression. All the vectors were purchased from Guangzhou
RiboBio Co., Ltd. When the fusion rate of H1975 and A549 cells
reached 70–80%, Cells were transfected with 40 nM vectors using
Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.) for 10
h at 37°C. After 48 h, the transfection efficiency was tested by
quantitative reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Before transfection, H1975 cells were
pretreated with 5 mM 2-deoxy-D-glucose (2-DG; Sigma-Aldrich; Merck
KGaA) for 8 h.
RT-qPCR
Total RNA was isolated from tissues and cells by
homogenization using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized using a PrimeScript RT
Reagent kit (Takara Bio, Inc.) according to the manufacturer's
protocols. The SYBR Green System (Takara Bio, Inc.) was used to
assess gene expression. The sequences of primers used for
amplification were as follows: HSPB7 forward,
5′-AACCACATCGAGCTGGCG-3′ and reverse, 5′-GAAAGGGAAGGGAGAGGCAC-3′;
MECOM forward, 5′-CTTCTTGACTAAAGCCCTTGGA-3′ and reverse,
5′-GTACTTGAGCCAGCTTCCAACA-3′; and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) forward, 5′-AATGGGCAGCCGTTAGGAAA-3′ and
reverse, 5′-GCCCAATACGACCAAATCAGAG-3′. The thermocycling conditions
involved initial denaturation at 95°C for 4 min; 40 cycles of
denaturation at 95°C for 15 sec, annealing at 60°C for 35 sec,
elongation at 72°C for 30 sec; and final extension at 72°C for 10
min. The 2−ΔΔCq method (20) was used to calculate the mRNA
expression level. Expression levels were normalized to the internal
reference gene, GAPDH.
Western blotting
Total protein from the cells was isolated and
quantified using radioimmunoprecipitation (RIPA) assay lysis buffer
and a bicinchoninic acid kit (both from Beyotime Institute of
Biotechnology), respectively. Samples (30 µg) were separated by 12%
polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride (PVDF) membranes. After blocking with 5%
skim milk for 1 h at room temperature, the membranes were incubated
overnight at 4°C with the following primary antibodies: HSPB7
monoclonal antibody (1:1,000; cat. no. ab248960; Abcam), E-cadherin
polyclonal antibody (1:1,000; cat. no. GB11868; Wuhan Servicebio
Technology Co., Ltd.), N-cadherin polyclonal antibody (1:1,000;
cat. no. GB111009; Wuhan Servicebio Technology Co., Ltd.), Snail
polyclonal antibody (1:1,000; cat. no. 13099-1-AP; Proteintech
Group, Inc.), lactate dehydrogenase A (LDHA) polyclonal antibody
(1:1,000; cat. no. 19987-1-AP; Proteintech Group, Inc.), hexokinase
2 (HK2) monoclonal antibody (1:1,000; cat. no. 2867; Cell Signaling
Technology, Inc.); pyruvate kinase muscle isoform 2 (PKM2)
polyclonal antibody (1:500; cat. no. SAB4200094; Sigma-Aldrich;
Merck KGaA) and GAPDH polyclonal antibody (1:1,000; cat. no. AC001;
ABclonal Biotech Co., Ltd.). The PVDF membranes were then incubated
with the HRP-conjugated secondary antibody goat anti-rabbit IgG
(1:5,000; cat. no. AS014; ABclonal Biotech Co., Ltd.) at room
temperature for 1 h. The bands were visualized using enhanced
chemiluminescence (Beijing Solarbio Science & Technology Co.,
Ltd.) and quantified using Quantity One® 4.1.1 gel
analysis software (Bio-Rad Laboratories, Inc.). GAPDH was used as
the loading control.
Immunohistochemistry (IHC)
analysis
The lung tissue and xenograft tumours sections were
deparaffinized with xylene and rehydrated in gradient ethanol at
room temperature. To eliminate endogenous peroxidase activity, the
sections (4 µm) were incubated with 3% H2O2
at room temperature for 5–10 min. Subsequently, the sections were
rinsed three times with distilled water and soaked in phosphate
buffered saline for 5 min. After blocking in 5–10% normal goat
serum (cat. no. SL038; Beijing Solarbio Science & Technology
Co., Ltd.) for 10 min, the sections were incubated with primary
antibodies against HSPB7 (1:200; cat. no. ab150390; Abcam) and Ki67
(1:200; cat. no. ab15580; Abcam) overnight at 4°C, followed by
HRP-conjugated secondary antibody (1:200) at 37°C for 1 h. Finally,
the sections were stained with the developer 3,3′-diaminobenzidine
(Beijing Solarbio Science & Technology Co., Ltd.) for 3–15 min
and observed under a light microscope (DMI3000 B; Leica
Microsystems GmbH).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2-Htetrazolium bromide
(MTT) assay
After transfection, H1975 and A549 cells
(1×103 cells/well) were seeded into a 96-well plate and
cultured at 37°C for 24, 48 and 72 h. Next, cells were treated with
10 µl MTT reagent (Beyotime Institute of Biotechnology) at 37°C for
4 h, dimethyl sulfoxide (Thermo Fisher Scientific, Inc.) was
applied to treat cells at room temperature for 10 min, and the
value of optical density was measured at 570 nm using a microplate
reader (BioTek Instruments, Inc.).
Colony formation
After transfection, H1975 and A549 cells
(5×102 cells/well) were seeded into six-well plates.
After ~14 d, visible clones appeared in the six-well plates.
Colonies (>50 cells) were fixed with 4% formaldehyde for 15 min
and stained with 0.1% crystal violet (both from Sigma-Aldrich;
Merck KGaA) for 25 min. Finally, the number of effective clones was
calculated using a microscope (DMI3000 B; Leica Microsystems
GmbH).
Wound healing assay
H1975 and A549 cells (4×105 cells/well)
were seeded in six-well cell culture plates. When the confluence of
the monolayer cells reached 70–80%, a new 200-µl pipette tip was
used to gently scratch a wound on the monolayer cells. Cells were
cultured in serum-free medium for 48 h. Images were captured by a
microscope (DMI3000 B; Leica Microsystems GmbH) at 0 and 48 h, and
the wound width was analyzed using ImageJ software v1.51 (National
Institutes of Health).
Transwell assay
A 6.5 mm Transwell with 8.0 µm Pore Polycarbonate
Membrane Insert, Sterile (Corning, Inc.) was used in invasion
assay. The transfected H1975 and A549 cells (1×105
cells/ml) were resuspended in serum-free DMEM in the upper chamber
with Matrigel (BD Biosciences) at 37°C for 30 min, while the lower
chamber was filled with 150 µl DMEM containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). After culturing at
37°C for 48 h, 800 µl methanol and 800 µl crystal violet were used
to fix for 15 min at room temperature and stain for 30 min at room
temperature cells. A total of five randomly selected fields were
observed under microscope (Leica Microsystems GmbH).
Glucose and lactate measurement
A Glucose Assay kit (cat. no. ab65333; Abcam) and a
Lactic Acid Assay kit (cat. no. MAK064, Sigma-Aldrich; Merck KGaA)
were used to measure glucose consumption and lactic acid
production, respectively, in H1975 and A549 cells. Glucose enzyme
mix specifically oxidizes glucose, to generate a product which
reacts with a dye to generate color (OD 570 nm). Glucose content in
the cell culture medium was determined and cells in each well were
counted to normalize glucose concentration. Glucose uptake was
determined indirectly by determining the remained glucose content
in cell culture medium. Lactate specifically reacts with an enzyme
mix to generate a product, which interacts with lactate probe to
produce color (OD 570 nm). Lactate production in the cell culture
medium was detected and cells in each well were counted to
normalize lactate concentration. Absorbance at OD 570 nm was
detected using a microplate reader (BioTek Instruments, Inc.).
Co-Immunoprecipitation (Co-IP)
Whole-cell extracts were lysed in RIPA buffer
(Beyotime Institute of Biotechnology). Lysates were clarified by
centrifugation at 14,000 g for 15 min at 4°C. Supernatant was
incubated with 20 µl/ml protein A/G sepharose beads (Beyotime
Institute of Biotechnology) at 4°C for 1 h to remove non-specific
hybrid proteins. A total of 5 µg IgG (cat. no. ab37415; Abcam),
anti-HSPB7 (cat. no. ab150390; Abcam) or anti-MECOM (cat. no.
23201-1-AP; Proteintech Group, Inc.) antibodies were added to
pre-cleared cell lysate and incubated at 4°C overnight and then
rotated at 4°C with a mixture of protein A/G sepharose beads (20
µl/ml) for 4 h. The beads were then washed 3 times with RIPA buffer
to obtain protein samples for western blot analysis.
Chromatin immunoprecipitation
(ChIP)
In brief, H1975 and A549 cells were first
cross-linked with formaldehyde at room temperature for 15 min, and
then cleaved by the Magna ChIP™ Protein G Kit
(Sigma-Aldrich; Merck KGaA) to obtain chromatin, followed by
ultrasonic fragmentation. MECOM antibody (cat. no. HPA046537;
Sigma-Aldrich; Merck KGaA) was used to recruit HSPB7 DNA overnight
at 4°C, and the protein-DNA complex was precipitated with protein G
magnetic beads for 2 h. After immunoprecipitation, the DNA in the
complex was purified, and enriched HSPB7 was quantified by PCR.
Tumorigenesis assay
Male nude mice aged 4–6 weeks (weight, 14–15 g;
GemPharmatech Biotechnology Co., Ltd.) were used, with five mice
per experimental group. All mice were maintained under controlled
temperature (22±1°C) and humidity (50±5%) in a 12/12-h light/dark
cycle with food and water available ad libitum. A total of
1×106 control cells and A549 cells with overexpression
of HSPB7 were resuspended in phosphate-buffered saline and injected
subcutaneously into the back flank of mice, and tumour volume was
recorded every 3 days. Tumor volume >2,000 mm3 was
considered the humane endpoint. The tumor volume was calculated as
follows: Volume=0.5× length × width2. At the end of the
experiments, the weight of the mice was 18–19 g. After 27 days,
mice were sacrificed by cervical dislocation under anesthesia
(pentobarbital sodium, 50 mg/kg, intraperitoneal injection) and
confirmed the sacrifice by cessation of heartbeat. Animal
experiments were approved (approval no. W202203060148) by the
Animal Ethics Committee of Jinan Central Hospital Affiliated to
Shandong First Medical University (Jinan, China).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 6 software (Dotmatics). All data are presented as
the mean ± SD. Each experiment was performed in triplicate.
Survival curves were analysed by the Kaplan-Meier method and
compared by the log-rank test. Comparisons were analysed using the
paired or unpaired Student's t-test or one-way ANOVA, followed by
Tukey's post hoc test. Pearson's correlation analysis was performed
to assess the correlation between MECOM and HSPB7 expression levels
in the tumour tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
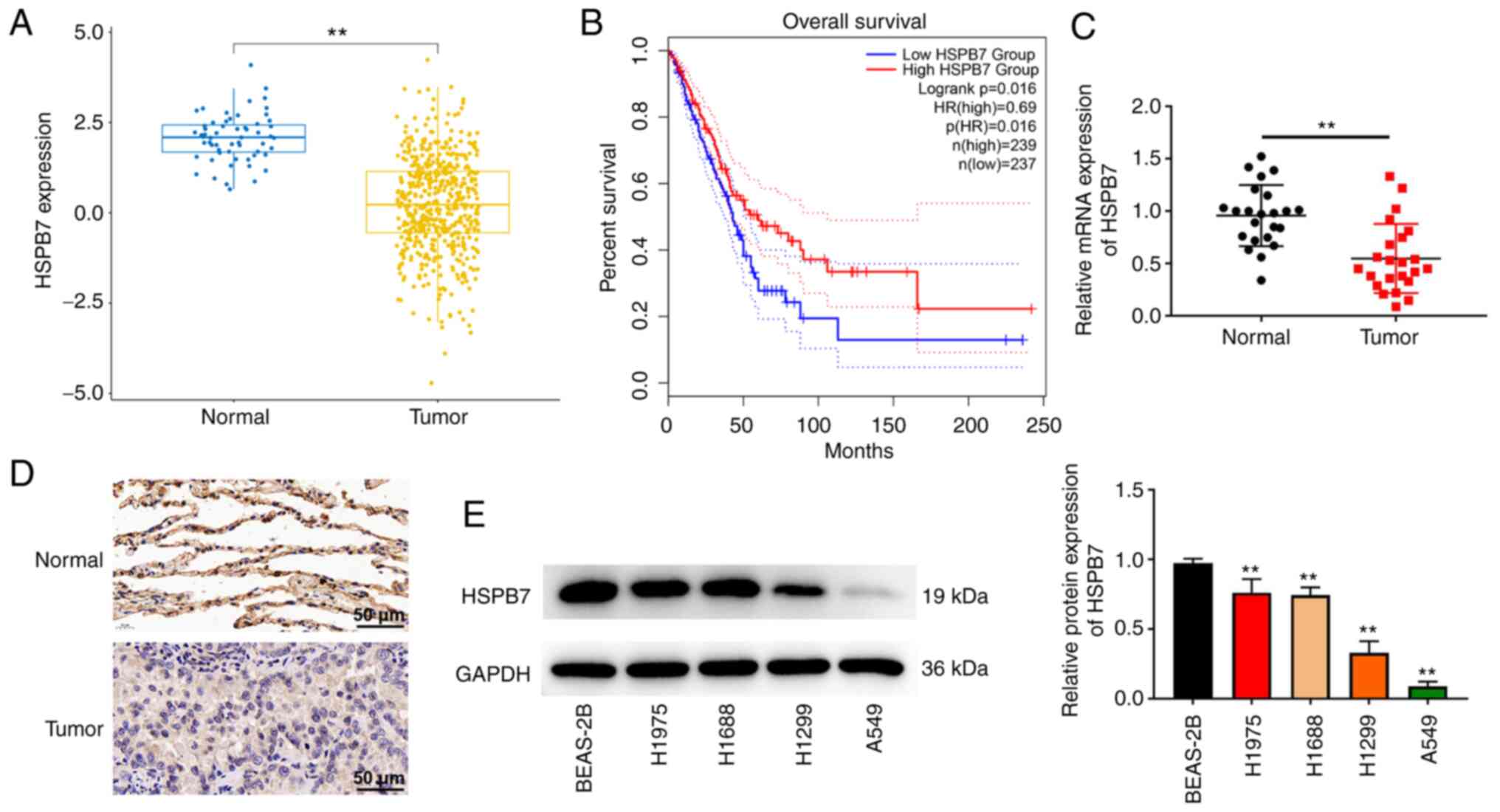
HSPB7 expression is downregulated in
LUAD tissues and cells
A total of 515 patients with LUAD were acquired from
TCGA-LUAD cohort datasets and analyzed to identify the HSPB7
expression in LUAD. The detailed clinical features of the patients
are listed in Table I. As revealed
in Fig. 1A, HSPB7 expression was
downregulated in LUAD tumour tissues compared with non-tumour
tissues. Survival analysis demonstrated that patients with high
HSPB7 expression had a higher survival rates (Fig. 1B). The detailed clinical features of
the 23 tumor tissues and paired normal tissues are presented in
Table II. The RT-qPCR and IHC data
indicated that HSPB7 was expressed at low levels in LUAD tumour
tissues compared with the paired normal tissues (Fig. 1C and D). In addition, the expression
of HSPB7 protein was detected in lung cancer cell lines. HSPB7
expression showed different degrees of reduction in the H1975,
H1688, H1299 and A549 cells compared with that in BEAS-2B cells
(Fig. 1E). To further clarify the
role of HSPB7 in LUAD, H1975 cells with the highest HSPB7
expression and A549 cells with the lowest expression were selected
among the four cell lines for a follow-up study.
 | Table I.Clinical characteristics of the lung
adenocarcinoma patients from The Cancer Genome Atlas. |
Table I.
Clinical characteristics of the lung
adenocarcinoma patients from The Cancer Genome Atlas.
| Clinical
characteristics | Number | Percentage, % |
|---|
| Age, years |
|
|
|
<65 | 239 | 46.4 |
|
≥65 | 276 | 53.6 |
| Sex |
|
|
|
Female | 277 | 53.8 |
|
Male | 238 | 46.2 |
| Stage |
|
|
| I | 275 | 53.4 |
| II | 122 | 23.7 |
|
III | 73 | 14.2 |
| IV | 11 | 2.1 |
| T
classification |
|
|
| T1 | 169 | 32.8 |
| T2 | 277 | 53.8 |
| T3 | 47 | 9.1 |
| T4 | 19 | 3.7 |
| TX | 3 | 0.6 |
| M
classification |
|
|
| M0 | 346 | 67.2 |
| M1 | 25 | 4.9 |
| MX | 140 | 27.2 |
| Missing
data | 4 | 0.8 |
| N
classification |
|
|
| N0 | 331 | 64.3 |
| N1 | 96 | 18.6 |
| N2 | 74 | 14.4 |
| N3 | 2 | 0.4 |
| NX | 11 | 2.1 |
| Missing
data | 1 | 0.2 |
| ICD-10 |
|
|
|
C34.0 | 2 | 0.4 |
|
C34.1 | 310 | 60.2 |
|
C34.2 | 20 | 3.9 |
|
C34.3 | 174 | 33.8 |
|
C34.8 | 4 | 0.8 |
|
C34.9 | 5 | 1.0 |
 | Table II.Clinical characteristics of the lung
adenocarcinoma patients from our hospital. |
Table II.
Clinical characteristics of the lung
adenocarcinoma patients from our hospital.
| Clinical
characteristics | Number | Percentage, % |
|---|
| Age, years |
|
|
|
<65 | 9 | 39.1 |
|
≥65 | 14 | 60.9 |
| Sex |
|
|
|
Female | 10 | 43.5 |
|
Male | 13 | 56.5 |
| Stage |
|
|
| I | 8 | 34.8 |
| II | 11 | 47.8 |
|
III | 3 | 13.0 |
| IV | 1 | 4.3 |
| T
classification |
|
|
| T1 | 7 | 30.4 |
| T2 | 13 | 56.5 |
| T3 | 2 | 8.7 |
| T4 | 1 | 4.3 |
| M
classification |
|
|
| M0 | 20 | 87.0 |
| M1 | 3 | 13.0 |
| N
classification |
|
|
| N0 | 19 | 82.6 |
| N1 | 3 | 13.0 |
| N2 | 1 | 4.3 |
| ICD-10 |
|
|
|
C34.1 | 15 | 65.2 |
|
C34.3 | 8 | 34.8 |
HSPB7 restricts LUAD cell
proliferation, migration, invasion and epithelial-mesenchymal
transition (EMT)
To improve understanding of the effects of HSPB7 on
LUAD cell behaviour, cell proliferation, migration, invasion and
EMT were evaluated. First, HSPB7 low expressing and overexpressing
cells were constructed, and RT-qPCR and western blotting results
revealed that the construction was successful (Fig. 2A and B). In the MTT and colony
formation assays, it was observed that knockdown of HSPB7 promoted
H1975 cell proliferation, while overexpression of HSPB7 inhibited
A549 cell proliferation (Fig. 2C and
D). In wound healing and Transwell experiments, the data also
revealed that low expression of HSPB7 promoted H1975 cell migration
and invasion, whereas overexpression of HSPB7 inhibited A549 cell
migration and invasion (Fig. 2E and
F). As western blotting results demonstrated, HSPB7 knockdown
significantly reduced E-cadherin protein expression and increased
the protein expression of N-cadherin and Snail in H1975 cells.
Conversely, overexpression of HSPB7 increased the expression of
E-cadherin protein and inhibited the protein expression of
N-cadherin and Snail in A549 cells (Fig. 2G).
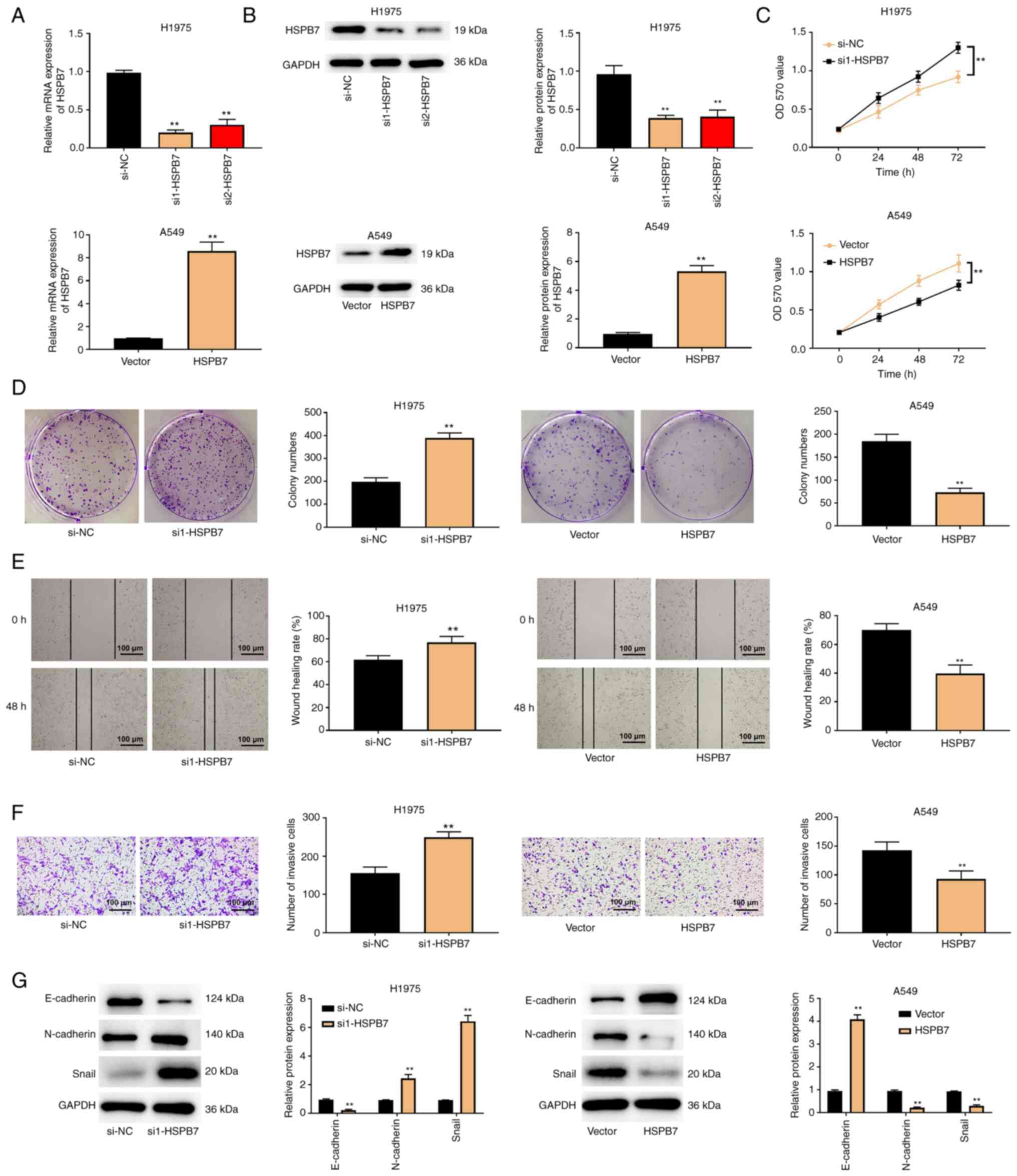 | Figure 2.HSPB7 restricts lung adenocarcinoma
cell proliferation, migration, invasion and EMT. (A) The H1975 and
A549 cells were transfected with si-NC, si1-HSPB7, si2-HSPB7,
vector, and HSPB7. HSPB7 expression in H1975 and A549 cells was
determined through reverse transcription-quantitative PCR. (B)
HSPB7 protein expression in H1975 and A549 cells was measured using
western blotting. The H1975 and A549 cells were transfected with
si-NC, si1-HSPB7, vector and HSPB7. (C) MTT assay, (D) colony
formation assay, (E) wound healing assay and (F) Transwell assay
were used to evaluate H1975 and A549 cell proliferation, migration
and invasion. (G) EMT-related proteins, including E-cadherin,
N-cadherin and Snail were detected in H1975 and A549 cells via
western blotting. Data are shown as the mean ± SD (n=3).
**P<0.01 vs. si-NC or vector group by unpaired Student's t test.
HSPB7, heat shock protein B7; EMT, epithelial-mesenchymal
transition; si-, small interfering; NC, negative control. |
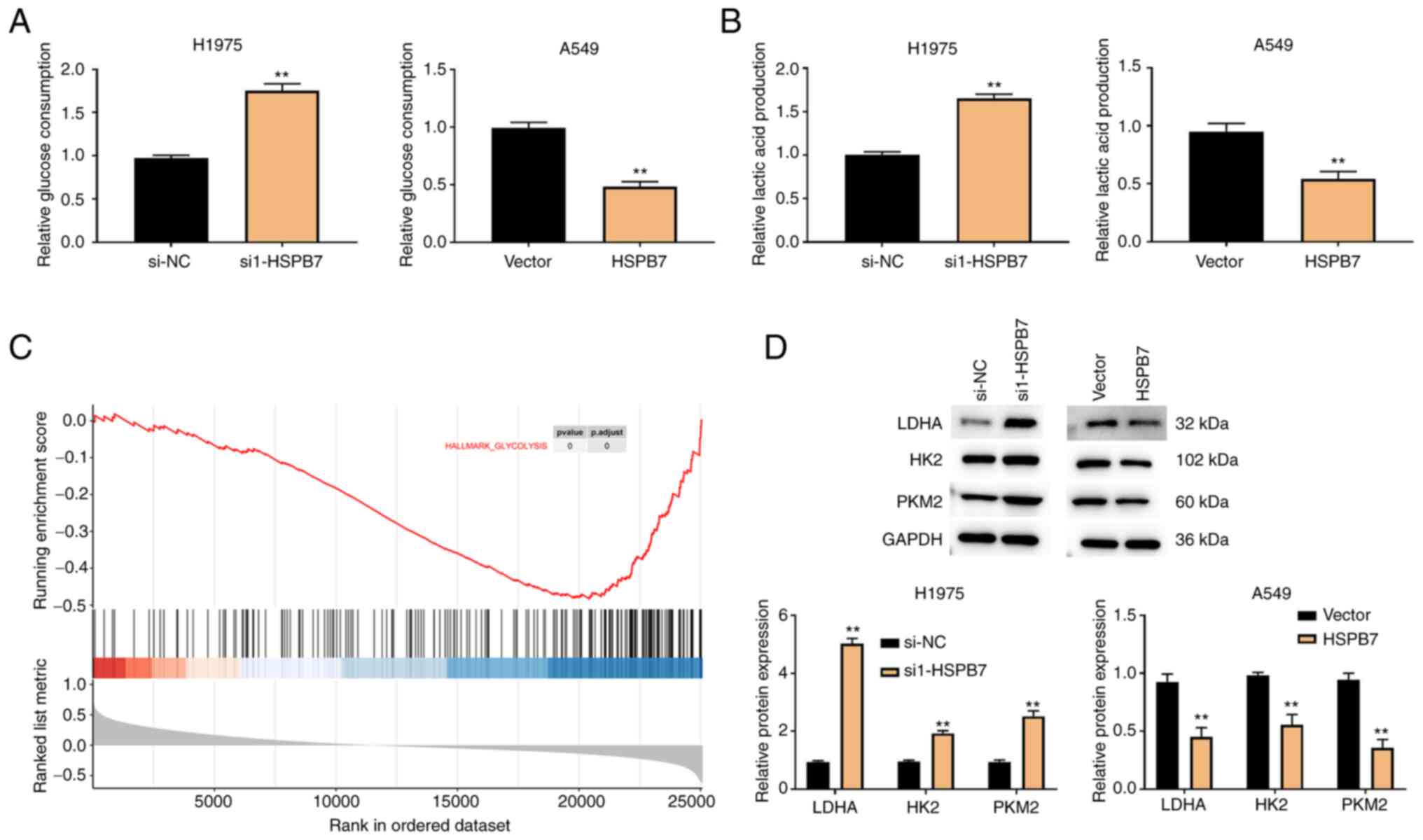
HSPB7 inhibits glycolysis
To support uncontrolled proliferation, migration and
invasion, LUAD cells can shift their glucose metabolism pattern to
aerobic glycolysis (21). It was
hypothesized that HSPB7 participates in the regulation of aerobic
glycolysis, thereby affecting LUAD progression. As revealed in
Fig. 3A and B, low HSPB7 expression
increased glucose consumption and lactic acid production in H1975
cells. On the contrary, when HSPB7 was overexpressed, A549 cells
reduced glucose consumption and lactic acid production.
Furthermore, GSEA revealed that HSPB7 was involved in glycolysis in
LUAD cells (Fig. 3C). Therefore, it
was hypothesized that HSPB7 participates in the regulation of LUAD
by inhibition of glycolysis. To test this hypothesis, the levels of
the glycolysis-associated proteins (LDHA, HK2 and PKM2) were
examined in the H1975 and A549 cells. The results of western
blotting indicated that HSPB7 knockdown increased the expression of
LDHA, HK2 and PKM2, whereas HSPB7 overexpression inhibited the
expression of LDHA, HK2 and PKM2 (Fig.
3D).
HSPB7 inhibits the proliferation,
migration, and invasion of LUAD cells by regulating glycolysis
To analyze the relationship among HSPB7, glycolysis
and tumour progression, the glycolysis inhibitor, 2-DG, was added
into the H1975 cells that silenced HSPB7 expression. 2-DG is a
glucose analogue that differs from glucose only by the removal of
an oxygen atom at the C-2 position, which prevents the
isomerization of glucose-6-phosphate to fructose-6-phosphate,
thereby inhibiting glycolysis (22). As shown in Fig. 4A, silencing of HSPB7 promoted H1975
proliferation, but the addition of 2-DG weakened the promoting
effect of si1-HSPB7 on cell proliferation. The cell migration and
invasion abilities were significantly reduced in the si1-HSPB7 +
2-DG group compared with si1-HSPB7 group (Fig. 4B and C). In addition, it was
observed that 2-DG significantly reduced the consumption of glucose
and production of lactic acid induced by HSPB7 knockdown in H1975
cells (Fig. 4D and E).
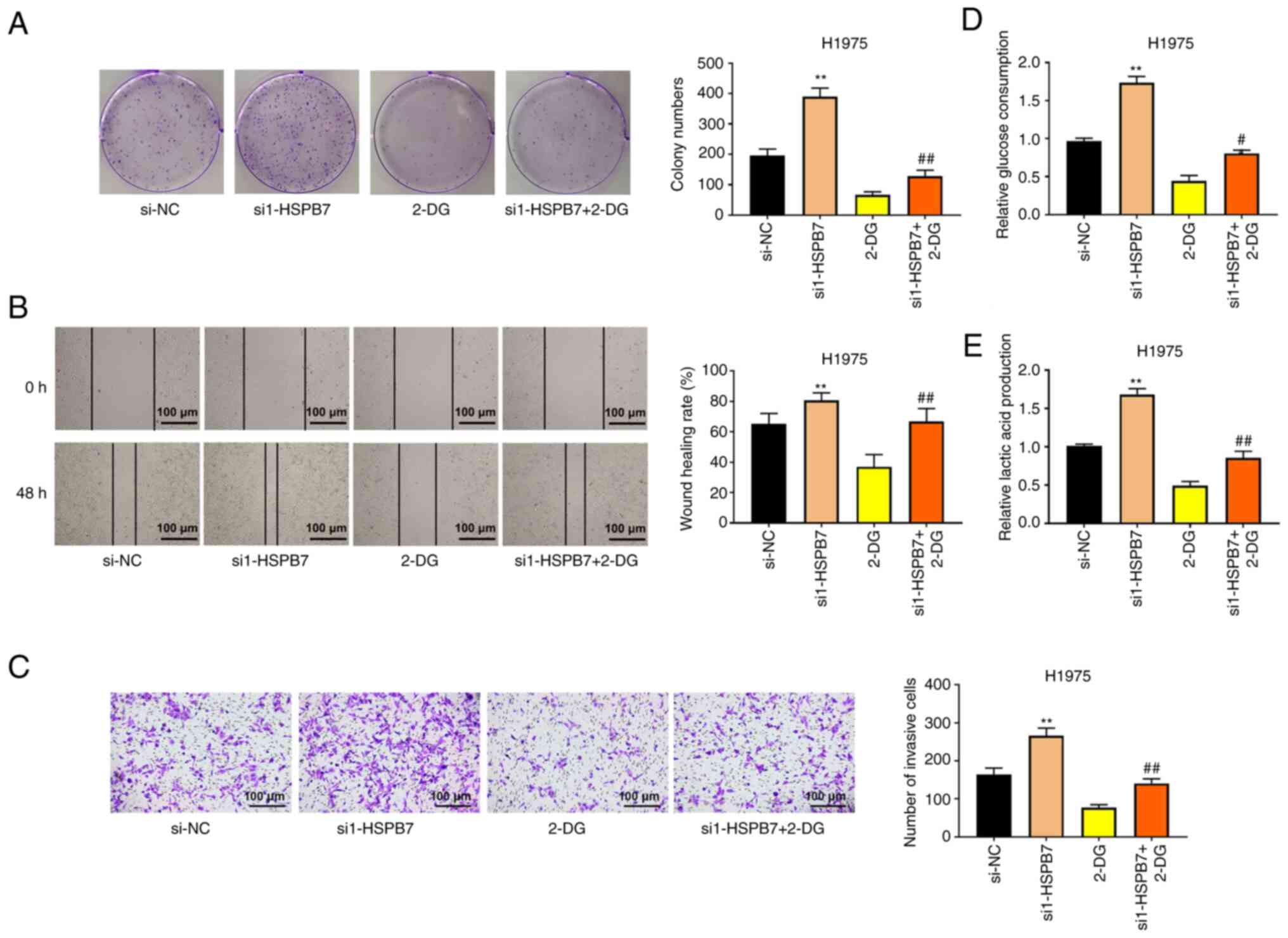 | Figure 4.Silencing HSPB7 promotes the
proliferation, migration, and invasion of lung adenocarcinoma cells
by regulating glycolysis. The H1975 cells were transfected with
si-NC or si1-HSPB7, followed by treating with or without glycolysis
inhibitor 2-DG. (A) Colony formation assay, (B) wound healing assay
and (C) Transwell assay were used to evaluate H1975 cell
proliferation, migration and invasion. (D) Glucose consumption in
H1975 cells was detected using Glucose Assay kit. (E) Lactic acid
production in H1975 cells was evaluated using Lactic Acid Assay
kit. Data are shown as the mean ± SD (n=3). **P<0.01 vs. si-NC
group; #P<0.05 vs. si1-HSPB7 group;
##P<0.01 vs. si1-HSPB7 group by one-way ANOVA test,
followed by Tukey's post hoc test. HSPB7, heat shock protein B7;
si-, small interfering; NC, negative control; 2-DG,
2-deoxy-D-glucose. |
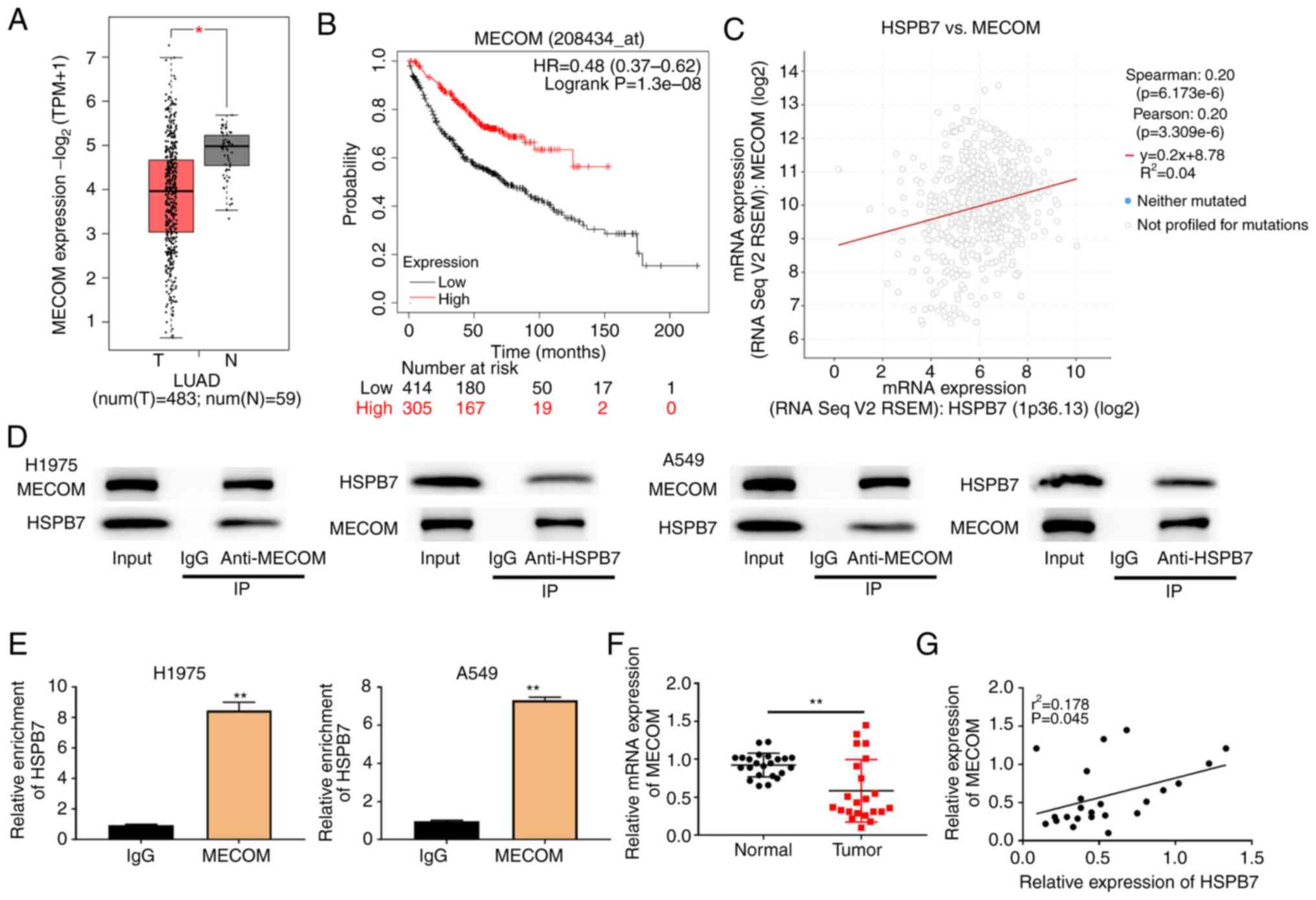
MECOM is a transcription factor of
HSPB7
TCGA dataset analysis identified that MECOM was
poorly expressed in LUAD tumour tissues, and the survival rate of
patients with low MECOM expression was also relatively low
(Fig. 5A and B). Furthermore, MECOM
levels were positively correlated with HSPB7 expression (Fig. 5C). Co-IP assays were conducted in
H1975 and A549 cells. The results of Co-IP revealed that HSPB7
could interact with MECOM (Fig.
5D). According to JASPAR website analysis, the binding site of
MECOM is located within 1 kb upstream of HSPB7. To verify this,
ChIP analysis was performed and it was found that HSPB7 was
significantly enriched in the MECOM group (Fig. 5E). The RT-qPCR results revealed that
MECOM was downregulated in tumour tissues, and there was a positive
correlation between the levels of MECOM and HSPB7 (Fig. 5F and G).
HSPB7 is regulated by the
transcription factor MECOM
The expression levels of MECOM mRNA in the lung
cancer cell lines were also measured. MECOM mRNA expression was
decreased in H1975, H1688, H1299 and A549 cells compared with
BEAS-2B cells (Fig. 6A). To clarify
the roles of HSPB7 and MECOM in LUAD, rescue experiments were
conducted in H1975 cells. As demonstrated in Fig. 6B, MECOM expression was increased in
H1975 cells transfected with MECOM overexpression plasmid, whereas
was decreased in H1975 cells transfected with si-MECOM.
Overexpression of MECOM increased HSPB7 protein expression, while
silencing of MECOM decreased HSPB7 protein expression (Fig. 6C). In terms of proliferation,
overexpression of MECOM decreased the colony number of H1975 cells,
but co-transfection of si1-HSPB7 reversed the inhibitory effect of
MECOM on cell proliferation (Fig.
6D). The migration and invasion abilities of H1975 cells in the
si1-HSPB7 + MECOM group were significantly higher than those in the
MECOM group (Fig. 6E and F). In
addition, the consumption of glucose and production of lactic acid
by the H1975 cells in the si1-HSPB7+MECOM group were increased
compared with MECOM group (Fig. 6G and
H).
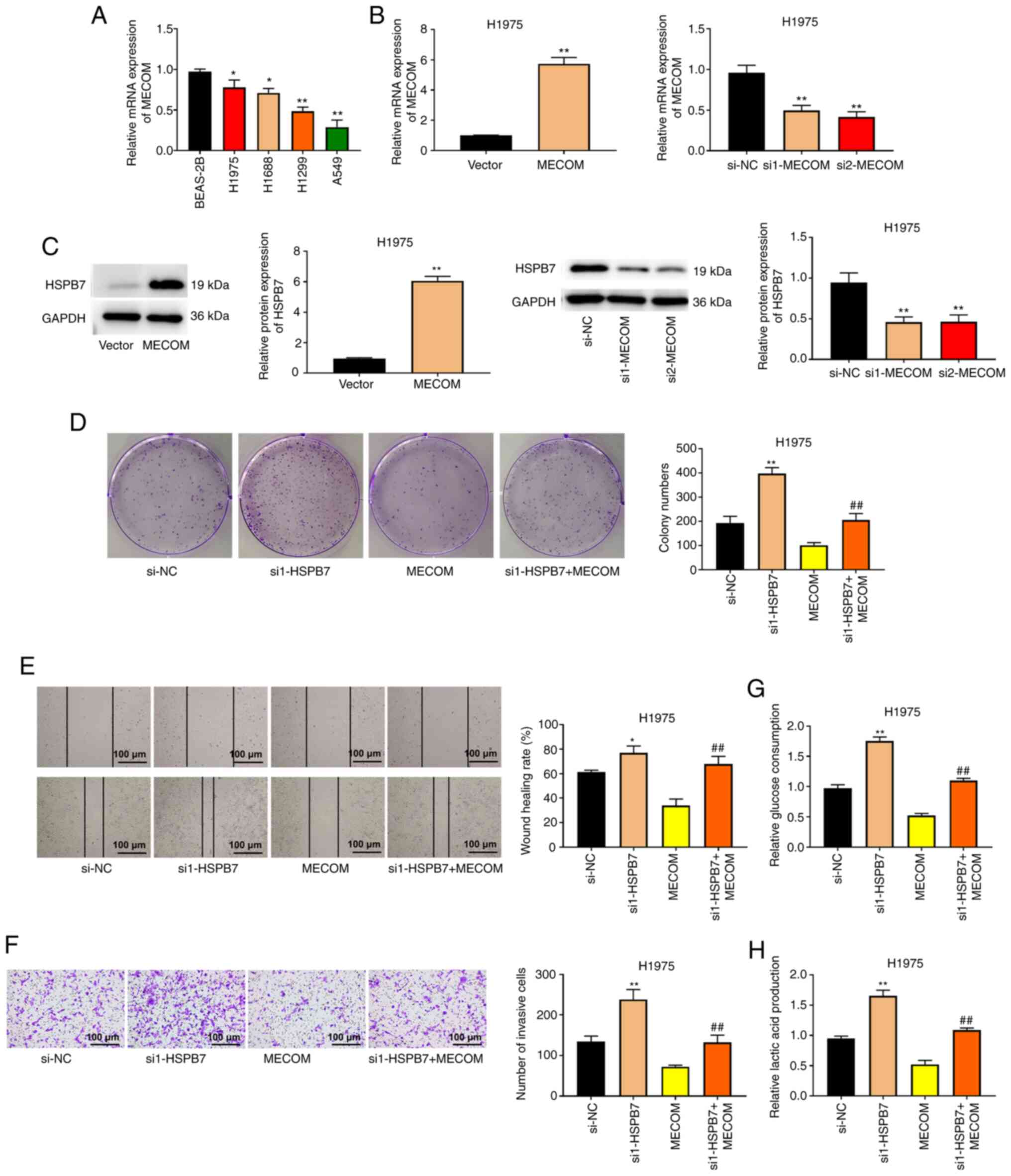 | Figure 6.HSPB7 is regulated by the
transcription factor MECOM. (A) The mRNA expression of MECOM in
human normal lung epithelial cells (BEAS-2B) and lung cancer cells
(H1975, H1688, H1299 and A549) was detected using RT-qPCR. The
H1975 cells were transfected with si-NC, si1-HSPB7, MECOM,
si1-MECOM, or si2-MECOM. (B) The mRNA expression of MECOM in H1975
cells was measured using RT-qPCR. (C) HSPB7 protein expression in
H1975 cells was measured using western blot analysis. (D) Colony
formation assay, (E) wound healing assay and (F) Transwell assay
were respectively to evaluate H1975 cell proliferation, migration
and invasion. (G) Glucose consumption in H1975 cells was detected
using Glucose Assay kit. (H) Lactic acid production in H1975 cells
was evaluated using Lactic Acid Assay kit. Data are shown as the
mean ± SD (n=3). *P<0.05 vs. BEAS-2B or si-NC group; **P<0.01
vs. BEAS-2B, si-NC, or vector group; ##P<0.01 vs.
MECOM group by one-way ANOVA test with Tukey's post hoc test.
HSPB7, heat shock protein B7; MECOM, myelodysplastic syndrome 1 and
ecotropic viral integration site 1 complex locus; RT-qPCR, reverse
transcription-quantitative PCR; si-, small interfering; NC,
negative control. |
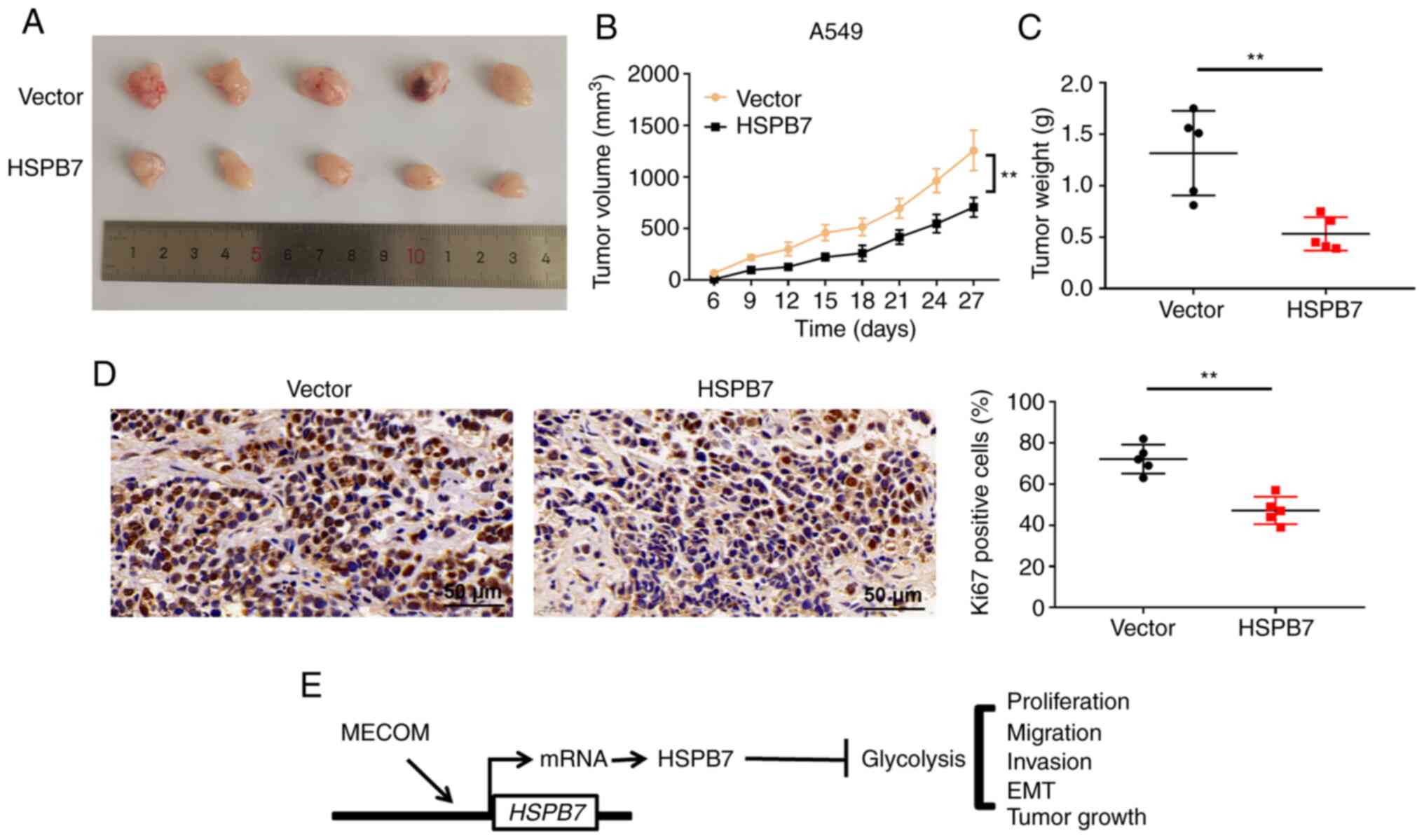
HSPB7 suppresses tumour growth
The effects of HSPB7 overexpression on the
tumorigenic ability of A549 cells derived from nude mice were
determined. Tumours derived from injected HSPB7 overexpression
cells were significantly smaller than those derived from
vector-transfected mice (Fig. 7A and
B). The average weight of tumours from nude mice injected with
HSPB7 overexpression cells was lower than that of tumours from nude
mice injected with control A549 cells (Fig. 7C). The number of Ki67 positive
cells, a marker of proliferation, in the HSPB7 overexpression group
decreased significantly (Fig. 7D).
These results suggested that overexpression of HSPB7 in A549 cells
inhibited their tumorigenic ability.
Discussion
In the present study, the influence and underlying
molecular mechanisms of HSPB7 on the biological behaviour of lung
cancer cells were investigated. The present findings revealed that
HSPB7 expression was downregulated in LUAD tissues and cells. MECOM
was a transcription factor of HSPB7. Knockdown of HSPB7 promoted
lung cancer cell proliferation, migration and invasion; all of
which were related to glycolysis (Fig.
7E).
Late diagnosis and complex clinical features lead to
poor prognosis in LUAD (23,24).
Precision medicine targeting the epidermal growth factor receptor,
anaplastic lymphoma kinase, and ROS proto-oncogene 1, receptor
tyrosine kinase (ROS1) has contributed significantly to the
effective treatment of LUAD (25,26).
Although targeted therapy has made some progress, an increasing
number of specific molecular biomarkers and therapeutic targets are
required to guide and improve the prognosis and treatment of LUAD.
A previous study reported that HSPB7 expression is downregulated by
methylation and that HSPB7 acts as a tumour suppressor regulated by
p53 in renal cell carcinoma (17).
In the present study, it was found that HSPB7 was significantly
downregulated in LUAD tissues and cells, and that its aberrant
expression correlated with survival in patients with LUAD. In
addition, silencing of HSPB7 promoted LUAD cell proliferation,
invasion and migration. HSPB7 acted as a tumour suppressor
regulated by MECOM in LUAD. Naderi (16) confirmed that knockdown of HSPB7
promotes the proliferation of human breast cancer cells. EMT is
closely related to primary invasion and secondary metastasis of a
variety of tumours and its inhibition could prevent cancer
progression (27). E-cadherin
transforms into N-cadherin during EMT, and expression of
E-cadherin, an epithelial cell marker, is downregulated (28). The E-cadherin suppressor, Snail,
facilitates cancer cell migration and invasion (29). In the present study, silencing of
HSPB7 promoted EMT in LUAD cells, which showed a decrease in
E-cadherin protein and an increase in N-cadherin and Snail
proteins; whereas, overexpression of HSPB7 led to the opposite
findings. Taken together, these results suggested that HSPB7 plays
a role in LUAD progression.
Increasing evidence has indicated that glycolysis
promotes cancer progression (30,31).
Therefore, it is a new strategy to delay tumor progression by
inhibiting glycolysis in cancer cells. In cancer cells, elevated
glycolysis promotes glucose uptake and lactic acid production to
meet metabolic needs, thereby increasing cell proliferation and
metastasis (7). Zhou et al
(31) demonstrated that silencing
of circRNA enolase 1 inhibits glucose uptake and lactic acid
production, thereby inhibiting glycolysis and ultimately LUAD
progression. Consistently, the present data demonstrated that HSPB7
reduced glucose consumption and lactic acid production in LUAD
cells. In addition, the inhibition of glycolysis by 2-DG weakened
the promoting effect of knockdown of HSPB7 on cell proliferation,
migration and invasion. LDHA, HK2 and PKM2 are important factors in
glycolysis and their overexpression is associated with poor
prognosis of patients with lung cancer (32,33).
The present study demonstrated that HSPB7 inhibits the expression
of LDHA, HK2 and PKM2 in lung cancer cells. Collectively, HSPB7
inhibited the proliferation, migration and invasion of LUAD cells,
all of which are related to glycolysis.
MECOM, also known as MDS1/EVI1, is located on
chromosome 3q26.2. It encodes the MDS1 and EVI1 complex locus
proteins and consists of 1051 amino acids (34). MECOM encodes zinc-finger
transcription factors and participates in a series of biological
processes by regulating downstream gene expression (35). In addition, MECOM is a tumour
suppressor that plays an important role in normal development and
tumourigenesis (36). Li et
al (37) reported that in LUAD,
the mRNA levels of MECOM are downregulated and that it may serve as
a potential prognostic biomarker. In the present study, MECOM
expression was downregulated in LUAD tissues. The ChIP assay
confirmed that MECOM directly regulated the transcription of HSPB7
in LUAD. Furthermore, overexpression of MECOM inhibited
proliferation, migration, invasion, glucose consumption and lactic
acid production in lung cancer cells; while knockdown of HSPB7
attenuated the inhibitory effect of MECOM on cell behaviour. Taken
together, these findings confirmed that HSPB7 was regulated by
MECOM and participated in the progression of LUAD.
There were certain limitations to the present study.
First, an in vivo metastatic assay was not performed.
Secondly, the effect of HSPB7 on apoptosis remains unclear. Future
studies could address these limitations.
In summary, HSPB7 was expressed at low levels in
LUAD tissues and cells, and overexpression of HSPB7 inhibited lung
cancer cell proliferation, invasion and migration. HSPB7 was
regulated by the MECOM and inhibited LUAD progression by inhibiting
glycolysis. The findings of the present study provide a new insight
into HSPB7 expression in patients with LUAD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZTC conceived and designed the study. PPL, LGS and
XYJ provided study materials. PPL, LGS and XYJ collected and
assembled the data. ZTC analyzed and interpreted the data. LGS and
XYJ confirm the authenticity of all the raw data. All authors
participated in manuscript writing, read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The protocols of human studies (W202203060147) and
animal experiments (W202203060148) were approved by the Ethics
Committee of Jinan Central Hospital Affiliated to Shandong First
Medical University (Jinan, China). Written informed consent was
provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MDS1
|
myelodysplastic syndrome 1
|
|
EVI1
|
ecotropic viral integration site 1
|
|
MECOM
|
MDS1 and EVI1 complex locus
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
HK2
|
hexokinase 2
|
|
HSPB7
|
heat shock protein B7
|
|
LDHA
|
lactate dehydrogenase A
|
|
LUAD
|
lung adenocarcinoma
|
|
PKM2
|
pyruvate kinase muscle isoform 2
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerdidani D, Chouvardas P, Arjo AR,
Giopanou I, Ntaliarda G, Guo YA, Tsikitis M, Kazamias G, Potaris K,
Stathopoulos GT, et al: Wnt1 silences chemokine genes in dendritic
cells and induces adaptive immune resistance in lung
adenocarcinoma. Nat Commun. 10:14052019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao F, Zhao Z, Han Y, Li S, Liu C and Jia
K: Baicalin suppresses lung cancer growth phenotypes via
miR-340-5p/NET1 axis. Bioengineered. 12:1699–1707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Sun D, Zhu Q and Liu X: The
screening of immune-related biomarkers for prognosis of lung
adenocarcinoma. Bioengineered. 12:1273–1285. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gettinger S and Lynch T: A decade of
advances in treatment for advanced non-small cell lung cancer. Clin
Chest Med. 32:839–851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Shi B, Zhao Y, Lu Q, Fei X, Lu C,
Li C and Chen H: HKDC1 promotes the tumorigenesis and glycolysis in
lung adenocarcinoma via regulating AMPK/mTOR signaling pathway.
Cancer Cell Int. 20:4502020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pagliarone AC, Castañeda ED, Santana JPP,
de Oliveira CAB, Robeldo TA, Teixeira FR and Borra RC:
Mitochondrial heat shock protein mortalin as potential target for
therapies based on oxidative stress. Photodiagnosis Photodyn Ther.
34:1022562021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henstridge DC, Whitham M and Febbraio MA:
Chaperoning to the metabolic party: The emerging therapeutic role
of heat-shock proteins in obesity and type 2 diabetes. Mol Metab.
3:781–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mymrikov EV, Riedl M, Peters C, Weinkauf
S, Haslbeck M and Buchner J: Regulation of small heat-shock
proteins by hetero-oligomer formation. J Biol Chem. 295:158–169.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muranova LK, Shatov VM, Bukach OV and
Gusev NB: Cardio-vascular heat shock protein (cvHsp, HspB7), an
unusual representative of small heat shock protein family.
Biochemistry (Mosc). 86 (Suppl 1):S1–S11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu T, Mu Y, Bogomolovas J, Fang X, Veevers
J, Nowak RB, Pappas CT, Gregorio CC, Evans SM, Fowler VM and Chen
J: HSPB7 is indispensable for heart development by modulating actin
filament assembly. Proc Natl Acad Sci USA. 114:11956–11961. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Juo LY, Liao WC, Shih YL, Yang BY, Liu AB
and Yan YT: HSPB7 interacts with dimerized FLNC and its absence
results in progressive myopathy in skeletal muscles. J Cell Sci.
129:1661–1670. 2016.PubMed/NCBI
|
|
14
|
Liu CC, Chou KT, Hsu JW, Lin JH, Hsu TW,
Yen DH, Hung SC and Hsu HS: High metabolic rate and stem cell
characteristics of esophageal cancer stem-like cells depend on the
Hsp27-AKT-HK2 pathway. Int J Cancer. 145:2144–2156. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu S, Yan B, Lai W, Chen L, Xiao D, Xi S,
Jiang Y, Dong X, An J, Chen X, et al: As a novel p53 direct target,
bidirectional gene HspB2/αB-crystallin regulates the ROS level and
Warburg effect. Biochim Biophys Acta. 7:592–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naderi A: SRARP and HSPB7 are
epigenetically regulated gene pairs that function as tumor
suppressors and predict clinical outcome in malignancies. Mol
Oncol. 12:724–755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin J, Deng Z, Tanikawa C, Shuin T, Miki
T, Matsuda K and Nakamura Y: Downregulation of the tumor suppressor
HSPB7, involved in the p53 pathway, in renal cell carcinoma by
hypermethylation. Int J Oncol. 44:1490–1498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al: An integrated TCGA pan-cancer clinical data resource to
drive high-quality survival outcome analytics. Cell.
173:400–416.e11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riaz SP, Horton M, Kang J, Mak V,
Lüchtenborg M and Møller H: Lung cancer incidence and survival in
England: An analysis by socioeconomic deprivation and urbanization.
J Thorac Oncol. 6:2005–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu XT, Wang YH, Cai XY, Dong Y, Cui Q,
Zhou YN, Yang XW, Lu WF and Zhang M: RNF115 promotes lung
adenocarcinoma through Wnt/β-catenin pathway activation by
mediating APC ubiquitination. Cancer Metab. 9:72021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sutula TP and Fountain NB: 2DG and
glycolysis as therapeutic targets for status epilepticus. Epilepsy
Behav. 140:1091082023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Wang H, Wu B, Zhang C, Yu H, Li X,
Wang Q, Shi X, Fan C, Wang D, et al: Long noncoding RNA LINC00551
suppresses glycolysis and tumor progression by regulating
c-Myc-mediated PKM2 expression in lung adenocarcinoma. Onco Targets
Ther. 13:11459–11470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhuo X, Chen L, Lai Z, Liu J, Li S, Hu A
and Lin Y: Protein phosphatase 1 regulatory subunit 3G (PPP1R3G)
correlates with poor prognosis and immune infiltration in lung
adenocarcinoma. Bioengineered. 12:8336–8346. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rocco D, Della Gravara L, Battiloro C,
Maione P and Gridelli C: The treatment of advanced lung
adenocarcinoma with activating EGFR mutations. Expert Opin
Pharmacother. 22:2475–2482. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamberti G, Andrini E, Sisi M, Rizzo A,
Parisi C, Di Federico A, Gelsomino F and Ardizzoni A: Beyond EGFR,
ALK and ROS1: Current evidence and future perspectives on newly
targetable oncogenic drivers in lung adenocarcinoma. Crit Rev Oncol
Hematol. 156:1031192020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bezdenezhnykh N, Semesiuk N, Lykhova O,
Zhylchuk V and Kudryavets Y: Impact of stromal cell components of
tumor microenvironment on epithelial-mesenchymal transition in
breast cancer cells. Exp Oncol. 36:72–78. 2014.PubMed/NCBI
|
|
28
|
Cervantes-Arias A, Pang LY and Argyle DJ:
Epithelial-mesenchymal transition as a fundamental mechanism
underlying the cancer phenotype. Vet Comp Oncol. 11:169–184. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Becker KF, Rosivatz E, Blechschmidt K,
Kremmer E, Sarbia M and Höfler H: Analysis of the E-cadherin
repressor Snail in primary human cancers. Cells Tissues Organs.
185:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou J, Zhang S, Chen Z, He Z, Xu Y and Li
Z: CircRNA-ENO1 promoted glycolysis and tumor progression in lung
adenocarcinoma through upregulating its host gene ENO1. Cell Death
Dis. 10:8852019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Zhang Z and Yu Z: Identification
of a novel glycolysis-related gene signature for predicting
metastasis and survival in patients with lung adenocarcinoma. J
Transl Med. 17:4232019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L, Li M, Yu X, Gao F and Li W:
Repression of hexokinases II-Mediated glycolysis contributes to
piperlongumine-induced tumor suppression in non-small cell lung
cancer cells. Int J Biol Sci. 15:826–837. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ripperger T, Hofmann W, Koch JC,
Shirneshan K, Haase D, Wulf G, Issing PR, Karnebogen M, Schmidt G,
Auber B, et al: MDS1 and EVI1 complex locus (MECOM): A novel
candidate gene for hereditary hematological malignancies.
Haematologica. 103:e55–e58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bleu M, Mermet-Meillon F, Apfel V, Barys
L, Holzer L, Bachmann Salvy M, Lopes R, Amorim Monteiro Barbosa I,
Delmas C, Hinniger A, et al: PAX8 and MECOM are interaction
partners driving ovarian cancer. Nat Commun. 12:24422021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Germeshausen M, Ancliff P, Estrada J,
Metzler M, Ponstingl E, Rütschle H, Schwabe D, Scott RH, Unal S,
Wawer A, et al: MECOM-associated syndrome: A heterogeneous
inherited bone marrow failure syndrome with amegakaryocytic
thrombocytopenia. Blood Adv. 2:586–596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Ren H, Zhang Y, Liu N, Fan M, Wang
K, Yang T, Chen M and Shi P: MECOM/PRDM3 and PRDM16 serve as
prognostic-related biomarkers and are correlated with immune cell
infiltration in lung adenocarcinoma. Front Oncol. 12:7726862022.
View Article : Google Scholar : PubMed/NCBI
|