Introduction
Bone defects are frequently observed and are a
significant issue in clinical practice. The repair of a bone with
extensive defects is a complex procedure. Massive bone defects
therefore constitute a major challenge in reconstructive surgery.
Currently, methods used to repair bone defects include bone
autografts, allografts and external fixation (1).
Although there are several ways to repair articular
cartilage defects, damage caused by degeneration of articular
cartilage, trauma and sport-associated injuries does not regenerate
(2). The rapid development of tissue
engineering techniques has enabled cartilage and bone regeneration
using scaffold material and stem cells. A previous study has
focused on the role of single growth factors in bone tissue
engineering (3). However, the
synergistic effects between various growth factors have rarely been
reported (4).
In the present study, beagle bone marrow mesenchymal
stem cells (BMSCs) were used as seed cells. Once induced, BMSCs
differentiate into osteoblasts and chondroblasts (5). BMSCs were first identified by
Fridenshtein (6) and are considered
to be optimal stem cells for the clinical treatment of bone and
cartilage defects, and have received widespread attention due to
their abundance, ease of access, vigorous growth and multi-lineage
differentiation (7). The present
study used platelet-rich plasma (PRP) as a source of growth
factors. PRP was generated by the resuspension of platelets derived
from autologous blood at a high concentration in a low volume of
plasma. The platelet count in PRP varies according to the
preparation technique, ranging from two- to several-fold increases
above physiological levels (8).
Activation of platelets in PRP by an agonist, such as thrombin,
leads to the release of numerous growth factors and other important
proteins, including basic fibroblast growth factor, fibronectin,
insulin-like growth factor-1 (IGF-1), osteocalcin, platelet-derived
growth factor, serotonin, transforming growth factor-b1,
thrombospondin-1, vascular endothelial growth factor and
Von-Willebrand factor (9). A
previous study revealed that the application of a single growth
factor was not sufficient to achieve the desired effect on BMSCs
(10). The aforementioned proteins
and growth factors have confirmed efficacy in the osteogenic
induction in BMSCs (11). Several of
these proteins are involved in wound healing and tissue
regeneration (12–15).
Tissue engineering scaffolds are 3D substrates that
provide the structural microenvironment required for the
cultivation of BMSCs (14).
Additionally, tissue engineering scaffolds are used in drug
delivery, absorbable sutures, surgical orthopedic devices and cell
tissue engineering applications (15). Porous bioceramics, such as
β-tricalcium phosphate (β-TCP), are excellent scaffolds for tissue
engineering; they are easy to combine with BMSCs, and are suitable
for the repair of bone defects in articular cartilage injury
(16). In the present study, BMSCs
and β-TCP scaffolds were used in a perfusion bioreactor to simulate
in vivo conditions that promote cell adhesion, proliferation
and differentiation. The current study may present a novel platform
for the generation of tissue-engineered bone that is suitable in
bone-repair applications.
Materials and methods
Animal studies
All animal experimental protocols were approved by
the local Institutional Animal Care and Use Committee of Shandong
University (Jinan, China) in compliance with the ‘Guide for the
Care and Use of Laboratory Animals’ published by the National
Academy Press (NIH Publication No. 85–23, revised 1996). Male
beagles (n=10; 4–10 months of age), weighing between 8 and 10 kg,
were obtained from the Experimental Animal Center of Shandong
Province (Jinan, China) and used in the present study. Animals were
kept in separate cages with an ambient temperature of 24°C and 45%
humidity, with a 14 h light/dark cycle, were fed a standard diet
with ad libitum access to food and water, and were allowed
to move freely during the study.
Isolation and cultivation of beagle
BMSCs
Following the administration of 3% pentobarbital
sodium (1 ml/kg; Solarbio Science & Technology Co., Ltd.,
Beijing, China), the tibia was shaved and treated with 1% povidone
iodine solution (Shandong Luxi Pharmaceutical Co., Ltd., Heze,
China). Bone marrow (5 ml) was aspirated with a sterile bone marrow
aspiration needle attached to a 10 ml syringe containing 1 ml
heparin solution (Shandong Lukang Pharmaceutical Group, Jining,
China). Following the addition of an equal volume (5 ml) of
phosphate-buffered saline (PBS) to the bone marrow serum, the
mixture was homogenized. The aspirate was centrifuged at a speed of
168 × g for 5 min at 20°C. The supernatant was removed and the
precipitate mixed with an equal volume of PBS (5 ml) and
homogenized again. The mixture was then centrifuged at a speed of
1,048 × g for 20 min at 20°C and the cloudy coat in the centre of
the centrifuge tube was harvested. This was combined with 5 ml PBS
in a centrifuge tube and separated at a speed of 2,500 rpm for 5
min. Subsequently, BMSCs were harvested from the bottom of the
centrifuge tube. The primary BMSCs were cultured in Dulbecco's
Modified Eagle's Medium (DMEM; HyClone, Rockford, IL, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C, in a humidified
incubator containing 5% CO2. After three passages, the
BMSCs were divided into osteoblast- and chondroblast-induced
groups. The osteoblast-induced group was cultured in osteogenic
differentiation medium containing DMEM supplemented with 10% fetal
bovine serum, 10 nM dexamethasone, 50 mg/l ascorbic acid and 10 mM
β-glycerophosphate (Wuhan Boster Biological Technology Co., Ltd.,
Wuhan, China). The chondroblast-induced group was cultured in
chondrogenesis medium containing TGF-β1 (10 ng/ml), IGF-1 (50 g/l)
and dexamethasone (40 g/l).
Cell identification
Cells were observed using a light microscope for
morphology and growth status daily. In the chondroblast-induced
group, type II collagen was detected by immunohistochemistry.
Antibodies were purchased from Wuhan Boster Biological Technology
Co., Ltd.. The cells were fixed with 3% paraformaldehyde for 10 min
and rinsed with Tris buffer, pH 7.4, at room temperature for 5 min.
The cells were then incubation with serum (1:20 in Tris buffer) at
room temperature for 10 min, incubated with primary antibodies
(anti-collagen II; cat. no. BA0533) in a moist chamber overnight at
4°C, washed with PBS and incubated with biotinylated goat
anti-rabbit immunoglobulin G secondary antibody (1:50 in Tris
buffer; cat. no. BA1003) at room temperature for 30 min, washed
with PBS and incubated with dual system bridge antibodies (1:50 in
Tris buffer; cat. no. BA1003) for 30 min at room temperature. The
cells were then washed with PBS and fuscin (Wuhan Boster Biological
Technology Co., Ltd.) was used for staining for 30 min at room
temperature. Subsequently, the specimens were washed with PBS and
dried, covered with glycerin/gelatin and examined under a light
microscope. In the osteoblast-induced group, cells were identified
with alkaline phosphatase (ALP) staining and Alizarin red staining
(Wuhan Boster Biological Technology Co., Ltd.) to observe the
activity of ALP and the number of mineral nodules.
3D scaffolds
β-TCP is a porous bio-ceramic 3D scaffold purchased
from Shanghai Bio-lu Biomaterials Co., Ltd. (Shanghai, China). The
diameter of the spherical pores was 500–600 µm and the porosity was
75±10% with >80% of the pores being spherical in shape. Using a
mold, a cylinder of β-TCP 5 mm in diameter and 5 mm in height was
constructed. The surface of the cylinder was smooth and was
sterilized using ethylene oxide.
Perfusion bioreactor
The perfusion bioreactor (Fig. 1) was designed by the East China
University of Science and Technology (Shanghai, China). The system
consists of a peristaltic pump, a device which pulls culture media
through silicone tubes, a perfusion column in which the
cell-scaffold composite can be implanted, two silicone tubes, an
air filter and a flask filled with cell culture media. The function
of the two silicone tubes is to enable the flow of culture media
into a peristaltic pump via the silicone tubes when the peristaltic
pump begins to rotate. This creates a circulatory flow to provide
mechanical stimulation to the cells.
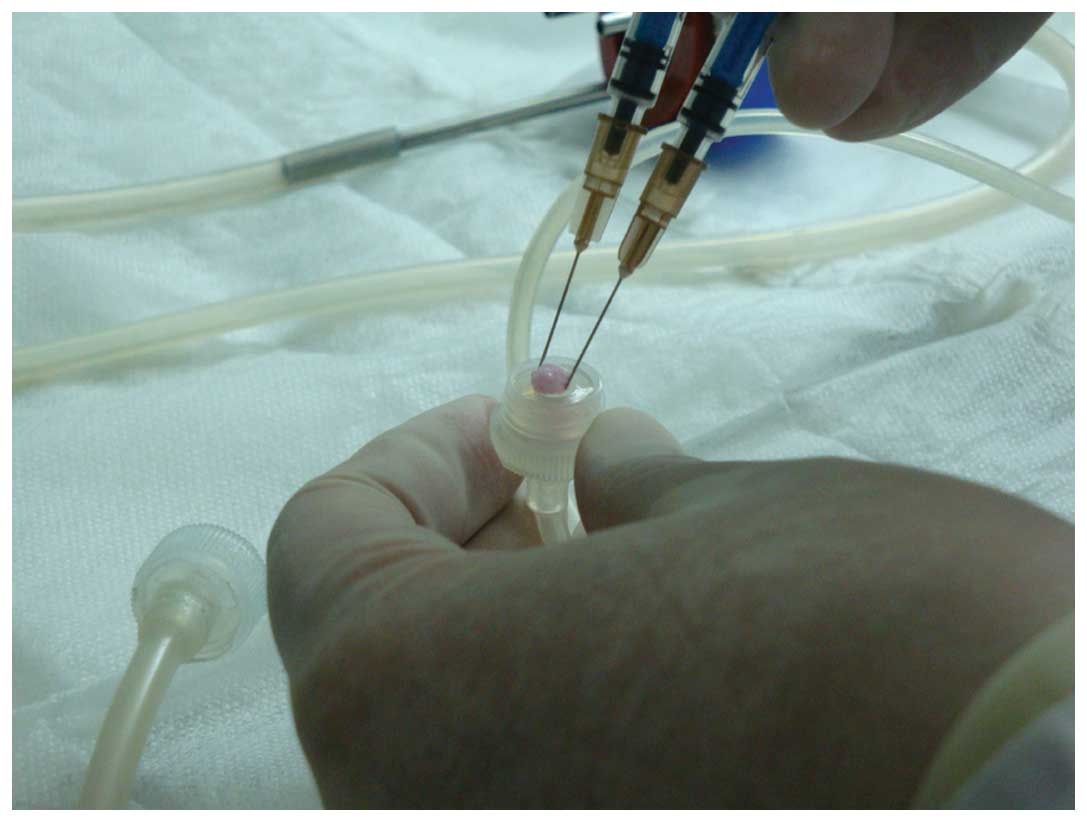
PRP was prepared according to a two-step
centrifugation method as previously described (13). The standard protocol involves the
preparation of PRP from autologous blood by a two-step
centrifugation process; a separation step and a concentration step
(17,18). For the preparation of PRP, ~10 ml of
blood was obtained from the femoral veins of 10 anesthetized (3%
pentobarbital sodium) beagles. The blood was collected in
sterilized tubes containing 1 ml of sodium citrate used as an
anticoagulant. The blood was then centrifuged twice; initially at
20 × g for 16 min at 20°C to remove red blood cells, and then at
1,500 × g for 12 min at 20°C to obtain the platelet pellet
(19). The pellet was resuspended in
platelet-poor plasma and the PRP from the 10 beagles was mixed and
stored at −80°C until required, and consequently allogenic PRP was
used in the subsequent experiments. The PRP was combined with 1 ml
coagulant (composed of 1 ml 10% CaCl2 and 1,000 U
thrombin) and homogenized. Platelet-rich plasma gel, a jelly-like
substance (Fig. 2), was then
harvested.
Tissue-engineered bone building
Once the cells had been passaged three times, BMSC
cell suspensions were prepared by trypsin (Wuhan Boster Biological
Technology Co., Ltd.) digestion prior to the addition to the β-TCP
scaffolds. One scaffold was prepared with an osteoblast-induced
suspension and another with a chondroblast-induced suspension.
Subsequently, 3 ml cell culture media was added to each cultivation
orifice plate. The BMSC-TCP composites were placed in an incubator
for 2 days at 37°C in an atmosphere containing 5% CO2
(Fig. 3). The composites were then
placed in the culture chamber of a perfusion bioreactor with a flow
rate of 3.5 ml/min for 3 weeks using culture media in the presence
or absence of PRP. The culture medium was changed every 2–3 days.
Adhesion, proliferation and growth of the BMSCs in the β-TCP
scaffold were examined under a scanning electron microscope (SEM).
The composites were divided into five groups as follows: i) Group
A, composite cultured with PRP in the bioreactor; ii) Group B,
composite cultured with PRP without the bioreactor; iii) Group C,
composite cultured in the bioreactor without PRP; iv) Group D,
composite cultured without PRP and the bioreactor and v) Group E,
β-TCP scaffold only (negative control group).
Surgical procedure
Models of arthrodial cartilage defects were
established in 20 joints of 10 beagles, with five defects in each
joint. The beagles were anesthetized with an intravenous injection
of 3% pentobarbital sodium. A median incision of 3 cm was made over
the knee joints, soft tissues were dissected and the bone was
exposed by gentle retraction of the muscles. Five defects with a
depth of 10 mm and a width of 5 mm were created in the distal
femoral articular surface with a hand brace.
Composite implantation
Once the defects had been irrigated with sterile
physiological saline solution, the BMSC-TCP composites were
implanted into the defects and press-fitted. The osteoblast-induced
BMSC-TCP composite (diameter, 5 mm; height, 5 mm) was implanted
into the innermost region of the bone defect, and the
chondroblast-induced BMSC-TCP composite of the same dimensions was
implanted into the remaining shallow region of the bone defect. The
osteoblast-induced BMSC-TCP composite was mechanically forced into
contact with the chondroblast-induced composite. Following this,
muscles, fascia and skin were separately closed over the regions of
the defects using 4–0 sterile sutures. The animals were kept in
standard conditions and were intramuscularly injected with 800,000
U penicillin (Wuhan Boster Biological Technology Co., Ltd.) each
day for 3 consecutive days following the surgical procedure. The
animals were then humanely sacrificed by anesthetic overdose (100
mg/kg) with pentobarbital sodium 3 months following the surgical
procedure and the composites with tissue cells nearby were removed
in order to perform reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and histological analysis.
Detection of ALP and BGLAP expression
levels using RT-qPCR
Template RNA used was from the total RNA of the
BMSC-TCP composites. Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The concentration of RNA was
determined using an ultraviolet spectrophotometer. QIAGEN
RNase-Free DNAse was purchased from Cyagen Biosciences (Santa
Clara, CA, USA). The cDNA sample was generated using the RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
Primers sequences used in RT-qPCR were as follows: Forward,
5′-TGTGCGGGGTCAAGGCTAAC-3′; reverse, 5′-GGCGTCCGAGTACCAGTTGC-3′ for
ALP; forward, 5′-CTCCTTACCCGGATCCCCTG3′; reverse,
5′-GTAGAAGCGCTGGTAGGCGT3′ for BGLAP2. Reaction Buffer, RiboLock
RNase Inhibitor, dNTP Mix, RevertAid M-MuLV Reverse Transcriptase
were provided by Cyagen Biosciences Inc.. A negative control was
included. Quantitative RT-PCR was performed using SYBR Green
Realtime PCR Master mix (Toyobo, Osaka, Japan) in an Applied
Biosystems AB7500 real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Amplification was performed under the
following thermal cycling conditions: 95°C for 60 sec followed by
40 cycles at 95°C for 15 sec and 60°C for 60 sec. GADPH was used as
a reference gene, and the 2−ΔΔCq method was used
(20). RT-qPCR was performed in
triplicate and the mean ± standard deviation was calculated.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using the SPSS, version 20.0 statistical software
package (IBM SPSS, Armonk, NY, USA). One-way analysis of variance
or paired Student's t-tests were used for comparisons between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
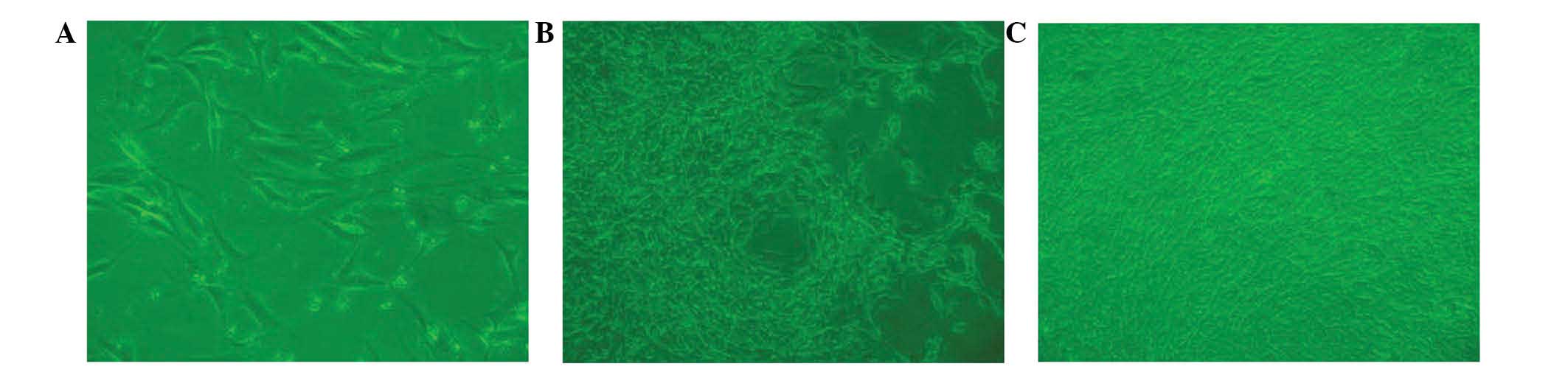
Cell culture observation
BMSCs from beagle bone marrow were harvested and
characterized microscopically in a cell culture. Following a
culture period of 3 days (Fig. 4A),
a small number of spindle-shaped primary cells were observed
growing against the wall of the cell culture flask. Cell colonies
formed after 7 days of culture (Fig.
4B). After 7 days of culture, the cells gathered into
swirl-shaped colonies (Fig. 4C).
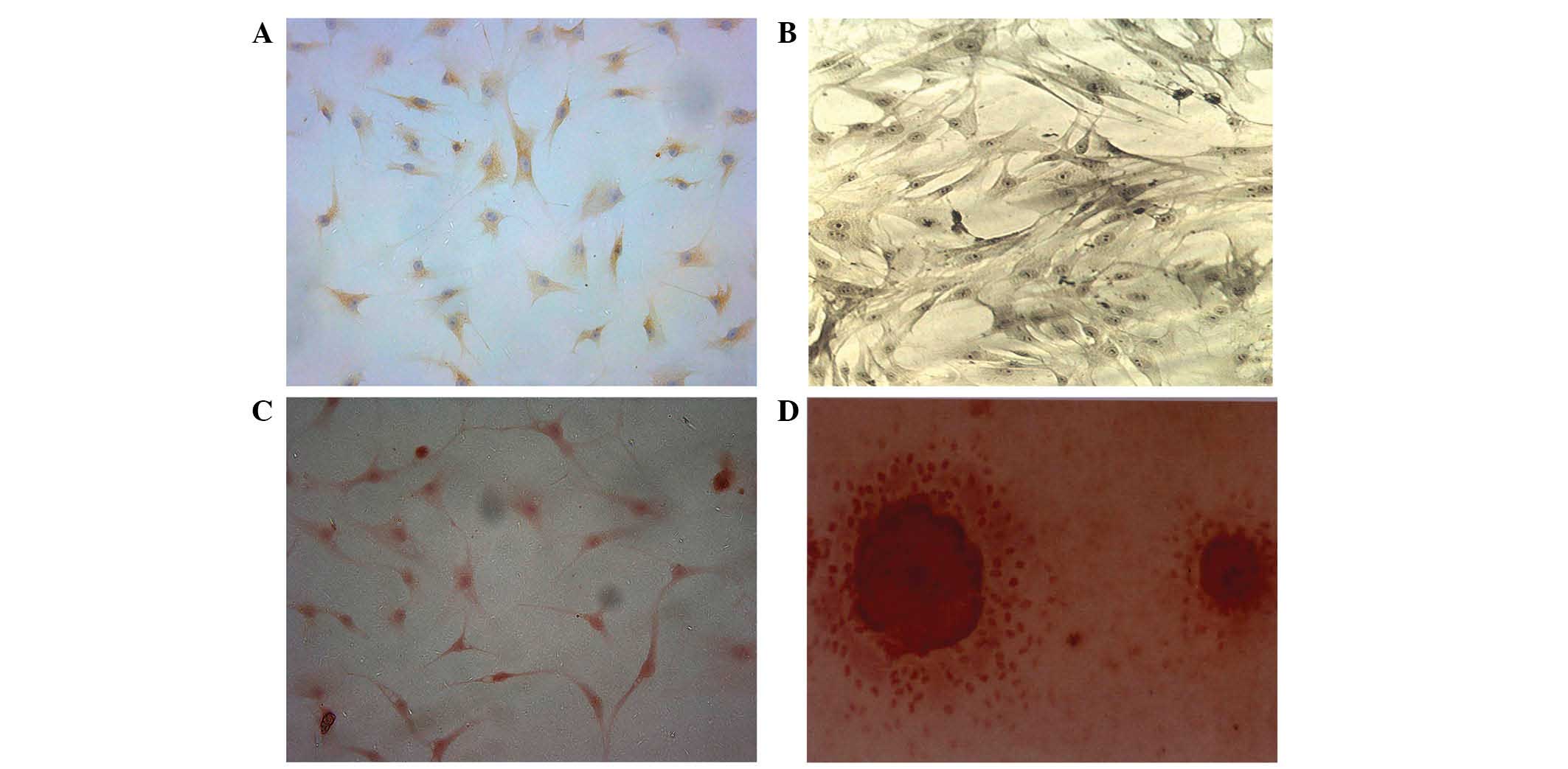
Characterization of BMSCs
BMSCs were induced to differentiate into
chondroblasts for 7 days. The cells displayed brownish yellow
cytoplasmic granules following type II collagen immunohistochemical
staining (Fig. 5A). Subsequent to 7
days of osteoblastic differentiation, alkaline phosphatase and
Alizarin red staining revealed a large quantity of gray-black
granules or black deposits (Fig.
5B). The aforementioned observations are consistent with the
initial stem cell phenotype, which was further confirmed by the
expression of CD29 and CD44 following differentiation. Alizarin red
staining revealed a large number of mineral nodes in the
osteoblast-induced group (Fig. 5C and
D).
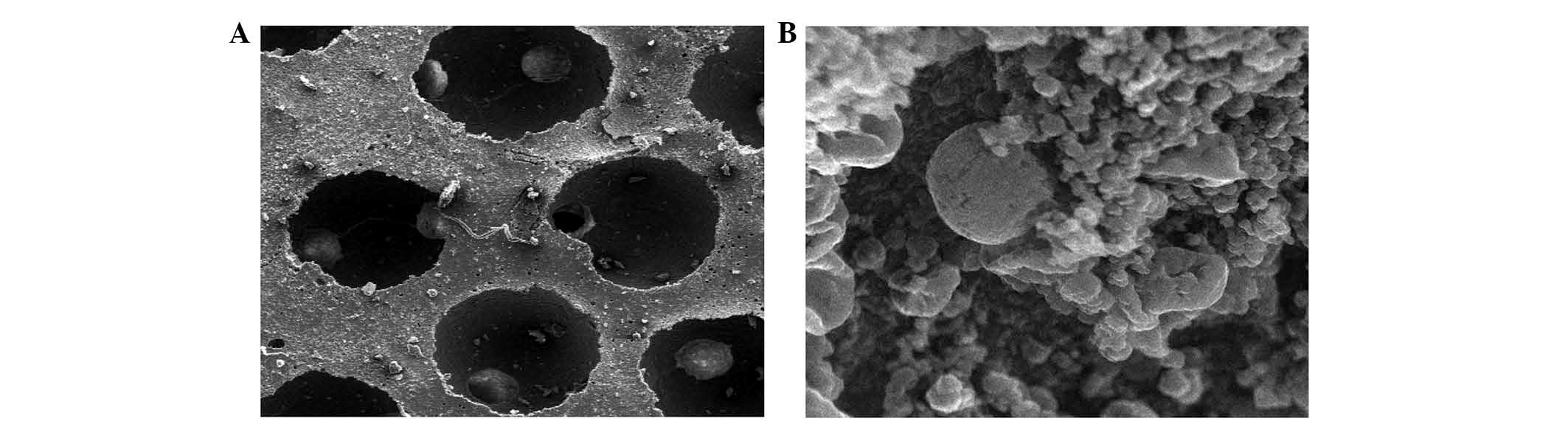
Examination of composites by SEM
Differentiated BMSCs were deposited on β-TCP
scaffolds and allowed to grow for 21 days. SEM revealed that cells
adhered to the scaffold and were widely distributed, secreting
abundant extracellular matrix onto the scaffold (Fig. 6). Cell proliferation and a stretched,
extended morphology was also observed, suggesting that the β-TCP
scaffold is a good substrate for BMSC growth (Fig. 6).
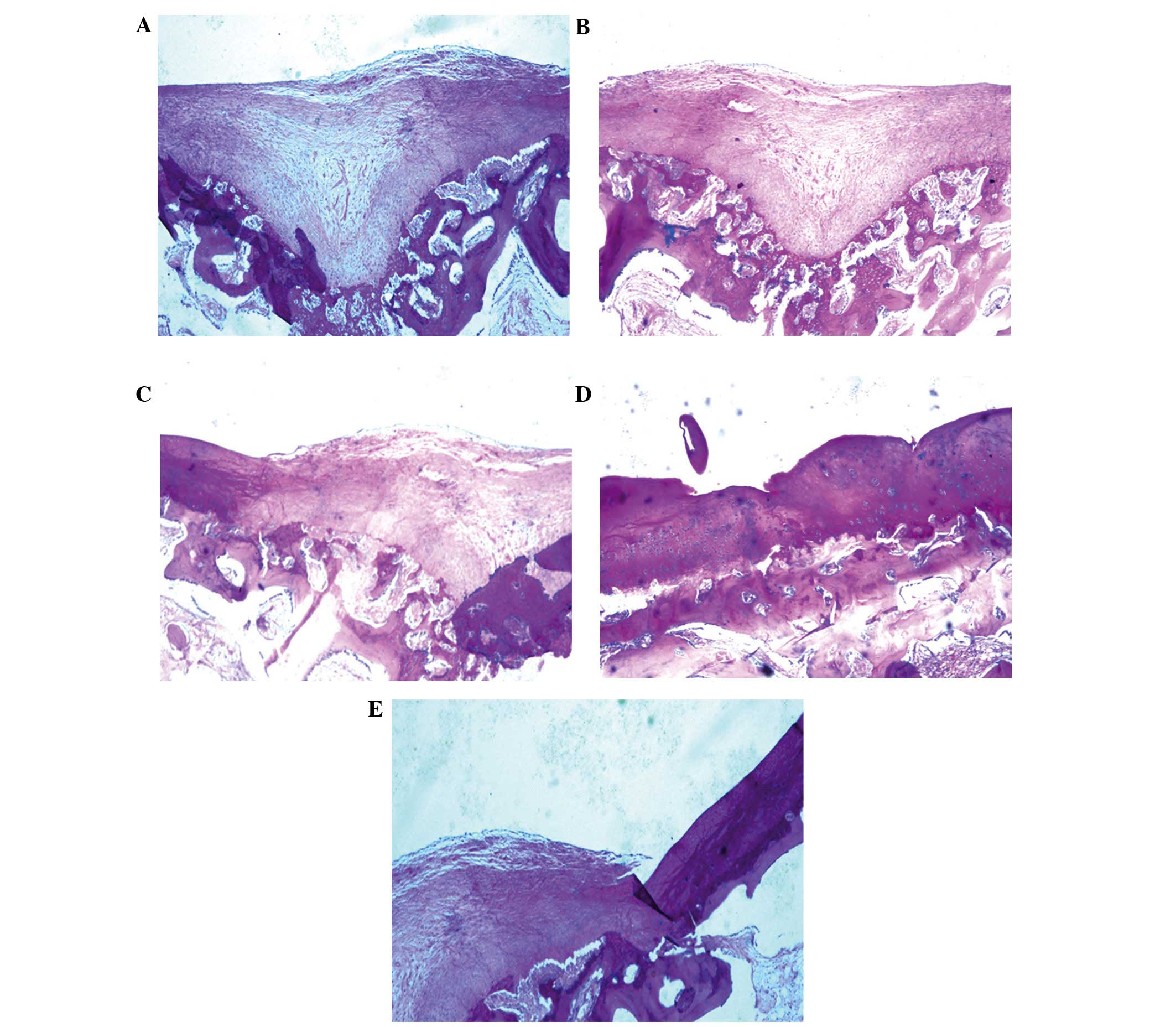
Histological analyses of cartilage
regeneration in vivo
Once the BMSCs had been cultured for 3 weeks in the
bioreactor, BMSC scaffolds (Group A) or controls (Groups B-E) were
implanted into an arthrodial cartilage defect model in beagles as
described in the Materials and methods section. Histological
examination demonstrated that several of the implanted BMSC
scaffolds regenerated cartilage tissue, and filled the defective
area. Numerous newly differentiated chondrocytes, in addition to
abundant bone formation, were observed with many chondrocytes and
osteoblasts present. Osteotylus was also observed and this was
regularly arranged and dispersed throughout the site of the defect
(Fig. 7A).
Several controls (bioreactor, PRP and BMSCs) were
performed to investigate the importance of each component of the
culture system. Implantation of BMSCs cultured on scaffolds without
the bioreactor led to the formation of high levels of hyaline
chondrocytes and cartilage lacunae, with the new cartilage
integrating with the existing articular cartilage. Conversely, in
Group A, new bone was not evenly distributed and the quantity of
hyaline chondrocytes was reduced (Fig.
7B). In Group C, in which BMSCs were cultured in the absence of
PRP, irregular, newly-formed osteotylus was present in the defect,
and chondrocytes and osteoblasts were randomly and loosely arranged
(Fig. 7C). In the absence of both
PRP and the bioreactor (Group D), a small number of newly-generated
chondrocytes and osteoblasts were detected in the defect. New bone
formation was minimal, with abundant fibrous tissues infiltrating
the damaged area (Fig. 7D). In the
negative control with no BMSCs (Group E), several fibroblasts were
observed to adhere to adjacent tissue on the defect border,
resulting in the proliferation of fibrous and scar tissue (Fig. 7E).
Expression levels of osteogenic
differentiation markers in BMSC implants
The expression levels of osteogenic differentiation
markers, ALP and BGLAP, in BMSC implants was determined by RT-qPCR
(Fig. 8). In experimental Groups A,
B, C and D, the expression levels of ALP and BGLAP were
significantly higher compared with the negative control group
(Group E; P<0.05). The expression levels of ALP and BGLAP were
significantly higher in Group A compared with Group C (P<0.05)
and in Group B compared with Group D (P<0.05). These results
therefore suggested that the presence of PRP promotes bone
regeneration. The expression levels of ALP were not significantly
higher in Group A compared with Group B, or in Group C compared
with Group D, (Group A compared with Group B, t=0.169, P>0.05;
Group C compared with Group D, t=0.646, P>0.05). The present
data supports the hypothesis of an osteogenic promoting effect of
the bioreactor. Consequently, a novel method for the efficient
generation of tissue-engineered cells for the repair of bone
defects has been developed in the present study.
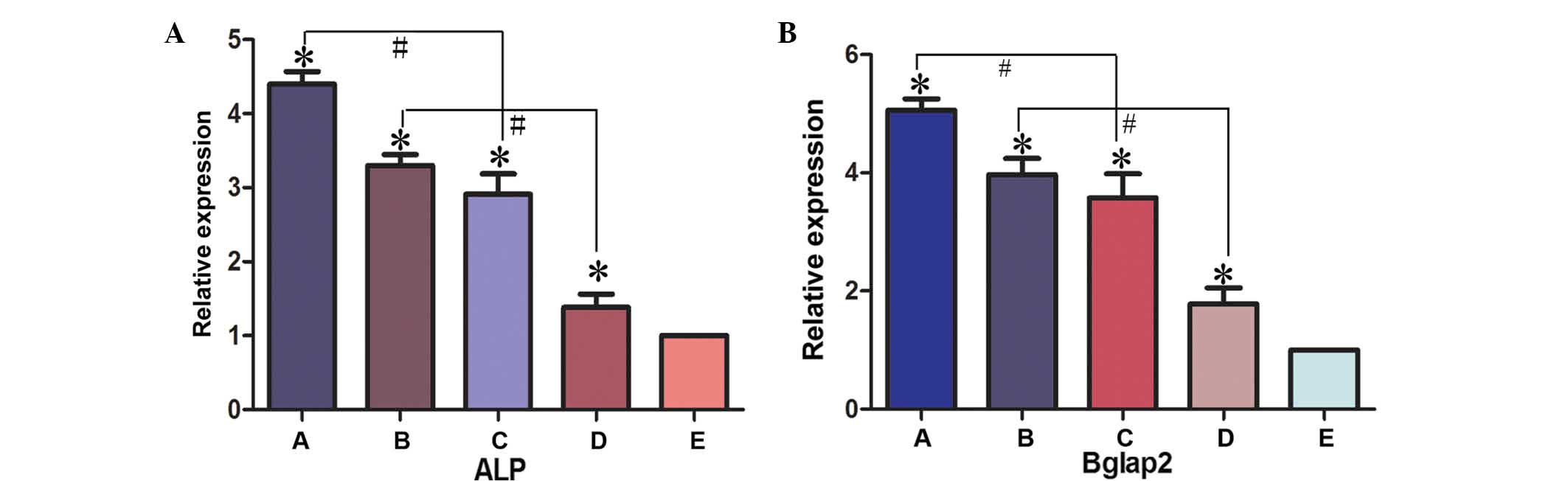 | Figure 8.Gene expression levels of (A) ALP and
(B) BGLAP2 were detected in the various experimental groups using
reverse transcription-quantitative polymerase chain reaction.
*P<0.05 vs. control group (group E); #P<0.05.
Group A, BMSCs cultured with PRP in the bioreactor; Group B, BMSCs
cultured with PRP without the use of the bioreactor; Group C, BMSCs
cultured in the bioreactor without PRP; Group D, BMSCs cultured
without PRP or use of the bioreactor; Group E, β-tricalcium
phosphate scaffold only. ALP, alkaline phosphatase; BGLAP2, bone
γ-carboxyglutamate protein 2; ALP, alkaline phosphatase; BGLAP2,
bone γ-carboxyglutamate protein; BMSC, bone marrow stem cell; PRP,
platelet-rich plasma. |
Discussion
In the present study, we proposed that addition PRP
and use of a bioreactor for the generation of BMSCs on scaffolds
would enhance the healing process of osteochondral defects.
Tissue-engineered bone was constructed by co-culturing BMSCs and
β-TCP scaffolds in a 3D perfusion bioreactor. The present study
revealed that BMSCs, PRP, β-TCP scaffolds and the use of a
bioreactor had positive effects on bone formation and were able to
enhance the healing of osteochondral defects.
In the present study, autologous PRP was used as a
cytokine source to promote bone formation. PRP is a concentrate of
platelets in a small volume of plasma. Once activated by an
agonist, growth factors contained in platelets may be released and
exert their biological functions. The initial clinical study using
PRP for bone reconstruction therapy was performed by Marx et
al (17). In this randomized
study, 88 patients with mandibular defects were treated with
autogenous cancellous bone grafts with or without the addition of
activated PRP. Both the radiographic and the histomorphometric
evaluation revealed a significantly greater percentage of bone
generated in the presence of PRP (17). PRP therefore appears to exert a
positive effect in the early phase of bone healing in combination
with autogenous cancellous bone grafts (21–23), and
in diabetic fracture healing (24).
Other studies support the hypothesis that PRP has the ability to
promote bone healing (25,26) and strongly advocate its use in bone
regenerative therapy (27,28). The identification of the potential of
PRP in the promotion of bone regeneration in recent years has
generated a novel field of research in orthopedics. The effect of
PRP in the treatment of periodontal intrabony defects in humans has
also been investigated (29) and its
beneficial effects in both periodontal and cosmetic surgical
procedures have been suggested (30–32).
In vitro studies have demonstrated the effects of platelet
concentration on the proliferation and differentiation of primary
osteoblasts and fibroblasts (33,34). In
two other in vivo studies (35,36),
goat PRP was prepared and activated with bovine thrombin and
calcium chloride. PRP is currently used clinically in several
countries and curative effects have been achieved (36). In addition, it has been demonstrated
that PRP may enhance the healing of osteochondral defects and that
the benefits may be detected at early stages of the healing process
(37,38). Froum et al (39) observed histomorphometric differences
in bone regeneration among the samples of three surgical cases.
Another study examining human PRP demonstrated upregulation of
collagen synthesis in osteoblasts (39). Growth factors and BMSCs attract other
progenitor cells or osteoclasts, and therefore initiate bone
remodeling (40,41). It is accepted that, subsequent to a
bone injury, growth factors and host precursors are released
(42). In addition, cell adhesion to
extracellular matrices is essential in the development, maintenance
and remodeling of osseous tissues (42,43). The
extracellular matrix component released by platelets is
fibronectin, and the latter mediates adhesive interactions and has
a central role in osteoblast survival, proliferation,
differentiation and matrix mineralization, in addition to bone
formation (44–46). The degranulation of PRP and release
of growth factors may be achieved by the addition of thrombin in
the presence of calcium chloride or by thawing the platelets
(47). Studies have demonstrated
that, in this combination, calcium sulfate acts as an activator of
platelets and also as a delivery system for the platelet-released
growth factors (48,49). Therefore, it may be concluded that
the endogenous growth factors released by platelets promote the
healing process.
In a previous study performed by the authors
(50), BMSCs were used as seed cells
and β-TCP was used as a scaffold material, and a tissue-engineered
osteochondral composite was successfully constructed in a perfusion
bioreactor. This composite was used in the repair of osteochondral
defects in beagles. It was found that certain grafts did not
completely integrate into the areas of bone cartilage defects,
indicating that the constructed osteochondral composite was not
completely vascularized. In the present study, in the process of
bone construction in the three-dimensional perfusion bioreactor,
autologous PRP was used as a cytokine source to promote bone
formation and vascularization. Autologous PRP is a convenient,
economical and sustainable source of high-quality cytokines. The
novel method for constructing tissue-engineered bone using BMSCs,
β-TCP scaffolds and PRP can construct efficient tissue-engineered
bone for cartilage repair.
A porous bio-ceramic 3D scaffold constructed of
β-TCP was used in the present study for its favorable mechanical
strength, high porosity ratio and ease of processing and molding.
SEM displayed good adhesion and distribution of BMSCs on the β-TCP
scaffold. Concurrently, extracellular matrix secretion was observed
on the scaffold. Favorable cell proliferation and morphology was
also detected using SEM, suggesting that the β-TCP scaffold has a
high affinity for BMSCs. In brief, β-TCP scaffolds display several
benefits in tissue engineering which as as follows: i) The β-TCP
scaffold is able to provide a 3D space that may support BMSC
growth, expose cells to shear force and improve unit expansion
rate, thus promoting the differentiation of cartilage and bone; ii)
a 3D structure may improve the biocompatibility of scaffolds and
promote adhesion of BMSCs; iii) bone inducing growth factors will
be gradually released by β-TCP in the process of cell culture due
to the characteristics of the material. Overall, the β-TCP scaffold
may be able to synchronously improve all three of the basic
components required for bone and cartilage engineering. However,
although the present study indicates that the β-TCP scaffold is an
efficient biomaterial for BMSC adhesion and chondrogenic and
osteogenic differentiation, further animal experiments are
necessary to fully assess the scaffolds.
The present study indicated that the use of a
bioreactor and PRP was able to synergistically enhance cell
proliferation and biosynthetic responses on scaffolds. Cell
behavior is influenced by shear stress, tension and hydrostatic
pressure stimulation. The continuous mechanical stimulation
provided by the bioreactor, including shear stress stimulation,
tension stimulation and hydrostatic pressure stimulation has
effects on the morphology and function of BMSCs (51,52). In
the present study, BMSCs were cultured on a 3D scaffold in a
perfusion bioreactor, a culture model that possesses several
advantages compared with traditional planar 2D culture models.
Firstly, it may provide mechanical stimulation to the BMSCs
promoting their functionality. Secondly, the use of a bioreactor
improves the distribution of nutrients within the scaffold. In
addition, using the bioreactor provided a 3D growth environment,
which promotes cell adhesion, growth and differentiation
simultaneously, thereby reducing the effect of contact inhibition
between the cells. Furthermore, the culture medium in a 3D
bioreactor does not need to be replaced frequently, while the
medium in a traditional 2D cell culture model requires intermittent
replacement. The predominant advantage of the system is a result of
the mechanical stimulation provided by the flowing fluid that may
synergistically enhance cell proliferation and biosynthetic
response on scaffolds, which is able to simulate a cell stress
environment highly similar to that observed in vivo
(51–53). In addition, cells may be fully
combined with, or adhered to, the scaffold materials in the
bioreactor, making cell culture closer to physiologic 3D growth
(54,55). The fluid shear stress provided by the
bioreactor has a range of effects on cellular morphological and
functional organization. It has previously been established that
the fluid shear stress provided by a bioreactor is able to enhance
the proliferation of MSCs (56).
Notably, in the present study, it was revealed that PRP and the
application of mechanical stimulation resulted in a greater number
of cells and total collagen present on scaffolds after 21 days,
compared with the use of PRP alone. The aforementioned findings
suggested that mechanical stimulation is able to synergistically
enhance cell proliferation and biosynthetic response on scaffolds.
In addition, mechanical stimulation maintained the expression
levels of ALP and BGLAP, the most important indicators of
osteogenic differentiation (57).
ALP has a critical role in the calcification process and has been
used as a conventional marker of early osteoblast differentiation.
BGLAP predominately appears in the mineralized formation as an
indicator of osteoblast maturation. The expression levels of ALP
and BGLAP therefore reflect the process of bone formation.
In conclusion, BMSCs, PRP, β-TCP scaffolds and the
use of a bioreactor all had positive effects on bone formation. In
the present study, a novel method for constructing
tissue-engineered bone using BMSCs, β-TCP scaffolds and PRP was
assessed. The tissue-engineered bone constructed using this method
displayed active biologic characteristics in morphology and in
function, and may therefore provide an improved approach for the
repair of osteochondral defects.
The novel method for constructing tissue-engineered
bone using BMSCs, β-TCP scaffolds and PRP detailed in the present
study, will initiate the design and building of increasingly
efficient tissue-engineered bone for cartilage repair.
References
|
1
|
Vacanti CA and Upton J: Tissue-engineered
morphogenesis of cartilage and bone by means of cell
transplantation using synthetic biodegradable polymer matrices.
Clin Plast Surg. 21:445–62. 1994.PubMed/NCBI
|
|
2
|
Liao J, Shi K, Ding Q, Qu Y, Luo F and
Qian Z: Recent developments in scaffold-guided cartilage tissue
regeneration. J Biomed Nanotechnol. 10:3085–3104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng L, Wu HEL, Wang D, Feng F, Dong Y,
Liu H and Wang L: Effects of vascular endothelial growth factor 165
on bone tissue engineering. PLoS One. 8:e829452013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou D, Zhang Z, Ye D, Tang A, Deng L, Han
W, Zhao J, Wang S, Zhang W, Zhu C, et al: Repair of critical-sized
rat calvarial defects using genetically engineered bone
marrow-derived mesenchymal stem cells overexpressing
hypoxia-inducible factor-1α. Stem Cells. 29:1380–1390.
2011.PubMed/NCBI
|
|
5
|
Sun S, Ren Q, Wang D, Zhang L, Wu S and
Sun XT: Repairing cartilage defects using chondrocyte and
osteoblast composites developed using a bioreactor. Chin Med J
(Engl). 124:758–763. 2011.PubMed/NCBI
|
|
6
|
Fridenshtein AI: Stromal bone marrow cells
and the hematopoietic microenvironment. Arkh Patol. 44:3–11.
1982.(In Russian). PubMed/NCBI
|
|
7
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Intini G: The use of platelet-rich plasma
in bone reconstruction therapy. Biomaterials. 30:4956–4966. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fréchette JP, Martineau I and Gagnon G:
Platelet-rich plasmas: Growth factor content and roles in wound
healing. J Dent Res. 84:434–439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lacoste E, Martineau I and Gagnon G:
Platelet concentrates: Effects of calcium and thrombin on
endothelial cell proliferation and growth factor release. J
Periodontol. 74:1498–1507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman R, Benz EJJ, Shattil SJ, Furie B,
Cohen HJ, Silberstein LE LE and McGlave P: Hematology: Basic
principles and practice. Churchill Livingstone (Philadelphia).
2000.
|
|
12
|
Thiede MA, Smock SL, Petersen DN, Grasser
WA, Thompson DD and Nishimoto SK: Presence of messenger ribonucleic
acid encoding osteocalcin, a marker of bone turnover, in bone
marrow megakaryocytes and peripheral blood platelets.
Endocrinology. 135:929–937. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Landesberg R, Moses M and Karpatkin M:
Risks of using platelet rich plasma gel. J Oral Maxillofac Surg.
56:1116–1117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie L, Yu H, Deng Y, Yang W, Liao L and
Long Q: Preparation, characterization and in vitro dissolution
behavior of porous biphasic α/β-tricalcium phosphate bioceramics.
Mater Sci Eng C Mater Biol Appl. 59:1007–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Fiqi A, Kim JH and Kim HW:
Osteoinductive fibrous scaffolds of biopolymer/mesoporous bioactive
glass nanocarriers with excellent bioactivity and long-term
delivery of osteogenic drug. ACS Appl Mater Interfaces.
7:1140–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Ye F, Yang R, Lu X, Shi Y, Li L,
Fan H and Bu H: Osteoinduction of hydroxyapatite/beta-tricalcium
phosphate bioceramics in mice with a fractured fibula. Acta
Biomater. 6:1569–1574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dwyer SD and Meyers KM: Anesthetics and
anticoagulants used in the preparation of rat platelet-rich-plasma
alter rat platelet aggregation. Thromb Res. 42:139–151. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasten P, Vogel J, Geiger F, Niemeyer P,
Luginbühl R and Szalay K: The effect of platelet-rich plasma on
healing in critical-size long-bone defects. Biomaterials.
29:3983–3992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiltfang J, Kloss FR, Kessler P, Nkenke E,
Schultze-Mosgau S, Zimmermann R and Schlegel KA: Effects of
platelet-rich plasma on bone healing in combination with autogenous
bone and bone substitutes in critical-size defects. An animal
experiment. Clin Oral Implants Res. 15:187–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dugrillon A and Klüter H: Topical
application of platelets for improved wound healing. Blood Ther
Med. 3:21–26. 2002.
|
|
23
|
Fennis JP, Stoelinga PJ and Jansen JA:
Mandibular reconstruction: A histological and histomorphometric
study on the use of autogenous scaffolds, particulate
cortico-cancellous bone grafts and platelet rich plasma in goats.
Int J Oral Maxillofac Surg. 33:48–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gandhi A, Dumas C, O'Connor JP, Parsons JR
and Lin SS: The effects of local platelet rich plasma delivery on
diabetic fracture healing. Bone. 38:540–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawase T, Okuda K, Wolff LF and Yoshie H:
Platelet-rich plasma-derived fibrin clot formation stimulates
collagen synthesis in periodontal ligament and osteoblastic cells
in vitro. J Periodontol. 74:858–864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada Y, Ueda M, Naiki T, Takahashi M,
Hata K and Nagasaka T: Autogenous injectable bone for regeneration
with mesenchymal stem cells and plateletrich plasma:
Tissue-engineered bone regeneration. Tissue Eng. 10:955–964. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marx RE: Platelet-rich plasma: Evidence to
support its use. J Oral Maxillofac Surg. 62:489–496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freymiller EG and Aghaloo TL:
Platelet-rich plasma: Ready or not? J Oral Maxillofac Surg.
62:484–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouyang XY and Qiao J: Effect of
platelet-rich plasma in the treatment of periodontal intrabony
defects in humans. Chin Med J (Engl). 119:1511–1521.
2006.PubMed/NCBI
|
|
30
|
Man D, Plosker H and Winland-Brown JE: The
use of autologous platelet-rich plasma (platelet gel) and
autologous platelet-poor plasma (fibrin glue) in cosmetic surgery.
Plast Reconstr Surg. 107:229–237; discussion 238–239. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mazor Z, Peleg M, Garg AK and Luboshitz J:
Platelet-rich plasma for bone graft enhancement in sinus floor
augmentation with simultaneous implant placement: Patient series
study. Implant Dent. 13:65–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kassolis JD, Rosen PS and Reynolds MA:
Alveolar ridge and sinus augmentation utilizing platelet-rich
plasma in combination with freeze-dried bone allograft: Case
series. J Periodontol. 71:1654–1661. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH
and Jung JH: Effect of platelet-rich plasma (PRP) concentration on
the viability and proliferation of alveolar bone cells: An in vitro
study. Int J Oral Maxillofac Surg. 34:420–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graziani F, Ivanovski S, Cei S, Ducci F,
Tonetti M and Gabriele M: The in vitro effect of different PRP
concentrations on osteoblasts and fibroblasts. Clin Oral Implants
Res. 17:212–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fennis JP, Stoelinga PJ and Jansen JA:
Mandibular reconstruction: A clinical and radiographic animal study
on the use of autogenous scaffolds and platelet-rich plasma. Int J
Oral Maxillofac Surg. 31:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Intini G, Andreana S, Intini FE, Buhite RJ
and Bobek LA: Calcium sulfate and platelet-rich plasma make a novel
osteoinductive biomaterial for bone regeneration. J Transl Med.
5:132007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cenni E, Perut F, Ciapetti G, Savarino L,
Dallari D, Cenacchi A, Stagni C, Giunti A, Fornasari PM and Baldini
N: In vitro evaluation of freeze-dried bone allografts combined
with platelet rich plasma and human bone marrow stromal cells for
tissue engineering. J Mater Sci Mater Med. 20:45–50. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lieberman JR, Daluiski A and Einhorn TA:
The role of growth factors in the repair of bone. Biology
andclinical applications. J BoneJoint Surg Am. 84:1032–1044.
2002.
|
|
39
|
Froum SJ, Wallace SS, Tarnow DP and Cho
SC: Effect of platelet-rich plasma on bone growth and
osseointegration in human maxillary sinus grafts: Three bilateral
case reports. Int J Periodontics Restorative Dent. 22:45–53.
2002.PubMed/NCBI
|
|
40
|
Bruder SP, Kraus KH, Goldberg VM and
Kadiyala S: The effect of implants loaded with autologous
mesenchymal stem cells on the healing of canine segmental bone
defects. J Bone Joint Surg Am. 80:985–996. 1998.PubMed/NCBI
|
|
41
|
Roldán JC, Jepsen S, Miller J, Freitag S,
Rueger DC, Açil Y and Terheyden H: Bone formation in the presence
of platelet-rich plasma vs. bone morphogenetic protein-7. Bone.
34:80–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Damsky CH: Extracellular matrix-integrin
interactions in osteoblast function and tissue remodeling. Bone.
25:95–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garcia AJ and Reyes CD: Bio-adhesive
surfaces to promote osteoblast differentiation and bone formation.
J Dent Res. 84:407–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weiss RE and Reddi AH: Role of fibronectin
in collagenous matrix-induced mesenchymal cell proliferation and
differentiation in vivo. Exp Cell Res. 133:247–254. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moursi AM, Damsky CH, Lull J, Zimmerman D,
Doty SB, Aota S and Globus RK: Fibronectin regulates calvarial
osteoblast differentiation. J Cell Sci. 109:1369–1380.
1996.PubMed/NCBI
|
|
46
|
Zimmerman D, Jin F, Leboy P, Hardy S and
Damsky C: Impaired bone formation in transgenic mice resulting from
altered integrin function in osteoblasts. Dev Biol. 220:2–15. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weibrich G, Gnoth SH, Otto M, Reichert TE
and Wagner W: Growth stimulation of human osteoblast-like cells by
thrombocyte concentrates in vitro. Mund Kiefer Gesichtschir.
6:168–174. 2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Intini G, Andreana S, Margarone JE III,
Bush PJ and Dziak R: Engineering a bioactive matrix by
modifications of calcium sulfate. Tissue Eng. 8:997–1008. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bateman J, Intini G, Margarone J III,
Goodloe S III, Bush P, Lynch SE and Dziak R: Platelet-derived
growth factor enhancement of two alloplastic bone matrices. J
Periodontol. 76:1833–1841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang D, Jiang H, Wang S, Li H, Zhang H,
Zhao L, Peng T, Cao Z and Sun S: Construction of tissue-engineered
bone using a bioreactor and platelet-rich plasma. Exp Ther Med.
8:413–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klein-Nulend J, van der Plas A, Semeins
CM, Ajubi NE, Frangos JA, Nijweide PJ and Burger EH: Sensitivity of
osteocytes to biomechanical stress in vitro. FASEB J. 9:441–445.
1995.PubMed/NCBI
|
|
52
|
Owan I, Burr DB, Turner CH, Qiu J, Tu Y,
Onyia JE and Duncan RL: Mechanotransduction in bone: Osteoblasts
are more responsive to fluid forces than mechanicalstrain. Am J
Physiol. 273:C8l0–C815. 1997.
|
|
53
|
Bakker AD, Soejima K, Klein-Nulend J and
Burger EH: The production of nitric oxide and prostaglandin E(2) by
primary bone cells is shear stress dependent. J Biomech.
34:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Y, Kim UJ, Blasioli DJ, Kim HJ and
Kaplan DL: In vitro cartilage tissue engineering with 3D porous
aqueous-derived silk scaffolds and mesenchymal stem cells.
Biomaterials. 26:7082–7094. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang W, Itaka K, Ohba S, Nishiyama N,
Chung UI, Yamasaki Y and Kataoka K: 3D spheroid culture system on
micropatterned substrates for improved differentiation efficiency
of multipotent mesenchymal stem cells. Biomaterials. 30:2705–2715.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song K, Wang H, Zhang B, Lim M, Liu Y and
Liu T: Numerical simulation of fluid field and in vitro
three-dimensional fabrication of tissue-engineered bones in a
rotating bioreactor and in vivo implantation for repairing
segmental bone defects. Cell Stress Chaperones. 18:193–201. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song Z, Wu C, Sun S, Li H, Wang D, Gong J
and Yan Z: Quantitative analysis of factors influencing
tissue-engineered bone formation by detecting the expression levels
of alkaline phosphatase and bone γ-carboxyglutamate protein 2. Exp
Ther Med. 9:1097–1102. 2015.PubMed/NCBI
|