Introduction
Idiopathic pulmonary fibrosis (IPF) is a
devastating, fibroproliferative chronic lung disorder (1,2), which
continues to exercise a heavy human, financial and social toll on
its victims and their family. Despite intense research efforts and
large multicenter clinical trials, IPF is gradually increasing
worldwide (3). Therefore, developing
new treatments for IPF that are safe, effective and tolerable is
now more challenging than ever. A growing number of investigations
of the therapeutic potential of mesenchymal stem cells (MSCs) in
experimental models of chronic lung diseases have expanded rapidly
(4). MSCs are stromal cells that can
be readily harvested from numerous tissues including bone marrow,
human umbilical cord-derived MSCs (HUC-MSCs) (5–7) and cord
blood (8,9). HUC-MSCs are widely available, with easy
proliferation and muti-differentiation, low immunogenicity, and can
be collected in a noninvasive manner; thus, therapy involving
HUC-MSCs is attracting increasing attention. However, only a few
cases of the clinical application in the treatment of IPF have been
reported.
In previous studies, we investigated the safety and
efficacy of HUC-MSCs in rat and human bone nonunion (10–12). In
the present study, HUC-MSCs were introduced into a patient of IPF.
The effect of HUC-MSCs on the pulmonary fibrosis was then assessed
in the following 12 months. The aim of this case report was to
provide useful clinical insights for future efficacy trials of stem
cell therapy in IPF.
Case report
We report a case of a 56-year-old Chinese man who
had previously smoked cigarettes (45 pack-years) in combination
with chronic obstructive pulmonary disease (COPD). Before his IPF
diagnosis our patient had been affected by COPD for 5 years. His
diagnosis of IPF was also confirmed by chest high-resolution
computed tomography (HRCT) showing areas of intralobular
interstitial and septal thickening with peripheral bilateral
distribution in addition to centrilobular emphysema of both the
lobes. In admission to our department, the patient exhibited
chronic respiratory failure, reporting dyspnea on exertion and dry
cough. Our patient received long-term oxygen therapy (LTOT) with
2.0 l/min of flow during 24 h as his peripheral capillary oxygen
saturation (SpO2) steeply declined when oxygen therapy
was discontinued (SpO2=70–75%). Based on the published
diagnostic criteria of ATS/ERS (2011), the patients' disease
severity was estimated with functional parameters including forced
vital capacity (FVC)=68.6% and diffusing capacity of the lung for
carbon monoxide (DLCO)=46.3% of the predicted normal values, the
spiro-metry showed a relatively mild mixed concurrent pulmonary
hypertension [pulmonary artery pressure (PAP), 60 mmHg] as
diagnosed by color Doppler echocardiography.
Basic principles and ethical
considerations
The protocol of the present study was approved by
the Institutional Review Board and the Ethics Committee of Siping
Hospital of China Medical University. The trial was conducted in
compliance with current Good Clinical Practice standards and in
accordance with the principles set forth under the Declaration of
Helsinki (1989).
Informed consent
The patient signed an informed consent form agreeing
to his treatment according to the Siping Hospital of China Medical
University. The general characteristics of the patient are shown in
Table I.
 | Table I.Cardiorespiratory and clinical tests
before and after HUC-MSC transplantation in the IPF patient. |
Table I.
Cardiorespiratory and clinical tests
before and after HUC-MSC transplantation in the IPF patient.
|
|
|
| Post-HUC-MSCs
transplantation |
|---|
|
|
|
|
|
|---|
| Test | Parameters | Before HUC-MSCs
transplantation | 6 months | 12 months |
|---|
| Respiratory
functional tests | FEV1/FVC | 62.3% | 70.5% | 79.9% |
| Spirometry | FEV1 | 68.6% of predicted
value | 80.3% of predicted
value | 82.7% of predicted
value |
|
| FVC | 63.7% of predicted
value | 73.3% of predicted
value | 75.6% of predicted
value |
| DLCO | DLCO | 46.3% of predicted
value | 63.1% of predicted
value | 78.6% of predicted
value |
| Arterial blood gas
analysis | PaO2 | 65.0 mmHg | 76.0 mmHg | 88.0 mmHg |
|
| PaCO2 | 40.0 | 38.0 | 35.0 |
| On oxygen 2.5
l/min | pH | 7.3 | 7.4 | 7.4 |
| mMRC |
| 4.0 | 3.6 | 2.0 |
| VAS |
| 8.0 | 6.5 | 5.0 |
| Borg scale |
| 5.0 | 3.6 | 3.0 |
| Transthoracic
two-dimensional echocardiography | PAP | 55 mmHg | 47 mmHg | 35 mmHg |
| Respiratory muscle
strength | PiMax | 40 mmHg | 46 mmHg | 53 mmHg |
|
| PeMax | 25 mmHg | 48 mmHg | 60 mmHg |
| 6MWT |
| 90 ma | 175 m | 210 m |
| Quality of life | Physical
activities | 60.7 | 77.6 | 87.6 |
| SGRQ | Impact | 70.4 | 57.3 | 32.6 |
|
| Total score | 73.9 | 62.8 | 51.5 |
| LTOT |
| Flow 2.0 l/min for 8
h | No need for
oxygen |
Physical and laboratory
examination
The patient underwent a detailed physical and
laboratory examination including arterial blood gases,
electrocardiogram (ECG), estimation of vital signs (blood pressure,
temperature, breaths and beats per minute) as well as routine
laboratory tests, including white blood cell count and differential
count, red blood cell count, liver and renal function and chest
HRCT, screening to evaluate the functional and radiological
severity of the disease and to localize the areas of the lungs.
Isolation, propagation and
confirmation of HUC-MSC phenotype
All of the HUC-MSC doses used in this trial were
derived from two donated umbilical cords obtained from healthy
mothers during routine term elective caesarean section birth. Fully
informed consent was obtained several weeks prior to delivery.
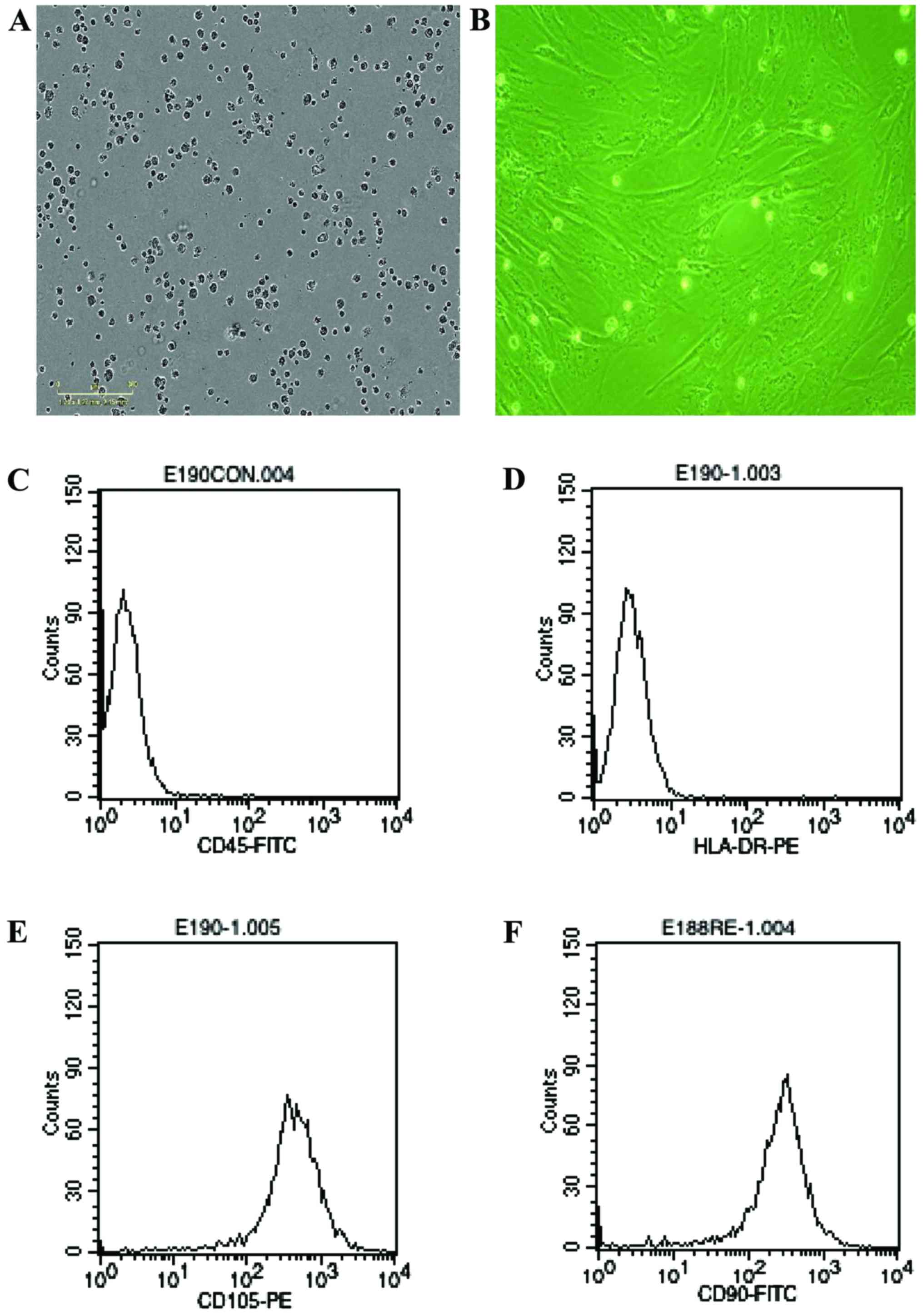
HUC-MSC were isolated and propagated as previously described
(10,11) were more than 90% double positive for
CD105 and CD90, negative for HLA-DR and CD45.
HUC-MSC intravenous infusion
The patient was placed in the supine position, The
HUC-MSCs were perfused into the right median cubital vein. HUC-MSCs
(10 ml) with a cell density of 5×106-1×107/ml
was intravenous infused at a rate no greater than
12.5×106/min and flushed with 20 ml saline to ensure
full cell dose delivery. Once the needle was fully withdrawn, the
puncture site was wrapped with sterilized dressing. The patients
remained in the supine decubitus on the operation bed for another
30 min before off-bed activities. Antibiotics were given to prevent
infection. The patient was monitored (temperature, blood pressure,
pulse and oxygen saturation) at 15, 30, 45 and 60 min, and then
hourly for a minimum of 4 h.
The patient was instructed to limit activities for 4
weeks and partial activities for the subsequent 8 weeks. Full
activities were achieved 6 months post-translation.
Clinical, functional and radiological
assessment
i) Primary safety assessments included monitoring
and recording of all adverse events and serious adverse events.
Arterial blood gases coupled with clinical [Medical Research
Council (MRC) dyspnea scale], ECG and monitoring of vital signs
(temperature, oxygen saturation, respiratory and heart rate) were
performed during the first 24 h after HUC-MSCs infusion. The
patient was then discharged 24-h post-transplantation given that he
was a febrile and hemodynamically stable, with no signs of
infection or any type of allergic reaction.
ii) As exploratory secondary end-points we
investigated whether stem cell infusion exerted any beneficial
effects as assessed by clinical [modified MRC (mMRC) dyspnea scales
functional (FVC, DL CO)], exercise capacity [six-minute walk test
(6MWT)] and quality of life [St. George's Respiratory Questionnaire
(SGRQ)] parameters, at baseline and at serial time-points (6 and 12
months post HUC-MSCs transplantation). Assessment of the disease
extent and severity as reflected by chest HRCT at 6 and 12 months
post-transplantation. The related parameters were supervised by an
exercise physiologist.
Pharmacological therapy protocol
Our patient's pharmacological therapy consisted of:
i) Oxygen therapy (shown above); ii) phosphodiesterase inhibitors:
Doxofylline, 0.3 g, once daily, intravenous infusion; iii)
antibacterial: Erythromycin, 0.9 g, once daily; and iv) improvement
of microcirculation: Danshen, 20 ml once daily.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (Chicago, IL, USA). Safety and exploratory efficacy
secondary endpoints was observed for the patient against the
baseline values. P<0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of HUC-MSCs
The cells derived from umbical cord were observed in
24 h after they were seeded (Fig.
1A), when part of the round mononuclear cells were adherent.
Three days after inoculation, small colonies of the adherent cells
with typical fibroblast-shaped morphology were obtained (Fig. 1B). The primary cells reached
monolayer confluence after planting for 5–6 days, when passaged for
the first time. Fifth passaged cells were analysis by flow
cytometry, they were strongly positive for CD105 and CD90, but
negative for CD45 and HLA-DR (Fig.
1C-F).
Functional analysis
The results of the evaluation by spirometry,
achieved before and after the HUC-MSC transplantation. The patient
was with severe impairment of air flow at the basic line, and
presented an increase in the forced expiratory volume in one second
(FEV1) and FVC values, immediately after the procedure (Table I). The FEV1 values at the end of 12
months after the procedure were presented relative to maintenance.
There was a marked decline in the FVC. The results of these two
parameters determined an increase in the post-transplantation
FEV1/FVC ratio, which increased from 62.3% in the pre-procedure
period to 70.5 and 79.9% in the following 6 and 12 months,
respectively. Thus, the isolated observation of these values shows
a close relation to the normal predicted values of FEV1/FVC
(>0.70 post-transplantation). The FVC was reduced by median 175
and 210 ml at 6 and 12 months, respectively, before returning to
baseline (Table I). The diffusing
capacity and 6MWD remained stable at 6 and 12 month time-points,
with a transient improvement in 6MWD at 3 months (Table I).
The arterial blood gas analysis showed a
pO2 increase of 11% and pCO2 decrease of 5.6%
on oxygen with a flow of 2.0 l/min in three days. The LTOT reduced
to a flow of 1.5 l/min for 24 h to compensate hypoxemia. The oxygen
requirement reduction observed may have been due to the
strengthening of his respiratory muscles, which allowed improvement
of his exercise capacity. Oxygen was not required at the end of 2
months. The pulmonary artery systolic pressure, estimated by
transthoracic two-dimensional echocardiography, improved by 9.3% in
terms of cardiac index and pulmonary vascular resistance. The 6MWT
showed an increase of distance walked by 144.4% at the end of 12
months post-transplantation. There was a marginal improvement in
DLCO (46.3 vs. 63.1%; 78.6% of predicted value) and fibrosis score
(9.8 vs. 4.6%) at the end of 6 and 12 months. The dyspnea scores
were reduced, the level of dyspnea at rest (the mMRC dyspnea scale)
decreased from 3.0 to 1.5, Borg scale during exercise was reduced
from 5.0 to 3.0, and his post-exercise visual analogue scale
decreased from 8.0 to 5.0; there was an increase in maximal
inspiratory pressure of 32.2%. In addition, the patient was in good
clinical condition and increased scores in indicators of quality of
life, namely SGRQ scoring values (70.4±3.6 vs. 57.3±2.9, and
32.6±3.1, P<0.05, respectively), both after 6 and 12 months,
were noted (Table I). Finally, our
results showed a trend towards increase in systolic pulmonary
arterial pressure (sPAP) at 6 and 12 months post-infusion.
Demographic and baseline patient data are listed in Table I.
Radiological analysis
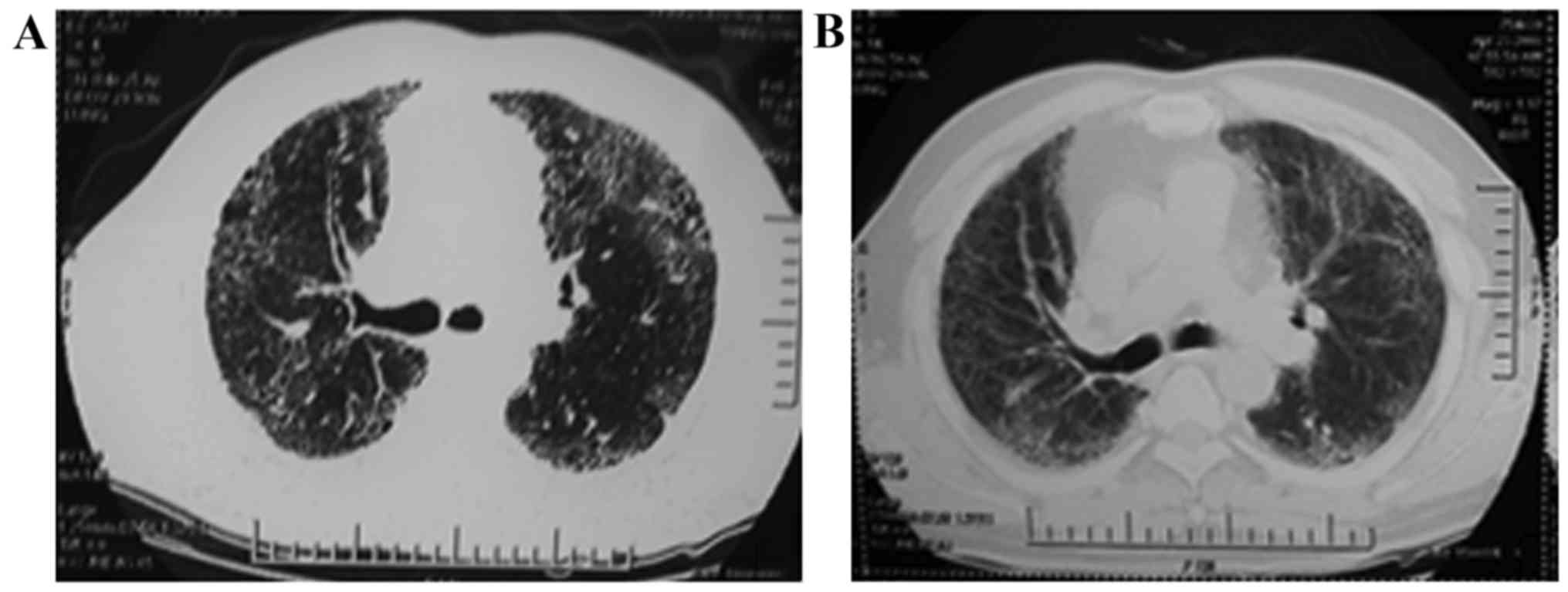
The chest HRCT scan showed (Fig. 2), there were multiple ‘harder’
patchy, peripheral, subpleural, and bibasilar reticular opacities
observed before HUC-MSCs. These images evolved into subpleural
multiple ‘softer’ ground-glass opacity at the end of 12 months
post-HUC-MSC transplantation, while alveolitis and fibrosis were
greatly reduced by HUC-MSC treatment.
Safety outcomes
No serious or clinically significant side effects
were observed during the entire study period. As shown in Table I, there were no side effects of minor
or medium severity, including allergic reactions, liver or renal
abnormalities, and oxygen desaturations, cardiac abnormalities such
as ECG or heart rate changes in 12 months.
Discussion
This case reported the safety and efficacy of
HUC-MSCs to treat IPF in 12 months follow-up duration. No adverse
events were observed, and at 12 months post-infusion, lung
function, 6MWD and CT fibrosis score were all increased from
baseline. Our data provided significant evidence for the long-term
safety of the cell therapy approach in at least moderate fibrotic
lung disease, and provided exciting and novel insights into lung
regeneration.
IPF is a refractory and lethal form of pulmonary
fibrosis characterized by fibroblast proliferation, extracellular
matrix deposition, and progressive lung scarring. With respect to
HUC-MSC therapy in IPF, our concerns focused on delaying or
reversing the process of lung fibrosis. As shown in Fig. 2, the fibrosis area was decreased in
12 months post-transplantation. Moreover, lung function and CT
scores were increased at the 12 month duration. Therefore, our data
show HUC-MSCs intravenous infusion was safe and beneficial in IPF.
In our previously studies (10–12), we
investigated the safety and efficacy of HUC-MSCs in rat liver
fibrosis (13), which reduced in 8
weeks post-translation. Taken together, our data suggest HUC-MSCs
are capable of attenuating fibrotic process.
Some studies of cell therapy for IPF have shown
different efficacy on IPF. Liu et al showed that MSCs
protects against bleomycin-induced liver injury and reduced
fibrosis (14); Ortiz et al
reported intravenous MSC derived from placenta to treat IPF is
feasible and has a good short-term safety profile in patients with
moderately severe IPF, while the efficacy of this kind of treatment
was not visibly (15). Other studies
also confirmed the safety of MSC therapy for IPF, however, the
efficacy of IPF was not obvious (16,17). For
these experimental results, we assume the reasons were: i)
Different cell source, i.e., although MSCs share some identical
phenotype, and meet the criterion of the International Society for
Cell Therapy (18,19), they have different characteristics
from each other. ii) The patients enrolled were different. iii)
Different auxiliary treatment companied with the MSC treatment. iv)
Improvement in the micro-environment of IPF at the same time.
Therefore, we suggested choosing different sources of MSCs to treat
diseases according to the specific condition of different patient.
Future studies will need to be designed to confirm safety and
efficacy over an even longer period, particularly if different
doses are employed.
In this case study, we elected to utilize HUC-MSCs
due to the convenience of isolating and propagating cells in
significant numbers relatively cheaply and easily from this tissue
source. Clinically relevant improvements and short-term benefits
were clearly demonstrated as a result of HUC-MSC transplantation.
In particular, we emphasized the improvement of microcirculation in
this treatment regimens. Future large-scale trials are likely to be
designed using cells from a similar source.
Acknowledgements
The present study was supported by the National High
Technology Research and Development Program (863 Program), nos.
2011AA020101 and 2012A020905.
Glossary
Abbreviations
Abbreviations:
|
DLCO
|
diffusing capacity of the lung for
carbon monoxide
|
|
FEV1
|
forced expiratory volume in one
second
|
|
FVC
|
forced vital capacity
|
|
HRCT
|
high-resolution computed
tomography
|
|
LTOT
|
long-term oxygen therapy
|
|
PaO2
|
partial arterial pressure of
oxygen
|
|
PaCO2
|
partial pressure of carbon dioxide
|
|
PAP
|
pulmonary artery pressure
|
|
PiMax
|
maximal inspiratory pressure
|
|
PeMax
|
maximal expiratory pressure
|
|
6MWT
|
six-minute walk test
|
|
VAS
|
visual analogue scale
|
References
|
1
|
Wuyts WA, Agostini C, Antoniou KM, Bouros
D, Chambers RC, Cottin V, Egan JJ, Lambrecht BN, Lories R, Parfrey
H, et al: The pathogenesis of pulmonary fibrosis: a moving target.
Eur Respir J. 41:1207–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis: An
official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis:
evidence-based guidelines for diagnosis and management. Am J Respir
Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raghu G, Weycker D, Edelsberg J, Bradford
WZ and Oster G: Incidence and prevalence of idiopathic pulmonary
fibrosis. Am J Respir Crit Care Med. 174:810–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss DJ, Bertoncello I, Borok Z, Kim C,
Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D
and Prockop DJ: Stem cells and cell therapies in lung biology and
lung diseases. Proc Am Thorac Soc. 8:223–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bailey MM, Wang L, Bode CJ, Mitchell KE
and Detamore MS: A comparison of human umbilical cord matrix stem
cells and temporomandibular joint condylar chondrocytes for tissue
engineering temporomandibular joint condylar cartilage. Tissue Eng.
13:2003–2010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conconi MT, Burra P, Di Liddo R, Calore C,
Turetta M, Bellini S, Bo P, Nussdorfer GG and Parnigotto PP:
CD105(+) cells from Wharton's jelly show in vitro and in
vivo myogenic differentiative potential. Int J Mol Med.
18:1089–1096. 2006.PubMed/NCBI
|
|
7
|
Lindenmair A, Hatlapatka T, Kollwig G,
Hennerbichler S, Gabriel C, Wolbank S, Redl H and Kasper C:
Mesenchymal stem or stromal cells from amnion and umbilical cord
tissue and their potential for clinical applications. Cells.
1:1061–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avanzini MA, Bernardo ME, Cometa AM,
Perotti C, Zaffaroni N, Novara F, Visai L, Moretta A, Del Fante C,
Villa R, et al: Generation of mesenchymal stromal cells in the
presence of platelet lysate: a phenotypic and functional comparison
of umbilical cord blood- and bone marrow-derived progenitors.
Haematologica. 94:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Divya MS, Roshin GE, Divya TS, Rasheed VA,
Santhoshkumar TR, Elizabeth KE, James J and Pillai RM: Umbilical
cord blood-derived mesenchymal stem cells consist of a unique
population of progenitors co-expressing mesenchymal stem cell and
neuronal markers capable of instantaneous neuronal differentiation.
Stem Cell Res Ther. 3:572012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu Z, Guo S, Fang G, Cui Z and Liu Y: AKT
pathway affects bone regeneration in nonunion treated with
umbilical cord-derived mesenchymal stem cells. Cell Biochem
Biophys. 71:1543–1551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Z, Guo L, Fang G, Cui Z, Guo S and Liu
Y: Biological characteristics and effect of human umbilical cord
mesenchymal stem cells (hUC-MSCs) grafting with blood plasma on
bone regeneration in rats. Cell Biochem Biophys. 63:171–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu Z, Fang G, Cui Z and Liu Y: Cell
therapy for bone nonunion: a retrospective study. Minerva Med.
106:315–321. 2015.PubMed/NCBI
|
|
13
|
Tzouvelekis A, Paspaliaris V, Koliakos G,
Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N,
Dardzinski B, Gritzalis D, et al: A prospective, non-randomized, no
placebo-controlled, phase Ib clinical trial to study the safety of
the adipose derived stromal cells-stromal vascular fraction in
idiopathic pulmonary fibrosis. J Transl Med. 11:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Shi ZL and Zhao Z: Transplantation
of human umbilical cord-derived mesenchymal stem cells improves
hepatic fibrosis in rats with carbon etrachloride-induced hepatic
cirrhosis. Chinese Tissue Engineering Res. 16:1837–1840. 2012.(In
Chinese). http://d.g.wanfangdata.com.cn/Periodical_xdkf201210028.aspx
|
|
15
|
Ortiz LA, Dutreil M, Fattman C, Pandey AC,
Torres G, Go K and Phinney DG: Interleukin 1 receptor antagonist
mediates the antiinflammatory and antifibrotic effect of
mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA.
104:11002–11007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chambers DC, Enever D, Ilic N, Sparks L,
Whitelaw K, Ayres J, Yerkovich ST, Khalil D, Atkinson KM and
Hopkins PM: A phase 1b study of placenta-derived mesenchymal
stromal cells in patients with idiopathic pulmonary fibrosis.
Respirology. 19:1013–1018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiss DJ, Casaburi R, Flannery R,
LeRoux-Williams M and Tashkin DP: A placebo-controlled, randomized
trial of mesenchymal stem cells in COPD. Chest. 143:1590–1598.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toonkel RL, Hare JM, Matthay MA and
Glassberg MK: Potential for Clinical Testing: Mesenchymal stem
cells and idiopathic pulmonary fibrosis. Potential for clinical
testing. Am J Respir Crit Care Med. 188:133–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|