Introduction
Neuroblastoma (NB) is the most common type of
extracranial solid tumor among children that arises in the
peripheral sympathetic nervous system. NB typically emerges in the
adrenal medulla or paraspinal ganglia during embryogenesis. NB
accounts for ~10% of pediatric cancer-associated mortalities, with
an annual incidence of ~650 novel cases in the USA (1) and it is a malignant tumor with a low
degree of differentiation. According to the degree of
histopathological differentiation, neuroblastic tumors can be
divided into NB, ganglioneuroblastoma and ganglioneuroma (2). The prognosis depends on the patients’
age, location, region and biological characteristics of NB, but it
is usually poor. In recent years, as methods of early diagnosis
have improved, the survival rate of children with NB has
significantly increased; however, patients with high-risk cases
still have a <40% chance of survival. Therefore, it is urgent to
search for a novel adjuvant agent suitable for the treatment of NB
with few side effects in order to increase the overall survival
rate.
Cucurbitacins are a group of tetracyclic triterpenes
isolated from Cucurbitaceae (3).
Previous studies have demonstrated that they have a broad range of
pharmacological effects, including anti-inflammatory (4,5),
anti-fertility (5), anti-viral
(6) and anticancer (4,7,8)
activities. Chemically, cucurbitacins are highly diverse and are
arbitrarily divided into 12 categories (5). Cucurbitacin B (CuB) is one of the
most abundant forms of cucurbitacins and the most widely used as an
anticancer agent. It has significant anti-inflammatory activity and
is used traditionally to treat hepatitis (9). Studies indicate that CuB is capable
of inhibiting the growth of a wide spectrum of malignant human
cells, including myeloid leukemia, breast cancer, glioblastoma
multiforme, pancreatic cancer, laryngeal cancer, melanoma and
osteosarcoma cells (10–16). The anticancer mechanism of CuB is
activated by different signaling pathways in different cancer
cells. Several studies have shown that CuB induces apoptosis by
inhibiting the Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) signaling pathway (13,14),
but others have indicated that CuB is a potent inhibitor of nuclear
factor κ-light-chain-enhancer of activated B cells activation
(17), and additionally exhibits
anticancer effects through wingless type signaling (11).
In previous years, researchers have searched for
novel effective drugs with few side effects suitable for the
treatment of NB. Gheeya et al (18) used a panel of drugs in order to
identify novel effective chemotherapeutics against NB, and they
demonstrated that cucurbitacin I inhibits cell growth through
inhibition of the STAT3 pathway. Although there is an increase in
the amount of evidence indicating that CuB is also an inhibitor of
the STAT3 pathway in several tumor cell lines (8,13,14,19),
whether CuB exhibits anticancer effects in NB cells and its exact
molecular mechanism remain to be elucidated. In the present study,
the effects of CuB on human NB SH-SY5Y cells were evaluated, and
the molecular mechanisms underlying CuB-induced apoptosis were
studied.
Materials and methods
Chemicals and reagents
CuB at 98% purity was purchased from Must
Biotechnology Co., Ltd. (Chengdu, China). MTT, dimethyl sulfoxide
(DMSO), PD98059, SP600125, SB203580 and interleukin (IL)-6 were
purchased from Sigma (St. Louis, MO, USA). Dulbecco’s modified
Eagle’s medium (DMEM) and fetal bovine serum (FBS) were acquired
from HyClone Laboratories (Logan, UT, USA). Propidium iodide (PI),
AG490 and bicinchoninic acid (BCA) Protein Assay kit were obtained
from Beyotime (Shanghai, China). An Annexin V-fluorescein
isothiocyanate (FITC)/PI apoptosis detection kit was purchased from
MultiSciences Biotech Co., Ltd (Hangzhou, China). The primary
antibodies specific to p53 (rabbit pAb), p21 (mouse mAb), B-cell
lymphoma 2 (Bcl-2; rabbit pAb), Bcl2-associated X protein (Bax;
rabbit pAb), phospho-JAK2 (Tyr 1007/1008; rabbit mAb), JAK2 (mouse
mAb), phospho-STAT3 (Tyr705; mouse mAb), phospho-extracellular
signal-regulated kinases (p-ERK; Thr202/Tyr204; rabbit pAb),
phospho-p38 (Thr180/Tyr182; rabbit pAb) and β-actin (mouse mAb)
were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The phospho-c-Jun N-terminal kinase (p-JNK; Thr183/Tyr185; rabbit
mAb) primary antibody was purchased from Cell Signaling Technology
(Danvers, MA, USA), cyclin-dependent kinase 1 (CDK1; mouse mAb),
Cyclin B1 (rabbit pAb), STAT3 (rabbit pAb), ERK (rabbit pAb), JNK1
and 2 (rabbit pAb), and p38 primary antibody (rabbit pAb) and
secondary polyclonal antibodies goat anti-mouse immunoglobulin (Ig)
G horseradish-peroxidase (HRP)-conjugate and goat anti-rabbit IgG
HRP-conjugate were obtained from Boster Biological Technology.,
Ltd. (Wuhan, China).
Cell line and culture
The human NB cell line (SH-SY5Y) was obtained from
the China Center for Type Culture Collection (Wuhan University,
Wuhan, China). The cells were cultured in DMEM supplemented with
10% FBS at 37°C in a humidified atmosphere containing 5%
CO2. All the experiments were performed one day after
the cells were seeded.
Cell viability assay
The effect of CuB on the growth and proliferation on
cancer cells was assessed by measuring the metabolic activity (MTT
assay). The cells were seeded in 96-well plates at a density of
2×104 cells/well with 200 μl culture medium per well for
24 h. On the next day, the medium was replaced with fresh medium
containing different concentrations of CuB (0–128 μM). Subsequent
to incubation for an additional 24 and 48 h, a total of 20 μl MTT
[5 mg/ml in phosphate-buffered saline (PBS)] solution was added to
each well and incubated at 37°C for 4 h to metabolize the MTT into
formazan. Next, the supernatant was discarded and 100 μl DMSO was
added to each well to terminate the reaction. The absorbance was
measured at 490 nm using a microplate reader (Model 680; Bio-Rad,
Richmond, VA, USA). Three independent experiments were performed in
triplicates. The percentage of proliferation was normalized
relative to the control.
Cell cycle analysis
The cell cycle parameters were analyzed using a
FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
The SH-SY5Y cells were cultured with the indicated concentrations
of CuB for 24 h. The cells were harvested by centrifugation, then
washed with cold PBS (pH 7.4) and fixed with 70% ice-cold ethanol
overnight at −20°C. Following fixation, the cells were harvested
and rinsed once with PBS (pH 7.4) and then incubated with 500 μl PI
staining solution (50 μg/ml PI, 100 μg/ml RNase A) for 1 h at room
temperature. The relative numbers of cells in the G1, S and G2/M
phases of the cell cycle were measured.
Annexin V-FITC/PI staining
The SH-SY5Y cells were exposed to the indicated
concentrations of CuB for 24 h. The samples of 1–5×105
cells were harvested and rinsed twice with cold PBS (pH 7.4). The
cells were resuspended in 500 μl binding buffer and stained with 5
μl Annexin-FITC and 10 μl of PI for 5 min in the dark at room
temperature. The stained apoptotic cells were counted using a
FACScan flow cytometer.
Western blot analysis
Following treatment with the indicated
concentrations of CuB, the SH-SY5Y cells were lysed and the protein
concentration was determined using a BCA protein assay kit. The
lysate containing 40 μg of protein was subjected to SDS-PAGE. The
protein was transferred to a nitrocellulose membrane, and the
membrane was blocked overnight with 1X Tris-Buffered Saline
containing 0.1% Tween-20 and 5% skimmed milk at 4°C. Following
blocking, the membrane was washed three times and incubated with
the respective primary antibodies for 2 h at room temperature.
Next, the membrane was washed three times and incubated with the
diluted HRP-conjugated secondary antibody (1:5,000) for 1.5 h at
room temperature. Subsequent to the three washes, the membrane was
detected using an enhanced chemiluminescence kit (Millipore,
Bedford, MA, USA).
Statistical analysis
All the values are expressed as the mean ± standard
deviation. Significant differences between the groups were
determined using Student’s t-test and the statistical significance
was expressed as *P<0.05 and **P<0.01.
All the figures shown represent the results from at least three
independent experiments.
Results
CuB inhibits proliferation of SH-SY5Y
cells
In order to investigate the growth inhibition
effects of CuB, the MTT assay and PI staining were performed to
evaluate the cell viability and cell cycle distribution,
respectively.
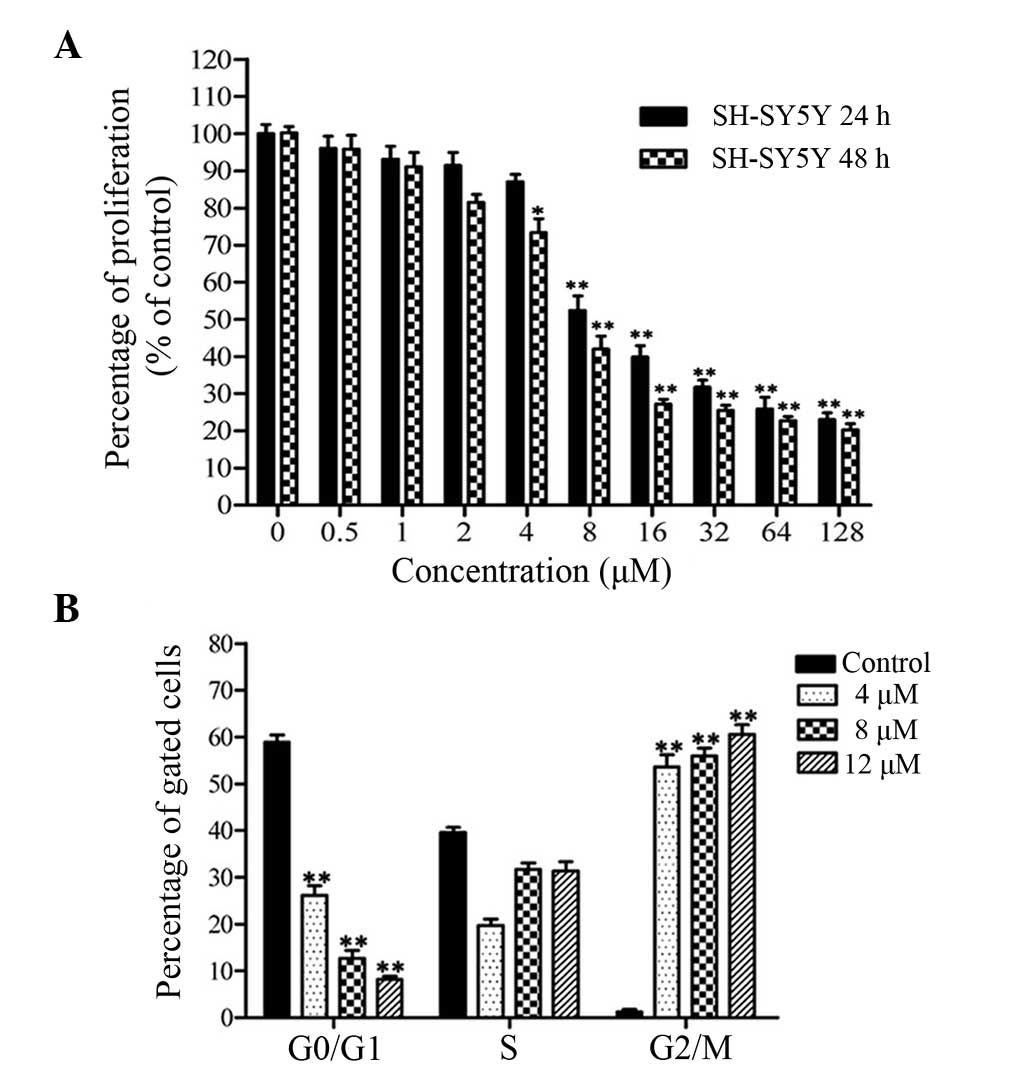
The viability of cancer cells treated with CuB was
investigated by the MTT assay. CuB inhibited cell proliferation in
SH-SY5Y cells in a time- and dose-dependent manner (Fig. 1A). The concentrations of 4, 8 and
12 μM CuB were used in subsequent experiments.
Cell cycle arrest caused by CuB for 24 h was
investigated by flow cytometry following PI staining. Evident
changes were found in the cell cycle distributions when treated
with CuB (Fig. 1B). The percentage
of cells in the G2/M phase was increased in a dose-dependent
manner. These data indicated that CuB treatment suppressed cell
proliferation through an increased accumulation of cells in G2/M
phase of the cell cycle.
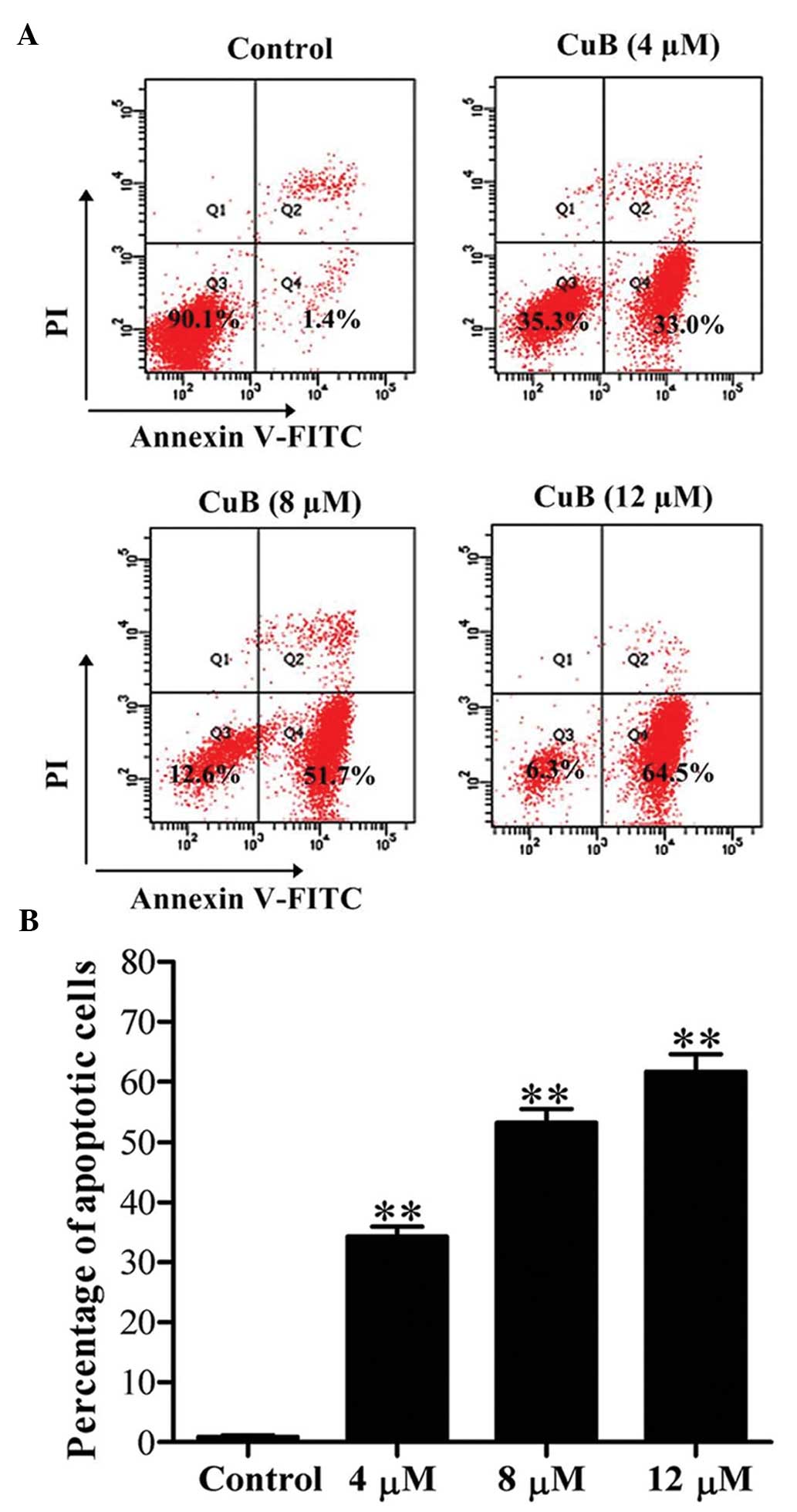
CuB induces early apoptosis in SH-SY5Y
cells
Apoptosis in SH-SY5Y cells was detected by flow
cytometry following Annexin V-FITC/PI double staining. The results
revealed that the early apoptotic rate (Annexin V-FITC-positive and
PI-negative cells) was significantly increased following CuB
treatment for 24 h and the rate increased in a dose-dependent
manner (Fig. 2).
CuB inhibits JAK2/STAT3 signaling
cascades
The phosphorylated forms of JAK2 and STAT3 were
assessed by western blot analysis in SH-SY5Y cells treated with CuB
for 24 h. Constitutive activation of JAK2 and STAT3 were suppressed
by CuB in a concentration-dependent manner (Fig. 3A). Next, the role of JAK2/STAT3 in
CuB-induced apoptosis by a JAK2 inhibitor (AG490) and an activator
of JAK2/STAT3 (IL-6) was determined. Western blot analysis and flow
cytometry were respectively employed to detect protein expression
and apoptosis with or without AG490 or IL-6 pretreatment. As
expected, the protein levels of p-JAK2 and p-STAT3 were regulated
by AG490 and IL-6, while the total protein levels of JAK2 and STAT3
did not exhibit any evident change. Compared with CuB treatment
alone, the protein levels of p-JAK2 and p-STAT3 were markedly
decreased and the rate of apoptotic cells was markedly increased
following the treatment of CuB and AG490. However, the alterations
induced by CuB could be attenuated partially by IL-6 (Fig. 3B and C).
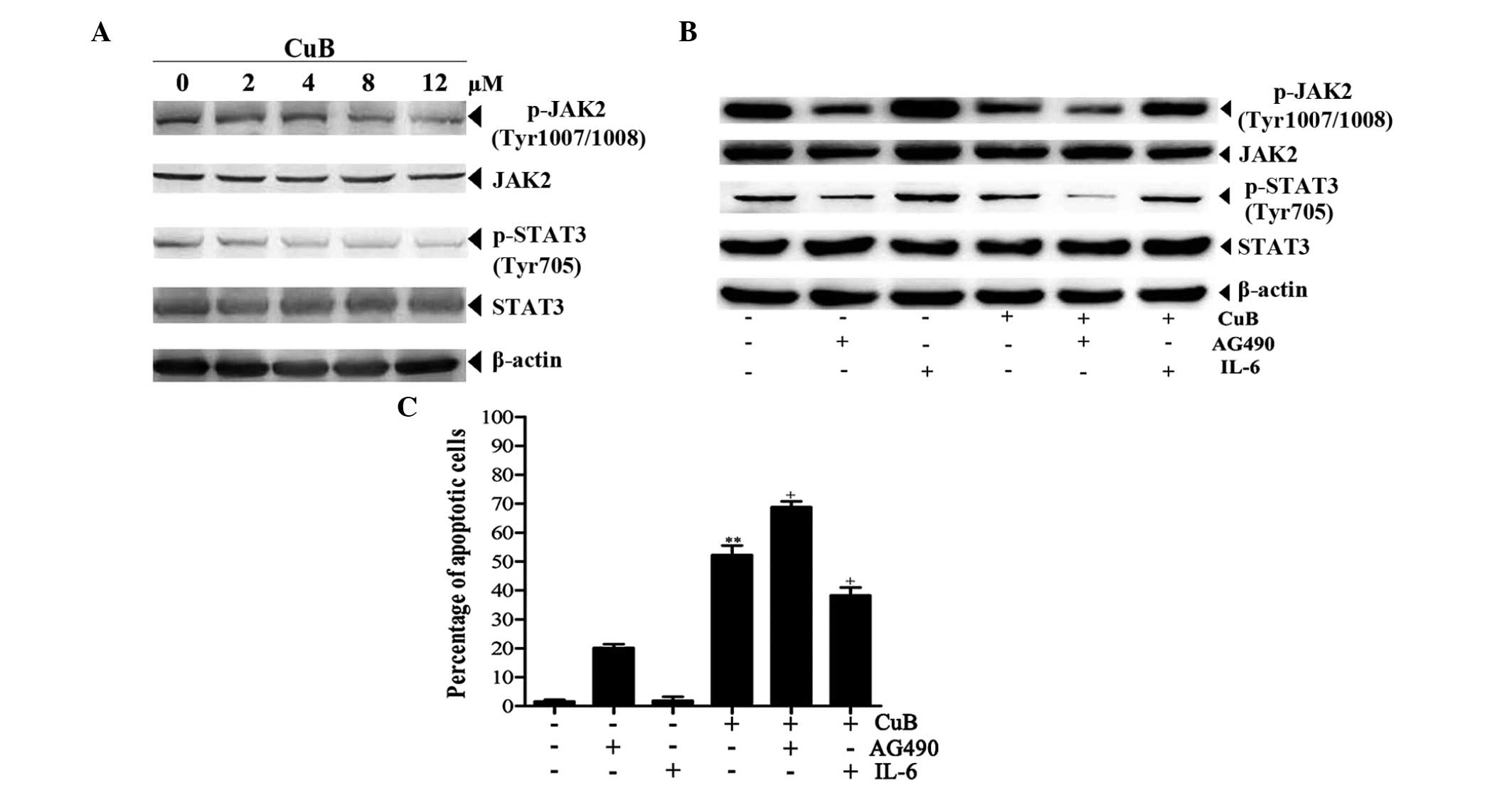 | Figure 3Effect of CuB on JAK2/STAT3 signaling
cascades. (A) Cell lysates from the SH-SY5Y cells treated with CuB
for 24 h were analyzed by western blot analysis using antibodies
against p-JAK2, JAK2, p-STAT3 and STAT3, and β-actin was used as a
loading control (bottom panel). (B) The cells were preincubated
with or without AG490 or IL-6 for 1 h and further incubated in the
presence or absence of 8 μM CuB for 24 h. Next, equal amounts of
protein were analyzed by western blot analysis. (C) The apoptotic
effects of CuB, AG490 and IL-6 on SH-SY5Y cells were detected by
flow cytometry. The values are provided as the mean ± SD of three
independent experiments. Significant differences are indicated by
**P<0.01 versus control and +P<0.05
versus CuB. CuB, cucurbitacin B; p-JAK2, phosphorylated Janus
kinase 2; STAT3, signal transducer and activator of transcription
3; IL-6, interleukin 6; SD, standard deviation. |
CuB induces JNK and p38 MAPK activation
and ERK inactivation in SH-SY5Y cells
Western blot analysis was performed to determine
whether CuB affects the activation of MAPK cascades, including ERK,
JNK and p38 MAPK in NB cells. As a result, CuB upregulated p-JNK
and p-p38 MAPK, and downregulated p-ERK in SH-SY5Y cells (Fig. 4A). Therefore, PD98059, SB203580 and
SP600125, which are specific inhibitors of ERK, p38 MAPK and JNK,
respectively, were used to examine the role of the MAPK signaling
pathway in CuB-treated cells. Upon pretreatment with the
inhibitors, the protein levels of p-ERK, p-JNK and p-p38 MAPK were
all decreased, and the decrease in p-ERK expression was significant
compared with CuB treatment alone. ERK, JNK and p38 MAPK did not
reveal evident changes (Fig. 4B).
Apoptosis analysis by flow cytometry revealed that the ERK
inhibitor PD98059 increased the percentage of apoptotic cells
induced by CuB, whereas the rates of apoptotic cells induced by CuB
were significantly abrogated by the p38 MAPK and JNK inhibitors
(Fig. 4C).
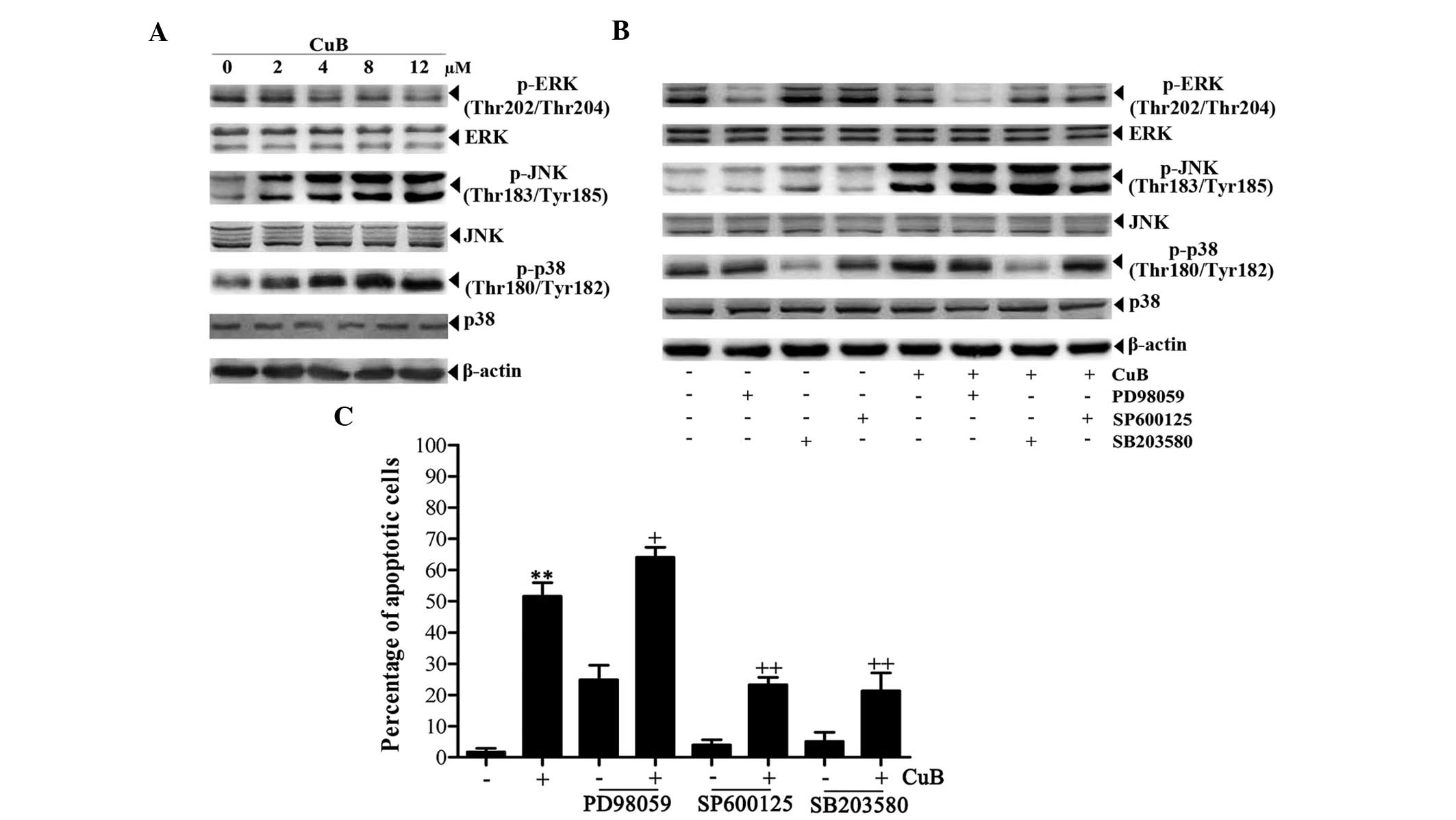 | Figure 4Effects of CuB on the MAPK signaling
pathway. (A) The cell lysates from SH-SY5Y cells treated with CuB
for 24 h were analyzed by western blot analysis using antibodies
against p-ERK, ERK, p-JNK, JNK, p-p38 and p38, and β-actin was used
as a loading control (bottom panel). (B) The cells were
preincubated with or without inhibitors of signaling molecules for
1 h and further incubated in the presence or absence of 8 μM CuB
for 24 h. The concentrations of the inhibitors are as follows:
PD98059, 100 μM; SP600125, 20 μM and SB203580, 20 μM. Next, equal
amounts of protein were analyzed by western blot analysis. (C) The
apoptotic effects of the inhibitors on SH-SY5Y cells were detected
by flow cytometry. The values are provided as the mean ± SD of
three independent experiments. Significant differences are
indicated by **P<0.01 versus control and
+P<0.05, ++P<0.01 versus CuB. CuB,
cucurbitacin B; MAPKs, mitogen-activated protein kinases; p-ERK,
phosphorylated extracellular signal-regulated kinases; p-JNK,
phosphorylated c-Jun N-terminal kinases; SD, standard
deviation. |
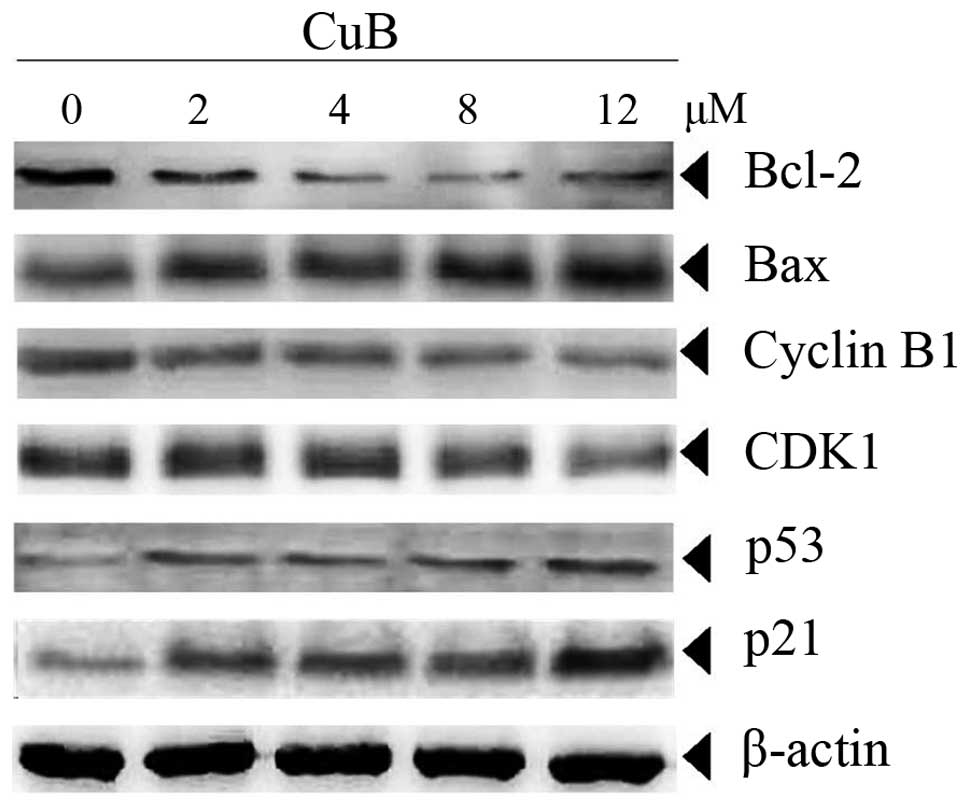
CuB alters expression of proteins
involved in proliferation and apoptosis
Bcl-2 and Bax have been implicated in apoptosis and
mitochondrial dysfunction. For this reason, the effects of CuB on
the expression of these two proteins were investigated. The data in
Fig. 5 demonstrated that CuB
downregulated the anti-apoptotic Bcl-2 and upregulated
pro-apoptotic Bax in a concentration-dependent manner in SH-SY5Y
cells. In addition, cell cycle proteins linked with the G2/M phase,
including cyclin B1 and CDK1, were also downregulated by CuB in a
dose-dependent manner. In multi-cellular organisms, p53 is involved
in the prevention of cancer. It acts as a tumor suppressor and was
reported to regulate the cell cycle through the control of the
expression of cyclin-dependent kinase inhibitor p21 (20). The results indicated that p53 and
p21 were also upregulated by CuB in a dose-dependent manner in
SH-SY5Y cells (Fig. 5).
Discussion
CuB is known for its ability to suppress the
proliferation and induce apoptosis in a wide variety of cancer cell
lines, and the mechanisms of CuB action differ among different
cancer cell lines. Identifying the molecular targets of an agent is
very important in the selection of anticancer agents with few side
effects on normal cells. The results of the present study indicated
that CuB could induce cell growth inhibition by G2/M phase arrest
and apoptosis.
JAK/STAT3 is the major anti-apoptotic pathway for
the transduction of a multitude of signals which are critical for
the development and homeostasis in mammals (21). JAK activation has a significant
role in cell proliferation, differentiation, migration and
apoptosis (21,22). Constitutive activation of STAT3 has
a critical role in cell growth and survival in human solid tumor
malignancies (23–25) and the upregulation of the
anti-apoptotic proteins in human cancer cells (23,26).
JAK2/STAT3 signaling has been extensively validated as a novel
molecular target for the agents against human solid tumors
(27–29). In the present study, it was
observed that CuB inhibited the JAK2/STAT3 signaling pathway by
markedly downregulating p-JAK2 and p-STAT3 protein expression. It
can be concluded that CuB may act as a JAK2/STAT3 inhibitor in
SH-SY5Y cells.
MAPKs are serine-threonine protein kinases and they
have a significant role in the regulation of numerous cellular
processes, including cell growth and proliferation, differentiation
and apoptosis (30,31). MAPKs consist of growth
factor-regulated ERKs, JNKs, p38 MAPK and ERK5 (32). ERK, the most widely studied MAPK
cascade, has been shown to be a major participant in the regulation
of cell growth and differentiation, and the activation of JNK and
p38 MAPK signaling cascades generally result in apoptosis (13,30,33).
In the present study, CuB was found to activate JNK and p38 MAPK
and inactivate ERK in NB cells in a concentration-dependent manner.
It can be concluded that CuB may also act as a MAPK regulator in
SH-SY5Y cells.
Bcl-2 and Bax belong to the Bcl-2 family and have a
significant role in cell apoptosis. Bcl-2 and Bax exhibit anti- and
pro-apoptotic activities (34).
The results of the present study revealed that Bcl-2 and Bax were
downregulated and upregulated, respectively, and further prompted
apoptosis. Flow cytometric analysis revealed that CuB induced cell
cycle arrest in the G2/M phase. Cyclin B1 and CDK1 are linked to
the G2/M phase progress. Consistent with the results of flow
cytometry, it was found that CuB inhibited the expression of Cyclin
B1 and CDK1 in a dose-dependent manner. p53 and p21 are anticancer
proteins that can induce apoptosis, inhibit Bcl-2 and cellular
inhibitor of apoptosis proteins and activate the activities of
pro-apoptosis proteins. In addition, the tumor suppressor p53 and
its downstream target p21 have been shown to induce cell cycle
arrest since they are potent cyclin-CDK inhibitors (35).
The present study demonstrated that human NB SH-SY5Y
cells undergo apoptosis in response to treatment with CuB, which
occurs through the JAK2/STAT3 and MAPK signaling pathways, and the
regulation of gene products that mediate tumor cell survival,
proliferation and apoptosis. The present study also reveals that
CuB can possibly be used as a novel potent therapeutic agent
against NB. However, since all these results were obtained from
in vitro experiments, in vivo studies are required in
order to validate these results for the therapeutic use of this
agent in humans.
Acknowledgements
This study was supported by funds from the Public
Science and Technology Research Funds Projects of Ocean (no.
201005013) and the Wuhan Municipal Science and Technology Project
(no. 201260523185).
References
|
1
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher JP and Tweddle DA: Neonatal
neuroblastoma. Semin Fetal Neonatal Med. 17:207–215. 2012.
View Article : Google Scholar
|
|
3
|
Zhang Y, Ouyang D, Xu L, Ji Y, Zha Q, Cai
J and He X: Cucurbitacin B induces rapid depletion of the G-actin
pool through reactive oxygen species-dependent actin aggregation in
melanoma cells. Acta Biochim Biophys Sin (Shanghai). 43:556–567.
2011. View Article : Google Scholar
|
|
4
|
Jayaprakasam B, Seeram NP and Nair MG:
Anticancer and antiinflammatory activities of cucurbitacins from
Cucurbita andreana. Cancer Lett. 189:11–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen JC, Chiu MH, Nie RL, Cordell GA and
Qiu SX: Cucurbitacins and cucurbitane glycosides: structures and
biological activities. Nat Prod Rep. 22:386–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dzubak P, Hajduch M, Vydra D, et al:
Pharmacological activities of natural triterpenoids and their
therapeutic implications. Nat Prod Rep. 23:394–411. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takasaki M, Konoshima T, Murata Y, et al:
Anticarcinogenic activity of natural sweeteners, cucurbitane
glycosides, from Momordica grosvenori. Cancer Lett. 198:37–42.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu T, Peng H, Zhang M, Deng Y and Wu Z:
Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling
pathway, enhances the chemosensitivity of laryngeal squamous cell
carcinoma cells to cisplatin. Eur J Pharmacol. 641:15–22. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Promkan M, Dakeng S, Chakrabarty S, Bögler
O and Patmasiriwat P: The effectiveness of cucurbitacin B in BRCA1
defective breast cancer cells. PLoS One. 8:e557322013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haritunians T, Gueller S, Zhang L, et al:
Cucurbitacin B induces differentiation, cell cycle arrest, and
actin cytoskeletal alterations in myeloid leukemia cells. Leuk Res.
32:1366–1373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dakeng S, Duangmano S, Jiratchariyakul W,
U-Pratya Y, Bögler O and Patmasiriwat P: Inhibition of Wnt
signaling by cucurbitacin B in breast cancer cells: reduction of
Wnt-associated proteins and reduced translocation of
galectin-3-mediated β-catenin to the nucleus. J Cell Biochem.
113:49–60. 2012.PubMed/NCBI
|
|
12
|
Yin D, Wakimoto N, Xing H, et al:
Cucurbitacin B markedly inhibits growth and rapidly affects the
cytoskeleton in glioblastoma multiforme. Int J Cancer.
123:1364–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thoennissen NH, Iwanski GB, Doan NB, et
al: Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT
pathway and potentiates antiproliferative effects of gemcitabine on
pancreatic cancer cells. Cancer Res. 69:5876–5884. 2009. View Article : Google Scholar
|
|
14
|
Liu T, Zhang M, Zhang H, Sun C and Deng Y:
Inhibitory effects of cucurbitacin B on laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 265:1225–1232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh H, Mun YJ, Im SJ, Lee SY, Song HJ, Lee
HS and Woo WH: Cucurbitacins from Trichosanthes kirilowii as the
inhibitory components on tyrosinase activity and melanin synthesis
of B16/F10 melanoma cells. Planta Med. 68:832–833. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee DH, Thoennissen NH, Goff C, et al:
Synergistic effect of low-dose cucurbitacin B and low-dose
methotrexate for treatment of human osteosarcoma. Cancer Lett.
306:161–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin HR, Jin X, Dat NT and Lee JJ:
Cucurbitacin B suppresses the transactivation activity of RelA/p65.
J Cell Biochem. 112:1643–1650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gheeya JS, Chen QR, Benjamin CD, et al:
Screening a panel of drugs with diverse mechanisms of action yields
potential therapeutic agents against neuroblastoma. Cancer Biol
Ther. 8:2386–2395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang M, Zhang H, Sun C, Shan X, Yang X,
Li-Ling J and Deng YH: Targeted constitutive activation of signal
transducer and activator of transcription 3 in human hepatocellular
carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol.
63:635–642. 2009. View Article : Google Scholar
|
|
20
|
Mirzayans R, Andrais B, Scott A and Murray
D: New insights into p53 signaling and cancer cell response to DNA
damage: implications for cancer therapy. J Biomed Biotechnol.
2012:1703252012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiu H and Nicholson SE: Biology and
significance of the JAK/STAT signalling pathways. Growth Factors.
30:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O’Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: new surprises in the Jak/Stat pathway.
Cell. 109(Suppl): S121–S131. 2002.PubMed/NCBI
|
|
23
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: a leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YY, Zheng Q, Fang B, et al: Germacrone
induces apoptosis in human hepatoma HepG2 cells through inhibition
of the JAK2/STAT3 signalling pathway. J Huazhong Univ Sci Technolog
Med Sci. 33:339–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Epling-Burnette PK, Liu JH,
Catlett-Falcone R, et al: Inhibition of STAT3 signaling leads to
apoptosis of leukemic large granular lymphocytes and decreased
Mcl-1 expression. J Clin Invest. 107:351–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nam S, Xie J, Perkins A, et al: Novel
synthetic derivatives of the natural product berbamine inhibit
Jak2/Stat3 signaling and induce apoptosis of human melanoma cells.
Mol Oncol. 6:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bill MA, Nicholas C, Mace TA, et al:
Structurally modified curcumin analogs inhibit STAT3
phosphorylation and promote apoptosis of human renal cell carcinoma
and melanoma cell lines. PLoS One. 7:e407242012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Um HJ, Min KJ, Kim DE and Kwon TK:
Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of
human renal carcinoma Caki cells. Biochem Biophys Res Commun.
427:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park KR, Nam D, Yun HM, et al:
β-Caryophyllene oxide inhibits growth and induces apoptosis through
the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated
MAPKs activation. Cancer Lett. 312:178–188. 2011.
|
|
31
|
Scuteri A, Galimberti A, Maggioni D, et
al: Role of MAPKs in platinum-induced neuronal apoptosis.
Neurotoxicology. 30:312–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boutros T, Chevet E and Metrakos P:
Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase
regulation: roles in cell growth, death, and cancer. Pharmacol Rev.
60:261–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishdorj G, Johnston JB and Gibson SB:
Cucurbitacin-I (JSI-124) activates the JNK/c-Jun signaling pathway
independent of apoptosis and cell cycle arrest in B leukemic cells.
BMC Cancer. 11:2682011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuster JJ, Sanz-González SM, Moll UM and
Andrés V: Classic and novel roles of p53: prospects for anticancer
therapy. Trends Mol Med. 13:192–199. 2007. View Article : Google Scholar : PubMed/NCBI
|