Introduction
Osteosarcoma is a cancerous bone tumor that usually
develops in adolescents (1).
Significant improvements in patient survival rates have been
achieved in recent years. Bioactive compounds derived from natural
products have been used for thousands of years for therapy,
pre-dating recorded history (2).
Berberine, an isoquinoline alkaloid derived from the Chinese herb
Huanglian, is used as a botanical drug (3). In China, berberine is commonly
prescribed for the treatment of gastrointestinal complaints,
diarrhea and other conditions. Accumulative evidence from in
vitro studies has demonstrated that berberine possesses
anticancer and anti-inflammatory activity in different types of
human cancer cells, including osteosarcoma (4), epidermoid carcinoma (5), lung cancer (6), melanoma (7), prostate cancer (8) and liver cancer (9) cells. Animal studies have demonstrated
that berberine is able to suppress chemical-induced carcinogenesis
(10), tumor formation (11) and tumor invasion (12,13).
Apoptosis and DNA damage have previously been revealed to be
effective in eliminating cancer cells. Numerous natural compounds,
including berberine, have been developed as preventive and
treatment agents against cancer. However, to the best of our
knowledge, few studies have examined the potential therapeutic
effects of berberine in osteosarcoma.
The present study examined the effects of berberine
on MG-63 cells in culture using DNA fragmentation analysis and flow
cytometry. It has previously been demonstrated that the formation
of DNA double-strand breaks induces γH2AX aggregations in nuclei,
and it has been suggested that γH2AX focus formation is a sensitive
method for detecting DNA double-strand breaks (14). A threshold of ≥4 γH2AX foci/cell
has been found to be optimal for the determination of DNA damage
(15). Thus the extent of DNA
damage was observed in berberine-treated cells, as determined by
measuring γH2AX focus formation.
Materials and methods
Drugs and materials
Berberine (purity, >98%) was purchased from
Tianping Pharmaceutical Co. (Shanghai, China). The compound was
dissolved in dimethyl sulfoxide (DMSO).
N-methyl-N′-nitro-N-nitrosoguanidine (MNNG), normal-melting
agarose, 4′,6-diamidino-2-phenylindole (DAPI),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Tween-20 and paraformaldehyde were obtained from Sigma Chemical Co.
(Silicon Valley, CA, USA). The apoptosis detection kit was obtained
from BD Pharmingen (San Diego, CA, USA). Triton X-100, fetal bovine
serum (FBS), xylene cyanol and bromophenol blue were obtained from
Sangon Biotech Shanghai Co., Ltd. (Shanghai, China). All other
chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China).
Cell culture
The MG-63 human osteosarcoma cell line (wild type)
was purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in Dulbecco’s modified Eagle’s medium supplemented with
10% heat-inactivated FBS, penicillin (100 U/ml) and streptomycin
(100 U/ml). The cells were incubated at 37°C in a 5% CO2
incubator. The medium was exchanged once every two days. Following
treatment, the cells were harvested by trypsinization.
Analysis of cytotoxicity
The cytotoxicity was determined using the MTT assay
(16). MG-63 cells were seeded at
a density of 1×104 cells/well in 100 μl of cell culture
medium and then placed in a 96-well plate. Following 12 h of
incubation, the cells were treated with 0–80 μM berberine for 12
and 24 h. MTT solution (5 mg/ml) was then added to each well and
the samples were incubated at 37°C for 4 h. Subsequently, the
supernatant was removed and replaced with 100 μl DMSO. The optical
density of the control and drug-treated wells was measured using an
automated microplate reader (Multiskan Ex; Ani Lab systems Ltd.,
Vantaa, Finland) at a test wavelength of 570 nm.
Flow cytometric analysis of
berberine-induced apoptosis in MG-63 cells
To determine the externalization of
phosphatidylserine by fluorescein isothiocyanate (FITC)-labeled
Annexin V and propidium iodide (PI), flow cytometry was used as
previously described (17).
Briefly, the cells were treated with berberine at concentrations of
20, 40, 60 and 80 μM for 12 and 24 h. The cells were washed twice
with cold phosphate-buffered saline (PBS) and resuspended in 500 μl
binding buffer at a concentration of 1×106 cells/ml.
Then, 5 μl Annexin V-FITC solution and 5 μl PI (1 mg/ml) were
added. The cells were incubated at 37°C for 30 min and analyzed by
flow cytometry within 1 h. The number of apoptotic cells were
counted and presented as a percentage of the total cell count.
DNA extraction and detection of DNA
fragmentation
The DNA ladder assay was performed as previously
described (18), with slight
modifications. After treating the cells with berberine and MNNG at
concentrations of 50 and 20 μM, respectively, for 24 h, pellets
containing 1×106 cells were lysed in lysis buffer [10 mM
Tris-HCl (pH 8.0), 25 mM ethylenediaminetetraacetic acid (EDTA),
0.5% sodium dodecyl sulfate, 100 mM NaCl and 400 g/ml protease K]
for 120 min at 56°C and then treated with 10 mg/ml RNase A for an
additional 50 min at 37°C. The lysates were centrifuged (12,000 × g
for 30 min at 4°C) and the supernatant was collected. The
fragmented DNA was extracted from the supernatant with a neutral
phenol:chloroform:isoamyl alcohol mixture (v/v/v; 25:24:1). The DNA
pellet was precipitated by adding isopropanol, washed with 75%
ethanol and dissolved in Tris-EDTA buffer (10 mM Tris-HCl and 1 mM
EDTA; pH 8.0). DNA fragmentation was detected by gel
electrophoresis and the bands were stained with ethidium bromide
for UV light visualization.
γH2AX focus staining
The phosphorylation of histone H2AX (a marker of DNA
double-strand breaks) was analyzed as previously described
(15), with slight modifications.
Briefly, 1×105 cells were seeded into 6-well culture
plates containing a glass cover slip in each well. Following
treatment, the cells were fixed in 4% paraformaldehyde for 15 min,
washed with PBS and permeabilized in 0.2% Triton X-100. Following
inhibition with blocking serum for 1.5 h, the samples were
incubated with a mouse monoclonal anti-H2AX antibody (1:1,000; Cell
Signaling Technology, Inc., Boston, MA, USA) for 2 h, followed by
incubation with FITC-conjugated goat anti-mouse secondary antibody
(1:500; Cell Signaling Technology, Inc.) for 1 h. For staining the
nuclei, DAPI was added to the cells and incubated for another 15
min. The cover slip was then removed from the plate, mounted on a
glass slide and observed using an Olympus BX53 fluorescent
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean of three independent experiments. The differences among
the treated groups and the negative control were compared by
one-way analysis of variance. The Newman-Keuls multiple comparisons
test was applied. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Cytotoxic effect of berberine on MG-63
cells
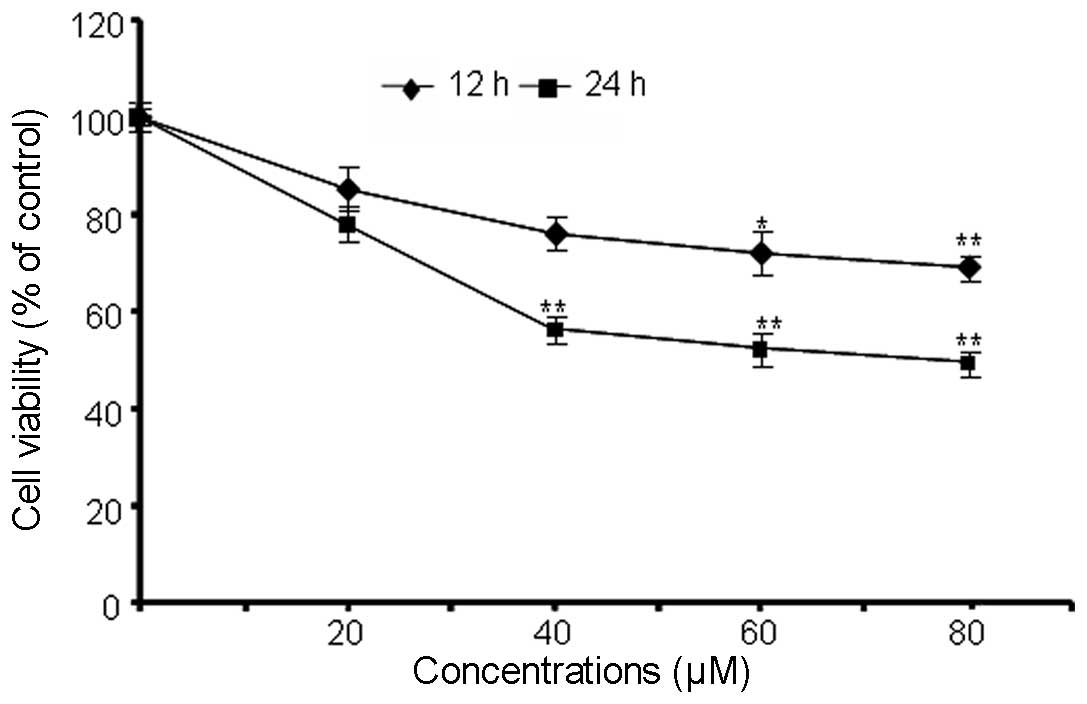
The results of the trypan MTT assay demonstrated
that berberine induced a concentration- and time-dependent decrease
in the viability of MG-63 cells compared with the control (Fig. 1), indicating that berberine has
cytotoxic effects on MG-63 cells.
Berberine induced apoptosis in MG-63
cells
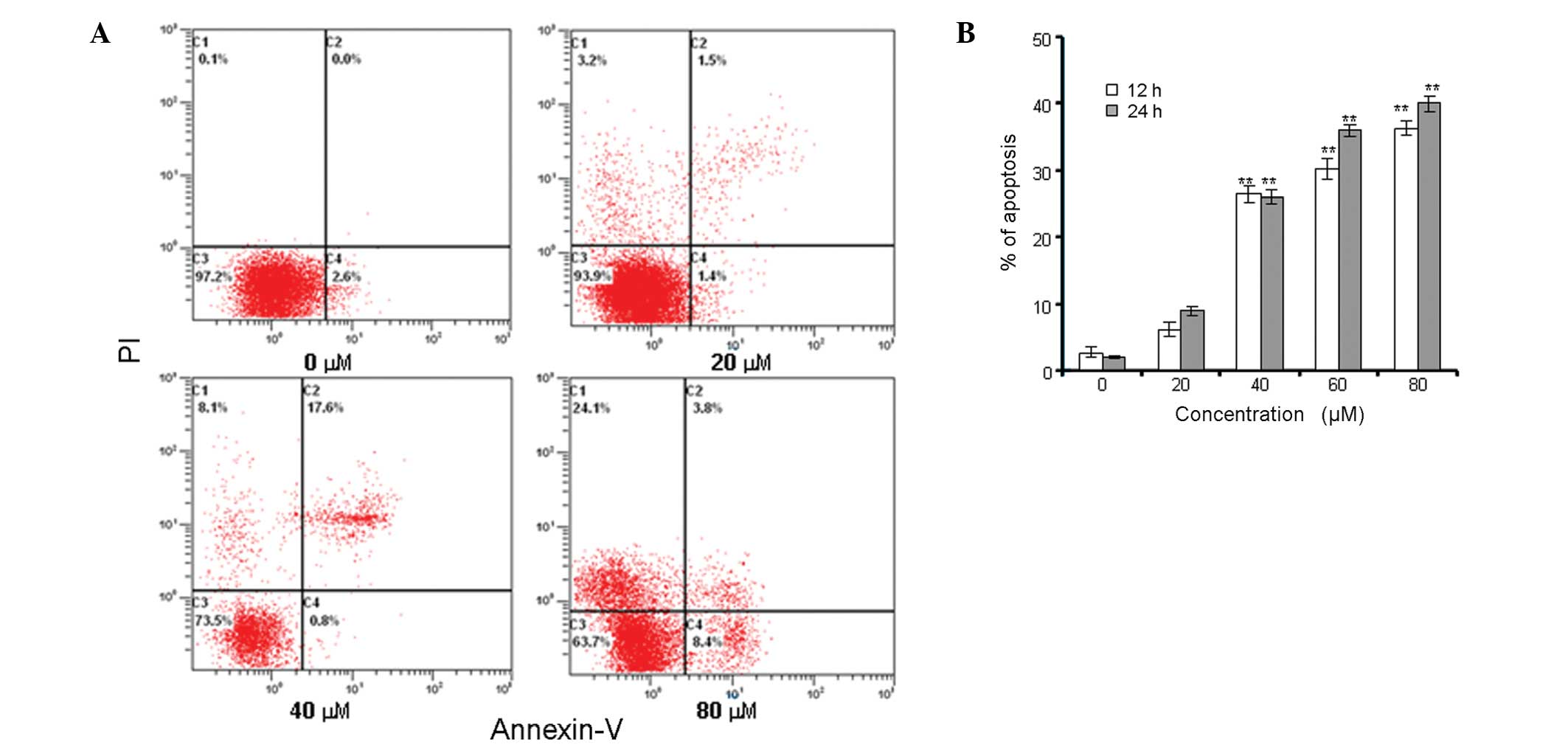
Annexin V/PI staining was used to analyze whether
the loss of cell viability induced by berberine was associated with
apoptosis. Fig. 2 shows the rate
of cell apoptosis detected by double-labeling flow cytometry with
Annexin V and PI. A concentration- and time-dependent increase was
observed in the apoptotic rate of MG-63 cells exposed to berberine.
The apoptotic rates of MG-63 cells treated with berberine at 20,
40, 60 and 80 μM increased to 6.1±1.1, 26.5±1.3, 30.2±2.8 and
36.3±1.0% following 12 h; 9.0±0.7, 26.1±1.5, 36.4±1.8 and 40.0±1.2%
following 24 h, respectively. By contrast, the control cells showed
apoptosis rates of only 2.8±0.8 and 2.0±0.2% following 12 and 24 h,
respectively (Fig. 2B).
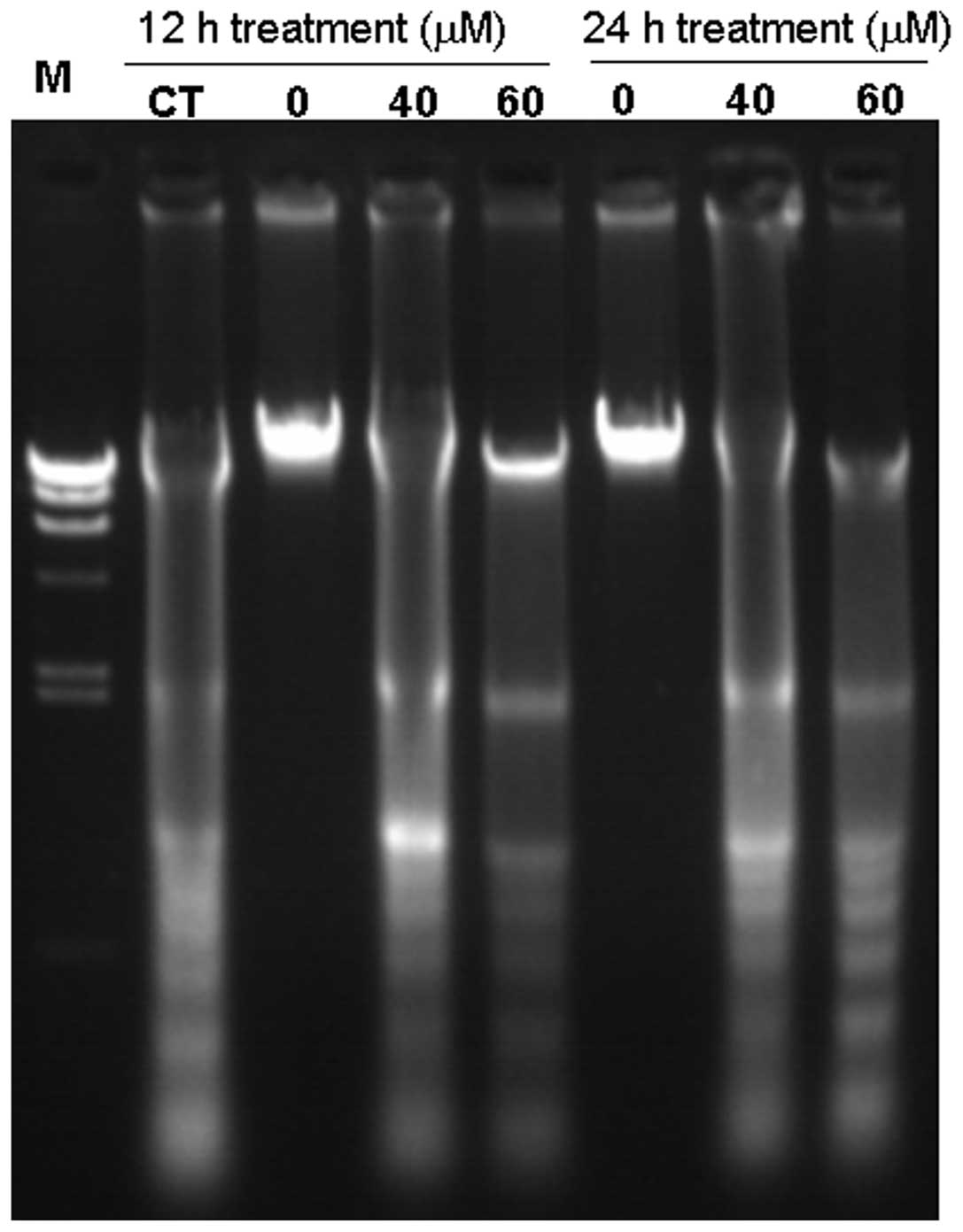
Furthermore, it was revealed that berberine induced significant DNA
fragmentation in MG-63 cells (Fig.
3). DNA fragmentation was observed in cells treated with
berberine at 40 and 60 μM following 12 and 24 h. These results
suggest that the anticancer activity of berberine involves the
induction of apoptosis.
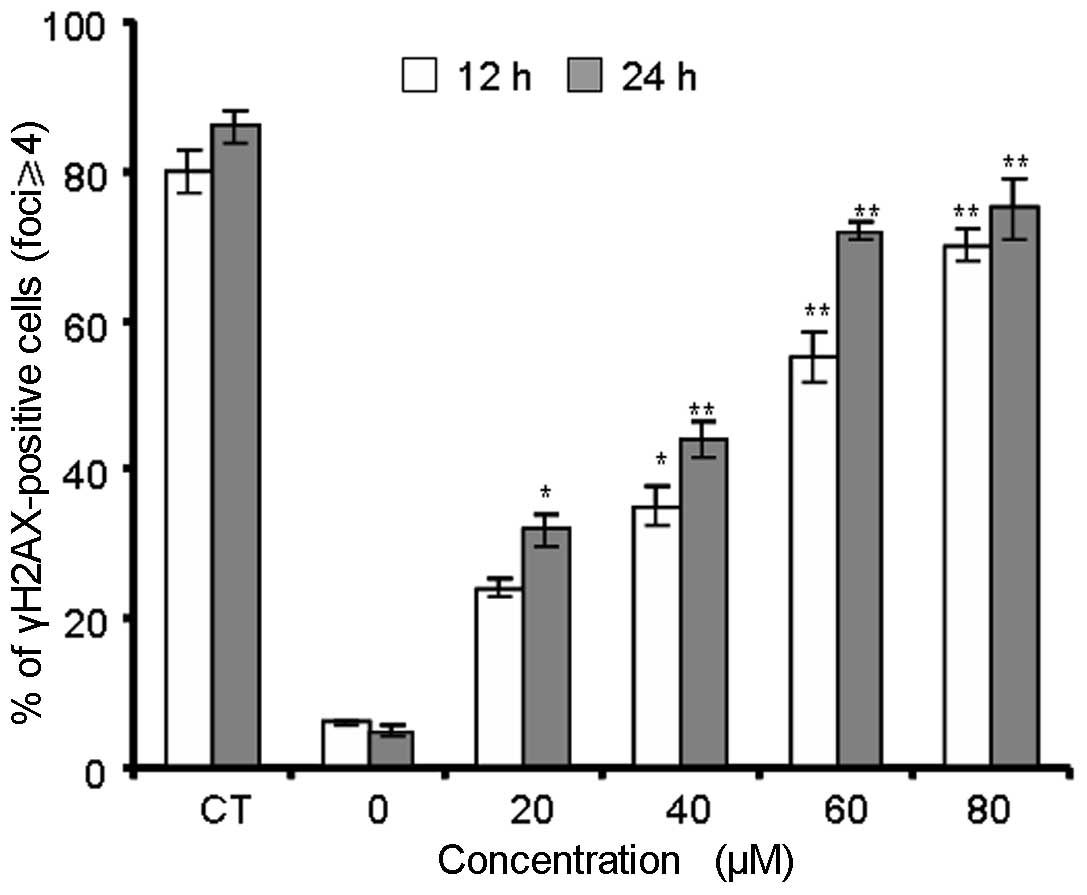
γH2AX foci show DNA double-strand breaks
are induced by berberine
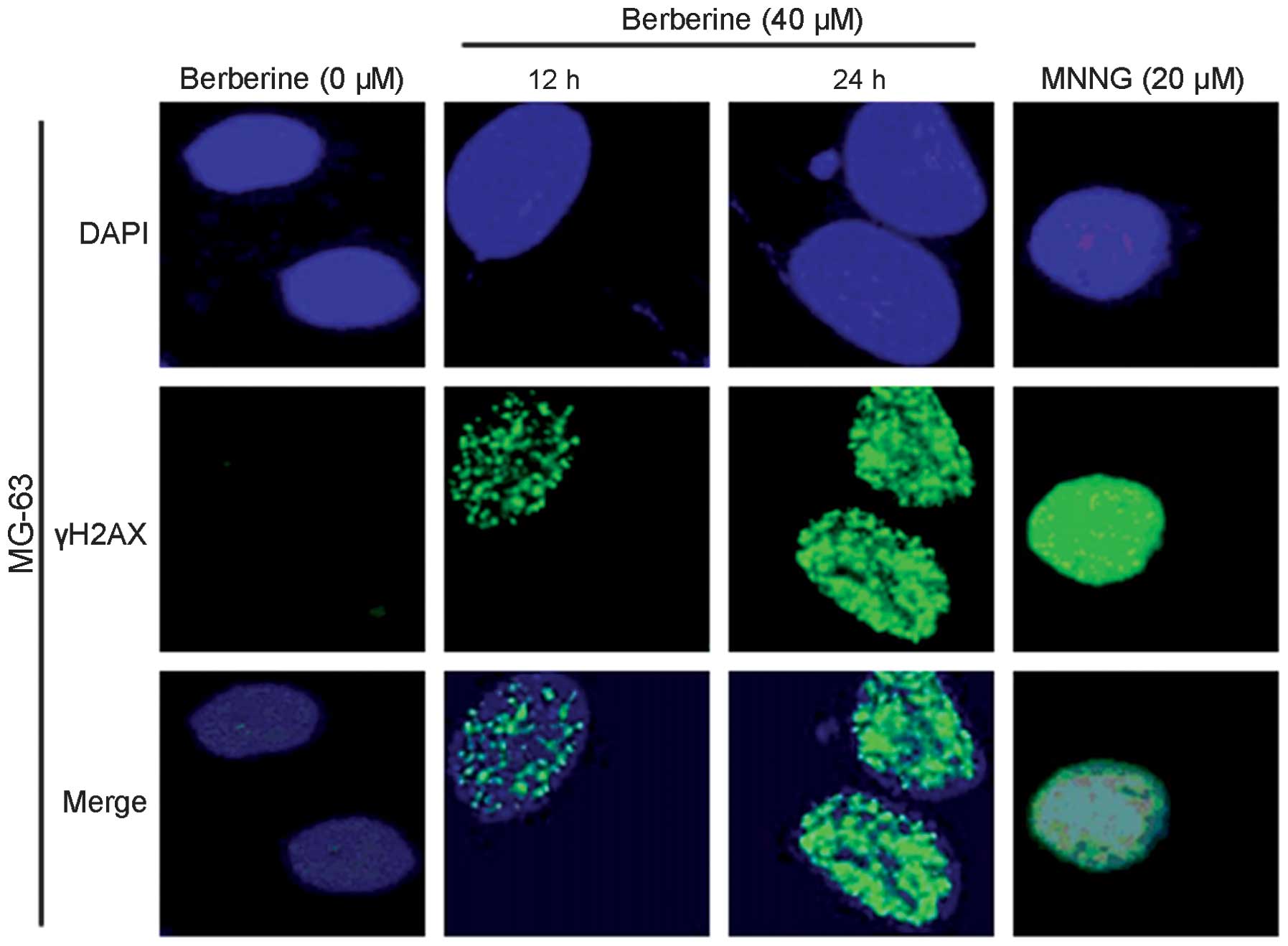
The immunofluorescent images of histone H2AX
phosphorylation in γH2AX-stained MG-63 cells are shown in Fig. 4. Treatment with berberine resulted
in time-dependent induction of γH2AX foci. In the control group,
MG-63 cells had few γH2AX foci in the nuclei, with only ~6% of
cells containing more than four foci (Fig. 5). Berberine and MNNG treatment
induced foci formation and increased the percentage of
γH2AX-positive cells. The data in Fig.
5 show that berberine and MNNG exhibited distinct
concentration- and time-dependent effects (P<0.01) on γH2AX foci
formation in MG-63 cells.
Discussion
Over the past decade, interest in the
pharmacological effects of bioactive compounds with respect to use
in cancer treatments and for cancer prevention has markedly
increased (19). Accumulating
evidence has demonstrated a correlation between natural compounds
and cancer prevention (5,6,9,20).
Thus, evaluation of ancient medicinal herbs may provide the basis
for the development of chemopreventive methods and strategies.
Berberine was used widely in ancient therapeutic medicinal
practices (3). It has been
demonstrated to exert numerous anticancer activities in various
types of cancer cells through different cytotoxic effects (21). Previous studies have demonstrated
that human osteosarcoma cells (U2OS, Saos-2 and HOS) treated with
berberine exhibited cell cycle arrest and apoptosis (4).
The present study found that berberine (20–80 μM)
inhibited growth of MG-63 cancer cells through induction of
apoptosis and DNA damage in vitro. Furthermore, berberine
was demonstrated to induce apoptosis and DNA damage of MG-63 cells
in a dose- and time-dependent manner. Therefore, berberine acts as
a potent genotoxin by inducing marked accumulation of DNA
double-strand breaks. The results indicated that treatment with
berberine triggered a cascade that includes DNA damage (Fig. 4).
Berberine induced double-strand DNA damage and
apoptosis in Ehrlich ascites carcinoma cells (20). Treatment with berberine in
vivo resulted in additive cytotoxicity and indicated that
berberine has potent antitumor activity against human and rat
malignant brain tumors (22). A
study involving Saccharomyces cerevisiae demonstrated that
berberine exhibited no cytotoxic, mutagenic or recombinogenic
activity in non-dividing cells. However, it had significant
cytotoxic and cytostatic effects on dividing cells (23). Notably, berberine is more toxic to
yeast mutants that are deficient in rad52-1, suggesting that
homologous recombination repair is required for the repair of
berberine-induced DNA damage. These results suggest that berberine
possesses recombinogenic activity. By contrast, coralyne, a close
derivative of berberine, has not been revealed to have detectable
mutagenic activity, when analyzed using the Ames test (24). Further studies are required to
evaluate the mutagenic activities of berberine.
Berberine has been widely prescribed for the
treatment of bacterial diarrhea and has potential applications in
several other diseases, including cancer. The findings of the
present study demonstrated that berberine causes DNA damage in
cultured cells, which raises concerns for its safety in clinical
use. However, the present study did not determine whether the
induction of apoptosis is directly associated with the genotoxicity
of berberine. The mechanisms underlying the genotoxicity of
berberine require further investigation to clearly demonstrate that
berberine causes genotoxocity and apoptosis. Furthermore, more
studies are required to understand the biological consequences of
DNA damage on exposure to berberine in vivo. Considering the
widespread clinical use of berberine, thorough evaluation of its
genotoxocity in vivo is warranted.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Craig WJ: Health-promoting properties of
common herbs. The Am J Clin Nutr. 70:491S–499S. 1999.PubMed/NCBI
|
|
3
|
Sun Y, Xun K, Wang Y and Chen X: A
systematic review of the anticancer properties of berberine, a
natural product from Chinese herbs. Anticancer Drugs. 20:757–769.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Liu Q, Xu B, et al: Berberine
induces p53-dependent cell cycle arrest and apoptosis of human
osteosarcoma cells by inflicting DNA damage. Mutat Res. 662:75–83.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine inhibits growth, induces G1 arrest and apoptosis in human
epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin
cascade, disruption of mitochondrial membrane potential and
cleavage of caspase 3 and PARP. Carcinogenesis. 27:2018–2027. 2006.
View Article : Google Scholar
|
|
6
|
Katiyar SK, Meeran SM, Katiyar N and
Akhtar S: p53 Cooperates berberine-induced growth inhibition and
apoptosis of non-small cell human lung cancer cells in vitro and
tumor xenograft growth in vivo. Mol Carcinog. 48:24–37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Letasiová S, Jantová S, Cipák L and
Múcková M: Berberine-antiproliferative activity in vitro and
induction of apoptosis/necrosis of the U937 and B16 cells. Cancer
Lett. 239:254–262. 2006.PubMed/NCBI
|
|
8
|
Meeran S and Katiyar S and Katiyar S:
Berberine-induced apoptosis in human prostate cancer cells is
initiated by reactive oxygen species generation. Toxicol Appl
Pharmacol. 229:33–43. 2008. View Article : Google Scholar
|
|
9
|
Hwang JM, Kuo HC, Tseng TH, Liu JY and Chu
CY: Berberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cells. Arch Toxicol. 80:62–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anis KV, Rajeshkumar NV and Kuttan R:
Inhibition of chemical carcinogenesis by berberine in rats and
mice. J Pharm Pharmacol. 53:763–768. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishino H, Kitagawa K, Fujiki H and
Iwashima A: Berberine sulfate inhibits tumor-promoting activity of
teleocidin in two-stage carcinogenesis on mouse skin. Oncology.
43:131–134. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng PL, Hsieh YS, Wang CJ, Hsu JL and
Chou FP: Inhibitory effect of berberine on the invasion of human
lung cancer cells via decreased productions of
urokinase-plasminogen activator and matrix metalloproteinase-2.
Toxicol Appl Pharmacol. 214:8–15. 2006. View Article : Google Scholar
|
|
13
|
Kim S, Choi JH, Kim JB, et al: Berberine
suppresses TNF-alpha-induced MMP-9 and cell invasion through
inhibition of AP-1 activity in MDA-MB-231 human breast cancer
cells. Molecules. 13:2975–2985. 2008. View Article : Google Scholar
|
|
14
|
Parry MC, Bhabra G, Sood A, et al:
Thresholds for indirect DNA damage across cellular barriers for
orthopaedic biomaterials. Biomaterials. 31:4477–4483. 2010.
View Article : Google Scholar
|
|
15
|
Sokolov MV, Smilenov LB, Hall EJ, Panyutin
IG, Bonner WM and Sedelnikova OA: Ionizing radiation induces DNA
double-strand breaks in bystander primary human fibroblasts.
Oncogene. 24:7257–7265. 2005. View Article : Google Scholar
|
|
16
|
Liu J, Yang F, Zhang Y and Li J: Studies
on the cell-immunosuppressive mechanism of Oridonin from Isodon
serra. Int Immunopharmacol. 7:945–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao YY, Shen X, Chao X, et al:
Ergosta-4,6,8(14), 22-tetraen-3-one induces G2/M cell cycle arrest
and apoptosis in human hepatocellular carcinoma HepG2 cells.
Biochim Biophys Acta. 1810.384–390. 2011.PubMed/NCBI
|
|
18
|
Guanggang X, Diqiu L, Jianzhong Y, et al:
Carbamate insecticide methomyl confers cytotoxicity through DNA
damage induction. Food Chem Toxicol. 53:352–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scott EN, Gescher AJ, Steward WP and Brown
K: Development of dietary phytochemical chemopreventive agents:
biomarkers and choice of dose for early clinical trials. Cancer
Prev Res (Phila). 2:525–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Letasiová S, Jantová S, Miko M, Ovádeková
R and Horváthová M: Effect of berberine on proliferation,
biosynthesis of macromolecules, cell cycle and induction of
intercalation with DNA, dsDNA damage and apoptosis in Ehrlich
ascites carcinoma cells. J Pharm Pharmacol. 58:263–270.
2006.PubMed/NCBI
|
|
21
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondria-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang RX, Dougherty DV and Rosenblum ML:
Laboratory studies of berberine used alone and in combination with
1,3-bis(2-chloroethyl)-1-nitrosourea to treat malignant brain
tumors. Chin Med J (Engl). 103:658–665. 1990.
|
|
23
|
Pasqual MS, Lauer CP, Moyna P and
Henriques JA: Genotoxicity of the isoquinoline alkaloid berberine
in prokaryotic and eukaryotic organisms. Mutat Res. 286:243–252.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng CC, Engle RR, Hodgson JR, et al:
Absence of mutagenicity of coralyne and related antileukemic
agents: structural comparison with the potent carcinogen
7,12-dimethylbenz[a]anthracene. J Pharm Sci. 66:1781–1783.
1977.PubMed/NCBI
|