Introduction
Diabetic cardiomyopathy (DCM) is a heart failure
disorder arising as a complication of diabetes mellitus; it is
described as a number of functional and structural changes in the
heart due to diabetes in the absence of other cardiac pathologies
(1). Since the prevalence of
diabetes increases worldwide, estimated to affect approximately 5%
of the global population by 2025 (2), the mortality due to DCM is also
likely to increase. In patients with diabetes, myocardial damage
involves both myocardial and cardiac interstitial cell damage. Most
research has concentrated on the damage to cardiac cells (3,4).
Myocardial fibrosis, excessive proliferation of cardiac fibroblasts
(CFs), and oversecretion and overexpression of collagen, are
considered the major pathological changes resulting from DCM. These
changes are associated with the risks of deterioration of the
cardiac function and cardiac decompensation (5,6).
The expression of numerous growth factors is higher
in patients with diabetes compared to healthy subjects. A newly
discovered adipocyte factor, visfatin, regulates blood sugar
levels, as well as insulin secretion (7). The plasma levels of visfatin are
associated with a wide range of diseases, including cardiovascular
diseases, endothelial dysfunction, insulin resistance, and the
occurrence and progression of diabetes. visfatin also plays a role
in vulnerable plaque formation and vascular proliferation and
inflammation, by exerting an insulin-like effect (8), facilitating adipocyte
differentiation, inhibiting cell apoptosis, and promoting cell
maturation and proliferation as a pro-inflammatory factor (9,10).
It has been suggested that visfatin is overexpressed in obese
patients and in patients with diabetes, causing myocardial
fibrosis, causing an increase in extracellular matrix synthesis, or
even leading to heart failure (11–13).
However, there are few studies on visfatin expression in cardiac
cells. To our knowledge, the high glucose-induced expression of
visfatin in CFs and visfatin synthesis in vitro have not
been reported to date.
Interstitial fibrosis is an important factor in the
occurrence and progression of DCM. Myocardial fibrosis in DCM
usually leads to ventricular diastole and contractile function
disorders, and ultimately to congestive heart failure (14). Myocardial fibrosis is tightly
linked to the high incidence and mortality associated with DCM.
Sufficient knowledge of the pathogenesis of DCM is the basis for
appropriate clinical treatment. Collagen is the major component of
the extracellular matrix, and the ratio of type I to type III
collagen indicates the severity of myocardial fibrosis. Increased
blood sugar levels induce the synthesis and secretion of myocardial
interstitial collagen. Type I collagen, which accounts for the
biggest proportion of total collagen, is more closely related to
myocardial stiffness than type III collagen. Patients with diabetes
are commonly at higher risk of myocardial fibrosis and greater
ventricular stiffness (15). A
study of the signal transduction pathways involved in myocardial
fibrosis is the basis for the identification of therapeutic targets
for DCM.
The Rho/Rho-associated protein kinase (ROCK)
signaling pathway has been shown to be an important pathway in
hypertension, cerebral apoplexy, and chronic heart failure
(16–18). The Rho/ROCK signaling pathway also
regulates contraction, adhesion, proliferation, and apoptosis of
cells. Dysfunctions of this pathway are predicted to lead to cell
function disorders and pathological changes. The Rho/ROCK signaling
pathway is implicated in chronic fibrosis of the liver and kidneys
(19,20). Previous studies showed that the
ROCK pathway is also activated in the cardiac cells of rats with
diabetes (21,22). The expression of the Ras homolog
gene family, member A (RhoA) protein was upregulated and its
activity was enhanced, resulting in increased polymerization of the
actin cytoskeleton. Rho kinase exists in the form of two isomers,
ROCK1 (or ROCKβ) and ROCK2 (or ROCKα), which are expressed in
vascular smooth muscle cells and cardiac cells. The ROCK2
mRNA is predominately detected in the brain and skeletal muscle. It
has been suggested that ROCK1 plays a key role in pathological
fibrosis of the organs, whereas ROCK2 is related to organ
hypertrophy (23,24). Other studies found that ROCK not
only increases the polymerization of the actin cytoskeleton, but
also induces the expression of fibrosis-related genes and proteins
(19,23,25,26).
Phrommintikul et al (23)
confirmed in the animal model of stress-induced cardiac hypertrophy
that the ROCK inhibitor reduces the synthesis of cardiac collagen,
and improves the diastolic function.
This study investigated visfatin and type I
procollagen expression, following high-glucose treatment at
different concentrations and for different durations, in CFs from
newborn rats, and examined the effects of these treatments on the
Rho/ROCK signaling pathway. Our results highlight a mechanism
involved in DCM that may be targeted in the context of new
therapeutic interventions.
Materials and methods
Animals
Clean-grade, male and female Sprague Dawley (SD)
rats, aged 2 to 3 days, were provided by the Experimental Animal
Center of the Hebei Medical University. The experiments were
carried out in accordance with the Hebei Animal Management
Regulations and all procedures and animal experiments were approved
by the Animal Ethics Committee of the Hebei Medical University. The
rats were housed before the experiment in room temperature and
humidity controlled cages with a 12-h day-night cycle. No narcotics
were used to avoid potential influences on the experimental
data.
Primary culture of CFs
The newborn SD rats were soaked with 70% alcohol for
10 sec, and then, sterile eye surgery scissors were used to cut the
anterior third of the ventricular portion of the heart, which was
placed on a Petri dish filled with 5 ml D-Hanks solution precooled
at 4°C. The ventricular muscles were cut into pieces of ~1–3 mm,
and then washed twice to remove blood cells. The non-adherent
myocardial cells were removed by enzymatic digestion and the
differential adhesion method as in (27). The tissue samples were digested
into single cell suspensions with 0.125% trypsin (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and 0.04% collagenase II
(Gibco Life Technologies, Carlsbad, CA, USA) at 37°C. The
supernatant was collected from each digestion, and an equal volume
of low-glucose Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) was added. After two
centrifugations at 1,200 × g for 5 min, the cells were resuspended,
filtered through a 200 mesh sieve, and seeded in 100 mm2
Petri dishes at 37°C, in a 5% CO2 incubator.
Non-adherent cardiomyocytes were removed using differential
adhesion after 90 min of culture. The cells were cultured in
low-glucose DMEM with 10% FBS at 37°C, in a 5% CO2
incubator. The cells from passages 2 and 3 were used in the
following experiments.
Immunocytochemical staining of CFs
The primary CFs were identified by
immunocytochemical staining (28)
for vimentin and α-smooth muscle actin (α-SMA). α-actin protein
levels were evaluated by immunocytochemical examination. Slides
were placed into 6-well culture plates and CFs at passages 2–3
(2×105 cells/well) were inoculated into each well.
Following attachment of CFs, slides were fixed in 4%
paraformaldehyde. Streptavidin-biotin complex immunocytochemical
staining for α-actin was performed. The α-actin content of the
cells cell was expressed as positive staining areas/the number of
positive cells from 10 random fields for each slide using the Motic
Med 6.0 digital medical image analysis system (Motic Incorporation,
Ltd., Causeway Bay, Hong Kong).
Study design
The extracted CFs were treated with high levels of
glucose (HG groups, 10, 30 and 50 mmol/l D-glucose; Sigma-Aldrich,
St. Louis, MO, USA), 5.5 mmol/l of D-glucose (normal glucose or NG
group), or 5.5 mmol/l D-glucose and 44.5 mmol/l mannitol (high
osmotic pressure or HOP group), and were further cultured for 48 h.
The concentration of 5.5 mmol/l D-glucose was used to simulate the
baseline sugar level of healthy human blood cells. The
concentration of 45.5 mmol/l mannitol (Sigma-Aldrich), combined
with 5.5 mmol/l D-glucose was used to establish equivalent osmosis
to that caused by 50 mmol/l D-glucose and avoid confusion with the
effects of osmosis on the expression of visfatin and type I
procollagen in the CFs. The CFs were also treated with 30 mmol/l
D-glucose for 6, 12, 24 and 48 h.
The CFs were cultured with 10 μmol/l of the ROCK
inhibitor Y27632, dissolved in 100% dimethyl sulfoxide (DMSO; both
from Santa Cruz Biotechnology, Inc.), or 0.1% DMSO for 30 min, and
then induced with 5.5 and 30 mmol/l D-glucose, respectively. They
were further cultured in DMEM containing 0.1% FBS for 24 h.
Determination of cardiac fibroblast
proliferation by the MTT assay
CFs at the logarithmic phase of growth were seeded
at a density of 5×103 cells/well (200 μl) in a 96-well
plate for 48 h. Blank wells were also prepared with culture medium
without cells. After culturing in serum-free medium for 24 h, the
cells were treated with different concentrations of D-glucose for
48 h, or 30 mmol/l D-glucose for 6, 12, 24 and 48 h, according to
the planned experiments. Next, the CFs were treated with 20 μl of 5
mg/ml HyClone™ 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Thermo Fisher Scientific, Logan, UT, USA) for 4 h.
DMSO (150 ml) was added to each well, and the plate was agitated
gently for 15 min. The absorbance was measured at 490 nm using the
Biochrom Anthos Zenyth 340rt microplate reader (Biochrom Ltd.,
Cambridge, UK).
RNA extraction and determination of
visfatin and type I procollagen mRNA expression by reverse
transcription-quantitative PCR (RT-qPCR)
The primers for the qPCR amplification of visfatin,
type I procollagen and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were designed by GeneCopoeia (catalog nos. RQP053337,
RQP054226 and RQP049537, respectively; Guangzhou, China). Total RNA
was extracted from CFs using the TRIzol reagent (SBS Genetech Co,
Ltd., Beijing, China), and the concentration and purity of the
extracted RNA were measured using a spectrophotometer (Beckman
Coulter, Miami, FL, USA). The integrity of RNA was assessed by
electrophoresis on 1% agarose gel. Total RNA was
reverse-transcribed into cDNA using oligo(dT) primers (Invitrogen
Life Technologies, Carlsbad, CA, USA). mRNA quantification was
performed by qPCR using the All-in-One™ qPCR mix (GeneCopoeia) in
an IQ5 Real-Time PCR system (Bio-Rad, Hercules, CA, USA), according
to the guidelines provided by the manufacturer. Forty cycles were
run with the following parameters: 95°C for 10 sec, 60°C for 20
sec, and 72°C for 15 sec. The dissociation curve was analyzed
immediately after qPCR. The mRNA expression level of the treated
group was calculated relative to that of the control group using
the ΔΔCT method (29), with the
2−ΔΔCt calculated based on the formula: ΔΔCt
= (Ct target gene−Ct GAPDH)treated
group − (Ct target gene−Ct
GAPDH)control group, where Ct denotes the
threshold cycle.
Determination of visfatin, type I
procollagen, and ROCK1 protein expression using western blot
analysis
The CFs were lysed with RIPA buffer (Promega,
Madison, WI, USA), and total protein was extracted by
centrifugation (4°C, 12,000 × g, 10 min). Proteins were quantified
using the Coomassie blue method according to the manufacturer’s
instructions (Life Technologies). Equal amounts of protein (80 μg)
were the loaded and separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis. The proteins were
electroblotted onto a polyvinylidene difluoride membrane (EMD
Millipore, Bedford, MA, USA). Next, the non-specific sites on each
blot were blocked with 5% non-fat milk diluted in TBS with 0.05%
Tween-20 (TBST) for 1 h. The membrane was then incubated at 4°C
overnight with the primary antibodies goat anti-rat anti-visfatin
and -procollagen type I (both from Santa Cruz Biotechnology, Inc.);
rabbit anti-rat anti-ROCK1 (Abbiotec, San Diego, CA, USA); and
rabbit anti-rat anti-GAPDH (Santa Cruz Biotechnology, Inc.). The
membranes were washed for 30 min with TBST at room temperature, and
then incubated with the secondary antibody (horseradish
peroxidase-labeled IgG) for 1 h. The chemiluminescence detection of
the antibody complex was carried out with the chemiluminescence kit
(Jei Daniel Biotech Corp., Shandong, China) and the Odyssey 9120
imaging system (LI-COR, Lincoln, NE, USA). The Gel-Pro Analyzer 3.1
(Media Cybernetics, Rockville, MD, USA) was used to measure the
absorbance. Protein expressions were normalized to that of
GAPDH.
Statistical analysis
All data were statistically analyzed using the SPSS
13.0 software (SPSS Inc., Chicago, IL, USA). The data were
expressed as means ± standard deviation (SD). Statistical
significance was evaluated by a one-way analysis of variance
(ANOVA) with Student-Newman-Keuls (SNK) test for post-hoc analysis.
The significance level was set at P<0.05.
Results
Identification of CFs
Immunocytochemical staining was positive for
vimentin (Fig. 1A), and negative
for α-SMA (Fig. 1B). The final
purity of CFs was ~95%, as indicated by the vimentin-positive
status of the cells.
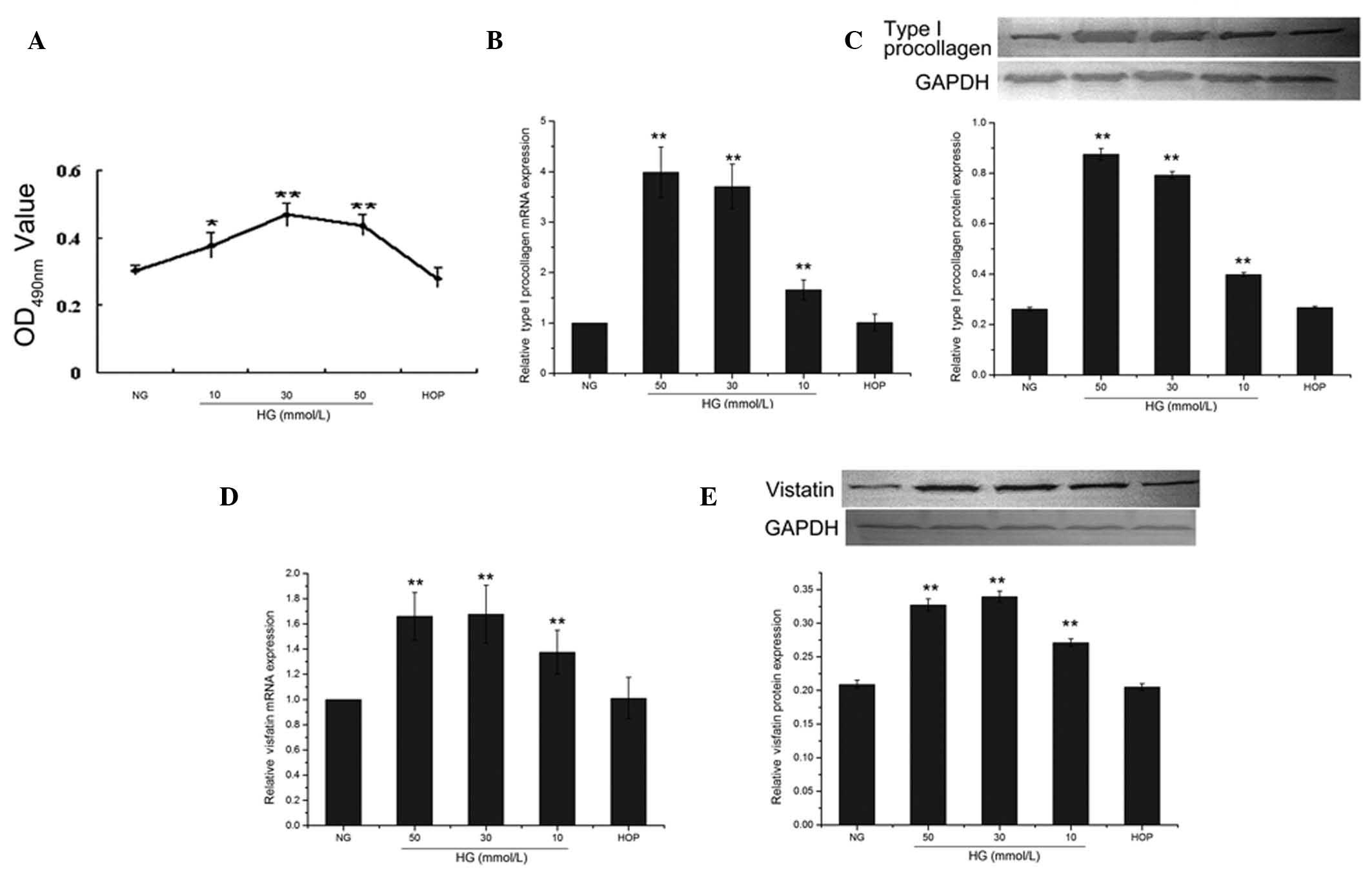
Effect of glucose concentration on the
proliferation of CFs
Cardiac fibroblast proliferation was reflected by
the absorbance value measured in the MTT assay. When the cells were
treated with different concentrations of D-glucose for 48 h, the
absorbance values of the 30 and 50 mmol/l D-glucose treatment
groups were significantly higher compared to the 5.5 mmol/l
D-glucose treatment group, serving as the control (both P<0.01).
The highest absorbance value was observed in the 30 mmol/l
D-glucose-treated group, while the absorbance value of the 50
mmol/l D-glucose-treated group was slightly lower; no significant
difference was identified between these two groups (P>0.05).
Compared to the control group, the absorbance value of the high
osmotic group was not significantly different (P>0.05) (Fig. 2A).
Effect of glucose concentration on mRNA
and protein expression of type I procollagen and visfatin in
CFs
When the cells were treated with different
concentrations of D-glucose, the mRNA and protein expression of
type I procollagen was increased in a dose-dependent manner
(Fig. 2B and C). The protein
expression of the 10, 30 and 50 mmol/l groups were significantly
different (0.398±0.008, 0.792±0.014, 0.875±0.023 and 0.261±0.007,
respectively, all P<0.01) from that of the control group (NG,
5.5 mmol/l). The expression of type I procollagen in the high
osmotic pressure group was not significantly different from that of
the control group (0.267±0.005, P>0.05) (Fig. 2C).
All high-glucose treatments (10, 30 and 50 mmol/l)
were accompanied by significantly increased (all P<0.01) mRNA
and protein expression of visfatin compared to the control group
(Fig. 2D and E). The 30 mmol/l
group reached the highest level of protein expression
(0.339±0.008), the 50 mmol/l group had a slightly lower level of
protein expression (0.271±0.0055), whereas the difference between
these two concentrations was not significant (P>0.05). The
protein expression of visfatin in the high osmotic pressure group
(0.205±0.0049) was not significantly different from that of the
control group (P>0.05) (Fig.
2E).
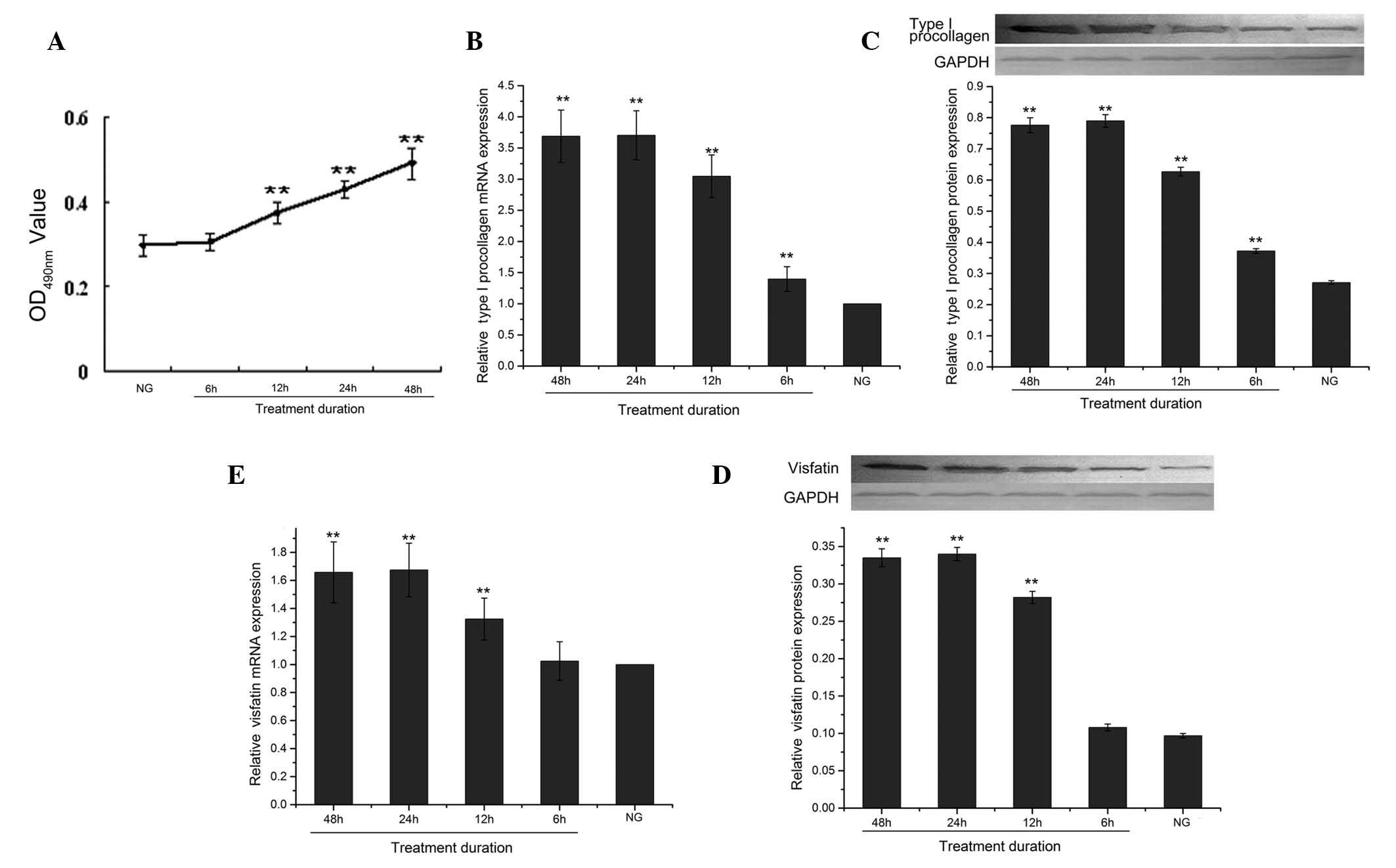
Effect of the duration of high-glucose
treatment on the proliferation of CFs
When the cells were treated with 30 mmol/l D-glucose
for different durations (6, 12, 24 and 48 h), the absorbance values
increased with treatment time (Fig.
3A).
Effect of high-glucose treatment duration
on mRNA and protein expression of type I procollagen and visfatin
in CFs
For all treatment durations (6, 12, 24 and 48 h)
with 30 mmol/l D-glucose, the mRNA and protein expression of type I
procollagen were significantly decreased (all P<0.01) compared
to the control group (Fig. 3B and
C). The protein expression of type I procollagen decreased in a
time-dependent manner until 24 h (3.70±0.39), while its expression
was even more decreased 48 h later (3.69±0.42); the difference
between 24 and 48 h was however not significant (P>0.05)
(Fig. 3C).
For all durations (6, 12. 24 and 48 h) of treatment
with 30 mmol/l D-glucose, the mRNA and protein expression of
visfatin were significantly decreased compared to the control
group, except for 6 h (Fig. 3D and
E). As shown in Fig. 3E, the
protein expression levels of the 12-, 24- and 48 h-treated groups
were significantly different from that of the control group
(0.282±0.008, 0.340±0.009, 0.335±0.001 and 0.097±0.003,
respectively, all P<0.01). The visfatin protein expression level
steadily decreased until 24 h and was further reduced after 48 h of
treatment, but the difference between these time-points was not
significant (P>0.05).
mRNA and protein expression levels of
type I procollagen and visfatin in the presence of a ROCK
inhibitor
Compared to the control group (5.5 mmol/l
D-glucose), the high-glucose (30 mmol/l)-treated group had a
significantly increased protein expression of ROCK1 (0.323±0.010
vs. 0.160±0.011, P<0.01). Pretreatment with 10 μmol/l Y27632
significantly reduced the ROCK1 protein expression compared to
high-glucose treatment (0.153±0.015 vs. 0.323±0.010, P<0.01),
but did not cause a significant change (P>0.05) compared to the
control group (Fig. 4E).
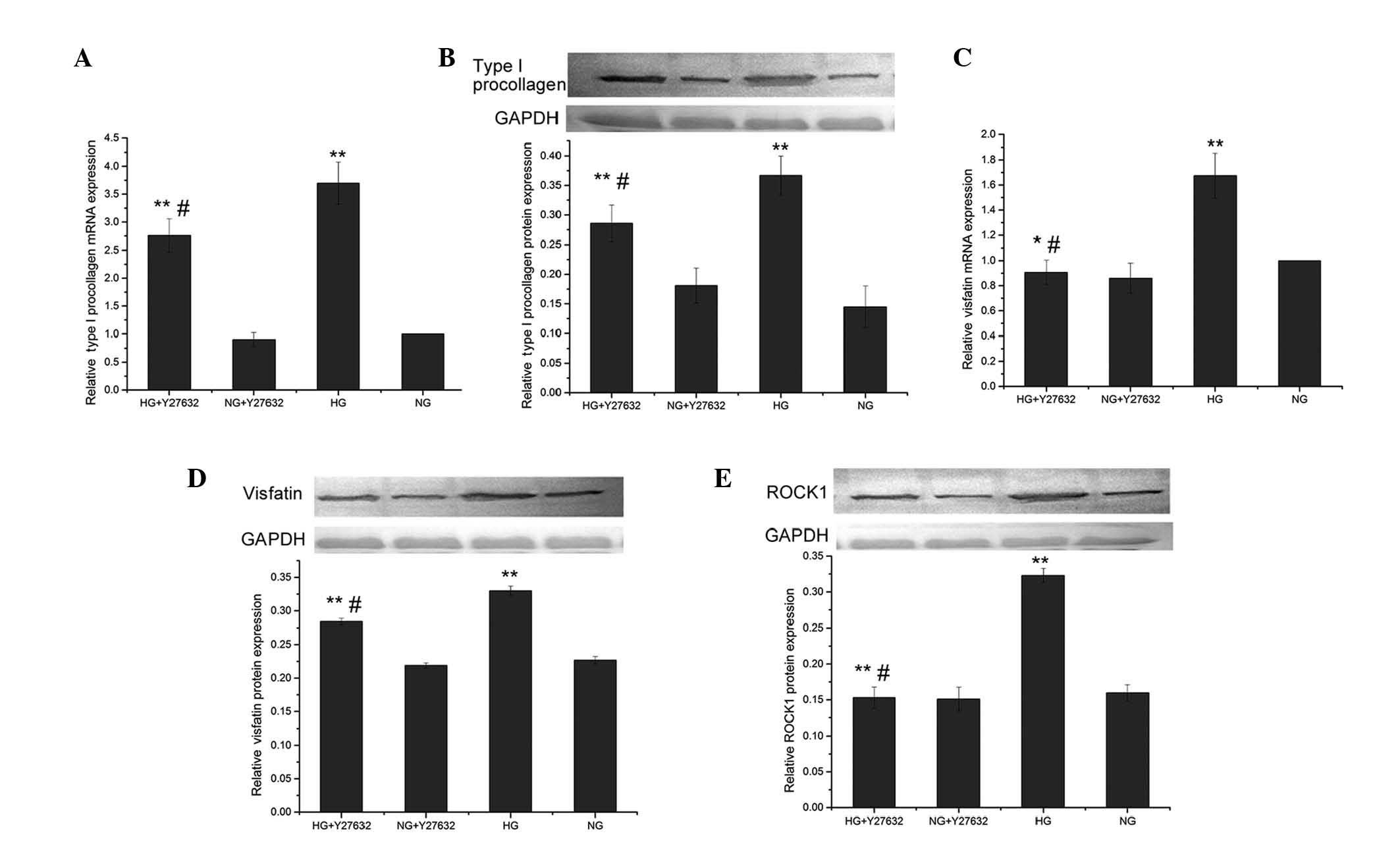 | Figure 4Effects of Y27632, a Rho-associated
kinase (ROCK) inhibitor, on the mRNA and protein expression of type
I procollagen, visfatin and ROCK1, induced by high glucose. Cardiac
fibroblasts (CFs) were cultured with 10 μmol/l Y27632 or 0.1%
dimethyl sulfoxide for 30 min, and then induced with 5.5 (normal
glucose; NG) and 30 mmol/l D-glucose (high glucose; HG) for 24 h,
respectively. (A) Type I procollagen mRNA and (B) protein
expression were determined by reverse transcription-quantitative
PCR and western blot analysis, respectively. Visfatin (C) mRNA and
(D) protein expression, and (E) ROCK1 protein expression.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control. The shown data represent means ± standard
deviation. *P<0.05 and **P<0.01, vs. NG
groups; #P<0.05 and ##P<0.01, HG +
Y27632 group vs. HG group. |
Pretreatment with Y27632 also significantly reduced
type I procollagen mRNA and protein expression induced by high
glucose (0.286±0.031 vs. 0.367±0.033 for the protein, P<0.05),
and the level of the protein in the HG+Y27632 group was
significantly higher compared to the NG group (0.145±0.035,
P<0.05) (Fig. 4A and B).
Pretreatment with Y27632 significantly reduced the
visfatin mRNA expression level induced by high-glucose treatment
(0.907±0.098 vs. 1.675±0.179, P<0.01), but the level of the HG +
Y27632 group did not significantly differ (P>0.05) from that of
the control group NG + Y27632 (Fig.
4C). Pretreatment with Y27632 also significantly reduced the
visfatin protein expression induced by high glucose (0.284±0.005
vs. 0.330±0.007, P<0.01), and the level of the HG + Y27632 group
was significantly different (P<0.05) from that of the NG +
Y27632 group (0.227±0.005) (Fig.
4D).
Discussion
The aim of this study was to enhance our
understanding on the pathological mechanism underlying DCM by
investigating whether high glucose affects the proliferation of CFs
and the expression levels of visfatin and type I procollagen in
CFs, and by further investigating whether these changes can be
inhibited by Y27632, a ROCK inhibitor. This study showed that high
glucose promotes cardiac fibroblast proliferation and induces
visfatin and type I procollagen expression in CFs, and Y27632
inhibited these effects.
Visfatin may be upregulated as a compensatory
mechanism to reduce high blood sugar levels. Previous studies
showed that visfatin promotes the proliferation of vascular smooth
muscle cells and endothelium (9,10)
and facilitates the synthesis of type I collagen (28,30).
There are relatively few studies on visfatin expression in cardiac
tissues and the pathological and physiological involvement of
visfatin in myocardial fibrosis in DCM remains a disputed
issue.
The CFs used in this study were extracted from
newborn rats. This was to avoid the interference of the in
vivo environment. qRT-PCR and western blot analysis showed that
the CFs of rats express visfatin. The high glucose concentration
affected the synthesis of visfatin and type I procollagen, which
may further cause insulin resistance and myocardial fibrosis in
patients with diabetes and DCM. Three different concentrations of
glucose (10, 30 and 50 mmol/l) were prepared for the high-glucose
treatmentand administered to the rats. The glucose concentration in
the control group was set at 5.5 mmol/l, which is the baseline
blood sugar level, while a high osmotic pressure control was also
set up to avoid the influence of high osmotic pressure on the
expression of visfatin and type I procollagen. The expression of
visfatin and type I procollagen in this group was not significantly
increased compared the control group. It should be noted that the
expression of visfatin and type I procollagen in the 10 and 30
mmol/l glucose treatment groups were significantly higher than
those in the control group. These results demonstrated that the
expression of visfatin and type I procollagen is significantly
different among HOP and NG groups, and was higher in the 10 and 30
mmol HG groups compared with NG groups, but not in the 50 mmol
group, while it decreased from 10 to 30 mmol HG, regardless of
osmotic pressure. We also found that the duration of the high
glucose treatment had an impact on the expression of visfatin and
type I procollagen. After CFs were treated with high glucose (30
mmol/l) for 6, 12 and 24 h, respectively, the expression of
visfatin and type I procollagen were all higher compared to the
control group. The peak occurred at 24 h, followed by a gradual
decline. The expression level at 48 h was slightly lower than that
at 24 h, not statistically significant. Within 24 h of treatment,
expression of visfatin and type I procollagen were increased
steadily as the glucose culture proceeded.
In order to develop a potential therapeutic target
for DCM, it is important to identify the relevant signaling
pathways. We hypothesized that the Rho/ROCK signaling pathway may
be involved in the increase in visfatin and type I procollagen
levels, since previous studies have shown a role for this pathway
in chronic fibrosis of the liver and kidney (19,25),
and that it is activated in the heart of diabetic rats (21,22).
The present study showed that high-glucose treatment increased the
expression levels of ROCK1, visfatin and type I procollagen in CFs
from newborn rats. Y27632, an inhibitor of the Rho/ROCK signaling
pathway, effectively inhibited the high glucose-induced expression
of ROCK1, visfatin and type I procollagen. This indicates that the
Rho/ROCK pathway is activated by high glucose, and this pathway,
once activated, may facilitate the synthesis of visfatin and type I
procollagen in CFs.
In conclusion, high-glucose treatment appears to
promotecardiac fibroblast proliferation and induce the expression
of visfatin, type I procollagen and ROCK1 in CFs. These findings
suggest that visfatin may be upregulated in order to compensate for
the glucose levels, that increased fibrosis may result from the
increase in type I procollagen levels, and that the above changes
may be mediated by the Rho/ROCK signalling pathway. Overall, these
results will contribute to the future development of therapeutic
agents for the treatment of DCM.
References
|
1
|
Chavali V, Tyagi SC and Mishra PK:
Predictors and prevention of diabetic cardiomyopathy. Diabetes
Metab Syndr Obes. 6:151–160. 2013.PubMed/NCBI
|
|
2
|
King H, Aubert RE and Herman WH: Global
burden of diabetes, 1995–2025: prevalence, numerical estimates, and
projections. Diabetes Care. 21:1414–1431. 1998.
|
|
3
|
Cai L and Kang YJ: Oxidative stress and
diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol.
1:181–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe K, Thandavarayan RA, Harima M,
Sari FR, Gurusamy N, Veeraveedu PT, Mito S, Arozal W, Sukumaran V,
Laksmanan AP, Soetikno V, Kodama M and Aizawa Y: Role of
differential signaling pathways and oxidative stress in diabetic
cardiomyopathy. Curr Cardiol Rev. 6:280–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Heerebeek L, Hamdani N, Handoko ML,
Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ,
Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden
J, Stienen GJ, Laarman GJ, Niessen HW and Paulus WJ: Diastolic
stiffness of the failing diabetic heart: importance of fibrosis,
advanced glycation end products, and myocyte resting tension.
Circulation. 117:43–51. 2008.
|
|
6
|
Mizushige K, Yao L, Noma T, Kiyomoto H, Yu
Y, Hosomi N, Ohmori K and Matsuo H: Alteration in left ventricular
diastolic filling and accumulation of myocardial collagen at
insulin-resistant prediabetic stage of a type II diabetic rat
model. Circulation. 101:899–907. 2000. View Article : Google Scholar
|
|
7
|
Tanaka T and Nabeshima Y:
Nampt/PBEF/Visfatin: a new player in beta cell physiology and in
metabolic diseases? Cell Metab. 6:341–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pfützner A and Forst T: Comment on: Haider
DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M (2006) the
release of the adipocytokine visfatin is regulated by glucose and
insulin. Diabetologia. 49:1909–1914
Diabetologia. 49:2795author reply 2796,
2006.
|
|
9
|
Adya R, Tan BK, Punn A, Chen J and Randeva
HS: Visfatin induces human endothelial VEGF and MMP-2/9 production
via MAPK and PI3K/Akt signalling pathways: novel insights into
visfatin-induced angiogenesis. Cardiovasc Res. 78:356–365. 2008.
View Article : Google Scholar
|
|
10
|
Kim SR, Bae SK, Choi KS, Park SY, Jun HO,
Lee JY, Jang HO, Yun I, Yoon KH, Kim YJ, Yoo MA, Kim KW and Bae MK:
Visfatin promotes angiogenesis by activation of extracellular
signal-regulated kinase 1/2. Biochem Biophys Res Commun.
357:150–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kannel WB, Hjortland M and Castelli WP:
Role of diabetes in congestive heart failure: the Framingham study.
Am J Cardiol. 34:29–34. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gottdiener JS, Arnold AM, Aurigemma GP,
Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE and Boineau
RC: Predictors of congestive heart failure in the elderly: the
Cardiovascular Health Study. J Am Coll Cardiol. 35:1628–1637. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nichols GA, Hillier TA, Erbey JR and Brown
JB: Congestive heart failure in type 2 diabetes: prevalence,
incidence, and risk factors. Diabetes Care. 24:1614–1619. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Falcão-Pires I, Hamdani N, Borbely A,
Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen
GJ, Niessen HW, Leite-Moreira AF and Paulus WJ: Diabetes mellitus
worsens diastolic left ventricular dysfunction in aortic stenosis
through altered myocardial structure and cardiomyocyte stiffness.
Circulation. 124:1151–1159. 2011.
|
|
15
|
Galderisi M: Diastolic dysfunction and
diabetic cardiomyopathy: evaluation by Doppler echocardiography. J
Am Coll Cardiol. 48:1548–1551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masumoto A, Hirooka Y, Shimokawa H,
Hironaga K, Setoguchi S and Takeshita A: Possible involvement of
Rho-kinase in the pathogenesis of hypertension in humans.
Hypertension. 38:1307–1310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shibuya M, Hirai S, Seto M, Satoh S and
Ohtomo E; Fasudil Ischemic Stroke Study Group. Effects of fasudil
in acute ischemic stroke: results of a prospective
placebo-controlled double-blind trial. J Neurol Sci. 238:31–39.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kishi T, Hirooka Y, Masumoto A, Ito K,
Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A and
Sunagawa K: Rho-kinase inhibitor improves increased vascular
resistance and impaired vasodilation of the forearm in patients
with heart failure. Circulation. 111:2741–2747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukushima M, Nakamuta M, Kohjima M, Kotoh
K, Enjoji M, Kobayashi N and Nawata H: Fasudil hydrochloride
hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen
production and enhances collagenase activity in hepatic stellate
cells. Liver Int. 25:829–838. 2005. View Article : Google Scholar
|
|
20
|
Rikitake Y, Oyama N, Wang CY, Noma K,
Satoh M, Kim HH and Liao JK: Decreased perivascular fibrosis but
not cardiac hypertrophy in ROCK1+/− haploinsufficient
mice. Circulation. 112:2959–2965. 2005.PubMed/NCBI
|
|
21
|
Lin G, Craig GP, Zhang L, Yuen VG, Allard
M, McNeill JH and MacLeod KM: Acute inhibition of Rho-kinase
improves cardiac contractile function in streptozotocin-diabetic
rats. Cardiovasc Res. 75:51–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soliman H, Craig GP, Nagareddy P, Yuen VG,
Lin G, Kumar U, McNeill JH and Macleod KM: Role of inducible nitric
oxide synthase in induction of RhoA expression in hearts from
diabetic rats. Cardiovasc Res. 79:322–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phrommintikul A, Tran L, Kompa A, Wang B,
Adrahtas A, Cantwell D, Kelly DJ and Krum H: Effects of a Rho
kinase inhibitor on pressure overload induced cardiac hypertrophy
and associated diastolic dysfunction. Am J Physiol Heart Circ
Physiol. 294:H1804–H1814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang YM, Bo J, Taffet GE, Chang J, Shi J,
Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ and Wei
L: Targeted deletion of ROCK1 protects the heart against pressure
overload by inhibiting reactive fibrosis. FASEB J. 20:916–925.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kolavennu V, Zeng L, Peng H, Wang Y and
Danesh FR: Targeting of RhoA/ROCK signaling ameliorates progression
of diabetic nephropathy independent of glucose control. Diabetes.
57:714–723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen XY, Dun JN, Miao QF and Zhang YJ:
Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses
5-hydroxytryptamine-induced pulmonary artery smooth muscle cell
proliferation via JNK and ERK1/2 pathway. Pharmacology. 83:67–79.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simpson P and Savion S: Differentiation of
rat myocytes in single cell cultures with and without proliferating
nonmyocardial cells. Cross-striations, ultrastructure, and
chronotropic response to isoproterenol. Circ Res. 50:101–116. 1982.
View Article : Google Scholar
|
|
28
|
Xie H, Tang SY, Luo XH, Huang J, Cui RR,
Yuan LQ, Zhou HD, Wu XP and Liao EY: Insulin-like effects of
visfatin on human osteoblasts. Calcif Tissue Int. 80:201–210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bubner B and Baldwin IT: Use of real-time
PCR for determining copy number and zygosity in transgenic plants.
Plant Cell Rep. 23:263–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song HK, Lee MH, Kim BK, Park YG, Ko GJ,
Kang YS, Han JY, Han SY, Han KH, Kim HK and Cha DR: Visfatin: a new
player in mesangial cell physiology and diabetic nephropathy. Am J
Physiol Renal Physiol. 295:F1485–F1494. 2008. View Article : Google Scholar : PubMed/NCBI
|