Introduction
Liver fibrosis is a serious health problem
worldwide, which induces portal hypertension, liver cirrhosis and
liver failure, and increases the risk of hepatocellular carcinoma
(1). The hepatic stellate cell
(HSC) is a key cell type contributing to liver fibrosis, and
transdifferentiates to become activated under conditions of liver
damage, including hepatitis viral infection, alcohol abuse,
nonalcoholic steatohepatitis or fatty liver disease (1). These activated HSCs secrete certain
profibrogenic cytokines, such as transforming growth factor-β
(TGF-β), and promote the development of liver fibrosis (2). Therefore, inhibition of the
activation of HSCs is hypothesized to be an effective strategy to
prevent the development of liver fibrosis.
Cell senescence involves normal cells losing the
ability to proliferate, despite the presence of sufficient space,
nutrients and growth factors in the medium (3). Senescent HSCs have been demonstrated
to exhibit a gene expression profile consistent with cell cycle
exit, reduced secretion of extracellular matrix (ECM) components,
enhanced secretion of ECM-degrading enzymes and enhanced immune
surveillance (4), which indicate
its value in protecting against liver fibrosis and cancer.
OSU-03012, a derivative of celecoxib lacking
cyclooxygenase-2 inhibitory activity, is able to inhibit the
activity of phosphomositide-dependent kinase-1 (PDK1) and induce
cell death in various types of cancer cell, including
hepatocellular carcinoma (5),
primary chronic lymphocytic leukemia (6), glioblastoma (7,8),
breast cancer (9) and pancreatic
cancer (10) cell lines. In
addition to the inhibition of PDK1/protein kinase B (AKT)
signaling, previous studies have suggested that OSU-03012 may be a
multitargeted inhibitor in a cell type-dependent manner (6,7,11).
However, the effect of this compound in hepatic fibrosis remains to
be fully elucidated. Thus, in the current study, the ability of
OSU-03012 to inhibit activated HSCs was investigated in order to
verify its potential as a drug against liver fibrosis. Mechanisms
underlying this inhibitory effect were also investigated.
Materials and methods
Cell lines and reagents
The LX2 human HSC cell line originated from the
American Type Culture Collection (Manassas, VA, USA) was purchased
from Shanghai Fuxiang Biotechnology Co., Ltd. (Shanghai, China). It
was cultured in high-glucose Dulbecco’s modified Eagle’s medium
(DMEM-h) supplemented with 10% fetal bovine serum (FBS) (Invitrogen
Life Technologies, Carlsbad, CA, USA) and maintained in a 5%
CO2 atmosphere at 37°C. OSU-03012 was purchased from
Selleck Chemicals (Houston, TX, USA) and was dissolved in dimethyl
sulfoxide (DMSO; Sangon Biotech, Co., Ltd., Shanghai, China) at a
concentration of 50 μM as stock solution for further use. The
rabbit anti-human antibodies against P15INK4B (#4822; polyclonal),
p16INK4A (#4824; polyclonal), p21Cip1 (#9932; monoclonal), P27Kip1
(#9932; monoclonal), AKT (#9272; polyclonal), p-AKT (activated AKT;
#9275; polyclonal) and β-actin (#4967; polyclonal) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA; 1:1,000
dilution) and diluted with Primary Antibody Dilution Buffer
(Beyotime Institute of Biotechnology).
Cell proliferation assay
The cell counting kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology, Shanghai, China) was used to evaluate
cell proliferation. LX2 cells were seeded in 96-well plates at a
density of 2×103 cells/well in DMEM-h supplemented with
10% FBS. Following a resting period of 24 h, cells were washed with
phosphate-buffered saline (PBS; Medicago AB, Uppsala, Sweden) and
exposed to either DMSO alone or a series of dilutions of OSU-03012
in DMSO (1, 5 or 15 μM). Subsequent to incubation for another 24,
48 or 72 h, the medium was replaced with fresh DMEM-h. A total of
10 μl CCK-8 was added to each well and the cells were incubated in
the presence of CCK-8 for 3 h. The absorbencies were determined
with a DTX 800 Multimode Detector (Beckman Coulter, Brea, CA, USA)
at a wavelength of 450 nm. The absorbance values were normalized by
subtracting blank values obtained from untreated cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following a resting period of 0, 24, 48 or 72 h
subsequent to the addition of 1 μM OSU-03012, the total RNA in the
LX2 cells was extracted using TRIzol reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions. The RNA
concentrations were assessed by spectrophotometry at a wavelength
of 260 nm using a Nano-100 spectrophotometer (Hangzhou Allsheng
Instruments Co., Ltd., Hangzhou, China). RNA was
reverse-transcribed using the PrimeScript RT-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China) and qPCR was performed
using the 7500 Real-Time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA). The PCR primer sequences used
were as follows: Type I collagen, F 5′-TGA CGA GAC CAA GAA CTG-3′
and R 5′-CCA TCC AAA CCA CTG AAA-3′; α-smooth muscle actin (α-SMA),
F 5′-TTC GTT ACT ACT GCT GAG CGT GAG A-3′ and R 5′-AAA GAT GGC TGG
AAG AGG GTC-3′; internal reference gene GAPDH, F 5′-ACC ACA GTC CAT
GCC ATC AC-3′ and R 5′-TCC ACC ACC CTG TTG CTG T-3′. The PCR
cycling conditions were as follows: 95°C for 30 sec, 40 cycles of
95°C for 5 sec and 60°C for 34 sec.
Apoptosis detection
Approximately 5×105 LX2 cells were seeded
onto 10-cm dishes and incubated at 37°C overnight. The cells were
treated with either DMSO alone or 1 μM OSU-03012 in DMSO for 48 or
72 h, and then suspended by treatment with trypsin 0.25%-EDTA
(Biosera, Boussens, France) and fixed with 70% ice-cold ethanol
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) overnight
at 4°C. Subsequent to washing the cells with PBS and resuspending
in the binding buffer (Beyotime Institute of Biotechnology)
containing Annexin V-fluorescein isothiocyanate and propidium
iodide (PI) according to the manufacturer’s instructions, apoptosis
was assessed by flow cytometry using a FACScan cytometer (Beckman,
Pasadena, CA, USA).
Senescence-associated β-galactosidase
(SA-β-Gal) staining
SA-β-Gal staining was performed using the
Senescence-Associated β-Galactosidase Staining kit (Beyotime
Institute of Biotechnology). Approximately 3×105 LX2
cells were seeded onto 6-cm dishes and incubated at 37°C overnight.
The cells were treated with 1 μM OSU-03012 in DMSO or DMSO alone
for 48 h or 72 h. The cells were then washed three times with PBS
and fixed with 4% paraformaldehyde (Beyotime Institute of
Biotechnology) for 15 min at room temperature. Next, the cells were
incubated overnight at 37°C in darkness with the working solution
containing 0.05 mg/ml X-gal (Beyotime Institute of Biotechnology)
and viewed under an optical microscope (TE2000-S; Nikon Corp.,
Tokyo, Japan).
Cell cycle analysis
Approximately 5×105 LX2 cells were seeded
onto 10-cm dishes and incubated at 37°C overnight. The cells were
treated with 1 μM OSU-03012 in DMSO or DMSO alone for 48 h or 72 h
and then suspended by treatment with trypsin and fixed with 70%
ice-cold ethanol overnight at 4°C. The cells were then stained with
PI in the presence of RNase A (Beyotime Institute of
Biotechnology). The DNA content was analyzed by flow cytometry
(FACScan cytometer; Beckman) and data were analyzed using Modfit LT
software version 3.2 (Verity Software House, Inc., Topsham, ME,
USA).
Western blot analysis
Following the above treatments, the LX2 cells were
lysed in protein extraction buffer (Beyotime Institute of
Biotechnology) followed by incubation at 95°C for 5 min. Samples
were separated using an SDS-PAGE system (MINI-P TET SYS/PPAC BASIC;
Bio-Rad Laboratories, Inc., Hercules, CA, USA), transferred to
polyvinylidene fluoride membranes (Bio-Rad Laboratories), blocked
with 5% nonfat milk in Tris-buffered saline/Tween-20 (Shanghai
BioScience Co., Ltd., Shanghai, China) for 1 h and probed with the
antibodies at 4°C overnight. The membranes were then incubated with
corresponding horseradish peroxidase (HRP)-conjugated polyclonal
goat anti-rabbit secondary antibody (#HA1001-100; 1:2000; Hangzhou
Huaan Biotechnology Co., Ltd., Hangzhou, China) for 1 h at room
temperature. The immunoblots were visualized using Immobilon
Western Chemiluminescent HRP Substrate (EMD Millipore, Billerica,
MA, USA).
Statistical analysis
All data were collected from a minimum of three
independent replicates. Analysis was performed using SPSS software,
version 11.0 (SPSS, Inc., Chicago, IL, USA). Comparisons between
groups were performed using Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
OSU-03012 inhibits the growth and
activation of LX2 cells
To examine whether OSU-03012 has an inhibitory
effect on LX2 cell proliferation, LX2 cells were treated with a
range of doses (0–15 μM) of OSU-03012 and assessed for viability
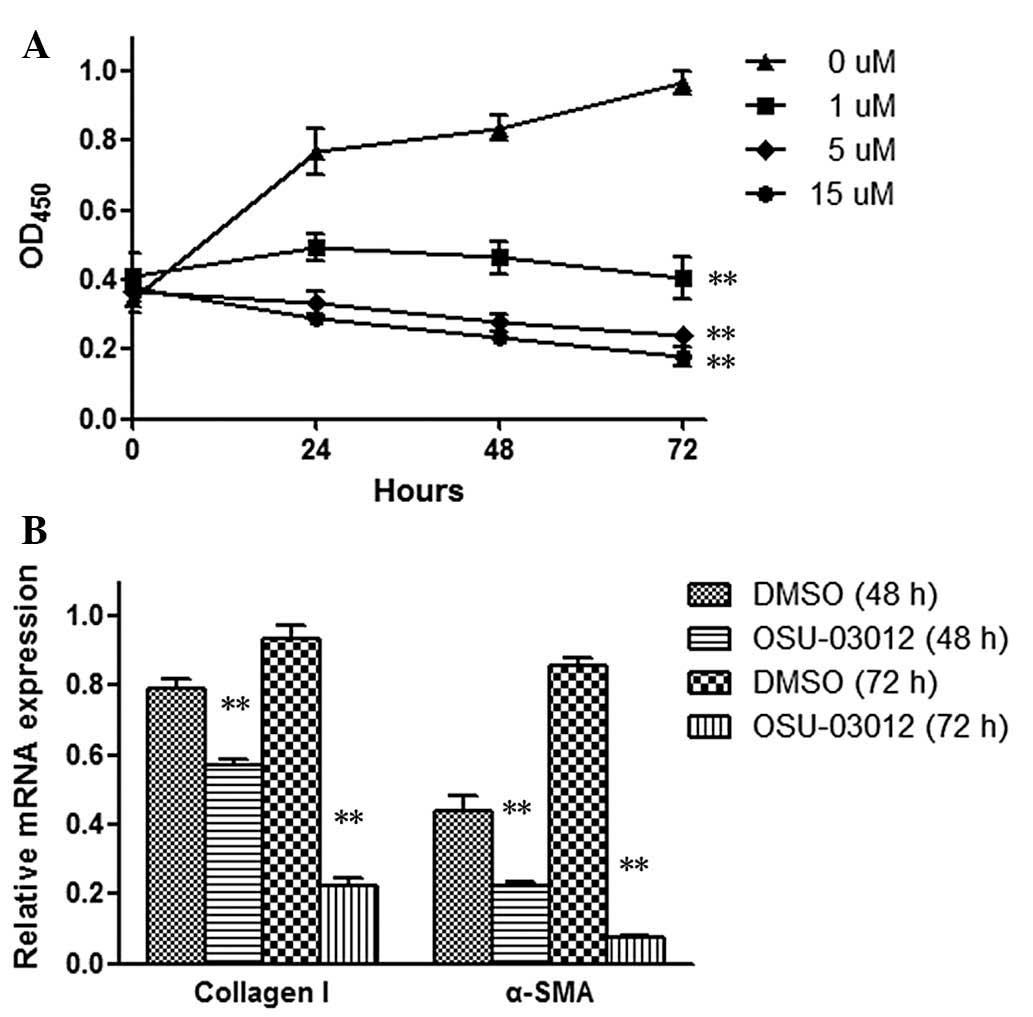
using the CCK-8 assay. As demonstrated in Fig. 1A, the growth of cells was
suppressed by OSU-03012 in a dose-dependent manner, indicating that
OSU-03012 may inhibit LX2 cell proliferation.
Activated HSCs express type I collagen and α-SMA,
which primarily contribute to liver fibrogenesis. To investigate
the effect of OSU-03012 on the activation of HSCs, the mRNA levels
of the hepatic profibrotic factors type I collagen and α-SMA were
analyzed using RT-qPCR. Following 48 or 72 h of exposure, a
reduction in the mRNA levels of type I collagen and α-SMA was
observed in OSU-03012 cells, as presented in Fig. 1B. These results demonstrate the
inhibitory effect of OSU-03012 on the activation of HSCs,
indicating its potential function against hepatic fibrosis.
OSU-03012 induces senescence, not
apoptosis, in LX2 cells
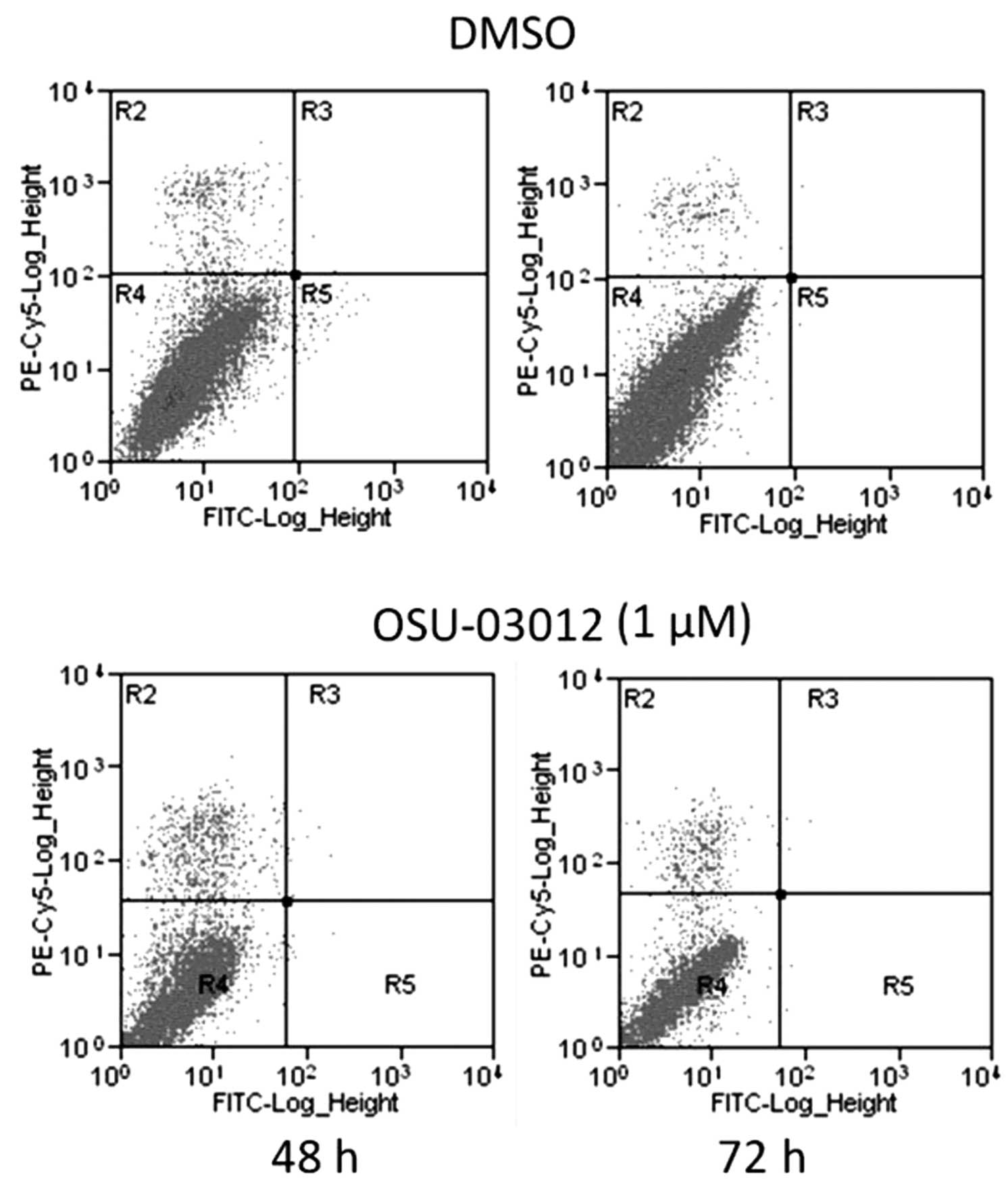
It was reported that the inhibitory effect of
OSU-03012 on cell proliferation was mediated via the induction of
cell apoptosis in certain cancer cells (6–11).
To verify this hypothesis, the ability of OSU-03012 to induce
apoptosis in LX2 cells was investigated by flow cytometric
analysis. As demonstrated in Fig.
2, apoptosis was not induced in LX2 cells treated with
OSU-03012, compared with those treated with DMSO. These results
indicated that OSU-03012 did not inhibit the growth of LX2 cells
via the induction of apoptosis.
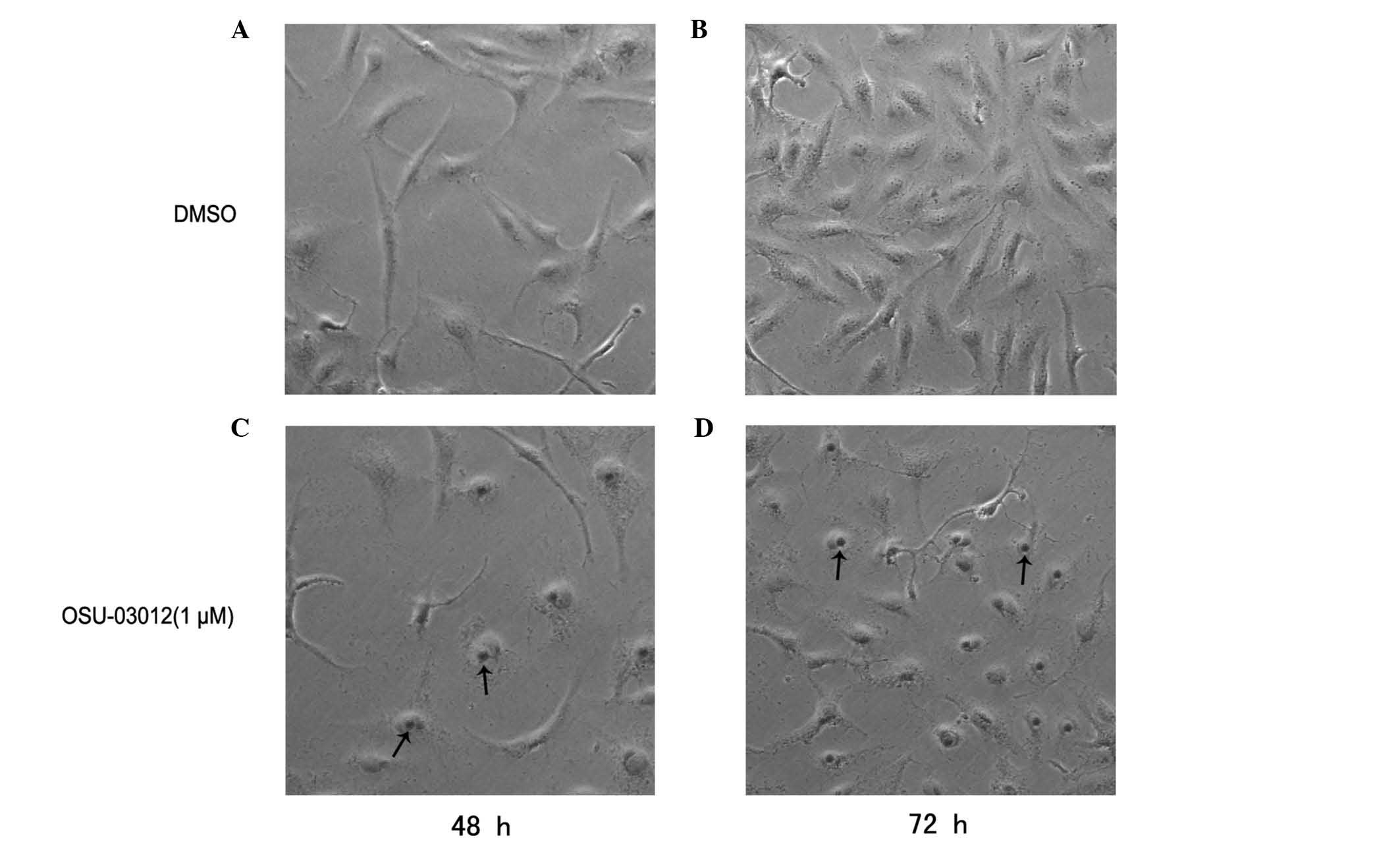
Cell senescence involves normal cells losing the
ability to proliferate, thus it was hypothesized that the
inhibition of LX2 cells by OSU-03012 is mediated by senescence. LX2
cells were exposed to OSU-03012 and assessed for senescence using
the SA-β-Gal assay 48 h later. The cells treated with OSU-03012
displayed a large increase in SA-β-Gal activity (P<0.02;
Fig. 3) compared with the control
(DMSO). Senescence-associated morphological alterations were
observed in the OSU-03012-treated group, with the cells frequently
becoming flat with pyknosis of the nucleus. These results
demonstrate that OSU-03012 may induce senescence in LX2 cells.
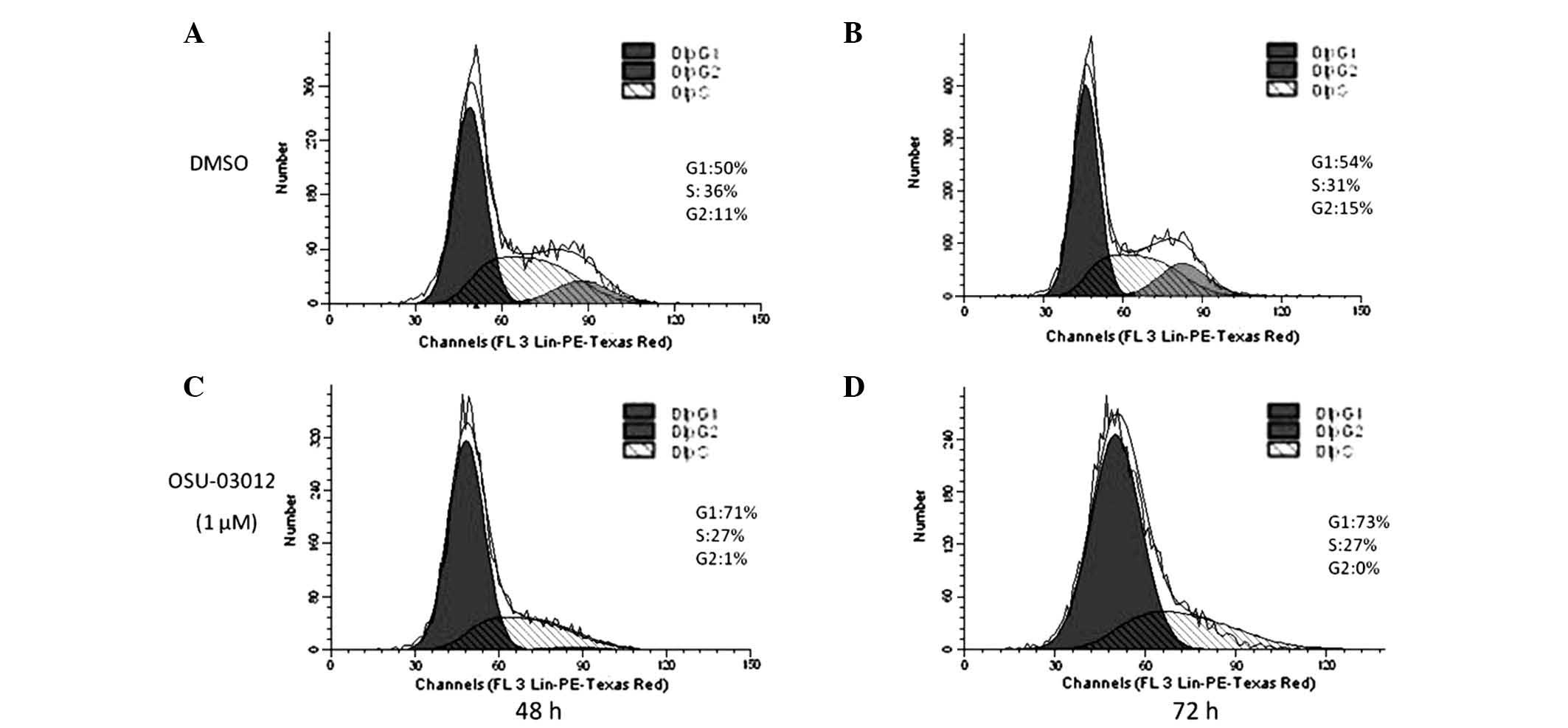
OSU-03012 induces G1 phase
arrest in LX2 cells
Cell senescence is a stable form of cell cycle
arrest in mitotic cells, usually at the G1 phase.
However, certain cells become senescent at the G2 or S
phase (3). Thus, the influence of
OSU-03012 on the cell cycle of LX2 cells was investigated. The
cells treated with OSU-03012 for 48 or 72 h were stained with PI
and then subjected to flow cytometry. As demonstrated in Fig. 4, LX2 cells treated with OSU-03012
exhibited a significant ~20× increase in G1-arrested
cells compared with those treated with DMSO (P<0.05). These
results suggest that OSU-03012 may induce G1 phase
arrest in HSCs.
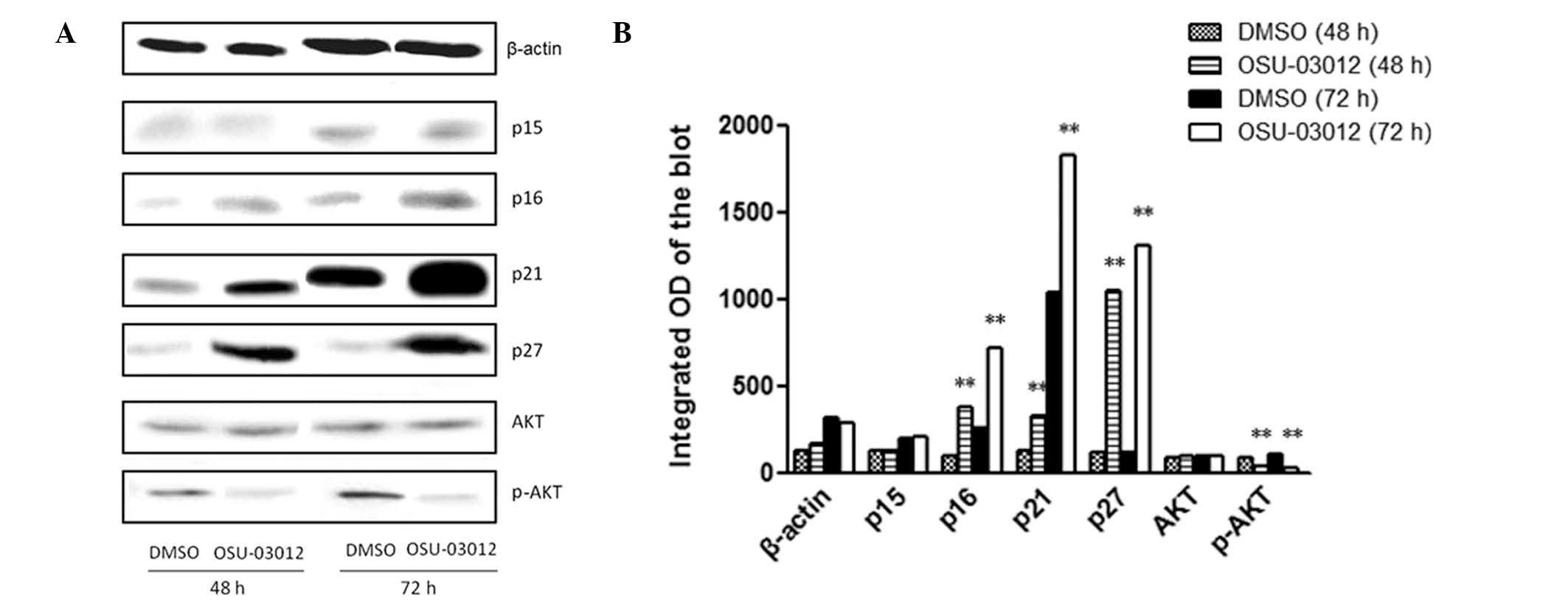
OSU-03012 induces senescence in LX2 cells
via the upregulation of p16, p21 and p27
There are several pathways known to be associated
with cell senescence. P53 promotes senescence by inhibiting
cyclin-dependent kinases 2 and 4 by transactivating p21 or p16
(INK4a) (3,12). In addition, p15 and p27 have been
identified to be associated with cell senescence (13,14).
Therefore, to elucidate the mechanism of OSU-03012 in the induction
of senescence in LX2 cells, total proteins were extracted from
cells treated with 1 μM OSU-03012 or DMSO, for 48 or 72 h and
analyzed by western blot analysis with the antibodies against p15,
p16, p21 and p27. As demonstrated in Fig. 5, at 48 and 72 h, OSU-03012
significantly increased the protein levels of p16, p21 and p27,
compared with DMSO-treated cells (P<0.01). No significant
difference was observed in the levels of p15 between OSU-03012- and
DMSO-treated cells at 48 or 72 h.
Furthermore, the PDK1/AKT signaling pathway, an
important senescence regulator, has been suggested to be involved
in the inhibition of proliferation in certain cells by OSU-03012
(15,16), but its role in LX2 cells remains
unclear. To investigate whether OSU-03012 acts via the inhibition
of the PDK1/AKT signaling pathway in LX2 cells, the levels of AKT
and p-AKT were also measured in the western blot analysis. As
demonstrated in Fig. 5, the level
of p-AKT was reduced following treatment with OSU-03012
(P<0.01). These results suggest that OSU-03012 induced
senescence of LX2 cells via p16, p21, p27 and the PDK1/AKT
signaling pathway.
Discussion
Liver fibrosis is a common developmental stage in
the majority of chronic liver diseases and may result in liver
cirrhosis, failure and in severe cases, hepatocellular carcinoma.
At present, there are no effective antifibrotic agents on the
market, however a previous study hypothesized that targeting the
senescence of activated HSCs may be a novel strategy to inhibit the
development of liver fibrosis (4).
In the current study OSU-03012, a novel celecoxib derivative, was
demonstrated to induce cell senescence in activated HSCs and thus,
inhibit cell proliferation and prevent the secretion of profibrotic
factors. The results indicated OSU-03012 as a potential agent
against liver fibrosis.
HSCs become activated following injury and are
crucial in the pathogenesis of liver fibrosis via the excessive
accumulation of α-SMA and ECM proteins, including type I, III and
IV collagen. The rates of synthesis and degradation of the ECM
determines the severity of liver fibrosis. Type I collagen
constitutes a high proportion of the ECM in hepatic fibrosis, while
α-SMA is also commonly known as a marker of fibrosis. In the
current study, the mRNA levels of type I collagen and α-SMA were
observed to be significantly reduced following treatment with
OSU-03012, indicating the role of OSU-03012 in the inactivation of
HSCs and reduction of ECM.
Apoptosis is a process of programmed cell death, in
which the chromatin condenses, DNA becomes fragmented and is
phagocytosed by the macrophages without inflammation. It was
reported that OSU-03012 may induce the apoptosis of non-small cell
lung cancer (14) and breast
cancer (17,5) cells. However, a previous study
observed that OSU-03012 was not able to induce apoptosis in liver
cancer cells (5), which is in
agreement with the observations in the present study in LX2 cells.
It is hypothesized that the different effects of OSU-03012 on
apoptosis are due to the varied cell types used. The underlying
molecular mechanism remains to be fully elucidated.
Cell senescence may induce irreversible arrest of
cell growth, which may be a novel strategy for inhibiting cell
proliferation. The hallmark of cell senescence is an inability to
progress through the cell cycle, which cannot be activated by any
known physiological stimulation (3). The results of the current study
demonstrate that OSU-03012 induced cell cycle arrest at the
G1 phase, which suppressed cell proliferation. Further
investigation to fully elucidate the effects of OSU-03012 treatment
on the cell cycle are necessary.
Additionally, the current study demonstrated that
OSU-03012 induced senescence in LX2 cells via the activation of
p16, p21 and p27. Previous studies have identified that OSU-03012
inhibits the PDK1/AKT signaling pathway (18) and that p-AKT (activated AKT) is one
of the important pathway molecules responsible for regulating cell
senescence (19). AKT is able to
downregulate p27 via the phosphorylation and inhibition of the FOXO
transcription factors (20) and
downregulate p21 via the activation of MDM2 (5,21).
Therefore, it was hypothesized in the present study that the
upregulation of p21 and p27 by OSU-03012 was via the inhibition of
the PDK1/AKT signaling pathway, which thus induced cell senescence.
The current study demonstrated the complexity of the underlying
mechanism of cell senescence induced by OSU-03012, which requires
further investigation.
In conclusion, the present study identified the
inhibitory effect of OSU-03012 on the proliferation and activation
of HSCs, which was mediated via cell senescence. The results of the
current study support the use of OSU-03012 against liver fibrosis,
however, further studies are required to confirm its effects in
vivo in an animal model of hepatic fibrosis.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81125001, 91129702,
81300327 and 81170405).
References
|
1
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campisi J and d’Adda di Fagagna F:
Cellular senescence: when bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krizhanovsky V, Yon M, Dickins RA, et al:
Senescence of activated stellate cells limits liver fibrosis. Cell.
134:657–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao M, Yeh PY, Lu YS, et al: OSU-03012, a
novel celecoxib derivative, induces reactive oxygen species-related
autophagy in hepatocellular carcinoma. Cancer Res. 68:9348–9357.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson AJ, Smith LL, Zhu J, et al: A
novel celecoxib derivative, OSU03012, induces cytotoxicity in
primary CLL cells and transformed B-cell lymphoma cell line via a
caspase- and Bcl-2-independent mechanism. Blood. 105:2504–2509.
2005. View Article : Google Scholar
|
|
7
|
Yacoub A, Park MA, Hanna D, et al:
OSU-03012 promotes caspase-independent but PERK-, cathepsin B-,
BID-, and AIF-dependent killing of transformed cells. Mol
Pharmacol. 70:589–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCubrey JA, Lahair MM and Franklin RA:
OSU-03012 in the treatment of glioblastoma. Mol Pharmacol.
70:437–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kucab JE, Lee C, Chen CS, et al: Celecoxib
analogues disrupt Akt signaling, which is commonly activated in
primary breast tumours. Breast Cancer Res. 7:R796–R807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Zhu J, Melvin WS, Bekaii-Saab TS,
Chen CS and Muscarella P: A structurally optimized celecoxib
derivative inhibits human pancreatic cancer cell growth. J
Gastrointest Surg. 10:207–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Suvannasankha A, Crean CD, et al:
OSU-03012, a novel celecoxib derivative, is cytotoxic to myeloma
cells and acts through multiple mechanisms. Clin Cancer Res.
13:4750–4758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collado M, Medema RH, Garcia-Cao I, et al:
Inhibition of the phosphoinositide 3-kinase pathway induces a
senescence-like arrest mediated by p27Kip1. J Biol Chem.
275:21960–21968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YC, Kulp SK, Wang D, et al: Targeting
endoplasmic reticulum stress and Akt with OSU-03012 and gefitinib
or erlotinib to overcome resistance to epidermal growth factor
receptor inhibitors. Cancer Res. 68:2820–2830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Passino MA, Adams RA, Sikorski SL and
Akassoglou K: Regulation of hepatic stellate cell differentiation
by the neurotrophin receptor p75NTR. Science. 315:1853–1856. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams PD: Healing and hurting: molecular
mechanisms, functions, and pathologies of cellular senescence. Mol
Cell. 36:2–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weng SC, Kashida Y, Kulp SK, et al:
Sensitizing estrogen receptor-negative breast cancer cells to
tamoxifen with OSU-03012, a novel celecoxib-derived
phosphoinositide-dependent protein kinase-1/Akt signaling
inhibitor. Mol Cancer Ther. 7:800–808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Huang JW, Tseng PH, et al: From the
cyclooxygenase-2 inhibitor celecoxib to a novel class of
3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer
Res. 64:4309–4318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iredale JP: Models of liver fibrosis:
exploring the dynamic nature of inflammation and repair in a solid
organ. J Clin Invest. 117:539–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayo LD and Donner DB: A
phosphatidylinositol 3-kinase/Akt pathway promotes translocation of
Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA.
98:11598–11603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|