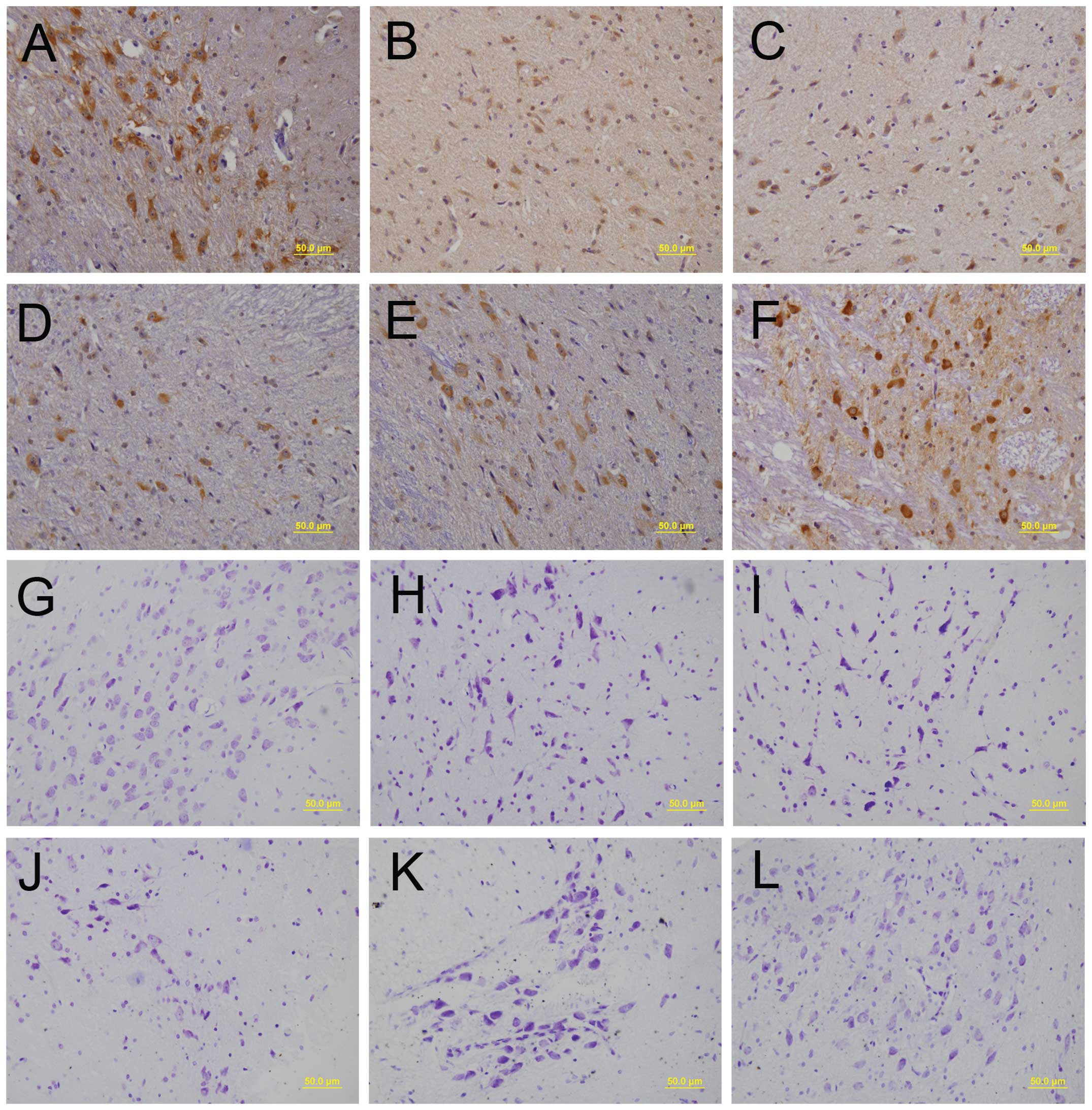
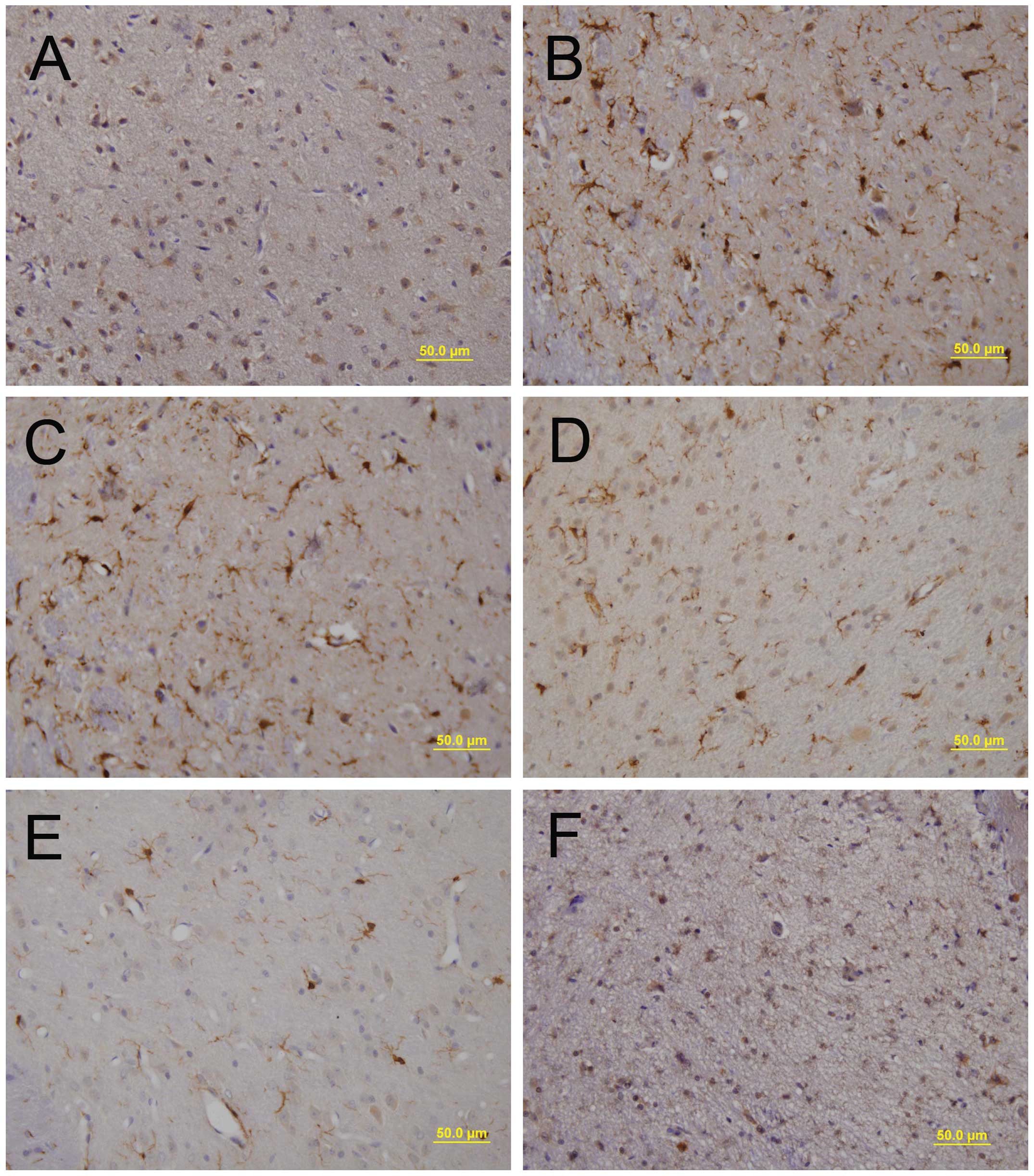
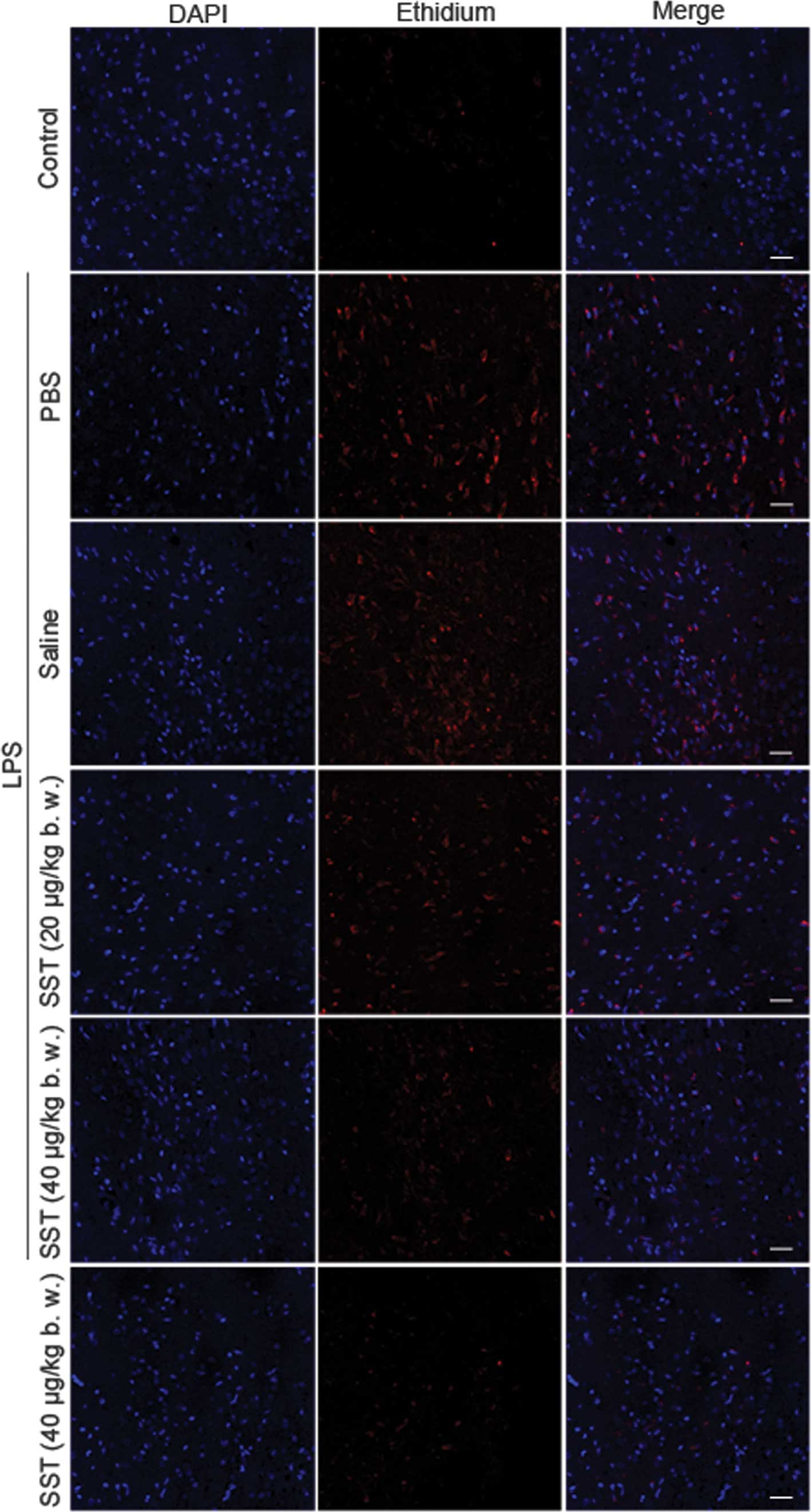
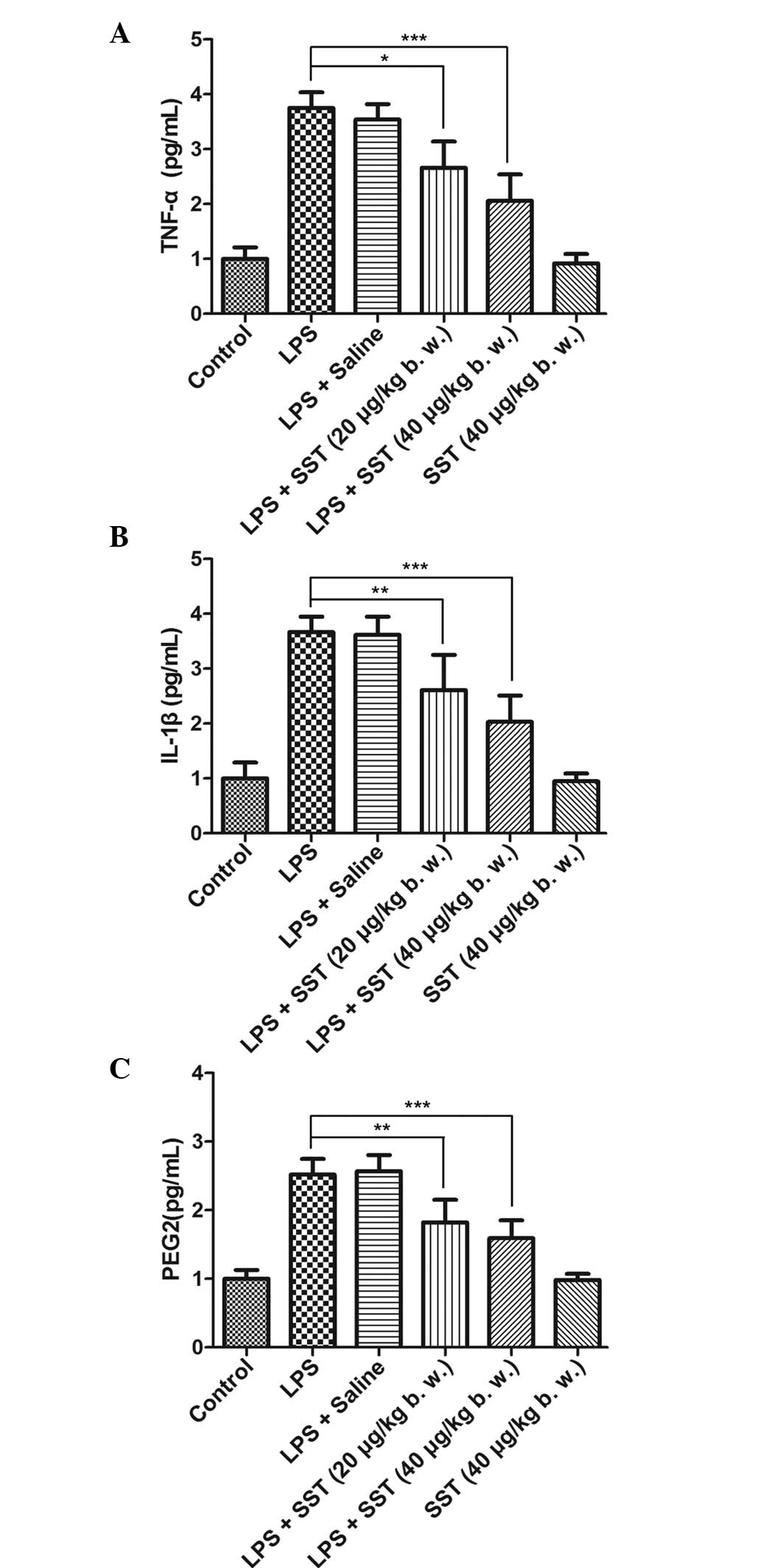
|
1
|
Ramos-Moreno T, Castillo CG and
Martinez-Serrano A: Long term behavioral effects of functional
dopaminergic neurons generated from human neural stem cells in the
rat 6-OH-DA Parkinson’s disease model. Effects of forced expression
of BCL-X(L). Behav Brain Res. 232:225–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golbe LI: Young-onset Parkinson’s disease:
A clinical review. Neurology. 41:168–173. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zanon A, Rakovic A, Blankenburg H, et al:
Profiling of Parkin-binding partners using tandem affinity
purification. PLoS One. 8:e786482013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi SH, Joe EH, Kim SU and Jin BK:
Thrombin-induced microglial activation produces degeneration of
nigral dopami-nergic neurons in vivo. J Neurosci. 23:5877–5886.
2003.PubMed/NCBI
|
|
5
|
Song DY, Yu HN, Park CR, et al:
Down-regulation of microglial activity attenuates axotomized nigral
dopaminergic neuronal cell loss. BMC Neurosci. 14:1122013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan L, Wu Y, Ren X, Liu Q, Wang J and Liu
X: Isoorientin attenuates lipopolysaccharide-induced
pro-inflammatory responses through down-regulation of ROS-related
MAPK/NF-κB signaling pathway in BV-2 microglia. Mol Cell Biochem.
386:153–165. 2014. View Article : Google Scholar
|
|
7
|
Epelbaum J, Guillou JL, Gastambide F,
Hoyer D, Duron E and Viollet C: Somatostatin, Alzheimer’s disease
and cognition: An old story coming of age? Prog Neurobiol.
89:153–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Viollet C, Lepousez G, Loudes C, Videau C,
Simon A and Epelbaum J: Somatostatinergic systems in brain:
Networks and functions. Mol Cell Endocrinol. 286:75–87. 2008.
View Article : Google Scholar
|
|
9
|
Epelbaum J, Ruberg M, Moyse E, Javoy-Agid
F, Dubois B and Agid Y: Somatostatin and dementia in Parkinson’s
disease. Brain Res. 278:376–379. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Espino A, Calopa M, Ambrosio S, Ortolà J,
Peres J and Navarro MA: CSF somatostatin increase in patients with
early parkinsonian syndrome. J Neural Transm Park Dis Dement Sect.
9:189–196. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuboly G and Vécsei L: Somatostatin and
cognitive function in neurodegenerative disorders. Mini Rev Med
Chem. 13:34–46. 2013. View Article : Google Scholar
|
|
12
|
Chung ES, Chung YC, Bok E, et al:
Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic
neurons by inhibiting microglia-mediated oxidative stress. Brain
Res. 1363:143–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung ES, Bok E, Chung YC, Baik HH and Jin
BK: Cannabinoids prevent lipopolysaccharide-induced
neurodegeneration in the rat substantia nigra in vivo through
inhibition of microglial activation and NADPH oxidase. Brain Res.
1451:110–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu M and Bing G: Lipopolysaccharide
animal models for Parkinson’s disease. Parkinson’s Dis.
2011:3270892011.
|
|
15
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neuro-toxicity: uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
16
|
Koppula S, Kumar H, Kim IS and Choi D-K:
Reactive oxygen species and inhibitors of inflammatory enzymes,
NADPH oxidase, and iNOS in experimental models of Parkinson’s
disease. Mediators Inflamm. 2012:8239022012. View Article : Google Scholar
|
|
17
|
Geng Y, Fang M, Wang J, et al: Triptolide
down-regulates COX-2 expression and PGE2 release by suppressing the
activity of NF-κB and MAP kinases in lipopolysaccharide-treated
PC12 cells. Phytother Res. 26:337–343. 2012.
|
|
18
|
Tansey MG and Goldberg MS:
Neuroinflammation in Parkinson’s disease: Its role in neuronal
death and implications for therapeutic intervention. Neurobiol Dis.
37:510–518. 2010. View Article : Google Scholar :
|
|
19
|
Minghetti L: Role of COX-2 in inflammatory
and degenerative brain diseases. Inflammation in the Pathogenesis
of Chronic Diseases. 42. Harris R, Bittman R, Dasgupta D, et al:
Springer; Netherlands: pp. 127–141. 2007
|
|
20
|
Broom L, Marinova-Mutafchieva L, Sadeghian
M, Davis JB, Medhurst AD and Dexter DT: Neuroprotection by the
selective iNOS inhibitor GW274150 in a model of Parkinson disease.
Free Radic Biol Med. 50:633–640. 2011. View Article : Google Scholar
|
|
21
|
Aoki E, Yano R, Yokoyama H, Kato H and
Araki T: Role of nuclear transcription factor kappa B (NF-kappaB)
for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced
apoptosis in nigral neurons of mice. Exp Mol Pathol. 86:57–64.
2009. View Article : Google Scholar
|
|
22
|
Dexter DT and Jenner P: Parkinson disease:
from pathology to molecular disease mechanisms. Free Radic Biol
Med. 62:132–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SR, Chung ES, Bok E, et al:
Prothrombin kringle-2 induces death of mesencephalic dopaminergic
neurons in vivo and in vitrovia microglial activation. J Neurosci
Res. 88:1537–1548. 2010.
|
|
24
|
Miller R, James-Kracke M, Sun G and Sun A:
Oxidative and inflammatory pathways in Parkinson’s disease.
Neurochem Res. 34:55–65. 2009. View Article : Google Scholar
|
|
25
|
Shen W, Qi R, Zhang J, et al: Chlorogenic
acid inhibits LPS-induced microglial activation and improves
survival of dopaminergic neurons. Brain Res Bull. 88:487–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park ES, Kim SR and Jin BK: Transient
receptor potential vanilloid subtype 1 contributes to mesencephalic
dopaminergic neuronal survival by inhibiting microglia-originated
oxidative stress. Brain Res Bull. 89:92–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lull M and Block ML: Microglial activation
and chronic neuro-degeneration. Neurotherapeutics. 7:354–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hancock D, Martin ER, Vance JM and Scott
WK: Nitric oxide synthase genes and their interactions with
environmental factors in Parkinson’s disease. Neurogenetics.
9:249–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Dai FR, Du XP, Yang QD and Chen Y:
Neuroprotection by silencing iNOS expression in a 6-OHDA model of
Parkinson’s disease. J Mol Neurosci. 48:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller JA, Trout BR, Sullivan KA, Bialecki
RA, Roberts RA and Tjalkens RB: Low-dose
1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine causes inflammatory
activation of astrocytes in nuclear factor-κB reporter mice prior
to loss of dopaminergic neurons. J Neurosci Res. 89:406–417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
More SV, Kumar H, Kim IS, Song SY and Choi
DK: Cellular and molecular mediators of neuroinflammation in the
pathogenesis of Parkinson’s disease. Mediators Inflamm.
2013:9523752013. View Article : Google Scholar
|
|
32
|
Cui YQ, Jia YJ, Zhang T, Zhang QB and Wang
XM: Fucoidan protects against lipopolysaccharide-induced rat
neuronal damage and inhibits the production of proinflammatory
mediators in primary microglia. CNS Neurosci Ther. 18:827–833.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teismann P: COX-2 in the neurodegenerative
process of Parkinson’s disease. Biofactors. 38:395–397. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Phani S, Loike JD and Przedborski S:
Neurodegeneration and inflammation in Parkinson’s disease.
Parkinsonism Relat Disord. 18(Suppl 1): S207–S209. 2012. View Article : Google Scholar
|
|
35
|
Kang CH, Jayasooriya RG, Choi YH, Moon SK,
Kim WJ and Kim GY: β-Ionone attenuates LPS-induced pro-inflammatory
mediators such as NO, PGE2 and TNF-α in BV2 microglial cells via
suppression of the NF-κB and MAPK pathway. Toxicol In Vitro.
27:782–787. 2013. View Article : Google Scholar
|