Introduction
Obesity has become increasingly common, and is
over-taking malnutrition and infectious diseases as a primary
contributor to morbidity (1,2).
Obesity is characterized by an increase in adipose tissue mass that
results from an increase in the size and/or the number of
adipocytes (3). In numerous recent
studies (4–6), the loss of adipocytes through
apoptosis was postulated as a factor contributing to the reduction
of body fat. Thus, the development of therapeutic agents, in
particular from natural products with low toxicity, which reduce
the number of adipocytes by inducing apoptosis in these cells, may
serve as a strategy by which to treat and prevent obesity, in
addition to related metabolic disorders (7).
Curcumin, a polyphenol compound extracted from
rhizomes of the curcuma species, has been shown to exhibit
beneficial effects in patients with diabetes, allergies, arthritis,
Alzheimer’s disease, cancer and multiple sclerosis (8–11).
Experimental evidence supports the role of curcumin in reducing the
incidence of obesity-related diseases through the suppression of
chronic inflammation (12). In
addition, a number of studies have indicated that the induction of
adipocyte apoptosis is a potential novel strategy with which to
treat obesity (4–6,13).
The present study thus hypothesized that curcumin may reduce
obesity and related diseases by mediating the induction of
apoptosis in adipocytes.
Apoptosis may be initiated through the activation of
two alternative signaling pathways. The first is the extrinsic
pathway, which acts on death receptors on the cell surface. The
second is the intrinsic pathway, which acts through the
mitochondria (14,15). Mitochondria are involved in the
regulation of a number of apoptotic processes (16). The chemical-induced apoptotic
pathway involving mitochondria has been shown to be regulated by
key proteins associated with apoptosis, such as Bax, Bcl-2 and
cytochrome c in the mitochondria pathway, with subsequent
activation of caspase-3 and poly (ADP) ribose polymerase (PARP)
(13,17). The SW872 human adipocyte cell line
was employed in the present study due to its widespread use as a
human adipocyte cell model in adipose cell biology research
(18,19).
In the current study, the efficacy of curcumin in
inducing apoptosis in SW872 adipocytes was examined. In addition,
the mechanisms underlying this effect were investigated by
measuring the Bax/Bcl-2 ratio, changes in cytochrome c
release, activation of caspase-3 and the cleavage of PARP.
Materials and methods
Materials
The SW872 human adipocyte cell line was obtained
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). Dulbecco’s modified Eagle’s medium with F12 (DMEM/F12), fetal
bovine serum (FBS) and phosphate-buffered saline (PBS) were
purchased from Gibco Life Technologies (Grand Island, NY, USA).
Curcumin (99%) and
3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
ApoStrand™ ELISA Apoptosis Detection kits were purchased from
Chemicon International (Temecula, CA, USA). Rabbit polyclonal
antibodies against PARP (1:1,000; cat. no. sc-25780), Bax (1:500;
cat. no. sc-493), Bcl-2 (1:500; cat. no. sc-492), caspase-3 (1:800;
cat. no. sc-7148), β-actin (1:800; cat. no. sc-1616-R) and
cytochrome c (1:500; cat. no. sc-7159) were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture of SW872
The SW872 cells were grown in DMEM/F12 (3:1)
containing 10% FBS, penicillin (100 U/ml) and streptomycin (100
U/ml) (Gibco Life Technologies) at 37°C in 5% CO2. SW872
cells that formed a confluent monolayer were induced to
differentiate using DMEM/F12 containing 1% bovine serum albumin
(BSA; Sigma-Aldrich) and 0.6 mol/l oleic acid for three days, until
>90% of the cells had reached maturity.
Cell viability assay
The cell viability of mature adipocytes was
evaluated using an MTT assay in 96-well plates. The mature SW872
adipocytes were treated with 10, 20, 40, 60 or 80 μmol/l of
curcumin, and were incubated for 24, 48 and 72 h (every 12-wells
was a group). The contents of each well was added into 20 μl
of the MTT dye (50 μg/ml) and incubated for 4 h at 37°C. The
medium was then discarded. Dimethyl sulfoxide (DMSO; 150 μl)
was added into each well, and the absorbance was measured at 620 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
4′,6-diamidino-2′-phenylindole
dihydrochloride (DAPI) staining
SW872 cells were cultured in 6-well plates and grown
to maturity, as described above. The cells were then treated with
or without 40 μmol/l curcumin for 24 h. Cells were washed
with PBS and then stained with 0.1 μg/ml DAPI for 30 min at
20°C. Images were acquired using an Olympus IX-70 inverted
fluorescent microscope (magnification, x400; Olympus Corporation,
Tokyo, Japan) and the percentage of cells that contained condensed
chromatin and/or fragmented nuclei was determined.
Single-stranded (ss)DNA ELISA assay
Tests were performed in 96-well plates. The SW872
cells were seeded (5,000 cells/well), grown to confluence, induced
to differentiate and grown to maturity. Curcumin (0, 10, 20 or 40
μmol/l) in 0.01% DMSO carrier was added for 24 h with the
wash buffer. Following washing, cells were incubated with 100
μl peroxide substrate for 1 h, and absorbance was read using
an ELISA plate reader (Spectra Max M2; Molecular Devices,
Sunnyvale, CA, USA) at 405 nm. The reaction was stopped by the
addition of 100 μl l% sodium dodecyl sulfate.
Western blotting
Mature SW872 adipocytes were incubated with 0, 10,
20 or 40 μmol/l of curcumin for 24 h, or with 40
μmol/l curcumin for 24, 48 or 72 h. Cells were washed twice
with cold PBS and then treated with ice-cold lysates containing 1
μg/ml aprotinin, 1 μg/ml leupeptin and 1 mmol/l PMSF
(20). The lysates were collected
following centrifugation at 15,000 × g for 15 min at 4°C. Once
samples had been stratified, protein content was isolated by
collecting the middle transparent liquid. Samples were prepared
with 2-mercaptoethanol and denatured by heating at 100°C for 6 min.
Protein samples were resolved by SDS-PAGE (Beijing Saichi
Biological Technology Co., Ltd., Beijing, China), transferred to
the nitrocellulose membrane (Bio-Rad Laboratories, Inc.) and
immunoblotted with primary antibodies against caspase-3, PARP, Bax,
Bcl-2 and cytochrome c. Subsequently, the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
polyclonal immunoglobulin G secondary antibodies (1:10,000; cat.
no. sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Images were acquired and quantified using a
ChampGel-3200 Digital Imaging system (Cell Biosciences, Inc., Santa
Clara, CA, USA).
Statistical analysis
Differences among groups were assessed using one-way
analysis of variance (ANOVA). Data are expressed as the mean ±
standard deviation. Statistical analyses were performed using the
SPSS 13.0 statistical program (version 13.01 S; Beijing Stats Data
Mining Co., Ltd., Beijing, China). P<0.05 was considered to
indicated a statistically significant difference.
Results
Curcumin inhibits population growth in
SW872 adipocytes
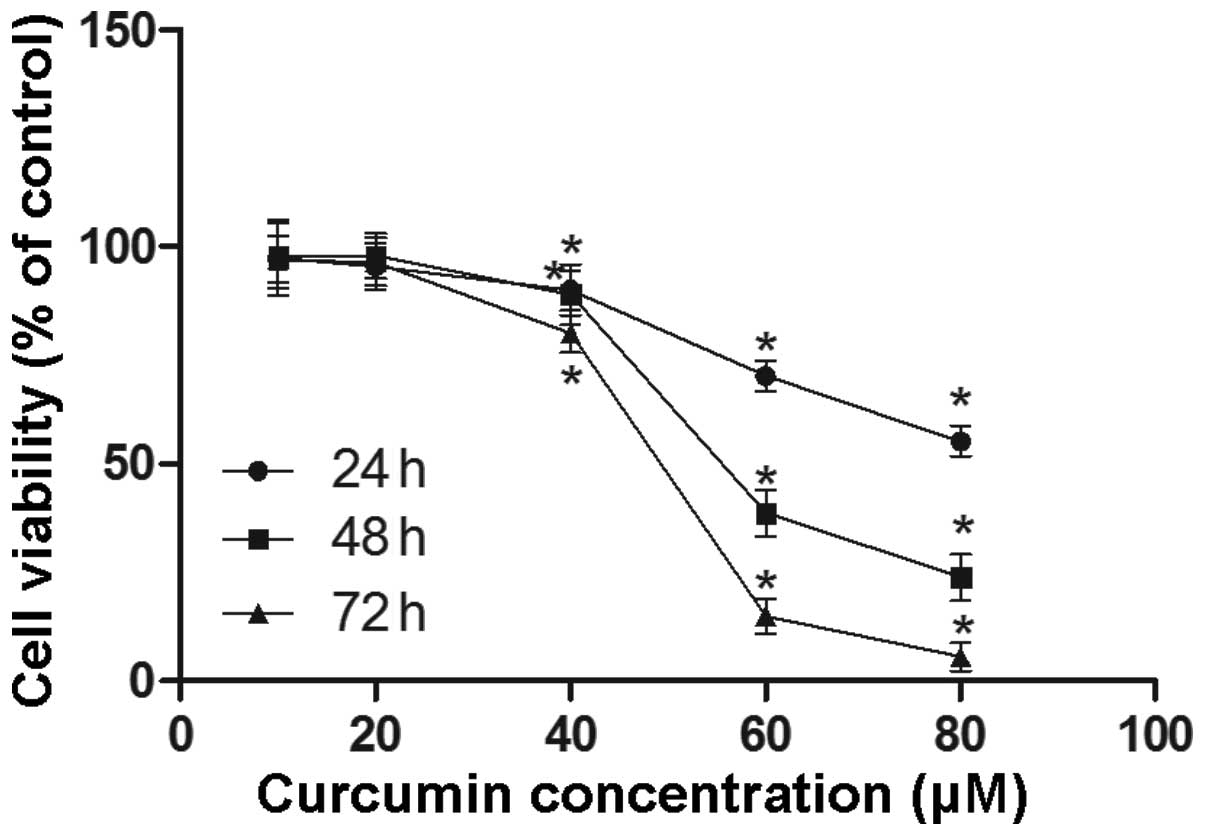
The effect of curcumin on cell viability was
determined in the mature adipocytes. The results are shown in
Fig. 1. A reduction in cell
viability was observed in mature adipocytes treated with 40, 60 and
80 μmol/l of curcumin, for 24, 48 and 72 h. Furthermore,
curcumin treatment reduced cell viability in a time- and
dose-dependent manner.
Curcumin induces apoptosis in SW872
mature adipocyte
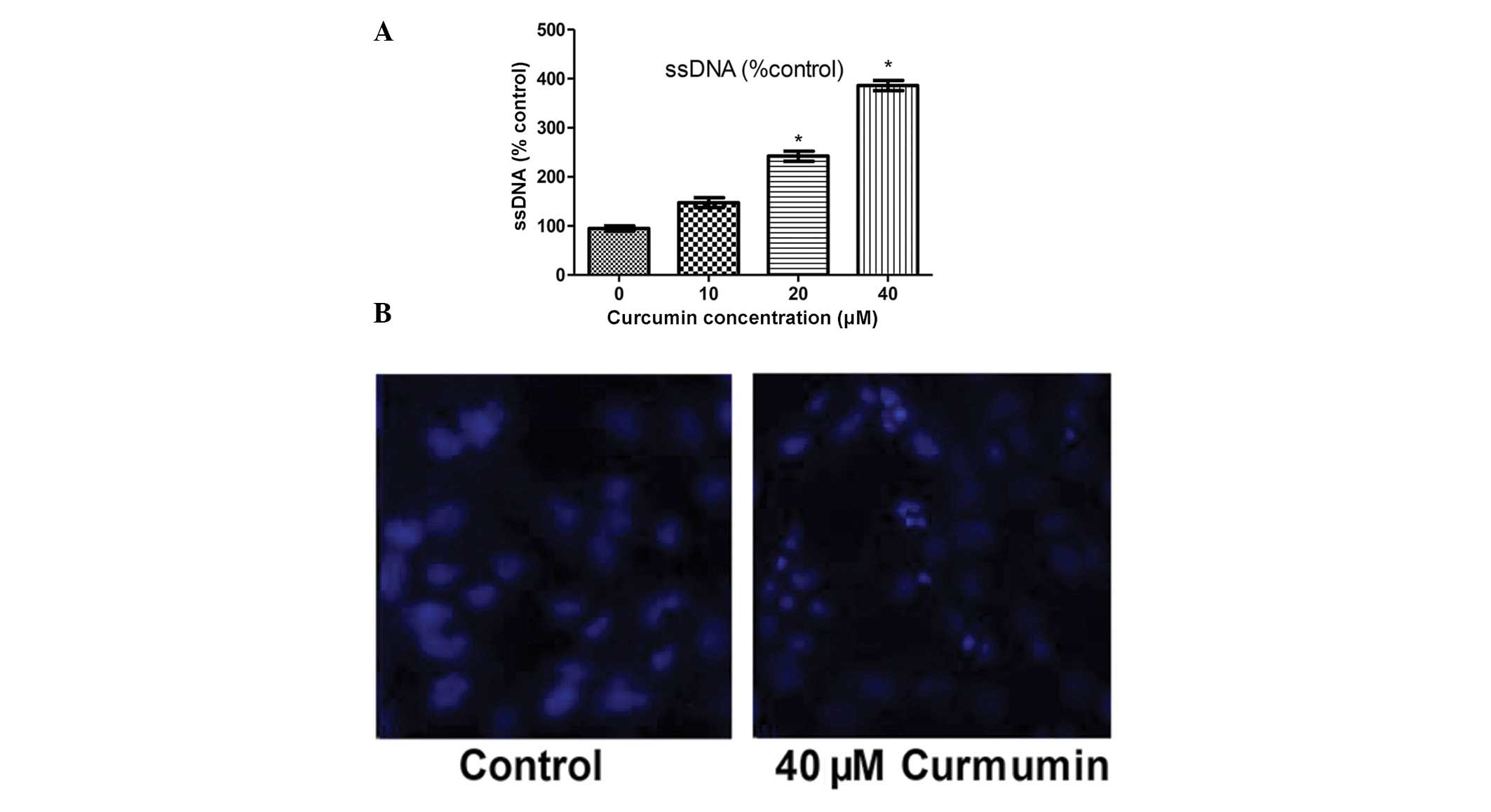
In order to assess whether the reduction in cell
number following treatment with curcumin was due to increased
apoptosis, an ssDNA ELISA kit was used to determine cell apoptosis.
As shown in Fig. 2A, exposure of
adipocytes to curcumin resulted in a dose-dependent increase in the
level of apoptosis. In order to determine the direct effect of
curcumin on nuclear morphology, DAPI staining was used to visualize
nuclear shrinkage and fragmentation (20). When the mature SW872 adipocytes
were treated with curcumin (40 μmol/l) for 48 h, the cells
exhibited morphological features characteristic of apoptotic cells,
such as bright nuclear condensation, DNA fragmentation and
perinuclear apoptotic bodies, as demonstrated by DAPI staining
(Fig. 2B).
Curcumin increases the Bax/Bcl-2
ratio
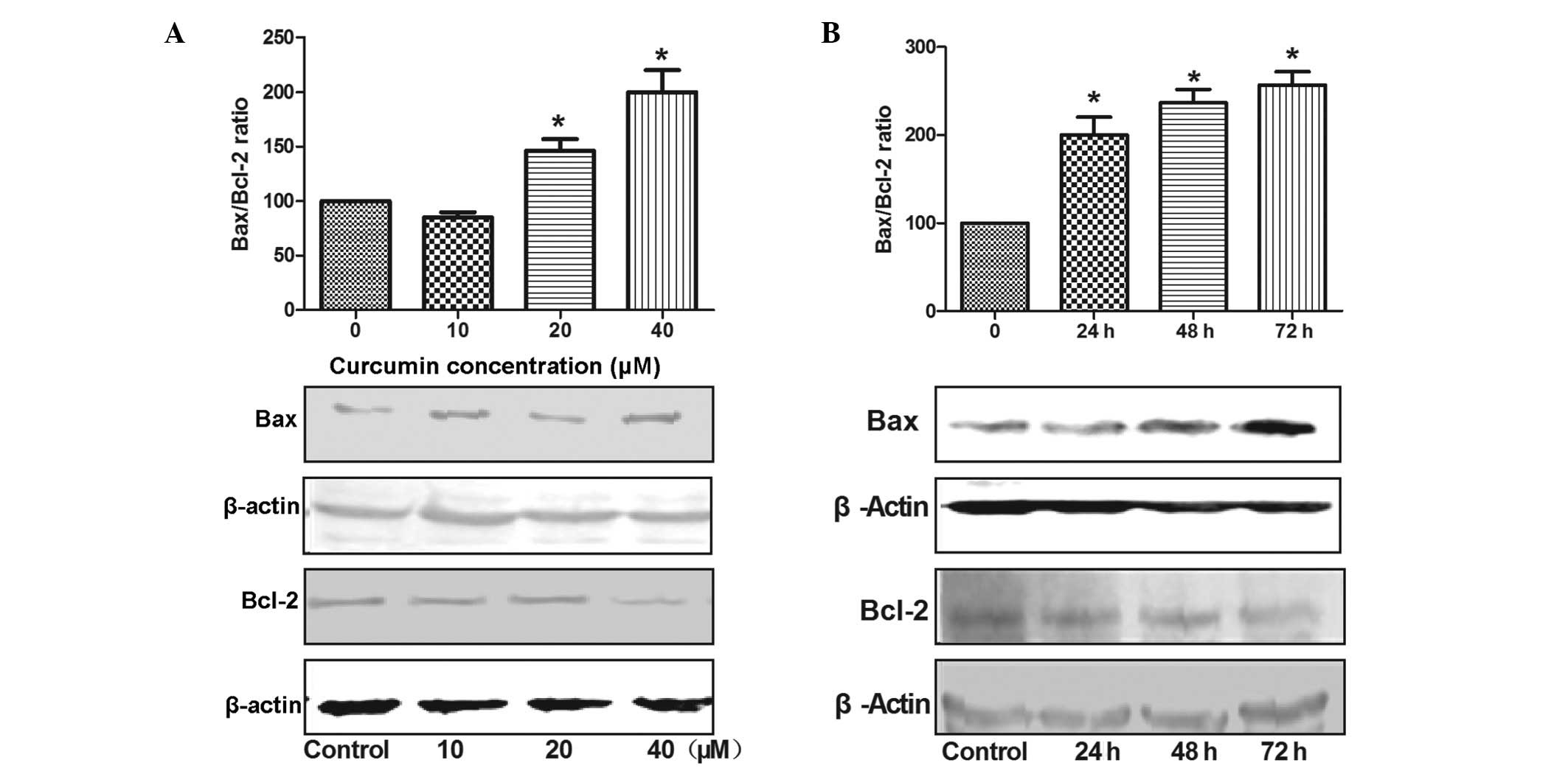
In order to investigate the effect of curcumin on
the Bcl-2 family in SW872 cells, western blotting was used to
detect the expression of the Bax (proapoptotic) and Bcl-2
(antiapoptotic) proteins, and thus evaluate the changes in the
Bax/Bcl-2 ratio (Fig. 3A). The
SW872 cells were treated with 20 or 40 μmol/l of curcumin
for 24 h, and with 40 μmol/l of curcumin for 24, 48 or 72 h,
and a time- and dose-dependent increase in Bax expression and a
decrease in Bcl-2 expression was observed (Fig. 3B). The Bax/Bcl-2 ratio was
significantly increased, by 146 and 220% following treatment with
20 and 40 μmol/l of curcumin, respectively, compared with
the control cells (P<0.05).
Curcumin causes release of cytochrome c
from mitochondria to cytoplasm
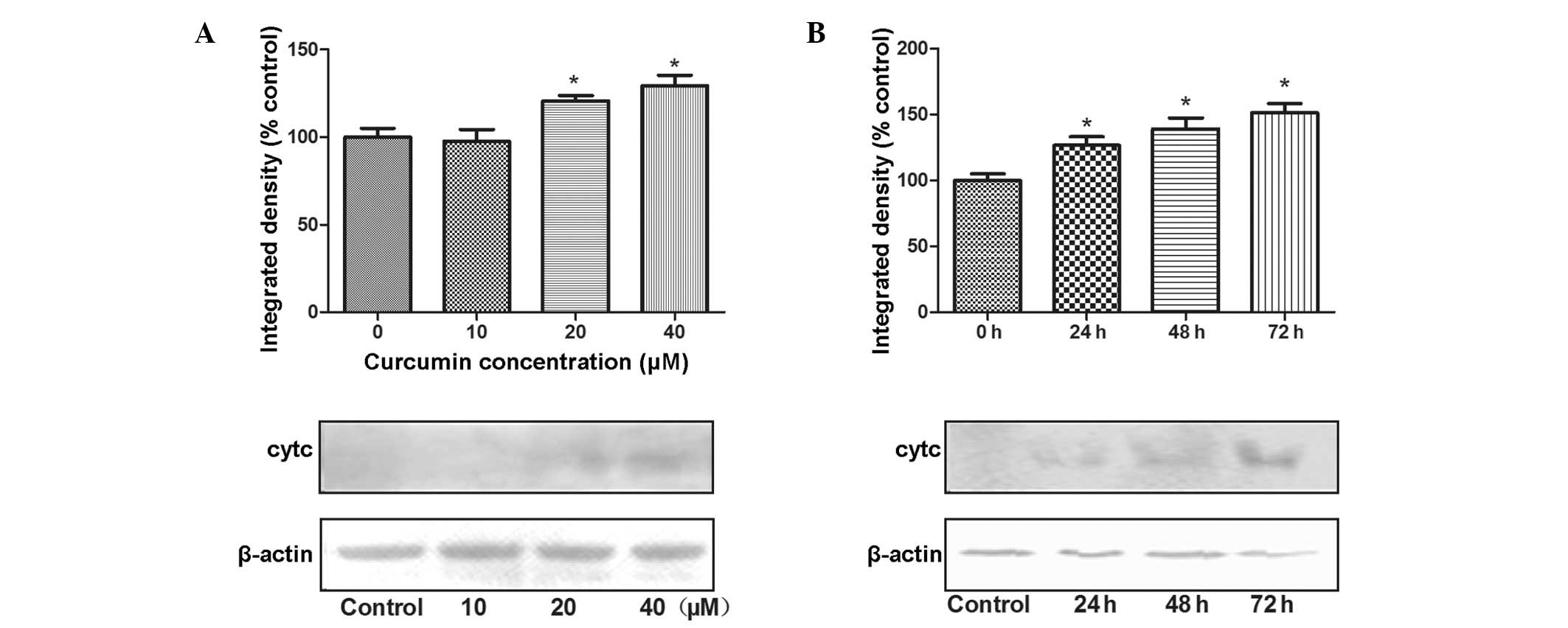
Following an increase in the Bax/Bcl-2 ratio in the
mitochondrial membrane, mitochondria release cytochrome c
into the cytosol, leading to the subsequent activation of caspase-3
for apoptosis (21). The effects
of curcumin on the protein expression of cytochrome c in
SW872 adipocytes are shown in Fig.
4. The expression of cytochrome c in the cytoplasm
significantly increased, of 121 and 129%, when SW872 cells were
treated with 20 and 40 μmol/l of curcumin, respectively for
24 h compared with that in the control cells (P<0.05; Fig. 4A). Furthermore, the expression of
cytochrome c also showed a significant increase, of 129,
139% and 151%, in cells treated with 40 μmol/l of curcumin
for 24, 48 and 72 h, respectively (all P<0.05; Fig. 4B).
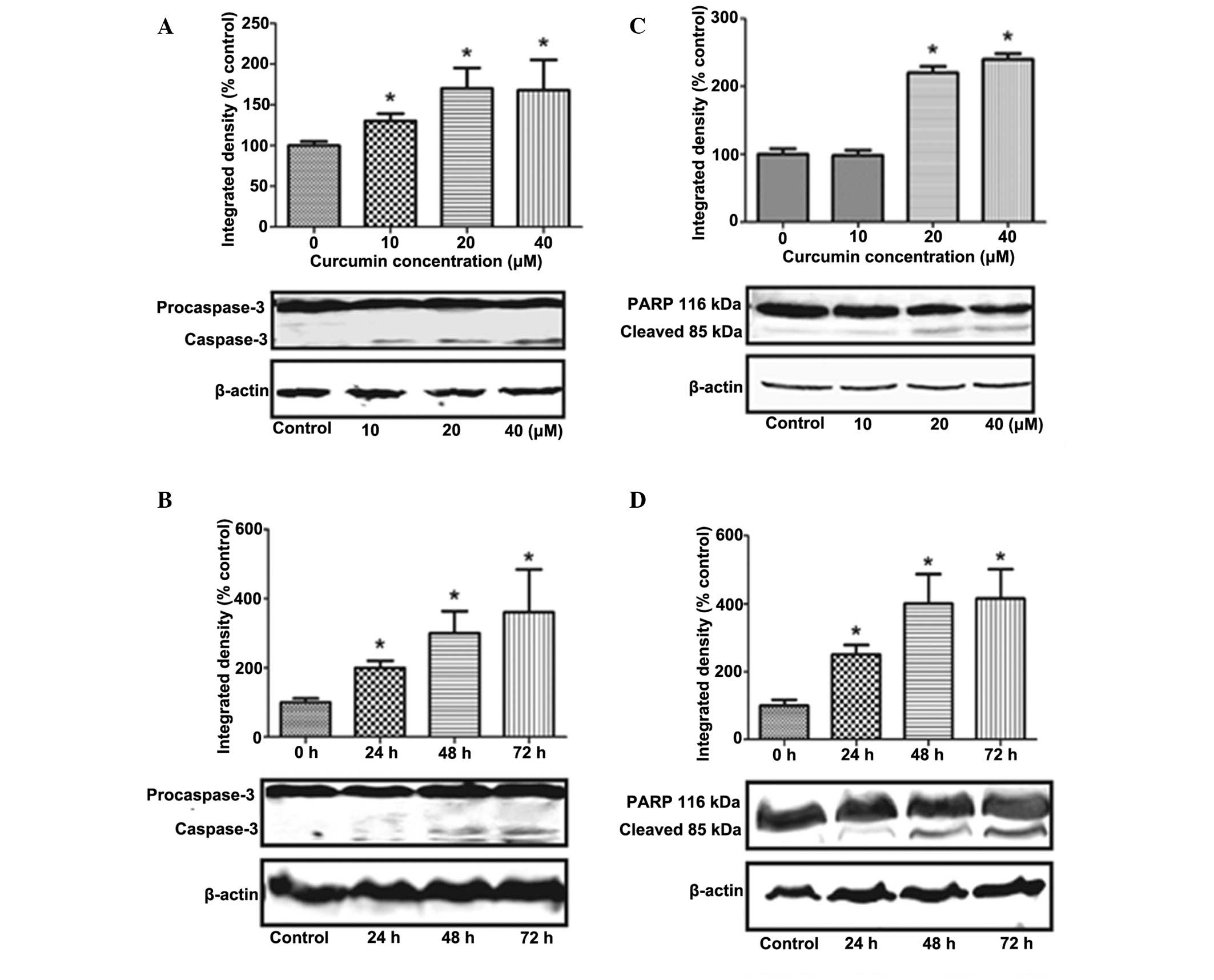
Curcumin triggers caspase-3 activation
and PARP cleavage
The release of Cytochrome c from mitochondria
into the cytosol is an important event in the activation of
caspase-3 (13). Treatment of
adipocytes with 40 and 20 μmol/l of curcumin for 24 h
(Fig. 5A), and with 40
μmol/l for 24, 48 and 72h (Fig.
5B) significantly stimulated caspase-3 expression in a time-
and dose-dependent manner (P<0.05), with a maximal increase of
17 kDa. However, the activation of caspase-3 results in the
cleavage of a number of proteins, the most important of which is
PARP. PARP is thought to have a multifunctional role in apoptosis,
DNA repair and recombination, as well as in the maintenance of
chromosomal stability (22).
Cleavage of this protein leads to its inactivation and thus
prevents the futile DNA repair cycle. Treatment of cells with 20 or
40 μmol/l of curcumin for 24 h (Fig. 5C), or with 40 μmol/l of
curcumin for 24, 48 and 72 h (Fig.
5D) significantly induced PARP cleavage, compared with the
control cells, with a maximal cleavage of 85kD (P<0.05).
Discussion
Curcumin is a natural compound existing in the
commonly-used spice turmeric. Although the potential therapeutic
activity of curcumin in the treatment of obesity and
obesity-related metabolic disorders has been widely reported, much
is unknown regarding its biological effects and mechanisms of
action in the cell microenvironment (12,23,24).
The 3T3-L1 mouse embryo fibroblasts and human SW872 adipocytes are
the primary cell lines used in studies investigating obesity
(18,19). The 3T3-L1 cell line is
characterized by its differentiation into mature adipocytes
following incubation with cocktail, including insulin,
dexamethasone and isobutylmethylxanthine (25). However, human SW872 adipocytes have
the advantages of being human in origin and of not requiring an
incubation cocktail for the induction of differentiation (26). Obesity is characterized by an
increased number and/or size of adipocytes (3). Adipocyte loss, as a result of the
induction of apoptosis, may be important for regulating adipocyte
numbers in strategies used to combat obesity (27). In the present study, the results
showed that curcumin induced apoptosis in SW872 adipocytes in a
dose-dependent manner (Fig. 2).
This is in accordance with the results from a study conducted by
Ejaz et al (28) in 3T3-L1
adipocytes, which indicates that curcumin induces adipocyte
apoptosis regardless of the species involved. However, the pathways
involved in curcumin-induced apoptosis in SW872 adipocyte remain
unclear.
The present results indicate that curcumin may
induce apoptosis by the pathway that involves the activation of
caspase-3 and PARP cleavage (Fig.
4), in a time- and dose-dependent manner. Caspase-3 is a member
of the caspase family and is the final common molecule involved in
the process of apoptosis (29).
Furthermore, the activation of downstream caspase-3 by the majority
of agents causes cleavage of the PARP protein (30). Although PARP is not essential for
cell death, the cleavage of PARP is an additional hallmark of
apoptosis (22). The current data
also demonstrated that curcumin induces cytochrome c release
from mitochondria into the cytosol fraction (Fig. 3C and D) in a time- and
dose-dependent manner. Cytochrome c, which is necessary for
the initiation of the apoptotic program, is ordinarily located in
the mitochondrial inter membrane space (31). The release of cytochrome c
from mitochondria is a key signaling mechanism in apoptosis
(32).
Cytochrome c release is regulated by a number
of Bcl-2 family proteins. Members of the Bcl-2 family are important
regulators of the apoptotic pathways (13). Bcl-2 is involved in cell survival
and also inhibits cell apoptosis, induced by a variety of stimuli,
indicating that Bcl-2 is a negative regulator of cell apoptosis
(33). Bax is a proapoptotic
protein, which resides in the outer mitochondrial membrane and
translocates to the mitochondria at an early stage of apoptosis,
suggesting that it is important for apoptotic signal transduction
(34). The ratio of the various
Bcl-2 family members has been hypothesized to predispose a cell to
either accelerated or suppressed apoptosis in response to external
stimuli (35). Therefore,
alterations in the relative levels of Bax and Bcl-2 are important
in determining whether cells will undergo apoptosis. The present
findings showed that curcumin upregulated proapoptotic Bax
expression and downregulated antiapoptotic Bcl-2 expression,
resulting in an elevation of the Bax/Bcl-2 ratio in mature SW872
adipocytes (Fig. 3A and B). These
results suggest that the cell apoptosis induced by treatment with
curcumin is dependent on alterations in the expression of Bcl-2
family proteins and cytochrome c, and is associated with the
mitochondrial pathway.
In conclusion, the results of the present study
suggest that curcumin induces adipocyte apoptosis, and provides
evidence for the induction of mitochondrial apoptotic events by
curcumin, which are associated with the regulation of the Bcl-2
family proteins, cytochrome c, caspase-3 and the cleavage of
PARP in SW872 adipocytes. These data reveal that curcumin may be a
promising therapeutic agent for obesity, by decreasing adipocyte
numbers through the induction of adipocyte apoptosis.
Acknowledgments
This study was supported by the National Science
Foundation of China (grant no. 81172650), Postdoctoral Science
Foundation of China (grant no. 2012M520774) and Heilongjiang
Postdoctoral Science Foundation (grant no. LBH-Z12156).
References
|
1
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
2
|
Kim SH, Park HS, Lee MH, et al: Vitisin A
inhibits adipocyte differentiation through cell cycle arrest in
3T3-L1 cells. Biochem Biophys Res Commun. 372:108–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sorisky A, Magun R and Gagnon AM: Adipose
cell apoptosis: death in the energy depot. Int J Obes Relat Metab
Disord. 24(Suppl 4): S3–S7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HK, Della-Fera MA, Hausman DB and
Baile CA: Effect of clenbuterol on apoptosis, adipogenesis, and
lipolysis in adipocytes. J Physiol Biochem. 66:197–203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Lee JH, Kim SY, et al:
Phosphatidylcholine induces apoptosis of 3T3-L1 adipocytes. J
Biomed Sci. 18:912011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y and Huang C: Targeting adipocyte
apoptosis: a novel strategy for obesity therapy. Biochem Biophys
Res Commun. 417:1–4. 2012. View Article : Google Scholar
|
|
7
|
Lin J, Della-Fera MA and Baile CA: Green
tea polyphenol epigallocatechin gallate inhibits adipogenesis and
induces apoptosis in 3T3-L1 adipocytes. Obes Res. 13:982–990. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chandran B and Goel A: A randomized, pilot
study to assess the efficacy and safety of curcumin in patients
with active rheumatoid arthritis. Phytother Res. 26:1719–1725.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Na LX, Zhang YL, Li Y, et al: Curcumin
improves insulin resistance in skeletal muscle of rats. Nutr Metab
Cardiovasc Dis. 21:526–533. 2011. View Article : Google Scholar
|
|
10
|
Ma C, Ma Z, Fu Q and Ma S: Curcumin
attenuates allergic airway inflammation by regulation of CD4+CD25+
regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized
mice. Fitoterapia. 87:57–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed T and Gilani AH: Therapeutic
potential of turmeric in Alzheimer’s disease: curcumin or
curcuminoids? Phytother Res. 28:517–525. 2014. View Article : Google Scholar
|
|
12
|
Bradford PG: Curcumin and obesity.
Biofactors. 39:78–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JY, Della-Fera MA and Baile CA:
Esculetin induces mitochondria-mediated apoptosis in 3T3-L1
adipocytes. Apoptosis. 11:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.PubMed/NCBI
|
|
15
|
Reed JC: Cytochrome c: can’t live with it
– can’t live without it. Cell. 91:559–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu CL and Yen GC: Effects of capsaicin on
induction of apoptosis and inhibition of adipogenesis in 3T3-L1
cells. J Agric Food Chem. 55:1730–1736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He YH, He Y, Liao XL, et al: The
calcium-sensing receptor promotes adipocyte differentiation and
adipogenesis through PPARγ pathway. Mol Cell Biochem. 361:321–328.
2012. View Article : Google Scholar
|
|
19
|
Izem L and Morton RE: Possible role for
intracellular cholesteryl ester transfer protein in adipocyte lipid
metabolism and storage. J Biol Chem. 282:21856–21865. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KA, Chae JI and Shim JH: Natural
diterpenes from coffee, cafestol and kahweol induce apoptosis
through regulation of specificity protein 1 expression in human
malignant pleural mesothelioma. J Biomed Sci. 19:602012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karmakar S, Banik NL and Ray SK: Curcumin
suppressed anti-apoptotic signals and activated cysteine proteases
for apoptosis in human malignant glioblastoma U87MG cells.
Neurochem Res. 32:2103–2113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koide T, Kamei H, Hashimoto Y, Kojima T
and Hasegawa M: Antitumor effect of hydrolyzed anthocyanin from
grape rinds and red rice. Cancer Biother Radiopharm. 11:273–277.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao W, Yu Z, Chiang Y, et al: Curcumin
prevents high fat diet induced insulin resistance and obesity via
attenuating lipogenesis in liver and inflammatory pathway in
adipocytes. PLoS One. 7:e287842012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aggarwal BB: Targeting
inflammation-induced obesity and metabolic diseases by curcumin and
other nutraceuticals. Ann Rev Nutr. 30:173–199. 2010. View Article : Google Scholar
|
|
25
|
Yang JY, Della-Fera MA, Hartzell DL,
Nelson-Dooley C, Hausman DB and Baile CA: Esculetin induces
apoptosis and inhibits adipogenesis in 3T3-L1 cells. Obesity
(Silver Spring). 14:1691–1699. 2006. View Article : Google Scholar
|
|
26
|
Wassef H, Bernier L, Davignon J and Cohn
JS: Synthesis and secretion of apoC-I and apoE during maturation of
human SW872 liposarcoma cells. J Nutr. 134:2935–2941.
2004.PubMed/NCBI
|
|
27
|
Prins JB and O’Rahilly S: Regulation of
adipose cell number in man. Clin Sci (Lond). 92:3–11. 1997.
|
|
28
|
Ejaz A, Wu D, Kwan P and Meydani M:
Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and
angiogenesis and obesity in C57/BL mice. J Nutr. 139:919–925. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakahira H, Enari M and Nagata S: Cleavage
of CAD inhibitor in CAD activation and DNA degradation during
apoptosis. Nature. 391:96–99. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sodhi RK, Singh N and Jaggi AS:
Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic
implications. Vascul Pharmacol. 53:77–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skemiene K, Rakauskaite G, Trumbeckaite S,
Liobikas J, Brown GC and Borutaite V: Anthocyanins block
ischemia-induced apoptosis in the perfused heart and support
mitochondrial respiration potentially by reducing cytosolic
cytochrome c. Int J Biochem Cell Biol. 45:23–29. 2013. View Article : Google Scholar
|
|
33
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Narita M, Shimizu S, Ito T, et al: Bax
interacts with the permeability transition pore to induce
permeability transition and cytochrome c release in isolated
mitochondria. Proc Natl Acad Sci USA. 95:14681–14686. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chresta CM, Masters JR and Hickman JA:
Hypersensitivity of human testicular tumors to etoposide-induced
apoptosis is associated with functional p53 and a high Bax:Bcl-2
ratio. Cancer Res. 56:1834–1841. 1996.PubMed/NCBI
|