Introduction
Colorectal cancer (CRC) was reported to be the
second most prevalent cause of cancer-associated mortality
worldwide in 2009 (1). Early-stage
colorectal cancer is treatable and may be cured through surgical
resection; however, the rate of recurrence remains high (1). Therefore, it is essential to develop
novel therapeutic strategies for the treatment of CRC.
Histone deacetylase inhibitors (HDACIs) have an
important role in the regulation of genes which are associated with
the cell cycle and apoptosis; in addition, HDACIs have been
suggested to have promising anti-cancer properties (2–4).
Trichostatin A (TSA), a pan-HDAC inhibitor, has been reported to
induce cell cycle arrest, promote cell differentiation and
apoptosis as well as inhibit metastasis (5) in numerous types of tumor (6). It was previously demonstrated that
TSA induced apoptosis in CRC cells through p53-dependent and
-independent pathways via B cell lymphoma 2 (Bcl-2)-associated X
protein (Bax)-dependent mechanisms (7). Ku70 is an important protein in DNA
damage repair, which has also been reported to participate in
apoptosis regulation through interacting with Bax in the cytoplasm
and inhibiting the translocation of Bax to the mitochondria upon
receiving an apoptosis signal (8).
In addition, it was reported that acetylation of Ku70 may disrupt
its association with Bax, therefore releasing Bax from the complex
and resulting in Bax-dependent mitochondrial apoptosis (9). However, the function of Ku70 in
TSA-induced apoptosis of CRC cells remains to be elucidated.
The aim of the present study was to investigate the
role of Ku70 in TSA-induced apoptosis in the CRC cell lines HCT116
and HT29.
Materials and methods
Cell culture and treatments
The CRC cell lines HCT116 and HT29 (American Type
Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL), 10 mg/ml
antibiotics (penicillin and streptomycin; Sigma-Aldrich, St Louis,
MO, USA) and 2 mmol/l L-glutamine at 37°C under a humidified
atmosphere with 5% CO2. TSA (Sigma-Aldrich) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) to a final
concentration of 1.0 μM, which was used to treat the cells
(3×105 cells/well in a six-well plate). An equal volume
of DMSO was used as vehicle control. The cells were incubated with
TSA for 15 h for Bax detection, and for 24 h for the detection of
the other proteins.
Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) staining
HCT116 and HT29 cells were cultured and treated with
TSA as described above; cells were then digested using trypsin
(Gibco-BRL), stained with 5 μl Annexin V-FITC (BD
Pharmingen, San Diego, CA, USA) and 5 μl PI (BD Pharmingen)
staining solution in the dark at room temperature (RT) for 15 min.
The cell samples were analyzed by flow cytometry using a FACScan
station with Cell Quest software (BD Biosciences, Franklin Lakes,
NJ, USA) using the fluorescent resolution FL1 and FL2 ranges for
Annexin V FITC and PI, respectively.
Western blot analysis and
co-immunoprecipitation
Cells were lysed in lysis buffer containing 150 mM
NaCl, 1% NP40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris (pH 8.0)
(Sigma-Aldrich) and 1:25 protease inhibitor cocktail. For cellular
fractionation, cells were washed with phosphate-buffered saline
(PBS) and lysed in cytosolic lysis buffer containing 250 mM
sucrose, 70 mM KCl, 137 mM NaCl, 4.3 mM
Na2HPO4, 1.4 mM KH2PO4
(pH 7.2), 200 μg/ml digitonin, 100 mM phenylmethylsulfonyl
fluoride and 1:25 protease inhibitor cocktail for 30 min on ice.
Cells were centrifuged at 16,873 × g for 15 min at 4°C. The
supernatants were stored as cytosolic protein extract and the
pellets were further dissolved with lysis buffer containing 1% SDS
as the mitochondrial fraction. Protein concentrations of the
lysates were determined using the Bradford protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA). In brief, equal amounts
of protein (30 μg protein/lane) were separated using
SDS-PAGE (5% stacking gel and 12% separation gel), transferred to
nitrocellulose membranes (Hybond C, GE Healthcare, Little Chalfont,
UK). Immunoblots were blocked with 5% skimmed milk in Tris-buffered
saline/Tween 20 (0.05%, v/v) (Sigma-Aldrich) for 1 h at RT. The
membranes were then incubated with the following primary antibodies
overnight at 4°C: Mouse monoclonal anti-poly-adenyl-ribosyl
polymerase (PARP; 1:2,000 dilution; cat. no. 556362; BD
Pharmingen), rabbit monoclonal anti-cytochrome c (1:1,000
dilution; cat. no. 3895-1; Epitomics, Burlingame, CA, USA), rabbit
polyclonal anti-cycloxygenase (COX) IV (1: 500 dilution; cat. no.
GTX101499; GeneTex, San Antonio, TX, USA), rabbit monoclonal
anti-Bax (1:1,000 dilution; cat. no. 1063-1; Epitomics), rabbit
monoclonal anti-Ku70 (1:1,000; cat. no. 2829-1; Epitomics), rabbit
polyclonal anti-active caspase 3 (1:800; cat. no. Ab2302; Abcam,
Cambridge, MA, USA) and mouse monoclonal anti-actin (1:5,000
dilution; cat. no. A1978; Sigma-Aldrich). The membranes were then
incubated with the corresponding secondary antibodies, including
polyclonal goat anti-mouse IgG-H&L (1:7,000 dilution; cat. no.
ab6787; Abcam) and polyclonal goat anti-rabbit IgG-H&L (1:7,000
dilution; cat. no. ab6721; Abcam) conjugated with horseradish
peroxidase at RT for 1 h. Blots were developed using an enhanced
chemiluminescence western blotting detection kit (GE Healthcare).
For co-immunoprecipitation, HCT116 cells were treated with TSA (1
μM) and the cell lysates were precipitated with anti-Ku70
antibodies. The bound proteins were then subjected to SDS-PAGE and
blotted for Bax, as previously described (10).
Ku70 small interfering (si)RNA
transfection
Ku70 siRNA and scrambled siRNA were purchased from
Santa Cruz Biotechnology Inc. A siPORT NeoFX transfection agent
(Ambion Life Techonolgies, Grand Island, NY, USA) was used for the
siRNA transfection. At 24 h post-transfection, cells were treated
with TSA for a further 24 h. Knockdown of Ku70 expression was
confirmed by western blot analysis.
Statistical analysis
All values are presented as the mean ± standard
deviation or a representative of ≥3 independent experiments.
Statistical comparisons were performed using the Student’s
t-test. SPSS version 11.0 (SPSS Inc., Chicago, IL, USA) was
used to perfom all statistical analyses. P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
TSA induces Ku70 acetylation and
apoptosis in CRC cell lines
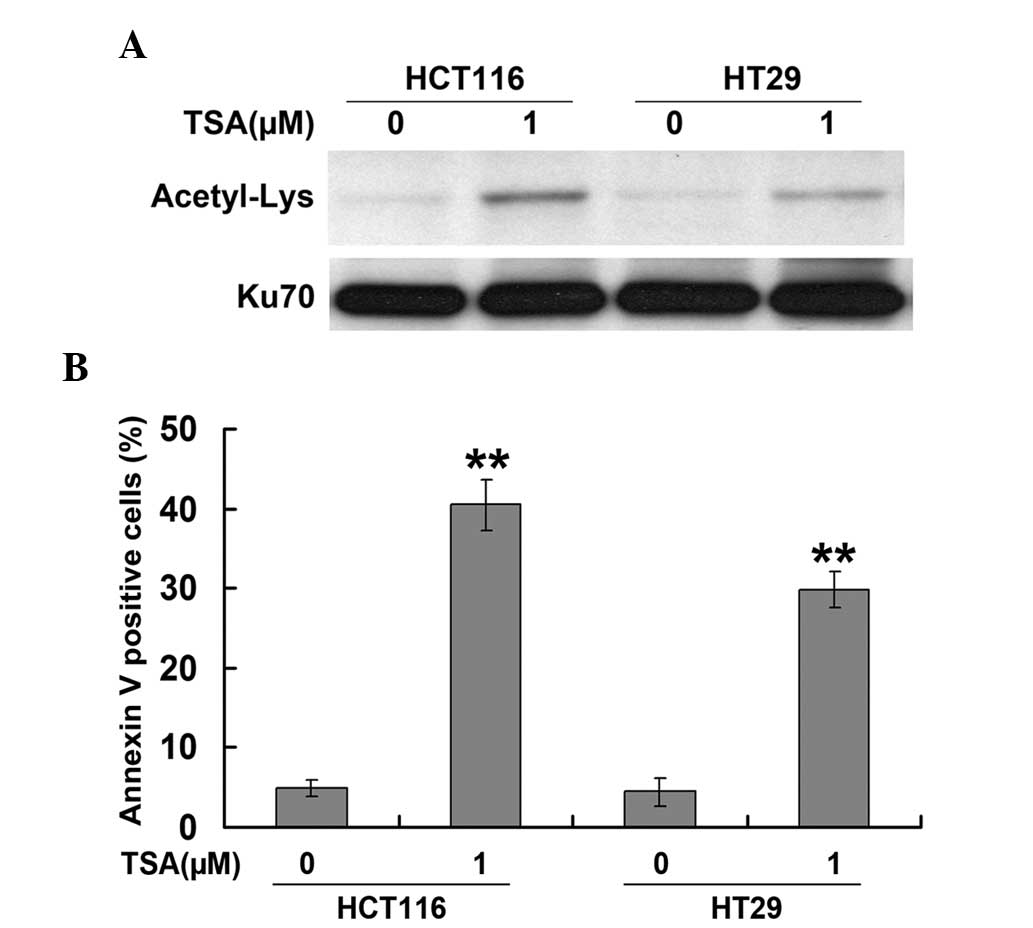
HCT116 and HT29 cells were exposed to various
concentrations of TSA (0.1, 1.0 and 5.0 μM) for 24 h. Ku70
acetylation was detected by western blot and immunoprecipitation
analysis. As shown in Fig. 1A, TSA
induced the acetylation of Ku70 in HCT116 and HT29 cells. In
addition, TSA was found to significantly enhance apoptosis, as
measured using Annexin-V-FITC/PI staining (Fig. 1B), which was in parallel with
changes in Ku70 acetylation levels. These results therefore
suggested that Ku70 acetylation was involved in the mechanism of
TSA-induced apoptosis in CRC cells.
TSA promotes Bax release from its
association with Ku70
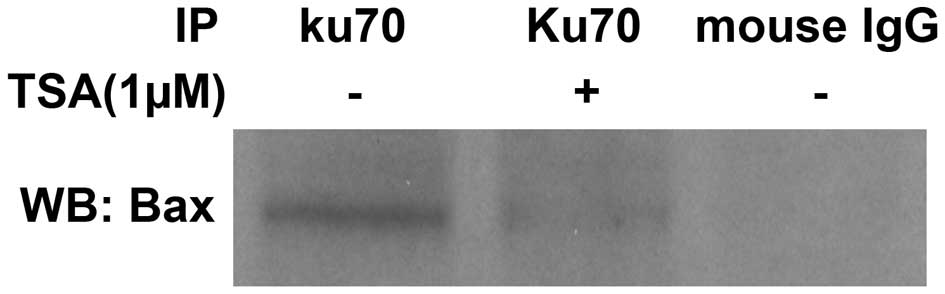
As TSA was found to induce Ku70 acetylation, it was
investigated whether TSA treatment impaired the interaction between
Ku70 and Bax. Co-immunoprecipitation analysis was performed, the
results of which revealed a weakened Bax band in TSA-treated cells
following protein lysate immunoprecipitation by the Ku70 antibody
compared with that of the untreated Bax band (Fig. 2). These results indicated that Bax
was released from its association with Ku70 upon TSA-mediated Ku70
acetylation.
TSA induces Bax translocation from the
cytoplasm to the mitochondria and caspase-3 activation
Bax translocation is an early event in Bax-dependent
apoptosis, which results in damage of the mitochondrial outer
membrane and the release of key apoptosis mediators, such as
cytochrome c, subsequently leading to the activation of
caspase-9 and the downstream cleavage of caspase-3 (11,12).
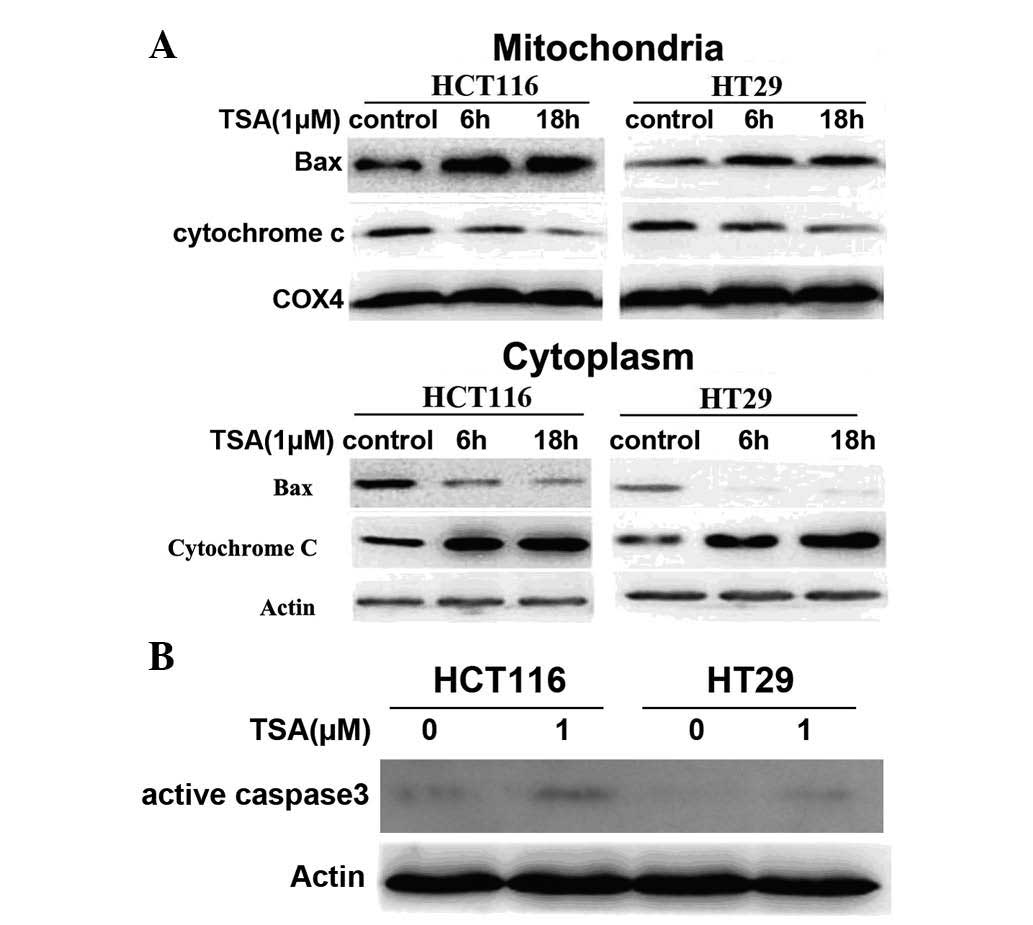
In the present study, Bax localization in the cytosol and
mitochondria was investigated following cell fractionation. This
was performed using immunoblotting of lysates of HCT116 and HT29
cells following exposure to 1 μM TSA. As shown in Fig. 3A, Bax levels were high in the
cytoplasm of control cells and declined following TSA treatment. By
contrast, Bax levels in the mitochondria were increased when
treated with TSA. In addition, cytochrome c levels in the
cytoplasm were increased following TSA treatment, which was
accompanied by a decrease in the mitochondrial fraction (Fig. 3A). Furthermore, western blot
analysis revealed that active caspase 3 expression was increased
following TSA treatment (Fig. 3B),
indicating that TSA enhanced Bax translocation from the cytosol
into the mitochondria, leading to the release of cytochrome
c into the cytosol, which resulted in activation of caspase
3.
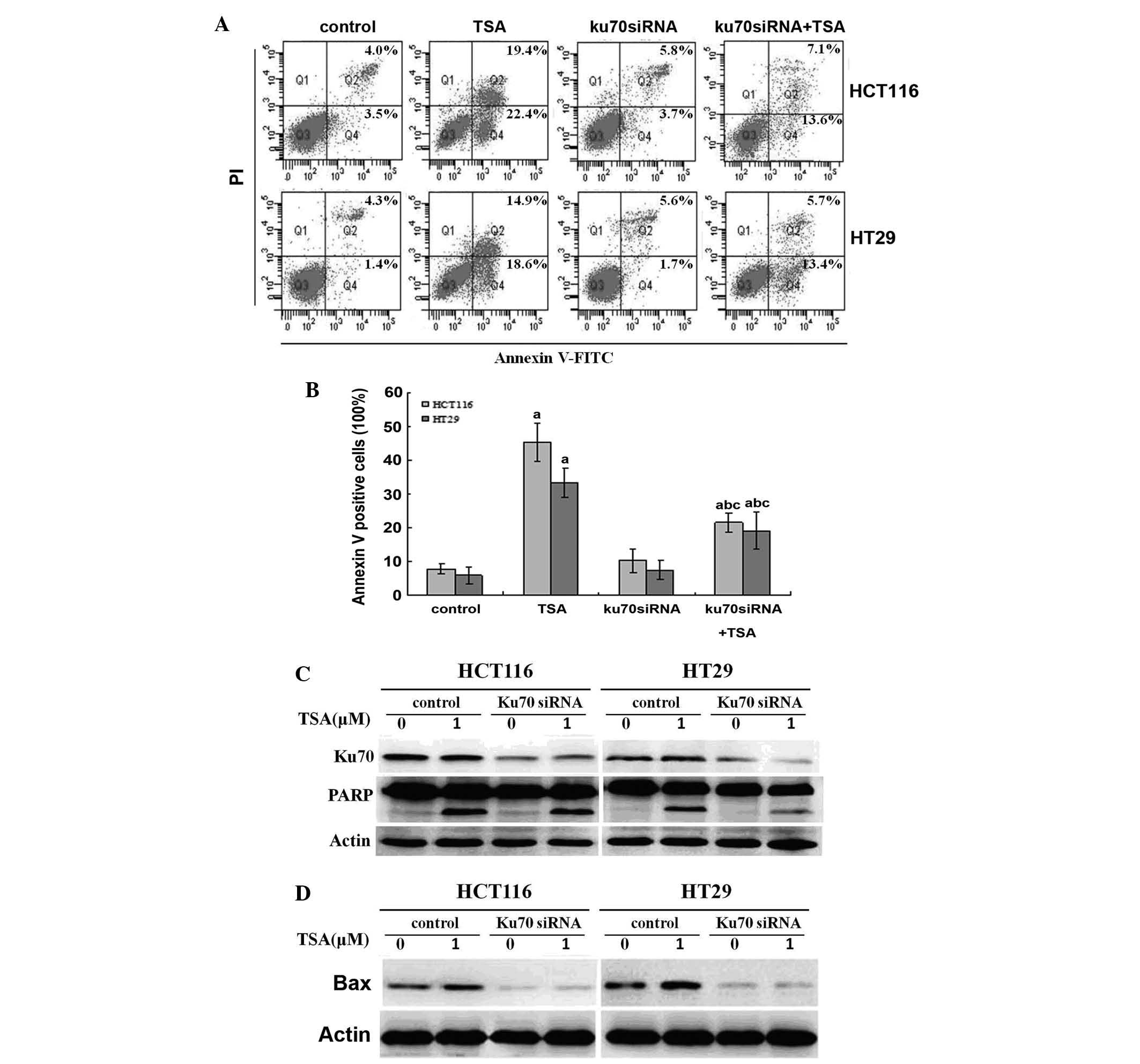
Knockdown of Ku70 via siRNA impairs
TSA-induced apoptosis
Ku70 has previously been reported to regulate
apoptosis through interacting with cytosolic Bax and inhibiting its
trans-location to the mitochondria (8). It was therefore hypothesized that
decreased Ku70 may increase the release of Bax, which translocates
to the mitochondria and initiates apoptosis upon TSA treatment. By
contrast, the results of the present study showed that Ku70 siRNA
impaired TSA-induced apoptosis in CRC cells, as determined by flow
cytometric and western blot analysis (Fig. 4A–C). In addition, this decrease in
apoptosis was accompanied with a decline in Bax expression, as
detected by western blot analysis (Fig. 4D).
Decreased apoptosis by Ku70 siRNA
following TSA treatment is rescued by pre-treating cells with
proteasome inhibitor MG132
The decreased Bax protein levels may be due to
proteasomal degradation, which increases the instability of the
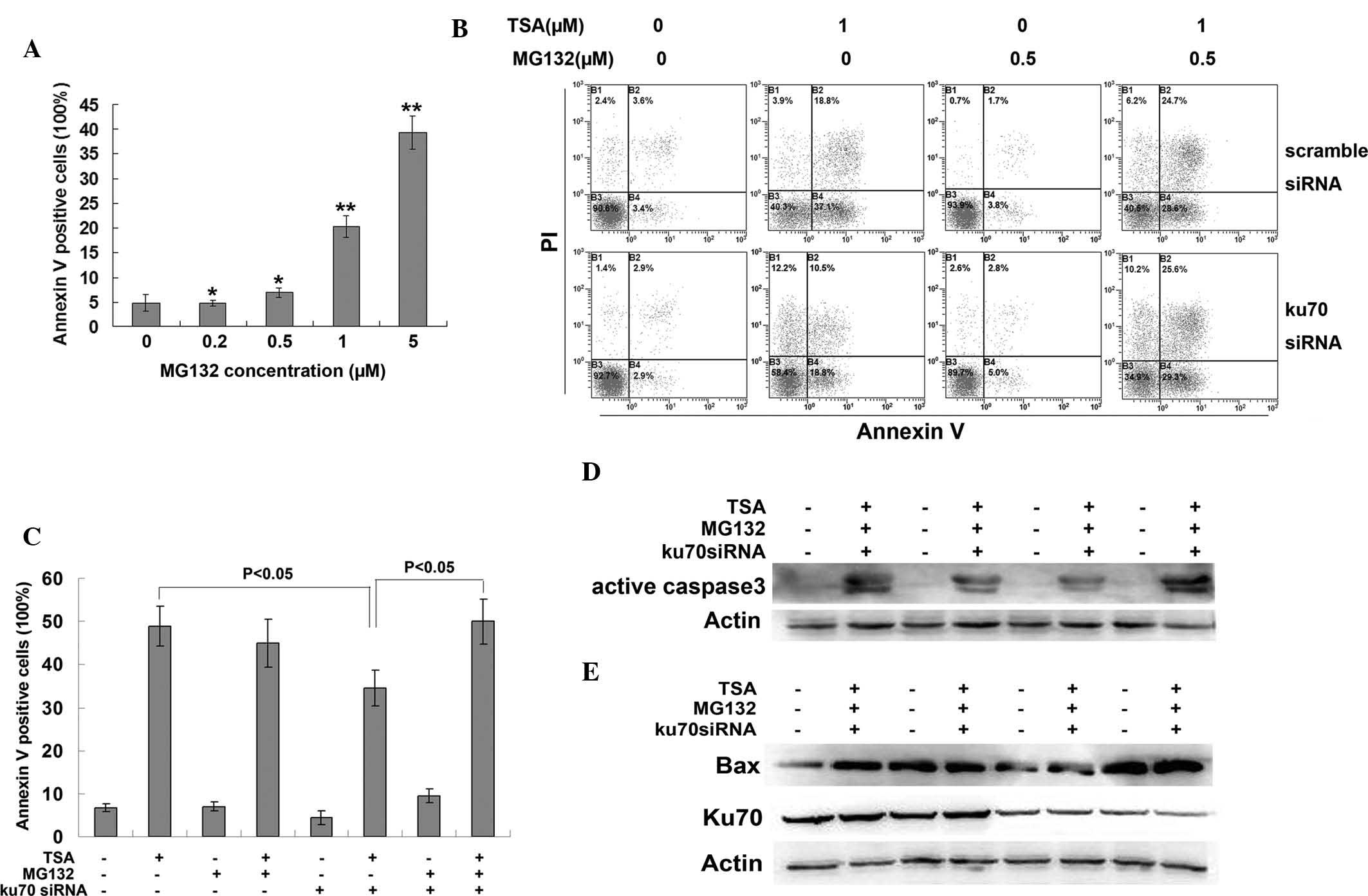
protein. MG132 is a potent proteasome inhibitor, which was found to
induce apoptosis in CRC cells in a dose-dependent manner (Fig. 5A). A low dose of MG132 (0.5
μM), which was shown to have little effect on apoptosis, was
selected for the pre-treatment of HCT116 cells prior to TSA
treatment. MG132 pre-treatment rescued the Ku70 siRNA-induced
decrease in apoptosis (Fig. 5B and
C), as well as decreased the protein expression levels of
activated caspase 3 (Fig. 5D) and
Bax (Fig. 5E). These results
demonstrated that Ku70 may have an important role in the protection
of Bax against proteasomal degradation.
Discussion
HDACIs have been reported to be a class of
relatively selective candidates of anti-cancer agents, which are
thought to induce cell growth arrest and trigger the apoptosis of
tumor cells (13). A previous
study demonstrated that TSA induced apoptosis in CRC cells via
Bax-dependent mechanisms. Bax is a pro-apoptotic protein, which is
essential for the initiation of mitochondria-mediated apoptosis,
the mechanism of which proceeds through permeabilizing the
mitochondrial outer membrane, resulting in cytochrome c
release from mitochondria, which then associates with the 47 kDa
procaspase-9/apoptotic protease activating factor 1 and activates
the caspase cascade, leading to apoptosis (14). It was reported that Bax-initiated
apoptosis was suppressed by Ku70, which interacted with Bax and
sequestering it from the mitochondria (8). Ku70 functions as a DNA repair protein
in the nucleus and as an anti-apoptotic protein through binding to
Bax in the cytoplasm (8). In
neuroblastoma cells, increased Ku70 acetylation was found to result
in the release of Bax from its complex with Ku70, therefore
triggering programmed cell death (15). The results of the present study
have shown that Ku70 acetylation and the subsequent release and
activation of Bax were also involved in TSA-induced apoptosis in
CRC cells; this therefore indicated that this may be an important
mechanism by which Bax-dependent apoptosis was mediated.
Ku70 is known to be a repair protein as well as an
inhibitor of apoptosis through its association with Bax (8). The results of the present study have
also demonstrated that TSA induced cell death in CRC cells through
increasing the acetylation of Ku70, a Bax-binding protein, which
therefore promoted Bax release and translocation from the cytoplasm
into mitochondria in order to stimulate apoptosis. These data
suggested that Ku70 is an inhibitor of Bax-dependent apoptosis. It
was hypothesized that the knockdown of Ku70 may increase
TSA-induced apoptosis. In the present study, Ku70 siRNA was used to
knockdown Ku70 expression in CRC cells followed by treatment with
TSA after 24 h. However, flow cytometric and western blot analyses
revealed a decrease in TSA-induced apoptosis following the
downregulation of Ku70.
Ku proteins, including Ku70 and Ku80, are subunits
of the DNA-dependent protein kinase (DNA-PK) complex, which are
essential for the DNA-binding activity of the complex following DNA
breakage in order to facilitate the DNA repair process (16–18).
It was previously demonstrated that cells which were defective in
any of the DNA-PK subunits, including DNA-PK catalytic subunits
Ku70 and Ku80, were highly sensitive to DNA damage as they were
unable to repair DNA double-strand breaks efficiently (19). Therefore, the role of Ku70 has been
linked to cell survival and carcinogenesis (20). The natural product justicidin A was
reported to induce the apoptosis of CRC cells through decreasing
cytoplasmic Ku70 and increasing the mitochondrial translocation of
Bax (21). In addition, Ku70
depletion was reported to increase sensitivity to x-ray and
radiation-induced caspase-dependent apoptosis in lung cells
(22). However, the results of the
present study demonstrated that decreased Ku70 levels may impair
rather than promote Bax-dependent apoptosis induced by TSA,
therefore suggesting that Ku70 has a complex role in the regulation
of apoptosis.
In order to elucidate the reason why Ku70 knockdown
impaired TSA-induced apoptosis in the present study, Bax protein
levels were detected by western blot analysis, the results of which
revealed decreased Bax expression accompanied with decreased Ku70
expression. This therefore indicated that the decreased stability
of the Bax protein occurred as a result of Ku70 depletion. A
previous study reported that Bax was ubiquitylated and that Ku70
promoted the deubiquitylation of Bax (23). Therefore, in the present study,
HCT116 CRC cells were treated with the proteosomal inhibitor MG132
following Ku70 siRNA transfection, but prior to TSA treatment. The
results showed that MG132 was able to rescue the decreased Bax
expression and apoptosis, which were induced by Ku70 knockdown
prior to TSA treatment. This therefore suggested that Ku70 had an
important role in maintaining the stability of the Bax protein and
Bax-initiated apoptosis.
In conclusion, the results of the present study
demonstrated that Ku70 acetylation mediated Bax-dependent
apoptosis, which was induced by TSA treatment in CRC cells. This
therefore indicated that Ku70 was essential for the protection of
Bax from proteosomal degradation.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 31171344,
81272587 and 81172223) and the General Financial Grant from the
China Postdoctoral Science Foundation (no. 2013M542505).
Abbreviations:
|
TSA
|
trichostatin A
|
|
CRC
|
colorectal cancer
|
|
HDACI
|
histone deacetylase inhibitor
|
References
|
1
|
Jernal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar
|
|
2
|
Johnstone RW: Histone-deacetylase
inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug
Discov. 1:287–299. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu X, Guo ZS, Marcu MG, et al: Modulation
of p53, ErbB1, ErbB2 and Raf-1 expression in lung cancer cells by
depsipeptide FR901228. J Natl Cancer Inst. 94:504–513. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu WG and Otterson GA: The interaction of
histone deacetylase inhibitors and DNA methyltransferase inhibitors
in the treatment of human cancer cells. Curr Med Chem Anti-Cancer
Agents. 3:187–199. 2003. View Article : Google Scholar
|
|
5
|
Meinke PT and Liberator P: Histone
deacetylase: a target for antipro-liferative and antiprotozoal
agents. Curr Med Chem. 8:211–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshida M, Kijima M, Akita M and Beppu T:
Potent and specific inhibition of mammalian histone deacetylase
both in vivo and in vitro by trichostatin A. J Biol Chem.
265:17174–17179. 1990.PubMed/NCBI
|
|
7
|
Meng J, Zhang HH, Zhou CX, Li C, Zhang F
and Mei QB: The histone deacetylase inhibitor trichostatin A
induces cell cycle arrest and apoptosis in colorectal cancer cells
via p53-dependent and -independent pathways. Oncol Rep. 28:384–388.
2012.PubMed/NCBI
|
|
8
|
Sawada M, Sun W, Hayes P, Leskov K,
Boothman DA and Matsuyama S: Ku70 suppresses the apoptotic
translocation of Bax to mitochondria. Nat Cell Biol. 5:320–329.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen HY, Lavu S, Bitterman KJ, et al:
Acetylation of the C terminus of Ku70 by CBP and PCAF controls
Bax-mediated apoptosis. Mol Cell. 13:627–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Bäumer N, Rode M, et al: The
inhibitor of growth protein 5 (ING5) depends on INCA1 as a
co-factor for its antiproliferative effects. PLoS One.
6:e215052011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroemer G: Mitochondrial implication in
apoptosis: towards an endosymbiont hypothesis of apoptosis
evolution. Cell Death Differ. 4:443–456. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reed JC: Cytochrome c: can’t live with
it-can’t live without it. Cell. 91:559–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindemann RK, Gabrielli B and Johnstone
RW: Histone-deacetylase inhibitors for the treatment of cancer.
Cell Cycle. 3:779–788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Giorgi F, Lartigue L, Bauer MK, et al:
The permeability transition pore signals apoptosis by directing Bax
translocation and multimerization. FASEB J. 16:607–609.
2002.PubMed/NCBI
|
|
15
|
Subramanian C, Hada M, Opipari AW Jr,
Castle VP and Kwok RP: CREB-binding protein regulates Ku70
acetylation in response to ionization radiation in neuroblastoma.
Mol Cancer Res. 11:173–181. 2013. View Article : Google Scholar :
|
|
16
|
Collis SJ, DeWeese TL, Jeggo PA, et al:
The life and death of DNA-PK. Oncogene. 24:949–961. 2005.
View Article : Google Scholar
|
|
17
|
He F, Li L, Kim D, et al:
Adenovirus-mediated expression of a dominant negative Ku70 fragment
radiosensitizes human tumor cells under aerobic and hypoxic
conditions. Cancer Res. 67:634–642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shintani S, Mihara M, Li C, et al:
Up-regulation of DNA- dependent protein kinase correlates with
radiation resistance in oral squamous cell carcinoma. Cancer Sci.
94:894–900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto M, Rao S, Tokuno O, et al:
DNA-PK: the major target for wortmannin-mediated radiosensitization
by the inhibition of DSB repair via NHEJ pathway. J Radiat Res.
44:151–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Com E, Lagadec C, Page A, et al: Nerve
growth factor receptor TrkA signaling in breast cancer cells
involves Ku70 to prevent apoptosis. Mol Cell Proteomics.
6:1842–1854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JC, Lee CH, Su CL, et al: Justicidin A
decreases the level of cytosolic Ku70 leading toapoptosis in human
colorectal cancer cells. Carcinogenesis. 26:1716–1730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koike M, Yutoku Y and Koike A: The defect
of Ku70 affects sensitivity to x-ray and radiation-induced
caspase-dependent apoptosis in lung cells. J Vet Med Sci.
75:415–420. 2013. View Article : Google Scholar
|
|
23
|
Amsel AD, Rathaus M, Kronman N and Cohen
HY: Regulation of the proapoptotic factor Bax by Ku70-dependent
deubiquitylation. Cell Biol. 105:5117–5122. 2008.
|