Introduction
Benign prostatic hyperplasia (BPH) is the most
common proliferative disease of the prostate in aged males.
Symptoms of BPH include urinary urgency, dysuria, nocturia and
increased frequency of urination, and, if left untreated, the
disease may lead to recurrent urinary tract infections and acute
urinary retention, and thereby affect the quality of life (1,2). BPH
has been defined as a progressive hyperplasia of glandular and
stromal tissues around the urethra. It is characterized by a
hyperplastic process predominantly of stromal cells, and was able
to be stimulated by local paracrine, autocrine growth factors as
well as inflammatory cytokines (3–5). One
of these cytokines is basic fibroblast growth factor (bFGF), a
member of the family of heparin-binding polypeptide growth factors,
which has been implicated in the pathogenesis of BPH by promoting
abnormal proliferation of stromal cells (6,7).
Despite the prevalence of BPH, its pathogenesis has
remained controversial. The androgenic hormones testosterones and
dihydrotestosterone have a significant role in this process, which
is at least of permissive nature (8). Growth factors and other hormones
including estrogens may also have a function in this process
(8). Multiple partially
overlapping and complementary theories have been proposed,
including embryonic reawakening, stem cell defects, chronic
inflammation, imbalance between androgen/estrogen signaling and
increased TGF-β signaling, all of which partly explain for the
abnormal growth observed in BPH. However, it is now widely accepted
that increases in the total number of stromal and epithelial cells,
resulting from excessive cell proliferation and/or reduction of
cell apoptosis, have a critical role in the development of BPH
(9–11).
The division and duplication of all cells, including
prostatic stromal and epithelial cells, is regulated by the cell
cycle. G1/S transition is one of the two main checkpoints used by
cells to regulate the cell cycle progress and thus the cell
proliferation. G1/S progression is tightly regulated by cyclin D1
and cyclin-dependent kinase 4 (CDK4) (12,13).
PCNA is an acidic nuclear protein that has been recognized as a
histological marker for the G1/S phase transition of the cell cycle
(14). Therefore, the expression
levels of PCNA, CDK4 and cyclin D1 reflect the proliferative state
of BPH cells to a certain extent.
To date, no completely effective treatment for BPH
has been developed. Besides prostatic surgery, alpha (1)- adrenergic-receptor antagonists [alpha
(1) ARAs] and 5 alpha-reductase
inhibitors (5ARIs) are two major drug classes which have remained
to be used in treating the BPH (15,16).
However, all these therapies may have side effects, including
orthostatic hypotension, decreased libido and erectile dysfunction.
Therefore, a number of herbal medicines that appear to have limited
adverse effects have gained increasing popularity in the use for
treating BPH (17). Saw palmetto,
Pygeum africanum and Ginkgo biloba leaf extract
(18–20) have long been used to treat BPH
successfully.
Qianliening capsules (QC) are a widely used
Traditional Chinese Medicinal formulation consisting of Rheum
palmatum L., Hirudo Medicinalis, Astragalus
membranaceus (Fisch.) Bunge, Achyranthes aspera and
Cuscuta chinensis Lam., which has long been used in the
clinic and has been shown to be effective in the treatment of BPH
(21–24). QC is able to obviously improve a
number of lower urinary tract symptoms (LUTS) in BPH patients,
including frequency of urination, urinary urgency, thin urine flow
and certain other voiding disorders. Previous in vivo and
in vitro studies by our group showed that QC significantly
decreased the prostatic volume and weight in BPH model rats via the
promotion of apoptosis, suppression of the EGFR/STAT3 signaling
pathway and regulation of the expression of sex hormones as well as
their receptors (21–24). However, the underlying mechanism of
its anti-BPH activity remains to be fully elucidated. Therefore,
the present study aimed to evaluate the therapeutic effect of QC on
a rat model of BPH, which was generated by castration and
subcutaneous injection with testosterone propionate, and the
underlying molecular mechanism of the anti-proliferative activity
of QC was investigated. In addition, a model of stromal hyperplasia
was generated by stimulation of the normal human prostate stromal
cell line WPMY-1, a myofibroblast stromal cell line derived from
stromal cells of a normal adult prostate, with bFGF, and this in
vitro model was used to further verify the anti-proliferation
mechanism of QC.
Materials and methods
Materials and reagents
QC was provided by the Academy of Pharmacology of
Fujian University of Traditional Chinese Medicine (Fuzhou, China;
FDA approval no. Z09104065). QC was extracted by
ultrasonic-assisted extraction. The high-performance liquid
chromatography (HPLC) analysis method of QC, which was previously
established by our group (25),
confirmed its identity and stability according to the drug
requirements of China (23). QC
were ground into powder, dissolved in distilled water and stored at
4°C. Testosterone propionate injection solution (25 mg/ml) was
obtained from the Shanghai General Pharmaceutical Co., Ltd. (batch
no. H31020524; Shanghai, China). Fetal bovine serum (FBS),
Dulbecco’s Modified Eagle’s Medium (DMEM) and TRIzol reagent were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
bFGF was obtained from Sigma-Aldrich (St. Louis, MO, USA).
SuperScript II reverse transcriptase was provided by Promega
(Madison, WI, USA). PCNA (#13110; rabbit monoclonal IgG), cyclin D1
(#2978; rabbit monoclonal IgG), CDK4 (#12790; rabbit monoclonal
IgG) and β-actin (#12790; rabbit monoclonal IgG) antibodies as well
as horseradish peroxidase (HRP)-conjugated secondary antibodies
(#7075) were obtained from Cell Signaling Technologies (Danvers,
MA, USA). Cell lysis buffer for western blot analysis,
Bicinchoninic Acid Protein Assay kit, SDS-PAGE gel preparation kit,
SDS-PAGE electrophoresis buffer, western transfer buffer,
polyvinylidene difluoride (PVDF) membrane and Beyo Enhanced
Chemiluminescence (ECL) Plus were all obtained from Beyotime
Institute of Biotechnology (Shanghai, China). A fluorescein
isothiocyanate (FITC)-conjugated annexin V apoptosis detection kit
was purchased from BD Biosciences (San Jose, CA, USA). All the
other chemicals used, unless otherwise stated, were obtained from
Sigma-Aldrich
Experimental animals
Thirty-two specific pathogen-free grade male adult
Sprague-Dawley (SD) rats (200–220 g; 8 weeks old) were purchased
from Shanghai Si-Lai-Ke Experimental Animal Ltd. (Shanghai, China).
The rats were housed in clean pathogen-free rooms in an environment
with controlled temperature (22°C), humidity and a 12-h light/dark
cycle with free access to water and a standard laboratory diet. All
animal treatments were strictly in accordance with international
ethical guidelines and the National Institutes of Health Guide
concerning the Care and Use of Laboratory Animals, and the
experiments were approved by the Institutional Animal Care and Use
Committee of Fujian University of Traditional Chinese Medicine
(Fuzhou, China).
In vivo BPH model construction and drug
administration
The testicles of 24 male SD rats were removed under
anaesthesia with intraperitoneal phenobarbital (50 mg/kg body
weight; New Asia Pharmaceutical Co., Ltd., Shanghai, China). The
remaining eight rats were incised above the pelvic region on the
ventral side and then sutured without cutting off the testicles,
which were allocated as the sham-operated group (Cont). Following
castration, the 24 rats were injected subcutaneously into the
abdomen with testosterone propionate (5 mg/kg) for 28 consecutive
days to induce the BPH, and the rats of the sham-operated group
were subcutaneously administered edible oil as a vehicle control.
Along with the construction of the BPH model (26,27),
the 24 castrated rats were randomly assigned to three experimental
groups with eight animals in each: The model group (Model), the
finasteride group (Finast; Merck & Co., Inc., Rahway, NJ, USA)
and the QC group (QC), which were intragastrically administered
with saline (10 ml/kg), finasteride (0.5 mg/kg) or QC (4.5 mg/kg),
respectively. After four weeks of treatment, all animals were
euthanized using intraperitoneal injection of pentobarbital (100
mg/kg body weight) the prostates from the rats in all groups were
removed, weighed and subjected to reverse transcription
quantitative polymerase chain reaction (RT-qPCR) assays and western
blot analysis.
Prostatic index (PI)
An analytical balance (ME3002E; Mettler-Toledo
International, Inc., Greifensee, Switzerland) was used to measure
the prostate weight (PW) and body weight (BW). The prostatic index
(PI) was calculated as: PW/BW ×100%.
Histological examination
The fixed prostatic tissue was dehydrated in a
graded ethanol series (Nanjing Chemical Reagent Co., Ltd., Nanjing,
China), embedded in paraffin, sliced into serial 5-µm
sections, deparaffinized in xylene (Beyotime Institute of
Biotechnology), rehydrated in a graded ethanol series and then
stained with hematoxylin and eosin (H&E; Beyotime Institute of
Biotechnology) for histological observation under a light
microscope (BX51T-PHD-J11; Olympus Corporation, Tokyo, Japan).
Preparation of cell culture
The human prostate stromal cell line WPMY-1 was
obtained from the cell bank of the Chinese Academy of Science
(Shanghai, China). The cells were grown in DMEM containing 5% (v/v)
FBS, 100 Units/ml penicillin and 100 µg/ml streptomycin in a
37°C humidified incubator with 5% CO2. The cells were
subcultured at 80–90% confluency.
Evaluation of cell viability by MTT
assay
Cell viability was assessed by the MTT colorimetric
assay. WPMY-1 cells were seeded into 96-well plates at a density of
1.0×104 cells/well in 0.1 ml medium. Following
incubation overnight, the cells were stimulated with bFGF, while
unstimulated cells served as a control. The bFGF-stimulated cells
were treated with various concentrations of QC (0, 1, 3 or 5 mg/ml)
for 24, 48 or 72 h, and unstimulated cells were either left
untreated or treated with QC (5 mg/ml) for 24, 48 and 72 h to test
the toxicity of QC. At the end of the incubation, 10 µl MTT
[Sigma-Aldrich; 5 mg/ml in phosphate-buffered saline (PBS; GE
Healthcare Life Sciences, Logan, UT, USA)] was added to each well,
and the samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 µl
dimethylsulfoxide. The absorbance was measured at 570 nm using an
ELISA reader (Model EXL800; BioTek, Winooski, VT, USA).
Observation of morphological changes
WPMY-1 cells were seeded into six-well plates at a
density of 1.0×105 cells/ml in 2 ml medium. Following
incubation overnight, the cells were stimulated with bFGF, while
unstimulated cells served as a control, and bFGF-stimulated cells
were treated with various concentrations of QC (0, 1, 3 or 5 mg/ml)
for 24 h. Cell morphology was observed using a phase-contrast
microscope (BX51T-PHD-J11; Olympus Corporation), with images
captured at a magnification of 20×.
Colony formation
WPMY-1 cells were seeded into six-well plates at a
density of 1×105 cells/well in 2 ml medium. Following
incubation overnight, the cells were stimulated with bFGF, while
unstimulated cells served as a control, and bFGF-stimulated cells
were treated with various concentrations of QC (0, 1, 3 or 5 mg/ml)
for 24 h. The cells were then collected and diluted in fresh medium
in the absence of QC as well as bFGF and then re-seeded into
six-well plates at a density of 1×103 cells/well.
Following incubation for eight days in a humidified incubator with
5% CO2 at 37°C, the colonies were observed using a
phase-contrast microscope at a magnification of 4×, and the
colonies consisting of ≥50 cells were counted.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry
using a FACSCalibur (BD Biosciences) and propidium iodide staining.
bFGF-stimulated WPMY-1 cells were treated with or without QC (3
mg/ml) for 24 h, and unstimulated cells were used as a control. All
the cells were collected and suspensions were adjusted to a
concentration of 1×106 cells/ml, and fixed in 70%
ethanol at 4°C overnight. The fixed cells were washed twice with
cold PBS and then incubated for 30 min with RNase (8 µg/ml;
Sigma-Aldrich) and PI (10 µg/ml; Sigma-Aldrich). The
fluorescent signal was detected through the FL2 channel and the
proportion of DNA in various phases was analyzed using ModfitLT
version 3.0 (Verity Software House, Topsham, ME, USA).
RNA extraction and RT-qPCR analysis
Total RNA from all samples collected from the in
vivo and in vitro studies was isolated with TRIzol
reagent according to the manufacturer’s instructions. Oligo
(dT)-primed RNA (1 µg; Shanghai Yingjun Biotechnology Co.,
Ltd., Shanghai, China) was reverse-transcribed with SuperScript II
reverse transcriptase according to the manufacturer’s instructions.
The obtained cDNA was used to determine the mRNA levels of PCNA,
cyclin D1 or CDK4 by PCR with Taq DNA polymerase (Fermentas; Thermo
Fisher Scientific, Waltham, MA, USA). GAPDH or β-actin was used as
an internal control. The sequences of the primers used for
amplification of PCNA, cyclin D1 and CDK4 in rats are as follows:
PCNA forward, 5′-GAC ACA TAC CGC TGC GAT CG-3′ and reverse, 5′-TCA
CCA CAG CAT CTC CAA TAT-3′; cyclin D1 forward, 5′-GGA GCA GAA GTG
CGA AGA-3′ and reverse, 5′-GGG TGG GTT GGA AAT GAA-3′; CDK4
forward, 5′-CTT CCC GTC AGC ACA GTT C-3′ and reverse, 5′-GGT CAG
CAT TTC CAG TAG C-3′; β-actin forward, 5′-ACT GGC ATT GTG ATG GAC
TC-3′ and reverse, 5′-CAG CAC TGT GTT GGC ATA GA-3′. The primers
used for PCR analysis of the human tissue-derived cell line were as
follows: Cyclin D1 forward, 5′-TGG ATG CTG GAG GTC TGC GAG GAA-3′
and reverse, 5′-GGC TTC GAT CTG CTC CTG GCA GGC-3′; CDK4 forward,
5′-CAT GTA GAC CAG GAC CTA AGC-3′ and reverse, 5′-AAC TGG CGC ATC
AGA TCC TAG-3′; GADPH forward, 5′-CGA CCA CTT TGT CAA GCT CA-3′ and
reverse, 5′-AGG GGT CTA CAT GGC AAC TG-3′. Samples were analyzed by
gel electrophoresis (1.5% agarose). Reactions were carried out in a
C1000 Thermal Cycler (BioRad Laboratories, Inc., Munich, Germany).
The cycling conditions were as follows: Initial denaturation at
95°C for 1 min (1 cycle), denaturation at 94°C for 45 sec and
annealing at 5°C below melting temperature for 30 sec (35 cycles),
extension at 72°C for 45 sec. A final extension step for 5 min at
72°C was followed by cooling to 4°C. The amplified fragments were
analyzed using ethidium bromide stained 1% agarose gels in 1X TBE
buffer (all from Beyotime Institute of Biotechnology) at 100 V,
until the dye was approximately 75–80% of the way down the gel. The
DNA bands were examined using a Gel Documentation System (Gel Doc
2000; Bio-Rad Laboratories, Hercules, CA, USA).
Western blot analysis
Samples collected from tissues or cells were lysed
with cold cell lysis buffer containing
phenylmeth-anesulfonylfluoride (Beyotime Institute of
Biotechnology) and subjected to SDS-PAGE. The proteins were then
electrophoretically transferred onto PVDF membranes, blocked, and
then exposed to primary antibodies against PCNA (1:1,000), cyclin
D1 (1:1,000) or CDK4 (1:1,000) overnight at 4°C. β-actin (1:1,000)
was also measured as an internal control for protein loading.
Membranes were then incubated with secondary HRP-conjugated
antibodies at 1:2,500 dilution for 2 h at room temperature followed
by enhanced chemiluminescence detection.
Statistical analysis
All values are expressed as the mean of three
determinations and data were analyzed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Statistical analysis of the data was performed
using Student’s t-test and analysis of variance. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
QC inhibits prostate growth and
ameliorates pathological changes of prostate tissue in a rat model
of BPH
The in vivo therapeutic efficacy of QC
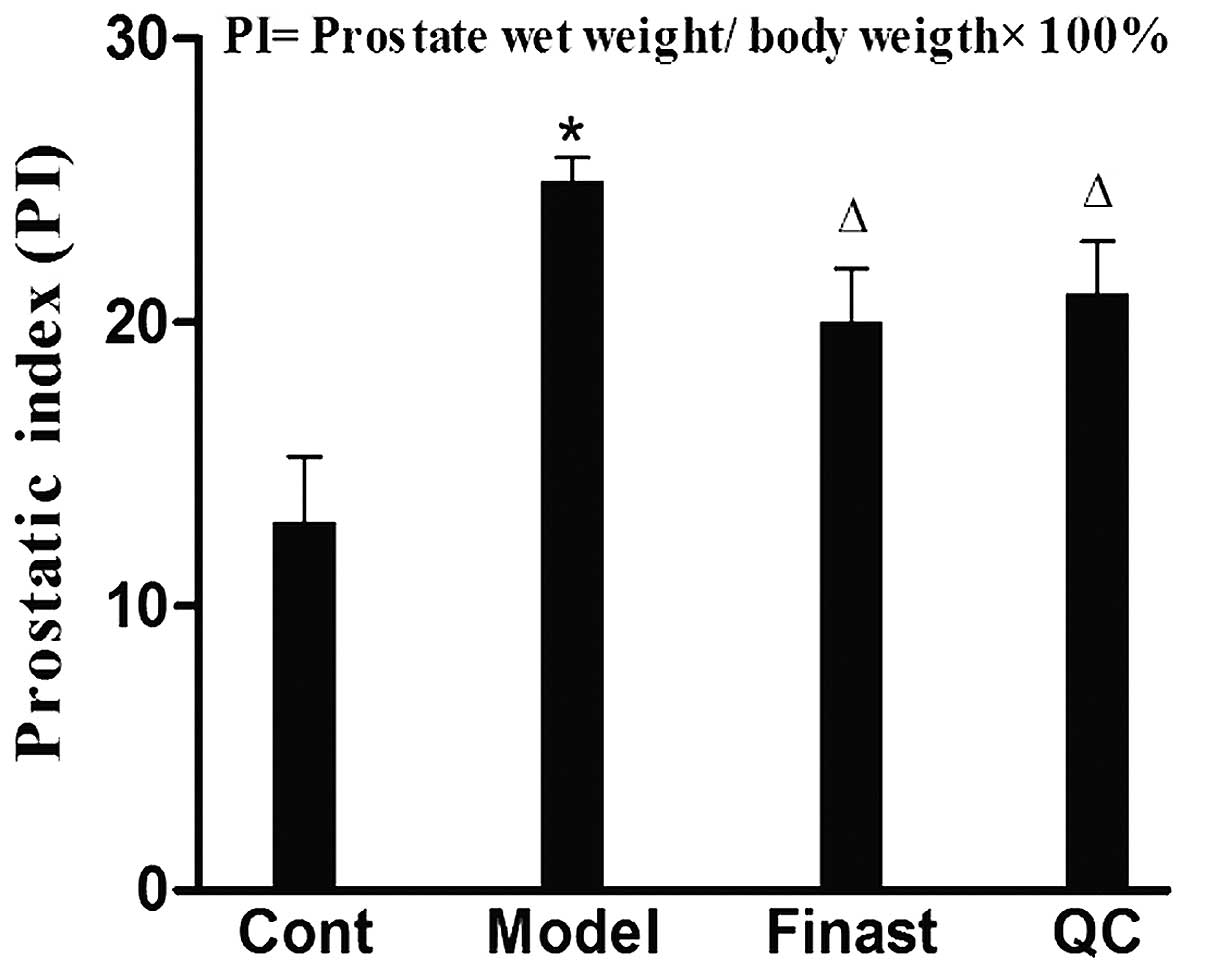
against BPH was evaluated by determining the PI in BPH rats. As
shown in Fig. 1, the mean PI in
the model group was significantly elevated when compared with that
in the control group (P<0.05). However, administration of either
QC or finasteride significantly reduced the PI in BPH rats
(P<0.05), demonstrating the anti-BPH efficacy of QC in
vivo.
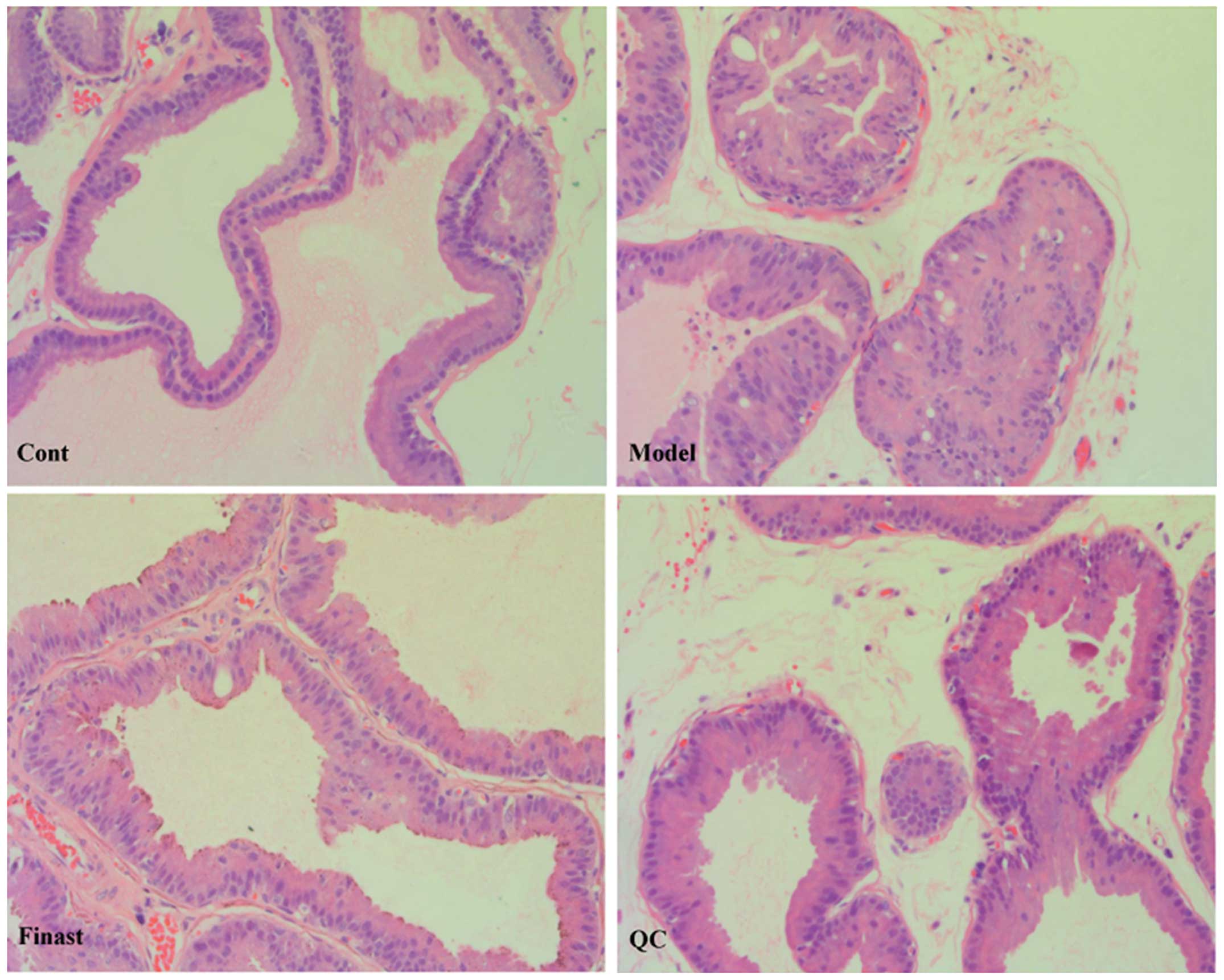
Histological changes in prostate tissue of BPH rats
were observed using light microscopy following H&E staining. As
shown in Fig. 2, low columnar
epithelial cells in the control group were arranged as a
single-layer secretory lumen that was filled with thin acidophilic
materials, whereas the epithelial cells in the model group clearly
proliferated to develop excessive glands and cells were arranged as
multiple unorganized layers. However, the prostate
histopathological damages in BPH rats were significantly
ameliorated by QC treatment finasteride (Fig. 2).
QC treatment inhibits mRNA and protein
expression of PCNA, cyclin D1 and CDK4 in prostatic tissue of BPH
rats
RT-qPCR and western-blot analysis showed that the
mRNA and protein expression levels of PCNA, cyclin D1 and CDK4 in
prostatic tissues of the model group were significantly higher than
those of the control group (P<0.05) (Fig. 3). Of note, treatment with QC or
finasteride profoundly inhibited the protein expression of PCNA,
cyclin D1 and CDK4 in the prostatic tissues of BPH rats (P<0.05)
(Fig. 3).
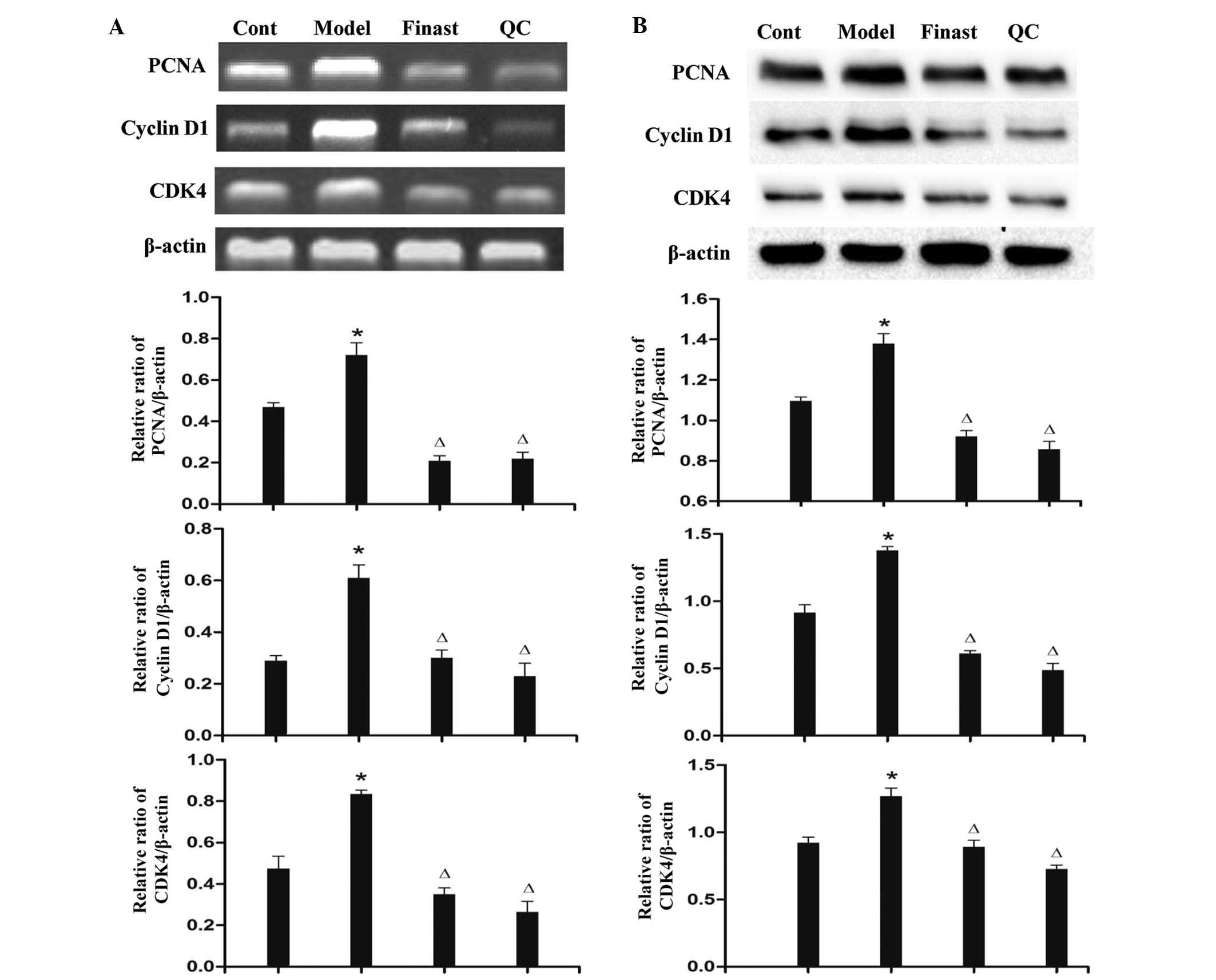 | Figure 3QC treatment inhibited the mRNA and
protein expression of PCNA, cyclin D1 and CDK4 in the prostatic
tissue of BPH rats. (A) The mRNA levels of PCNA, cyclin D1 and CDK4
in different groups were determined by reverse transcription
quantitative polymerase chain reaction. (B) The protein expression
levels of PCNA, cyclin D1 and CDK4 were analyzed by western
blotting. The results were evaluated by densitometric analysis with
β-actin as a loading control. *P<0.05 vs. control group;
∆P<0.05 vs. Model group. Cont, control group; Model,
model group; Finast, finasteride group; BPH, benign prostatic
hyperplasia; QC, qianliening capsules; PCNA, proliferating cell
nuclear antigen; CDK4, cyclin-dependent kinase 4. |
QC suppresses the proliferation ability
of bFGF-stimulated WPMY-1 cells
Histological changes in BPH rats showed that the
main effect of dihydrotestosterone was the induction of glandular
epithelial hyperplasia in the rat prostate, which was consistent
with results from a study by Wang et al (28). However, it is well documented that
BPH is a proliferative process of the stromal as well as epithelial
elements (8–11), and this process was able to be
stimulated by local paracrine and autocrine growth factors
(3–5). Therefore, in the present study, bFGF
was used to stimulate the normal human prostate stromal cell line
WPMY-1, a myofibroblast stromal cell line derived from stromal
cells of the normal adult prostate, to mimic stromal hyperplasia
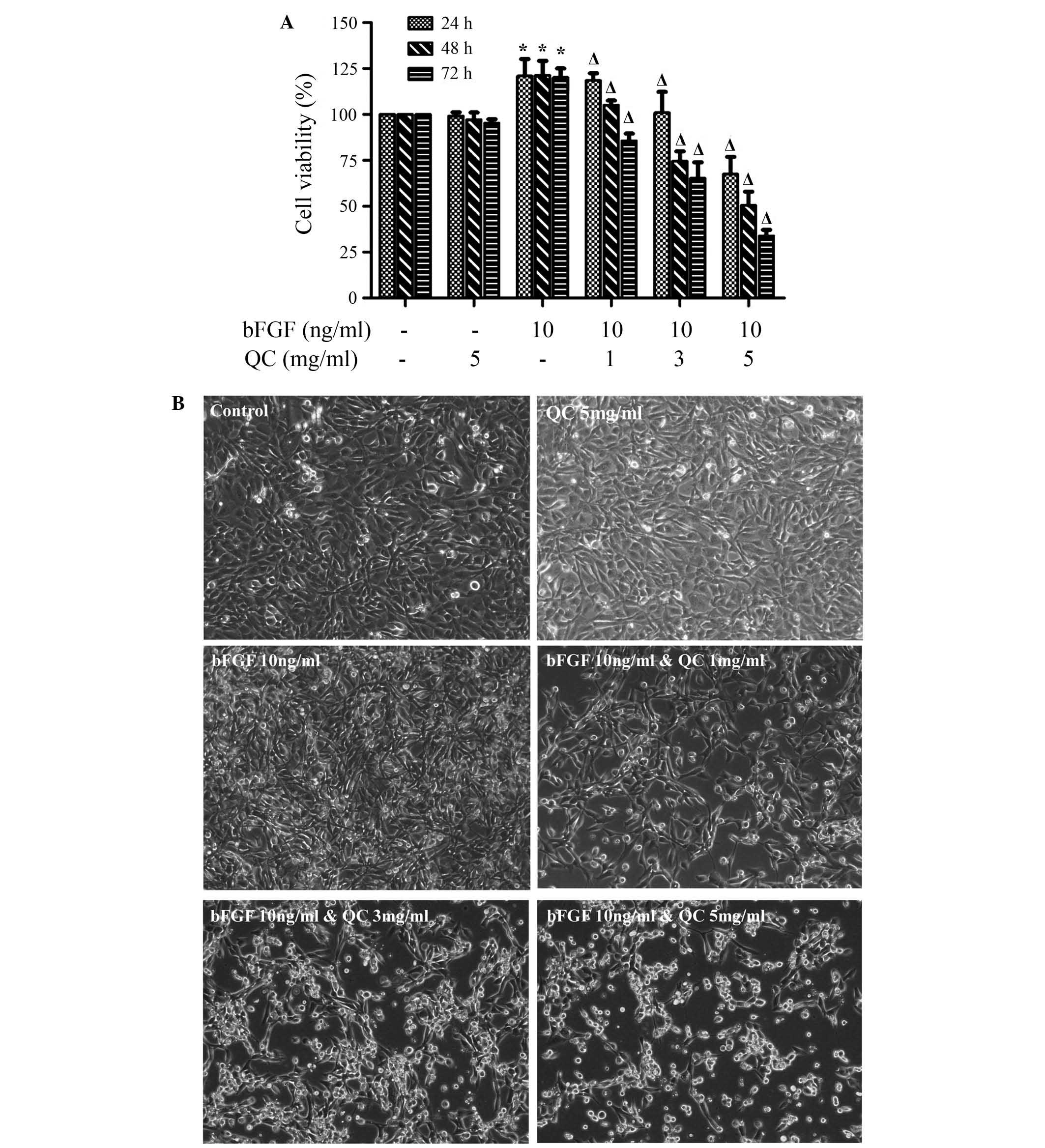
in vitro. The effect of QC on the viability of
bFGF-stimulated and non-bFGF-stimulated WPMY-1 cells was determined
by MTT and colony formation assays. As shown in Fig. 4A, treatment with 1, 3 and 5 mg/ml
QC for different periods of time (24, 48 and 72 h) dose-dependently
and time-dependently reduced the bFGF-induced cell viability
increase in WPMY-1 cells (P<0.05). However, WPMY-1 cells which
were not stimulated with bFGF and treated with 5 mg/ml QC for
different periods of time (24, 48 and 72 h) showed no significantly
change in viability compared to that of untreated control cells
(Fig. 4A). Morphologic observation
by phase-contrast microscopy further verified these results. As
shown in Fig. 4B, bFGF-stimulated
WPMY-1 cells without QC treatment showed an excessive cell density
and cell number compared to those of unstimulated WPMY-1 cells.
However, treatment with 1, 3 and 5 mg/ml QC for 24 h decreased the
cell density and cell number of bFGF-stimulated WPMY-1 cells,
accompanied by morphological changes, including cell shrinkage as
well as round and floating cells. However, no morphological changes
were observed in unstimulated WPMY-1 cells which were treated with
5 mg/ml QC. These results suggested that QC inhibited the growth
and viability of bFGF-stimulated WPMY-1 cells in a dose- and
time-dependent manner, as described previously (21). Furthermore, QC showed no toxic or
proliferative effects on normal WPMY-1 cells which were not
stimulated with bFGF.
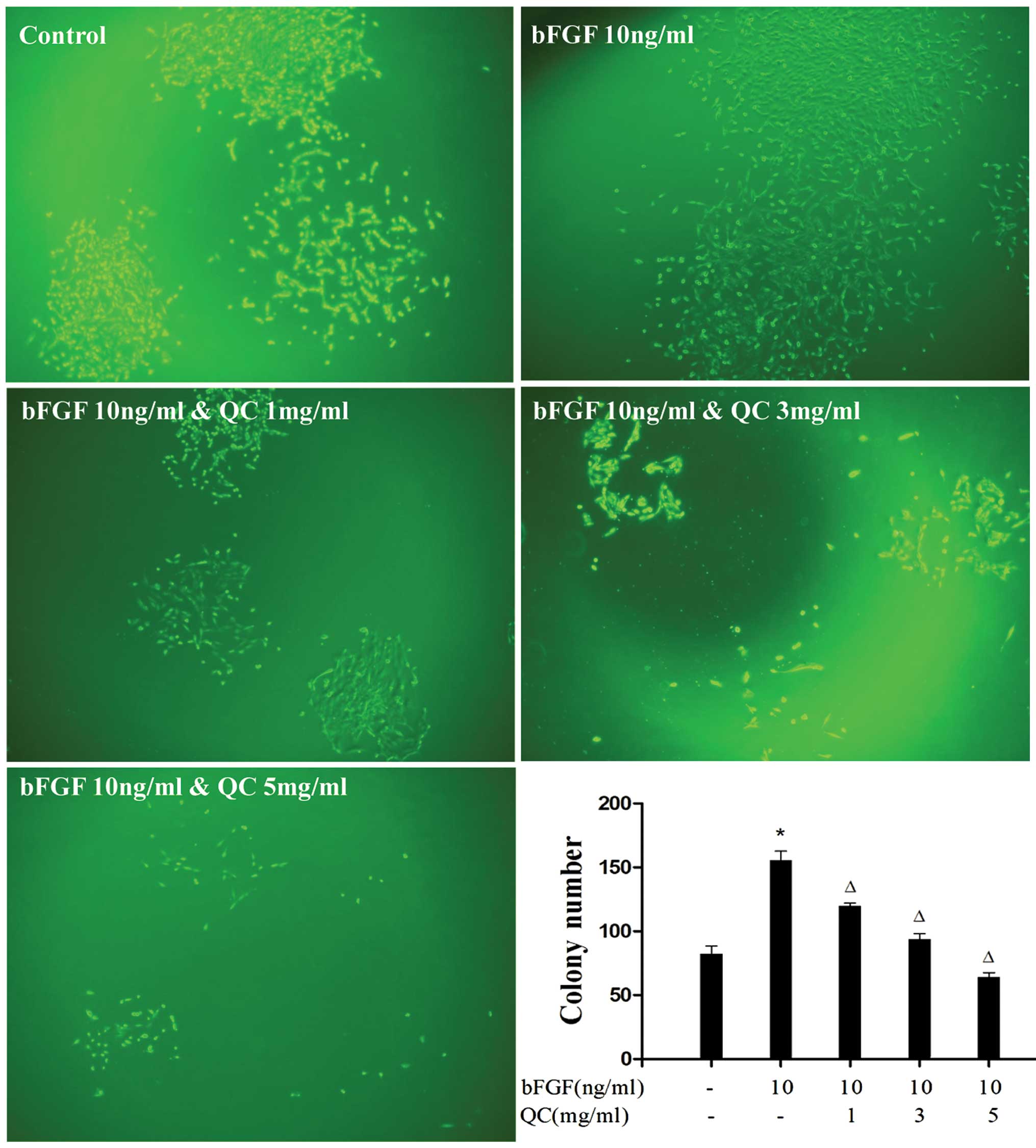
In addition, the present study examined the effect
of QC on the proliferation ability of bFGF-stimulated WPMY-1 cells
by performing a colony formation assay. As shown in Fig. 5, treatment with 1, 3 and 5 mg/ml of
QC for 24 h profoundly suppressed colony numbers (P<0.05) as
well as the colony size in a dose-dependent manner, indicating that
QC suppressed the bFGF-induced WPMY-1 cell proliferation.
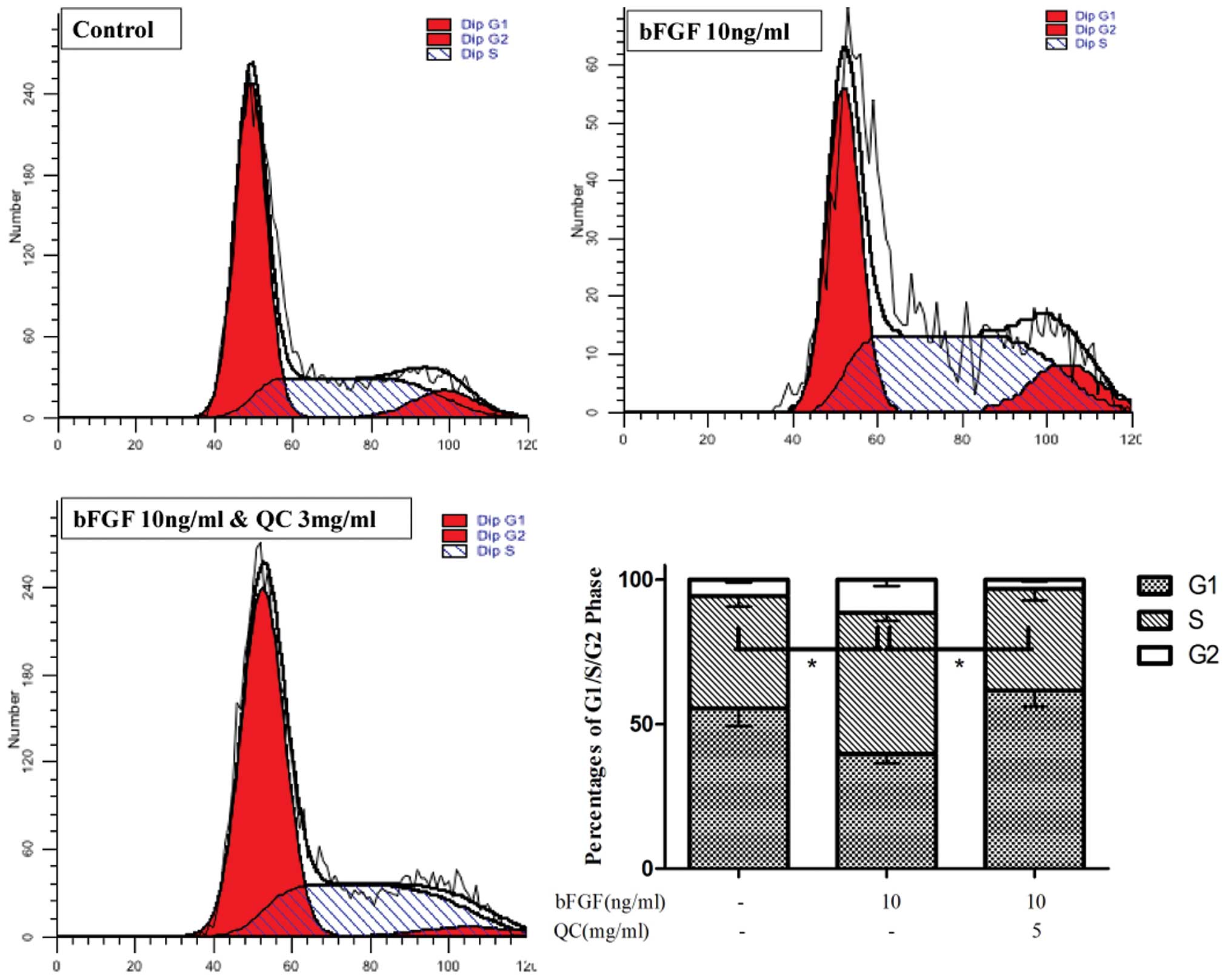
QC blocks G1/S progression of
bFGF-stimulated WPMY-1 cells
G1/S transition is one of the two main checkpoints
used by cells to regulate cell cycle progression and thus, the cell
proliferation. The present study therefore investigated the effect
of QC on the G1 to S progression in bFGF-stimulated WPMY-1 cells
via propidium iodide staining followed by fluorescence-assisted
cell sorting (FACS) analysis. As shown in Fig. 6, the percentage of cells in S-phase
following bFGF stimulation was 48.73±2.59%, compared with
38.86±3.68% in the unstimulated group (P<0.05). Furthermore,
treatment with 5 mg/ml QC resulted in an S-phase population of
35.23±4.11%, which was significantly different from that in the
bFGF-stimulated group without QC treatment (P<0.05). These
results indicated that QC inhibits the proliferation of
bFGF-induced WPMY-1 cells by blocking the G1 to S-phase
transition.
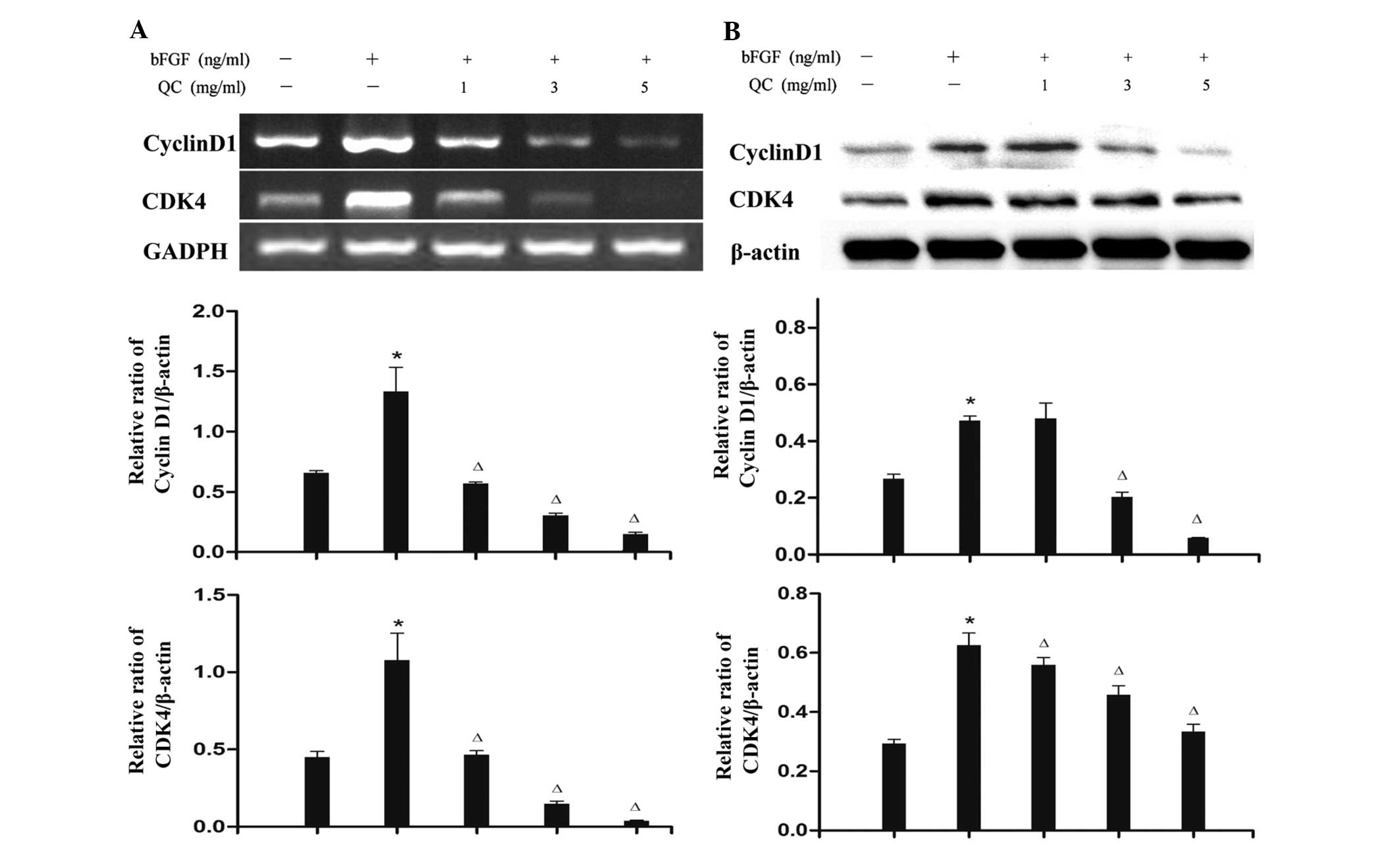
QC regulates the expression of cyclin D1
and CDK4 in bFGF-stimulated WPMY-1 cells
To further verify the mechanism of the
anti-proliferative activity of QC, RT-PCR and western blot analyses
were performed to examine the mRNA and protein expression levels of
cyclin D1 and CDK4 in WPMY-1 cells. As shown in Fig. 7, QC treatment profoundly and
dose-dependently reduced the expression of cyclin D1 and CDK4 at
the transcriptional as well as the translational level.
Discussion
As therapeutic approaches for BPH, alpha (1)ARAs, 5ARIs or surgery are widely
prescribed to decrease functional or mechanical outlet obstruction.
However, as these therapies have side effects, numerous patients
seek herbal remedies for BPH, which may generate less negative
effects and display therapeutic efficacy. As a traditional Chinese
herbal formulation which has long been used in clinical practice,
QC has been shown to be effective in the treatment of BPH (21–24).
In the in vivo experiment of the present study, QC and
finasteride significantly reduced the PI, and ameliorated
histopathological changes and damage of prostate tissue in BPH
rats, which validated the clinical effect of QC. Previous in
vivo and in vitro studies by our group showed that QC
exhibited activity against BPH via the promotion of apoptosis,
suppression of the EGFR/STAT3 signaling pathway and regulating the
expression of sex hormones as well as their receptors (21–24).
However, the mechanism of its anti-proliferative activity still
remained to be fully elucidated.
BPH is considered to be a proliferative process of
the stromal as well as epithelial elements. Cell proliferation is
highly regulated by the cell cycle, which consists of the following
phases: G1 phase, S phase (DNA synthesis phase) and G2/M phase
(mitosis). G1/S transition is one of the two major checkpoints of
the cell cycle (29), and is
responsible for initiation and completion of DNA replication. G1/S
progression is precisely regulated by cyclin D1, which exerts its
function via forming an active complex with its CDK major catalytic
partners (CDK4/6) (30,31). An unchecked or hyper-activated
cyclin D1/CDK4 complex may be responsible for enhanced cellular
proliferation and the alteration of cyclin D1/CDK4 complexes is
increasingly considered to be a possible target for
anti-proliferative therapies (32–34).
PCNA is a 36-kD DNA polymerase delta auxiliary protein involved in
proliferation and it is specifically expressed in proliferating
cell nuclei. PCNA has been recognized as a histological marker for
the G1/S phase of the cell cycle (35). In the present study, it was found
that the expression of PCNA, cyclin D1 and CDK4 was significantly
increased in the BPH model group, which, however, could be
significantly inhibited by QC treatment, as evidenced by RT-PCR and
western blot analyses. Considering the marked epithelial changes in
prostates of BPH rats, the in vivo experiment of the present
study suggested that QC can inhibit prostate cell proliferation as
well as the G1/S transition in prostate epithelial cells of BPH
rats by regulating cyclin D1 and CDK4 expression.
However, it is well documented that BPH is
considered to be a proliferative process of the stromal and
epithelial elements (8–11), and this process was able to be
stimulated by local paracrine and autocrine growth factors
(3–5). Therefore, bFGF was used in the
present study to stimulate the normal human prostate stromal cell
line WPMY-1, a myofibroblast stromal cell line derived from stromal
cells of normal adult prostate, to mimic stromal hyperplasia in
vitro. As expected, QC treatment inhibited the proliferation of
bFGF-stimulated WPMY-1 cells, which was evaluated by cell viability
assay and morphological observation. In addition, a colony
formation assay showed that QC had an inhibiting effect on
bFGF-induced WPMY-1 cell proliferation. Furthermore, cell cycle
analysis showed that QC treatment repressed the G1 to S-phase
transition in bFGF-stimulated WPMY-1 cells. Consistent with the
inhibitory effect of QC on G1/S transition, RT-qPCR and western
blot analyses indicated that QC treatment suppressed the mRNA and
protein expression of the G1/S checkpoint proteins cyclin D1 and
CDK4 in bFGF-stimulated WPMY-1 cells.
In conclusion, the in vivo and vitro
results of the present study suggested that QC exhibits activity
against BPH not only by targeting epithelial cells, but also
stromal cells of the prostate. The underlying mechanism of the
effect of QC against BPH is the inhibition of cell proliferation by
blocking G1 to S-phase transition, which is mediated via
suppression of cell cycle checkpoint proteins.
Acknowledgments
This work was supported by the Nature Science
Foundation of China (grant nos. 81072927 and 81173433).
Abbreviations:
|
QC
|
Qianliening capsule
|
|
BPH
|
benign prostatic hyperplasia
|
|
PI
|
prostatic index
|
|
bFGF
|
basic fibroblast growth factor
|
|
LUTS
|
lower urinary tract symptoms
|
|
alpha (1) ARAs
|
alpha (1)-adrenergic-receptor
antagonists
|
|
5ARIs
|
5 alpha-reductase inhibitors
|
|
DHT
|
5-dihydrotestosterone
|
References
|
1
|
Kirby RS: The natural history of benign
prostatic hyperplasia: what have we learned in the last decade?
Urology. 56(Suppl 5): 3–6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Djavan B: Lower urinary tract
symptoms/benign prostatic hyperplasia 1: fast control of the
patient’s quality of life. Urology. 62(Suppl 3): 6–14. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lucia MS and Lambert JR: Growth factors in
benign prostatic hyperplasia: basic science implications. Curr Urol
Rep. 9:272–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori H, Maki M, Oishi K, et al: Increased
expression of genes for basic fibroblast growth factor and
transforming growth factor type beta 2 in human benign prostatic
hyperplasia. Prostate. 16:71–80. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giri D and Ittmann M: Interleukin-8 is a
paracrine inducer of fibroblast growth factor 2, a stromal and
epithelial growth factor in benign prostatic hyperplasia. Am J
Pathol. 159:139–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartsch G, Brüngger A, Schweikert U,
Hintner H, Höpfl R and Rohr HP: Benign prostatic hyperplasia: a
stromal disease. Urologe A. 28:321–328. 1989.In German. PubMed/NCBI
|
|
7
|
Djavan B, Remzi M, Erne B and Marberger M:
The pathophysiology of benign prostatic hyperplasia. Drugs Today
(Barc). 38:867–876. 2002. View Article : Google Scholar
|
|
8
|
Roehrborn CG: Pathology of benign
prostatic hyperplasia. Int J Impot Res. 20(Suppl 3): 11–18. 2008.
View Article : Google Scholar
|
|
9
|
Zhang MD, Zhao YN and An LW: B-cell
lymphoma/leukemia-2 and benign prostatic hyperplasia. Chin J
Androl. 15:452–454. 2009.In Chinese.
|
|
10
|
Kyprianou N, Tu H and Jacobs SC: Apoptotic
versus proliferative activities in human benign prostatic
hyperplasia. Hum Pathol. 27:668–675. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Claus S, Berges R, Senge T and Schulze H:
Cell kinetic in epithelium and stroma of benign prostatic
hyperplasia. J Urol. 158:217–221. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Robles AI, Martinez LA, Liu F,
Gimenez-Conti IB and Conti CJ: Expression of G1 cyclins,
cyclin-dependent kinases and cyclin-dependent kinase inhibitors in
androgen-induced prostate proliferation in castrated rats. Cell
Growth Differ. 7:1571–1578. 1996.PubMed/NCBI
|
|
13
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
14
|
Zhong W, Peng J, He H, et al: Ki-67 and
PCNA expression in prostate cancer and benign prostatic
hyperplasia. Clin Invest Med. 31:E8–E15. 2008.PubMed/NCBI
|
|
15
|
McVary KT: A review of combination therapy
in patients with benign prostatic hyperplasia. Clin Ther.
29:387–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schulman CC: Lower urinary tract
symptoms/benign prostatic hyperplasia: minimizing morbidity caused
by treatment. Urology. 62(3 Suppl 1): 24–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lieber MM: Pharmacologic therapy for
prostatism. Mayo Clin Proc. 73:590–596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilt T, Ishani A, Stark G, MacDonald R,
Mulrow C and Lau J: Serenoa repens for benign prostatic
hyperplasia. Cochrane Database Syst Rev. CD0014232000.PubMed/NCBI
|
|
19
|
McPartland JM and Pruitt PL: Benign
prostatic hyperplasia treated with saw palmetto: a literature
search and an experimental case study. J Am Osteopath Assoc.
100:89–96. 2000.PubMed/NCBI
|
|
20
|
Peng CC, Liu JH, Chang CH, et al: Action
mechanism of Ginkgo biloba Leaf extract intervened by exercise
therapy in treatment of benign prostate hyperplasia. Evid Based
Complement Alternat Med. 2013:4087342013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong ZF, Lin JM, Zhong XY, et al:
Qianliening capsule inhibits human prostate cell growth via
induction of mitochondrion-dependent cell apoptosis. Chin J Integr
Med. 18:824–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Zhou J, Xu W, Zhong X, Hong Z and
Peng J: Qianliening capsule treats benign prostatic hyperplasia via
suppression of the EGF/STAT3 signaling pathway. Exp Ther Med.
5:1293–1300. 2013.PubMed/NCBI
|
|
23
|
Zheng H, Xu W, Lin J, Peng J and Hong Z:
Qianliening capsule treats benign prostatic hyperplasia via
induction of prostatic cell apoptosis. Mol Med Rep. 7:848–854.
2013.PubMed/NCBI
|
|
24
|
Zhou J, Lin J, Wei X, Zhong X, Zheng Y,
Hong Z and Peng J: Qianliening capsule treats benign prostatic
hyperplasia through regulating the expression of sex hormones,
estrogen receptor and androgen receptor. Afr J Pharm Pharmacol.
6:173–180. 2012. View Article : Google Scholar
|
|
25
|
Xu W, Li H, Lin J, et al: Optimization of
the chitosan purification process of Qianleining capsule by
orthogonal test. J Zhejiang Univ Trad Chin Med. 35:571–574.
2011.
|
|
26
|
Shin IS, Lee MY, Ha HK, Seo CS and Shin
HK: Inhibitory effect of Yukmijihwangtang, a traditional herbal
formula against testosterone-induced benign prostatic hyperplasia
in rats. BMC Complement Altern Med. 12:482012. View Article : Google Scholar
|
|
27
|
Wang Y, Shao JC and Zhang SW:
Histomorphological studies on hyperplastic prostate of castrated
rat caused by androgen. Zhonghua Nan Ke Xue. 8:190–193. 2002.In
Chinese.
|
|
28
|
Wang Y, Shao JC and Zhang SW:
Histomorphological studies on hyperplastic prostate of castrated
rat caused by androgen. Chin J Androl. 3:190–193. 2002.In
Chinese.
|
|
29
|
Nurse P, Masui Y and Hartwell L:
Understanding the cell cycle. Nat Med. 4:1103–1106. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nurse P: A long twentieth century of the
cell cycle and beyond. Cell. 100:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Day PJ, Cleasby A, Tickle IJ, et al:
Crystal structure of human CDK4 in complex with a D-type cyclin.
Proc Natl Acad Sci USA. 106:4166–4170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sridhar J, Akula N and Pattabiraman N:
Selectivity and potency of cyclin-dependent kinase inhibitors. AAPS
J. 8:E204–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park KI, Park HS, Kang SR, et al: Korean
Scutellaria baicalensis water extract inhibits cell cycle G1/S
transition by suppressing cyclin D1 expression and
matrix-metalloproteinase-2 activity in human lung cancer cells. J
Ethnopharmacol. 133:634–641. 2011. View Article : Google Scholar
|
|
35
|
Bantis A, Giannopoulos A, Gonidi M, et al:
Expression of p120, Ki-67 and PCNA as proliferation biomarkers in
imprint smears of prostate carcinoma and their prognostic value.
Cytopathology. 15:25–31. 2004. View Article : Google Scholar : PubMed/NCBI
|