Introduction
Lipopolysaccharide (LPS) from Gram-negative bacteria
is a potent stimulus for the inflammatory response by inducing the
upregulation and release of cytokines (1). Previous studies have demonstrated
that LPS can induce inflammation and apoptosis in cardiomyocytes
(2,3). Sepsis, characterized by a systemic
inflammatory response to bacterial infection and progressive
multi-organ dysfunction, is the leading contributor to morbidity
and mortality rates in healthcare administrations worldwide
(4). Sepsis causes cardiac
contractile dysfunction, which is a major cause of morbidity and
mortality in patients with sepsis (5). Pro-inflammatory cytokines, induced by
bacterial LPS endotoxin, contributes to myocardial dysfunction
during sepsis (6–8). Previous reports have identified that
a reduced incidence of sepsis improves outcomes and reduces the
mortality rates in patients with heart failure (9,10).
It has been demonstrated that a reduced systemic inflammatory
response improves cardiac function (11).
Pachymic acid (PA), a lanostane-type triterpenoid
and major component of Poria cocos alcoholic extracts, has
been reported to possess anti-inflammatory and anticancer
properties in various models of cancer and inflammation (12). Triterpenoids, extracted from
plants, are usually used as a type of medicine in a number of Asian
countries (13) for its sedative,
diuretic and tonic effects. Previous studies have revealed that PA
induces apoptosis in prostate cancer cells and suppresses breast
cancer metastasis (14). Another
previous study demonstrated that PA stimulates glucose uptake by
improving the expression and trans-location of glucose transporter
type 4 (15). However, the effect
of PA on LPS-induced H9c2 cell apoptosis and inflammation remains
to be elucidated. The present study investigated whether PA
affected LPS-induced H9c2 cell apoptosis and inflammation and the
possible mechanisms underlying these effects.
Materials and methods
Cell culture
The H9c2 cell line was obtained from the Cell Bank
of Academy of Science (Shanghai, China). The cells were cultured at
a density of 1×106 cells/well in standard Dulbecco’s
modified Eagle’s medium (#C11995; Gibco Life Technologies,
Carlsbad, CA, USA), supplemented with 10% fetal bovine serum
(#10099; Gibco Life Technologies) and 1% penicillin (100 U/ml;
#15140; Gibco Life Technologies) and streptomycin (100 mg/ml;
#15140; Gibco Life Technologies), and were cultured in a
CO2 incubator (Sanyo 18 M; Sanyo, Osaka, Japan) with 5%
CO2 at 37°C (16). The
cells cultured between passages three and five were selected for
use in the subsequent experiments. Cells were seeded at a density
of 1×106 cells per well onto 6-well culture plates for
RT-PCR and 5×105 cells per well onto six-well culture
plates for TUNEL analysis.
Chemicals
PA was obtained from Shanghai Winherb Medical
S&T Development Co. Ltd. (Shanghai, China) and was up to 98% in
purity. The PA was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) at 50 μM and diluted into
DMEM containing <0.1% DMSO, which was also present in the
corresponding controls (normal saline instead of LPS or PA).
Cell viability assay
The cell viability was evaluated using a Cell
Counting kit-8 (CCK-8), according to the manufacturer’s
instructions (17). Briefly,
following the indicated treatment, 10 μl CCK-8 solution was
added to each well of a 96-well plate and, following 4 h incubation
with 5% CO2 at 37°C, the absorbance was measured at 450
nm using a Synergy HT microplate reader (Bio-Tek Instruments, Inc.,
Winooski, VT, USA). The effect of PA on cell viability was
expressed as the percentage cell viability compared with the
control group, which was set at 100%.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL was performed using the in situ Cell
Death Detection kit (Roche Diagnostics, Indianapolis, IN, USA),
according to the manufacturer’s instructions (18). The cells were grown on cover slips
in a 24-well plate and, following treatment, the cells were fixed
in 4% paraformaldehyde (Amresco, LLC, Solon, OH, USA) and
permeabilized using 0.1% Triton X-100 (Amresco, LLC). The cells
were incubated in 13 μl (per cover slip) TUNEL reaction
mixture (Roche, Basel, Switzerland) for 1 h at 37°C. The nuclei
were labeled with 13 μl (per cover slip)
4′,6-diamidino-2-phenyl-indole (DAPI; Invitrogen Life Technologies,
Carlsbad, CA, USA) for 30 sec, and DNA fragmentation was quantified
using a DX51 microscope (Olympus Corporation, Tokyo, Japan) at
high-power magnification (x200). The percentages of TUNEL-positive
cells relative to the DAPI-positive cells were counted by an
investigator in a blinded-manner. Image analysis was performed
using Image Pro-Plus version 6.0 software (Media Cybernetics,
Silver Spring,. MD, USA).
Western blot analysis
Western blotting was performed to determine the
activation states of the mitogen-activated protein kinase (MAPK)
signaling and apoptotic proteins. Cells were lysed in RIPA buffer
(Beyotime Institute of Biotechnology, Shanghai, China). The protein
quantities from each of the samples were determined by creating a
standard curve using a Bicinchoninic Protein Acid assay kit
(#23227; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
followed by normalization of the protein concentration prior to
western blotting. The extracted protein (50 μg) from each
sample were separated on 8–12% SDS-PAGE gels (Google Biological
Technology, Wuhan, China), and the proteins were transferred onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk powder (Yili
Group, Hohhot, China) and were then incubated with the following
primary antibodies overnight at 4°C: Monoclonal rabbit GAPDH
(1:1,000; #5174); phospho-Erk1/2 (1:2,000; #4370); and phospho-p38
(1:1,000; #4511) (Cell Signaling Technology, Inc., Danvers, MA,
USA); polyclonal rabbit p38 (1:200; ab7952; Abcam, Cambridge, UK);
and phospho-p90RSK (1:1,000; #9341; Cell Signaling Technology,
Inc.); monoclonal rabbit p90RSK (1:1,000; ab32203; Abcam); and
cleaved caspase-3 (1:1,000; #9664); polyclonal rabbit cleaved
caspase-9 (1:1,000; #9509); cleaved caspase-8 (1:1,000; #9429)
(Cell Signaling Technology, Inc.); and Bad (1:1,000; ab90435;
Abcam); monoclonal rabbit Bcl-2 (1:1,000; #3498); and polyclonal
rabbit Bcl-xL (1:1,000; #2762) (Cell Signaling Technology, Inc.).
The membranes were subsequently incubated with secondary antibody
(Goat-anti-Rabbit IRDye 800CW or Goat-anti-Mouse IRDye 800CW; Licor
Biosciences, Lincoln, NE, USA) at 25°C for 1 h (18). The signals were detected using an
Odyssey infrared imaging system (Licor Biosciences). The specific
protein expression levels were normalized against the expression of
GAPDH.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using a TRIzol kit
(#15596-026; Invitrogen Life Technologies), according to
manufacturer’s instructions. The RNA concentration was determined
spectrophotometrically (Nanodrop 2000/2000C; Thermo Fisher
Scientific, Inc.). The cDNA synthesis was performed using a cDNA
Synthesis kit (#04896866001; Roche) and 2 μg total RNA. The
PCR amplifications were quantified using a LightCycler 480 SYBR
Green Master mix (#04707516001; Roche) and the results were
normalized against GAPDH. The relative mRNA expression levels of
interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)α were
assessed.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. The differences in the data between two groups was
determined using Student’s t-test. Comparisons between the groups
were assessed by one-way analysis of variance followed by a Tukey’s
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
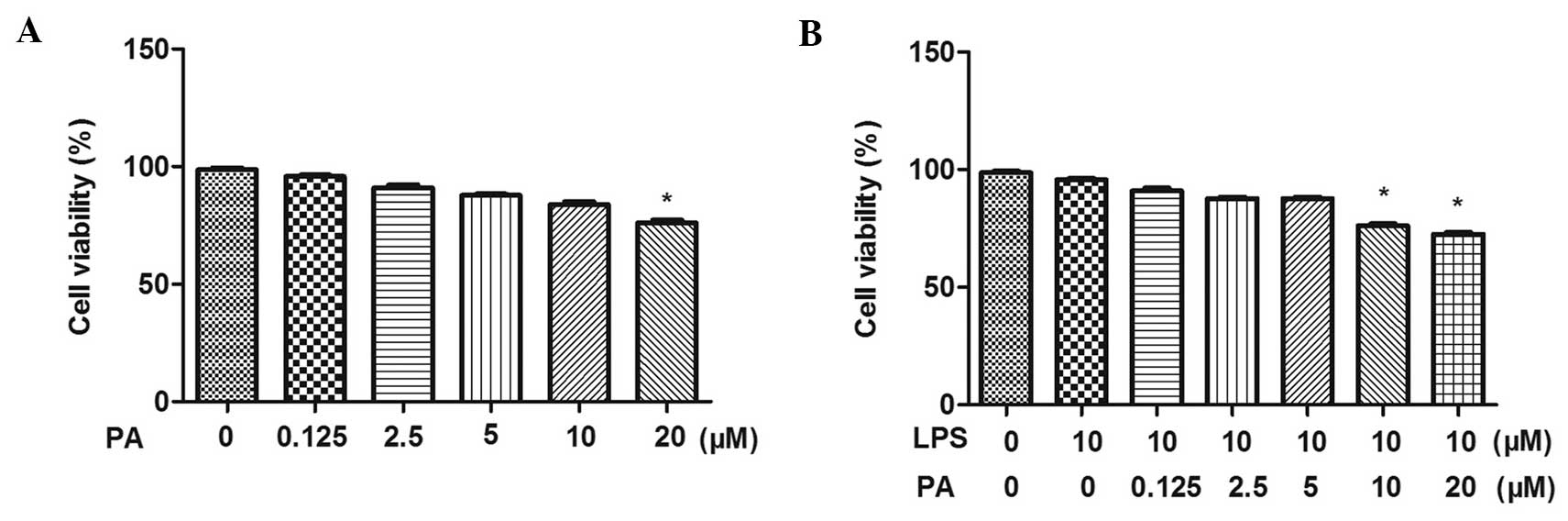
Effect of PA on the viability of
LPS-treated H9c2 cells
The present study determined the effect of PA on
H9c2 cell viability using a CCK-8 assay. As shown in Fig. 1, following incubation with
different concentrations of PA (0.125, 2.5, 5, 10 and 20
μM), with or without LPS for 12 h, the H9c2 cells in three
concentrations of PA (0.125, 2.5 and 5 μM) exhibited no
differences in viability compared with the control cells. This
indicated that neither 0.125, 2.5 or 5 μM PA were not
cytotoxic to the H9c2 cells. Therefore, these three concentrations
of PA were selected for use in the subsequent experiments.
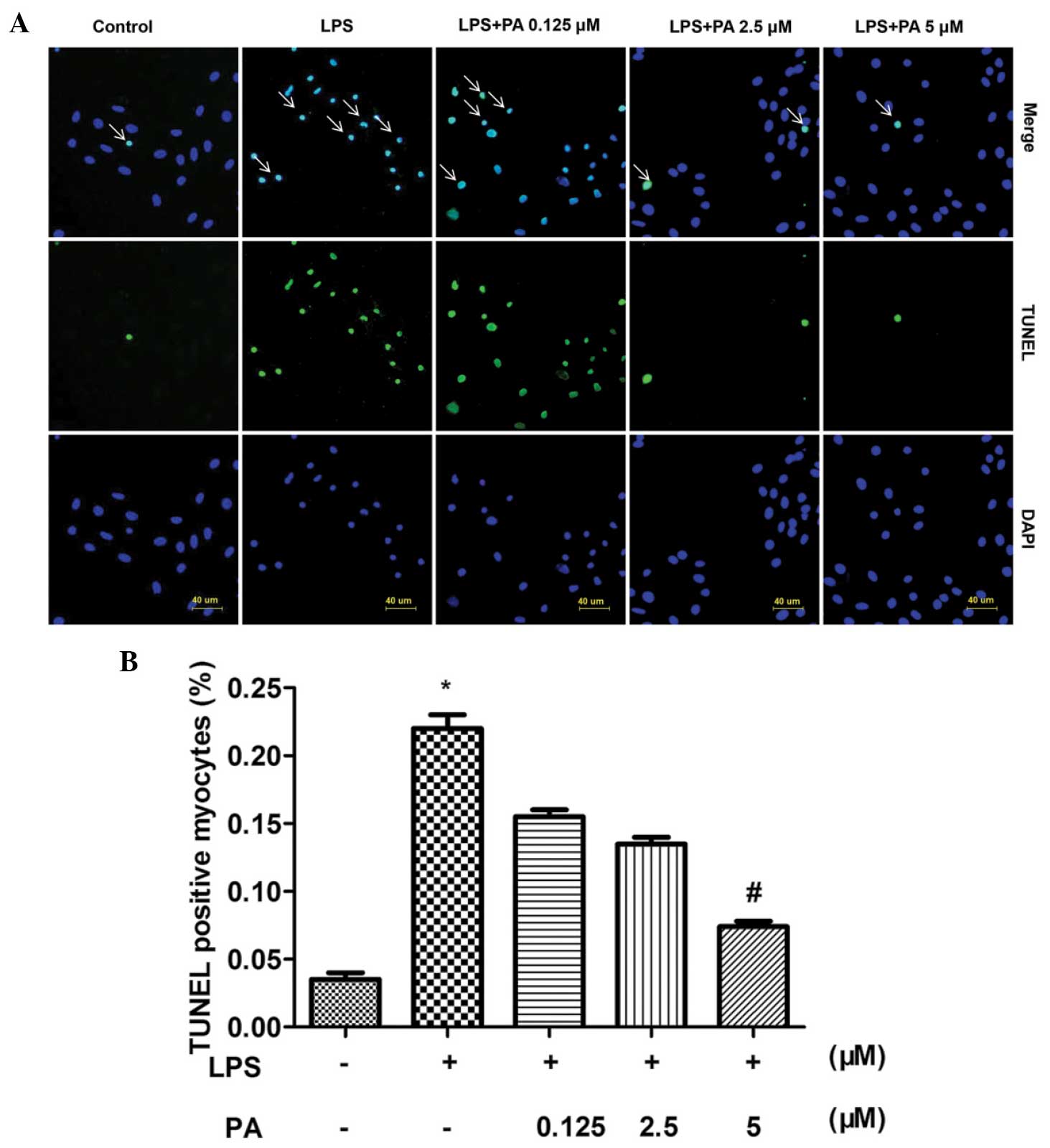
Effect of PA on LPS-induced apoptosis in
H9C2 cells
To investigate the protective role of PA in the
LPS-induced apoptosis of H9c2 cells, TUNEL was used to stain the
apoptotic nuclei. A noticeable increase in cardiomyocyte apoptosis
was observed in the H9c2 cells induced by LPS, and treatment with
PA reduced the LPS-induced apoptosis of cardiomyocytes (Fig. 2).
PA attenuates the LPS-induced increased
mRNA expression levels of IL-1, IL-6 and TNFα
Treatment of the H9c2 cardiomyocytes with LPS (10
μM for 12 h) upregulated the mRNA expression levels of
inflammatory markers, including IL-1, IL-6 and TNFα. The induction
of IL-1, IL-6 and TNFα in response to LPS (10 μM) was
restrained to different extents by different concentrations (0.1,
2.5 and 5 μM) of PA. This indicated that treatment with PA
led to a significant reduction in the mRNA expression levels of the
IL-1, IL-6 and TNFα inflammatory markers in a
concentration-dependent manner. The H9c2 cells were subsequently
co-incubated with PA (5 μM) and LPS (10 μM) for
different durations (0, 1, 6, 12 and 24 h). PA exhibited
significant time-dependent inhibition of the LPS-induced activation
of IL-1, IL-6 and TNFα (Fig.
3).
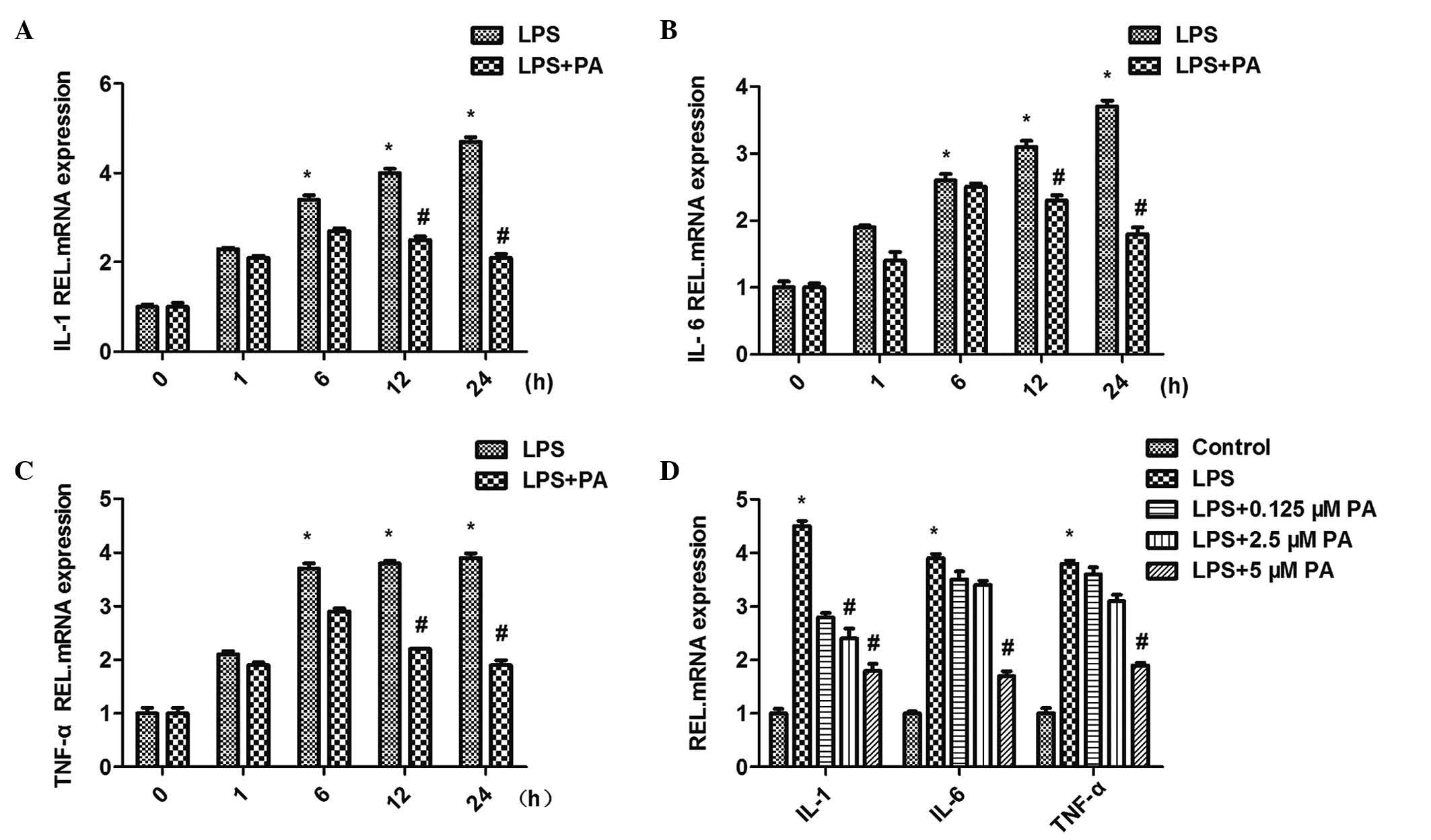 | Figure 3Effect of PA on the mRNA expression
levels of inflammatory mediators, (A) IL-1, (B) IL-6 and (C) TNF-α.
(D) PA decreased LPS-induced expression levels of TNF-α, IL-1β and
IL-6 in a concentration- and time-dependent manner
(*P<0.05, vs. control; #P<0.05, vs.
LPS). Data are expressed as the mean ± standard error of the mean.
PA, pachymic acid; LPS, lipopolysaccharide; IL, interleukin; TNF,
tumor necrosis factor; REL, relative. |
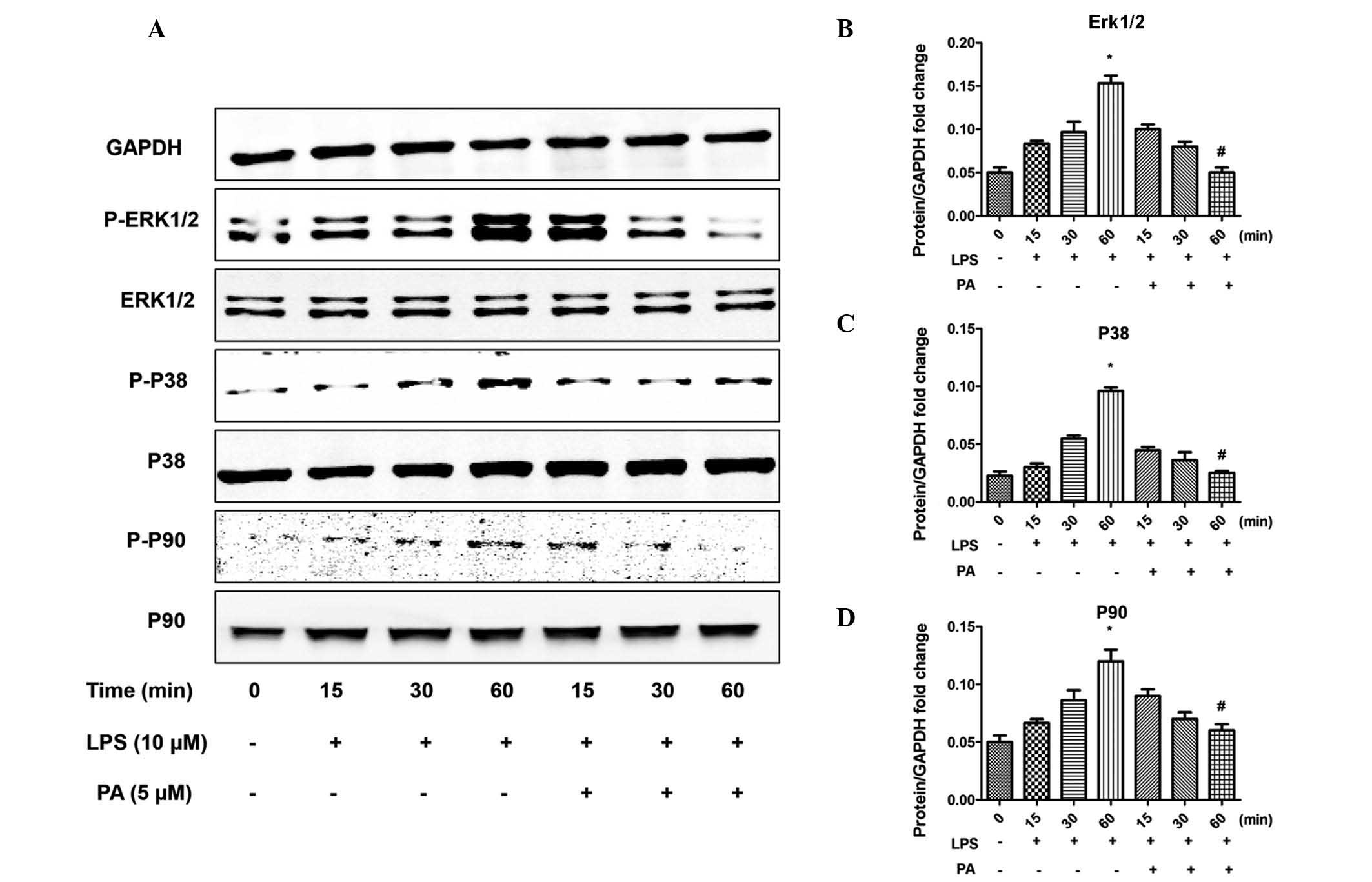
PA regulates the apoptotic pathway and
MAPK signaling
To investigate the molecular mechanisms underlying
the anti-inflammatory and anti-apoptotic effects of PA, the
apoptotic pathway and MAPK signaling pathways were examined. LPS
increased the phosphorylation of extracellular-regulated kinase
(Erk)1/2, p38 and p90, the downstream target of Erk1/2, and
increased the activation of mediators of apoptosis, including
caspase 3, 8 and 9. Notably, PA significantly attenuated these
effects, as shown in Figs. 4 and
5. In addition, the LPS-induced
increased expression of pro-apoptotic B-cell lymphoma (Bcl)-2
family member, Bcl-2-associated agonist of cell death (Bad), was
attenuated by PA, and the opposite result was observed for the
anti-apoptotic Bcl-2 family members, Bcl-2 and Bcl-extra large
(xL).
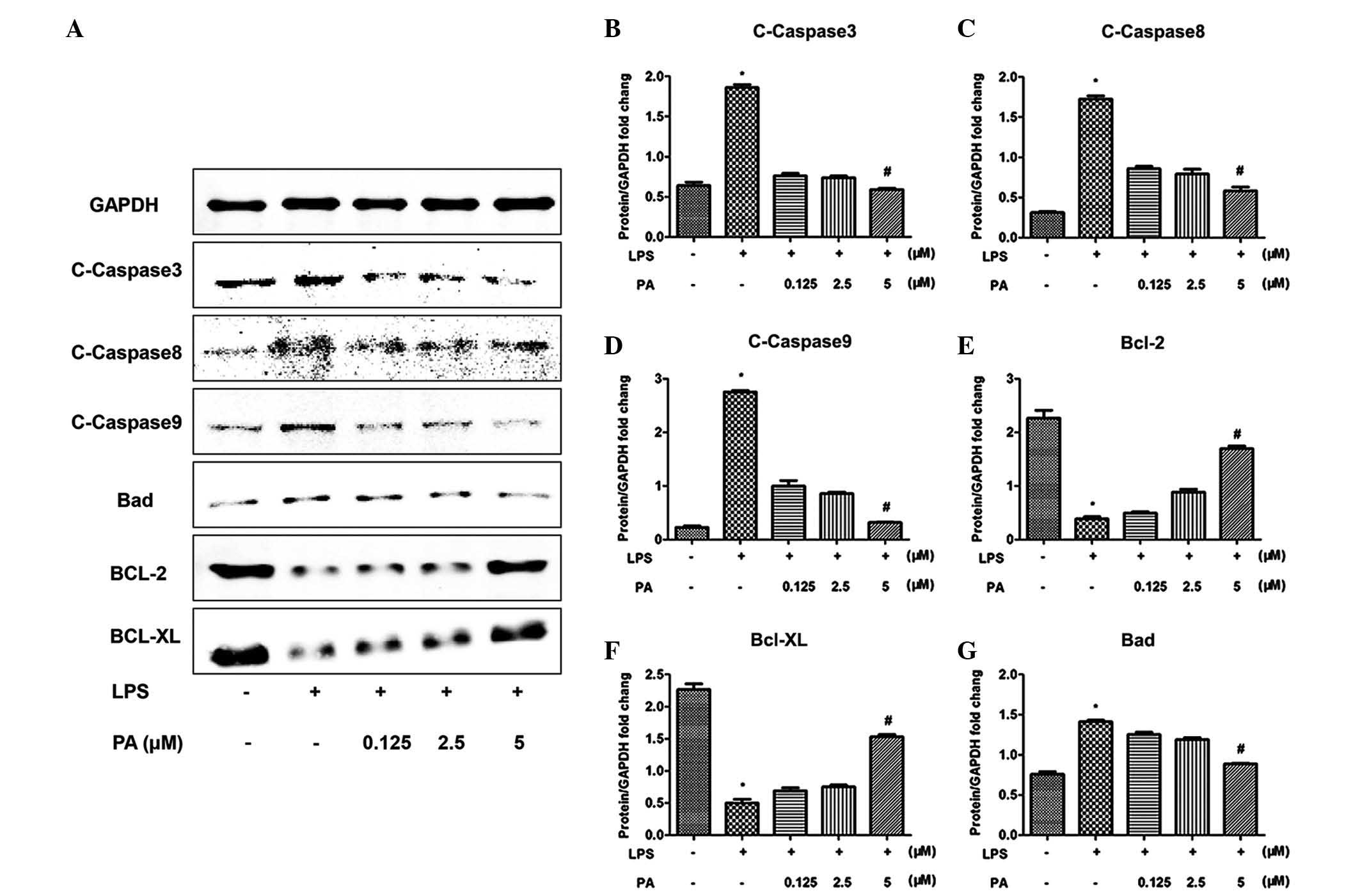 | Figure 5Effect of PA on the activation of
apoptotic signaling pathways. PA decreased the levels of
C-caspase-3, -8 and -9. PA decreased the expression of Bad and
increased the expression of of Bcl-2/Bcl-xL in response to LPS. (A)
Representative western blotting and (B–G) quantitative results
(*P<0.05, vs. control; #P<0.05, vs.
LPS). Data are expressed as the mean ± standard error of the mean.
PA, pachymic acid; C-caspase, cleaved-caspase; LPS,
lipopolysaccharide; Bcl, B-cell lymphoma; Bcl-X, Bcl-2 extra large;
Bad, Bcl-2-associated death promoter; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
Discussion
The PA from P. cocos has been demonstrated in
previous studies to possess anticancer and chemopreventive
properties (12–14). The present study demonstrated that
PA attenuated the LPS-induced apoptosis and inflammation in H9C2
cardiomyocytes. The data suggested that PA effectively attenuated
LPS-stimulated apoptosis in the cardiomyocytes via suppression of
the Erk and p38 signaling pathways. In addition, the present study
demonstrated that the anti-inflammatory property of PA may be
associated with the P38 signaling pathway.
Previous studies have suggested that the plasma
concentrations of LPS are increased in patients with chronic heart
failure, and controlling inflammation may assist in improving
cardiac dysfunction and reducing sepsis-associated mortality
(6). The inflammatory responses
induced by LPS in cardiomyocytes lead to the activation of
intracellular signaling pathways and transcription factors, and the
induction of inflammatory mediators (19), including TNF-α, IL-1 and IL-6.
These mediators may be involved in the depression of cardiac
function (20). PA, a
lanostane-type triterpenoid and a major component of Poria
cocos alcoholic extracts, has been reported to possess
anti-inflammatory and anticancer properties in various models of
cancer and inflammation (12,20–22).
In the present study, PA exhibited anti-inflammatory ability. The
expression levels of TNF-α, IL-1 and IL-6 in the H9c2 cells
following LPS stimulation were examined, and the data revealed that
the increased mRNA expression levels of these factors, induced by
LPS, were attenuated following treatment with PA, indicating the
anti-inflammatory property of PA in cardiomyocytes.
Loss of cardiomyocytes via apoptosis is considered a
contributing factor in progressive deterioration of the
hypertrophied left ventricle, ultimately leading to end-stage
cardiomyopathy (23,24). There has been increased interest in
the effects of apoptosis on cardiomyocytes (25) following establishment that the
cardioprotective effects of apoptosis are decreased by LPS. In the
present study, PA downregulated the expression of Bad and
upregulated the expression of Bcl-2 in the LPS-stimulated H9c2
cells, which may mediate the anti-apoptotic effect of PA. In
addition, the increased expression levels of cleaved-caspase 3, 8
and 9 were attenuated by treatment with PA, suggesting that PA
attenuated the LPS-induced cardiomyocyte apoptosis, including
endogenous and exogenous apoptosis. These findings indicated that,
through its anti-apoptotic property, PA may be beneficial in
certain heart diseases.
Based on the results of the present study that PA
attenuated the LPS-induced inflammatory response and apoptosis in
H9c2 cells, the mechanisms underlying the beneficial effect of PA
were further investigated. Kim et al demonstrated that PA
inhibits oxidative stress induced inflammation in oral diseases
treated with LPS by inhibiting the translocation of nuclear factor
(NF)-κB (12). Ling et al
(13) revealed that PA suppresses
the IL-1β-induced activation of MAPKs and inhibits the IL-1-induced
activation of MAPs and NF-κB signaling pathways in human A549
non-small cell lung cancer cells. It has also been observed that
production of the IL-1, IL-6 and TNFα inflammatory mediators,
contribute to the LPS-induced activation of p38, which belongs to
the MAPK family and is important in the inducting the inflammatory
response and apoptosis (26). It
is possible that the anti-inflammatory property of the PA may be
closely associated with the p38 signaling pathway. It was
demonstrated that LPS activated Erk1/2 and p38, which may lead to
the phosphorylation and activation of p90. However, several
previous studies have demonstrated that p90 activates Bad kinase,
which is a proapoptotic Bcl-2 family protein involved in the
mitochondrial pathway of apoptosis (27). Bad promotes apoptosis by binding to
anti-apoptotic Bcl-2 proteins, including Bcl-xL and Bcl-2, in the
outer mitochondrial membrane, which results in the release of
cytochrome c from the intermembrane space of the
mitochondria into the cytoplasm This promotes the formation of an
apoptosome complex, leading to the cleavage and activation of
procaspase 9 (28,29). Initiator caspases are closely
coupled to pro-apoptotic signals, including Erk1/2 and p38. Once
activated, these caspases cleave and activate downstream effector
caspases, including caspase-3, which lead to apoptosis by cleaving
cellular proteins. In the present study, PA treatment decreased the
LPS-induced activation of Erk1/2 and p38, attenuated the activation
of p90 and Bad, and decreased the expression levels of
cleaved-caspase 3, 8 and 9, suggesting that PA attenuated
LPS-induced apoptosis by inhibiting the Erk1/2 and p38 MAPK
signaling pathways.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, the effect of PA on
LPS-induced inflammation and apoptosis in H9c2 cardiomyocytes,
which may negatively feedback to the Erk1/2 and p38 pathways. These
results provided experimental evidence for the application of PA in
the treatment of inflammatory injury of cardiovascular
diseases.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81270303).
References
|
1
|
Yang S, Li R, Qu X, Tang L, Ge G, Fang W,
et al: Fosinoprilat alleviates lipopolysaccharide (LPS)-induced
inflammation by inhibiting TLR4/NF-κB signaling in monocytes. Cell
Immunol. 284:182–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng YC, Chen LM, Chang MH, Chen WK, Tsai
FJ, Tsai CH, et al: Lipopolysaccharide upregulates uPA, MMP-2 and
MMP-9 via ERK1/2 signaling in H9c2 cardiomyoblast cells. Mol Cell
Biochem. 325:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Z, Liu Y, Deng W, Dai J, Li F, Yuan
Y, et al: Hesperetin attenuates mitochondria-dependent apoptosis in
lipopolysaccharide-induced H9C2 cardiomyocytes. Mol Med Rep.
9:1941–1946. 2014.PubMed/NCBI
|
|
4
|
Sammon JD, Klett DE, Sood A, et al: Sepsis
after major cancer surgery. J Surg Res. 193:788–794. 2015.
View Article : Google Scholar
|
|
5
|
Yang P, Han Y, Gui L, Sun J, Chen YL, Song
R, et al: Gastrodin attenuation of the inflammatory response in
H9c2 cardio-myocytes involves inhibition of NF-κB and MAPKs
activation via the phosphatidylinositol 3-kinase signaling. Biochem
Pharmacol. 85:1124–1133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ronco C: Lipopolysaccharide (LPS) from the
cellular wall of Gram-negative bacteria, also known as endotoxin,
is a key molecule in the pathogenesis of sepsis and septic shock.
Preface Blood Purif. 37(Suppl 1): 12014. View Article : Google Scholar
|
|
7
|
Song Y, Dou H, Gong W, Liu X, Yu Z, Li E,
et al: Bis-N-norgliovictin, a small-molecule compound from marine
fungus, inhibits LPS-induced inflammation in macrophages and
improves survival in sepsis. Eur J Pharmacol. 705:49–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alazawia W, Heath H, et al: Stat2 loss
leads to cytokine-independent, cell-mediated lethality in
LPS-induced sepsis. Proc Natl Acad Sci USA. 110:8656–8661. 2013.
View Article : Google Scholar
|
|
9
|
Hagiwara S, Iwasaka H, Matsumoto S and
Noguchi T: Effect of enteral versus parenteral nutrition on
LPS-induced sepsis in a rat model. J Surg Res. 145:251–256. 2008.
View Article : Google Scholar
|
|
10
|
Hoesel LM, Niederbichler AD and Ward PA:
Complement-related molecular events in sepsis leading to heart
failure. Mol Immunol. 44:95–102. 2007. View Article : Google Scholar
|
|
11
|
Li YP, Huang J, Huang SG, et al: The
compromised inflammatory response to bacterial components after
pediatric cardiac surgery is associated with cardiopulmonary
bypass-suppressed Toll-like receptor signal transduction pathways.
J Crit Care. 29:312.e7–312.e13. 2014. View Article : Google Scholar
|
|
12
|
Kim TG, Lee YH, Lee NH, Bhattarai G, Lee
IK, Yun BS, et al: The antioxidant property of pachymic acid
improves bone disturbance against AH plus-induced inflammation in
MC-3T3 E1 cells. J Endod. 39:461–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ling H, Jia X, Zhang Y, Gapter LA, Lim YS,
Agarwal R, et al: Pachymic acid inhibits cell growth and modulates
arachidonic acid metabolism in nonsmall cell lung cancer A549
cells. Mol Carcinog. 49:271–282. 2010.
|
|
14
|
Gapter L, Wang Z, Glinski J and Ng KY:
Induction of apoptosis in prostate cancer cells by pachymic acid
from Poria cocos. Biochem Biophys Res Commun. 332:1153–1161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YC, Chang WL, Huang SF, Lin CY, Lin
HC and Chang TC: Pachymic acid stimulates glucose uptake through
enhanced GLUT4 expression and translocation. Eur J Pharmacol.
648:39–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du M, Huang K, Gao L, Yang L, Wang WS,
Wang B, et al: Nardosinone protects H9c2 cardiac cells from
angiotensin II-induced hypertrophy. J Huazhong Univ Sci Technolog
Med Sci. 33:822–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D,
et al: Exogenous hydrogen sulfide protects against
doxorubicin-induced inflammation and cytotoxicity by inhibiting
p38MAPK/NFκB pathway in H9c2 cardiac cells. Cell Physiol Biochem.
32:1668–1680. 2013.
|
|
18
|
Chang YM, Tsai CT, Wang CC, Chen YS, Lin
YM, Kuo CH, et al: Alpinate oxyphyllae fructus (Alpinia Oxyphylla
Miq) extracts inhibit angiotensin-II induced cardiac apoptosis in
H9c2 cardiomyoblast cells. Biosci Biotechnol Biochem. 77:229–234.
2013.PubMed/NCBI
|
|
19
|
Angeloni C and Hrelia S: Quercetin reduces
inflammatory responses in LPS-stimulated cardiomyoblasts. Oxid Med
Cell Longev. 2012:8371042012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan MJ, Huang-Liu R, Shen CY, Ju DT, Lin
YM, Pai P, et al: Reduction of TLR4 mRNA stability and protein
expressions through inhibiting cytoplasmic translocation of huR
transcription factor by E (2) and/or ERα in LPS-treated H9c2
cardiomyoblast cells. Chin J Physiol. 57:8–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong R, Shen MH, Xie XH and Ruan SM:
Inhibition of breast cancer metastasis via PITPNM3 by pachymic
acid. Asian Pac J Cancer Prev. 13:1877–1880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee YH, Lee NH, Bhattarai G, Kim GE, Lee
IK, Yun BS, et al: Anti-inflammatory effect of pachymic acid
promotes odontoblastic differentiation via HO-1 in dental pulp
cells. Oral Dis. 19:193–199. 2013. View Article : Google Scholar
|
|
23
|
Kazama K, Okada M, Yamawaki H, et al:
Adipocytokine, omentin inhibits doxorubicin-induced H9c2
cardiomyoblasts apoptosis through the inhibition of mitochondrial
reactive oxygen species. Biochem Biophys Res Commun. 457:602–607.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Picatoste B, Ramirez E, Caro-Vadillo A,
Iborra C, Ares-Carrasco S, Egido J, et al: Sitagliptin reduces
cardiac apoptosis, hypertrophy and fibrosis primarily by
insulin-dependent mechanisms in experimental type-II diabetes.
Potential roles of GLP-1 isoforms. PLoS One. 8:e783302013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Huang H, Fan Y, Kong B, Hu H, Hu
K, et al: Effects of downregulation of microRNA-181a on
H2O -induced H9c2 cell apoptosis via the mitochondrial
apoptotic pathway. Oxid Med Cell Longev. 1–16. 2014:2014.
|
|
26
|
Wang W, Tang L, Li Y and Wang Y: Biochanin
A protects against focal cerebral ischemia/reperfusion in rats via
inhibition of p38-mediated inflammatory responses. J Neurol Sci.
348:121–125. 2015. View Article : Google Scholar
|
|
27
|
Lee KW, Kim SG, Kim HP, Kwon E, You J,
Choi HJ, et al: Enzastaurin, a protein kinase C beta inhibitor,
suppresses signaling through the ribosomal S6 kinase and bad
pathways and induces apoptosis in human gastric cancer cells.
Cancer Res. 68:1916–1926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
29
|
Laulier C and Lopez BS: The secret life of
Bcl-2: Apoptosis-independent inhibition of DNA repair by Bcl-2
family members. Mutat Res. 751:247–257. 2012. View Article : Google Scholar : PubMed/NCBI
|