Introduction
Hepatic fibrosis, a prominent pathological feature
at the end stage of chronic liver disease, is a dynamic process and
develops due to an increase in extracellular matrix (ECM) synthesis
and deposition, along with insufficient remodeling, however,
fibrosis may be reversible prior to the establishment of advanced
architecture (1–3). Matrix metalloproteinase-2 (MMP-2) may
be involved in the formation and reversal of hepatic fibrosis
(4–6). MMP-2 promotes the development of
fibrosis in the early stage, but increased MMP-2 activity can
induce the reversal of fibrosis following removal of the pathogenic
factors (4,6). However, whether MMP-2 has an effect
on the formation of hepatic fibrosis by directly examining hepatic
stellate cells (HSCs) remains to be elucidated, and no clinical
antifibrotic drugs based on MMP-2 have been approved for preventing
fibrosis (5,7).
RNA interference (RNAi) is a strategy, which
involves specifically degrading homogenous mRNA to suppress its
gene expression. Compared with other techniques for inhibiting gene
expression, this method is not only specific, effective and
persistent, but is also simple and safe. However, the vectors for
siRNA have certain bottlenecks, including low transfer efficiency
and inevitable prominent toxicity. Among these vectors, cationic
liposomes are notable due to their lack of immunogenicity and
natural degradation, however, specific targeting remains a
challenge (8).
Several studies have demonstrated that hepatic
stellate cells (HSCs) are crucial mediators of fibrosis (3,9,10).
HSCs reside in the perisinusoidal space and store vitamin A (VitA)
in their quiescent state (11).
The present study selected HSCs as target cells of fibrosis to
analyze the effect of VitA-coupled liposomes (VitA-lips) combined
with MMP-2 siRNA, which may aid further investigations examining
specific fibrosis prophylactic drug treatments.
Materials and methods
Materials
Cholesterol (Aobox, Peiking, China), 3β-[N
-(N′,N′-dimethylaminoethane) carbamoyl] cholesterol (DC-Chol;
Sigma-Aldrich, St. Louis, MO, USA),
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE;
Sigma-Aldrich), MMP-2-siRNA (GenePharma, Shanghai, China),
4′,6-diamidino-2-phenylindole (DAPI) staining solution (Beyotime
Institute of Biotechnology, Haimen, China), mouse anti-human α-SMA
monoclonal antibody (cat. no. ZM-0003), rabbit anti-mouse type I
collagen monoclonal antibody (cat. no. ZA-0616) and
immunohistological reagent kits (Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China), gelatin zymography assay
kits (Applygen Technologies, Inc., Beijing, China), H2600
transmission electronic microscope (TEM; JEM-200CX; JEOL, Ltd.,
Tokyo, Japan), Zeta Sizer 3000 laser particle size analyzer
(Malvern Instruments Ltd., Malvern, UK), high-pressure homogenizer
(Avestin, Ottawa, Canada), and fluorescent microscope (TI-S; Nikon
Corporation, Tokyo, Japan) and an inverted fluorescent microscope
(TE2000-U, Nikon Corporation). were all used. The hepatic stellate
cell line HSC-T6 was purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China).
Cell culture
The HSC-T6 rat hepatic stellate cells were grown in
Dulbecco's modified Eagle's medium (DMEM, Gibco Life Technologies,
Carlsbad, CA, USA), supplemented with 10% (v/v) fetal bovine serum
(Gibco Life Technologies) and antibiotics (100 U/ml streptomycin
and 100 U/ml penicillin) in plastic culture flasks or dishes at
37°C in a humidified incubator under a 5% CO2
atmosphere, and the culture medium was replaced with fresh medium
every other day. The confluent cells were then harvested with 0.25%
trypsin-EDTA solution.
Preparation of cationic liposomes
DC-Chol, DOPE and cholesterol (4:3:3, molar ratio)
were mixed in round-bottom flasks, and
CCl3-CH3OH (3:2, v/v) was added to the
mixture, followed by sonication for 5 min in a water bath prior to
evaporation in 37°C to remove organic reagents. Finally, homogenous
thin films were obtained from the internal walls of the flasks. The
films were resolved with 20 ml phosphate-buffered saline (PBS; pH
7.2), followed by centrifugation for 30 min at room temperature
(RT). When the films were completely stripped down, they were
sonicated for 1 min and then squeezed three times through 220 nm
filters (Merck Millipore, Darmstadt, Germany) to reduce their size.
Simultaneously, VitA-cephalin was synthesized using the
dicyclohexylcarbodiimide-1, 3-diaminopentane (DCC-DAMP) method.
Briefly, 50 mg VitA (Zhengzhou Lion Biological Technology Co.,
Ltd., Henan, China) and 100 mg phycoerythrin (Sigma-Aldrich) were
dissolved in 5 ml dimethyl sulfoxide (DMSO; Sigma-Aldrich), and
mixed with 100 μl DAMP (Sinopharm Chemical Reagent Beijing
Co., Ltd., Beijing, China), followed by the activation at 4°C for
30 min. Subsequently, DCC (Sinopharm Chemical Reagent Beijing Co.,
Ltd.) solution (50 mg in 1 ml chloroform) was slowly added to the
above mixture, and agitated for 24 h at RT in the dark. After
standing for 2 h at RT, the supernatant were mixed with 20 ml cold
acetone (Sinopharm Chemical Reagent Beijing Co., Ltd.), and
centrifuged for 10 min at 9,279 x g at RT. The precipitate was
washed with cold acetone for three times and dried at RT, then the
products were analyzed using a Fourier Transform Infrared
spectrometer (Avatar 370 Thermo Nicolet; Thermo-Nicolet
Corporation, Madison, WI, USA).
Characterization of cationic
liposomes
Cationic liposomes were diluted and dropped onto
copper gauze with a membrane for morphological characterization.
The particle size was measured using a Zeta Sizer 3000 laser
particle size analyzer and processed using Dynamic Light Scattering
software (Malvern Instruments Ltd.). The average particle size and
polydispersity index (PDI) were recorded. No aggregation or
deposition were observed in the cationic liposomes on being
maintained at RT for 2 months, and the stability was further
examined using TEM. Briefly, a few drops of the liposome solution
were dropped onto a TEM grid, dried and absorbed the extra solution
with filter paper prior to analysis. TEM images were photographed
using a field emission JEM-200CX TEM equipped with a charge-coupled
device camera.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The VitA-lip-MMP-2 siRNA complex (mass ratio of
cationic liposome to MMP-2-siRNA, 5:1; siRNA, 30 nM) was used to
transfect the HSC-T6 cells at a density of 70–80%. Subsequent to
the cells being transfected for 36 h at 37°C, the total RNA was
extracted using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA), and the mRNA expression of MMP-2
was analyzed using RT-qPCR. Briefly, 1 μg total RNA was
reverse transcribed in a reaction mix containing 2 ml
dithiothreitol (0.1 M), 1 μl dNTPs (100 mM), 2 μg
random hexamers, 1 μl (200 units) superscript II reverse
transcriptase and 1 μl (40 units) RNAse inhibitor (GE
Healthcare, Chalfont, UK) for 1 h at RT, then the synthesized cDNA
was used for RT-qPCR. The sequences of the sense and antisense
primers for MMP-2 were 5′-CATCGTACTCCTCGTTGCTGATCCACAT-3′ and
5′-CTCCCTCATGCCATCCTGCGTCTG-3′, respectively. To normalize the
loading samples, a GAPDH control was produced using sense
5′-GAAGGGCTCATGACCACAGT-3′ and antisense 5′-GGATGCAGGGATGATGTTCT-3′
primers. The primers were synthesized by Yingjun Biotechnogy Co.,
Ltd. (Shanghai, China). The PCR amplification cycles were as
follows: 94°C 5 min, followed by 35 cycles of 94°C for 30 sec,
62.5°C for 30 sec and 72°C for 30 sec, with a final extension step
for 10 min at 72°C. The product was detected using electrophoresis
and a 2% agarose gel (Sigma-Aldrich). The bands were scanned to
analyze the gray values using a Smart Biological Electrophoretic
Image Analyzer (Shanghai Furi Science & Technology Co. Ltd.,
Shanghai, China). The expression of MMP-2 was normalized to the
corresponding GAPDH band (117 bp). The interference efficiency of
MMP-2 was calculated according to the following formula:
Interference efficiency = (1 - MMP-2 expression in the siRNA
group/MMP-2 expression in the untreated group) × 100%.
Analysis of siRNA-binding ability
The cationic liposomes were mixed with siRNA (0.01
g/l) at various mass ratios of cationic liposome to siRNA (0:1,
1:1, 3:1, 5:1, 7:1, 9:1 and 15:1) and incubated at RT for 30 min to
allow for complete electrostatic interaction between the liposomes
and siRNA. Subsequently, the complexes were obtained and loaded
into an agarose gel to evaluate the siRNA-binding ability of the
cationic liposomes. The bands were scanned to analyze the gray
values using a Smart Biological Electrophoretic Image Analyzer. The
binding abilities at each mass ratio were calculated according to
the following formula: (Gray value at 0:1 - gray value at other
mass ratio)/(gray value of 0:1) × 100%.
Cell viability assay
An MTT assay was used to examine the cellular
toxicity of the synthesized liposomes on HSC-T6 cells. The cells in
the logarithmic growth phase were seeded on a 96-well plate
(5×103/well) and cultured for 24 h. Experimental wells
were used for cationic liposome/siRNA complexes with different mass
ratios, whereas wells containing fresh DMEM media and Lipofectamine
2000 were used as negative and positive controls, respectively. For
each group, six parallel wells were prepared. Following incubation
for 72 h at 37°C, MTT solution 20 μl (5 g/l) was added to
each well and after 4 h, the culture reaction was terminated using
150 μl DMSO. The optical density values were read at 492 nm
using a microplate reader (Thermo Multiskan MK3; Thermo Fisher,
Vantaa, Finland), with the viability of the cells presented as the
percentage compared with the negative control cells.
Evaluation of transfection
efficiency
The HSC-T6 cells were plated onto a 6-well plate
(3×105/well). At a density of ~50–60%, 0.2 ml cationic
liposome/carboxyfluorescein (FAM)-siRNA complex with different mass
ratios (0:1, 1:1, 3:1, 5:1, 7:1, 9:1, and 15:1, the concentration
of siRNA was always 0.01 mg/ml) were added to the cells and then
supplemented with 1.8 ml DMEM. Triplicate wells for the same
condition were prepared. Lipofectamine 2000 and FAM-siRNA was used
as a positive control, and FAM-siRNA alone was used as a negative
control. After 6 h, the transfer efficiency was estimated using a
fluorescent microscope.
Gelatin zymography
The gelatin zymography procedure was described in
detail in a previous study (12).
Briefly, electrophoresis plates for 8% SDS-PAGE, including 1 g/l
gelatin, were prepared (Sigma-Aldrich). The samples from the
cultured supernatants were prepared using dialysis bags, with a
molecular weight cut-off of 35 kDa, and incubated in
polyethyleneglycol (Sigma-Aldrich) for 40 min at RT to concentrate
the samples. Subsequently, 15 g concentrated supernatant from each
sample was loaded for electrophoresis onto the prepared gel, which
was run at 100 V. Subsequently, the gel was rinsed twice with
Zymogram A buffer for 30 min at RT and incubated with Zymogram B
buffer overnight. Finally, the gel was stained with Coomassie
Brilliant Blue R-250 (0.2% Coomassie Brilliant Blue R-250, 20%
methanol and 10% acetic acid) for 2 h at 37°C and destained in
methanol to obtain clear bands. The gray values of the bands were
analyzed using a Smart Biological Electrophoretic Image Analyzer.
The MMP-2 activity was presented as a percentage of the activity of
the 12 h control group.
DAPI staining
The cells were fixed in cold acetone for 20 min,
followed by staining with DAPI staining buffer for 10 min at RT.
Following washing with PBS, the cellular morphology was analyzed
and images were captured using a fluorescent microscope.
Immunocytochemistry staining
The cells were fixed in cold acetone, and
nonspecific antibody binding was blocked with goat serum. This was
followed by incubation with the primary antibody, α-SMA, or type I
collagen monoclonal antibody (1:100) and a secondary antibody,
conjugated to biotin and immunoperoxidase with streptavidin at 4°C.
Subsequently, the cells were visualized using DAB and
H2O2, followed by counter-staining with
hematoxylin (Sigma-Aldrich), and the intensity of staining was
observed under a fluorescent microscope. The negative control was
treated with PBS buffer instead of a monoclonal antibody. A total
of five images were captured in the left, middle, right, upper and
lower fields of each slide. The protein expression levels of α-SMA
or type I collagen was calculated as the average percentage of
positive cells (number of positive cells/number of total cells ×
100%).
Statistical analysis
All experiments were performed at least three times,
and the numerical data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS 13.0
software. The difference between two groups were evaluated using
Student's t-test for independent samples. P<0.05 was considered
to indicate a statistically significant difference.
Results
Physicochemical properties of
liposomes
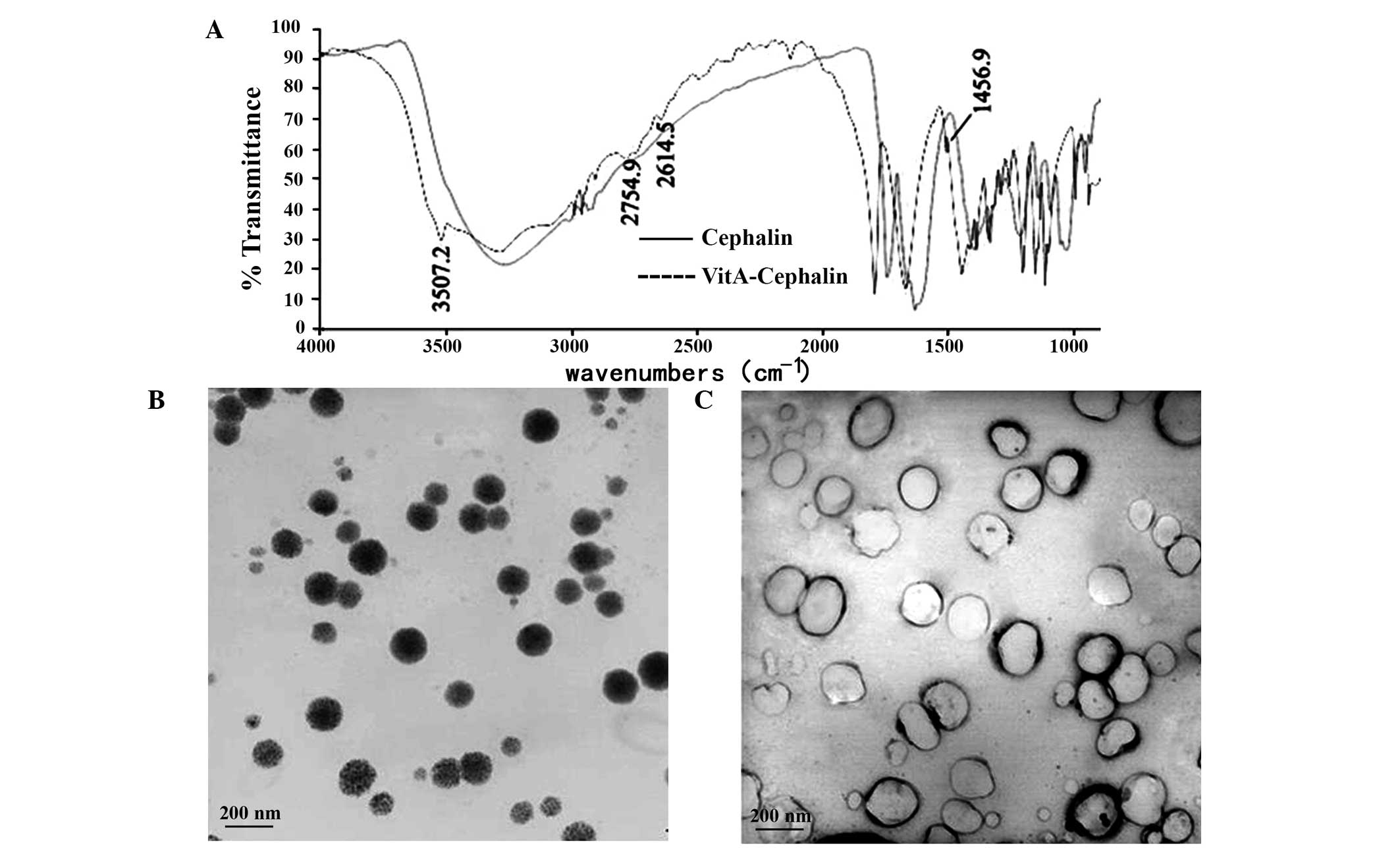
The present study used infrared spectra to examine
whether cephalin was bound by VitA. The spectra of cephalin and
VitA-cephalin were the same, with the exception of the vibration
benzene peak at 1,456.9 cm−1 and an NH-stretching
vibration characteristic peak of an amide bond at 3,507.2
cm−1 for VitA-cephalin, which confirmed that VitA bound
cephalin via an amide bond (Fig.
1A).
Subsequently, the morphology and stability of the
liposomes were examined. The results revealed that unmodified
cationic liposomes appeared as white emulsions with light blue
opalescence. They dispersed well, and the majority were single
chamber and spherical-like, with particles sizes distributed
between 100 nm and 200 nm (Fig.
1B). No flocculation or sediment were observed on being
maintained at RT for 2 months, however, their diameter increased
without prominent fusion. Following modification by VitA, the
particle size of the cationic liposomes increased, and they
exhibited mild aggregation (Fig.
1B). These findings were further confirmed by the results from
the Zeta Sizer 3000 laser particle size analyzer. The data
indicated that the average size of the cationic liposomes was
148.2±0.3 nm and the surface charge, based on the zeta potential
measurement, was +41.67 mV, with these values changing to 227.3±4.1
nm and +44.67 mV, respectively following modification (Table I). The PDI values revealed an
almost monodisperse particle size (Table I). These findings demonstrated that
the VitA-lips exhibited a positive charges with good stability.
 | Table ICharacterization of liposomes. |
Table I
Characterization of liposomes.
| Method | TEM
| Zeta sizer 3000 laser
particles analyzer
|
|---|
| Size distribution
(nm) | Z-average diameter
(nm) | Polydispersity | Zeta potential
(mV) |
|---|
| Liposome | 114.34±36.94 | 148.2±0.3 | 0.162±0.014 | +41.67 |
| VitA-lips | 155.77±39.00 | 2273±41 | 0.171±0.025 | +44.67 |
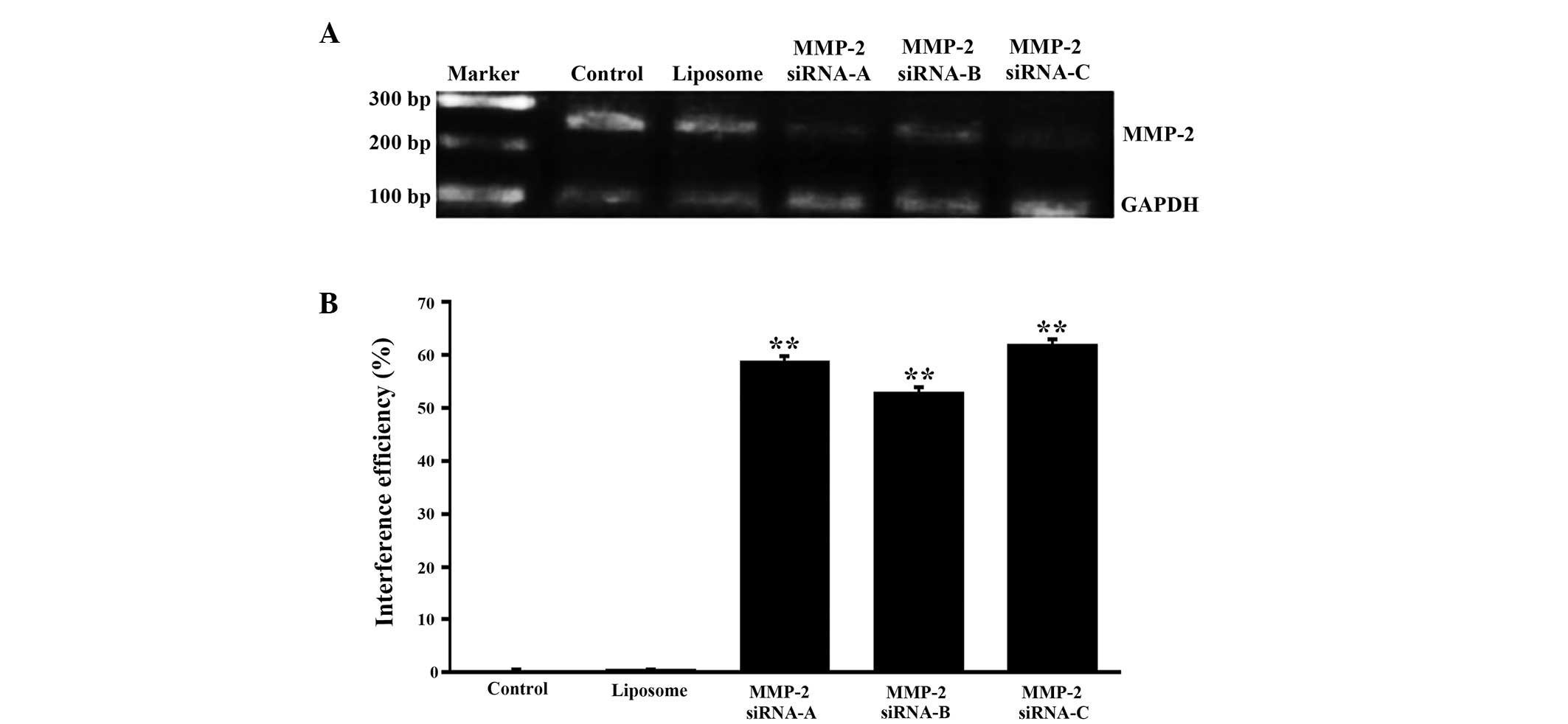
Identification of MMP-2 siRNA
interference efficiency
To obtain higher gene silence efficiency, three
interference sequences and corresponding random sequences of MMP-2
were designed. At 36 h post-transfection of the cells with the
VitA-lip-MMP-2 siRNA complexes, the cells were used to evaluate the
mRNA expression of MMP-2 using RT-qPCR. The data demonstrated that
the interference efficiencies of MMP-2 siRNA-A, siRNA-B and siRNA-C
were 58.76, 52.87 and 61.84%, respectively, and no interference
effects were observed in the cells treated with VitA-lip only
(Fig. 2). These results indicated
that the mRNA expression levels of the target gene in the three
interference groups were significantly different compared with that
in the untreated control or VitA-lip group. Among these, MMP-2
siRNA-C exhibited the highest silencing efficiency and was used for
the subsequent experiments.
siRNA-binding capacity of
theliposomes
To determine the siRNA-binding capability of the
liposomes, agarose gel electrophoresis was performed. This process
demonstrated that the cationic liposomes were bound to more siRNA
when the ratio of cationic liposome to siRNA was increased. The
cationic liposomes completely encapsulated MMP-2 siRNA when the
ratio of cationic liposomes to siRNA was ≥1. Following
modifiication by VitA, the cationic liposomes effectively
integrated with siRNA when the ratio was ≥5. Therefore, the
cationic liposomes exhibited stable binding capability, which was
efficient following modification by VitA (Fig. 3).
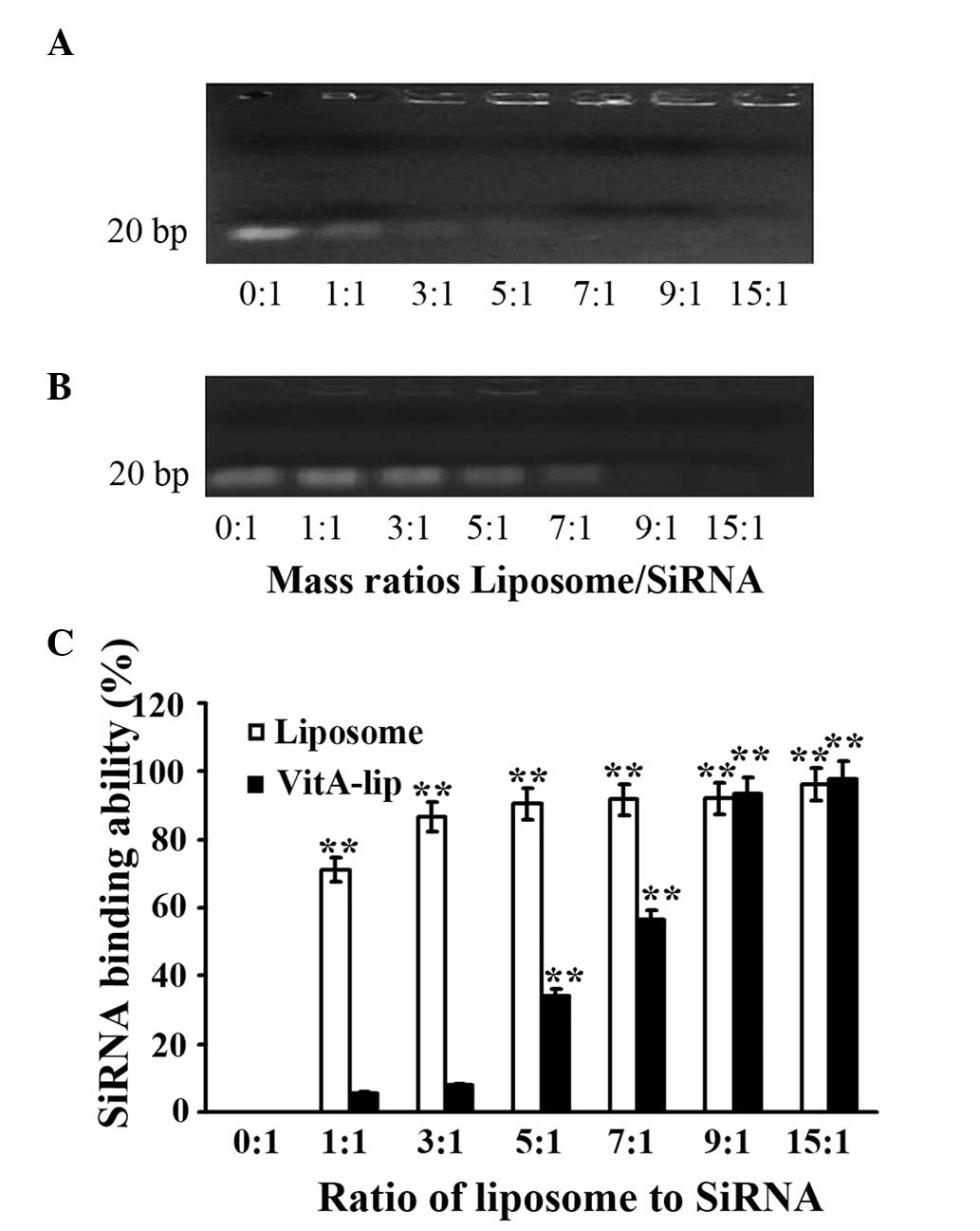 | Figure 3siRNA-binding capacity of cationic
liposomes. Different mass ratios of cationic liposomes or VitA-Lips
were mixed with siRNA, and a gel retardation assay was performed.
(A) cationic liposomes; (B) VitA-Lips; (C) Quantitative analysis of
densitometry in A and B. The mass ratios of liposome/siRNA were
0:1, 1:1, 3:1, 5:1, 7:1, 9:1 and 15:1 in lanes 1–7, respectively.
The data are representative of three replicate experiments. Data
are expressed as the mean ± standard
deviation.**P<0.01, compared with the control (mass
ratio, 0:1). siRNA, small interference RNA; VitA, vitamin A. |
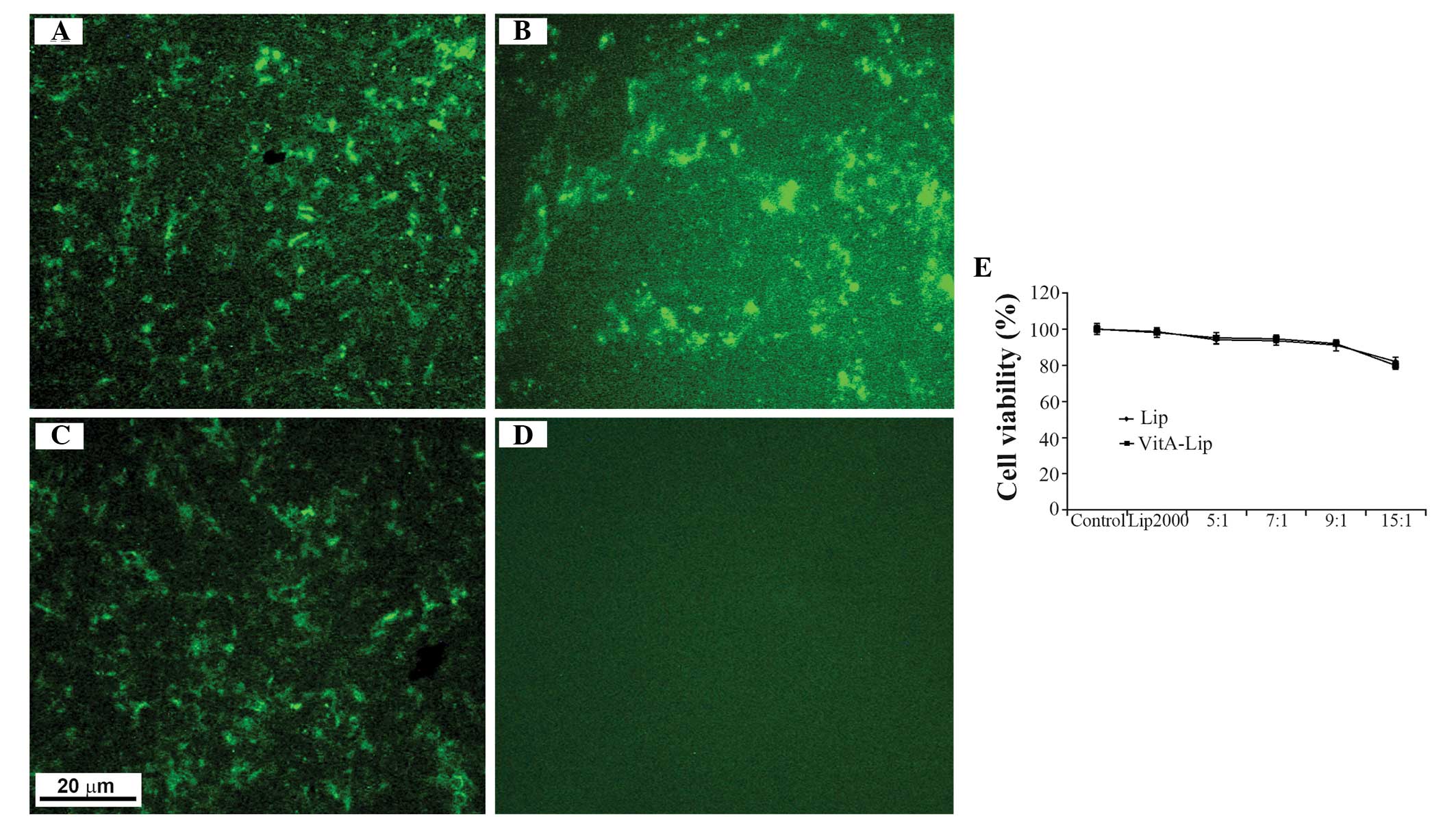
Transfection efficiency of VitA-lip-MMP-2
siRNA
Prior to examining the transfection efficiency of
the VitA-lip-MMP-2 siRNA complexes, their cellular toxicity was
examined using an MTT assay. Following treatment of the cells with
pure liposomes or with the complexes containing the various mass
ratios of liposome to siRNA (5:1, 10:1 and 15:1), no cytotoxicity
was observed in the HSC-T6 cells whether the complexes contained
the liposomes or VitA-lips (Fig.
4E). As expected, the cytotoxicity was liposome
concentration-dependent (Fig. 4E).
Although decreased cell viability was observed in the HSC-T6 cells
transfected with the complexes, they remained at ~80%, despite a
liposome/siRNA ratio of 15:1 (Fig.
4E).
Subsequently, the cells were transfected with
cationic liposome/MMP-2 siRNA complexes with different mass ratios
and, after 6 h, green fluorescence was observed inside the cells
exposed to the complexes, however no fluorescence was observed when
cells were treated with MMP-2 siRNA alone (Fig. 4A–D). Among the cell groups, the
highest transfection efficiency was detected when the ratio of
liposome/siRNA was 5:1 prior to modification. Following
modification with VitA, the most efficient transfection was
achieved at a liposome/siRNA ratio of 7:1 (data not shown). These
findings indicated that the transfection efficiency increased
markedly following modification. However, the cellular toxicity was
dependent on the mass ratio of liposome/siRNA, of which a ratio of
7:1 was selected for the subsequent experiments.
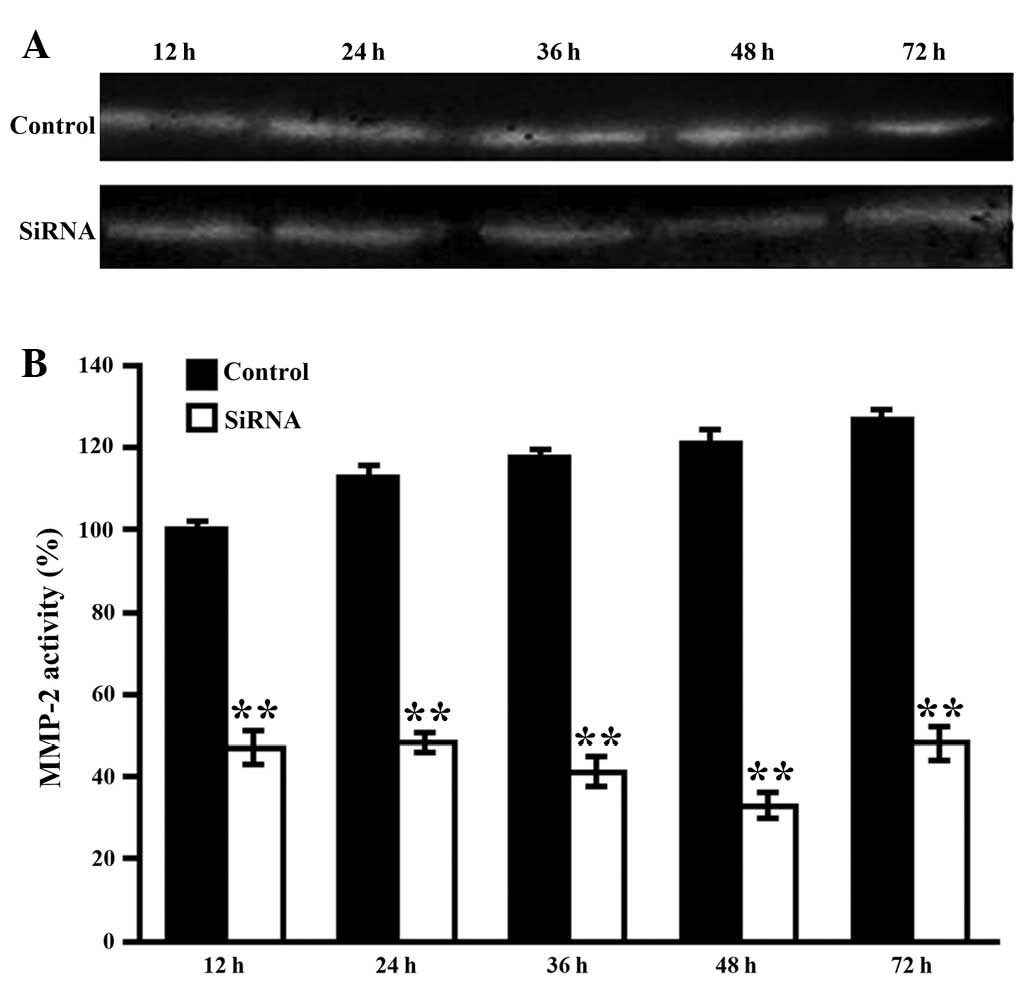
Silencing efficiency of MMP-2 induced by
VitA-lip-MMP-2 siRNA
To further optimize the MMP-2 interference, the HSCs
cells were transfected with VitA-lip-MMP-2 siRNA for 12, 24, 36, 48
and 72 h. The activities of MMP-2 in the supernatants were then
detected using zymography. As shown in Fig. 4, the gene interference gradually
increased between 12 and 48 h, peaked at 48 h and weakened at 72 h
(Fig. 5).
The effect of VitA-lip-MMP-2 siRNA on the
cellular behavior of HSCs
To investigate the effect of VitA-lip-MMP-2 siRNA
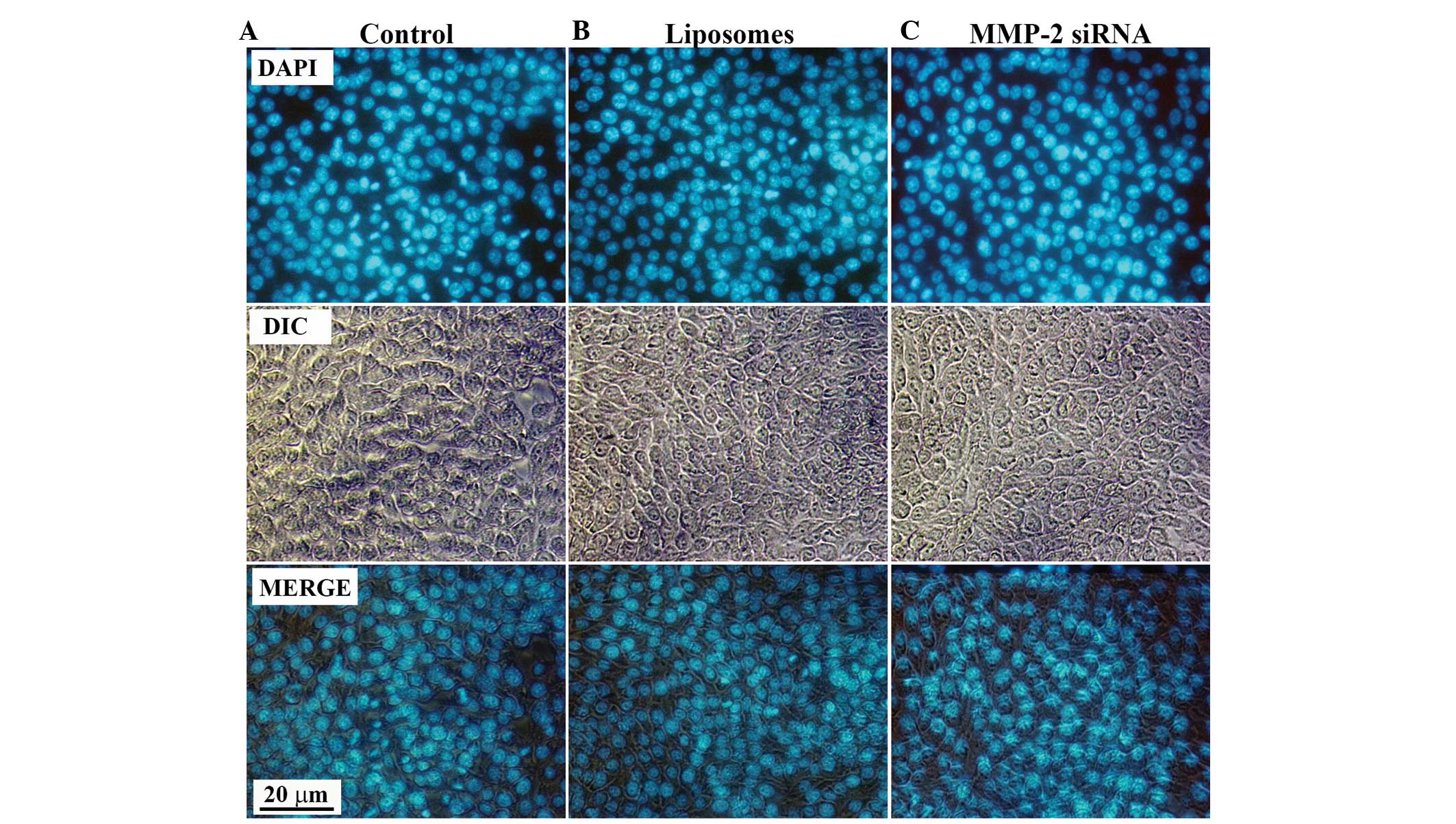
complexes on the cellular behavior of HSC-T6 cells, DAPI staining
was performed to examine whether apoptosis was induced. Following
transfection of the HSCs with VitA-lip-MMP-2 siRNA complexes for 48
h, the cells were stained with DAPI staining solution. No
differences in apoptosis were observed in the cells in the
interference groups, compared with those of the control groups
(Fig. 6), which suggested that the
decreased viability of the HSC-T6 cells was due to the
downregulated cell activation, and not apoptosis.
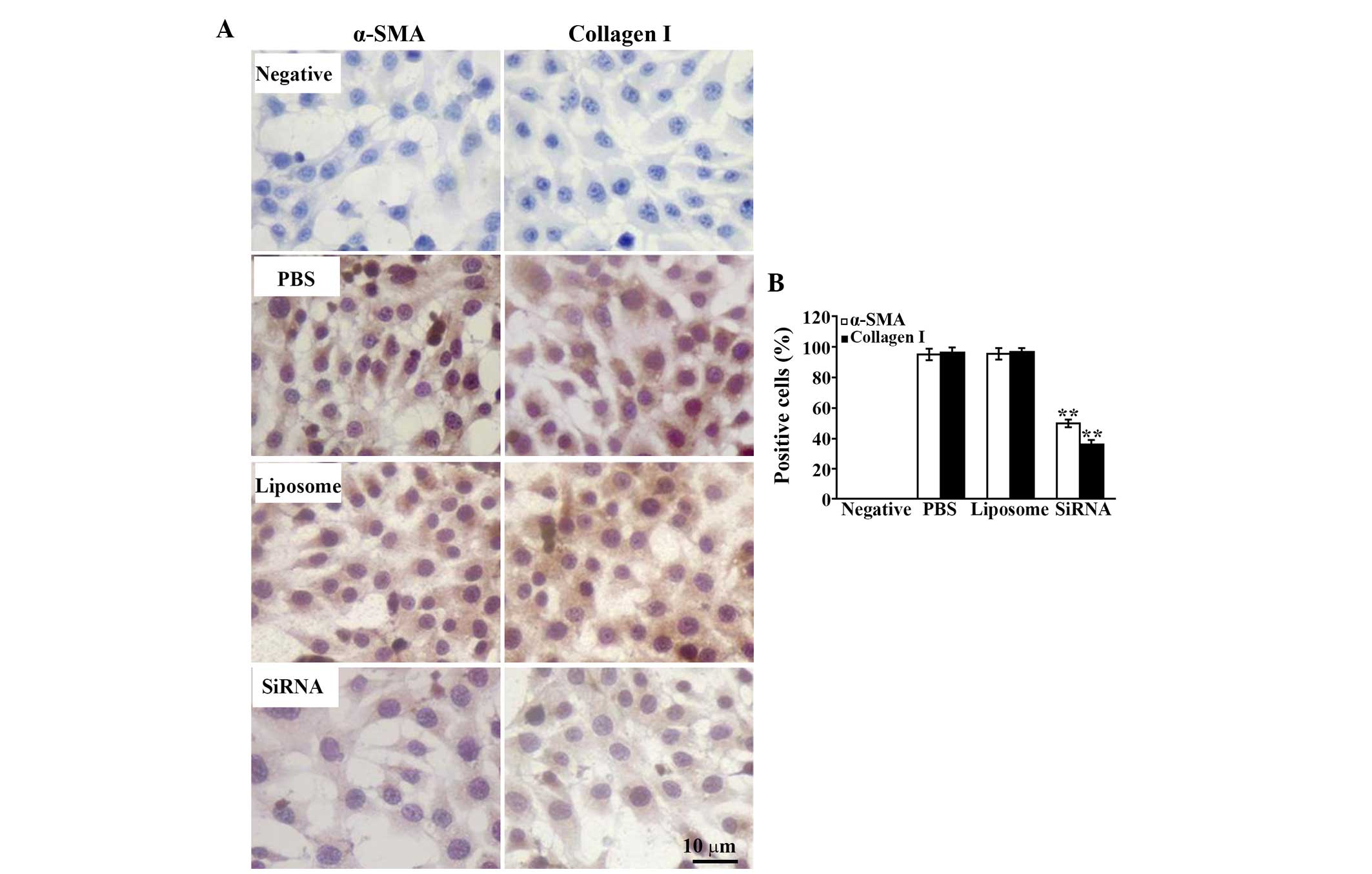
To detect the activation of the HSC-T6 cells, the
present study performed immunostaining with specific antibodies to
α-SMA and type I collagen, with positive cells presenting diffusely
distributed brown-yellow granules in the cytoplasm. The results
demonstrated that the expression of α-SMA was significantly
decreased and the number of positive cells was reduced following
treatment with MMP-2 siRNA, compared with that of the control
groups (Fig. 7). The same
expression pattern was observed for type I collagen (Fig. 7).
Discussion
Hepatic fibrosis is the final stage of all chronic
hepatic diseases and may develop into cirrhosis and hepatic
carcinoma, and finally induce liver failure (13). The sustained secretion of ECM is a
prerequisite of hepatic fibrogenesis, and HSCs are vital cells,
which produce ECM during the development of hepatic fibrosis
(13,14). The activation of HSCs has been
considered an important step for the formation and development of
fibrosis (3,9,11,15).
In the healthy liver, HSCs are in a quiescent state and are
involved in the metabolism of VitA. Under physiological conditions,
HSCs do not express α-SMA, and exhibit low proliferative ability
and collagen secretion (10,11,16).
However, when exposed to injury, HSCs are activated and transformed
into a fibroblast phenotype (10,11).
HSCs lose VitA in the cytoplasm, but express cytokines, receptors,
α-SMA and ECM, including type I collagen, and proliferate rapidly
(11,16,17).
HSCs are not only key cells in ECM secretion, they also crucial
cells in the generation of MMP (18). Previous reports suggest that
hepatic fibrosis is reversible (1–3,6,14).
However, no effective drugs have been developed against the
formation of fibrosis in chronic liver disease due to the shortage
of specific targets and drugs, and inevitable side effects.
MMP-2 is an important collagenase of the matrix
metalloproteinase family and is produced by several types of cell,
including activated HSCs (19). At
the early stage of fibrosis, HSCs secrete substantial MMP-2 and
degrade abundant type IV collagen around the HSCs, which promotes
the further activation and proliferation of HSCs (11,12,16,20,21).
The activated HSCs secrete more collagen and MMP-2, following which
MMP-2 conversely accelerates HSC activation, which constitutes a
malignant feedback loop. MMP-2 also contributes to angiogenesis,
vascular remodeling and hepatic sinusoid capillarization, which
aggravates the progression of fibrosis (20). However, in the naturally decaying
stage, MMP-2 activity is increased (22). This indicates that MMP-2 promotes
the development of fibrosis in the early stage, but induces the
elimination of fibrosis in the late stage. These findings can
assist in developing specific drugs for fibrosis by regulating the
expression and activity of MMP-2 during different periods of
hepatic fibrosis.
RNAi is a gene silencing tool of substantial
functionality. To silence a specific gene, specific homogenous mRNA
is degraded using double-stranded RNA. This technique has the
advantage of high efficiency and specificity and is widely used for
investigating prophylaxis and treatment of diseases (23). Considering the vital functions of
activated HSCs in the development of hepatic fibrosis, several
studies have examined the regulation of gene expression by siRNA
interference, which further inhibits activation and proliferation,
promotes apoptosis and increases degradation of the ECM in HSCs.
The findings of these studies suggest that HSCs are promising
targets in identifying effective drugs against hepatic fibrosis.
However, the shortage of specificity in RNAi in vivo has
limited its application. Thus, based on the specific receptor and
regulating sequences on the cell surface, a number of vectors
targeting HSCs have been identified, including mannose-targeted
liposomes and folic acid-targeted liposomes (24). These vectors specifically deliver
siRNA to HSCs, which protects healthy hepatocytes. Furthermore, the
higher efficiency of siRNA transfer results in more effective
therapy. Large numbers of VitA receptors are expressed on the
cellular membrane of HSCs (11),
and Sato et al confirmed its specificity and effectiveness
in vivo (25). Therefore,
the present study constructed VitA-coupled cationic liposomes to
deliver MMP-2 siRNA, and the data confirmed that the vector
efficiently conveyed siRNA into the HSCs and inhibited the gene
expression of MMP-2. However, the specificity of the vector
requires further investigation in vivo.
The HSC-T6 cell line, an activated HSC model, has
been used as a target cell in several studies on hepatic fibrosis.
HSC-T6 cells present the features of hepatic stellate cells and
activation phenotypes, including vigorous proliferation, abundant
expression levels of α-SMA and type I collagen, and fibroblast-like
morphology (3,26). A study by Kawada (27) found that MMP-2 promotes the
activation and proliferation of HSCs, activates the expression of
α-SMA, a marker of HSC activation, and produces increased ECM,
predominantly type I collagen. In the present study, HSCs
activation and the expression of type I collagen were reduced
following treatment with MMP-2 siRNA. The results demonstrated that
the MMP-2 siRNA-transfected HSCs were smaller, and the ratio of
nucleus to plasma was reduced. In addition, the cell viability was
downregulated, which suggested that decreased activity and
expression of MMP-2 reduced the the proliferation rate of the HSCs,
however, these findings were not associated with apoptosis.
Therefore, the present study hypothesized that the reduction in the
viability of the HSCs may have been caused by MMP-2 siRNA-induced
phenotype transversion of the activated HSCs. The immunostaining
confirmed this hypothesis, as the protein expression levels of
α-SMA and type I collagen were significantly decreased in MMP-2
siRNA-treated HSCs. However, whether the weakened viability of the
HSCs is associated with the induction of senescence requires
further investigation.
In conclusion, the present study successfully
constructed a VitA-coupled cationic liposome vector, which
effectively delivered MMP-2 siRNA to the HSCs. Following
transfection with the vector, the expression and activity of MMP-2
in the HSCs were prominently downregulated. Based on these changes,
the activation and proliferation of HSCs were decreased, and the
secretion of type I collagen in the HSCs cells was significantly
reduced (Fig. 8). These findings
present a novel direction in the targeted prevention of hepatic
fibrosis. However, further investigations are required to elucidate
the application of this system in vivo.
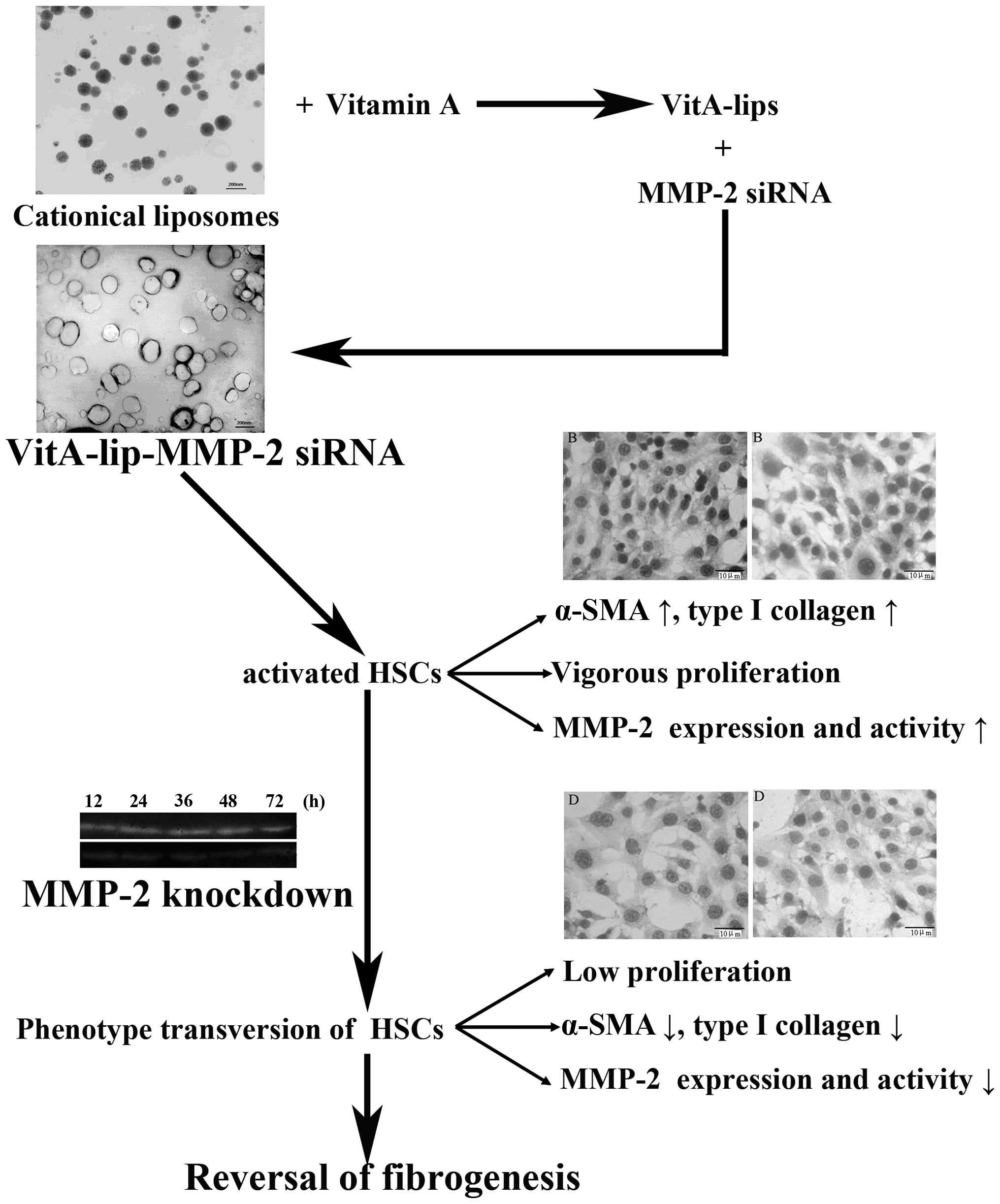 | Figure 8Schematic illustration of the
association among HSCs, MMP-2 and fibrogenesis. At the fibrogenesis
stage, hepatic stellate cells proliferate rapidly, secrete abundant
MMP-2 and express markers of activated HSCs (α-SMA and type I
collagen). However, when MMP-2 was downregulated by the constructed
liposomes carrying MMP-2 siRNA, the viability of the HSCs decreased
and expression levels of α-SMA and type I collagen were reduced.
These results indicated that silencing MMP-2 reversed fibrogenesis.
HSCs, hepatic stellate cells; PBS, phosphate-buffered saline; VitA,
vitamin A; siRNA, small interference RNA; SMA, smooth muscle
actin. |
Acknowledgments
This study was supported by grants from the National
Basic Research Program, People's Republic of China (973 Program;
grant. no. 2011CB933404), the National Natural Science Foundation,
People's Republic of China (grant. no. 30470780), and the
Specialized Research Fund for the Doctoral Program of Higher
Education (grant. no. 20090092110053).
References
|
1
|
Povero D, Busletta C, Novo E, et al: Liver
fibrosis: a dynamic and potentially reversible process. Histol
Histopathol. 25:1075–1091. 2010.PubMed/NCBI
|
|
2
|
Ismail MH and Pinzani M: Reversal of
hepatic fibrosis: pathophysiological basis of antifibrotic
therapies. Hepat Med. 3:69–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novo E, Cannito S, Paternostro C, Bocca C,
Miglietta A and Parola M: Cellular and molecular mechanisms in
liver fibro-genesis. Arch Biochem Biophys. 548:20–37. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu YB, Li DG and Lu HM: Modified synthetic
siRNA targeting tissue inhibitor of metalloproteinase-2 inhibits
hepatic fibrogenesis in rats. J Gene Med. 9:217–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mormone E, George J and Nieto N: Molecular
pathogenesis of hepatic fibrosis and current therapeutic
approaches. Chem Biol Interact. 193:225–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radbill BD, Gupta R, Ramirez MC, et al:
Loss of matrix metalloproteinase-2 amplifies murine toxin-induced
liver fibrosis by upregulating collagen I expression. Dig Dis Sci.
56:406–416. 2011. View Article : Google Scholar
|
|
7
|
Kong D, Zhang F, Zhang Z, Lu Y and Zheng
S: Clearance of activated stellate cells for hepatic fibrosis
regression: molecular basis and translational potential. Biomed
Pharmacother. 67:246–250. 2013. View Article : Google Scholar
|
|
8
|
Balazs DA and Godbey W: Liposomes for use
in gene delivery. J Drug Deliv. 2011:326497–326508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kocabayoglu P and Friedman SL: Cellular
basis of hepatic fibrosis and its role in inflammation and cancer.
Front Biosci (Schol Ed). 5:217–230. 2013.
|
|
10
|
Puche JE, Saiman Y and Friedman SL:
Hepatic stellate cells and liver fibrosis. Compr Physiol.
3:1473–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Senoo H, Yoshikawa K, Morii M, Miura M,
Imai K and Mezaki Y: Hepatic stellate cell (vitamin A-storing cell)
and its relative-past, present and future. Cell Biol Int.
34:1247–1272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan RH, Chen PS, Zhao D and Zhang WD:
Hypoxia induced by CoCl2 influencing the expression and the
activity of matrix metalloproteinase-2 in rat hepatic stellate
cells. Zhonghua Gan Zang Bing Za Zhi. 15:654–657. 2007.In Chinese.
PubMed/NCBI
|
|
13
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar M and Sarin SK: Is cirrhosis of the
liver reversible? Indian J Pediatr. 74:393–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gressner AM: Transdifferentiation of
hepatic stellate cells (Ito cells) to myofibroblasts: a key event
in hepatic fibrogenesis. Kidney Int Suppl. 54(Suppl): 39–45.
1996.
|
|
16
|
Senoo H, Hata R, Nagai Y and Wake K:
Stellate cells (vitamin A-storing cells) are the primary site of
collagen synthesis in non-parenchymal cells in the liver. Biomed
Res. 5:451–458. 1984.
|
|
17
|
Enzan H, Himeno H, Iwamura S, et al:
Immunohistochemical identification of Ito cells and their
myofibroblastic transformation in adult human liver. Virchows Arch.
424:249–256. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arthur MJ, Friedman SL, Roll FJ and
Bissell DM: Lipocytes from normal rat liver release a neutral
metalloproteinase that degrades basement membrane (type iv)
collagen. J Clin Invest. 84:1076–1085. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Niu JZ, Wang JF, Li Y and Tao XH:
Pathological mechanisms of alcohol-induced hepatic portal
hypertension in early stage fibrosis rat model. World J
Gastroenterol. 11:6483–6488. 2005.
|
|
21
|
Li J, Fan R, Zhao S, et al: Reactive
oxygen species released from hypoxic hepatocytes regulates MMP-2
expression in hepatic stellate cells. Int J Mol Sci. 12:2434–2447.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Domitrović R, Jakovac H, Marchesi VV and
Blažeković B: Resolution of liver fibrosis by isoquinoline alkaloid
berberine in CCl4-intoxicated mice is mediated by suppression of
oxidative stress and upregulation of MMP-2 expression. J Med Food.
16:518–528. 2013. View Article : Google Scholar
|
|
23
|
Schroeder A, Levins CG, Cortez C, Langer R
and Anderson DG: Lipid-based Nano therapeutics for siRNA delivery.
J Intern Med. 267:9–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wasungu L and Hoekstra D: Cationic lipids,
lipoplexes and intracellular delivery of genes. J Control Release.
116:255–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato Y, Murase K, Kato J, et al:
Resolution of liver cirrhosis using vitamin A-coupled liposomes to
deliver siRNA against a collagen specific chaperone. Nat
Biotechnol. 26:431–442. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vogel S, Piantedosi R, Frank J, et al: An
immortalized rat liver stellate cell line (HSC-T6): a new cell
model for the study of retinoid metabolism in vitro. J Lipid Res.
41:882–893. 2000.PubMed/NCBI
|
|
27
|
Kawada N: Evolution of hepatic fibrosis
research. Hepatol Res. 41:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|