Introduction
It is well known that glioma accounts for 44.6% of
tumors of the central nervous system and has the characteristics of
a high recurrence rate and high mortality rate (1). Although certain surgical
comprehensive treatments can significantly improve the survival
rates of patients with glioma, the prognosis remains poor due to
the occurrence of chemotherapy drug resistance in glioma therapy
(2).
Matrix metalloproteinases (MMPs) are reported to
interact with the cellular adhesive molecular degradation of the
extracellular matrix, promoting the growth of tumor cells into the
surrounding brain tissue (3). Cell
proliferation may be affected by regulating MMP-9 activity, and
MMP-9 activity is controlled by several factors, including the
quantity and activation of enzymes (4). MMP-9 is correlated with glioma and it
has been demonstrated that the expression levels of MMP-9 directly
reflect the prognosis of patients with glioma and is the preferred
predictor of invasive glioma cell growth (5,6).
MicroRNAs (miRNAs) are a series of non-coding, small
molecule RNAs, which regulates gene expression via sequence
complementation. These small RNAs consist of between 19 and 25
nucleotides (7). Following
transcription, they exert inhibitory effects on gene expression and
are involved in several physiological processes, including cell
differentiation, apoptosis and metabolism (8). Previous studies have demonstrated
that miR-16 is important in tumors of various origins (9–11).
In glioma growth and invasiveness, the importance of miR-16 as a
tumor suppressor gene and a novel mechanism of miR-16 regulation
through inhibition the nuclear factor (NF)-κB1/MMP-9 signaling
pathway has been reported (11).
Osthole is a natural coumarin isolated from
umbelliferae plant monomers (ripe fruit). A previous modern
pharmacological investigation demonstrated that osthole has
anti-inflammatory, antioxidant and other pharmacological properties
(12,13). In addition, it was also revealed
that osthole markedly decreases the activity and protein content of
MMP-9, suggesting that this protective effect at the molecular
level may be due to downregulation of the MMP-9 pathway (14). The aim of the present study was to
investigate the anticancer potential of osthole against glioma
cells and examine whether this mechanism is dependent on the
upregulation of miR-16 and downregulation of MMP-9 expression.
Materials and methods
Reagents and chemicals
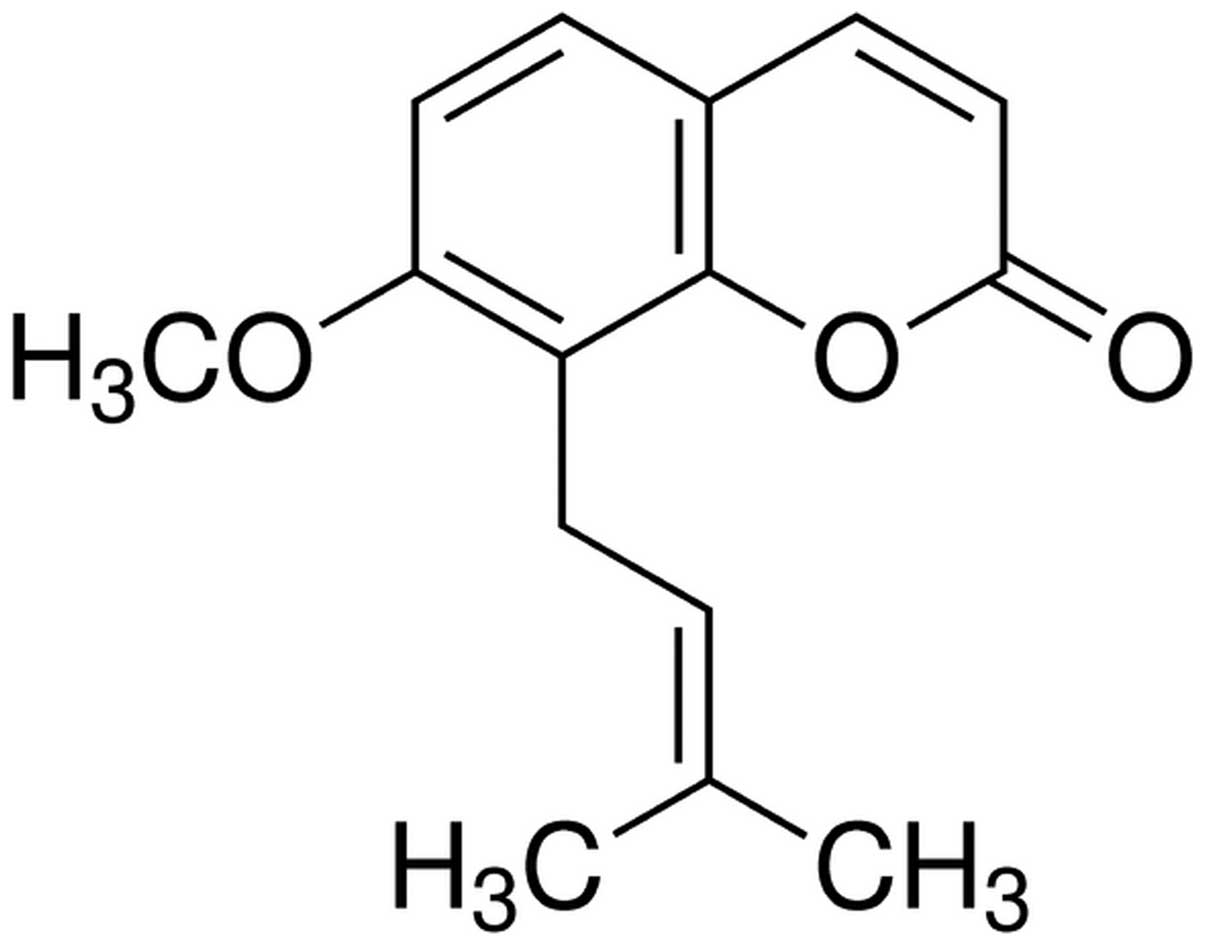
The chemical structure of osthole (Sigma-Aldrich,
St. Louis, MO, USA; purity, ≥95%) is shown in Fig. 1. Osthole was dissolved in
physiological saline (50–200 µM). Roswell Park Memorial
Institute-1640 (RPMI-1640) medium, fetal calf serum (FCS) and
Lipofectamine 2000 were purchased from Invitrogen Life Technologies
(Carlsbad CA, USA).
3-(4,5-dimethylthiazol-2-thiazolyl)-2,5-diphenyl-tetrazolium
bromide (MTT) was purchased from Beyotime Institute of
Biotechnology, (Haimen, China). The Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) Double Staining kit was
purchased from BestBio (Shanghai, China).
Cancer cell lines
The U87 glioma cell line was purchased from the
Animal Experiments of Clinical School of Taishan Medical University
(Taian, China). The U87 cells were cultured in RPMI-1640 medium,
supplemented with 10% FCS, 100 U/ml penicillin and 100 mg/ml
streptomycin, at 37°C and 5% CO2.
MTT viability assay
The effect of osthole on the proliferation of U87
cells was measured using an MTT assay. The U87 cells
(5.0×103 cells/well) were seeded into 96-well culture
plates at 95% confluence and incubated at 37°C and 5%
CO2 in a humidified incubator for 24 h. Following
incubation, the cells were treated with different concentrations of
osthole (0, 50, 100 or 200 µΜ) for 0, 24, 48 or 72 h. MTT
(~10 µl of 10 mg/ml) was added into each well and incubated
at 37°C and 5% CO2 for 4 h. Subsequently, 150 µl
dimethyl sulfoxide was added to each well and incubated for 20 min
at room temperature with agitation. The absorbance of the plates
was detected using an CM2600d spectrometer (Bio-Tek Instruments,
Inc., Winooski, VT, USA) at 570 nm.
Measurement of caspase-3 activity
The activity of caspase-3 in the cells was measured
using a Colorimetric Caspase-3 Assay kit (Beyotime Institute of
Biotechnology). Following treatment with 100 µM osthole for
48 h, the U87 cells (1.0–2.0×106 cells/well) were
centrifuged at 12,300 × g for 20 min at 4°C. The supernatant was
collected and the protein concentration was quantified using a
bicinchoninic acid (BCA) protein assay kit (Sangon Biotech,
Shanghai, China). The protein extract (~50 µg) was incubated
and added to a reaction buffer containing 90 µl 1X assay
buffer and 10 µl Ac-DEVD-pNA caspase-3 substrate at 37°C for
6 h. The protein extract (~50 µg) was incubated at 37°C and
added to a reaction buffer containing 90 µl 1X assay buffer
(Beyotime Institute of Biotechnology) and 10 µl Ac-DEVD-pNA
caspase-3 substrate (Beyotime Institute of Biotechnology) at 37°C
for 6 h. The change was calculated using a CM2600d spectrometer at
a wavelength of 405 nm (Bio-Tek Instruments, Inc.).
Annexin V/PI flow cytometric
analysis
The apoptotic rates of the U87 cells were determined
using flow cytometric analysis (BD Biosciences, Franklin Lakes, NJ,
USA) using an Annexin V-FITC/PI apoptosis kit. Following treat ment
with 100 µM osthole for 48 h, the U87 cells were collected
and washed twice with phosphate-buffered saline. Annexin V-FITC (10
µl) was added to the U87 cells, following which the cells
were stained with binding buffer (BestBio) for 30 min in the dark,
according to the manufacturer's instructions. PI (10 µl) was
added to the glioma cell samples, which were then incubated for 30
min at room temperature in the dark. Immediately following
incubation, the samples were analyzed using flow cytometry.
MMP-9 measurement
To determine whether 100 µM osthole induced
the expression of MMP-9 in the U87 cells, gelatin zymography assays
were used. Following treatment with osthole for 48 h at 37°C, the
U87 cells were harvested and the concentration of protein was
determined using a BCA protein assay kit (Sangon Biotech). Equal
quantities of protein were extracted and were subsequently
electrophoresed on 10% SDS-PAGE gels (Beyotime Institute of
Biotechnology), containing 1% gelatin. Following electrophoresis,
the gel was rinsed in 2% Triton X-100 (Nanjing Senbeijia Biotech
Company, Nanjing, China) for 1 h and subsequently washed in water.
The gels were incubated in radioimmunoprecipitation assay buffer
(pH 8.0; Sigma-Aldrich) at 37°C for 12 h and the gel was stained
with 0.2% Coomassie Blue R-250 (Beyotime Institute of
Biotechnology) for 1 h. The protein expression levels of MMP-9 were
quantified using a MiniBis system (DNR Bio-Imaging Systems Ltd.,
Jerusalem, Israel) and prestained SDS-PAGE standards (Houbio Tech
Co., Ltd., Fan Ling, Hong Kong).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of the expression of
miR-16
The present study investigated whether 100 µM
osthole induced the expression of miR-16 in the U87 cells using
RT-qPCR. Following treatment with osthole for 48 h at 37°C, the
total RNA was extracted from the cells using TRIzol reagent
(Invitrogen Life Technologies), according to manufacturer's
instructions. Approximately 1 µg total RNA from U87
cells-treated was used to perform the first-strand cDNA synthesis.
Subsequently, 2 ml RNA was transcribed to cDNA using random
hexamers (Promega, Madison, WI, USA) according to the
manufacturer's instructions. The cycling conditions were as
follows: 10 min at 95°C, 40 cycles of 45 sec at 95°C, 45 sec at
58°C and 45 sec at 72°C. The quantification of the miR-16 level was
conducted by real- time PCR using TransStart™ SYBR Green qPCR
Supermix (TransGen Biotech, Beijing, China) and U6 small nuclear
RNA was regarded as an endogenous reference gene. The U6 primer
sequence was as follows: Forward 5′-CTCGCTTCGGCAGCACA-3′ and
reverse 5′-AACGCTTCACGAATTTGCGT-3′. The miR-16 primer sequence was
as follows: Forward 5′-TTCCATGCTGTTTTGGTCCC-3′ and reverse
5′-TGGGTGGAGGTTTGTTCGGA-3′. Primers were provided by Sangon Biotech
Co., Ltd. (Shanghai, China). Relative quantification was carried
out using the 2−ΔΔCt cycle threshold method.
miR-16 and anti-miR-16 transfection
The miR-16 precursor and the anti-miR-16 were
obtained from KeyGen Biotech Co., Ltd. (Nanjing, China). The U87
cells (5×105 cells/well) were cultured in six-well
plates and transfected with either miR-16 precursor or anti-miR-16
using Lipofectamine 2000 for 6 h at 37°C. The transfection media
was replaced with RPMI-1640 containing 10% FCS without antibiotic
in a humidified atmosphere at 37°C with 5% CO2 for 18
h.
Statistical analysis
All data are presented as the mean ± standard
deviation. The data were analyzed using Student's t-test.
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Osthole suppresses proliferation and
increases caspase-3 activity in the U87 cells
To determine whether there is an association between
osthole and U87 cells, the viability of U87 cells following
treatment with different concentrations of osthole (0, 50, 100 or
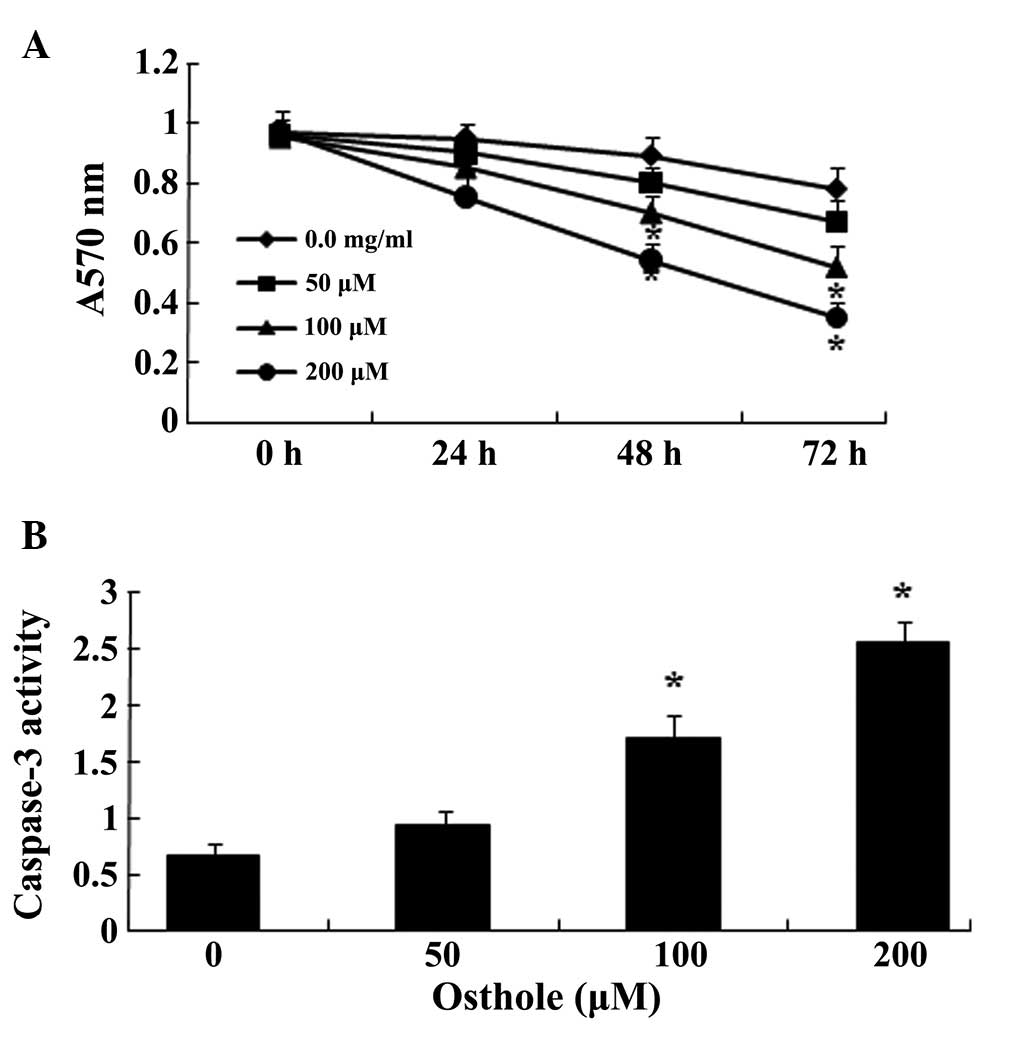
200 µΜ) was determined using an MTT assay. As shown in
Fig. 2A, the analysis revealed
that treatment with 50 µΜ osthole for 72 h significantly
reduced U87 cell viability, and treatment with 100 and 200
µΜ for 48 h or 72 h significantly reduced U87 cell
viability. In addition, the decrease in U87 cell viability was
found to occur in a time- and concentration-dependent manner in the
osthole-treated cells. The activity of caspase-3 in the U87 cells
was analyzed following treatment with osthole (0, 50, 100 or 200
µΜ) using a caspase-3 assay. Following treatment with 100 or
200 µΜ osthole for 48 h, the activity of caspase-3 was
significantly increased in the U87 cells (P<0.05), and this
increase in the activity of caspase-3 occurred in a
concentration-dependent manner in the osthole-treated cells
(Fig. 2B).
Flow cytometric analysis for the
detection of cellular apoptosis
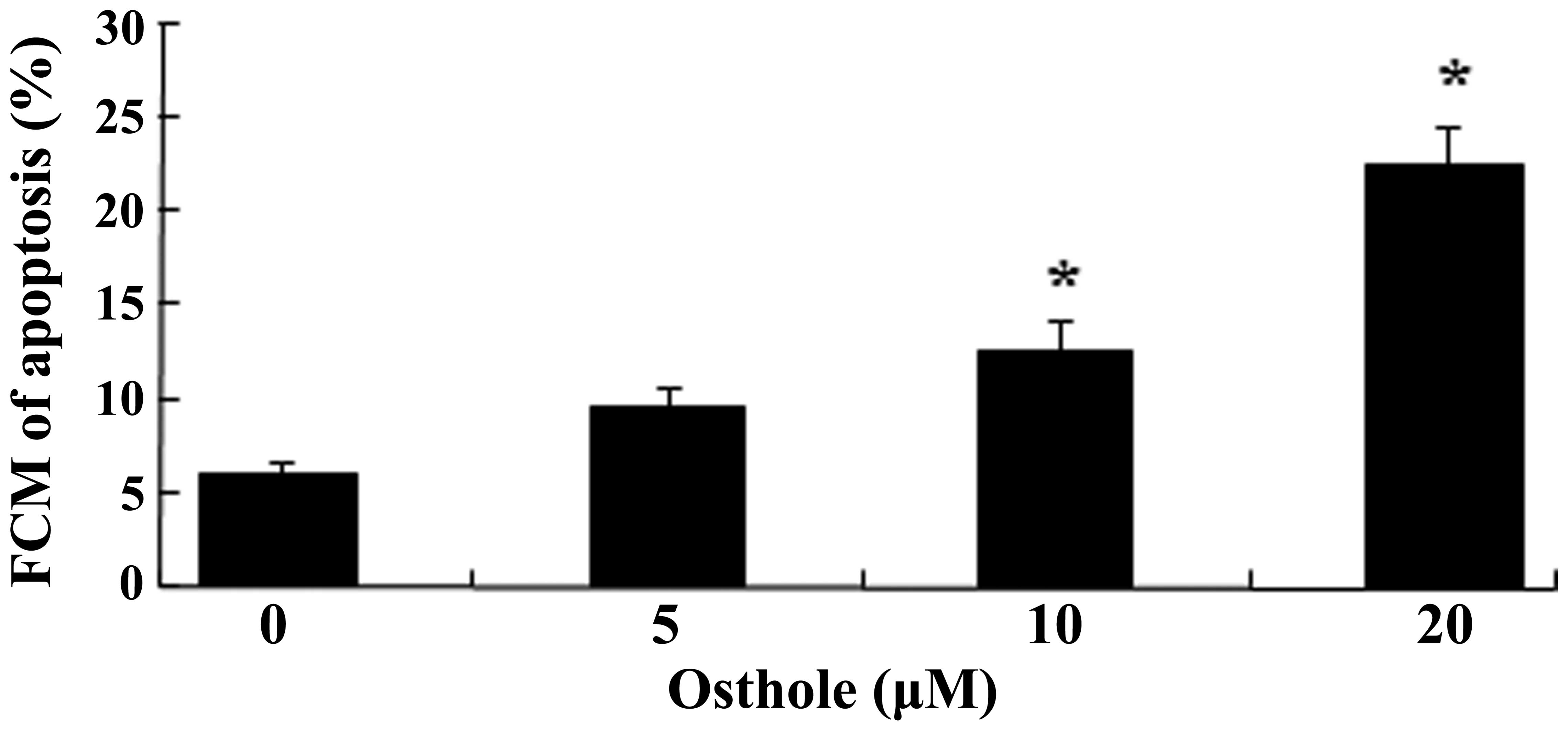
The U87 cells were exposed to osthole at
concentrations of 0, 50, 100 or 200 µΜ for 48 h, and the
effect of osthole on the augmentation of apoptosis was
investigated. The results of this analysis using flow cytometry
demonstrated that osthole inhibited the growth of the U87 cells in
a dose-dependent manner (Fig. 3).
Treatment with 100 and 200 µΜ osthole for 48 h significantly
increased the apoptosis of U87 cells.
Inhibition of MMP-9 by osthole
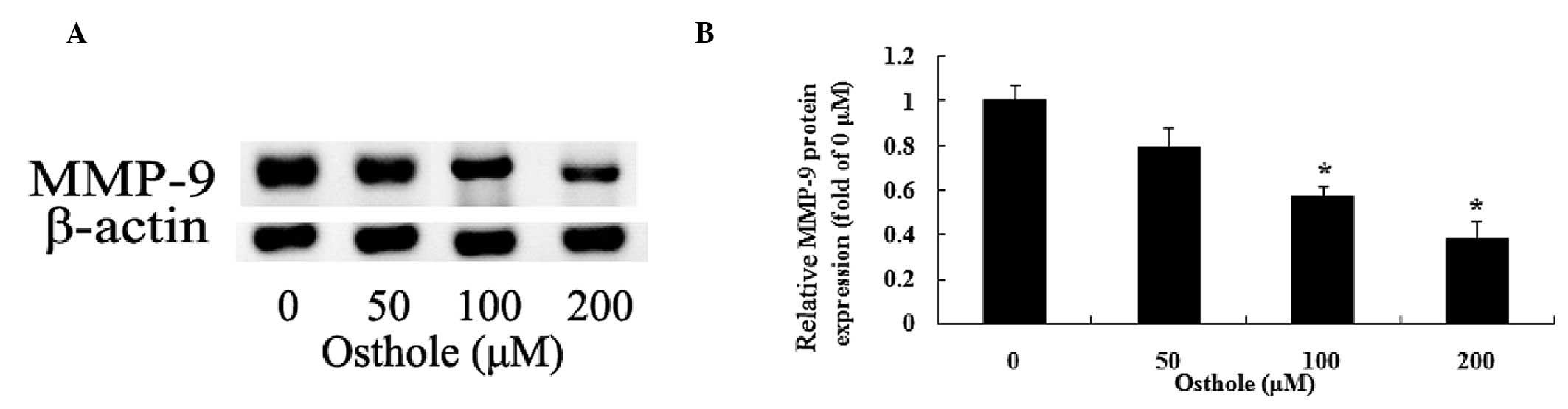
To examine a potential association between the
effect of osthole (0, 50, 100 of 200 µΜ) on the U87 cells
and MMP-9, the protein expression levels of MMP-9 were determined
using gelatin zymography assays. The data indicated that osthole
inhibited the protein expression levels of MMP-9 in a
dose-dependent manner (Fig. 4A).
As shown in Fig. 4B, treatment
with 100 and 200 µΜ of osthole for 48 h significantly
reduced the protein expression levels of MMP-9 in the U87
cells.
Osthole activates the expression of
miR-16
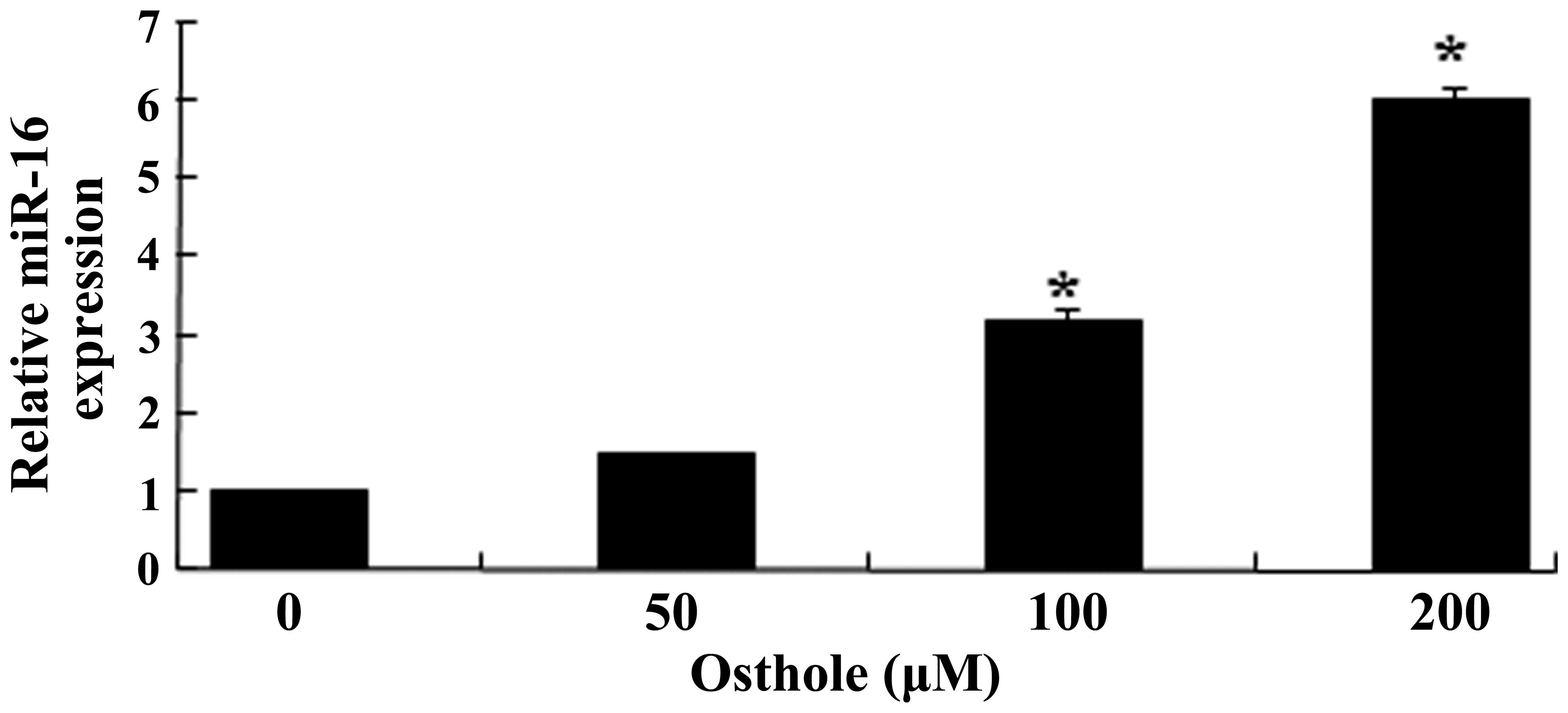
The present study aimed to determine the possible
correlations between osthole (0, 50, 100 or 200 µΜ) and the
expression levels of miR-16, which was assessed using RT-qPCR. The
data indicated that osthole promoted the expression levels of
miR-16 in a dose-dependent manner. As shown in Fig. 5, treatment with 100 and 200
µΜ osthole for 48 h significantly promoted the expression of
miR-16 in the U87 cells (P<0.05).
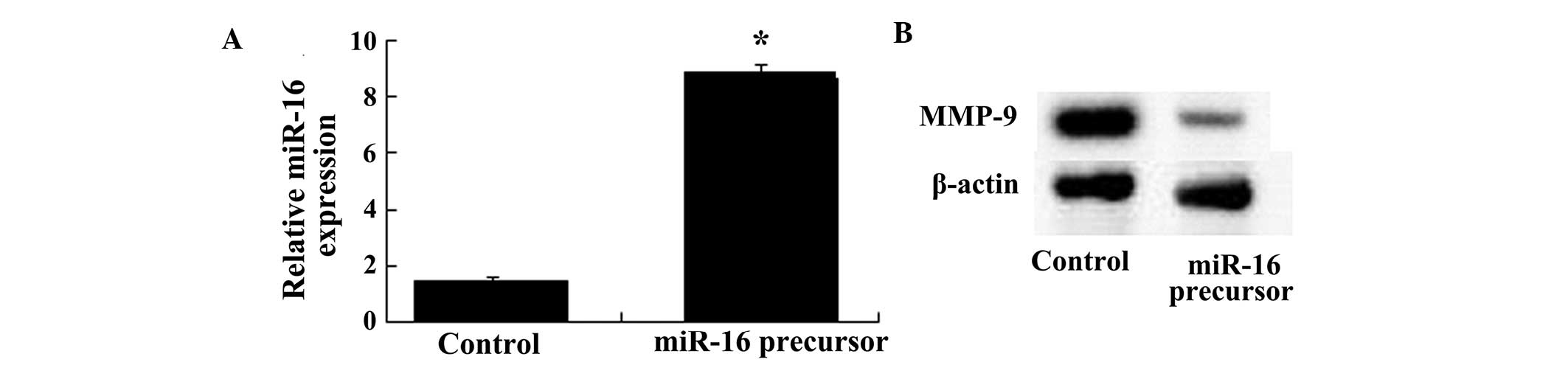
Overexpression of miR-16 inhibits the
protein expression of MMP-9
The protein expression levels of MMP-9 were examined
following transfection of the U87 cells with miR-16. The results
demonstrated that the protein expression levels of MMP-9 were
reduced by overexpression of miR-16 in the U87 cells treated with
100 µΜ osthole. As shown in Fig. 6A and B, the miR-16 overexpressing
cells inhibited the protein expression of MMP-9.
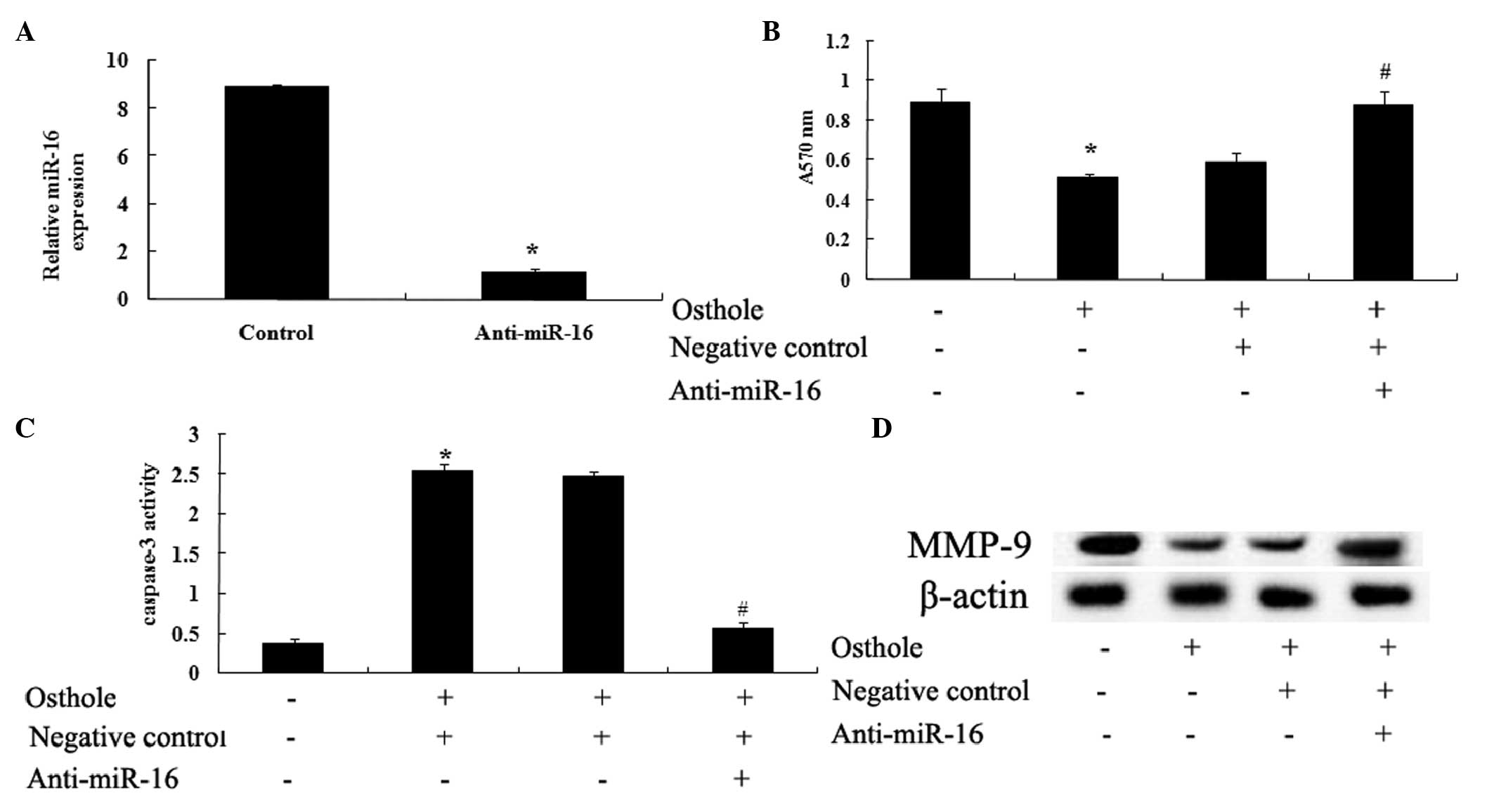
Anti-miR-16 reverses the effect of
osthole
To confirm the functional role of osthole and of
miR-16 in mediating the effects on the viability of the U87 cells,
the present study examined the activity of caspase-3 and the
protein expression levels of MMP-9 following transfection of the
cells with anti-miR-16. The results indicated that transfection
with the anti-miR-16 antibody efficiently penetrated into the U87
cells and significantly reduced the expression of miR-16 in the U87
cells (Fig. 7A). Following
treatment with 100 µΜ osthole for 48 h, transfection with
the anti-miR-16 antibody neutralized the effect of miR-16 on the
U87 cells (Fig. 7B), inhibited the
apoptotic effect on the U87 cells, indicated by downregulation in
capsase-3 activity (Fig. 7C), and
neutralized the inhibitory effect of osthole through downregulating
the expression of MMP-9 (Fig.
7D).
Discussion
Glioma comprises 44.6% of tumors of the central
nervous system and exhibit characteristics of a high recurrence
rate and high mortality rate. With the development of molecular
biology techniques, investigating glioma resistance mecha nism has
been achieved at the molecular gene level and, through increased
investigation, a deeper understanding of the resistance mechanism
of glioma has been demonstrated (15). Several studies have reported
certain natural chemical and compound products, which exert
anticancer effects on a wide variety of tumor types (16,17).
A previous study reported that osthole induces cell apoptosis and
weakens cell migration in brain tumors (18). However, the detailed mechanisms
underlying the anticancer effects of osthole on glioma remain to be
fully elucidated. The present study demonstrated that osthole
suppressed the proliferation and increased the activity of
caspase-3 in the U87 cells. These results were consistent with
those of previous studies (19–21).
For example, Ding et al (19) demonstrated that osthole inhibits
the proliferation, migration and invasion, and induces the
apoptosis of glioma cells.
Osthole, isolated from the rumbrelliferae plant
monomers of the ripe fruit, Cnidium monnieri, possesses
anti-inflammatory properties, improves learning and memory, and
inhibits thrombosis (22–24). In the present study, treatment of
the U87 cells with osthole significantly increased the apoptosis of
the cells. Osthole was previously observed to suppress cell
proliferation and induce cellular apoptosis in NCI-H460 lung
carcinoma cells, which corresponded with the present study
(25).
The degradation of the extracellular matrix by MMPs
via type II collagenase AB is associated with the invasive cell
growth of glioma, with MMP-9 being the most closely associated with
glioma and, to a certain extent, its expression can reflect the
extent of glioma invasion within the body (26,27).
The mRNA expression level of MMP-9 is closely associated with the
degree of malignant glioma, and it has been reported that the
intensity of MMP-9-positive staining is closely associated with the
pathological levels of glioma (28). These reports indicate that MMP-9
can be used to determine the human glioma malignant phenotype
(29). Using a nude mouse model to
investigate the growth of glioma, it was determined that increased
expression levels of MMP-9 are more marked, compared with the
expression level of MMP-2, and indicates that MMP-9 is important in
the growth of glioma (30).
Consistently, the results of the present study demonstrated that
treatment with osthole significantly reduced the protein expression
levels of MMP-9. It was previously revealed that osthole suppresses
the levels of MMP-2 and MMP-9 in A549 human lung cancer cells
(31). In addition, osthole has
been observed to inhibit NF-κB-MMP-9 in human lung adenocarcinoma
(14).
In the present study, RT-qPCR was used to identify
possible correlations between osthole (0, 50, 100 or 200 µΜ)
and the expression of miR-16. The data indicated that osthole
promoted the expression of miR-16 in a dose-dependent manner. The
expression levels of miR-16-1 were markedly reduced in the human
glioma cell lines, which suggested that miR-16-1 is involved in the
proliferative, migratory and invasive abilities of highly-invasive
glioma cells (32). The present
study demonstrated that upregulation of the expression of miR-16
reduced the protein expression levels of MMP-9 in the (U87 cells.
In addition, the results revealed that downregulation of the
expression of miR-16, via anti-miR-16 transfection, promoted the
protein expression of MMP-9 in the U87 cells and reduced the effect
of osthole on the proliferation and apop-tosis of the U87 cells. In
conclusion, the results of the present study suggested that osthole
suppressed the proliferation and accelerated the apoptosis of U87
human glioma cells via upregulation of the expression of miR-16 and
downregulation of the expression of MMP-9.
Acknowledgments
This study was supported by the Twelve-Five National
Science and Technology Support Program (grant no.
2011BAI08B04).
References
|
1
|
Sun YC, Wang J, Guo CC, Sai K, Wang J,
Chen FR, Yang QY, Chen YS, Wang J, To TS, et al: MiR-181b
sensitizes glioma cells to teniposide by targeting MDM2. BMC
Cancer. 14:6112014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunha LC, Del Bel E, Pardo L, Stühmer W
and Titze-DE-Almeida R: RNA interference with EAG1 enhances
interferon gamma injury to glioma cells in vitro. Anticancer Res.
33:865–870. 2013.PubMed/NCBI
|
|
3
|
Yang X, Lv S, Liu Y, Li D, Shi R, Tang Z,
Fan J and Xu Z: The clinical utility of matrix metalloproteinase 9
in evaluating pathological grade and prognosis of glioma patients:
a meta-analysis. Mol Neurobiol. Aug 10–2014.
|
|
4
|
Sun C, Wang Q, Zhou H, Yu S, Simard AR,
Kang C, Li Y, Kong Y, An T, Wen Y, et al: Antisense MMP-9 RNA
inhibits malignant glioma cell growth in vitro and in vivo.
Neurosci Bull. 29:83–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asuthkar S, Velpula KK, Chetty C, Gorantla
B and Rao JS: Epigenetic regulation of miRNA-211 by MMP-9 governs
glioma cell apoptosis, chemosensitivity and radiosensitivity.
Oncotarget. 3:1439–1454. 2012.PubMed/NCBI
|
|
6
|
Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S,
Ge R, Jiang S, Li G, Chen Y, et al: microRNA-146b inhibits glioma
cell migration and invasion by targeting MMPs. Brain Res.
1269:158–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Y, Bai Y, Wang XD, Liu B, Zhao Z and
Wang LS: Differential expression of miRNA in rat myocardial tissues
under psychological and physical stress. Exp Ther Med. 7:901–906.
2014.PubMed/NCBI
|
|
8
|
Yan X, Liang H, Deng T, et al: The
identification of novel targets of miR-16 and characterization of
their biological functions in cancer cells. Mol Cancer. 12:922013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Xia Y, Niu H and Chen Y: MiR-16
induced the suppression of cell apoptosis while promote
proliferation in esophageal squamous cell carcinoma. Cell Physiol
Biochem. 33:1340–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar
|
|
11
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-kappaB1/MMP9 signaling pathway. Cancer Sci.
105:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XY, Dong WP, Bi SH, Pan ZG, Yu H,
Wang XW, Ma T, Wang J and Zhang WD: Protective effects of osthole
against myocardial ischemia/reperfusion injury in rats. Int J Mol
Med. 32:365–372. 2013.PubMed/NCBI
|
|
13
|
Sun F, Xie ML, Zhu LJ, Xue J and Gu ZL:
Inhibitory effect of osthole on alcohol-induced fatty liver in
mice. Dig Liver Dis. 41:127–133. 2009. View Article : Google Scholar
|
|
14
|
Kao SJ, Su JL, Chen CK, Yu MC, Bai KJ,
Chang JH, Bien MY, Yang SF and Chien MH: Osthole inhibits the
invasive ability of human lung adenocarcinoma cells via suppression
of NF-kappaB-mediated matrix metalloproteinase-9 expression.
Toxicol Appl Pharmacol. 261:105–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Lv S, Zhou X, Liu Y, Li D, Shi R,
Kang H, Zhang J and Xu Z: The clinical implications of transforming
growth factor beta in pathological grade and prognosis of glioma
patients: A meta-analysis. Mol Neurobiol. Aug 23–2014.
|
|
16
|
Lu DY, Chang CS, Yeh WL, Tang CH, Cheung
CW, Leung YM, Liu JF and Wong KL: The novel phloroglucinol
derivative BFP induces apoptosis of glioma cancer through reactive
oxygen species and endoplasmic reticulum stress pathways.
Phytomedicine. 19:1093–1100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai CF, Yeh WL, Huang SM, Tan TW and Lu
DY: Wogonin induces reactive oxygen species production and cell
apoptosis in human glioma cancer cells. Int J Mol Sci.
13:9877–9892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai CF, Yeh WL, Chen JH, Lin C, Huang SS
and Lu DY: Osthole suppresses the migratory ability of human
glioblastoma multiforme cells via inhibition of focal adhesion
kinase-mediated matrix metalloproteinase-13 expression. Int J Mol
Sci. 15:3889–3903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding D, Wei S, Song Y, Li L, Du G, Zhan H
and Cao Y: Osthole exhibits anti-cancer property in rat glioma
cells through inhibiting PI3K/Akt and MAPK signaling pathways. Cell
Physiol Biochem. 32:1751–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Peng Y, Shi K, et al: Osthole
inhibits proliferation of human breast cancer cells by inducing
cell cycle arrest and apoptosis. J Biomed Res. 29:132–138.
2015.PubMed/NCBI
|
|
21
|
Lin YC, Lin JC, Hung CM, et al: Osthole
inhibits insulin-like growth factor-1-induced epithelial to
mesenchymal transition via the inhibition of PI3K/Akt signaling
pathway in human brain cancer cells. J Agric Food Chem.
62:5061–5071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Zhang W, Zhou L, Wang X and Lian Q:
Anti-inflammatory effect and mechanism of osthole in rats. Zhong
Yao Cai. 28:1002–1006. 2005.
|
|
23
|
Zhang Q, Qin L, He W, Van Puyvelde L, Maes
D, Adams A, Zheng H and De Kimpe N: Coumarins from Cnidium monnieri
and their antiosteoporotic activity. Planta Med. 73:13–19. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luszczki JJ, Andres-Mach M, Cisowski W,
Mazol I, Glowniak K and Czuczwar SJ: Osthole suppresses seizures in
the mouse maximal electroshock seizure model. Eur J Pharmacol.
607:107–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu XM, Zhang Y, Qu D, Liu HB, Gu X, Jiao
GY and Zhao L: Combined anticancer activity of osthole and
cisplatin in NCI-H460 lung cancer cells in vitro. Exp Ther Med.
5:707–710. 2013.PubMed/NCBI
|
|
26
|
Singh MK, Bhattacharya D and Chaudhuri S,
Acharya S, Kumar P, Santra P, Basu AK and Chaudhuri S: T11TS
inhibits glioma angio-genesis by modulation of MMPs, TIMPs, with
related integrin alphav and TGF-β1 expressions. Tumour Biol.
35:2231–2246. 2014. View Article : Google Scholar
|
|
27
|
Kim MS, Kwak HJ, Lee JW, Kim HJ, Park MJ,
Park JB, Choi KH, Yoo H, Shin SH, Shin WS, et al:
17-Allylamino-17-demethoxygeldanamycin down-regulates hyaluronic
acid-induced glioma invasion by blocking matrix metalloproteinase-9
secretion. Mol Cancer Res. 6:1657–1665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang HC, Huang CY, Lin-Shiau SY and Lin
JK: Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma
invasion through suppressing the association ZIP/p62 with PKC-zeta
and downregulating the MMP-9 expression. Mol Carcinog. 48:517–531.
2009. View
Article : Google Scholar
|
|
29
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Downregulation of uPA, uPAR and MMP-9 using
small, interfering, hairpin RNA (siRNA) inhibits glioma cell
invasion, angiogenesis and tumor growth. Neuron Glia Biol.
1:165–176. 2004. View Article : Google Scholar
|
|
30
|
Pagliara V, Adornetto A, Mammi M, Masullo
M, Sarnataro D, Pietropaolo C and Arcone R: Protease Nexin-1
affects the migration and invasion of C6 glioma cells through the
regulation of urokinase Plasminogen Activator and Matrix
Metalloproteinase-9/2. Biochim Biophys Acta. 1843:2631–2644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu XM, Zhang Y, Qu D, Feng XW, Chen Y and
Zhao L: Osthole suppresses migration and invasion of A549 human
lung cancer cells through inhibition of matrix metalloproteinase-2
and matrix metallopeptidase-9 in vitro. Mol Med Rep. 6:1018–1022.
2012.PubMed/NCBI
|
|
32
|
Li X, Ling N, Bai Y, Dong W, Hui GZ, Liu
D, Zhao J and Hu J: MiR-16-1 plays a role in reducing migration and
invasion of glioma cells. Anat Rec (Hoboken). 296:427–432. 2013.
View Article : Google Scholar
|