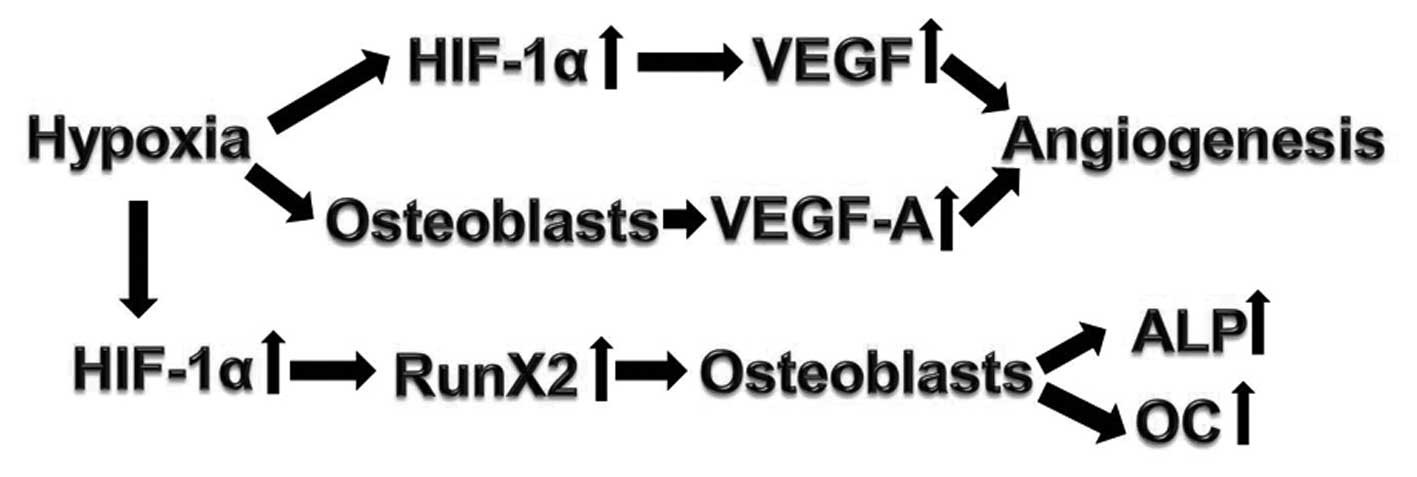Introduction
Traumatic fractures are the most common type of
injury in daily life. In the majority of clinical cases, the most
simple fractures heal with minimal intervention; however, in severe
fractures and in certain patient populations, including diabetics
and patients with splintered fractures, impaired fracture healing
and bone defects occur (1,2). In spite of numerous advances in every
discipline of medicine, patients with complex bone injuries of the
upper and lower extremities and are required to undergo prolonged
reconstructive procedures for retrieval of their limb functions
(2,3).
It has been reported that the hypoxia-inducible
factor (HIF) pathway is the central pathway for sensing and
responding to alterations in local oxygen levels in a wide variety
of organisms (4). Activation of
the HIF-1α pathway can act as a critical mediator of
neoangiogenesis, which is required for skeletal regeneration; thus,
it is suggested that the application of HIF activators may be used
as therapies to improve bone healing (4). An increasing number of studies
suggested that hypoxia may be a powerful stimulus for bone cells
via the mediation of angiogenesis [vascular endothelial growth
factor (VEGF)], cellular metabolism (glucose transporter) and the
recruitment of mesenchymal cells (MSCs) to areas of matrix damage
(5–7). A more thorough understanding of
hypoxia in bone healing will lead to the elucidation of cellular
and molecular mechanisms that may aid in the development of
protective therapies. CoCl2, a mimic of hypoxia,
directly enhances HIF-1α stabilization and downstream target genes
by inhibiting prolyl hydroxylase enzymes (8). An improved understanding of the
alterations in gene expression that occur during fracture healing
induced by CoCl2 in vivo may lead to improvements
in treatment.
In the present study, it was hypothesized that
tibiae treated with CoCl2 may exhibit accelerated
fracture healing. To determine whether systemic application of
CoCl2 enhances the rates of fracture healing in a
pre-clinical model, the effects of CoCl2 on callus
formation and fracture healing using radiographic evaluation as
well as callus mechanical strength and integrity using three-point
bending and key gene expression were examined in vivo.
Materials and methods
Animal care
The present study was performed in accordance with
the regulations of Xuanwu Hospital Affiliated to Capital Medical
University (Beijing, China) and approved by the local animal
research committee. All Sprague-Dawley rats were obtained from the
Laboratory Animal Center of Capital Medical University.
Animal experiments
Sprague-Dawley (SD) rats, 6 weeks of age, were
maintained under humidity (50–60%) and temperature (23–25°C)
controlled conditions with a 12-h light/dark cycle at the Central
Animal Facility, Capital Medical University (Beijing, China). The
animals were allowed 1 week acclimatization to local vivarium
conditions and had free access to untreated tap water and standard
rat chow. A total of 48 female SD rats were randomly assigned to
two groups: i) Control animals treated with saline (n=24) and ii)
animals with 15 mg/kg CoCl2 treatment per day,
administered by intraperitoneal injection (n=24) prior to fracture.
Subsequent to anesthesia with intraperitoneal ketamine
hydrochloride (80 mg/kg body weight) and xylazine (10 mg/kg body
weight) both purchased from Fujian Furuta Pharmaceutical Co., Ltd.
(Fujian, China), surgery was performed with a fracture device, as
described previously (9). In
brief, fractures were created at the tibial tuberosity using a
blunt guillotine driven by a drop weight, and a steel K-wire was
inserted into the medullary canal. Radiographs were captured
immediately to confirm the extent of fractures.
Subsequent to capturing the radiographs, the rats
were sacrificed by cervical dislocation at 7, 28 and 42 days
following fracture (n=8 for each time-point). The K-wire was
removed, the tibiae were dissected, and the fracture zone was
prepared for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), western blotting and immunohistochemistry
Radiological analysis
Radiographic analysis was performed to assess
healing parameters using a Faxitron x-ray machine (MX-20 Specimen
Radiography System; Faxitron Bioptics, LLC, Tucson, AZ, USA).
Radiographs were captured at multiple time-points post-fracture (7,
28 and 42 days) and were assessed blindly by three independent
investigators using the scoring scale described previously
(10). The scale was obtained
according to rebridgement (no rebridgement, partial or complete)
and the results were expressed as a percentage alteration from
saline-treated groups (saline-treated =100%).
RNA extraction and RT-qPCR
Total RNA was extracted from fresh bone tissues of
each group using the TRIzol reagent according to the manufacturer's
instructions (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA
quantity and quality was determined by using the NanoDrop 2000
spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA).
Total RNA (500 ng) was reverse-transcribed using the ReverTra Ace
following the manufacturer's instructions (Toyobo Co., Ltd., Osaka,
Japan). RT-qPCR was performed to measure mRNA expression levels
relative to β-actin (ACTB) mRNA expression with the iCycle iQ
real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA,
USA) using SYBR Green Master Mix (Toyobo, Co., Ltd.). The primers
used were as follows: HIF-1α, 5′-CCCCTACTATGTCGCTTTCTTGG-3′
(forward) and 5′-GGTTTCTGCTGCCTTGTATGG-3′ (reverse); VEGF,
5′-CGACAAGGCAGACTATTCAACG-3′ (forward) and
5′-GGCACGATTTAAGAGGGGAAT-3′ (reverse); runt-related transcription
factor 2 (Runx2), 5′-CCCACGAATGCACTATCCAG-3′ (forward) and
5′-GGCTTCCATCAGCGTCAACA-3′ (reverse); ALP,
5′-GGACGGTGAACGGGAGAAC-3′ (forward) and 5′-CCCTCAGAACAGGGTGCGTAG-3′
(reverse); osteocalcin (OC), 5′-CGGACCACATTGGCTTCCAG-3′ (forward)
and 5′-GCTGTGCCGTCCATACTTTCG-3′ (reverse); and ACTB,
5′-CCGTAAAGACCTCTATGCCAACA-3′ (forward) and
5′-CGGACTCATCGTACTCCTGCT-3′ (reverse). Primers were synthesized by
Sangon Biotech (Shanghai, China). The PCR thermal cycling
conditions were as follows: 95°C for 5 min followed by 40 cycles of
95°C for 15 sec and 60°C for 1 min. All experiments were performed
in triplicate and were repeated a minimum of three times. The
qRT-PCR results were expressed relative to gene expression levels
at the threshold cycle (Ct) and were related to the control.
Immunohistochemistry
Sections were prepared and processed using standard
techniques following a previously described method (11). In brief, tissues generated from the
fracture site were cut and mounted on slides, and following
de-paraffinization and hydration, antigen retrieval was performed
by incubating with 10 mmol/l sodium citrate (pH 6.0) and followed
by 3% H2O2 in methanol for 10 min to inhibit
endogenous peroxide. The slides were incubated with primary
antibodies in the blocking solution in a humidified chamber at 4°C
overnight. To determine the expression of activated forms of VEGF,
ALP and OC proteins, rabbit polyclonal VEGF antibody (cat. no.
ABS82, 1:100, EMD Millipore, Temecula, California, USA), rabbit
polyclonal ALP antibody (cat. no. ab84401, 1:100, Abcam, Cambridge,
MA, USA) and mouse monoclonal OC antibody (cat. no. ab13418, 1:100,
Abcam) were used. Finally, the sections were incubated with the
secondary antibody for 10 min. Subsequent to washing with
phosphate-buffered saline, the sections were then incubated with
streptavidin-peroxidase conjugate for 10 min. The final staining
was performed in diaminobenzidine tetrahydrochloride (Ventana
Medical Systems, Inc., Tucson, AZ, USA) solution and were then
counterstained with hematoxylin, dehydrated and mounted. Negative
controls included replacement of the primary antibody with normal
polyclonal mouse immunoglobulin G of the same concentration.
The score was assessed by two independent observers,
under a light microscope (Olympus BX61; Olympus, Melville, NY,
USA). The percentages of stained cells and staining intensity were
taken into account in order to obtain the score. Staining intensity
was scored as follows: 0, no staining; 1, weak intensity; 2,
moderate intensity; and 3, high intensity. The number of positive
cells was evaluated as follows: 0 (negative), <10% positive
cells; 1 (weak), <30% positive cells; 2 (moderate), <50%
positive cells; and 3 (strong), >70% positive cells.
Biomechanical analysis
Hydrated tibiae were assessed in regard to torsion
using previously published methods (12). The rat tibiae from each group were
tested to failure by three-point bending using a material testing
system (ELF 3400; EnduraTEC, Minnetonka, MN, USA). Biomechanical
parameters, including breaking force (maximum load), stiffness
(average slope of linear portion of the curve before yielding) and
work-to-fracture (bend strain at maximum and bend strength at
maximum) were calculated from the force displacement data.
Western blot analysis
Radioimmunoprecipitation assay buffer containing
protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA) was used to
prepare tissue lysates with 1% SDS. Protein quantification was
performed using a Bicinchoninic Acid Protein Assay kit (Thermo
Fisher Scientific). Total proteins (50 µg) were resolved on
10% SDS-PAGE and electrotransferred onto polyvinylidene difluoride
membranes (EMD Millipore, Bedford, MA, USA). The membranes were
blocked in 5% skimmed milk in Tris-buffered saline containing 0.1%
Tween 20 (TBST) for 1 h and incubated overnight at 4°C with primary
rabbit polyclonal antibodies against HIF-1α (cat. no. PA1-16601,
Thermo Fisher Scientific), VEGF (cat. no. ABS82; EMD Millipore),
ALP (cat. no. ab84401; Abcam), Runx2 (cat. no. H00000860-M04;
Abnova, Taipei, Taiwan), OC (cat. no. ab13418; Abcam) and mouse
monoclonal β-actin (cat. no. sc-47778; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). All antibodies were diluted 1:1,000 in
Tris-buffered saline. Blots were washed in TBST and labeled with
the horseradish peroxidase-conjugated secondary antibody (Cell
Signaling Technology, Inc, Danvers, MA, USA). Bands and band
intensity were detected and calculated using chemiluminescence
(Thermo Fisher Scientific) and Image Quant LAS4000 (GE Healthcare
Life Sciences, Little Chalfont). The protein expression levels were
expressed relative to β-actin levels.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical differences between groups were evaluated
using Student's two-tailed t-test and P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed with SPSS 15.0 software (SPSS, Inc.,
Chicago, IL, USA). All experiments were repeated a minimum of three
times, independently.
Results
CoCl2 accelerates the
formation and remodeling of bone during fracture repair
Previous studies have demonstrated that hypoxia
affects the fracture healing by MSCs (5,13);
however, the underlying cellular and molecular mechanisms have
remained to be fully elucidated. To further examine the effects of
hypoxia during fracture repair, callus formation was performed for
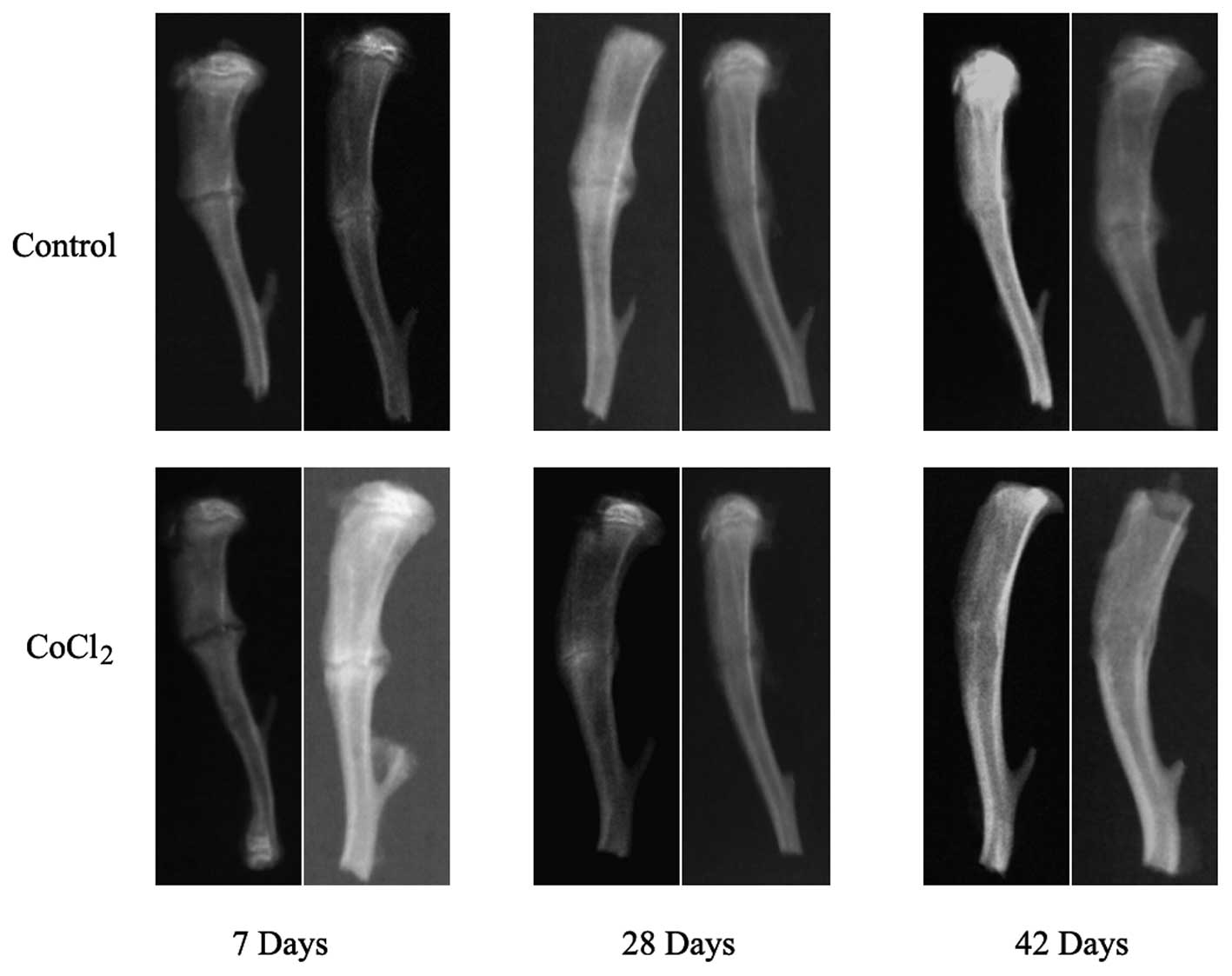
the radiographic examination on days 7, 28 and 42 in all animals.
Treatment of rats with CoCl2, a hypoxia mimic, markedly
strengthened new bone formation during the course of fracture
repair (Fig. 1). Of note,
fractured tibiae exhibited enhanced repair at 7 days compared with
vehicle-treated animals and displayed near-complete healing of the
CoCl2-treated tibiae at 42 days, whereas incomplete
bridging of cortical bone was clearly visible in the
vehicle-treated animals. These results indicated that
CoCl2 may serve an important role in fracture
healing.
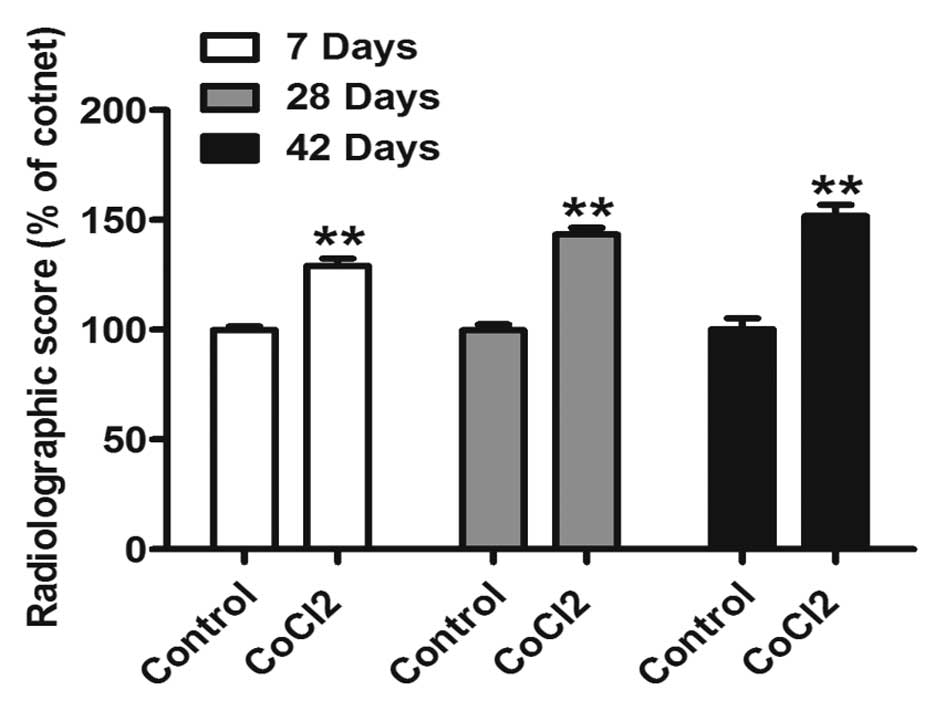
In order to confirm this observation, re-bridgement
of the cortices and acceleration of healing was analyzed using a
grading scale (10). Radiological
evaluation of animals treated with CoCl2 at 7, 28 and 42
days suggested a significant increase in the healing rate (Fig. 2). These results indicated that
CoCl2 may promote fracture repair.
The HIF-1 pathway is functional and
mediates hypoxia-induced gene expression during fracture repair
under hypoxia
Hypoxia is one of the most important pathological
features of numerous diseases and it is well known that
CoCl2 is able to mimic the effects of HIF-1α. The mRNA
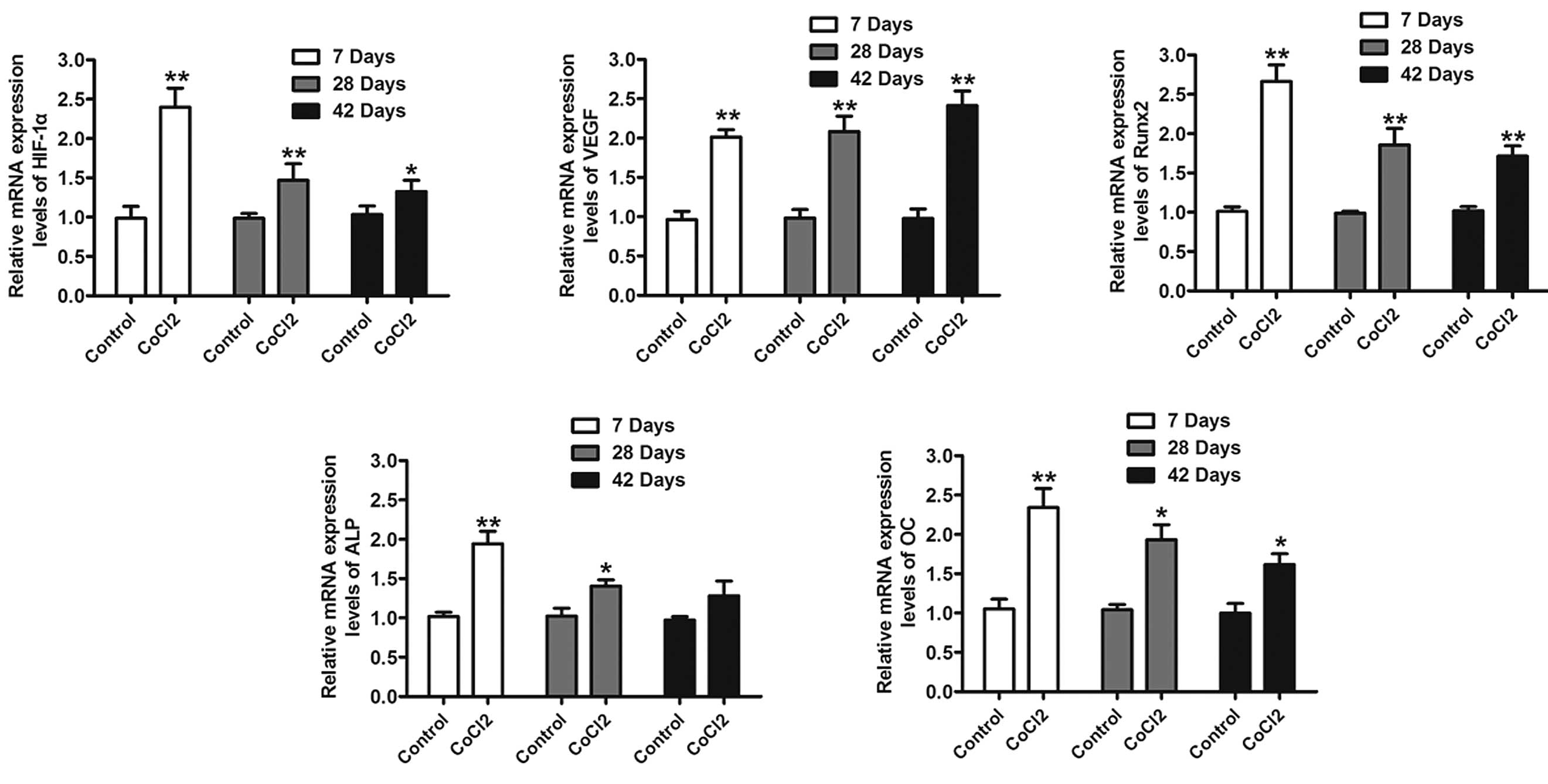
levels of HIF-1α were analyzed using RT-qPCR. As presented in
Fig. 3, rats with CoCl2
exhibited a significantly increased HIF-1α expression. In addition,
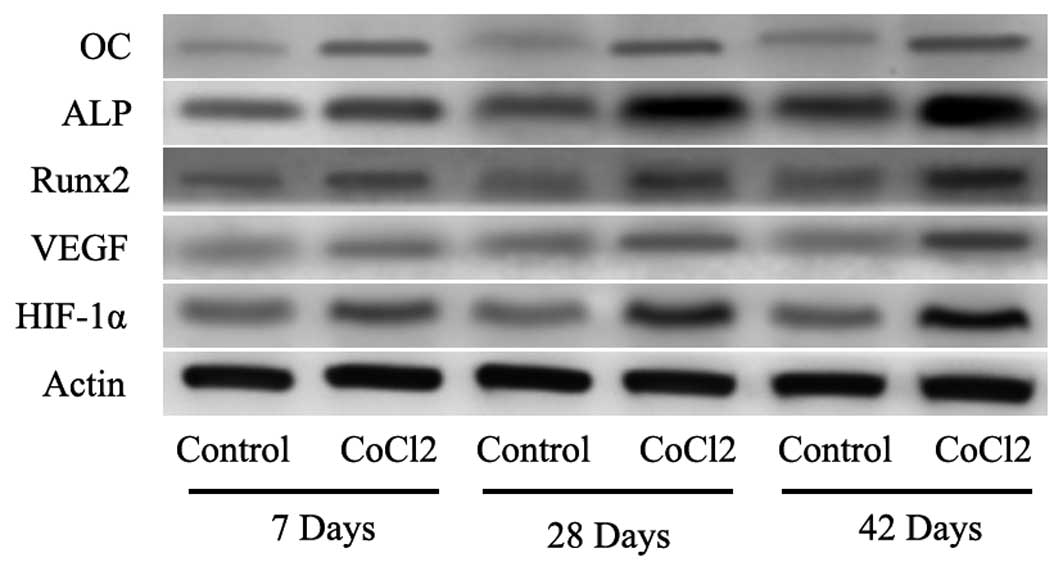
it was observed that the protein levels of HIF-1α were increased at
the measured time-points (Fig. 4).
To further evaluate whether HIF-1α had a direct functional role in
this process, the expression of downstream genes of HIF-1α was
investigated using RT-qPCR and western blot analysis. The results
also indicated that CoCl2 significantly increased VEGF,
Runx2, ALP and OC mRNA and protein levels (Figs. 3 and 4). These results suggested that the
effect of CoCl2 on fracture healing partly involved the
activation of the HIF-1α signaling pathway.
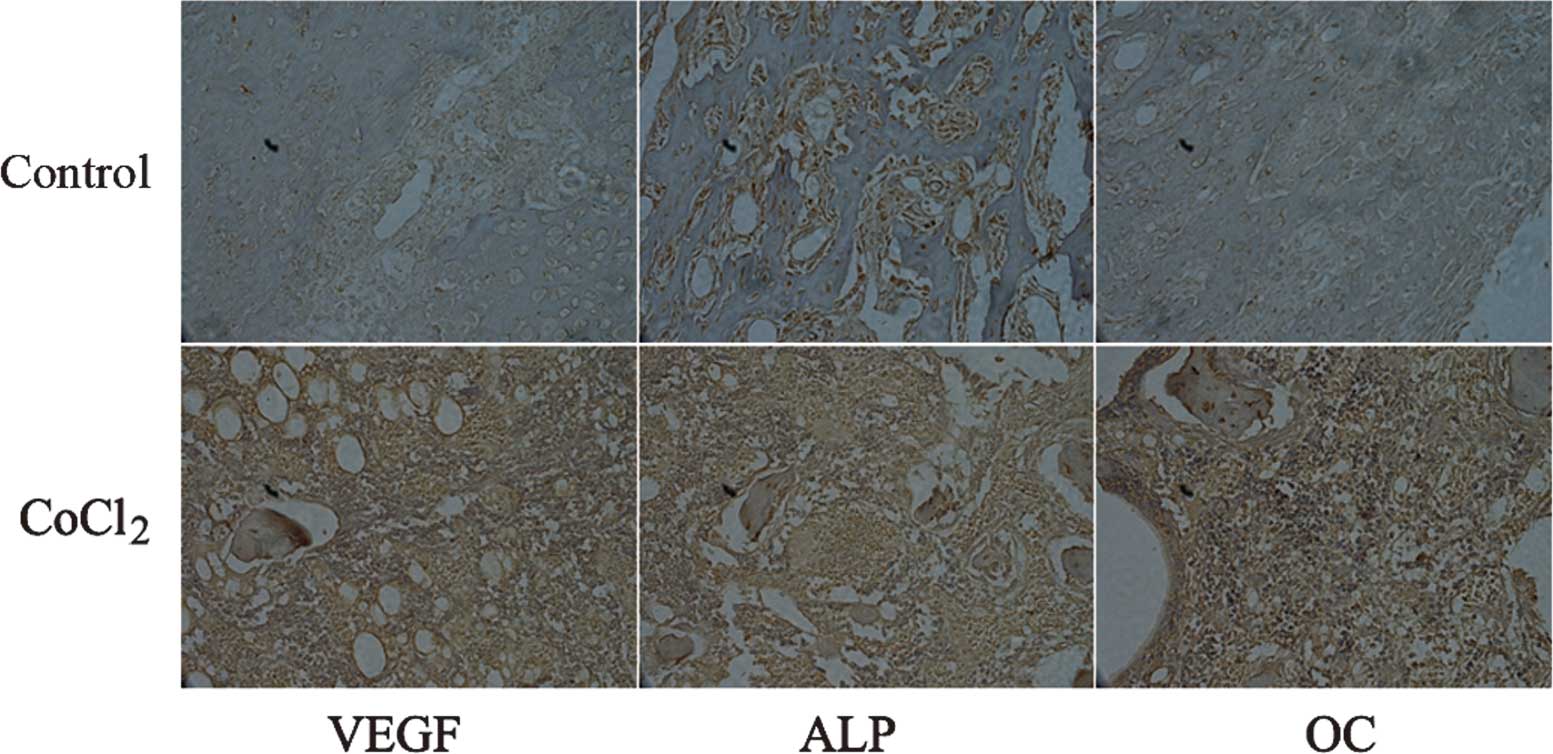
Immunohistochemical analysis
Runx2 is considered to be an osteoblast-specific
transcriptional factor and is involved in chondrocyte maturation
and osteoblast differentiation (14,15).
Osteoblast-specific expression of genes, including ALP and OC, is
an important characteristic of bone healing (16). Therefore, the expression of VEGF,
ALP and OC was assessed in bone tissues by immunohistochemistry at
42 days following fracture. An increase in the expression of VEGF,
ALP and OC was observed in the groups treated with CoCl2
compared with that in the vehicle-treated animals (Fig. 5). These results further suggested
that CoCl2 induces fracture healing through the
activation of HIF-1α and its target genes.
Biomechanical analysis: Three-point
bending
To further assess the functional features of
fracture healing, the influence of CoCl2 on the
mechanical properties of tibiae was evaluated in rats. All
specimens were tested according to the guidelines of the American
Society for Testing and Materials for the uniaxial strength
testing. The tibiae of the animals that had been administered
CoCl2 were significantly stronger than those of the
control animals (Table I). Of
note, the force required to break the bone and the structural
stiffness of the fractured tibia was 55.4 and 50.4% greater,
respectively, than that of the vehicle-treated controls at 42
days.
 | Table IBiomechanical testing. |
Table I
Biomechanical testing.
| Parameter | Control group
| CoCl2
group
|
|---|
| 28 days | 42 days | 28 days | 42 days |
|---|
| Maximum force
(N) | 68.9±3.4 | 116.5±6.7 | 82.9±5.4a | 181.3±12.7b |
| Stiffness (N/mm) | 193.8±13.6 | 307.2±24.6 | 237.1±16.2a | 462.5±20.8b |
| Work to fracture
(N-mm) | 18.7±0.6 | 32.8±2.7 | 22.4±1.8 | 41.6±3.4a |
Discussion
Normal fracture healing is a complex process
involving cellular recruitment, specific gene expression and
synthesis of compounds that regenerate native tissue to restore the
mechanical integrity, and thus the function, of damaged bone
(17–19). Treatments for fracture healing have
addressed numerous aspects, including biological, nutritional,
physical and genetic factors. Previous studies have demonstrated
that hypoxia may induce MSC recruitment to areas of matrix damage
by upregulating the osteopontin/CD44 pathway (5). VEGF serves an important role in
physiological and pathological neovascularization (angiogenesis)
and has been demonstrated to stimulate bone healing in animal
models (6). In the present study,
it was established that hypoxia is able to promote fracture healing
and it was demonstrated that an osteoblast-specific transcriptional
factor, Runx2, as well as the osteoblast-specific genes ALP and OC
were upregulated. This provides a potential strategy for the
regeneration of bone tissue.
As CoCl2 is a well-known mimic of
hypoxia, it was hypothesized that this chemical may have a delayed
pre-conditioning effect in fracture healing. The fracture models
were first identified by radiological evaluation. Consistent with a
previous study, administration of CoCl2 was able to
mimic the effect of hypoxia and promote fracture healing (5). Subsequently, the expression of HIF-1α
was detected at mRNA and protein levels, and it was identified that
the expression of HIF-l1 was significantly upregulated throughout
the process. HIF-a is known to interact with the core DNA sequence
5′-[AG]CGTG-3′ at the hypoxia response element target gene
promoters, resulting in the upregulation of numerous
hypoxia-sensitive genes, including VEGF (20). Of note, CoCl2 has been
observed to enhance the expression of Runx2, an essential
osteoblast transcription factor, as well as ALP and OC, which are
required for osteogenesis in vivo (21–23).
In addition to the significant alterations in gene expression
observed, the biomechanical properties of the healing construct can
be attributed to the density and to the amount of tissue. The
results of the biomechanical assessment indicated that
CoCl2 was able to significantly increase the maximum
load, stiffness and energy absorption. These results indicated that
pretreatment with CoCl2 may support the restoration of
cartilage and bone during fracture healing in vivo.
Previous studies have indicated that MSCs can be
recruited by osteocytes under hypoxia, and genetic activation of
the HIF-1α pathway increases neoangiogenesis and promotes bone
regeneration (13). The present
study identified that CoCl2 was able to significantly
increase the expression of Runx2, ALP and OC. In spite of
inadequate blood supply caused by hypoxia being a major cause of
delayed union or non-union during fracture healing (24), an appropriate amount of hypoxia may
aid in bone healing, providing a novel strategy for the treatment
of bone fractures. A potential pathway for these processes is
proposed in Fig. 6. A mouse
fracture model treated with hypoxia results in upregulated HIF-1α,
which activates VEGF and Runx2, leading to the osteogenic
differentiation and angiogenesis.
In conclusion, the results of the present study
demonstrated that CoCl2 enhances fracture healing. It
was demonstrated that CoCl2 induces bone and cartilage
formation, increases tissue vascularization and may activate the
HIF-1α pathway.
References
|
1
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giannoudis PV, Jones E and Einhorn TA:
Fracture healing and bone repair. Injury. 42:549–550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou W, Kong W, Zhao B, Fu Y, Zhang T and
Xu J: Posterior internal fixation plus vertebral bone implantation
under navigational aid for thoracolumbar fracture treatment. Exp
Ther Med. 6:152–158. 2013.PubMed/NCBI
|
|
4
|
Wan C, Gilbert SR, Wang Y, Cao X, Shen X,
Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC,
et al: Activation of the hypoxia-inducible factor-1alpha pathway
accelerates bone regeneration. Proc Natl Acad Sci USA. 105:686–691.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raheja LF, Genetos DC and Yellowley CE:
Hypoxic osteocytes recruit human MSCs through an OPN/CD44-mediated
pathway. Biochem Biophys Res Commun. 366:1061–1066. 2008.
View Article : Google Scholar
|
|
6
|
Street J, Bao M, DeGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loboda A, Jazwa A, Wegiel B, Jozkowicz A
and Dulak J: Heme oxygenase-1-dependent and -independent regulation
of angiogenic genes expression: Effect of cobalt protoporphyrin and
cobalt chloride on VEGF and IL-8 synthesis in human microvascular
endothelial cells. Cell Mol Biol (Noisy-Le-Grand). 51:347–355.
2005.
|
|
8
|
Ho VT and Bunn HF: Effects of transition
metals on the expression of the erythropoietin gene: Further
evidence that the oxygen sensor is a heme protein. Biochem Biophys
Res Commun. 223:175–180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen W, Wildemann B, Pufe T, Raschke M
and Schmidmaier G: The angiogenic peptide pleiotrophin (PTN/HB-GAM)
is expressed in fracture healing: An immunohistochemical study in
rats. Arch Orthop Trauma Surg. 124:603–607. 2004. View Article : Google Scholar
|
|
10
|
Garrett IR, Gutierrez GE, Rossini G, Nyman
J, McCluskey B, Flores A and Mundy GR: Locally delivered lovastatin
nanoparticles enhance fracture healing in rats. J Orthop Res.
25:1351–1357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerstenfeld LC, Wronski TJ, Hollinger JO
and Einhorn TA: Application of histomorphometric methods to the
study of bone repair. J Bone Miner Res. 20:1715–1722. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutierrez GE, Edwards JR, Garrett IR,
Nyman JS, McCluskey B, Rossini G, Flores A, Neidre DB and Mundy GR:
Transdermal lovastatin enhances fracture repair in rats. J Bone
Miner Res. 23:1722–1730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wagegg M, Gaber T, Lohanatha FL, Hahne M,
Strehl C, Fangradt M, Tran CL, Schönbeck K, Hoff P, Ode A, et al:
Hypoxia promotes osteogenesis but suppresses adipogenesis of human
mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent
manner. PLoS One. 7:e464832012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inada M, Yasui T, Nomura S, Miyake S,
Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, et al:
Maturational disturbance of chondrocytes in Cbfa1-deficient mice.
Dev Dyn. 214:279–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lian JB, Javed A, Zaidi SK, Lengner C,
Montecino M, van Wijnen AJ, Stein JL and Stein GS: Regulatory
controls for osteoblast growth and differentiation: Role of
Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 14:1–41. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mimori K, Komaki M, Iwasaki K and Ishikawa
I: Extracellular signal-regulated kinase 1/2 is involved in
ascorbic acid-induced osteoblastic differentiation in periodontal
ligament cells. J Periodontol. 78:328–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komatsu DE and Warden SJ: The control of
fracture healing and its therapeutic targeting: Improving upon
nature. J Cell Biochem. 109:302–311. 2010.
|
|
18
|
Zhang L, Wang H, Wang T, Jiang N, Yu P,
Liu F, Chong Y and Fu F: Potent anti-inflammatory agent escin does
not affect the healing of tibia fracture and abdominal wound in an
animal model. Exp Ther Med. 3:735–739. 2012.PubMed/NCBI
|
|
19
|
Chen GQ, Wang S and Hu SY: Osteoporosis
increases chondrocyte proliferation without a change in apoptosis
during fracture healing in an ovariectomized rat model. Mol Med
Rep. 5:202–206. 2012.
|
|
20
|
Formenti F, Constantin-Teodosiu D,
Emmanuel Y, Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM,
Humphreys SM, Lappin TR, McMullin MF, et al: Regulation of human
metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA.
107:12722–12727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lian JB, Javed A, Zaidi SK, Lengner C,
Montecino M, van Wijnen AJ, Stein JL and Stein GS: Regulatory
controls for osteoblast growth and differentiation: Role of
Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 14:1–41. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murshed M, Harmey D, Millan JL, McKee MD
and Karsenty G: Unique coexpression in osteoblasts of broadly
expressed genes accounts for the spatial restriction of ECM
mineralization to bone. Genes Dev. 19:1093–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu C, Saless N, Wang X, Sinha A, Decker S,
Kazakia G, Hou H, Williams B, Swartz HM, Hunt TK, et al: The role
of oxygen during fracture healing. Bone. 52:220–229. 2013.
View Article : Google Scholar
|