Introduction
Coronary artery disease (CAD) is the leading
contributor to morbidity and mortality rates worldwide, and results
in a mortality rate of >7,000,000 per year (1). Atherosclerotic plaques are key in the
pathophysiology of CAD, in which macrophages initially become foam
cells and initiate plaque formation. The imbalance of cholesterol
uptake and efflux in macrophages may cause excessive accumulation
of cholesterol and foam cell formation, which is a hallmark in the
development of atherosclerosis (2). Cholesterol efflux from macrophages is
a critical step in reverse cholesterol transport, which may prevent
macrophages from becoming foam cells and thus reduce
atherosclerosis (3,4). Free cholesterol is transported from
macrophages in the arterial atherosclerotic plaque to an
extracellular acceptors, including apoliporotein (apo) A-1 and
high-density lipoprotein (HDL), and then back to the liver for bile
acid synthesis and excretion (5).
Previously, Khera et al reported a reverse correlation
between cholesterol efflux capacity and atherosclerosis (6). To understand the underlying mechanism
regulating cholesterol efflux, it is important to develop a
reliable assay for measuring cholesterol efflux in
vitro.
Traditionally, radioisotope-labeled cholesterols
have been used as probes in cholesterol efflux assays. However, the
procedure is lengthy and labor-intensive, and the radioactivity
disposal procedure can limit the use of this assay in
high-throughput screening. By contrast, fluorescent cholesterol
analogs have demonstrated potential as suitable alternatives for
radioisotope-labeled cholesterols (7). For decades, fluorescent cholesterol
analogs have been used in membrane biophysics for the investigation
of lipid trafficking and the membrane organization of native
cholesterol (8,9). Until recently, fluorescent sterols
were also used in choles-terol efflux assays as an alternative for
radioisotope-labeled cholesterol (10,11).
Commonly used fluorescent analogs of cholesterol include
N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)
amino)-23,24-bisnor-5-xholen-3β-ol (NBD)-cholesterol,
boron-dipyrromethene (BODIPY)-cholesterol and dehydroergosterol
with intrinsic fluorescence (8).
The analogs bearing the NBD fluorophore have demonstrated
advantageous properties for membrane investigations. There are
three versions of NBD-cholesterol: The NBD flurophore attached to
the 3β-OH group of cholesterol, NBD linked to carbon 22 in the
sterol side chain (22-NBD-cholesterol) and NBD linked to carbon 25
(25-NBD-cholesterol).
The THP-1 human monocytic leukemia cell line is a
differentiated monocytic cell line, which exhibites macrophage-like
characteristics when stimulated with phorbol ester (12,13).
Notably, THP-1-derived macrophages have been widely used as a
suitable in vitro model for investigating cholesterol efflux
(14–16). At present, there is a lack of
information regarding the use of fluorescent cholesterol analogs,
including NBD-cholesterol, in efflux assays in THP-1-derived
macrophages. The present study aimed to characterize a cholesterol
efflux assay using fluorescent NBD-cholesterol as a probe in
THP-1-derived macrophages, as well as in human peripheral blood
mononuclear cells (PBMCs). The results may assist in the
development of an in vitro model for cholesterol efflux,
which can be used as a high-throughput screening assay.
Materials and methods
Reagents and materials
THP-1 cells were obtained from the Cell Bank of
Basic Research Institute of the Chinese Academy of Medicine
Sciences (Beijing, China). RPMI 1640 medium and Triton X-100 were
purchased from Gibco Life Technologies (Carlsbad, CA, USA). Phorbol
myristate acetate (PMA) and macrophage colony stimulating factor
(M-CSF) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
22-(N-nitrobenz-2-oxa-1,3-diazol-4-yl-amino)-23,
24-bisnor-5-cholen-3-ol (NBD)-cholesterol was obtained from
Molecular Probes (Eugene, OR, USA). The fluorescent cholesterol was
diluted in ethanol, with a final concentration of 0.5% ethanol.
Purified human high-density lipoprotein (HDL) and apolipoprotein A1
(apoA-1) were obtained from CalBiochem (San Diego, CA, USA).
Ficoll-Paque Premium was purchased from Haoyang Biosciences
(Tianjing, China). Ox-LDL was purchased from the Basic Research
Institute of the Chinese Academy of Medicine Sciences (cells were
preloaded with 50 µg/ml Ox-LDL for 48 h).
Isolation of human PBMCs
The human PBMCs were isolated from 13 volunteers.
All the volunteers were healthy men (average age, 32 years)
screened in Peking Union Medical College Hospital (Beijing, China).
A total of 30 ml of blood was collected by venipuncture following
12 h fasting, which was anticoagulated with heparin vacutainer
tubes (BD Biosciences, San Jose, CA, USA). The procedures of the
present study were approved by the Human Ethics Committee of Peking
Union Medical College Hospital (Beijing, China; registration no.
ChiCTR-RCH-10000748). Each participant provided written informed
consent.
The isolation of PBMCs was performed by gradient
centrifugation at room temperature for 40 min at 400 × g, by
layering 30 ml heparinized (10 U/ml) blood over 15 ml NycoPrep
(Invitrogen Life Technologies, Carlsbad, CA, USA). Following
centrifugation at room temperature for 40 min at 400 × g on a
Ficoll-Paque density gradient, the resting human PBMCs were
collected from the interface and washed with 0.9% (w/v) sodium
chloride (17). The viability and
purity of the monocytes were determined using flow cytometric
analysis (CD14 staining), which confirmed a purity of at least 85%
in all the experiments. The culture conditions were set at 37°C in
a humidified atmosphere with 5% CO2. The cells were
cultured in a polystyrene 12-well plate at a density of
5×106 cells/ml in serum-free RPMI 1640 medium containing
25 mmol/l HEPES (Sigma-Aldrich) and 10 ng/ml human M-CSF for 4-6 h.
Subsequently, the cells were cultured in RPMI 1640 containing 10%
autologous serum for 7 days. The macrophages were serum-starved for
6 h and washed with phosphate-buferred saline (PBS) prior to
incubation with NBD-cholesterol.
Cell culture and treatment
The cells of the THP-1 human monocytic cell line
were cultured in RPMI 1640 medium supplemented with 20%
heat-inactivated fetal bovine serum (FBS) and 100 µg/ml
penicillin-streptomycin in 5% CO2 at 37°C. The THP-1
cells were seeded at a density of 2×106 in a 12-well
plate (Costar; Corning Incorporated, New York, NY, USA) and
differentiated into macrophages by treatment with 100 ng/ml PMA for
72 h (18). To assess
NBD-cholesterol uptake, the differentiated THP-1 cells were
incubated at 37°C with various concentrations of NBD-cholesterol
(0.1, 1, 5 and 10 µmol/l) in basic phenol red-free RPMI 1640
medium. Following incubation, the supernatant was removed and the
cells were washed three times with PBS. Cellular cholesterol was
then extracted using 0.1% Triton X-100. The cells were harvested at
different time-points for the detection of fluorescence intensity
(FI), and images were captured using a fluorescence microscope
(EVOS-fl; Thermo Fisher Scientific, Waltham, MA, USA). Cellular
NBD-cholesterol uptake was also evaluated under microscopy.
FI detection
The FI of NBD-cholesterol in the medium and cell
lysate was measured in a black polystyrene 96-well plate (Costar;
Corning Incorporated) using a microplate spectrophotometer
(EnVision®; PerkinElmer, Inc., Waltham, MA, USA) at a
wavelength of 469 nm for excitation and a wavelength of 537 nm for
emission. The parameter of sensitivity was set to 100 and the final
volume for the assay was 200 µl.
Cholesterol efflux assays
For the [3H]-cholesterol assay, the cells
were washed with cold PBS three times and were then labeled with 1
µCi/ml [3H]-cholesterol (NEN Life Science
Products, Inc., Boston, MA, USA) in 10% FBS RPMI 1640 medium for 12
h. Following incubation at 37°C, the cells were washed with PBS and
equilibrated for an additional 2 h at 37°C in medium containing 2%
FBS, apoA-1 or HDL, used as a lipid acceptor, in which the cells
were incubated for an additional 4 h at 37°C. The same
concentrations of apoA-1 and HDL were used in the NBD-cholesterol
efflux assay. The medium was collected, and the cells were lysed in
0.1% Triton X-100 for at least 1 h at room temperature. The cell
lysate and medium were each mixed with scintillation fluid and
incubated for 24 h at room temperature, protecting the cells from
light prior to scintillation counting (LS 6500 Scintillation
Counter; Beckman Coulter, Brea, CA, USA). Medium radioactivity was
taken for 3H radioactivity determination in the
scintillation counter. The cholesterol efflux rate was expressed as
the percent efflux calculated by disintegrations per minute of
[3H] cholesterol in medium/([3H] cholesterol
in medium + [3H] cholesterol in cell lysates) ×100.
To assess NBD-cholesterol efflux, macrophages were
incubated in phenol red-free RPMI 1640 medium containing 5
µmol/l NBD-cholesterol for 4 h at 37°C. Following
incubation, the cells were washed with PBS three times and were
then incubated with HDL or apoA-1, as lipid acceptors. To determine
the correlation, various concentrations of HDL (5, 20, 50 and 100
µg/ml) and apoA-1 (10, 20, 50 and 100 µg/ml) were
used for the [3H]-cholesterol and NBD-cholesterol efflux
assays. HDL concentrations ranged between 5 and 100 µg/ml,
and apoA-1 ranged between 10 and 100 µg/ml. Subsequently,
the cells were harvested after 4 h, and the medium and cell lysate
were collected for the detection of FI. Triton X-100 (0.1%) was
used to lyse the cells in a 12-well plate, and the cells lysate was
homogenized by pipetting up and down several times. A total volume
of 600 µl was then aliquoted into three wells of a 96-well
plate for the measurement of FI. The percentage of NBD-cholesterol
efflux was calculated by dividing the FI in the medium by the sum
of the FI in the medium and cell lysate. All data were from three
independent experiments, each performed in triplicate.
Statistical analysis
Data were analyzed using Prism software version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). The determination of
correlation was performed using Deming's regression. All data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
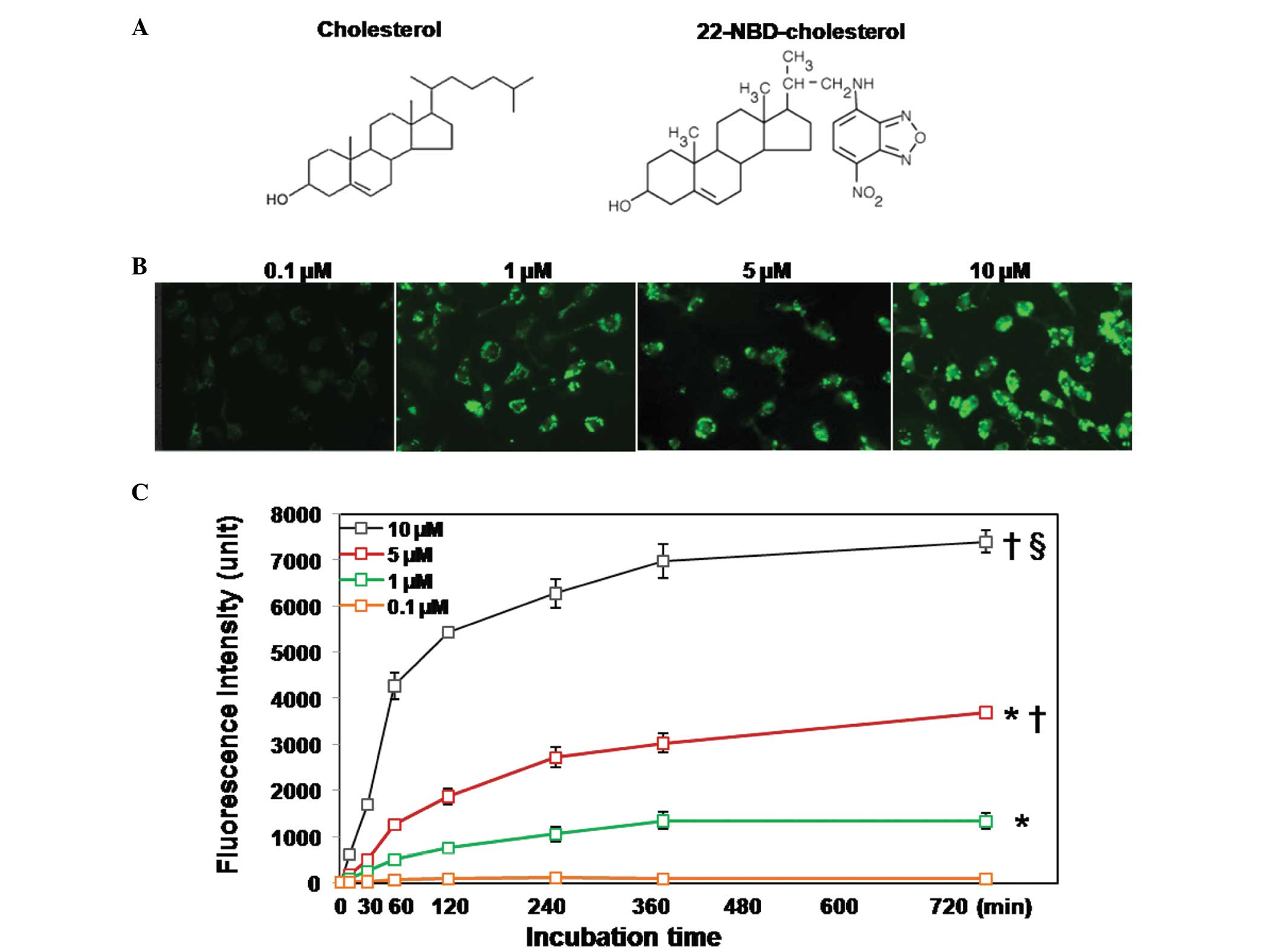
NBD-cholesterol uptake and metabolism in
THP-1-derived macrophages
The THP-1-derived macrophages were incubated with
0.1-10 µM 22-NBD-cholesterol and then harvested at different
time-points for the determination of FI. Using a fluorescent
microscope, real-time images were captured of the monolayer cells
following 4 h incubation with NBD-cholesterol. The results revealed
that NBD-cholesterol uptake from the medium occurred in a
concentration- and time-dependent manner (Fig. 1C). When 0.1 µM of
NBD-cholesterol was used, which represents a concentration reported
in several previous studies (10,19,20),
for up to 12 h of incubation, real-time imaging yielded dim
fluorescent images. This finding was confirmed by quantitative FI
detection, in which the FI of the cell lysate was undetectable
following 12 h incubation. The FI increased with increasing
concentrations of NBD-cholesterol, reaching the highest levels at
an NBD-cholesterol concentration of 10 µM. Similar results
were obtained in the fluorescent imaging (Fig. 1B). NBD-cholesterol uptake in the
THP-1 cells also exhibited a time-dependent effect. The FI was
markedly increased within the first 30 min of incubation and
reached a plateau following incubation for 6 h, which was observed
at all concentrations, with the exception of 0.1 µM.
Real-time imaging revealed that NBD-fluorescence was distributed
equally inside the cell, but not in the nucleus.
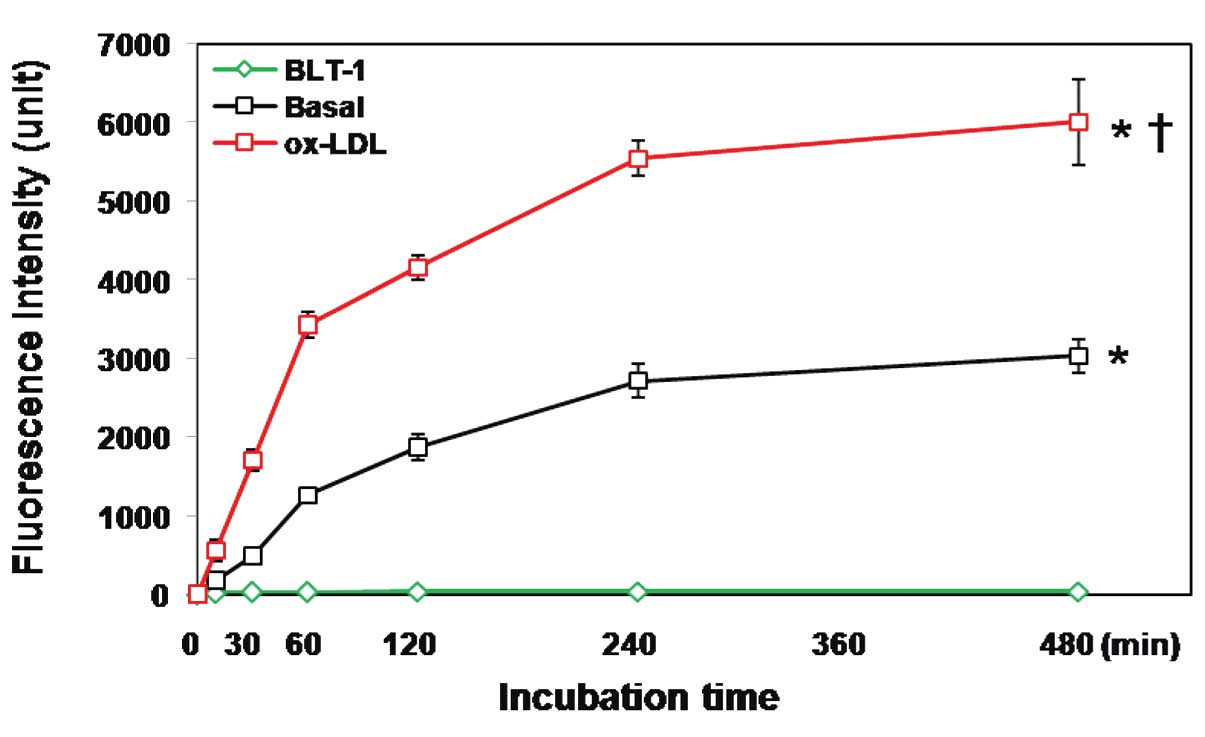
Oxidized LDL (ox-LDL) increases
NBD-cholesterol uptake by macrophages
Exogenous cholesterol is taken up by macrophages
through the LDL receptor-mediated pathway, as well as by the
HDL-mediated pathway (21). The
present study investigated the role of ox-LDL in NBD-cholesterol
uptake. The THP-1-derived macrophages were incubated with 5
µM NBD-cholesterol for up to 8 h in the presence and absence
of 50 µg/ml ox-LDL. The FI of the cell lysate was then
measured at different time-points. The results demonstrated that
ox-LDL significantly increased NBD-cholesterol uptake by the
macrophages (Fig. 2).
Optimization of the NBD-cholesterol
efflux assay in THP-1-derived macrophages
When using 2% FBS to induce NBD-cholesterol efflux,
the present study found that the percentage of NBD-cholesterol
efflux increased in a time-dependent manner and had a marked
increase within the first 30 min, reaching a plateau after 8 h
(Fig. 3A). Subsequently,
NBD-cholesterol efflux was measured using either HDL (50
µg/ml) or apoA-1 (50 µg/ml) as lipid acceptors. The
results revealed that the percentage of NBD-cholesterol efflux was
increased in a similar time-dependent manner. However, when apoA-1
was used as an inducer, the percentage of efflux was markedly lower
than that observed when HDL was used as an inducer (Fig. 3B and C). Based on these results, a
duration of 4 h was set as the efflux assay duration for the
subsequent NBD-cholesterol efflux assays.
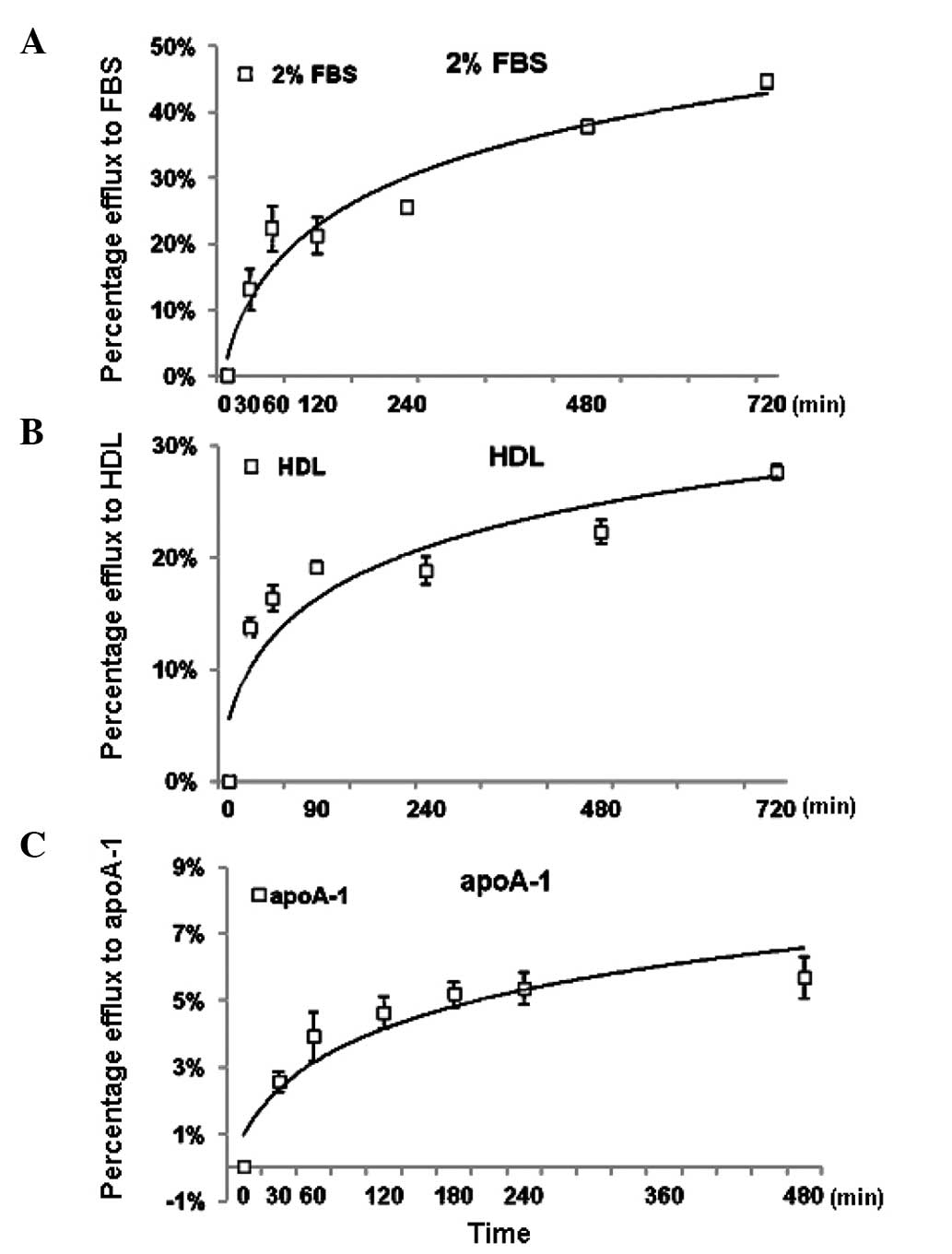 | Figure 3Optimization of the NBD-cholesterol
efflux assay in THP-1 cells. Following labeling with
NBD-cholesterol for 4 h, the THP-1-derived macro-phages were
incubated with (A) 2% FBS, (B) 50 µg/ml HDL and (C) 50
µg/ml apoA-1, as lipid acceptors for the cholesterol efflux
assay. The percentage of NBD-cholesterol efflux was measured at the
indicated times and was calculated, as described in the Materials
and methods. The values are presented as the mean ± standard
deviation of triplicate wells and the data were plotted using
non-linear regression. The results are representative of three
independent experiments. NBD-cholesterol,
N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)
amino)-23,24-bisnor-5-xholen-3β-ol-cholesterol; FBS, fetal bovine
serum; HDL, high-density lipoprotein; apoA-1, apolopoprotein
A-1. |
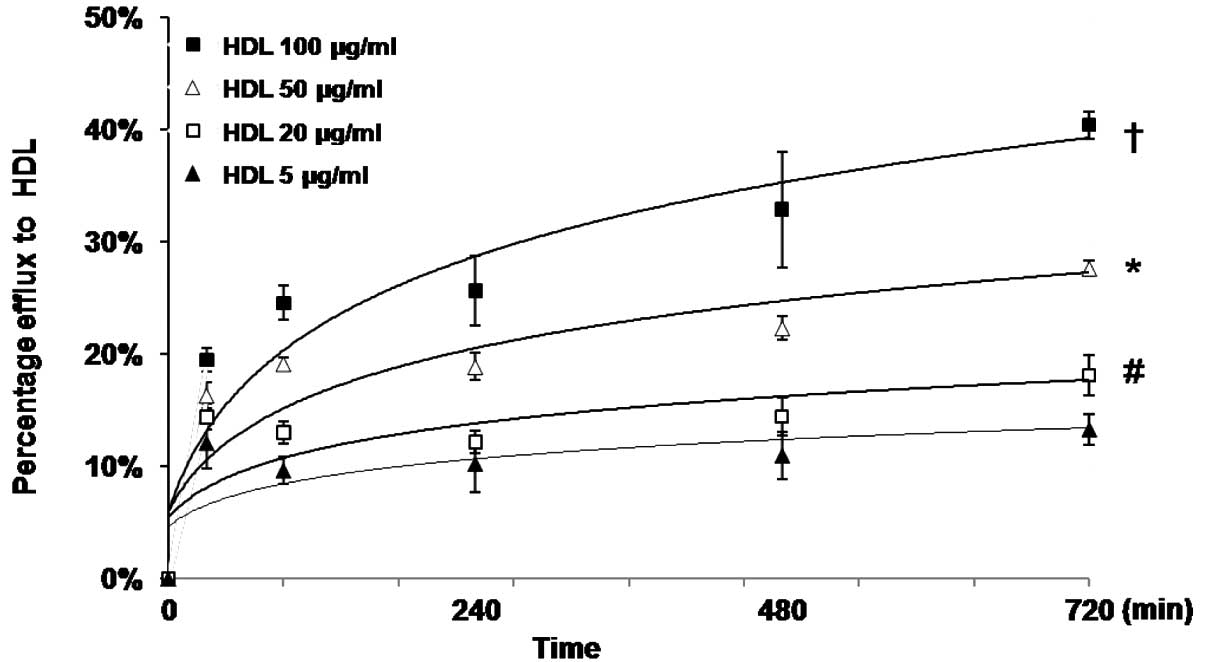
In addition, the present study characterized the
dose-response effect of HDL on NBD-cholesterol efflux in the THP-1
cells, using various concentrations of HDL (5–100 µg/ml) as
a lipid acceptor. The results indicated that the percentage efflux
of NBD-cholesterol increased, also in a time-dependent manner, at
different concentrations of HDL (Fig.
4).
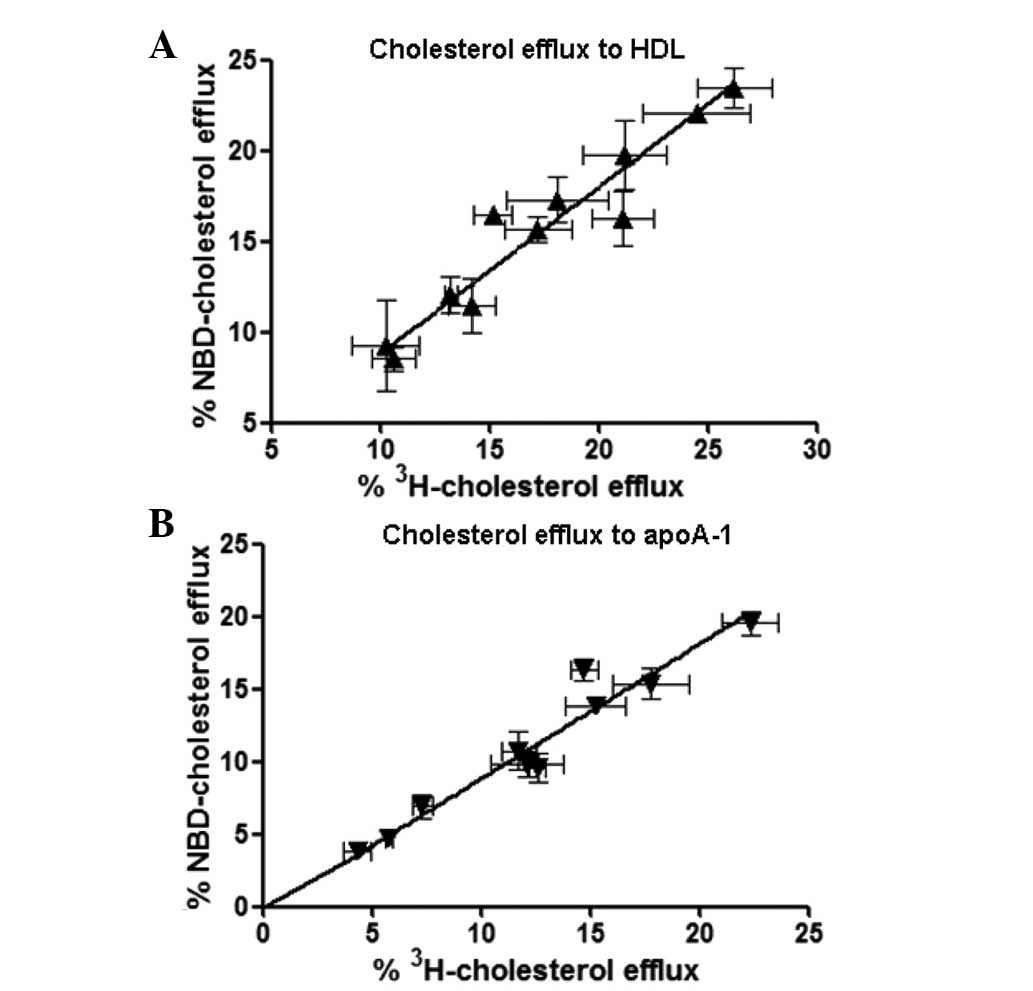
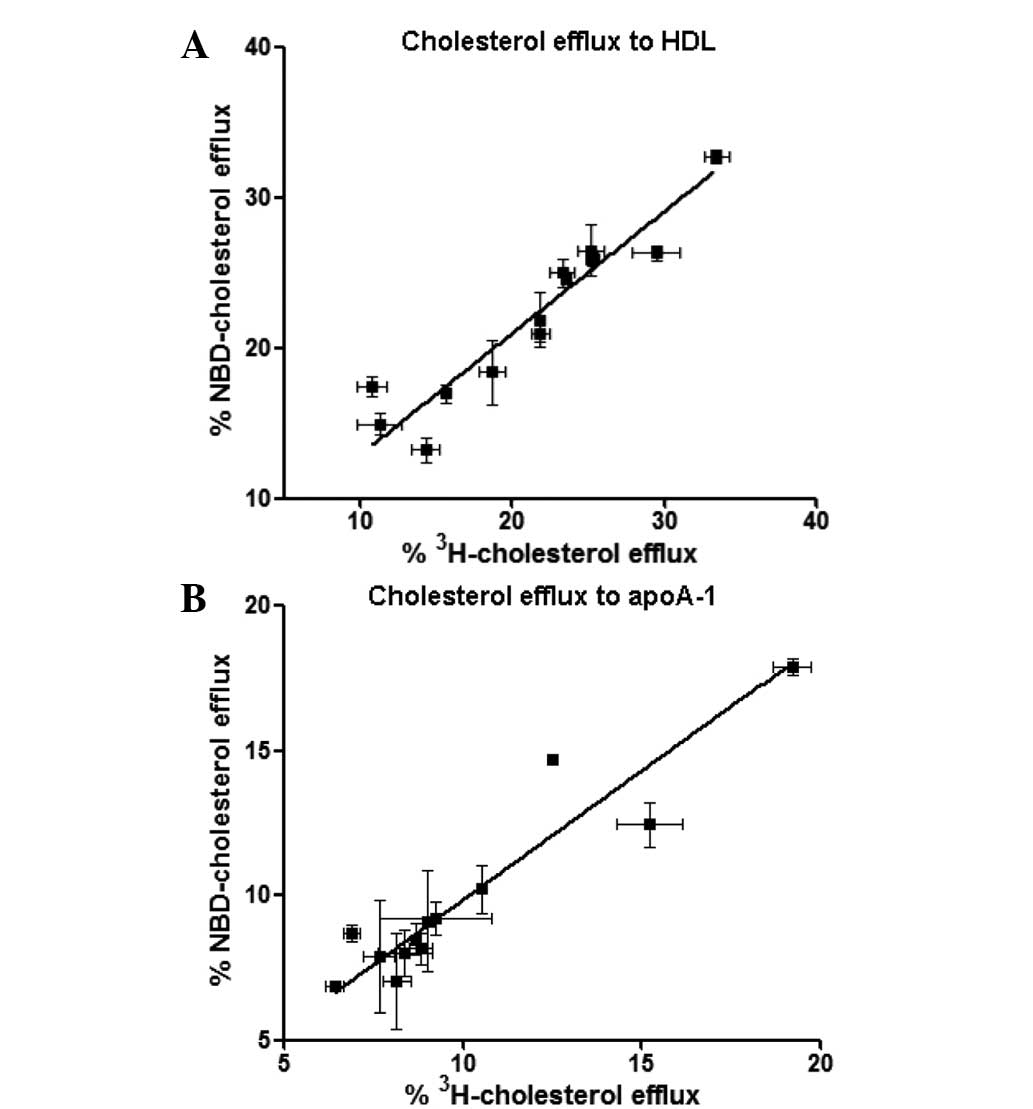
Correlation of NBD-cholesterol and
[3H]-cholesterol efflux in THP-1-derived
macrophages
To determine the correlation between the percentage
of efflux of NBD-cholesterol and [3H]-cholesterol in the
THP-1-derived macrophages, the present study used various
concentrations of HDL and apoA-1 as lipid acceptors for the
[3H]-cholesterol and NBD-cholesterol efflux assays. The
HDL concentrations ranged between 5 and 100 µg/ml, and the
apoA-1 concentrations ranged between 10 and 100 µg/ml
(Fig. 5). Correlation coefficiency
was determined using Deming regression. The results demonstrated a
significant correlation between NBD-cholesterol efflux and
[3H]-cholesterol efflux in the HDL-
(R2=0.876; P<0.001) and apoA-1-mediated cholesterol
efflux (R2=0.837; P<0.001) in the THP-1 cells,
suggesting that NBD-cholesterol may be a suitable substitute for
[3H]-cholesterol in the cholesterol efflux assay
(Fig. 5). These results also
indicate a potential in vitro model for developing a
sensitive, high-throughput screening method for investigating the
regulation of cholesterol efflux in macrophages.
Correlation of NBD-cholesterol and
[3H]-cholesterol efflux in PBMCs
To further assess this hypothesis, the presents
study compared NBD-cholesterol with [3H]-cholesterol in
the efflux assay in human macrophages isolated from 13 healthy
volunteers. HDL (50 µg/ml) and apoA-1 (50 µg/ml) were
used as lipid acceptors for the [3H]-cholesterol and
NBD-cholesterol efflux assays. The results demonstrated a
significant correlation between NBD-cholesterol efflux and
[3H]-cholesterol efflux in HDL- (R2=0.887;
P<0.001) and apoA-1- (R2=0.882; P<0.001) mediated
cholesterol efflux in the PBMCs (Fig.
6), which was consistent with the data obtained from the THP-1
cells.
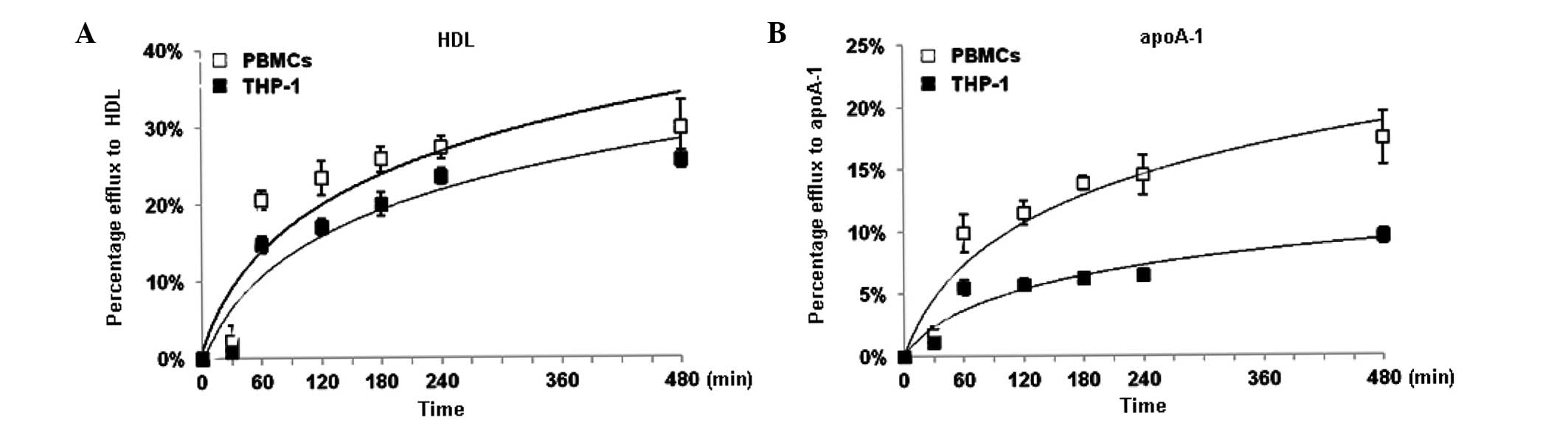
Comparison of NBD-cholesterol efflux in
THP-1-derived macrophages and PBMCs
THP-1 cells resemble human macrophages in certain
biological characteristics. To validate the efficiency of these
cells as macrophages in the NBD-cholesterol efflux assay, the
present study further compared the THP-1 cells with the PBMCs
isolated from volunteers. HDL and apoA-1 were used as lipid
acceptors. The percentages of NBD-cholesterol efflux in the THP-1
cells and PBMCs were markedly increased within the first hour, and
continuously increased between 1 and 4 h (Fig. 7). The percentage of efflux of
NBD-cholesterol reached a peak at 8 h with either HDL or apoA-1 as
an inducer. Although the percentage of NBD-cholesterol efflux in
the THP-1 cells appeared lower than that in the PBMCs,
NBD-cholesterol efflux in the THP-1 cells exhibited a similar trend
to that in the PBMCs, suggesting that THP-1 cells may be a suitable
alternative model in this NBD-cholesterol efflux assay (Fig. 7).
Discussion
Fluorescent cholesterol analogs, which can closely
mimic the properties of natural cholesterol, whilst permitting
detection by microscopic techniques, have been widely used to
investigate the intracellular distribution and membrane
organization of cholesterol. These fluorescent cholesterol analogs
have also demonstrated significant potential as substitutes for
traditional radioisotope-labeled cholesterol for investigating
lipid trafficking, including cholesterol efflux (7,22).
Cholesterol efflux may prevent the development of atherosclerosis
by reducing the accumulation of cholesterol in the artery wall
(2,23) and is a key step in RCT.
Understanding the regulation of cholesterol efflux can be useful
therapeutically and merits in-depth investigation. Previously,
Zhang et al developed fluorescent cholesterol bearing the
Pennsylvania Green fluorophore for use as a molecular probe for the
cholesterol efflux assay. This fluorescent sterol exhibits good
correlation with radioisotope-labeled cholesterol in the efflux
assay in THP-1 cells (24).
Sankaranarayanan et al demonstrated that another fluorescent
sterol, bearing a BODIPY fluorophore, can be used as a sensitive
probe in the cholesterol efflux assay in J774 mouse macrophages
(11). NBD-cholesterol is a
commercially available and widely used fluorescent analog of
cholesterol, which may be used in cholesterol efflux assays
(25-27). Frolov et al used
22-NBD-cholesterol in an investigation of HDL-mediated cholesterol
efflux in mouse L-cells (21).
Storey et al also used this fluorescent sterol in an
investigation of HDL-mediated cholesterol efflux in cultured
primary mouse hepatocytes (10).
Although these studies demonstrated the potential application of
NBD-cholesterol, there remains a lack of data regarding
NBD-cholesterol uptake and efflux in THP-1 cells, which is a more
physiologically relevant human cell line that exhibits
macrophage-like characteristics. In the present study, the
cholesterol efflux assay was characterized and optimized using
fluorescent NBD-cholesterol in THP-1-derived macrophages.
The initial step of a cholesterol efflux assay is to
pre-incubate cells with NBD-cholesterol, which is a prerequisite
for the assay (22). Therefore,
the present study first characterized NBD-cholesterol uptake in the
THP-1-derived macrophages and optimized the experimental
conditions. The cells were incubated with various concentrations of
NBD-cholesterol, and the cellular FI was measured at different
time-points. The results revealed that NBD-cholesterol uptake in
the THP-1 cells was increased over time and reached a plateau after
4 h incubation, suggesting that intracellular NBD-cholesterol
metabolism may reach equilibrium over time. The results also
demonstrated that pre-incubation of the cells with 5 µM
NBD-cholesterol resulted in optimal outcomes in fluorescence
microscopy and scintillation counting. Thus, the optimized
conditions for cellular NBD-cholesterol uptake were established for
the subsequent experiments. Previously, Storey et al
investigated cholesterol uptake in cultured primary mouse
hepatocytes using 0.1 µM NBD-cholesterol (10), which was a lower level than that
used in the present study. In another study, Portioli Silva et
al analyzed cholesterol incorporation in rat macrophages using
2 µM NBD-cholesterol, which was close to the concentration
used in our study (28). Notably,
the present study used ethanol as a specific solvent for
NBD-cholesterol solubilization, which was also used in the study by
Portioli Silva et al, but not in the study by Storey et
al. The use of a solvent is important for the fluorescence
quantum yield and extinction coefficient, and may be the cause of
the difference between the results of the studies (8).
The present study also demonstrated that by treating
the cells with ox-LDL, NBD-cholesterol uptake in the THP-1 cells
was significantly increased. Previous studies have reported that
ox-LDL is a specific ligand for peroxisome proliferator-activated
receptors and, upon binding, may increase its target gene
expression and regulate lipid homeostasis (29,30).
The results of the present study demonstrated that NBD-cholesterol
absorption in macrophages was increased by ox-LDL treatment, which
was similar to native cholesterol.
Subsequently, the present study investigated whether
NBD-cholesterol efflux was well-correlated with
[3H]-labeled cholesterol efflux in the THP-1-derived
macrophages. [3H] radioisotope-labeling is a classical
labeling method for investigating intracellular cholesterol
trafficking and metabolism due to its sensitivity and specificity.
In the present study, [3H]-cholesterol efflux and
fluorescent NBD-cholesterol efflux were measured in the THP-1 cells
using various concentrations of HDL or apoA-1 as lipid acceptors.
The data revealed a significant correlation between NBD-cholesterol
efflux and [3H]-cholesterol efflux in the THP-1 cells.
In addition, NBD-cholesterol efflux and [3H]-cholesterol
effluxwere examined in PBMCs isolated from healthy volunteers. A
significant correlation between NBD-cholesterol efflux and
[3H]-cholesterol efflux was also observed in the
PBMCs.
Cellular cholesterol transportation to extracellular
acceptors either occurs through aqueous diffusion or is mediated by
the ABCA1 or ABCG1 transmembrane proteins, which are essential in
the process (31-33). Previous studies have revealed that
cellular cholesterol is transported to either lipid-depleted apoA-1
through the ABCA1-mediated pathway or HDL through the
ABCG1-mediated pathway (34-36).
Therefore, the present study used apoA-1 and HDL as extracellular
acceptors for evaluating cellular cholesterol efflux. The resulting
data demonstrated that the percentage of NBD-cholesterol efflux was
significantly correlated to that of [3H]-cholesterol in
the different cholesterol efflux pathways in macrophages. Thus,
these findings support NBD-cholesterol as a sensitive and specific
probe, which may be used efficiently for cholesterol efflux
measurement. Several other studies have reported that fluorescent
BODIPY-cholesterol and Pennsylvania Green-cholesterol can also be
used in cholesterol efflux assay as substitutes for
[3H]-cholesterol (11,24).
Further investigation to compare different fluorescent analogs in
parallel with [3H]-cholesterol may assist in determining
which fluorescent analog can mimic native cholesterol efflux most
closely in the assay.
The THP-1 cells used in the present study are a
human monocytic leukemia cell line, which can be differentiated
into macrophage-like cells. Therefore, THP-1 cells were used as an
in vitro model relevant to human macrophages for the
NBD-cholesterol efflux assay. It is important to determine whether
NBD-cholesterol metabolism and efflux in THP-1 cells is similar to
that in PBMCs. In the present study, NBD-cholesterol efflux in
THP-1 cells was compared with that in PBMC. NBD-cholesterol efflux
in the THP-1 cells exhibited a similar trend to that in PBMCs,
suggesting that THP-1 is a suitable model for assessing
NBD-cholesterol efflux in macrophages. By contrast, with regard to
the lower percentage of NBD-cholesterol efflux observed in THP-1
cells than in the PBMCs, it is possible that the expression levels
of lipid transporters, including ABCA1 may be lower in THP-1 cells
than in PBMCs, which may be responsible for a lower efflux
percentage. Further investigation on the comparison between THP-1
cells and PBMCs, in terms of the expression and function of ABCA1
amay answer this important question.
In conclusion, the present study demonstrated a
simpler procedure with a similar efficiency for performing
fluorescent NBD-cholesterol efflux assays, compared with
[3H] radioisotope-labeled cholesterol. The results may
assist in the development of a rapid sensitive high-throughput
screening assay for investigating macrophage cholesterol
efflux.
Abbreviations:
|
22-NBD-cholesterol
|
22-(7-nitrobenz-2-oxa-1,
3-diazol-4-yl-amino)-23, 24-bisnor-5-cholen-3β-ol
|
|
PMA
|
phorbol myristate acetate
|
|
FBS
|
fetal bovine serum
|
|
RCT
|
reverse cholesterol transport
|
|
PBMCs
|
human peripheral blood mononuclear
cells
|
|
BODIPY
|
boron-dipyrromethene
|
|
DHE
|
dehydroergosterol
|
|
apoA-1
|
apolipoprotein A-1
|
|
HDL
|
high-density lipoprotein
|
|
Ox-LDL
|
oxidized low-density lipoprotein
|
|
[3H]-cholesterol
|
tritium-labeled cholesterol
|
|
ABCA1
|
ATP binding cassette transporter
A1
|
|
ABCG1
|
ATP binding cassette transporter
G1
|
|
SR-B1
|
scavenger receptor class B type I
|
Acknowledgments
The present study was supported by a grant from
Pfizer Inc. (New York, NY, USA; grant. no. WS554487).
References
|
1
|
Yusuf S, Reddy S, Ounpuu S and Anand S:
Global burden of cardiovascular diseases: Part I: General
considerations, the epidemiologic transition, risk factors and
impact of urbanization. Circulation. 104:2746–2753. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fielding CJ and Fielding PE: Cellular
cholesterol efflux. Biochim Biophys Acta. 1533:175–189. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tall AR, Costet P and Wang N: Regulation
and mechanisms of macrophage cholesterol efflux. J Clin Invest.
110:899–904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuchel M and Rader DJ: Macrophage reverse
cholesterol transport: Key to the regression of atherosclerosis?
Circulation. 113:2548–2555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khera AV, Cuchel M, de la Llera-Moya M,
Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage
ML, Wilensky RL, et al: Cholesterol efflux capacity, high-density
lipoprotein function and atherosclerosis. N Engl J Med.
364:127–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Cai S, Peterson BR, Kris-Etherton
PM and Heuvel JP: Development of a cell-based, high-throughput
screening assay for cholesterol efflux using a fluorescent mimic of
cholesterol. Assay Drug Dev Technol. 9:136–146. 2011. View Article : Google Scholar :
|
|
8
|
Wustner D: Fluorescent sterols as tools in
membrane biophysics and cell biology. Chem Phys Lipids. 146:1–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramirez DM, Ogilvie WW and Johnston LJ:
NBD-cholesterol probes to track cholesterol distribution in model
membranes. Biochim Biophys Acta. 1798:558–568. 2010. View Article : Google Scholar
|
|
10
|
Storey SM, Atshaves BP, McIntosh AL,
Landrock KK, Martin GG, Huang H, Ross Payne H, Johnson JD,
Macfarlane RD, Kier AB and Schroeder F: Effect of sterol carrier
protein-2 gene ablation on HDL-mediated cholesterol efflux from
cultured primary mouse hepatocytes. Am J Physiol Gastrointest Liver
Physiol. 299:G244–G254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sankaranarayanan S, Kellner-Weibel G, de
la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R and Rothblat
GH: A sensitive assay for ABCA1-mediated cholesterol efflux using
BODIPY-cholesterol. J Lipid Res. 52:2332–2340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuchiya S, Yamabe M, Yamaguchi Y,
Kobayashi Y, Konno T and Tada K: Establishment and characterization
of a human acute monocytic leukemia cell line (THP-1). Int J
Cancer. 26:171–176. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuchiya S, Kobayashi Y, Goto Y, Okumura
H, Nakae S, Konno T and Tada K: Induction of maturation in cultured
human monocytic leukemia cells by a phorbol diester. Cancer Res.
42:1530–1536. 1982.PubMed/NCBI
|
|
14
|
Liu XH, Xiao J, Mo ZC, Yin K, Jiang J, Cui
LB, Tan CZ, Tang YL, Liao DF and Tang CK: Contribution of D4-F to
ABCA1 expression and cholesterol efflux in THP-1 macrophage-derived
foam cells. J Cardiovasc Pharmacol. 56:309–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Larrede S, Quinn CM, Jessup W, Frisdal E,
Olivier M, Hsieh V, Kim MJ, Van Eck M, Couvert P, Carrie A, et al:
Stimulation of cholesterol efflux by LXR agonists in
cholesterol-loaded human macrophages is ABCA1-dependent but
ABCG1-independent. Arterioscler Thromb Vasc Biol. 29:1930–1936.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel S, Drew BG, Nakhla S, Duffy SJ,
Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D,
et al: Reconstituted high-density lipoprotein increases plasma
high-density lipoprotein anti-inflammatory properties and
cholesterol efflux capacity in patients with type 2 diabetes. J Am
Coll Cardiol. 53:962–971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Almeida MC, Silva AC, Barral A and
Barral Netto M: A simple method for human peripheral blood monocyte
isolation. Mem Inst Oswaldo Cruz. 95:221–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Wang W, Zhang L, Qi LP, Li LY, Chen
LF, Fang Q, Dang AM and Yan XW: A polymorphism in the ABCG1
promoter is functionally associated with coronary artery disease in
a Chinese Han population. Atherosclerosis. 219:648–654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan J, Rone MB and Papadopoulos V:
Translocator protein 2 is involved in cholesterol redistribution
during erythropoiesis. J Biol Chem. 284:30484–30497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki H and White SH: A novel fluorescent
probe that senses the physical state of lipid bilayers. Biophys J.
96:4631–4641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frolov A, Petrescu A, Atshaves BP, So PT,
Gratton E, Serrero G and Schroeder F: High density
lipoprotein-mediated cholesterol uptake and targeting to lipid
droplets in intact L-cell fibroblasts. A single- and multiphoton
fluorescence approach. J Biol Chem. 275:12769–12780. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sengupta B, Narasimhulu CA and
Parthasarathy S: Novel technique for generating macrophage foam
cells for in vitro reverse cholesterol transport studies. J Lipid
Res. 54:3358–3372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohashi R, Mu H, Wang X, Yao Q and Chen C:
Reverse cholesterol transport and cholesterol efflux in
atherosclerosis. QJM. 98:845–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Cai S, Peterson BR, Kris-Etherton
PM and Heuvel JP: Development of a cell-based, high-throughput
screening assay for cholesterol efflux using a fluorescent mimic of
cholesterol. Assay Drug Dev Technol. 9:136–146. 2011. View Article : Google Scholar :
|
|
25
|
Ohashi M, Murata M and Ohnishi S: A novel
fluorescence method to monitor the lysosomal disintegration of low
density lipoprotein. Eur J Cell Biol. 59:116–126. 1992.PubMed/NCBI
|
|
26
|
Adams MR, Konaniah E, Cash JG and Hui DY:
Use of NBD-cholesterol to identify a minor but NPC1L1-independent
cholesterol absorption pathway in mouse intestine. Am J Physiol
Gastrointest Liver Physiol. 300:G164–G169. 2011. View Article : Google Scholar :
|
|
27
|
Takahashi M, Murate M, Fukuda M, Sato SB,
Ohta A and Kobayashi T: Cholesterol controls lipid endocytosis
through Rab11. Mol Biol Cell. 18:2667–2677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Portioli Silva EP, Peres CM, Roberto
Mendonca J and Curi R: NBD-cholesterol incorporation by rat
macrophages and lymphocytes: A process dependent on the activation
state of the cells. Cell Biochem Funct. 22:23–28. 2004. View Article : Google Scholar
|
|
29
|
Nagy L, Tontonoz P, Alvarez JG, Chen H and
Evans RM: Oxidized LDL regulates macrophage gene expression through
ligand activation of PPARgamma. Cell. 93:229–240. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boullier A, Bird DA, Chang MK, Dennis EA,
Friedman P, Gillotre-Taylor K, Hörkkö S, Palinski W, Quehenberger
O, Shaw P, et al: Scavenger receptors, oxidized LDL and
atherosclerosis. Ann N Y Acad Sci. 947:214–222; discussion 222–223.
2001. View Article : Google Scholar
|
|
31
|
Yvan-Charvet L, Ranalletta M, Wang N, Han
S, Terasaka N, Li R, Welch C and Tall AR: Combined deficiency of
ABCA1 and ABCG1 promotes foam cell accumulation and accelerates
atherosclerosis in mice. J Clin Invest. 117:3900–3908.
2007.PubMed/NCBI
|
|
32
|
Adorni MP, Zimetti F, Billheimer JT, Wang
N, Rader DJ, Phillips MC and Rothblat GH: The roles of different
pathways in the release of cholesterol from macrophages. J Lipid
Res. 48:2453–2462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Collins HL, Ranalletta M, Fuki IV,
Billheimer JT, Rothblat GH, Tall AR and Rader DJ: Macrophage ABCA1
and ABCG1, but not SR-BI, promote macrophage reverse cholesterol
transport in vivo. J Clin Invest. 117:2216–2224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang N, Lan D, Chen W, Matsuura F and Tall
AR: ATP-binding cassette transporters G1 and G4 mediate cellular
cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci
USA. 101:9774–9779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tall AR, Yvan-Charvet L, Terasaka N,
Pagler T and Wang N: HDL, ABC transporters and cholesterol efflux:
Implications for the treatment of atherosclerosis. Cell Metab.
7:365–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yvan-Charvet L, Wang N and Tall AR: Role
Of HDL, ABCA1 and ABCG1 transporters in cholesterol efflux and
immune responses. Arterioscler Thromb Vasc Biol. 30:139–143. 2010.
View Article : Google Scholar :
|