Introduction
Age-associated hearing loss, also known as
presbycusis, is characterised by an age-dependent decline of
auditory function associated with loss of sensory hair cells,
spiral ganglion neurons and stria vascularis cells in the cochleae
of the inner ear (1,2). However, the exact pathogenesis of
age-related hearing loss remains to be elucidated.
As the cochleae tissue is not acquirable from humans
during life, and the genetic and environmental background of
individuals with hearing loss is heterogenous, the investigation of
presbycusis is relatively limited. Natural aging can be
experimentally modelled by the chronic administration of
D-galactose (D-gal). Animals treated in this way exhibit a
reduction in the activity of antioxidant enzymes (3–5),
dysfunctional mitochondria (6–8),
increased apoptosis (9,10) and neurotoxicity (7,11,12).
Consequently, these animals exhibit a shortened lifespan (13), poor learning and memory (14–16)
and an attenuated immune response (17–19).
These characteristics are considered to be associated with an
increase in oxidative stress caused by a metabolic disturbance.
Previous studies have established a mimetic aging model in the
cochleae of rats following 8 weeks of D-gal treatment, and
demonstrated that the activity levels of antioxidant enzymes
decreased and those of lipid peroxidation increased in this model
(20–22). Furthermore, the levels of
mitochondrial DNA (mtDNA) common deletion (CD) were significantly
increased in the cochleae of the D-gal-treated rats (20–24).
However, the sources of reactive oxygen species (ROS) and the
effects of mtDNA CD in the cochleae of rats from this model remain
to be fully elucidated.
In addition to mitochondria, the NADPH oxidase (NOX)
system is one of the predominant ROS-generating sites, and it is
now clear that NOX is not restricted to the immune system, and that
alternative isoforms may be active in several other cell types as
essential components of cellular signalling, gene expression
regulation and cell differentiation (25). These enzymes share the capacity to
transport electrons across the plasma membrane and to generate
superoxide and other downstream ROS (25). A previous study reported that the
expression levels of NOX3 are higher in the cochleae than in any
other tissue (26). NOX3 forms a
functional complex with P22phox to produce superoxide
(27). Previous studies have
demonstrated that NOX3 is a relevant source of ROS generation in
the cochleae, and that NOX3-dependent ROS generation may contribute
to hearing loss in response to ototoxic drugs (26,28–30).
Apoptosis may be important in the age-related
decline of physiological function in several organs (31), including aging in the cochleae
(32,33). A previous investigation
demonstrated that D-gal-induced apoptotic cells are significantly
increased in the cochlear section of newborn rats (34). Previous studies have also reported
that apoptotic cells immediately increase in the central auditory
system of adult rats following 8 weeks of treatment with D-gal
(35,36). Du et al (37) reported that apoptotic cells
increase in the peripheral auditory system of D-gal-treated aging
rats following 12 months of treatment. However, whether 8 weeks of
treatment with D-gal immediately causes apoptosis in the cochleae
of adult rats has not been investigated. In the present study, the
accumulation of mtDNA CD, mitochondrial ultrastructural changes and
changes in the expression levels of 8-OHdG, NOX3,
P22phox and cleaved caspase 3 (C-cas3) were
investigated, as well as the occurrence of apoptosis in the
cochleae of rats exposed to D-gal for 8 weeks. Furthermore, the
present study also investigated the possible mechanism underlying
presbycusis using D-gal-induced aging rats.
Materials and methods
Animals and treatments
A total of 60 1 month old male Sprague-Dawley rats
were obtained from the Experimental Animal Centre of the Guangxi
Medical University (Guangxi, China). The rats were individually
housed in a temperature-controlled (20–22°C) room with a 12 h
light/dark cycle, and were provided with free access to food and
drinking water. The body weights of the experimental animals were
monitored during the experiment as a general measure of health. The
injection of D-gal (Sigma-Aldrich, St. Louis, MO, USA) to induce
aging was administered, according to an established method
(37). Following acclimation for 2
weeks, the rats were randomly divided into three groups: (1) D-gal(H) group, injected subcutaneously
with 500 mg/kg D-gal once a day for 8 weeks; (2) D-gal(L) group, injected subcutaneously
with 150 mg/kg D-gal once a day for 8 weeks; (3) control group, which were administered
with an equal volume of vehicle (0.9% saline) for 8 weeks.
Following the experimentation period, the rats were anaesthetised
with intraperitoneally injected ketamine (30 mg/kg; Maijin
Biotechnology, Hubei, China) and intramuscular injected
chloropromazine (15 mg/kg; Maijin Biotechnology), and blood samples
(6 ml/rat) were obtained from the heart. Serum was obtained by
centrifugation at 800 × g for 15 min at 4°C, and stored at −80°C
until the assessments of H2O2, total
superoxide dismutase (T-SOD) activity and malondialdehyde (MDA)
levels were performed. The cochleae were dissected and used for the
extraction of total RNA, genomic DNA and protein. Alternatively,
the cochleae were perfused with 2.5% glutaraldehyde (Maijin
Biotechnology) for morphological investigation using transmission
electron microscopy (TEM), or with 4% paraformaldehyde (Maijin
Biotechnology) for immunohistochemical analysis and terminal
deoxynucleotidyl transferase-mediated deoxyuridine triphosphate
nick-end-labelling (TUNEL) staining. All experiments were conducted
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The protocol was approved by the Committee on the Ethics of
Animal Experiments of Guangxi Medical University.
Serum H2O2, T-SOD
activity and MDA assays
Using the serum from 30 rats (n=10 per group), the
levels of H2O2, T-SOD activity and MDA were
quantified using H2O2 Assay, T-SOD Assay and
MDA Assay kits, respectively (Nanjing Jiancheng Chemical Industrial
Co., Ltd, Nanjing, China), according to the manufacturer's
instructions.
DNA isolation and determination of mtDNA
CD
Following the final injection, 18 rats (n=6 per
group) were euthanised under deep anaesthesia with chlorpromazine
(15 mg/kg; Maijin Biotechnology) and ketamine hydrochloride (30
mg/kg; Maijin Biotechnology), and the cochlea from both sides of
each rat were rapidly removed. The soft tissue samples were then
harvested from the cochleae using an anatomical microscope (Nikon
Corporation, Tokyo, Japan). Samples were stored at −80°C until
experimentation. The cochlea from one side was used for mtDNA
analysis and that from the other side was used for RNA extraction.
Total DNA was extracted using a Genomic DNA Purification kit
(Tiangen Biotech Co., Ltd, Beijing, China), according to the
manufacturer's instructions. The DNA concentration of each specimen
was measured using a GeneQuant pro DNA/RNA Calculator (BioChrom,
Cambridge, UK). The quantity of the mtDNA CD was determined using a
TaqMan polymerase chain reaction (PCR) assay kit (Takara
Biotechnology Co., Ltd., Dalian, China). Due to the fact that the
D-Loop region is rarely deleted, it can represent the conserved
segment. Primers and probes for the mtDNA D-loop and the mtDNA CD
have previously been described (38), and were as follows: Forward,
5′-GGTTCTTACTTCAGGGCCATCA-3′; reverse,
5′-GATTAGACCCGTTACCATCGAGAT-3′ for the mtDNA D-loop primers and
5′-FAM-TTGGTTCATCGTCCATACG TTCCCCTTA-TAMRA-3′ for the probe; and
forward, 5′-AAGGACGAACCTGAGCCCTAATA-3′; reverse,
5′-CGAAGTAGATGATCCGTATGCTGTA-3′ for the mtDNA CD primers and
5′-FAM-TCACTTTAATCGCCAC ATCCATAACTGCTGT-TAMRA-3′ for the probe. The
PCR amplification was performed on a StepOnePlus™ Real-Time PCR
system (Applied Biosystems Life Technologies, Foster City, CA, USA)
in a 20 µl reaction volume consisting of 10 µl 2X
TaqMan PCR mix (Takara Biotechnology Co., Ltd.), 0.4 µl 50X
ROX reference dye, 0.4 µl of each forward and reverse primer
(10 µM), 0.2 µl of each probe (10 µM), 4
µl of the sample DNA (10 ng/µl) and 4.6 µl
distilled water. The cycling conditions comprised an initial phase
at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and at
60°C for 30 sec. The cycle number at which a significant increase
in the normalised fluorescence was first detected was designated as
the threshold cycle (Ct). The ratio of mtDNA CD to mtDNA was
calculated using the following equation: ΔCt = CtmtDNA
deletion − CtmtDNA D-loop. The relative expression
(RE) was calculated to indicate the factorial difference in the
deletions between the experimental groups and the control group.
The RE was calculated using the 2−ΔΔCt method, where
ΔΔCt = ΔCtmtDNA deletion in experimental group −
ΔCtmtDNAdeletion in control group.
TEM
The ultrastructure of the mitochondria in the spiral
ganglion cell (SGC) of the cochleae was observed using TEM. A total
of 12 rats (n=4 per group) were sacrificed, and both cochleae from
each rat were removed, treated with 2.5% glutaraldehyde and fixed
overnight at 4°C. The following day, the cochleae were washed with
0.1 M phosphate-buffered saline (PBS) and placed in 10%
ethylenediaminetetraacetic acid solution (EDTA; Maijin
Biotechnology) for decalcification for 3 days. The spiral ganglion
(SG) was carefully dissected and harvested from the cochleae using
an anatomical microscope. Following post-fixation in 1% osmium
tetroxide (Maijin Biotechnology) for 2 h at room temperature, the
SG was dehydrated using graded ethanol or acetone (50, 70, 80, 90
and 100%), immersed in an acetone/Epon 812 mixture (1:1) for 2 h,
followed by immersion in Epon 812 for 2 h and final embedding in
Epon 812 for 10 h at 80°C. Serial ultrathin sections (50 nm) were
collected on copper grids and stained with uranyl acetate and lead
citrate. The ultrastructure of the stained sections were examined
using a FEI TecnaiG212 TEM (Philips, Amsterdam,
Netherlands).
RNA preparation and reverse
transcription-quantitative (RT-q) PCR
The mRNA expression levels of NOX3 and
P22phox were determined using RT-qPCR. Total RNA was
extracted using TRIzol® reagent (Takara Biotechnology
Co., Ltd.), according to the manufacturer's instructions. cDNA was
reverse transcribed using a PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd.). The RNA and cDNA of each sample were
analysed using a GeneQuant pro DNA/RNA calculator to assess the
concentrations and purity. The cDNA samples (n=6/group) were stored
at −20°C until further use. RT-qPCR was performed using real-time
SYBR Green PCR technology with a StepOnePlus™ Real-Time PCR system
(Applied Biosystems Life Technologies). The primer pairs for NOX3,
P22phox and the internal standard (β-actin) were as
follows: NOX3, forward 5′-TCGACGAATGGCAGGAAGC-3′ and reverse
5′-ATGGATGGGCACTGGATAAAG-3′; P22phox, forward
5′-ACCGTCTGCTTGGCCATTG-3′ and reverse 5′-TCAATGGGAGTCCACTGCTCAC-3′;
and β-actin, forward 5′-CCTGGAGAAGAGCTATGAGC-3′; and reverse
5′-ACAGGATTCCATACCCAGG-3′. The amplification thermocycling
conditions were as follows: 30 sec at 95°C, 40 cycles of 5 sec at
95°C, 30 sec at 60°C and 30 sec at 72°C. An internal standard was
used to normalise the relative gene expression levels. Subsequent
melting curve analysis was performed for each gene, and the
specificity and integrity of the PCR products were confirmed by the
presence of a single peak. The relative expression levels were
calculated from the variations in Ct values between the target mRNA
and the internal standard (β-actin). Changes in the relative mRNA
expression levels between the experimental and control groups were
analysed using the 2−ΔΔCt method, as previously reported
(39).
Immunohistochemical analysis
The expression of 8-hydroxy-2-deoxyguanosine
(8-OHdG) expression was analysed using immunohistochemistry. A
total of 12 rats (n=4 per group) were sacrificed, and the cochleae
from each rat removed and fixed with 4% buffered-paraformaldehyde
overnight, followed by decalcification with 10% EDTA in PBS for 2
weeks, dehydration and embedding in paraffin wax. The cochlea from
one side was used for immunohistochemical analysis, and the cochlea
from the other side was used for the TUNEL assay. A 5 µm
section was deparaffinised in xylene and rehydrated through graded
concentrations of ethanol. The samples were incubated with mouse
monoclonal anti-8-OHdG antibody (1:4,000; Abcam, Cambridge, MA,
USA) overnight at 4°C. The samples were then incubated with
CY3-labelled goat anti-mouse secondary antibody (1:200; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) for 30 min at
room temperature. The nuclei were counterstained with DAPI staining
solution (Beyotime Institute of Biotechnology, Haimen, China) for 5
min at room temperature. For immunofluorescence imaging, the slides
were visualised using a laser scanning confocal microscope (Nikon
Corporation, Tokyo, Japan) and analysed using Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Western blot analysis
The protein expression levels of NOX3,
P22phox and C-cas3 were determined using western blot
analysis. A total of 18 rats (n=6 per group) were sacrificed, and
soft tissue samples (~500 µg) of the cochleae from each rat
were dissected. Total protein was extracted using
Radioimmunoprecipitation Assay Lysis buffer (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions.
Protein concentrations were determined using an Enhanced
Bicinchoninic Acid Protein assay kit (Beyotime Institute of
Biotechnology). A total of 30 µg of each protein lysate was
separated by 12% SDS-PAGE (Maijin Biotechnology) and transferred
onto polyvinylidene difluoride membranes (Maijin Biotechnology).
The membranes were incubated for 1 h in a blocking solution,
Tris-buffered saline (TBS; Maijin Biotechnology) containing 5%
skimmed milk, and then washed briefly in TBS. The membranes were
subsequently incubated overnight at 4°C with the appropriate
dilution of rabbit polyclonal anti-NOX3 (1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit polyclonal
anti-p22phox (1:100; Wuhan Boster Biological Technology,
Ltd.) and rabbit monoclonal anti-C-cas3 (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibodies. Following membrane
washing with TBS, to remove excess primary antibody, the membranes
were incubated for 1 h at room temperature with the appropriate
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:5,000; Santa Cruz Biotechnology, Inc.). The membranes
were visualised using BeyoECL Plus (Beyotime Institute of
Biotechnology). Quantification of the detected bands was performed
using Image-Pro Plus 6.0 software. β-actin served as an internal
control.
TUNEL assay
Apoptotic cells were detected in situ using a
TUNEL POD assay kit (Roche Diagnostics GmbH, Mannheim, Germany).
Briefly, the tissue sections were deparaffinized through a
concentration gradient of xylene and rehydrated with distilled
water. Following treatment with proteinase K (20 µg/ml;
Beyotime Institute of Biotechnology) in 10 mM Tris-HCl (pH 7.6) for
10 min at 37°C, the sections were washed in PBS, and the labelling
reaction was performed using labelling solution containing terminal
deoxynucleotidyl transferase, its buffer, and fluorescein dUTP at
37°C for 60 min in a humidity chamber. The nuclei were
counterstained using DAPI staining solution for 5 min at room
temperature. Following washing with PBS, the sections were examined
using a laser scanning confocal microscope (C1si; Nikon
Corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical significance was determined using a one-way
analysis of variance, and a least significant difference
post-hoc test was used to evaluate the statistical
differences between groups. Analyses were performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Oxidative stress is induced by D-gal
The serum levels of H2O2,
T-SOD and MDA from the rats are summarised in Table 1. Following 8 weeks D-gal exposure,
the serum levels of H2O2 and MDA were
significantly higher, and the serum activity levels of T-SOD were
markedly lower, compared with the control group.
 | Table ISerum levels of
H2O2, T-SOD activity and MDA following
treatment with D-gal. |
Table I
Serum levels of
H2O2, T-SOD activity and MDA following
treatment with D-gal.
| Compound | Control | D-gal (L) | D-gal (H) |
|---|
|
H2O2
(µmol/ml) | 14.65±1.78 | 19.37±1.82a | 27.88±3.31a |
| T-SOD (U/ml) | 146.99±7.10 | 118.11±3.95a | 97.59±3.81a |
| MDA (nmol/ml) | 2.44±0.56 | 5.04±0.93a | 7.65±1.09a |
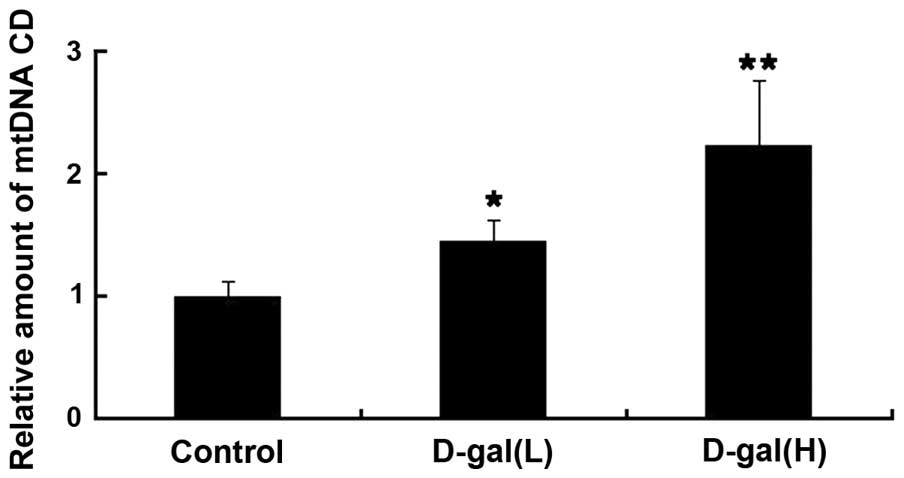
Age-associated accumulation of mtDNA CD
is induced by D-gal
To evaluate the level of mtDNA damage induced by
D-gal in the cochleae, the levels of mtDNA CD were determined using
RT-qPCR with a TaqMan probe. The dual-labelled fluorescent DNA
probe used was specific for the novel fusion sequence, which was
present only in mutant mtDNA, which contained the CD. As shown in
Fig. 1, the levels of mtDNA CD
were significantly higher in the D-gal group, compared with the
control group. Compared with the control group, the accumulation of
mtDNA CD in the D-gal(L) group and in the D-gal(H) group were
increased by 1.45- and 2.23-fold, respectively.
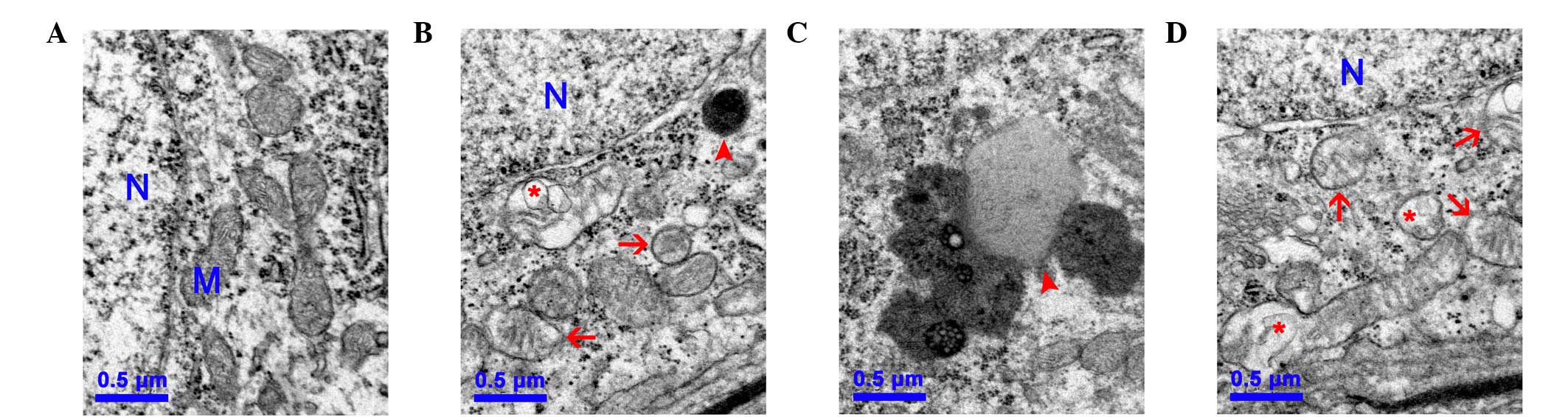
Mitochondrial ultrastructural damage is
induced by D-gal
To further investigate the mitochondrial damage
induced by D-gal in the cochleae, changes to the mitochondrial
ultrastructure in the SGC of the cochleae were observed using TEM.
In the control group, numerous round and oval mitochondria with
lamellar cristae were present, predominantly around the nucleus of
the SGC (Fig. 2A). By contrast,
the mitochondria in the SGC of the D-gal groups were swollen with
reduced electron density in the matrix or exhibiting severe
degeneration. Furthermore, the lipofuscins were also deposited in
the SGC of the D-gal groups, indicating structural decay (Figs. 2B–C).
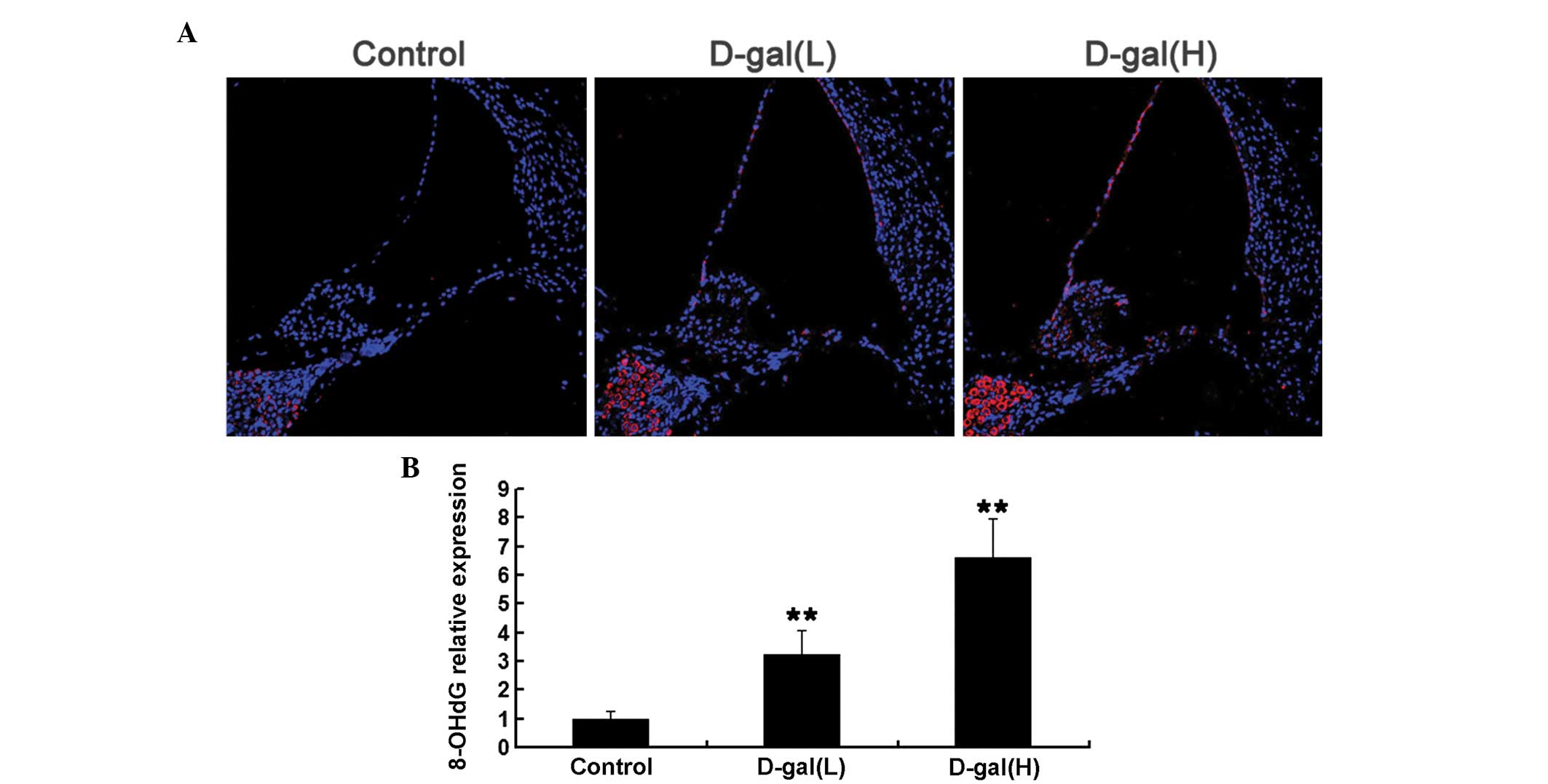
Oxidative mtDNA damage is induced by
D-gal
To determine whether increased mtDNA CD was
associated with increased oxidative stress induced by D-gal in the
cochleae, the expression levels of 8-OHdG, a biomarker of DNA
oxida-tive damage, were analysed using immunohistochemical analysis
(Fig. 3). As shown in Fig. 3A, the expression levels of 8-OHdG
were markedly increased in the cytoplasm of the cochleae cells from
the D-gal-induced aging rats, compared with those of the control
rats, which suggested that D-gal increased oxidative mtDNA damage
in the cochleae. Compared with the control group, the
immunohistochemical analysis indicated that the expression levels
of 8-OHdG in the D-gal(L) and the D-gal(H) groups increased by
3.24- and 6.59-fold, respectively (Fig. 3B).
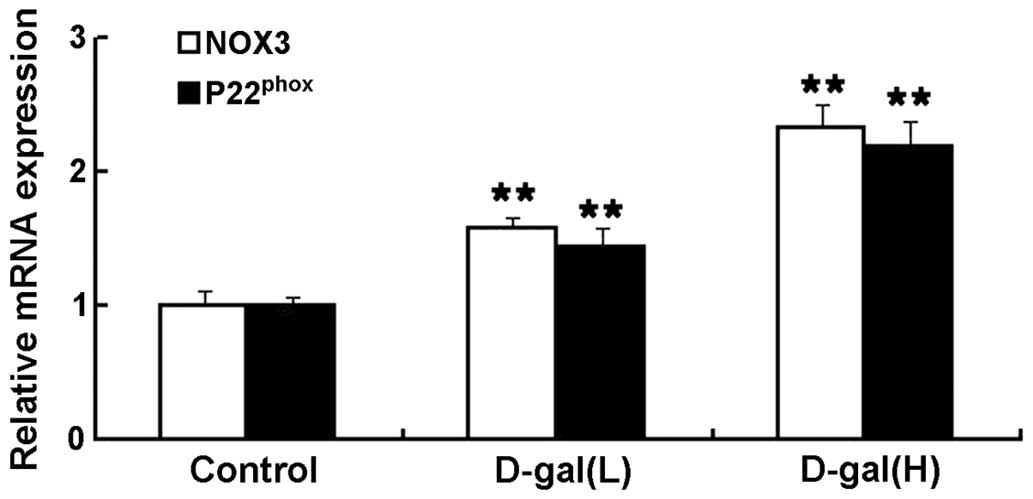
Increased mRNA expression levels of NOX3
and P22phox are induced by D-gal
To investigate the effects of NOX3-associated
oxidative stress on the mtDNA damage induced by D-gal in the
cochleae, the mRNA expression levels of NOX3 and P22phox
were determined using an RT-qPCR assay. As shown in Fig. 4, the mRNA expression levels of NOX3
and P22phox were significantly higher in the D-gal
groups, compared with the control group. Compared with the control
group, the mRNA expression levels of NOX3 in the D-gal(L) and
D-gal(H) group increased by 1.57- and 2.33-fold, respectively. The
mRNA expression levels of P22phox in the D-gal(L) and
D-gal(H) group increased by 1.43- and 2.19-fold, respectively.
Increased protein expression levels of
NOX3, P22phox and C-cas3 are induced by D-gal
To examine the protein expression levels of NOX3,
P22phox and C-cas3 in the cochleae, western blot
analysis was performed. As shown in Fig. 5A, the protein expression levels of
NOX3, P22phox and C-cas3 were markedly increased
following treatment with D-gal. Compared with the control group,
the protein expression levels of NOX3, P22phox and
C-cas3 in the D-gal(L) group increased by 1.92-, 2.25-and
4.18-fold, respectively. The protein expression levels of NOX3,
P22phox and C-cas3 in the D-gal(H) group increased by
3.63-, 3.87- and 6.59-fold, respectively (Fig. 5B).
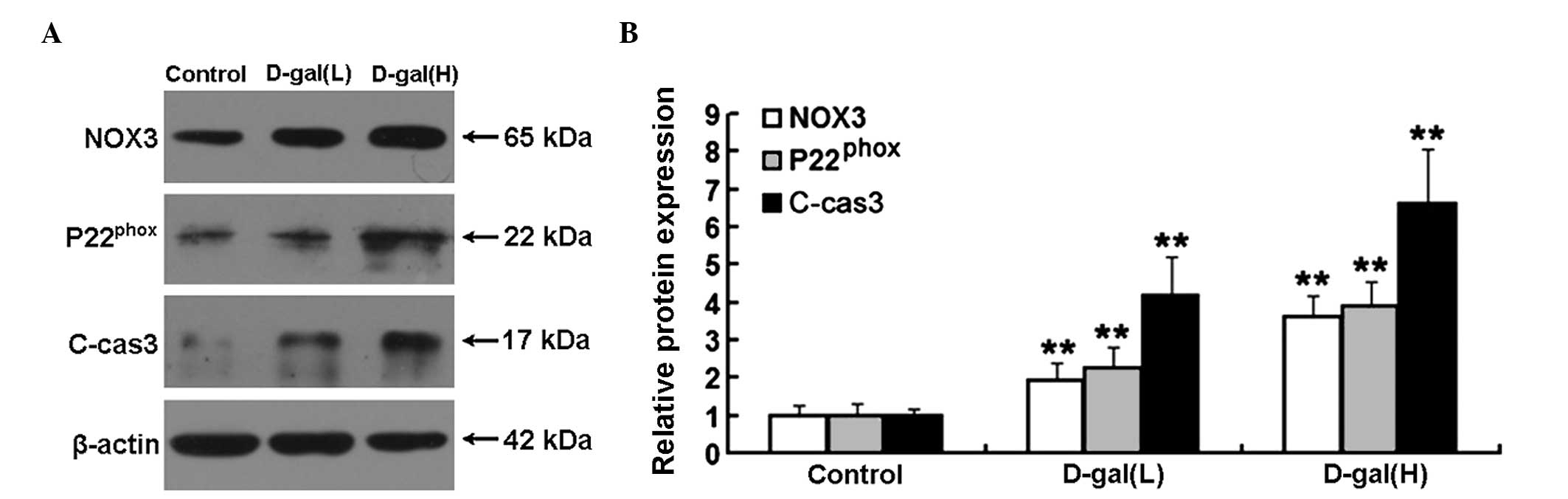 | Figure 5Western blot analysis and
densitometric analysis of the expression levels of NOX3,
P22phox and C-cas3 in the cochleae. (A) Expression
levels of NOX3, P22phox and C-cas3 in the different
treatment groups, determined using western blot analysis. (B)
Relative protein expression levels of NOX3, P22phox and
C-cas3 were significantly increased in the D-gal groups, compared
with the control group. The data are expressed as the mean ±
standard deviation of six rats per group. **P<0.01,
vs. control group. C-cas3, cleaved caspase 3; D-gal, D-galactose;
H, 500 mg/kg; L, 150 mg/kg; NOX3, NADPH oxidase 3. |
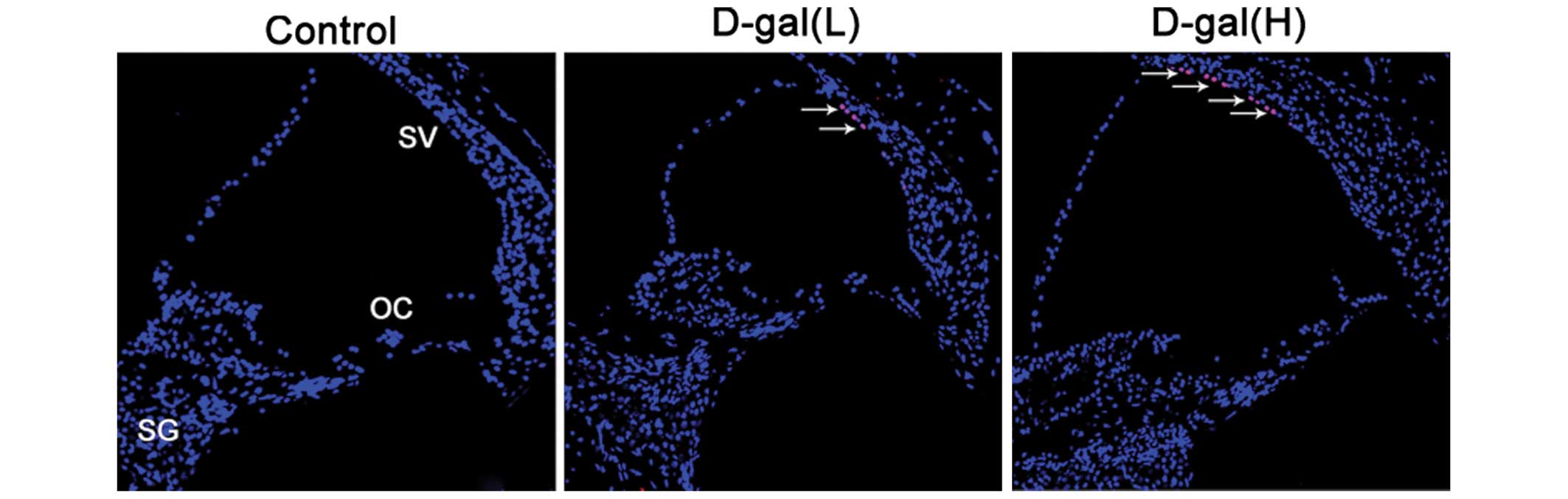
Cell apoptosis is induced by D-gal
To further understand the occurrence of apoptosis
induced by D-gal in the cochleae, the numbers of apoptotic cells
were determined using TUNEL staining. As shown in Fig. 6, TUNEL-positive cells were located
only in the cochleae of the D-gal-treated rats. A small number of
TUNEL-positive cells were limited to the SV of the basal turn of
the cochleae.
Discussion
The results of the present study demonstrated that
the levels of H2O2 and MDA increased, and the
activity of T-SOD decreased in the blood of rats following 8 weeks
of D-gal exposure, which indicated that an animal model of mimetic
aging was successfully established by D-gal (40). The results also indicated that the
accumulation of mtDNA CD was significantly increased in the
cochleae following treatment with D-gal, which is concordant with
the results of previous studies (20–24).
Mitochondria are one of the predominant generators of ROS within
the cell (41,42). The mitochondrial theory of aging
states that ROS generated inside mitochondria damage key
mitochondrial components, including mtDNA and respiratory chain
complex proteins. This damage accumulates with time and ultimately
leads to permanent age-associated mitochondrial dysfunction, which
in turn contributes to the aging phenotypes (43,44).
The mtDNA 4977 bp deletion in humans, also known as the CD, and the
corresponding mtDNA 4834-bp deletion in rats, is the most frequent
age-associated mtDNA damage, therefore, CD has been used as a
biomarker for aging (38,45,46).
An association between elevated mtDNA CD and presbycusis has been
observed in several studies (35,47–49).
Although no significant difference is observed in elevation of the
auditory brainstem response (ABR) threshold between rats with mtDNA
CD induced by D-gal and control rats, the hearing threshold in the
rats carrying the mtDNA CD increases significantly following
aminoglycoside antibiotic injection, compared with the control rats
(20). These results indicate that
the mtDNA CD may not directly lead to hearing loss, but rather act
as a predisposing factor that enhances the sensitivity of the
cochleae to aminoglycoside antibiotics (20). To further evaluate D-gal-induced
mitochondrial damage in the cochleae, the present study
investigated changes in the mitochondrial ultrastructure using TEM.
The results indicated that numerous mitochondria were degenerated
in different cells of the cochleae in rats following 8 weeks of
D-gal exposure. Notably, increased accumulation of mtDNA CD and
mitochondrial ultrastructural damage in the cochleae of
D-gal-treated rats significantly correlated with increased
expression levels of 8-OHdG, a biomarker of DNA oxidative damage
(50,51). Therefore, these findings suggested
that chronic D-gal treatment and the elicited oxidative stress
inside mitochondria may contribute to the increased frequency of
mtDNA CD and mitochondrial ultrastructural damage in the cochleae
of D-gal-treated rats.
The NADPH oxidase system is another important source
of ROS production (52). The
expression of NOX3 is almost restricted to the cochleae (26), and NOX3-dependent super-oxide
production is dependent on P22phox (27). Previously, the involvement of NOX3
in cisplatin-induced hearing loss. Knockdown of NOX3 using small
interfering (si)RNA inhibited cisplatin ototoxicity, as evidenced
by the protection of the outer hair cells from damage, and reduced
threshold shifts in ABR in the rat (29,30).
Furthermore, transtympanic administration of NOX3 siRNA reduced the
expression of B cell lymphoma 2 (Bcl-2)-associated protein X (Bax),
reversed the decreased expression of Bcl-2 and attenuated the
apoptosis induced by cisplatin in the cochleae (29). The results of the present study
demonstrated that the expression levels of NOX3 and
P22phox were significantly increased in the cochleae of
rats in the D-gal groups, compared with those in the control group.
The overexpression of NOX3 and P22phox may partly
explain the mitochondrial oxidative damage in the cochleae and the
occurrence of apoptosis in the SV of the cochleae, in the rats of
the D-gal groups.
Apoptosis was also induced by the accumulation of
mtDNA mutations (50). In the
mitochondrial signalling pathway of apoptosis, mitochondrial
dysfunction can lead to permeabilization of the mitochondrial outer
membrane, the release of cytochrome c into the cytosol and
the activation of key effector protease, caspase-3, by proteolytic
cleavage (53,54). To determine whether increased
expression levels of C-cas3 is a feature in the cochleae of
D-gal-induced aging rats, soft tissue samples from the cochleae of
rats in the treatment groups were examined using western blot
analysis. The results indicated that D-gal significantly increased
the protein expression levels of C-cas3. Apoptosis is also
associated with nuclear DNA fragmentation. The present study
examined sections of the cochleae using a TUNEL assay, which
detects apoptotic cells in situ. Although a small number of
apoptotic cells were located in the SV of the basal turn of the
cochleae from the D-gal-induced aging rats, the region of damage in
the SV of cochleae may not be sufficient to cause hearing loss
(55).
In conclusion, the findings of the present study
demonstrated that a marked increase in the expression of NOX3 was
involved in the accumulation of mtDNA mutations and in the
activation of caspase-3-dependent apoptosis in the cochleae of
D-gal-induced aging rats. NOX3 may serve as a useful therapeutic
target to prevent or reduce the rate of development of
presbycusis.
Acknowledgments
The present study was supported by funding from the
Science and Technology Development Foundation of Shenzhen, China
(grant no. JCYJ20140411092351692), the Medical Scientific Research
Foundation of Guangdong Province, China (grant no. B2014370) and
the Science and Technology Development Foundation of Shenzhen
Nanshan District, China (grant no. 2012014).
Abbreviations:
|
ABR
|
auditory brainstem response
|
|
C-cas3
|
cleaved caspase 3
|
|
CD
|
common deletion
|
|
D-gal
|
D-galactose
|
|
MDA
|
malondialdehyde
|
|
mtDNA
|
mitochondrial DNA
|
|
NOX3
|
NADPH oxidase 3
|
|
8-OHdG
|
8-hydroxy-2-deoxyguanosine
|
|
OC
|
organ of Corti
|
|
ROS
|
reactive oxygen species
|
|
SG
|
spiral ganglion
|
|
SGC
|
spiral ganglion cell
|
|
SV
|
stria vascularis
|
|
T-SOD
|
total superoxide dismutase
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate
nick-end-labelling
|
References
|
1
|
Gates GA and Mills JH: Presbycusis.
Lancet. 366:1111–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamasoba T, Someya S, Yamada C, Weindruch
R, Prolla TA and Tanokura M: Role of mitochondrial dysfunction and
mitochondrial DNA mutations in age-related hearing loss. Hear Res.
226:185–193. 2007. View Article : Google Scholar
|
|
3
|
Lu J, Zheng YL, Wu DM, Luo L, Sun DX and
Shan Q: Ursolic acid ameliorates cognition deficits and attenuates
oxidative damage in the brain of senescent mice induced by
D-galactose. Biochem Pharmacol. 74:1078–1090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM,
Shan Q and Hu B: Purple sweet potato color attenuates oxidative
stress and inflammatory response induced by d-galactose in mouse
liver. Food Chem Toxicol. 47:496–501. 2009. View Article : Google Scholar
|
|
5
|
5Liu CM, Ma JQ and Lou Y: Chronic
administration of troxerutin protects mouse kidney against
D-galactose-induced oxidative DNA damage. Food Chem Toxicol.
48:2809–2817. 2010. View Article : Google Scholar
|
|
6
|
Chen CF, Lang SY, Zuo PP, Yang N, Wang XQ
and Xia C: Effects of D-galactose on the expression of hippocampal
peripheral-type benzodiazepine receptor and spatial memory
performances in rats. Psychoneuroendocrinology. 31:805–811. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hua X, Lei M, Zhang Y, Ding J, Han Q, Hu G
and Xiao M: Long-term D-galactose injection combined with
ovariectomy serves as a new rodent model for Alzheimer's disease.
Life Sci. 80:1897–1905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar A, Prakash A and Dogra S: Naringin
alleviates cognitive impairment, mitochondrial dysfunction and
oxidative stress induced by D-galactose in mice. Food Chem Toxicol.
48:626–632. 2010. View Article : Google Scholar
|
|
9
|
Lu J, Wu DM, Zheng YL, Hu B and Zhang ZF:
Purple sweet potato color alleviates D-galactose-induced brain
aging in old mice by promoting survival of neurons via PI3K pathway
and inhibiting cytochrome C-mediated apoptosis. Brain Pathol.
20:598–612. 2010. View Article : Google Scholar
|
|
10
|
Zhang ZF, Lu J, Zheng YL, Hu B, Fan SH, Wu
DM, Zheng ZH, Shan Q and Liu CM: Purple sweet potato color protects
mouse liver against d-galactose-induced apoptosis via inhibiting
caspase-3 activation and enhancing PI3K/Akt pathway. Food Chem
Toxicol. 48:2500–2507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei M, Hua X, Xiao M, Ding J, Han Q and Hu
G: Impairments of astrocytes are involved in the
d-galactose-induced brain aging. Biochem Biophys Res Commun.
369:1082–1087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh HM, Wu WM and Hu ML: Soy isoflavones
attenuate oxidative stress and improve parameters related to aging
and Alzheimer's disease in C57BL/6J mice treated with D-galactose.
Food Chem Toxicol. 47:625–632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W
and Liu J: D-galactose-caused life shortening in Drosophila
melanogaster and Musca domestica is associated with oxidative
stress. Biogerontology. 5:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei H, Li L, Song Q, Ai H, Chu J and Li W:
Behavioural study of the D-galactose induced aging model in
C57BL/6J mice. Behav Brain Res. 57:245–251. 2005. View Article : Google Scholar
|
|
15
|
Zhang XL, An LJ, Bao YM, Wang JY and Jiang
B: d-galactose administration induces memory loss and energy
metabolism disturbance in mice: Protective effects of catalpol.
Food Chem Toxicol. 46:2888–2894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian Y, Zou B, Yang L, Xu SF, Yang J, Yao
P and Li CM: High molecular weight persimmon tannin ameliorates
cognition deficits and attenuates oxidative damage in senescent
mice induced by D-galactose. Food Chem Toxicol. 49:1728–1736. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng HB, Cui DP, Jiang JM, Feng YC, Cai NS
and Li DD: Inhibiting effects of Achyranthes bidentata
polysaccharide and Lycium barbarum polysaccharide on nonenzyme
glycation in D-galactose induced mouse aging model. Biomed Environ
Sci. 16:267–275. 2003.PubMed/NCBI
|
|
18
|
Deng HB, Cheng CL, Cui DP, Li DD, Cui L
and Cai NS: Structural and functional changes of immune system in
aging mouse induced by D-galactose. Biomed Environ Sci. 19:432–438.
2006.
|
|
19
|
Uddin MN, Nishio N, Ito S, Suzuki H and
Isobe K: Toxic effects of D-galactose on thymus and spleen that
resemble aging. J Immunotoxicol. 7:165–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong WJ, Hu YJ, Wang Q, Wang Y, Han YC,
Cheng HM, Kong W and Guan MX: The effect of the mtDNA4834 deletion
on hearing. Biochem Biophys Res Commun. 344:425–430. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC and
Liu J: The relation between D-galactose injection and mitochondrial
DNA 4834 bp deletion mutation. Exp Gerontol. 41:628–634. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng W, Hu Y, Zhong Y, Chen B, Sun Y, Yang
Y and Kong W: Protective roles of alpha-lipoic acid in rat model of
mitochondrial DNA4834bp deletion in inner ear. J Huazhong Univ Sci
Technolog Med Sci. 30:514–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong Y, Hu YJ, Chen B, Peng W, Sun Y,
Yang Y, Zhao XY, Fan GR, Huang X and Kong WJ: Mitochondrial
transcription factor A overexpression and base excision repair
deficiency in the inner ear of rats with D-galactose-induced aging.
FEBS J. 278:2500–2510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong Y, Hu YJ, Yang Y, Peng W, Sun Y,
Chen B, Huang X and Kong WJ: Contribution of common deletion to
total deletion burden in mitochondrial DNA from inner ear of
d-galactose-induced aging rats. Mutat Res. 712:11–19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bánfi B, Malgrange B, Knisz J, Steger K,
Dubois-Dauphin M and Krause KH: NOX3, a superoxide-generating NADPH
oxidase of the inner ear. J Biol Chem. 279:46065–46072. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ueno N, Takeya R, Miyano K, Kikuchi H and
Sumimoto H: The NADPH oxidase Nox3 constitutively produces
superoxide in a p22phox-dependent manner: Its regulation by oxidase
organizers and activators. J Biol Chem. 280:23328–23339. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukherjea D, Whitworth CA, Nandish S,
Dunaway GA, Rybak LP and Ramkumar V: Expression of the kidney
injury molecule 1 in the rat cochlea and induction by cisplatin.
Neuroscience. 139:733–740. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mukherjea D, Jajoo S, Kaur T, Sheehan KE,
Ramkumar V and Rybak LP: Transtympanic administration of short
interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects
against cisplatin-induced hearing loss in the rat. Antioxid Redox
Signal. 13:589–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mukherjea D, Jajoo S, Sheehan K, Kaur T,
Sheth S, Bunch J, Perro C, Rybak LP and Ramkumar V: NOX3 NADPH
oxidase couples transient receptor potential vanilloid 1 to signal
transducer and activator of transcription 1-mediated inflammation
and hearing loss. Antioxid Redox Signal. 14:999–1010. 2011.
View Article : Google Scholar :
|
|
31
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
32
|
Someya S, Yamasoba T, Weindruch R, Prolla
TA and Tanokura M: Caloric restriction suppresses apoptotic cell
death in the mammalian cochlea and leads to prevention of
presbycusis. Neurobiol Aging. 28:1613–1622. 2007. View Article : Google Scholar
|
|
33
|
Someya S, Yamasoba T, Kujoth GC, Pugh TD,
Weindruch R, Tanokura M and Prolla TA: The role of mtDNA mutations
in the pathogenesis of age-related hearing loss in mice carrying a
mutator DNA polymerase gamma. Neurobiol Aging. 29:1080–1092. 2008.
View Article : Google Scholar
|
|
34
|
Yu F, Hao S, Zhao Y, Yang H, Fan XL and
Yang J: In utero and lactational β-carotene supplementation
attenuates D-galactose-induced hearing loss in newborn rats. Food
Chem Toxicol. 49:1697–1704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: Comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen B, Zhong Y, Peng W, Sun Y, Hu YJ,
Yang Y and Kong WJ: Increased mitochondrial DNA damage and
decreased base excision repair in the auditory cortex of
D-galactose-induced aging rats. Mol Biol Rep. 38:3635–3642. 2011.
View Article : Google Scholar
|
|
37
|
Du Z, Yang Y, Hu Y, Sun Y, Zhang S, Peng
W, Zhong Y, Huang X and Kong W: A long-term high-fat diet increases
oxidative stress, mitochondrial damage and apoptosis in the inner
ear of d-galactose-induced aging rats. Hear Res. 287:15–24. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nicklas JA, Brooks EM, Hunter TC, Single R
and Branda RF: Development of a quantitative PCR (TaqMan) assay for
relative mitochondrial DNA copy number and the common mitochondrial
DNA deletion in the rat. Environ Mol Mutagen. 44:313–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
40
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
43
|
Loeb LA, Wallace DC and Martin GM: The
mitochondrial theory of aging and its relationship to reactive
oxygen species damage and somatic mtDNA mutations. Proc Natl Acad
Sci USA. 102:18769–18770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hiona A and Leeuwenburgh C: The role of
mitochondrial DNA mutations in aging and sarcopenia: Implications
for the mitochondrial vicious cycle theory of aging. Exp Gerontol.
43:24–33. 2008. View Article : Google Scholar :
|
|
45
|
Yowe DL and Ames BN: Quantitation of
age-related mitochondrial DNA deletions in rat tissues shows that
their pattern of accumulation differs from that of humans. Gene.
209:23–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meissner C, Bruse P, Mohamed SA, Schulz A,
Warnk H, Storm T and Oehmichen M: The 4977 bp deletion of
mitochondrial DNA in human skeletal muscle, heart and different
areas of the brain: a useful biomarker or more. Exp Gerontol.
43:645–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bai U, Seidman MD, Hinojosa R and Quirk
WS: Mitochondrial DNA deletions associated with aging and possibly
presbycusis: A human archival temporal bone study. Am J Otol.
18:449–453. 1997.PubMed/NCBI
|
|
48
|
Ueda N, Oshima T, Ikeda K, Abe K, Aoki M
and Takasaka T: Mitochondrial DNA deletion is a predisposing cause
for sensorineural hearing loss. Laryngoscope. 108:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Markaryan A, Nelson EG and Hinojosa R:
Quantification of the mitochondrial DNA common deletion in
presbycusis. Laryngoscope. 119:1184–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kujoth GC, Hiona A, Pugh TD, Someya S,
Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA,
et al: Mitochondrial DNA mutations, oxidative stress, and apoptosis
in mammalian aging. Science. 309:481–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma Y, Mehta SL, Lu B and Li PA: Deficiency
in the inner mitochondrial membrane peptidase 2-like (Immp21) gene
increases ischemic brain damage and impairs mitochondrial function.
Neurobiol Dis. 44:270–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lambeth JD: Nox enzymes, ROS, and chronic
disease: An example of antagonistic pleiotropy. Free Radic Biol
Med. 43:332–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pauler M, Schuknecht HF and White JA:
Atrophy of the stria vascularis as a cause of sensorineural hearing
loss. Laryngoscope. 98:754–759. 1988. View Article : Google Scholar : PubMed/NCBI
|