Introduction
Pancreatic cancer is the fourth leading cause of
cancer-associated mortality worldwide (1). This cancer type is characterized by
early metastasis, and pronounced resistance to chemotherapy and
radiation (2–4). Although systemic treatment, including
gemcitabine and erlotinib, has been used for advanced pancreatic
cancer, the effect of current chemotherapy is only a small survival
advantage (5–7). Therefore, identification of novel
therapeutic targets and approaches are required against pancreatic
cancer to improve patient prognosis.
Yes-associated protein (YAP) overexpression has been
reported for several human tumor entities, including prostate,
ovarian, colon, liver, lung and pancreatic cancer (8–10).
Several previous studies have suggested that dysregulation of the
YAP cascade may be critically involved in the development of
several tumor types (11–15). In addition, the expression of YAP
correlates with poor prognosis in different cancer types, including
colorectal, esophageal, gastric, hepatocellular, lung and ovarian
(9,16–20).
The Hippo pathway is important in tumorigenesis (21) and YAP was first noted as an
oncogene from a previous study of the Hippo/YAP pathway, which
regulates the balance between cell proliferation and apoptosis
(22). It also has been confirmed
in a previous study that YAP functions as a critical
transcriptional switch downstream of the oncogenic
KRAS-mitogen-activated protein kinase pathway in pancreatic cancer
(15).
The present study revealed that YAP overexpression
promoted the epithelial-mesenchymal transition (EMT) of pancreatic
cancer cells and increased drug resistance. The role of YAP on the
sensitivity of pancreatic cancer cells to gemcitabine was
investigated and the present study explored the mechanisms, which
may mediate such an effect. The findings of the present study
suggested that YAP induces the EMT and regulates the sensitivity of
pancreatic cancer cells to gemcitabine by activating AKT and raises
the possibility that YAP may be a promising target to improve the
efficacy of therapy for pancreatic cancer.
Materials and methods
Cells and clinical samples
The pancreatic cancer cell lines, PANC-1, MIA
PaCa-2, BxPC-3, Capan-1, T3M4 and colo357, were purchased from
American Type Culture Collection (Rockville, MD, USA). The BxPC-3
cells were grown in RPMI-1640 medium, containing 10% fetal bovine
serum (FBS) and penicillin/streptomycin (all Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The PANC-1, MIA PaCa-2, T3M4
and colo357 cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.), containing 10% FBS
and penicillin/streptomycin.
Fresh-frozen specimens of human normal pancreatic
tissues and primary pancreatic cancer tissues were obtained along
with written informed consent and pathology reports from the Henan
Provincial People's Hospital (Henan, China), and were used for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blotting. Sample collection was performed
following approval from the institutional Ethics Review Committee
of the Henan Provincial People's Hospital. No patient had undergone
chemotherapy prior to surgery.
Western blotting
The cells were lysed in cell lysis buffer for
western and IP (Beyotime Institute of Biotechnology, Haimen, China)
to obtain the total cellular protein. The protein concentrations
were determined using an Enhanced BCA protein assay kit (Beyotime
Institute of Biotechnology) and were subsequently boiled for 10 min
at 100°C. The protein samples (2 µg/µl; 30 µg)
were separated by 12% SDS-PAGE (Beyotime Institute of
Biotechnology) and were subsequently transferred onto
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were rinsed in Tris-buffered
saline, containing 0.1% Tween-20 and blocked with 5% bovine serum
albumin (Beyotime Institute of Biotechnology) for 2 h at room
temperature. Following blocking, the membranes were incubated with
the following primary antibodies at 4°C overnight: Mouse monoclonal
anti-YAP (1:500; cat. no. sc-376830; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), rabbit monoclonal anti-E-cadherin (1:1,000;
cat. no. 3195; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit monoclonal anti-N-cadherin (1:1,000; cat. no. 13116; Cell
Signaling Technology, Inc.), rabbit monoclonal anti-snail (1:1,000;
cat. no. 3879; Cell Signaling Technology, Inc.), rabbit monoclonal
anti-phosphorylated (p)-AKT (1:1,000; cat. no. 4060; Cell Signaling
Technology, Inc.) and mouse monoclonal anti-β-actin (1:1,000; cat.
no. sc-130065; Santa Cruz Biotechnology, Inc.). The membranes were
rinsed in phosphate-buffered saline containing 0.1% Tween-20 and
incubated with horseradish peroxidase-conjugated goat anti-mouse
(cat. no. A0216) and goat anti-rabbit (cat. no. A0208) secondary
antibodies (1:1,000; Beyotime Institute of Biotechnology) for 2 h
at room temperature. Following washing, the proteins were detected
using enhanced chemiluminescence (Beyotime Institute of
Biotechnology).
Ipatasertib-induced AKT inhibition
The cells were treated with the AKT inhibitor
ipatasertib (0.5 µM; Anpei, Nanjing, China) for 24 h.
Subsequently, cell lysates were prepared and western blotting was
performed.
Transwell migration and invasion
assay
Cell migration and invasion were investigated using
a Transwell migration assay and a matrigel invasion assay (8
µm pore size; BD Falcon, San Jose, CA, USA), as described
previously (23). Briefly, for the
Transwell migration assay, 5×104 cells were suspended in
200 µl serum-free DMEM and placed in the cell culture insert
of a Transwell plate, and warmed culture medium supplemented with
10% FBS was placed in the well. Cells in serum-free DMEM were
seeded in the upper chamber and medium containing FBS was seeded in
the lower chamber. For the matrigel invasion assay,
2×105 cells were suspended in 200 µl DMEM without
FBS and then placed in the cell culture insert precoated with 1
µg/µl Matrigel (BD Biosciences). Warmed culture
medium containing 10% FBS was added to the well. The cells were
cultured for 12 h at 37°C in an atmosphere containing 5%
CO2 and were fixed in 4% paraformaldehyde and stained
with 0.1% crystal violet (Sigma-Aldrich). The number of migrated
cells in five randomly selected fields was counted under a light
microscope (magnification, ×100; Olympus, Tokyo, Japan).
Drug sensitivity assay
To determine drug sensitivity, the cells were seeded
into 96-well plates at a density of 2×103 cells/well.
Following incubation for 24 h, the cells were placed in complete
medium, containing different concentrations of gemcitabine (0.2, 1,
5, 25, 125 µM; Jiangsu Hansoh Pharmaceutical Co., Ltd.,
Lianyugang, China). Following incubation for a further 72 h, the
sensitivity of the cells to gemcitabine was measured using a cell
counting kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan).
Lentivirus production and transduction of
target cells
The YAP and YAP short hairpin (sh)RNA expression
lentivirus were purchased from Shanghai GeneChem Co., Ltd.
(Shanghai, China) and the target shRNA sequences were as follows:
5′-CTC AGG ATG GAG AAA TTTA-3′ and 5′-CGT GCC CCA GAC CGT GCCC-3′.
The lentiviral vector was transfected into cells, as described
previously (24), cancer cells
were infected with lentivirus plus 6 µg/ml polybrene
(Sigma-Aldrich) for 24 h, and transfection was confirmed by
immunoblotting.
Statistical analysis
Statistical analysis was performed using SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA). The data are expressed as
the mean ± standard deviation. The data were examined using
analysis of variance and the least significant differences method
for multisample comparisons, or Student's t-test for two-sample
comparisons. Kaplan-Meier curves were plotted to assess the effects
of YAP expression on the progression-free survival. Survival curves
were compared using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
YAP expression is upregulated in
pancreatic cancer tissues and this expression correlates with
cancer progression
To explore the role of YAP in pancreatic cancer
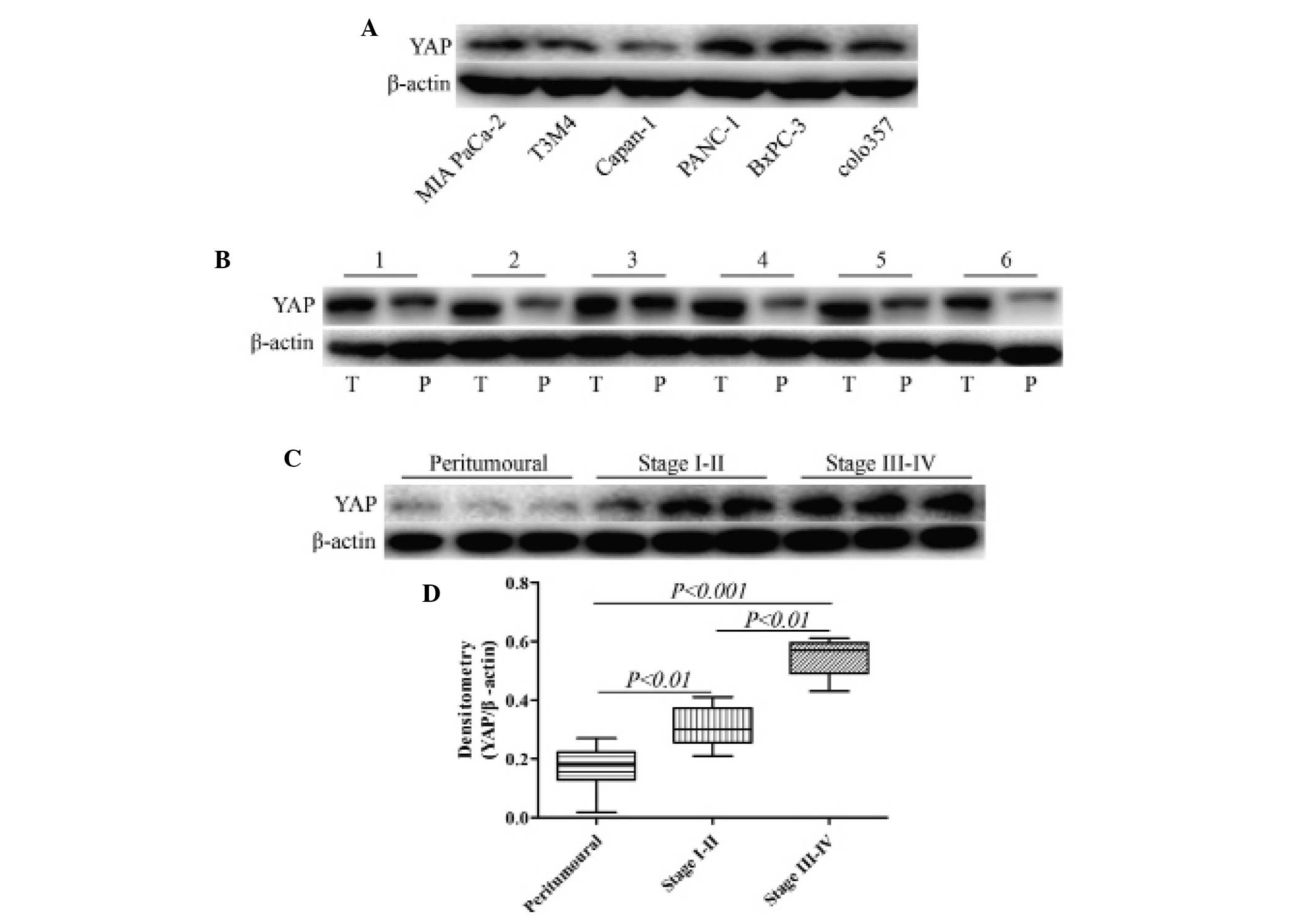
progression, the expression of YAP was assessed in various human
pancreatic cancer cell lines, pancreatic cancer and matched
peritumoral tissues. The expression of YAP in 30 pancreatic cancer
and matched peritumoral tissues was analyzed by western blotting.
Compared with the peritumoral samples, semi-quantitative analysis
revealed that the protein expression levels of YAP were markedly
higher in the cancer tissues (Fig. 1A
and B). By contrast, in normal pancreatic tissues, little YAP
expression was observed. YAP expression in early-stage (I-II) and
advanced-stage (III-IV) pancreatic cancer tissues was significantly
higher compared with that in normal pancreatic tissues (P<0.01).
In addition, YAP expression in advanced-stage (III-IV) was
significantly higher compared with in early-stage (I-II) pancreatic
cancer tissues (P<0.01; Fig.
1C).
YAP is involved in pancreatic cancer cell
invasion in vitro
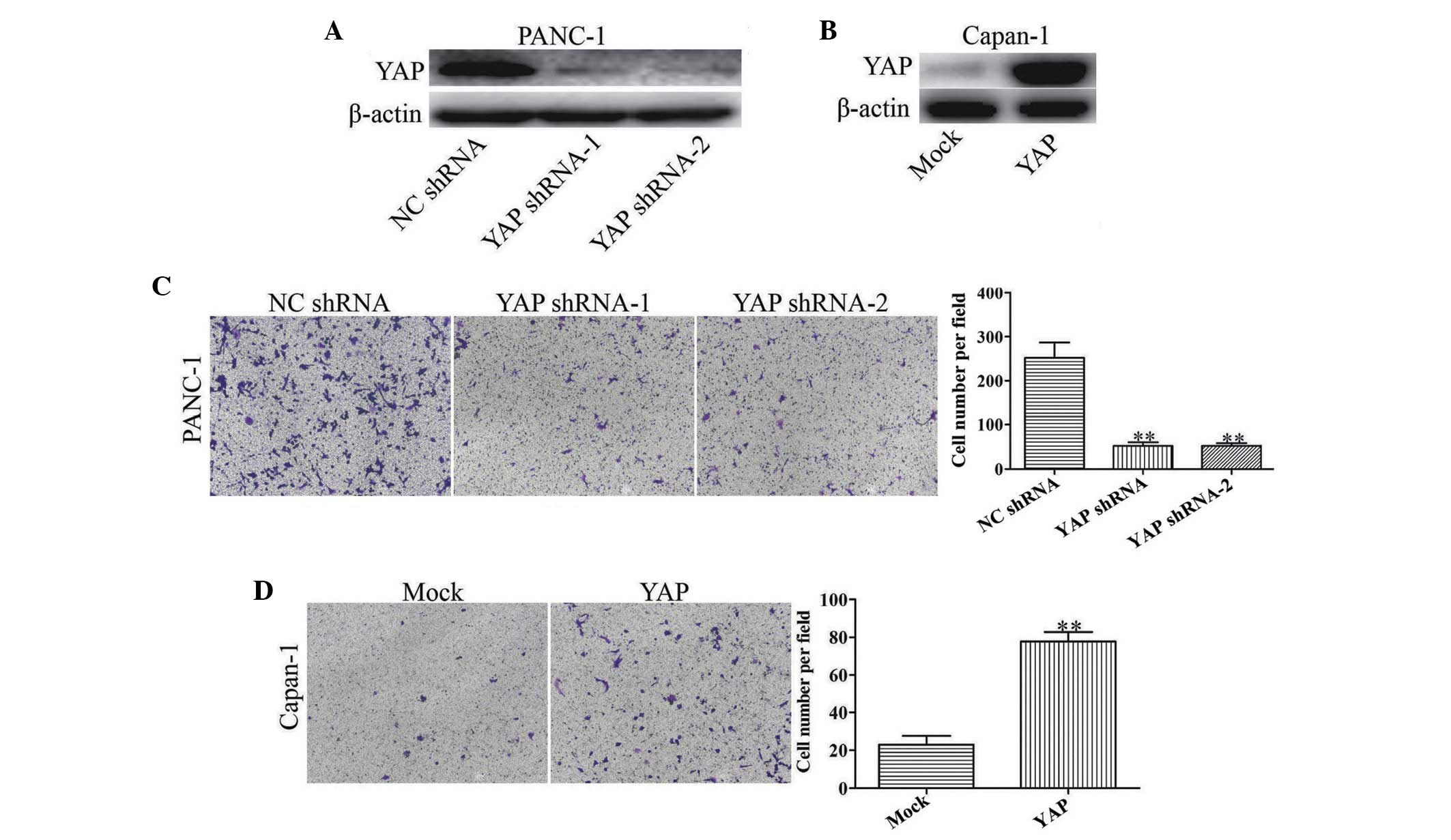
To elucidate the role of YAP in pancreatic cancer
progression, YAP shRNAs were used to reduce the expression of YAP
in the human PANC-1 pancreatic cancer cells, which exhibit a high
level of YAP protein expression (Fig.
1A). YAP shRNAs significantly reduced the expression of YAP, as
well as the invasion of PANC-1 cells (P<0.01; Fig. 2A and C). To further evaluate
whether YAP upregulation promoted tumor invasion,
lentivirus-mediated delivery of YAP cDNA was used to increase the
expression of YAP in human Capan-1 pancreatic cancer cells, which
exhibit low protein expression of YAP (Fig. 1A). Upregulation of the regulation
of YAP was observed in YAP infectants (Fig. 2B). YAP upregulation significantly
increased the invasion ability of Capan-1 cells compared with the
mock control (Fig. 2D).
Collectively, the data from the in vitro assays revealed
that YAP significantly contributed to tumor invasion of pancreatic
cancer.
YAP regulates the EMT phenotypes in
pancreatic cancer cells
Based on the association between the expression of
YAP and the invasion of pancreatic cancer in vitro, and
since that the EMT is considered a striking feature of most cancer
types and has a vital role in cancer migration and invasion, the
present study compared the expression of epithelial and mesenchymal
markers, as well as other molecules thought to induce EMT in cancer
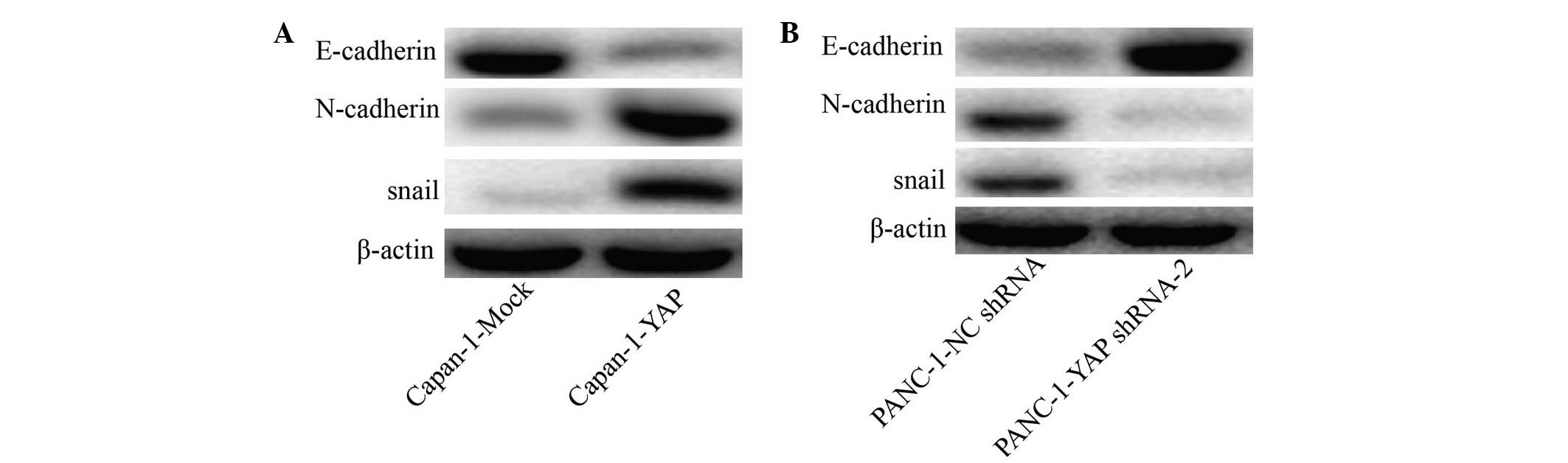
cells. As shown in Fig. 3A,
Capan-1-YAP cells expressed a lower level of the epithelial gene,
E-cadherin, compared with Capan-1-Mock cells. The mesenchymal
genes, snail and N-cadherin, were significantly upregulated in
Capan-1-YAP cells compared with Capan-1-Mock cells. Notably, the
level of E-cadherin was higher in PANC-1-YAP shRNA compared with
PANC-1-NC shRNA cells, while mesenchymal-associated genes, snail
and N-cadherin, were downregulated in PANC-1-YAP shRNA cells
(Fig. 3B).
YAP-mediated EMT occurs through the
activation of the AKT signaling pathway
It has been previous confirmed that the induction of
the EMT may be an important mechanism of constitutive AKT signaling
activation in various cancer types. To further understand whether
the YAP-mediated EMT process in pancreatic cancer cells was
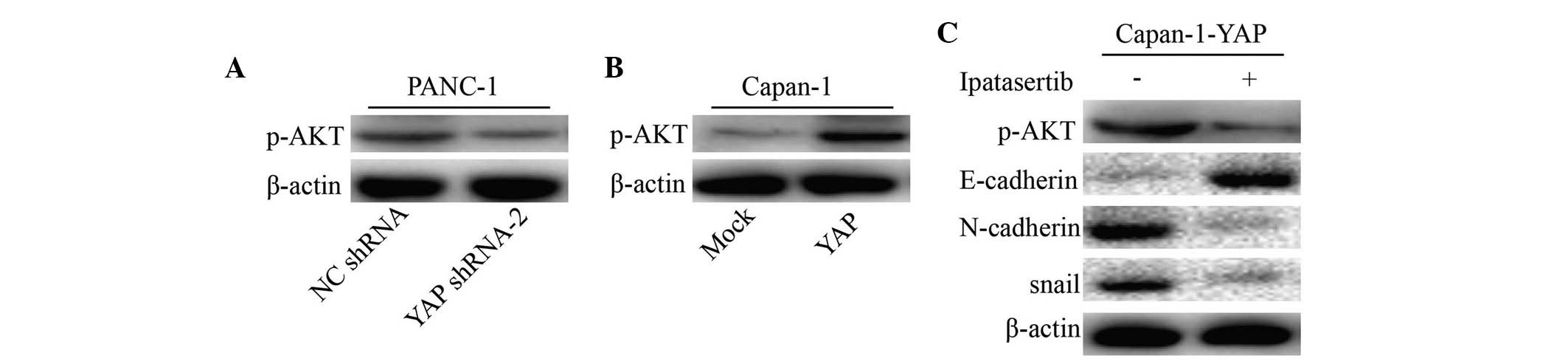
dependent on the activation of the AKT pathway, western blotting
analysis was performed to assess the activation of the components
of the AKT pathway in YAP-knockdown or -overexpressing pancreatic
cancer cells. The results indicated that shRNA-mediated YAP
downregulation in PANC-1 cells markedly reduced the expression of
p-AKT (Fig. 4A), whereas YAP
overexpression in Capan-1 cells increased the expression of p-AKT
(Fig. 4B). Finally, the present
study analyzed the effect of ipatasertib-mediated p-AKT inhibition
on the expression levels of E-cadherin, N-cadherin and snail in
pancreatic cancer cells. Notably, the expression levels of
N-cadherin and snail were markedly downregulated, while the
expression of E-cadherin was markedly upregulated in cancer cells
treated with ipatasertib (Fig.
4C). These results indicated that YAP induced the EMT by way of
hyperactivation of AKT signaling in pancreatic cancer cells.
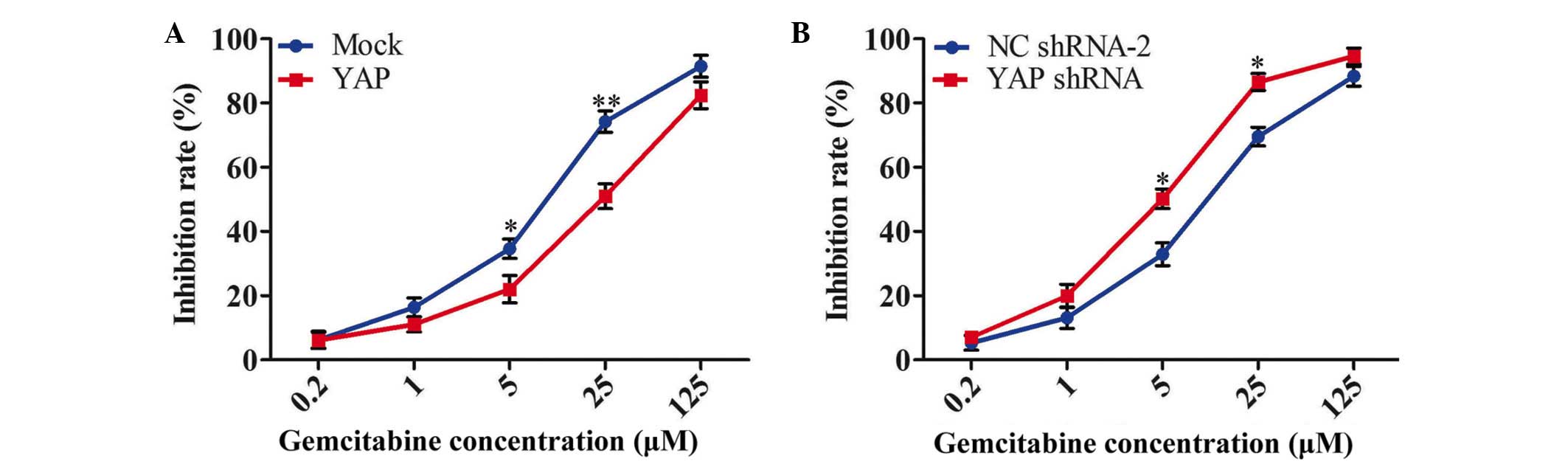
YAP modulates the chemoresistance of
human pancreatic cancer cells
The present study further investigated whether
increasing or inhibiting the expression of YAP modulated the
sensitivity of pancreatic cancer cells to gemcitabine, which is
currently used as the first line treatment for pancreatic cancer.
Following exogenous expression of YAP in Capan-1 cells, the cells
were treated with a series of concentrations of gemcitabine (0.2,
5, 25 and 125 µM). The effect of YAP on the chemoresistance
of Capan-1 cells is shown in Fig.
5A. The half-maximal inhibitory concentrations
(IC50) of gemcitabine on Capan-1-Mock and Capan-1-YAP
cells were 8.52±1.88 and 21.56±3.03 µM, respectively
(P<0.05). These results indicated that the introduction of YAP
notably reduced the chemosensi-tivity of Capan-1 cells to
gemcitabine. In addition, the inhibition of PANC-1 cell growth with
gemcitabine was significantly increased by transfection with YAP
shRNA. The IC50 values of gemcitabine on PANC-1-NC
shRNA-2 and PANC-1-YAP shRNA cells were 14.22±1.45 and 4.88±0.61
µM, respectively (P<0.05; Fig. 5B).
Discussion
YAP is a multifunctional molecule that regulates
cell survival, proliferation, migration and differentiation in
several human cancer types (25–27).
In the present study, based on depletion and overexpression
experiments in vitro, it was revealed that YAP has a crucial
role in regulating pancreatic cancer invasion and chemoresistance
to gemcitabine.
Increasing evidence from experimental and clinical
studies suggest that the EMT is important in tumor invasion,
migration and metastasis (28–30).
The EMT is observed in a series of cancer cells undergoing
phenotypic conversion for invasion and metastasis, and is
characterized by the gain of mesenchymal markers, including snail
and N-cadherin, and the loss of epithelial cell junction proteins,
including E-cadherin (31). The
present study reported that cells, which express high levels of
YAP, expressed high levels of snail, N-cadherin and low levels of
E-cadherin, suggesting that YAP may be a potent inducer of the EMT,
which may result in increased invasion and migration of pancreatic
cancer cells. Therefore, the YAP-induced EMT may be a major
contributing factor to the invasion of pancreatic cancer cells.
In models of chemotherapy resistant cancer types,
EMT gene signatures have been hypothesized to be involved in the
presence of chemotherapy resistance, and regulation of EMT
transcriptional regulators modulates resistance to chemotherapeutic
agents (32,33). Emerging evidence suggests that the
EMT is involved in cancer progression, and targeting the EMT can
reverse the resistance of antitumor drugs (34). Furthermore, it was also confirmed
in previous studies that hyperactivation of AKT signaling is
involved in the chemo-resistance of pancreatic cancer (35,36).
The present findings demonstrated that the gemcitabine resistance
of pancreatic cancer was due, in part, to the presence of YAP. YAP
significantly increased the activation of AKT, which can enhance
gemcitabine resistance in pancreatic cancer.
In conclusion, the results of the present study
revealed that YAP is expressed in pancreatic cancer tissues and is
positively correlated with tumor progression. The overexpression of
YAP may contribute to the invasiveness of pancreatic cancer cells.
Additionally, the present study provided evidence of a molecular
and phenotypic association between the YAP-induced EMT phenotype
and gemcitabine-resistance of pancreatic cancer cells. YAP
expression reduces the sensitivity to gemcitabine in pancreatic
cancer cells. Taken together, YAP is important for the pathogenesis
pancreatic cancer and may be a biomarker for predicting response to
gemcitabine treatment.
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
YAP
|
Yes-associated protein
|
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maheshwari V and Moser AJ: Current
management of locally advanced pancreatic cancer. Nat Clin Pract
Gastroenterol Hepatol. 2:356–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D and Sarkar FH: Pancreatic cancer: Understanding and
overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 8:27–33.
2011. View Article : Google Scholar
|
|
4
|
Goodman KA and Hajj C: Role of radiation
therapy in the management of pancreatic cancer. J Surg Oncol.
107:86–96. 2013. View Article : Google Scholar
|
|
5
|
Mahalingam D, Kelly KR, Swords RT, Carew
J, Nawrocki ST and Giles FJ: Emerging drugs in the treatment of
pancreatic cancer. Expert Opin Emerg Drugs. 14:311–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham D, Chau I, Stocken DD, Valle
JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J,
et al: Phase III randomized comparison of gemcitabine versus
gemcitabine plus capecitabine in patients with advanced pancreatic
cancer. J Clin Oncol. 27:5513–5518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrmann R, Bodoky G, Ruhstaller T,
Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A,
Pestalozzi B, et al: Gemcitabine plus capecitabine compared with
gemcitabine alone in advanced pancreatic cancer: A randomized,
multicenter, phase III trial of the Swiss group for clinical cancer
research and the central European cooperative oncology group. J
Clin Oncol. 25:2212–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kapoor A, Yao W, Ying H, Hua S, Liewen A,
Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al: Yap1 activation
enables bypass of oncogenic kras addiction in pancreatic cancer.
Cell. 158:185–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KP, Lee JH, Kim TS, Kim TH, Park HD,
Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM and Lim DS: The
Hippo-Salvador pathway restrains hepatic oval cell proliferation,
liver size, and liver tumorigenesis. Proc Natl Acad Sci USA.
107:8248–8253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang XY, Ke AW, Shi GM, Zhang X, Zhang C,
Shi YH, Wang XY, Ding ZB, Xiao YS, Yan J, et al: αB-crystallin
complexes with 14-3-3ζ to induce epithelial-mesenchymal transition
and resistance to sorafenib in hepatocellular carcinoma.
Hepatology. 57:2235–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng X, Degese MS, Iglesias-Bartolome R,
Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino
G, Sodhi A, et al: Hippo-Independent Activation of YAP by the GNAQ
uveal melanoma oncogene through a trio-regulated rho GTPase
signaling circuitry. Cancer Cell. 25:831–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Nandakumar N, Shi Y, Manzano M,
Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et
al: Downstream of mutant KRAS, the transcription regulator YAP is
essential for neoplastic progression to pancreatic ductal
adenocarcinoma. Sci Signal. 7:ra422014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muramatsu T, Imoto I, Matsui T, Kozaki K,
Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T and Inazawa J: YAP
is a candidate oncogene for esophageal squamous cell carcinoma.
Carcinogenesis. 32:389–398. 2011. View Article : Google Scholar
|
|
17
|
Wang LJ, Shi SJ, Guo ZY, Zhang X, Han S,
Yang A, Wen W and Zhu Q: Overexpression of YAP and TAZ Is an
independent predictor of prognosis in colorectal cancer and related
to the proliferation and metastasis of colon cancer cells. Plos
One. 8:e655392013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang W, Tong JH, Chan AW, Lee TL, Lung RW,
Leung PP, So KK, Wu K, Fan D, Yu J, et al: Yes-associated protein 1
exhibits oncogenic property in gastric cancer and its nuclear
accumulation associates with poor prognosis. Clin Cancer Res.
17:2130–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Dong Q, Zhang Q, Li Z, Wang E and
Qiu X: Overexpression of yes-associated protein contributes to
progression and poor prognosis of non-small-cell lung cancer.
Cancer Sci. 101:1279–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Du YC, Zhou XJ, Liu H and Tang SC:
The dual functions of YAP-1 to promote and inhibit cell growth in
human malignancy. Cancer Metastasis Rev. 33:173–181. 2014.
View Article : Google Scholar
|
|
23
|
Wang H, Zhou M, Shi B, Zhang Q, Jiang H,
Sun Y, Liu J, Zhou K, Yao M, Gu J, et al: Identification of an exon
4-deletion variant of epidermal growth factor receptor with
increased metastasis-promoting capacity. Neoplasia. 13:461–471.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH,
Sun J, Yi Y, Shi JY, Shi GM, Ding ZB, et al: CXCR6 upregulation
contributes to a proinflammatory tumor microenvironment that drives
metastasis and poor patient outcomes in hepatocellular carcinoma.
Cancer Res. 72:3546–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang JH, Pores Fernando AT, Faure N,
Andrabi S, Adelmant G, Hahn WC, Marto JA, Schaffhausen BS and
Roberts TM: Polyoma small T antigen interacts with Yes-associated
protein to regulate cell survival and differentiation. J Virol.
88:12055–12064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao J, Calvisi DF, Ranganathan S, Cigliano
A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger
S, et al: Activation of β-catenin and Yap1 in human hepatoblastoma
and induction of hepatocarcinogenesis in mice. Gastroenterology.
147:690–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu D, Lv X, Hua G, He C, Dong J, Lele SM,
Li DW, Zhai Q, Davis JS and Wang C: YAP regulates cell
proliferation, migration, and steroidogenesis in adult granulosa
cell tumors. Endoc Relat Cancer. 21:297–310. 2014. View Article : Google Scholar
|
|
28
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang TH, Tsai MF, Su KY, Wu SG, Huang CP,
Yu SL, Yu YL, Lan CC, Yang CH, Lin SB, et al: Slug confers
resistance to the epidermal growth factor receptor tyrosine kinase
inhibitor. Am J Respir Crit Care Med. 183:1071–1079. 2011.
View Article : Google Scholar
|
|
33
|
Saxena M, Stephens MA, Pathak H and
Rangarajan A: Transcription factors that mediate
epithelial-mesenchymal transition lead to multidrug resistance by
upregulating ABC transporters. Cell Death Dis. 2:e1792011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D,
Banerjee S and Sarkar FH: Targeting miRNAs involved in cancer stem
cell and EMT regulation: An emerging concept in overcoming drug
resistance. Drug Resist Updat. 13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nath S, Daneshvar K, Roy LD, Grover P,
Kidiyoor A, Mosley L, Sahraei M and Mukherjee P: MUC1 induces drug
resistance in pancreatic cancer cells via upregulation of multidrug
resistance genes. Oncogenesis. 2:e512013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu H, Gu Y, Qian Y, Hu B, Zhu C, Wang G
and Li J: DNA-PKcs is important for Akt activation and gemcitabine
resistance in PANC-1 pancreatic cancer cells. Biochem Biophys Res
Commun. 452:106–111. 2014. View Article : Google Scholar : PubMed/NCBI
|