Introduction
The most successful treatment option for
hepatocellular carcinoma (HCC) is hepatectomy. However, the
long-term survival rates following hepatectomy for HCC remain
unsatisfactory due to the high rate of postoperative intrahepatic
recurrence (1,2). Despite this, in these patients,
repeat hepatectomy has been reported to be a more effective
therapeutic strategy for the treatment of recurrent hepatic tumors
(3,4). Even multiple bilobar metastases from
colorectal cancer or carcinomas of other organs, which were
previously considered a contraindication for hepatectomy, are now
considered curable by planned two-staged hepatectomy under certain
circumstances (5).
Following extensive hepatectomy, the regenerative
capacity of the remnant liver is essential for patient survival
(6). Accordingly, a novel
therapeutic strategy is required for protection against liver
dysfunction and for the enhancement of regenerative capacity.
Fortunately, investigations into mesenchymal stem cells (MSCs) has
offered a potential therapeutic tool in the field of liver
regeneration (7). MSCs are an
adult stem cells population with powerful proliferative and
differentiation potential, which present an attractive tool for the
establishment of successful stem cell-based therapy for liver
diseases (8). Notably, several
studies have focused on the role of MSCs in the liver regeneration
process, and have reported that MSCs cam attenuate liver injuries
and promote liver regeneration following partial hepatectomy (PH)
(9–12). However, few studies have
investigated the role of MSCs in liver regenerative following
repeat partial hepatectomy (R-PH).
The aim of the present study was to investigate
whether autologous adipose tissue-derived mesenchymal stem cell
(ADSC) transplantation promoted the regeneration of the remaining
liver tissues in a rat model of R-PH.
Materials and methods
Animals
Male Wistar rats (n=60) aged 11 weeks and weighing
250–300 g were obtained from the Academy of Military Medical
Science [Beijing, China; certificate no. SCXK (JUN) 2007–004].
These animals were maintained in a standard animal laboratory with
free activity and free access to water and rodent chow. They were
maintained in a temperature-controlled environment at 22–24°C with
a 12-h light-dark cycle. The rats were fasted for 12 h prior to
surgery, and were provided with free access to 10% glucose water
following surgery. All the surgical procedures were performed under
sterile conditions, and all experiments were performed according to
the National Institutes of Health Guide for Care and Use of
Laboratory Animals (13) and were
approved by the ethics committee of Tianjin First Central Hospital,
Tianjin Medical University (Tianjin, China).
Establishment of the 70% PH and R-PH
models
For the introduction of 70% PH, the 60 rats were
anesthetized with isoflurane inhalation (Lunan Pharmaceutical Co.,
Ltd., Shandong, China) via an isoflurane vaporizer (Matrx VMR;
Midmark corporation, Dayton, OH, USA), and 70% of the liver of each
rat, comprising the left lateral and median lobes, was excised,
using the technique described by Saito et al (14). Suturing of the peritoneum and skin
were performed independently. The remaining 30% of the liver
started to grow for 7 days, following which R-PH was performed, in
which 40 of 60 rats were anesthetized and the right lateral lobe
was ligated and excised.
Isolation and culture of autologous
ADSCs
To obtain adequate cells and avoid the requirement
for a long duration following establishment of the PH model, the
autologous ADSCs were isolated and expanded 2 weeks prior to
surgery. Adipose tissue cells were isolated from all 60 rats using
a described previously method (15). Briefly, the rats were anesthetized
via inhalational isoflurane. The hemi-inguinal fat pads were
carefully excised and minced into pieces of ~1 mm3. The
adipose tissue was digested in collagenase type I solution
(Sigma-Aldrich, St. Louis, MO, USA) for 60 min at 37°C with
constant agitation (100 rpm). The stromal cells were separated from
the floating adipocytes by centrifugation at 200 g for 5 min at
room temperature. The cells released were then resuspended in
Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and then sieved through
70 µm mesh (BD Biosciences, Franklin Lakes, NJ, USA). The
resulting ADSCs were cultivated in DMEM/F12 medium containing 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.). Following in
vitro culture for 14 days at 37°C, 5% CO2 and 95%
humidity, a sufficient number of ADSCs were obtained for the
autologous transplantation. The ADSCs (3×106) from each
experimental rat were cryopreserved in liquid nitrogen (Air
Products and Chemicals (Tianjin) Co., Ltd., Tianjin, China) with
cell name marked on tube prior to injection.
Cell surface antigen profile of
ADSCs
The expression levels of cell surface antigen were
evaluated using flow cytometry. When cultures reach >80%
confluency at 37°C, 5% CO2 and 95% humidity, adherent
cells were removed from the tissue culture polystyrene flasks via
trypsinization (Invitrogen; Thermo Fisher Scientific, Inc.) and
washed twice with DMEM/F12. All cells were incubated with
fluorescein isothiocyanate-conjugated mouse anti-rat monoclonal
antibodies against rat CD45 (cat. no. 554877), CD73 (cat. no.
551123), CD90 (cat. no. 554894) all obtained from BD Biosciences),
CD34 (cat. no. sc-7324; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) and CD105 (cat. no. ab11414; Abcam, Cambridge, MA, USA)
for 40 min at room temperature at a dilution of 1:50. Following
antibody incubation, data were acquired using a FACSCalibur flow
cytometer (BD Biosciences) and analyzed using CellQuest 6.0
software (BD Biosciences).
Multidifferentiation ability of
ADSCs
The differentiation of the cells into osteogenic and
adipogenic lineages, and subsequent detection were performed using
established methodologies (15).
Briefly, the ADSCs were seeded in medium at 2×104
cells/cm2 in six-well tissue culture plates. When the
cells reached 100% confluency, DMEM/F12 was subsequently replaced
with osteogenic inducer medium containing 100 nmol/l dexamethasone
(Sigma-Aldrich), 10 mmol/l β-sodium glycerophosphate
(Sigma-Aldrich) and 50 µg/ml vitamin C (Sigma-Aldrich), or
adipogenic inducer medium containing 1 µmol/l dexamethasone,
0.5 mmol/l 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 5 mg/l
insulin (Sigma-Aldrich) and 100 µmol/l indomethacin
(Sigma-Aldrich), in DMEM/F12. Cells were maintained at 37°C in a 5%
CO2 incubator and the medium was changed every 3 days.
Following a 14 day induction period, the cells were assayed for
mineral content by Von Kossa staining (Shanghai Genmed Gene
Pharmaceutical Technology Co., Ltd., Shanghai, China) and for lipid
accumulation using Oil Red O staining (Sigma-Aldrich).
Experimental groups, cell transplantation
and sample collection
The rats were divided into the following three
groups: PH (n=20); R-PH (n=20), subjected to a R-PH and treated
with saline by portal vein injection; and R-PH/ADSC group (n=20),
subjected to R-PH and treated with autologous ADSCs
(2×106 cells/rat) by portal vein injection. Subsequent
to these procedures, five animals in each group were sacrificed
using anesthesia, as described above, at 24, 72 and 168 h following
hepatectomy, respectively. Blood samples (2 ml) were collected by
puncturing the vena cava, and the residual liver lobes were then
rapidly excised and weighed. The livers were fixed in formalin
(Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China)
overnight, prior to processing and embedding in paraffin wax
(Tianjin Kemiou Chemical Reagent Co., Ltd.). Sections (5-µm
thick) were deparaffinized and fixed. The sections were stained
with hematoxylin and eosin (H&E; Sigma-Aldrich) and observed
using a Nikon Ni-U fluorescence microscope (Nikon Corporation,
Tokyo, Japan) Additional samples were stored in liquid
nitrogen.
Liver mass and function recovery
For each time point, the total body weight of each
of the fasted rats were weighed prior to sacrifice. The
regenerating ratio of the liver following hepatectomy was
calculated as the liver wet weight to body weight ratio (LBR),
rather than the weight of the remnant lobes alone. The 2 ml blood
samples were centrifuged at 4,000 × g for 10 min at room
temperature prior to serum collection. The serum concentrations of
alanine aminotransferase (ALT), aspartate aminotransferase (AST)
and total bilirubin (TBIL) were measured using an automatic
biochemical analyzer (Hitachi 7600; Hitachi, Ltd., Tokyo, Japan)
24, 72, and 168 h postoperatively in the R-PH/ADSC, R-PH and PH
groups.
Proliferating cell nuclear antigen
(PCNA)-labeling index
The expression level of PCNA, determined by
immunohistochemistry, correlates with the degree of cell
proliferation (16). Briefly,
following fixation with formalin and paraffin embedding, the liver
tissue sections were incubated with rabbit anti-rat polyclonal
antibody against PCNA (cat. no. GTX100539; 1:500 dilution; Genetex
Inc. Irvine, CA, USA) at 4°C overnight, and subsequently with a
3′,3-diaminobenzidine kit (Beyotime Institute of Biotechnology,
Haimen, China). The proliferation index of the PCNA-stained cells
was measured by counting the number of positive nuclei of
hepatocytes under Ni-U fluorescence microscope, with data expressed
as the percentage of PCNA-stained hepatocytes of the total number
of hepatocytes.
mRNA expression of hepatocyte growth
factor (HGF)
The mRNA levels of HGF in liver tissue were measured
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis. Total RNA was extracted from the frozen remnant
lobe samples using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and then subjected to RT using a High Capacity
cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR was performed using an ABI 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using 1 µl cDNA template and 1X SYBR-Green I (Takara
Bio, Inc., Tokyo, Japan) in a 25 µl reaction mixture (Takara
Bio, Inc.; 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 200
µM dNTP mix, 0.2 µM of each primer and 1 unit of Taq
DNA polymerase). Primer Premier V5.0 software was used to design
the primers, according to HGF gene sequences (GenBank; www.ncbi.nlm.nih.gov/genbank). Primers were
synthesized by Integrated DNA Technologies (Coralville, IA, USA).
The primer sequences were as follows: HGF, sense 5′-ACA
GCTTTTTGCCTTCGAGCTA-3′ and anti-sense 5′-CATCAAAGCCCTTGTCGGGATA-3′;
β-actin, sense, 5′-ATATCGCTGCGCTCGTCGTC-3′ and anti-sense
5′-TCTTGCTCTGGGCCTCGTC-3′. The conditions for each qPCR reaction
were as follows: 30 sec at 95°C, followed by 40 cycles of
denaturation for 5 sec at 95°C, annealing for 30 sec at 58°C and
extension for 30 sec at 72°C. The level of expression was
calculated using the 2−ΔCq method, in which ΔCq was
calculated as Cq of target molecule − Cq of β-actin (17).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences in parameters were analyzed using one-way analysis of
variance. Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
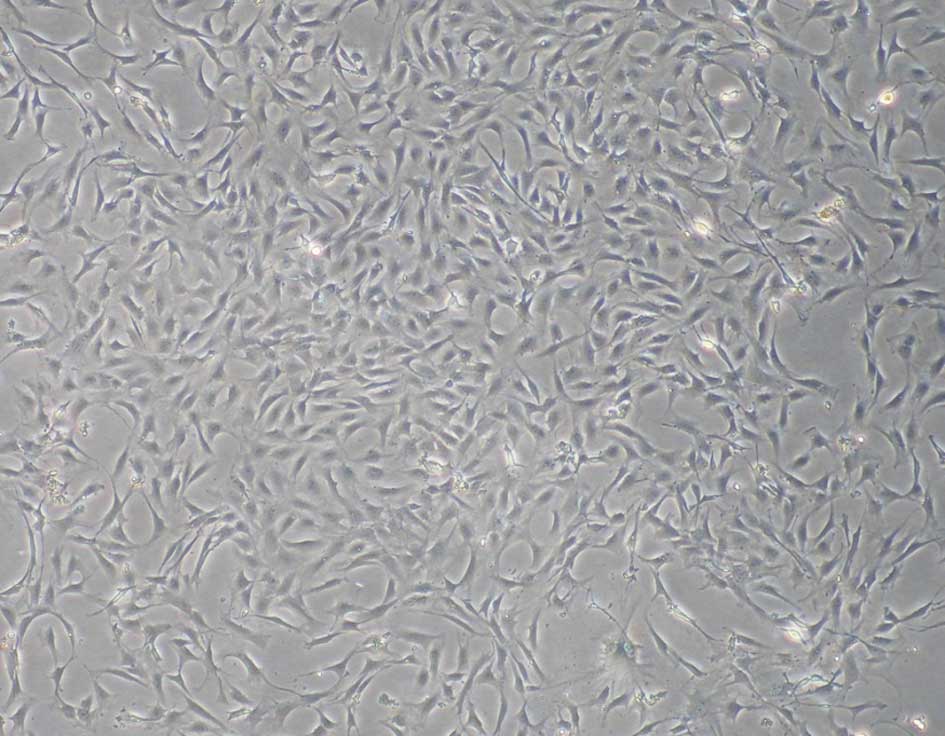
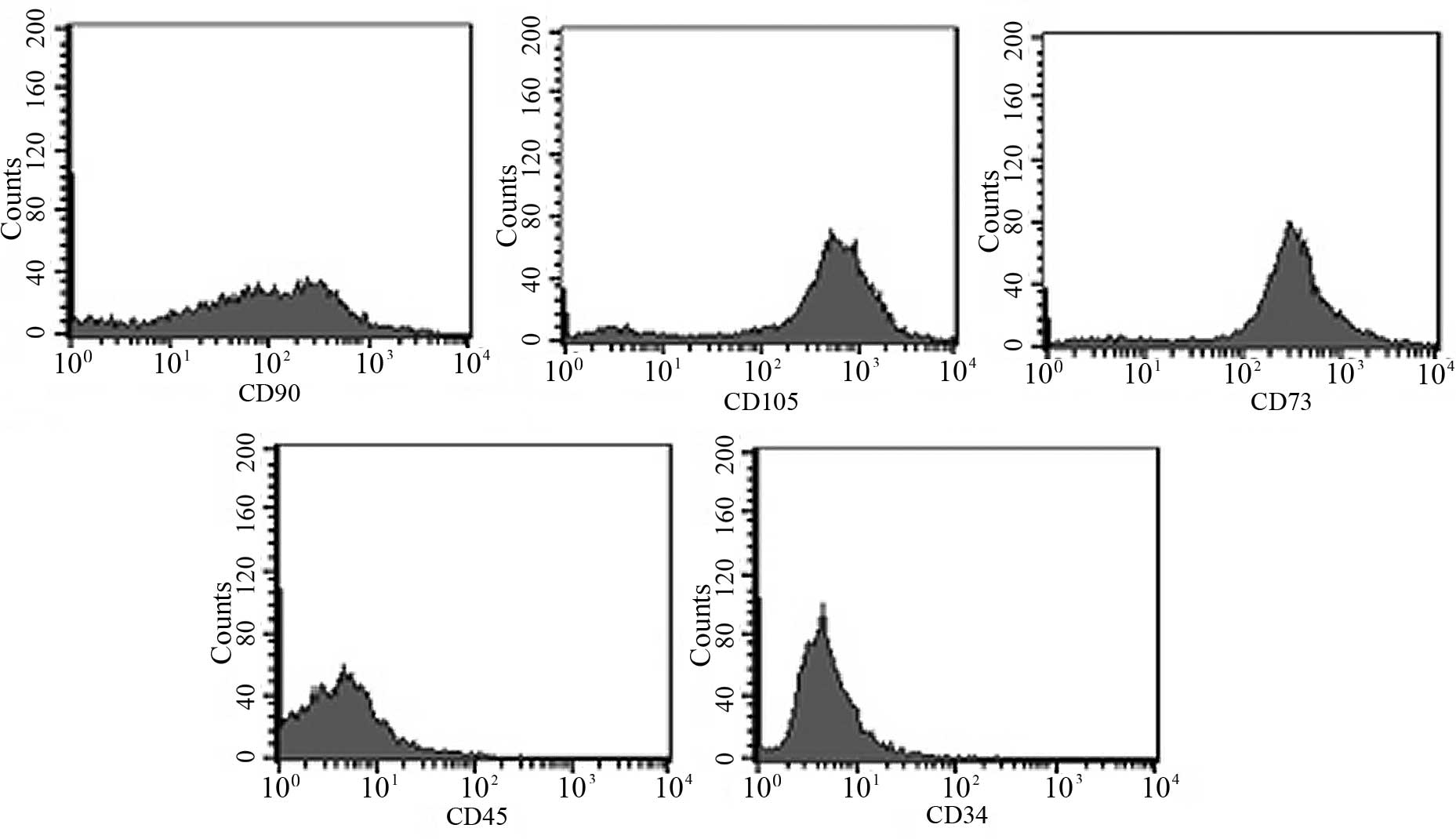
Characterization of rat ADSCs
The ADSCs were verified by analyzing the expression
surface markers and multipotent differentiation of the cells. At
passage three, the cultured ADSCs exhibited a fibroblast-like
morphology (Fig. 1). The data
showed that the ADSCs were positive for CD90, CD105 and CD73, but
were negative for CD34 and CD45 when analyzed using flow cytometric
analyses (Fig. 2). In addition,
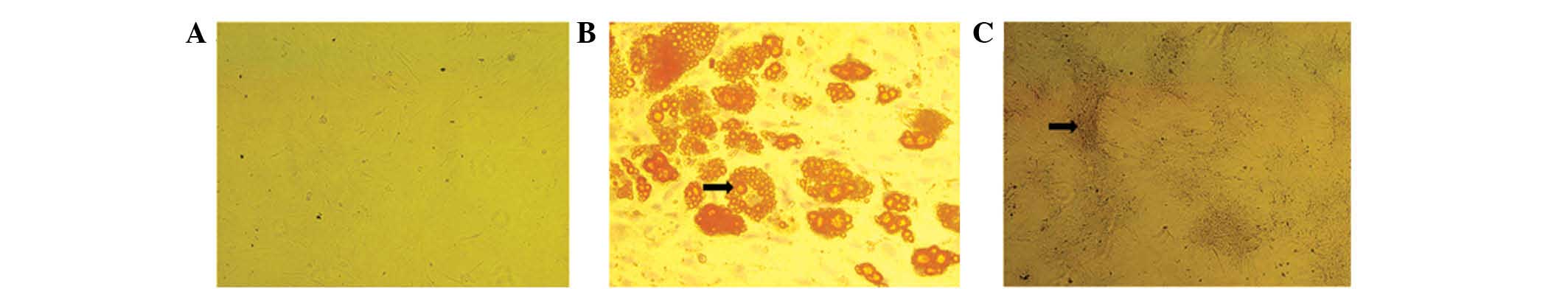
the ADSCs at passage three exhibited potential for osteogenic and
adipogenic differentiation following culture in osteogenic and
adipogenic growth media. Positive ALP and oil red O staining
confirmed the cells as osteogenic and adipogenic, respectively
(Fig. 3).
Effect of ADSCs on remnant liver
regeneration
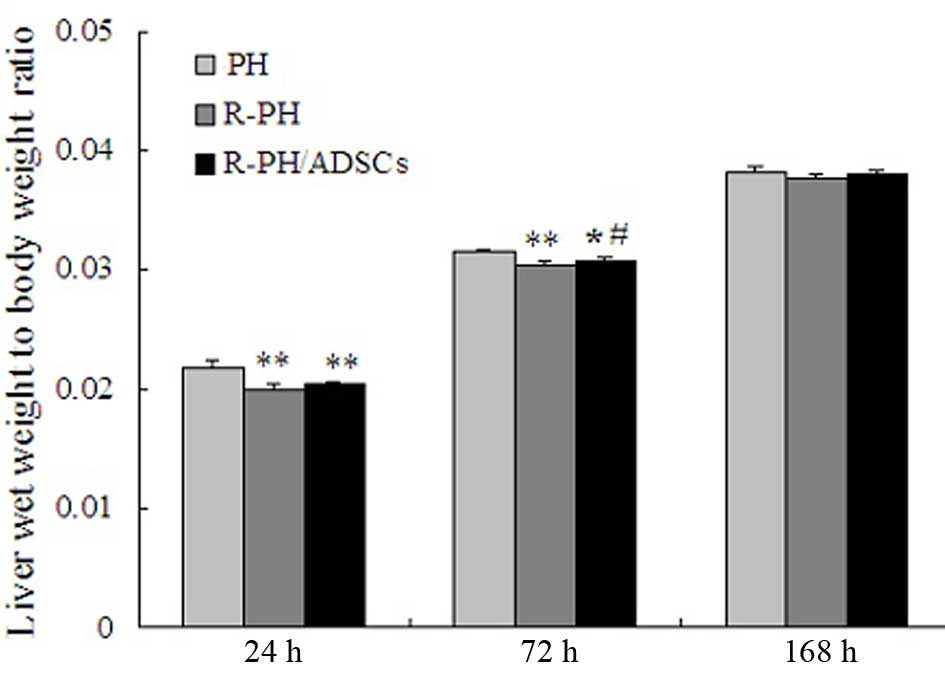
The outcomes in the 60 rats subjected to PH were
examined in the present study. The LBR at 24 and 72 h
post-hepatectomy was significant decreased in the R-PH group,
compared with that in the PH group (P<0.01; Fig. 4). However, the LBR in the R-PH rats
that received ADSC transplantation, was significantly higher,
compared with that in the R-PH rats 72 h postoperatively
(P<0.05). Furthermore, the LBR increased steadily in the three
groups, and no further differences among the groups were observed
168 h postoperatively (Fig.
4).
Effects of ADSC transplantation on liver
histopathology
At ~24 h post-hepatectomy, the sections of the
remnant lobes were stained with H&E and examined under a light
microscope. The histopathological analysis revealed that no liver
cell inflammation or necrosis was present in any of the samples. In
the PH group, the arrangement of hepatocytes was deranged, with
microvesicular fatty degeneration observed in the cytoplasm and
mild dilatation of the sinusoids (Fig.
5A). The remnant lobe in the R-PH group exhibited prevalent
vacuolization degeneration in the hepatocytes, and dilatation of
the sinusoids was more marked, compared with that in the PH group
(Fig. 5B). Similarly, in the
R-PH/ADSC group, vacuolization and sinusoidal dilatation was
similar, but to a lesser extent, compared with the R-PH group
(Fig. 5C).
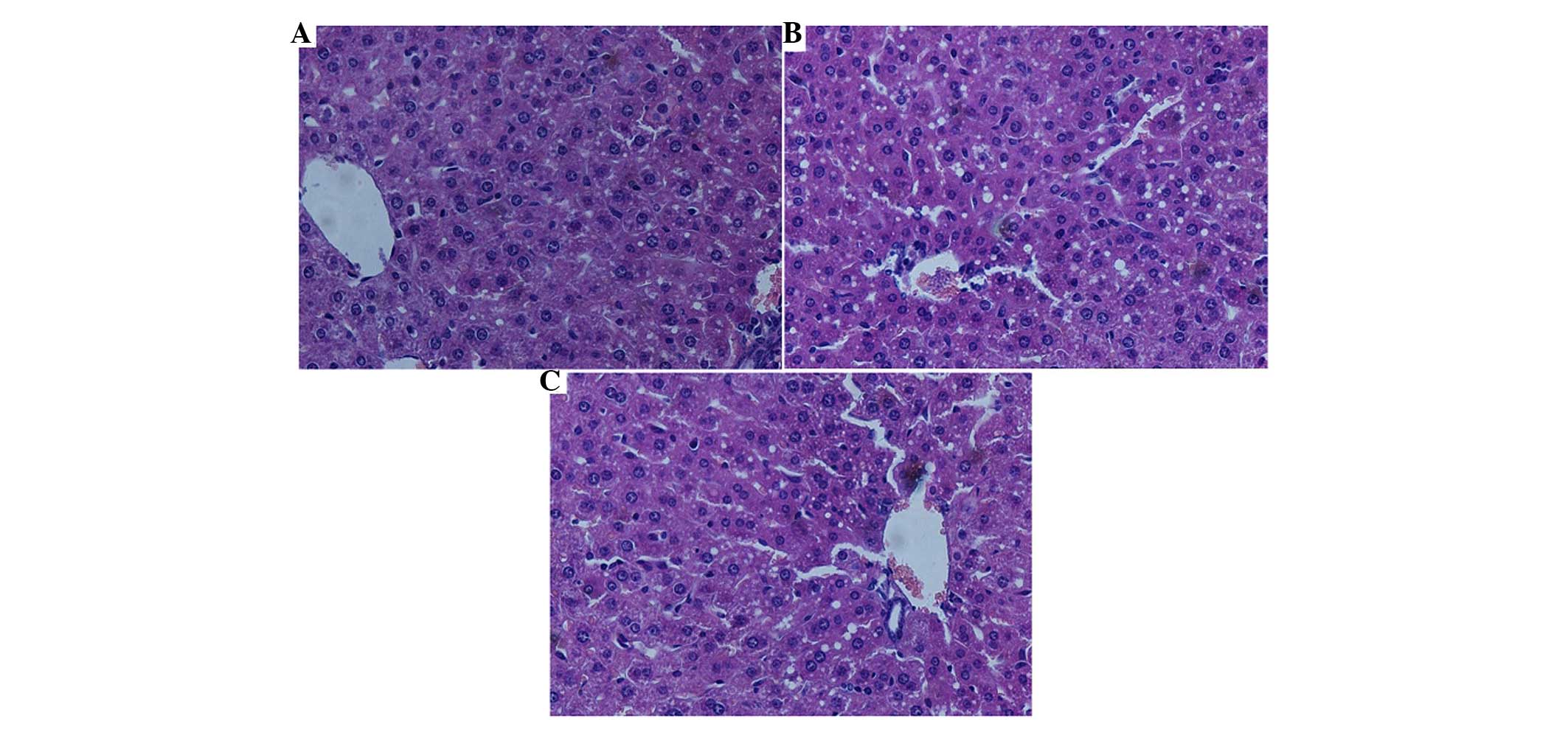 | Figure 5Effects of ADSC transplantation on
liver histopathology at 24 h postoperatively. (A) In the PH group,
derangement of liver cells and mild sinusoidal dilatation were
evident, and small vacuoles were present in the hepatocytes. (B) In
the R-PH group, vacuolization was prevalent in the hepatocytes and
sinusoidal dilatation was evident. (C) In the R-PH/ADSC group,
vacuolization degeneration and sinusoidal dilatation were observed,
but to a lesser extent, compared with the PH and R-PH groups.
(Magnification, ×200). ADSCs, adipose tissue-derived mesenchymal
stem cells; PH, partial hepatectomy; R-PH, repeat PH. |
Effects of ADSC transplantation on liver
function following 70% PH
In the R-PH/ADSC group, the levels of ALT and TBIL
were substantially lower 24 h following hepatectomy, compared with
those in the R-PH group, whereas the levels of AST were
significantly lower 24 and 72 h postoperatively, compared with
those in the R-PH group. Although a significant protective effect
was observed in the rats transplanted with ADSCs, the liver
function remained significantly higher in the R-PH group, compared
with that in the PH group 24 h postoperatively. At 72 h
postoperatively, the serum levels of ALT and TBIL in the three
groups were similar. Furthermore, the liver function gradually
decreased to the basal level in the three groups at 168 h
post-hepatectomy (Fig. 6).
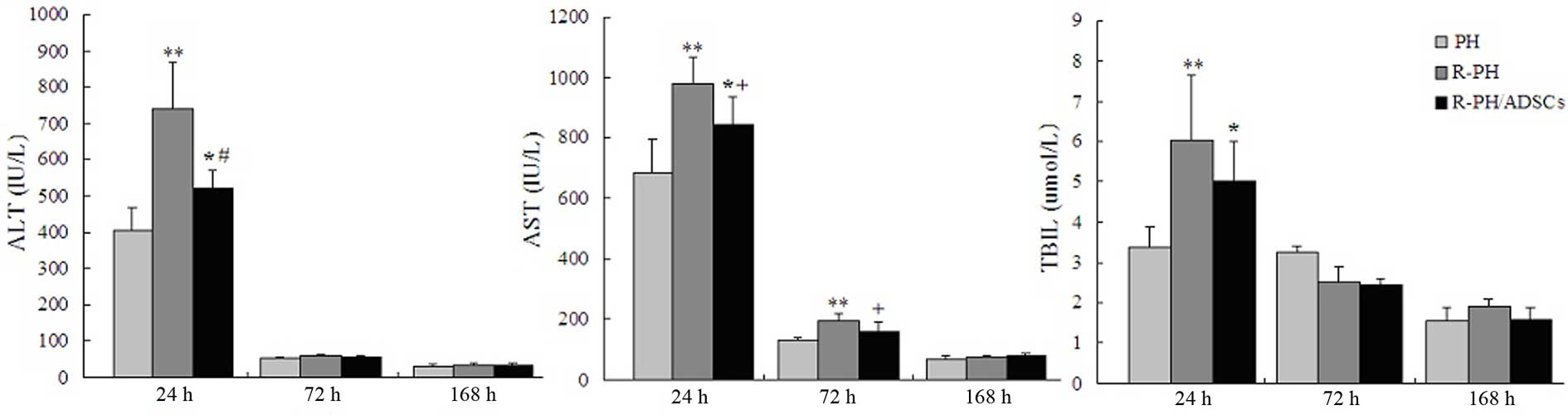 | Figure 6Effects of ADSC transplantation on
liver function at 24, 72, and 168 h postoperatively. Levels of ALT,
AST and TBIL were analyzed as indicators of liver function. Data
are expressed as the mean ± standard deviation of the results from
five animals. *P<0.05 and **P<0.01, vs.
PH group; +P<0.05, vs. R-PH group;
#P<0.01, vs. R-PH group. ADSC, adipose tissue-derived
mesenchymal stem cell; PH, partial hepatectomy; R-PH, repeat PH;
ALT, alanine aminotransferase; AST, aspartate aminotransferase;
TBIL, total bilirubin. |
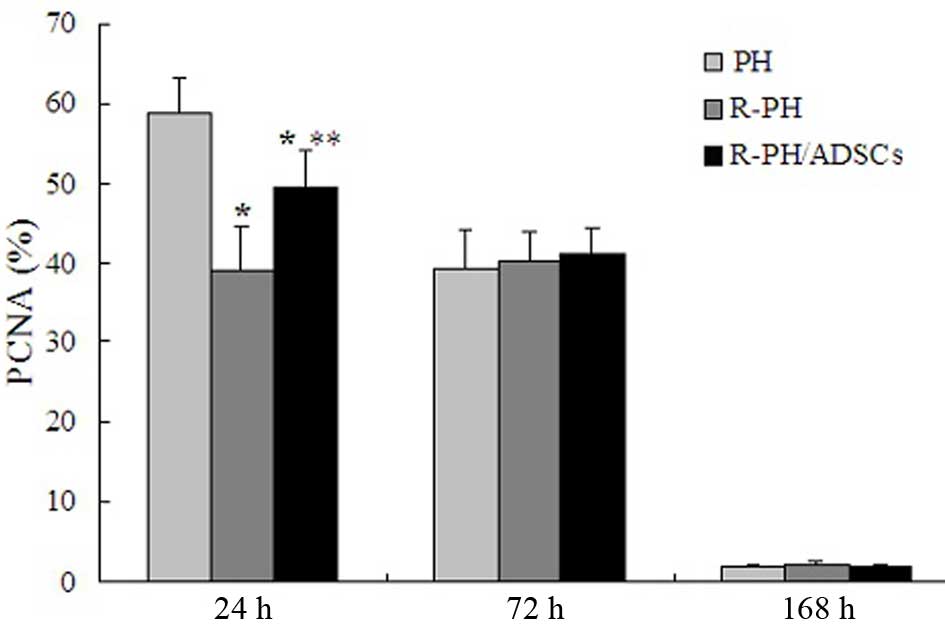
Effect of ADSCs on the PCNA-labeling
index
To evaluate whether ADSC transplantation enhanced
the proliferation of hepatocytes in remnant lobes, the expression
levels of PCNA were assessed. Although the number of PCNA-positive
hepatocytes was significantly reduced in the R-PH rats transplanted
with ADSCs, compared with the PH rats (P<0.01; Fig. 7), the R-PH rats transplanted with
ADSCs exhibited a significant increase in the number of
PCNA-positive hepatocytes at 24 h postoperatively, compared with
the R-PH rats, indicating that ADSCs exerted hepatoprotective
effects by promoting liver regeneration in the early phase. No
statistically significant differences in the PCNA-labeling index
were observed among three groups at 72 and 168 h (P>0.05;
Fig. 7).
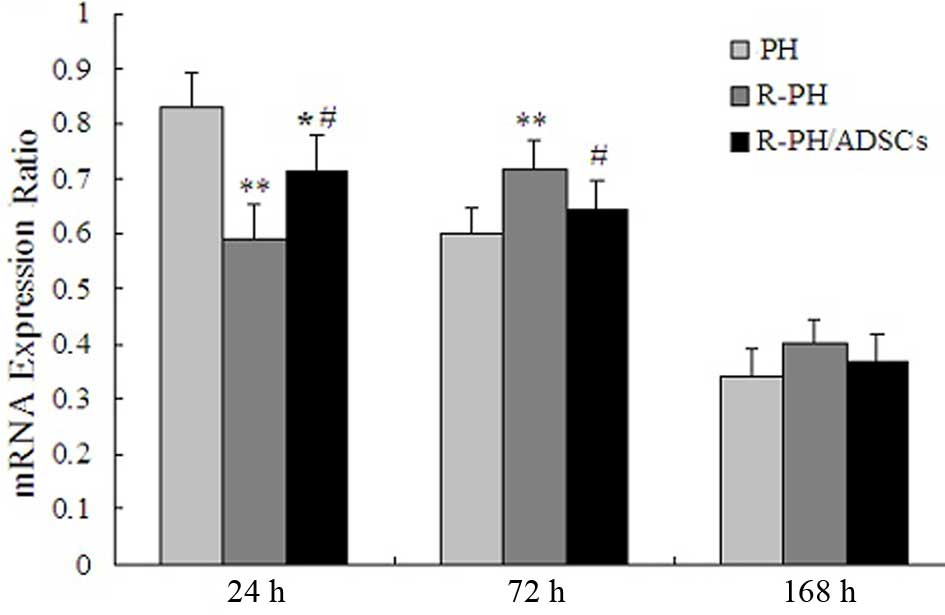
Effect of ADSCs on the mRNA levels of
HGF
The mRNA levels of HGF peaked within 24 h in the PH
group and R-PH/ADSC group, and then gradually decreased after 72 h.
However, the mRNA levels of HGF in the R-PH group decreased
significantly after 24 h, and peaked at 72 h, compared with PH and
R-PH/ADSC groups, indicating that hepatic regeneration following
R-PH was inhibited. The postoperative course of the mRNA levels of
HGF are shown in Fig. 8.
Discussion
HCC is the fifth most common type of cancer
worldwide, and has a mortality rate of 500,000 globally every year
(18). The long-term survival
rates of patients following hepatectomy remain unsatisfactory due
to the high incidence of recurrence. Clinically, repeat hepatectomy
can be performed safely, and is associated with long-term survival
rates in a subset of patients with recurrent HCC (19). In order to avoid liver dysfunction,
also termed small-for-size syndrome, the regenerative capacity of
the remnant liver is essential for patient survival (20). ADSCs have been considered as an
attractive and readily available type of adult MSC, and are
becoming increasingly popular for use in regenerative cell therapy
as they are readily accessible through minimally invasive methods
and can be used for autologous transplantation (21). In the present study, autologous
ADSC transplantation significantly enhanced liver regenerative
capacity following R-PH in rats, as indicated by an increased LBR
and PCNA-label index. In addition, autologous ADSC transplantation
alleviated R-PH-induced liver injury, as evidenced by inhibition in
the elevated serum levels of ALT, AST and TBIL, and the improvement
of pathological changes.
As a initial step in the present study, the LBR was
assessed as the liver growth kinetics of regeneration
postoperatively, which is the most direct index for evaluating
liver regeneration (22). The
results showed that regeneration in the liver tissue began from the
first day following PH. The mean LBR increased progressively among
three groups postoperatively. However, the LBR decreased
significantly in the R-PH group at 24 and 72 h postoperatively,
compared with the PH group, indicating that the hepatic
regeneration response following R-PH was significantly attenuated
at the initial stage, which is in agreement with previous data
(23). Furthermore, no
statistically significant differences were observed between PH and
R-PH at 168 h postoperatively, indicating that 7 days was a
sufficient period of time for these animals to recover from the
surgical stresses of a R-PH. Of note, the LBR in the R-PH rats
which received ADSC transplantation was significantly higher,
compared with that in the R-PH rats, indicating that the ADSCs
promoted the rapid regeneration of hepatocyte numbers in the
initial stage (24). Consistent
with the above results, the administration of conventionally
cultured autologous ADSCs in the present study also resulted in
proliferation of remnant hepatocytes 24 h following R-PH in the
rats, which was reflected by the elevated expression of
PCNA-positive cells, suggesting that the beneficial effects of
ADSCs on hepatic regeneration was more active at the cellular level
than following R-PH alone. In addition, the expression levels of
PCNA-positive cells were gradually decreased at 72 and 168 h among
three groups, which suggested that hepatocyte proliferation
occurred at a faster rate initially, and more slowly during
progression.
The present study also demonstrated that the
duration of recovery of liver volume closely coincided with that of
hepatocellular damage. The present study showed that the rats
subjected to ADSC transplantation via the portal vein following
R-PH showed increased improvement of liver function at 24 h,
compared with the corresponding liver function in the R-PH group.
However, the liver function in the R-PH/ADSC group remained
inferior to that in the PH group, as demonstrated by the serum
levels of ALT, AST and TBIL. Therefore, the improvement of liver
function by ADSC transplantation was partially contributed to
through the enhancement of hepatocyte proliferation. This is
consistent with the histopathological finding, in which the R-PH
rats exhibited derangement of hepatocyte structure, extensive lipid
vacuolization of hepatocytes and sinusoid dilatation in the remnant
lobe at 24 h postoperatively. However, vacuolization and sinusoidal
dilatation, was apparent to a lesser extent in the R-PH/ADSC group,
suggesting that ADSC transplantation may attenuate liver injury and
lead to the earlier reconstitution of residual liver tissue.
The concept of stem cell transplantation exerting a
paracrine proliferative effect on endogenous hepatocytes is gaining
support. The present study examined the expression levels of HGF,
which has been demonstrated to be the most potent stimulator of
hepatocyte growth and DNA synthesis in vitro, as well as one
of the key regulators of liver regeneration following PH or hepatic
injury (25,26). In the present study, the mRNA
levels of HGF peaked within 24 h in the PH and R-PH/ADSC groups,
and then gradually decreased after 72 h. Of note, the mRNA levels
of HGF in the R-PH group were significantly decreased after 24 h,
following which they increased to peak at 72 h post-operatively,
compared with the PH and R-PH/ADSC groups. This is in agreement
with a report by Saito et al, who observed that R-PH rats
exhibit inhibited hepatic regeneration during the early
postoperative phase due to the depressed expression of HGF
(14). It has been shown that MSCs
synthesize a wide variety of growth factors and cytokines, exerting
a paracrine effect on local cellular dynamics (27). Therefore, the results of the
present study support the viewpoint that the predominant advantage
provided by ADSCs transplanted via the portal vein is the
acceleration in the production of HGF in the early period through
paracrine effects, supporting this method as an effective treatment
strategy in liver regeneration. However, the follow-up period in
the present study was limited, therefore, it is not possible to
exclude that, in a longer period of observation, ADSCs as an
efficient alternative source can undergo hepatogenic
differentiation. However, in previous experiments involving the
administration of bone marrow MSCs in CCl4-treated mice, for 4
weeks, only a small percentage of the MSCs underwent
hepatocyte-like differentiation (28).
In conclusion, the results of the present study
suggested that the transplantation of autologous ADSCs reduced
liver injury and promoted hepatocyte proliferation, particularly
during the first 24 h following R-PH. The upregulation of HGF may
mediate the therapeutic effects of these transplanted ADSCs.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470982 and
81402322), the State-funded Construction Projects-Key Specialized
Subject of Clinical Laboratory Medicine (grant no. 2013-544),
Tianjin Research Program of Application Foundation and Advanced
Technology (grant no. 13JCYBJC23000) and the Technology Foundation
of Tianjin Municipal Health Bureau (grant no. 2014KZ028).
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubo S, Takemura S, Uenishi T, Yamamoto T,
Ohba K, Ogawa M, Hai S, Ichikawa T, Kodai S, Shinkawa H and Tanaka
H: Second hepatic resection for recurrent hepatocellular carcinoma
in patients with chronic hepatitis C. World J Surg. 32:632–638.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J
and Liu Z: Repeat hepatectomy for recurrent hepatocellular
carcinoma: A local experience and a systematic review. World J Surg
Oncol. 8:552010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wicherts DA, de Haas RJ, Salloum C,
Andreani P, Pascal G, Sotirov D, Adam R, Castaing D and Azoulay D:
Repeat hepatectomy for recurrent colorectal metastases. Br J Surg.
100:808–818. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tralhão JG, Abrantes AM, Hoti E, Oliveiros
B, Cardoso D, Faitot F, Carvalho C, Botelho MF and Castro-Sousa F:
Hepatectomy and liver regeneration: From experimental research to
clinical application. ANZ J Surg. 84:665–671. 2014. View Article : Google Scholar
|
|
7
|
Drosos I and Kolios G: Stem cells in liver
regeneration and their potential clinical applications. Stem Cell
Rev. 9:668–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du Z, Wei C, Cheng K, Han B, Yan J, Zhang
M, Peng C and Liu Y: Mesenchymal stem cell-conditioned medium
reduces liver injury and enhances regeneration in reduced-size rat
liver transplantation. J Surg Res. 183:907–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li DL, He XH, Zhang SA, Fang J, Chen FS
and Fan JJ: Bone marrow-derived mesenchymal stem cells promote
hepatic regeneration after partial hepatectomy in rats.
Pathobiology. 80:228–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Zhu J, Ma K, Liu N, Feng K, Li X,
Wang S and Bie P: Autologous bone marrow-derived mesenchymal stem
cell transplantation promotes liver regeneration after portal vein
embolization in cirrhotic rats. J Surg Res. 184:1161–1173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koellensperger E, Niesen W, Kolbenschlag
J, Gramley F, Germann G and Leimer U: Human adipose tissue derived
stem cells promote liver regeneration in a rat model of toxic
injury. Stem Cells Int. 2013:5342632013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaibori M, Adachi Y, Shimo T, Ishizaki M,
Matsui K, Tanaka Y, Ohishi M, Araki Y, Tokuhara K, Okumura T, et
al: Bone marrow cells enhance liver regeneration after massive
hepatectomy in mice. Dig Dis Sci. 59:1484–1489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: The 1996 Guide for the Care and Use of Laboratory
Animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar
|
|
14
|
Saito S, Togo S, Morioka D, Matsuo K,
Yoshimoto N, Nagano Y, Tanaka K, Kubota T, Nagashima Y and Shimada
H: A rat model of a repeat 70% major hepatectomy. J Surg Res.
134:322–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YL, Li G, Zou XF, Chen XB, Liu T and
Shen ZY: Effect of autologous adipose-derived stem cells in renal
cold ischemia and reperfusion injury. Transplant Proc.
45:3198–3202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding L, Yang Y, Qu Y, Yang T, Wang K, Liu
W and Xia W: Bile acid promotes liver regeneration via farnesoid X
receptor signaling pathways in rats. Mol Med Rep. 11:4431–4437.
2015.PubMed/NCBI
|
|
17
|
Wang Y, Wang Y, Mu H, Liu T, Chen XB and
Shen ZY: Enhanced specific antitumor immunity of dendritic cells
transfected with glypican 3 gene and co-cultured with
cytokine-induced killer cells against hepatocellular carcinoma
cells. Mol Med Rep. 11:3361–3367. 2015.PubMed/NCBI
|
|
18
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan DL, Morris DL and Chua TC: Clinical
efficacy and predictors of outcomes of repeat hepatectomy for
recurrent hepatocellular carcinoma-a systematic review. Surg Oncol.
22:e23–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Serenari M, Cescon M, Cucchetti A and
Pinna AD: Liver function impairment in liver transplantation and
after extended hepatectomy. World J Gastroenterol. 19:7922–7929.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishikawa T, Banas A, Hagiwara K, Iwaguro H
and Ochiya T: Stem cells for hepatic regeneration: The role of
adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res
Ther. 5:182–189. 2010. View Article : Google Scholar
|
|
22
|
Dusabineza AC, Van Hul NK, Abarca-Quinones
J, Starkel P, Najimi M and Leclercq IA: Participation of liver
progenitor cells in liver regeneration: Lack of evidence in the
AAF/PH rat model. Lab Invest. 92:72–81. 2012. View Article : Google Scholar
|
|
23
|
Aoki T, Murakami M, Niiya T, Murai N,
Shimizu Y, Kato H and Kusano M: Capacity of hepatic regeneration
following a second partial hepatectomy in rats. Hepatol Res.
21:228–241. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salomone F, Barbagallo I, Puzzo L, Piazza
C and Li Volti G: Efficacy of adipose tissue-mesenchymal stem cell
transplantation in rats with acetaminophen liver injury. Stem Cell
Res. 11:1037–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun J, Yuan Y, Qin H, Ying C, Liu W, Zhang
J, He Y and Liu Z: Serum from hepatectomized rats induces the
differentiation of adipose tissue mesenchymal stem cells into
hepatocyte-like cells and upregulates the expression of hepatocyte
growth factor and interleukin-6 in vitro. Int J Mol Med.
31:667–675. 2013.PubMed/NCBI
|
|
26
|
Nejak-Bowen K, Orr A, Bowen WC Jr and
Michalopoulos GK: Conditional genetic elimination of hepatocyte
growth factor in mice compromises liver regeneration after partial
hepatectomy. PLoS One. 8:e598362013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nikoozad Z, Ghorbanian MT and Rezaei A:
Comparison of the liver function and hepatic specific genes
expression in cultured mesenchymalstem cells and hepatocytes. Iran
J Basic Med Sci. 17:27–33. 2014.PubMed/NCBI
|
|
28
|
Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L,
Zhang J, Wang L, Han Z, Han Y and Fan D: In vivo tracking and
comparison of the therapeutic effects of MSCs and HSCs for liver
injury. PLoS One. 8:e623632013. View Article : Google Scholar : PubMed/NCBI
|