Introduction
Pituitary adenomas (PAs) are common benign neoplasms
and ~10–25% being intracranial neoplasms. Cross-sectional studies
from Switzerland, Belgium and the UK have shown that PAs have a
prevalence of 78–94 cases/100,000 inhabitants (1). PAs are formed due to
hypersecretion/hyposecretion of a number of or all of the pituitary
hormones and/or due to local tumor compression (2). The vast majority of PAs occur
sporadically; however, familial cases are now increasingly
recognized (2). Presently used
drugs, including metyrapone (3),
ketoconazole (4) and mitotane
(5), inhibit the secretion and
synthesis of cortisol, which is associated with PAs in the adrenal
gland. However, due to their side effects and moderate efficacy,
these drugs have limitations in PA treatment (5–7).
Therefore, it is required to elucidate the underlying molecular
mechanisms of PAs in order to discover novel targets and potential
drugs for their treatment.
It is commonly thought that the occurrence and
development of PAs is due to abberant gene expression in pituitary
cells as well as hypothalamic dysfunction (8). It has been reported that Gli1,
which is activated by the hedgehog (Hh) signal transduction
cascade, has a crucial role in the pathogenesis of PAs by
modulating adult stem cell fate or tumor-initiating stem cell
function in the adult pituitary gland and its neoplasms (9). In addition, Cazabat et al
(10) suggested that aryl
hydrocarbon receptor-interacting protein, which is a
ligand-activated transcription factor found in the cytoplasm, is
associated with the occurrence of PAs. Furthermore, a study
reported that the neuroactive ligand-receptor interaction signaling
pathway is associated with the development of PAs (11). Although great efforts have been
made to explore the pathogenesis of PAs and discover novel target
genes for PA treatment, the current knowledge is insufficient.
To obtain a systematic perspective for understanding
the underlying mechanisms of PAs and to discover novel therapeutic
targets for PA treatment, the present study utilized bioinformatics
methods to analyze gene expression profiles and performed
functional analysis of differentially expressed genes (DEGs)
between PA samples and normal controls. Furthermore, the
protein-protein interaction (PPI) network was constructed to
identify hub genes associated with PAs and small molecular drugs
with associated mechanisms were screened. The present study
provided a basis for exploring the potential underlying molecular
mechanism of PAs and to discover candidate target genes for PA
treatment.
Materials and methods
Affymetrix microarray data and data
pre-processing
The array dataset GSE4488 was downloaded from the
Gene Expression Omnibus (GEO) database from the national center of
biotechnology information (http://www.ncbi.nlm.nih.gov/geo/), which was deposited
by Vierimaa et al (12).
The expression profiles analyzed in the present study had been
obtained from nine blood samples from patients with PAs and seven
blood samples from healthy controls. The platform via which the
data had been obtained was the GPL570 (Affymetrix Human Genome U133
Plus 2.0 Array; Affymetrix, Inc., Santa Clara, CA, USA). All of the
array data were pre-processed using the robust multi-array average
algorithm (13). Normalization was
performed at probe level. Whenever multiple probes corresponded to
the same gene, the mean value was calculated as the gene expression
value for this gene.
Screening of DEGs and hierarchical
clustering analysis
The Limma package (14) in R language was used to screen
DEGs. DEGs with |log 2 fold change (FC)|>1 and P<0.05 were
considered to be significant.
Hierarchical cluster analysis produces a unique set
of nested categories or clusters by sequentially pairing variables,
clusters, or variables and clusters (15). The gene expression profiles of the
selected DEGs was subjected to two-dimensional hierarchical
clustering analysis based on Euclidean distance using the
'pheatmap' package in R language (16) and then the heat map was
generated.
Gene ontology (GO) and pathway enrichment
analysis
GO analysis is a commonly used approach for
functional studies of genomic or transcriptomic data (17). In order to analyze the DEGs at the
function level, GO annotation for DEGs was performed using the
online software Database for Annotation, Visualization and
Integration Discovery (18). The
DEGs were classified into three GO categories, including molecular
function, biological process and cellular components. P<0.05 was
set as the threshold value.
The Kyoto Encyclopedia of Genes and Genomes (KEGG)
knowledge database is used for classification of correlating gene
sets into their respective pathways (19). The Web-based Gene Set Analysis
Toolkit (http://bioinfo.vanderbilt.edu/webgestalt/,WebGestalt)
(20,21) was applied for the enrichment tests
of KEGG pathways. P<0.05 was selected as the threshold.
PPI network construction
The Search Tool for the Retrieval of Interacting
Genes (STRING) (22) is an online
database resource, which collects comprehensive information of
predicted and experimental interactions of proteins. The
interactions of protein pairs in the STRING database were displayed
with a combined score >0.4. The PPI network with significant
gene pairs was then visualized using Cytoscape software (23).
Identification of candidate agents
The Connectivity Map (cMap) (24,25)
database collects the gene expression profiles from cultured human
cells treated with small molecules. The DEGs were converted into a
probe set on the GPL570 platform and mapped onto the cMap database.
Small molecules mechanistically associated with PAs were then
identified as candidate agents using thresholds of |connectivity
score|>0.8 and P<0.05.
Results
Screening of DEGs and hierarchical
clustering analysis
According to the cut-off criteria of P<0.05 and
|log2 FC|>2.0, a total of 389 DEGs were obtained, including 49
downregulated and 340 upregulated DEGs.
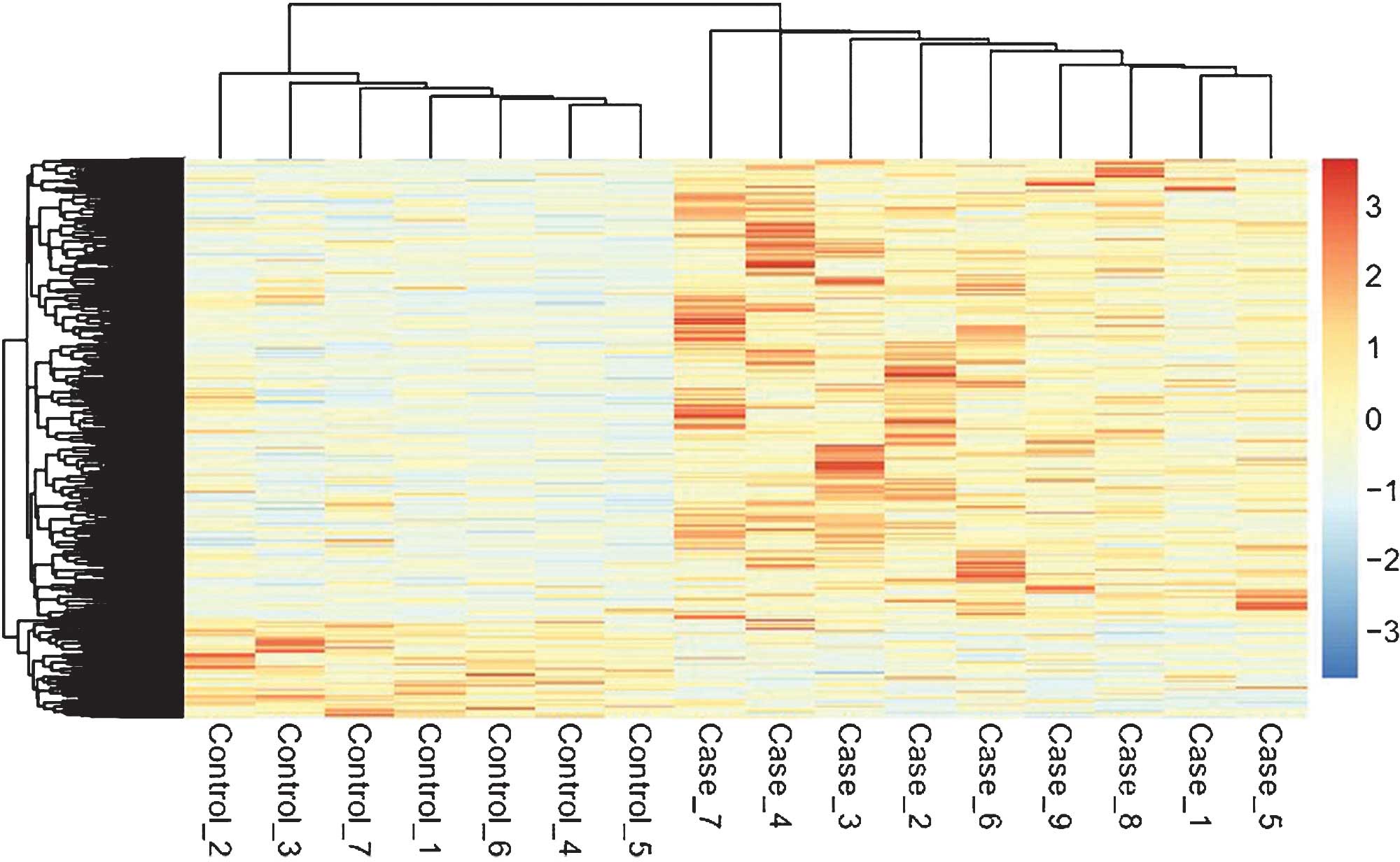
The heat map of hierarchical clustering analysis for
the DEGs is shown in Fig. 1, which
clearly demonstrated the obvious differences in expression between
PAs and normal controls. The expression values for the same gene in
the two groups were significantly different, rendering the DEGs
suitable for distinguishing PAs from normal controls.
GO and pathway enrichment analysis
The results of the GO analysis are shown in Table I. The upregulated DEGs were mainly
enriched in ectodermal development, epithelial development,
response to radiation and skeletal system development, while the
downregulated DEGs were mainly involved in responses to stimuli,
immune system-associated processes and gene expression.
 | Table IGene ontology enrichment analysis for
differentially expressed genes. |
Table I
Gene ontology enrichment analysis for
differentially expressed genes.
| Gene ontology
term | Function | Count | P-value |
|---|
| Downregulated | | | |
| 0048584 | Positive regulation
of response to stimulus | 4 |
1.79×10−2 |
| 0002684 | Positive regulation
of immune system process | 4 |
1.83×10−2 |
| 0002521 | Leukocyte
differentiation | 3 |
3.82×10−2 |
| 0050926 | Regulation of
positive chemotaxis | 2 |
4.40×10−2 |
| 0050927 | Positive regulation
of positive chemotaxis | 2 |
4.40×10−2 |
| 0010628 | Positive regulation
of gene expression | 5 |
4.68×10−2 |
| Upregulated | | | |
| 0007588 | Excretion | 5 |
9.52×10−3 |
| 0060429 | Epithelium
development | 9 |
1.67×10−2 |
| 0007398 | Ectoderm
development | 8 |
2.47×10−2 |
| 0009314 | Response to
radiation | 8 |
2.53×10−2 |
| 0007200 | Activation of
phospholipase C activity | 4 |
3.84×10−2 |
| 0045664 | Regulation of
neuron differentiation | 6 |
4.26×10−2 |
| 0015698 | Inorganic anion
transport | 5 |
4.47×10−2 |
| 0009416 | Response to light
stimulus | 6 |
4.86×10−2 |
Furthermore, only three significant pathways of the
screened DEGs were enriched, including extracellular matrix
(ECM)-receptor interaction [Homo sapiens (hsa)04512], the Hh
signaling pathway (hsa04340) and neuroactive ligand-receptor
interaction (hsa04080) (Table
II).
 | Table IIEnriched Kyoto Encyclopedia of Genes
and Genomes pathway of differentially expressed genes. |
Table II
Enriched Kyoto Encyclopedia of Genes
and Genomes pathway of differentially expressed genes.
| ID | Pathway | P-value |
|---|
| hsa04512 | ECM-receptor
interaction |
2.47×10−3 |
| hsa04340 | Hedgehog signaling
pathway |
1.33×10−2 |
| hsa04080 | Neuroactive
ligand-receptor interaction |
2.44×10−2 |
PPI network construction and functional
analysis of DEGs
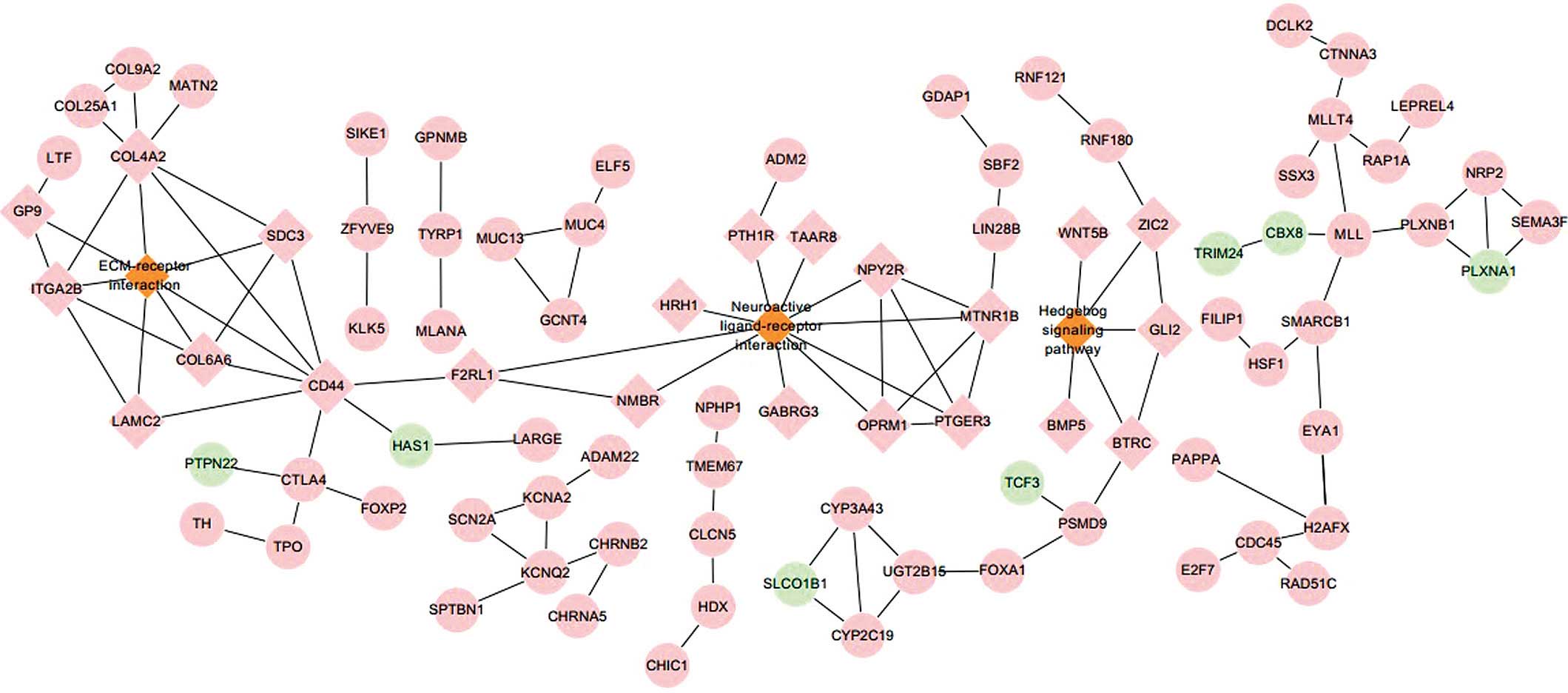
Based on the STRING database, a total of 101 gene
pairs with a combined score >0.4 were obtained. The PPI network
shown in Fig. 2 contained three
pathways comprising 117 nodes and 123 edges. The hub proteins of
cluster of differentiation 44 (CD44) and laminin, gamma 2 (LAMC2)
were involved in the ECM-receptor interaction pathway, while
prostaglandin E receptor 3 (PTGER3) was associated with the
neuroactive ligand-receptor interaction pathway. Glioma-associated
oncogene 2 (Gli2), which took part in the hedgehog signaling
pathway, was also a hub protein in the PPI network.
Screening of small molecular drug
candidates
After mapping of DEGs onto the cMap database, a
total of 13 potentially mechanistically associated small molecules
were obtained, which are listed in Table III. The small molecule depudecin
(connectivity score, −0.935) had the highest negative score.
 | Table IIIEnriched significant small
molecules. |
Table III
Enriched significant small
molecules.
| cMap name | Enrichment | P-value |
|---|
| Depudecin | −0.935 |
8.71×10−3 |
|
Sulfamonomethoxine | −0.863 |
6.40×10−4 |
|
Sulfadimethoxine | −0.807 |
6.20×10−4 |
| Prestwick-692 | −0.806 |
2.71×10−3 |
|
Podophyllotoxin | −0.803 |
2.88×10−3 |
| Cefamandole | −0.801 |
3.06×10−3 |
| Verteporfin | 0.802 |
1.58×10−2 |
| Thioguanosine | 0.803 |
2.84×10−3 |
| Fluorocurarine | 0.804 |
2.80×10−3 |
| Anabasine | 0.811 |
1.36×10−2 |
| Blebbistatin | 0.858 |
4.11×10−2 |
| Imidurea | 0.918 |
1.14×10−3 |
| Spiperone | 0.973 |
1.11×10−3 |
Discussion
PAs are a common type of benign intracranial
neoplasm (26). In previous
studies, gene expression profiling has been used to identify
germline mutations associated with the pre-disposition to pituitary
adenoma (26). KEGG pathway
enrichment analysis performed in the present study showed that DEGs
were significantly enriched in ECM-receptor interaction and Hh
signaling pathways, including CD44 and Gli2, which
were hub proteins in the PPI network. Furthermore, the
small-molecule depudecin was identified as a potential drug for the
to treatment of PAs.
The present study identified that CD44 was a hub
protein in the PPI network and had a significant role in the
ECM-receptor interaction pathway (27). CD44 is a cell-surface glycoprotein
(28). It is a receptor for
hyaluronic acid (HA) and can interact with ligands such as
osteopontin, collagens and matrix metalloproteinases (29–31).
It is involved in cellular functions, including cell-cell
interactions, cell adhesion and cell migration (31). Upregulation of CD44 may induce
signaling events that promote anchorage-independent tumor-cell
growth, survival and migration, thereby increasing metastatic
spread (32,33). Previous studies have reported that
CD44 is expressed in PAs (34) and
that its expression levels are significantly upregulated in PAs
(35). However, CD44 is also an
ECM receptor, which has a crucial role in tumorigenesis. It has
been reported that pituitary tumorigenesis involves remodeling of
the ECM (36). The CD44-ECM
interaction can contribute to malignant transformation in an
indirect manner, for instance by regulating sensitivity to
inflammation (37). The cleavage
of CD44 and ECM can promote cell migration of pituitary adenoma
(38); therefore, CD44 may induce
signaling events and interact with the ECM to promote the
tumorigenesis of pituitary adenoma.
Gli2, which has a key role in pituitary development,
belongs to the C2H2-type zinc finger protein sub-class of the Gli
family. The members of the Gli family are mediators of the Sonic
hedgehog (Shh) signaling pathway. A previous study has shown that
loss-of-function mutations in the human Gli2 gene are
associated with PAs (39).
Furthermore, Devine et al (40) indicated that dysregulation of
Gli2 function may contribute to PAs. Hh signaling is
necessary for the induction and functional patterning of the
pituitary placode (40). The
pathway analysis of the present study showed that Gli2 is an
important member of this pathway. A previous study showed that
downregulation of Shh, which is a member of the Hh family,
increases the proliferation of PA cells and may be involved in the
pathogenesis of PAs (41).
Gli2 is the most important mediator of Shh signaling in
vertebrates (42). It is required
for repressing Shh gene expression posteriorly in the pars
intermedia (40). Accordingly,
Gli2 takes part in the development of PAs through the Hh
signaling pathway.
In addition, the present study discovered candidate
small molecules which may be implicated the development of PAs
(positive cMAP enrichment factor) or which may be suitable drugs
for the treatment of PAs (negative cMAP enrichment factor).
Depudecin was identified to be small molecule drug with the most
significant reverse mechanistic association with PAs. Depudecin is
a fungal metabolite containing two epoxide groups (43). It was reported to have
anti-angiogenic activity (44),
regulate the assembly of the actin microfilament components of the
cytoskeleton in mammalian cells (45) and to induces morphological
reversion of transformed fibroblasts (46). Depudecin has been patented as a
histone deacetylase inhibitor for the treatment of neuroendocrine
tumors (47). The present study
indicated a close reverse mechanistic association of depudecin with
PAs, therefore suggesting that it may be a suitable drug for its
treatment; however, the efficacy of depudecin against PAs as well
as its mechanism of action remain to be elucidated in future
studies.
In conclusion, the present study identified enriched
pathways in PAs, generated a PPI network and virtually screened
candidate small molecules associated with PAs. The genes CD44 and
Gli2, which are involved in ECM-receptor interactions and Hh
signaling, were shown to have significant roles in the development
of PAs. In addition, depudecin may be a candidate drug for treating
PAs. The present study provided a systematic perspective to
elucidate the underlying mechanism of PAs, including molecular
targets for their treatment. However, the present study was
performed using bioinformatics methods and the conclusions remain
to be confirmed by corresponding experiments. Therefore, further
study is required to verify the underlying mechanisms of metastatic
PAs.
References
|
1
|
Karavitaki N: Prevalence and incidence of
pituitary adenomas. Ann Endocrinol (Paris). 73:79–80. 2012.
View Article : Google Scholar
|
|
2
|
Aflorei ED and Korbonits M: Epidemiology
and etiopathogenesis of pituitary adenomas. J Neurooncol.
117:379–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kokshoorn NE, Romijn JA, Roelfsema F,
Rambach AH, Smit JW, Biermasz NR and Pereira AM: The use of an
early postoperative CRH test to assess adrenal function after
transsphenoidal surgery for pituitary adenomas. Pituitary.
15:436–444. 2012. View Article : Google Scholar :
|
|
4
|
Feelders RA, de Bruin C, Pereira AM,
Romijn JA, Netea-Maier RT, Hermus AR, Zelissen PM, van Heerebeek R,
de Jong FH, van der Lely AJ, et al: Pasireotide alone or with
cabergoline and ketoconazole in Cushing's disease. N Engl J Med.
362:1846–1848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biller BM, Grossman AB, Stewart PM, Melmed
S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR,
Hofland LJ, et al: Treatment of adrenocorticotropin-dependent
Cushing's syndrome: A consensus statement. J Clin Endocrinol Metab.
93:2454–2462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newell-Price J, Bertagna X, Grossman AB
and Nieman LK: Cushing's syndrome. Lancet. 367:1605–1617. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tritos NA, Biller BM and Swearingen B:
Management of Cushing disease. Nat Rev Endocrinol. 7:279–289. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong J, Diao B, Yao GJ, Liu Y and Xu GZ:
Analysis of regulatory networks constructed based on gene
coexpression in pituitary adenoma. J Genet. 92:489–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lampichler K, Ferrer P, Vila G, et al: The
role of GLI1 in pituitary tumor formation and pituitary cell
survival. 2013.
|
|
10
|
Cazabat L, Bouligand J and Chanson P: AIP
mutation in pituitary adenomas. N Engl J Med. 364:1973–1974. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riester A, Issler O, Spyroglou A, Rodrig
SH, Chen A and Beuschlein F: ACTH-dependent regulation of microRNA
as endogenous modulators of glucocorticoid receptor expression in
the adrenal gland. Endocrinology. 153:212–222. 2012. View Article : Google Scholar
|
|
12
|
Vierimaa O, Georgitsi M, Lehtonen R,
Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM,
Salmela PI, Paschke R, et al: Pituitary adenoma predisposition
caused by germline mutations in the AIP gene. Science.
312:1228–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article3. 2004.
|
|
15
|
BRIDGES JRCC: Hierarchical cluster
analysis. Psychological reports. 18:851–854. 1966. View Article : Google Scholar
|
|
16
|
Team RC: R: A language and environment for
statistical computing. 2012.
|
|
17
|
Hulsegge I, Kommadath A and Smits MA:
Globaltest and GOEAST: Two different approaches for gene ontology
analysis. BMC Proc. 3(Suppl 4): S102009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altermann E and Klaenhammer TR:
PathwayVoyager: Pathway mapping using the kyoto encyclopedia of
genes and genomes (KEGG) database. BMC Genomics. 6:602005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
An integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33:W741–W748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duncan D, Prodduturi N and Zhang B:
WebGestalt2: An updated and expanded version of the Web-based Gene
Set Analysis Toolkit. Bmc Bioinformatics. 11(Suppl 4): P102010.
View Article : Google Scholar
|
|
22
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–D568. 2011. View Article : Google Scholar :
|
|
23
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar
|
|
24
|
Lamb J: The Connectivity Map: A new tool
for biomedical research. Nat Rev Cancer. 7:54–60. 2007. View Article : Google Scholar
|
|
25
|
Lamb J, Crawford ED, Peck D, Modell JW,
Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, et
al: The Connectivity Map: Using gene-expression signatures to
connect small molecules, genes and disease. Science. 313:1929–1935.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vierimaa O, Georgitsi M, Lehtonen R,
Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM,
Salmela PI, Paschke R, et al: Pituitary adenoma predisposition
caused by germline mutations in the AIP gene. Science.
312:1228–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagano O and Saya H: Mechanism and
biological significance of CD44 cleavage. Cancer Sci. 95:930–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsukita S, Oishi K, Sato N, Sagara J,
Kawai A and Tsukita S: ERM family members as molecular linkers
between the cell surface glycoprotein CD44 and actin-based
cytoskeletons. J Cell Biol. 126:391–401. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lesley J and Hyman R: CD44 can be
activated to function as an hyaluronic acid receptor in normal
murine T cells. Eur J Immunol. 22:2719–2723. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weber GF, Ashkar S, Glimcher MJ and Cantor
H: Receptor-ligand interaction between CD44 and osteopontin
(Eta-1). Science. 271:509–512. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toole BP: Hyaluronan: From extracellular
glue to pericellular cue. Nat Rev Cancer. 4:528–539. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaracz S, Chen J, Kuznetsova LV and Ojima
I: Recent advances in tumor-targeting anticancer drug conjugates.
Bioorg Med Chem. 13:5043–5054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frank S, Rihs H-P, Stöcker W, Müller J,
Dumont B, Baur X, Schackert HK and Schackert G: Combined detection
of CD44 isoforms by exon-specific RT-PCR and immunohistochemistry
in primary human brain tumors and brain metastases. Biochem Biophys
Res Commun. 222:794–801. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan Bo ZH and Li Xinjian: Expression and
relationship between CD44 and Ki-67 in invasive pituitary adenomas.
Cancer Res Prev Treat. 33:490–492. 2006.
|
|
36
|
Rubinfeld H, Cohen-Kaplan V, Nass D, Ilan
N, Meisel S, Cohen ZR, Hadani M, Vlodavsky I and Shimon I:
Heparanase is highly expressed and regulates proliferation in
GH-secreting pituitary tumor cells. Endocrinology. 152:4562–4570.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan Y, Han C, Wang C, Hu G, Luo C, Gan X,
Zhang F, Lu Y and Ding X: ADAM10 promotes pituitary adenoma cell
migration by regulating cleavage of CD44 and L1. J Mol Endocrinol.
49:21–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roessler E, Du YZ, Mullor JL, Casas E,
Allen WP, Gillessen-Kaesbach G, Roeder ER, Ming JE, Ruiz i Altaba A
and Muenke M: Loss-of-function mutations in the human GLI2 gene are
associated with pituitary anomalies and holoprosencephaly-like
features. Proc Natl Acad Sci USA. 100:13424–13429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Devine CA, Sbrogna JL, Guner B, Osgood M,
Shen MC and Karlstrom RO: A dynamic Gli code interprets Hh signals
to regulate induction, patterning and endocrine cell specification
in the zebrafish pituitary. Dev Biol. 326:143–154. 2009. View Article : Google Scholar
|
|
41
|
Vila G, Theodoropoulou M, Stalla J, Tonn
JC, Losa M, Renner U, Stalla GK and Paez-Pereda M: Expression and
function of sonic hedgehog pathway components in pituitary
adenomas: Evidence for a direct role in hormone secretion and cell
proliferation. J Clin Endocrinol Metab. 90:6687–6694. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ruiz i Altaba A, Palma V and Dahmane N:
Hedgehog-Gli signalling and the growth of the brain. Nat Rev
Neurosci. 3:24–33. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsumoto M, Matsutani S, Sugita K,
Yoshida H, Hayashi F, Terui Y, Nakai H, Uotani N, Kawamura Y and
Matsumoto K: Depudecin: A novel compound inducing the flat
phenotype of NIH3T3 cells doubly transformed by ras-and
src-oncogene, produced by Alternaria brassicicola. J Antibiot
(Tokyo). 45:879–885. 1992. View Article : Google Scholar
|
|
44
|
Oikawa T, Onozawa C, Inose M and Sasaki M:
Depudecin, a microbial metabolite containing two epoxide groups,
exhibits anti-angiogenic activity in vivo. Biol Pharm Bull.
18:1305–1307. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shimada J, Kwon HJ, Sawamura M and
Schreiber SL: Synthesis and cellular characterization of the
detransformation agent, (−)-depudecin. Chem Biol. 2:517–525. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwon HJ, Owa T, Hassig CA, Shimada J and
Schreiber SL: Depudecin induces morphological reversion of
transformed fibroblasts via the inhibition of histone deacetylase.
Proc Natl Acad Sci USA. 95:3356–3361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen H and Kunnimalaiyaan M: Modulating
notch1 signaling pathway for treating neuroendocrine tumors. US
Patent 8338482 B2. Filed July 20, 2007; issued December 25,
2012.
|