Introduction
Diabetes mellitus (DM) is a type of chronic
metabolic disease involving the interaction of genetic and
environmental factors. It is characterized by dysinsulinism,
insulin resistance (IR) and disorders of the blood sugar, lipid and
protein metabolisms (1). An
increase in the prevalence rate of DM in China is reported to be
due to the economic development and increase in the average life
expectancy (2). The first national
census of DM demonstrated that the prevalence rate in China was 1%
in 1979 and 2.02% in 1989 (3). In
addition, the prevalence rate of type-I DM was 0.00057% and type-II
DM was 3.21% in 1996 (4). At
present, there are 40 million patients with DM in China (4), however, in developed cities, such as
Shanghai, the prevalence rate of DM may be as high as 10% (5). The number of obese individuals,
particularly those suffering from abdominal obesity, has increased
due to the change of living standards, resulting from the increased
levels of sugar in their diet, smoking and alcohol consumption.
Therefore, the higher prevalence rate of DM observed may be due to
abdominal obesity, as it had been reported to be the basis of IR
(6).
DM is characterized by chronic complications
resulting in pain for the patients and a financial burden to their
families (7). Diabetic
cardiovascular disease (DCD) is one of the leading causes of death
in patients with DM. The cardiovascular complications may occur at
an early stage in DM and have a progressive development (8). The risk of stroke in patients with DM
is 2 to 4 times higher compared with the risk of patients with
cardiovascular diseases and without DM (7,8).
Furthermore, patients suffering from DM and myocardial infarction
have reduced cardiac output compared with patients with myocardial
infarction and without DM (7).
Thymoquinone is the main active monomer extracted
from black cumin in Middle Eastern countries (9). A previous study demonstrated that
thymoquinone acts as a tumor suppressor and inhibits the growth of
various types of cancer cells (10). The aim of the present study was to
identify whether the protective effect of thymoquinone improves
cardiovascular function in diabetic rats and to explore its
possible mechanisms.
Materials and methods
Reagents
Streptozotocin (STZ) and thymoquinone (purity
>98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Malondialdehyde (MDA), superoxide dismutase (SOD), cyclooxygenase
(COX)-2, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)
ELISA kits and caspase-3 activity kits were purchased from
Jiancheng Biotech Co., Ltd. (Nanjing, China).
Animals
A total of 40 healthy male albino Wistar rats (6–8
weeks old; 230–300 g) were purchased from the Animal Experiment
Center of Dalian Medical University (Dalian, China) and maintained
under standard conditions (22±2°C with a 12-h dark-light cycle) and
experiments were conducted in accordance with the Guide for the
Care and Use of Laboratory Animals adopted by the Institutional
Animal Care and Use Committee (11). All rats were fed with regular chow
and given free access to water.
Model and grouping
All rats were randomly assigned into four groups
(n=10): Sham group (Sham), thymoquinone group (THY), DM group (DM)
and DM + thymoquinone group (DM + THY). STZ was dissolved in
citrate buffer (Beijing Tiandz Biological Technology, Inc.,
Beijing, China; 60 mg/kg of body weight, pH 4.5) and DM was induced
by intravenous injection of STZ into the tail vein of the rats. The
sham group was injected with the same volume of citrate buffer
only, and the thymoquinone group was injected with 50 mg/kg
thymoquinone for 30 days. The DM and DM + THY groups were injected
with STZ + citrate buffer to induce DM. The DM group was then
treated with citrate buffer only and the DM + THY group was
injected with 50 mg/kg thymoquinone for 30 days.
Biochemical and body weight
measurements
Body weight was determined for all groups prior and
subsequent to DM-induction and treatments. Blood samples were
collected from the tail vein of rats and glucose levels were
measured using the glucose oxidase method (Bpc BioSed Srl, Capena,
Italy). A Glucose Oxidase ELISA kit (Elabscience Biotechnology Co.,
Ltd., Wuhan, China) and Insulin ELISA kit (Elabscience
Biotechnology Co., Ltd.) were used to determine insulin levels.
Insulin levels were measured using the Multiskan EX microplate
photometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Tail-cuff blood pressure (BP)
measurement
The CODA 8-channel high throughput non-invasive tail
cuff blood pressure system (Kent Scientific Corporation,
Torrington, CT, USA) was used to measure the BP of rats by
determining the tail blood volume. All rats were placed into a
designated platform maintained at 37°C and the system provided
measurements of four BP parameters: Systolic, diastolic and mean BP
and heart rate for 10 min. The proximal occlusion cuff constricts
the tail artery, while the distal cuff detects alterations in the
tail artery volume when the blood flow resumes following the
deflation of the occlusion cuff. Measurements of an average of
three sessions, each consisting of 15 cycles, were used for
statistical analysis.
Determination of oxidative stress, COX-2
and inflammation
Following thymoquinone treatment, blood was
collected from the tail vein and centrifuged at 8,000 × g, for 10
min at room temperature. The serum was used to measure the MDA,
SOD, COX-2, TNF-α and IL-6 levels in accordance with the
manufacturer's instructions (Jiancheng Biotech Co., Ltd.).
Determination of caspase-3 activity
Following thymoquinone treatment, rats were
sacrificed by decollation under anesthesia (chloral hydrate;
Sigma-Aldrich) and cardiac tissue specimens were collected and
prepared in lysis buffer (Beijing Applygen Gene Technology Co.,
Ltd., Beijing, China). Samples were then centrifuged at 8,000 × g
for 10 min at 4°C and protein concentration was determined with the
BCA protein assay kit (Bio-Rad Laboratories, Ltd., Hemel Hempstead,
UK) according to the manufacturer's instructions. The caspase-3
activity was blended by chromogenic caspase substrates and
Ac-DEVD-pNA according to the manufacturer's instructions (Jiancheng
Biotech Co., Ltd.), and measured at 405 nm with a Multiskan EX
microplate photometer (Thermo Fisher Scientific, Inc.).
Western blot analysis
Following thymoquinone treatment, protein
concentrations were determined as described above using the BCA
protein assay kit (Bio-Rad Laboratories, Ltd.). Proteins (50 mg)
were separated by sodium dodecyl sulfate polyacrylamide gel (100 v;
Beijing Tiandz Biological Technology, Inc.) electrophoresis in a
8–12% polyacrylamide gel, and transferred to polyvinyl difluoride
membranes (Millipore, Billerica, MA, USA). Following blocking with
5% non-fat milk, membranes were incubated overnight with monoclonal
rabbit anti-endothelial nitric oxide synthase (eNOS; cat. no. 9572;
1:500, Cell Signaling Technology, Inc., Danvers, MA, USA),
monoclonal rabbit anti-phosphorylated-protein kinase B (p-Akt; cat.
no. 9271; 1:500; Cell Signaling Technology, Inc.) and monoclonal
β-actin (cat. no. sc-1616; 1:500; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) primary antibodies. Membranes were then
incubated with horseradish peroxidase-conjugated goat anti-biotin
secondary antibody (cat. no. 7075; 1:500; Cell Signaling
Technology, Inc.) for 2 h at room temperature, and proteins were
visualized using Hybond enhanced chemiluminescence (Amersham
International; GE Healthcare Life Sciences, Chalfont, UK). Protein
expression was quantified using ImageJ software (version 1,37;
National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using analysis of variance,
followed by a post hoc Newman-Keuls test (InStat software version
3.06; Graphpad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
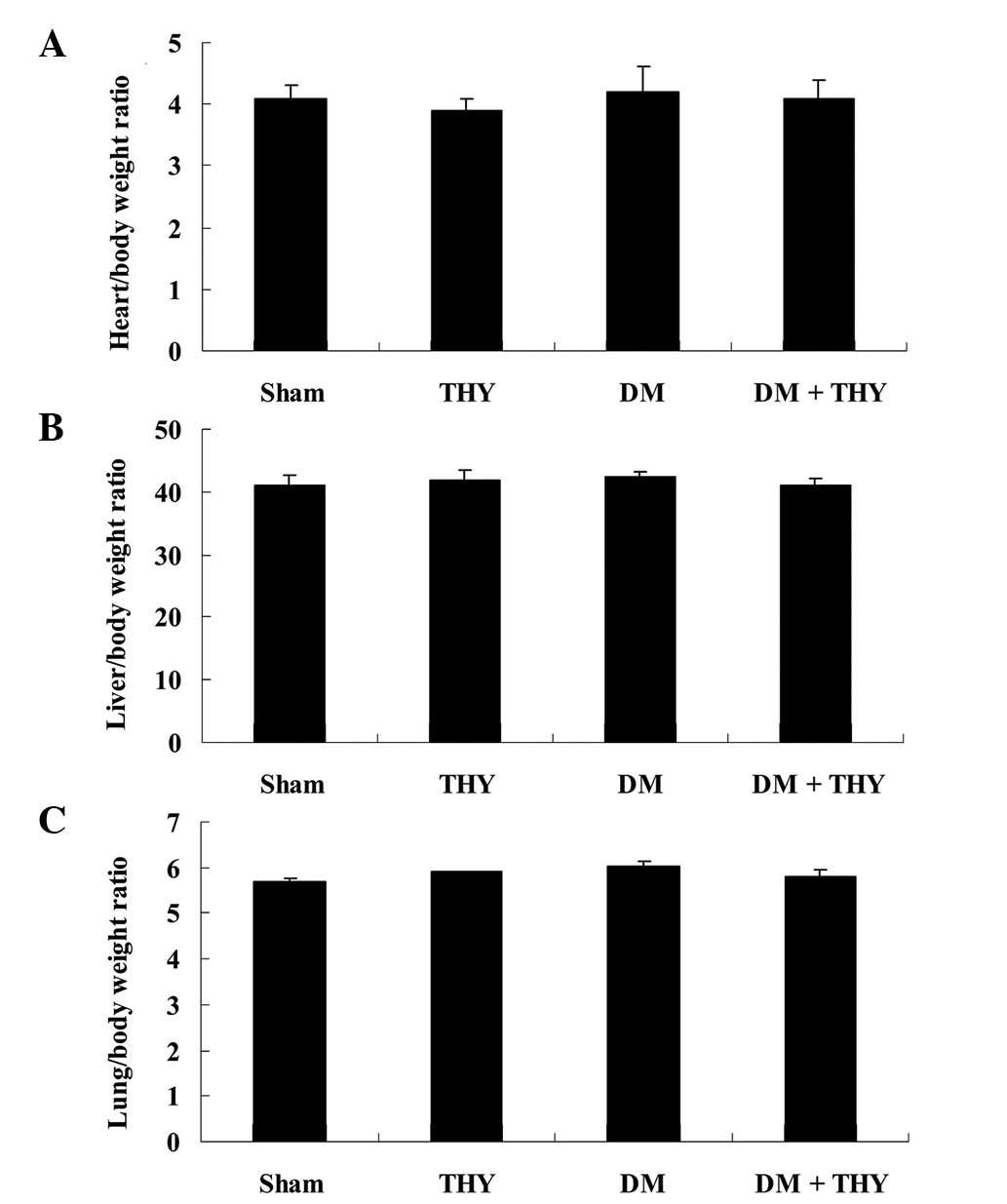
Effect of thymoquinone treatment on the
ratios of heart, liver and lung to body weight
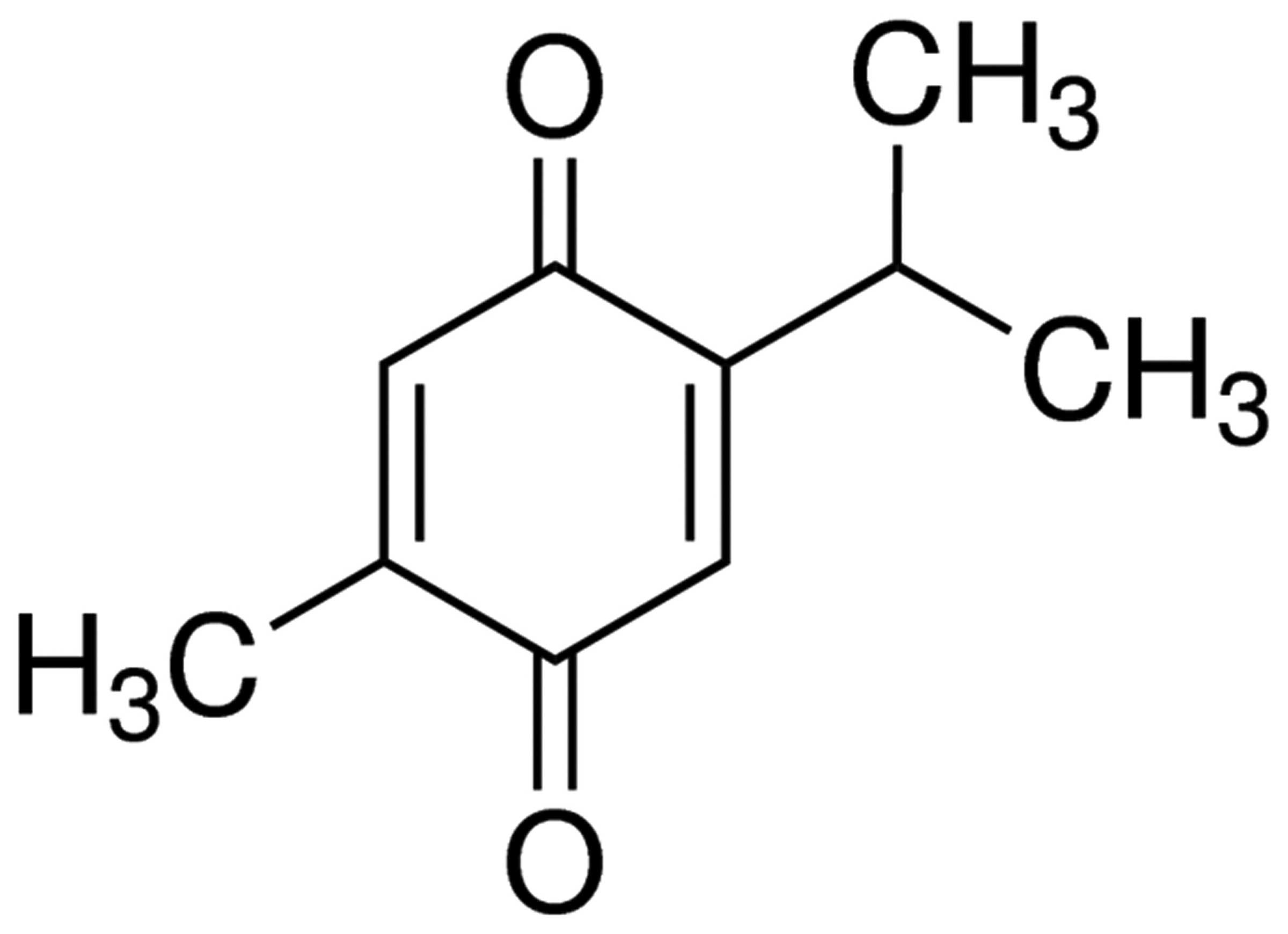
The chemical structure of thymoquinone is presented
in Fig. 1. As indicated in
Fig. 2, the ratios of heart, liver
and lung to body weight were not significantly different among the
experimental groups.
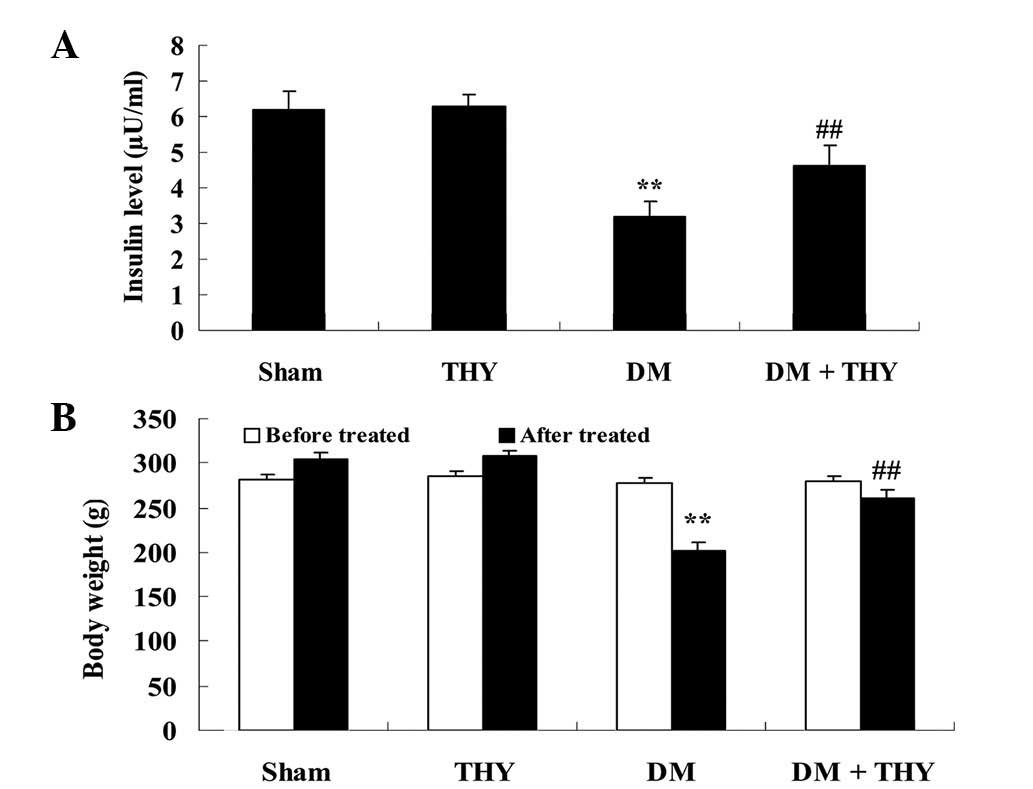
Effect of thymoquinone treatment on the
insulin levels and body weight
As demonstrated in Fig.
3, the insulin levels and body weight in the DM-induced group
were lower compared with the sham (P<0.01) and THY-treated
groups. In addition, administration of thymoquinone resulted in an
increase in the insulin levels and body weight of the DM +
THY-treated group compared with the DM-induced group (P<0.01;
Fig. 3).
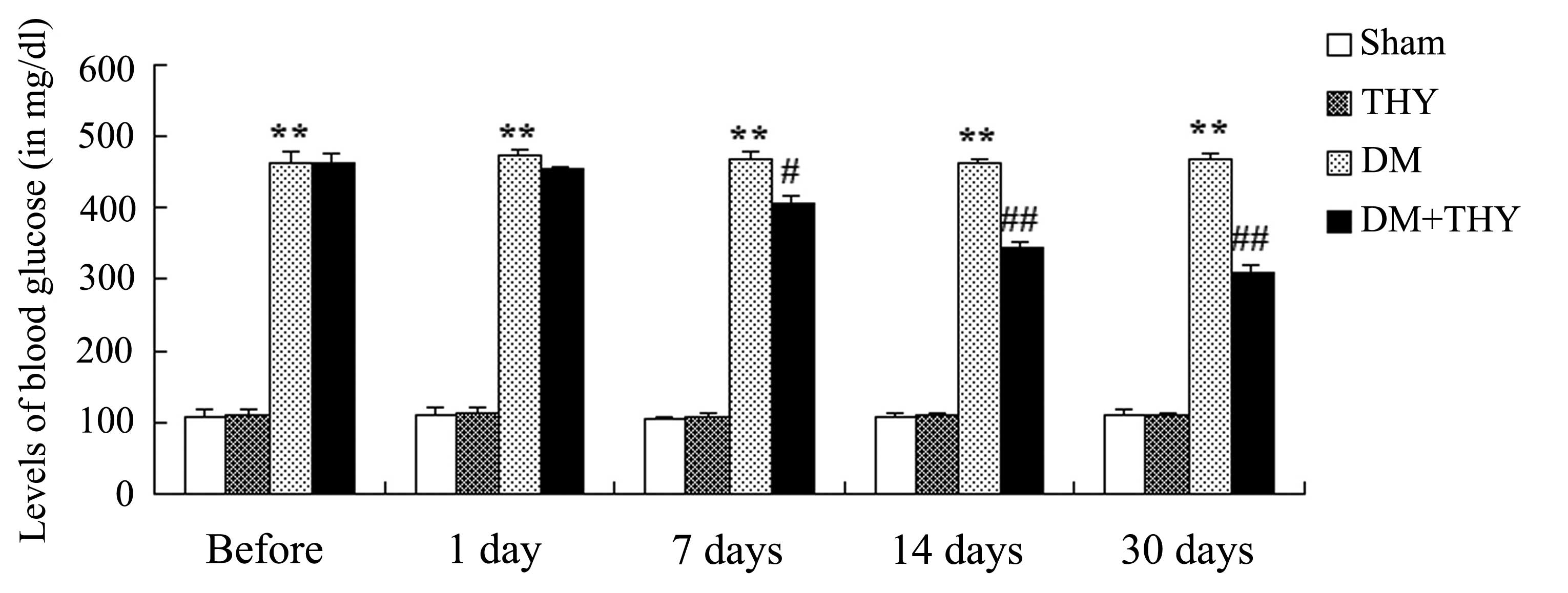
Effect of thymoquinone treatment on the
blood glucose levels
As demonstrated in Fig.
4, the levels of blood glucose were significantly increased in
the DM-induced groups compared with the sham (P<0.01) and
THY-treated groups during the 30-day experiment. Administration of
thymoquinone resulted in a reduction in the blood glucose levels of
the DM-induced rats compared with the DM rats that had not received
the treatment (P<0.01; Fig.
4).
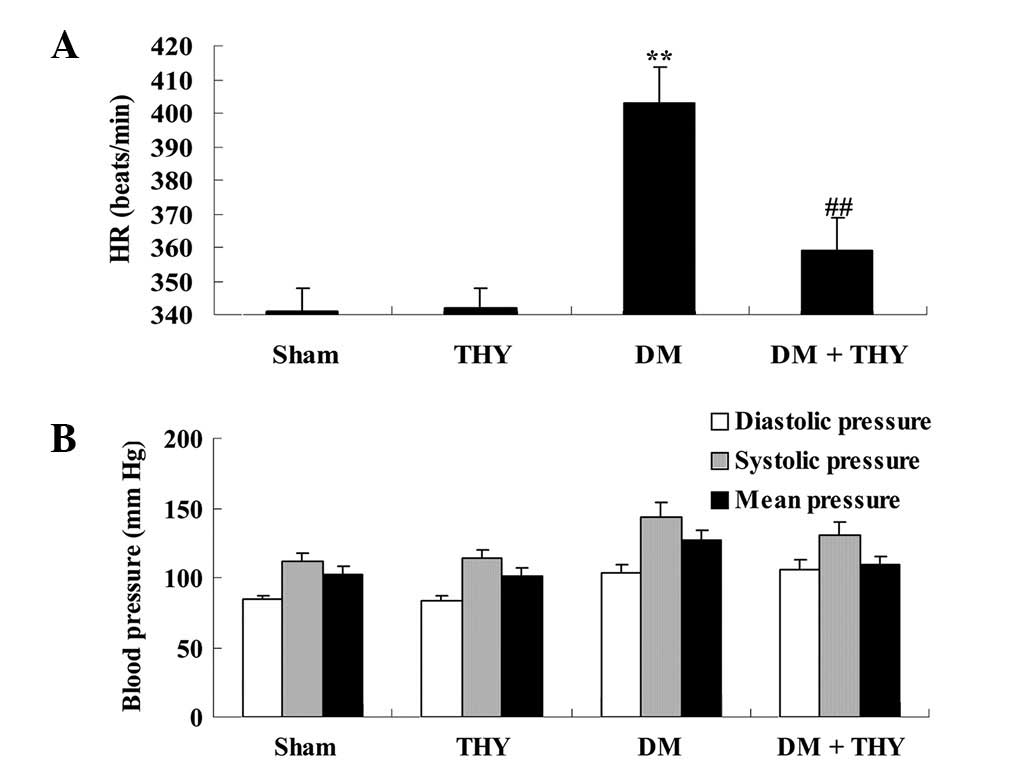
Effect of thymoquinone treatment on BP
and heart rate
A significant increase in the heart rate of
DM-induced rats was observed compared with the sham (P<0.01) and
THY-treated groups (Fig. 5A).
Following treatment with thymoquinone, the heart rate levels of the
DM + THY group were significantly reduced compared with the DM
group (P<0.01; Fig. 5A).
However, BP levels did not exhibit a significant difference among
the experimental groups (Fig.
5B).
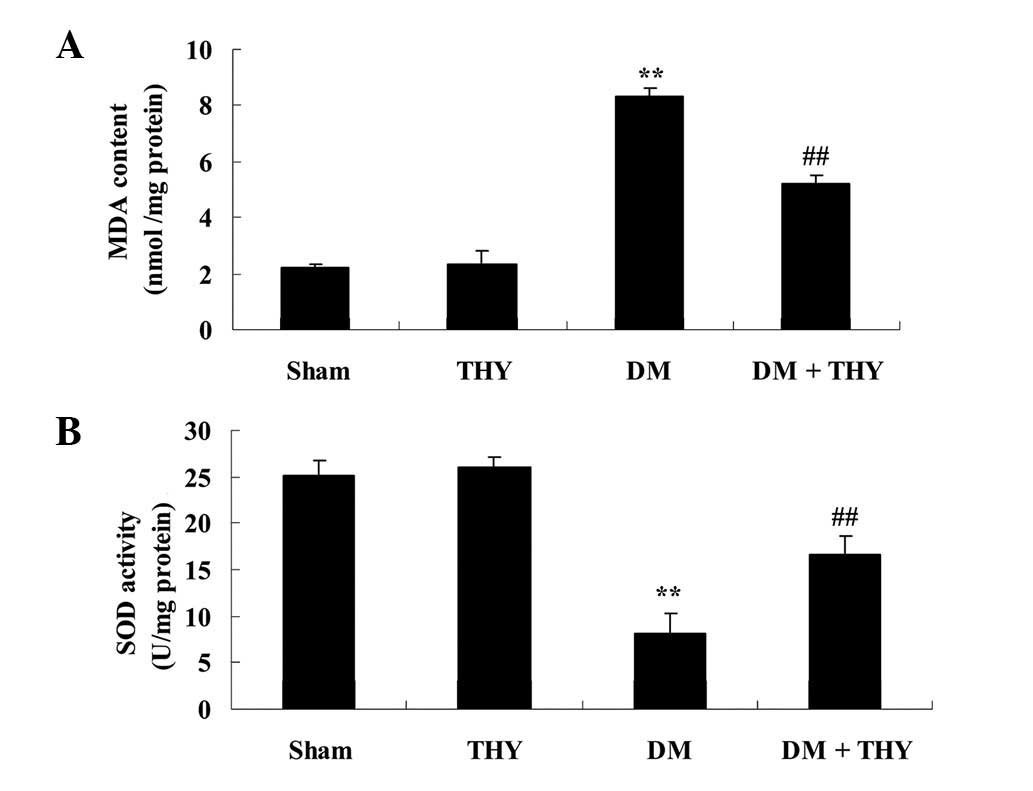
Effect of thymoquinone treatment on
oxidative stress levels
Following DM-induction in rats, MDA levels were
significantly increased and SOD levels were reduced compared with
the sham (P<0.01; Fig. 6) and
THY-treated groups (Fig. 6).
Furthermore, administration of thymoquinone significantly reduced
the oxidative stress damage in the DM rats, compared with the DM
group without the treatment (P<0.01; Fig. 6).
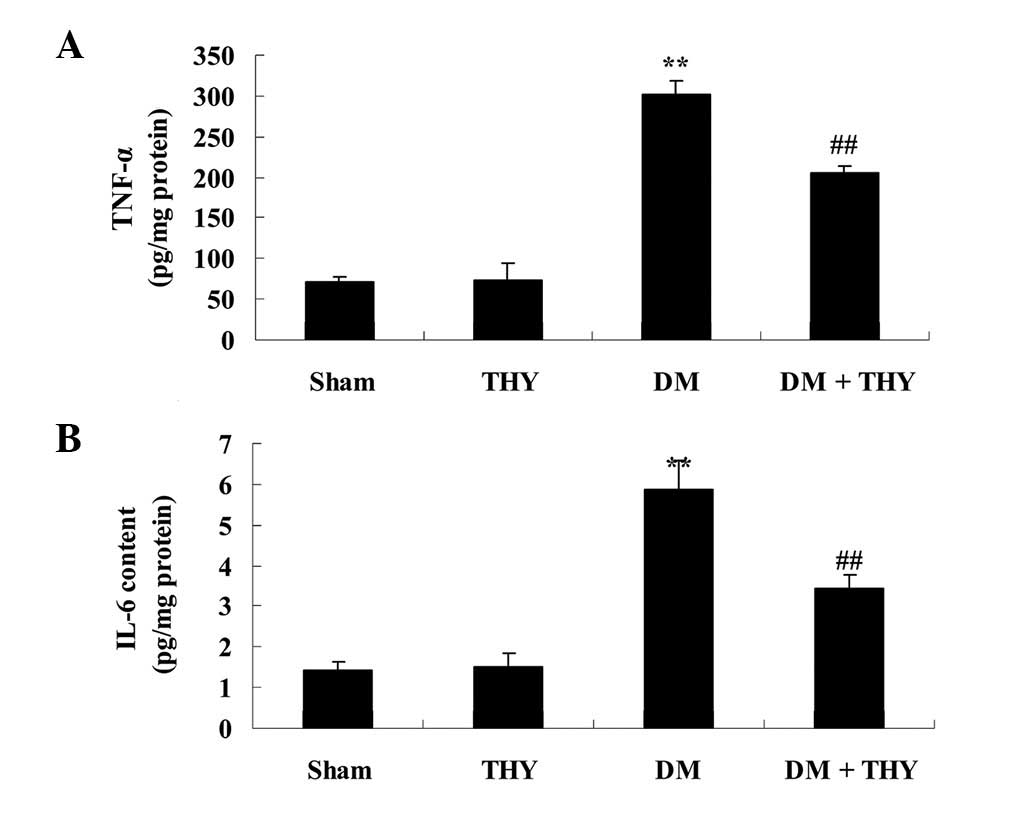
Effect of thymoquinone treatment on TNF-α
and IL-6 inflammatory factors
As demonstrated in Fig.
7, TNF-α and IL-6 levels were significantly increased compared
with the sham (P<0.01) and THY-treated groups. In addition, the
levels of TNF-α and IL-6 in the DM-induced rats were observed to
return to normal levels following treatment with thymoquinone,
compared with the DM group (P<0.01; Fig. 7).
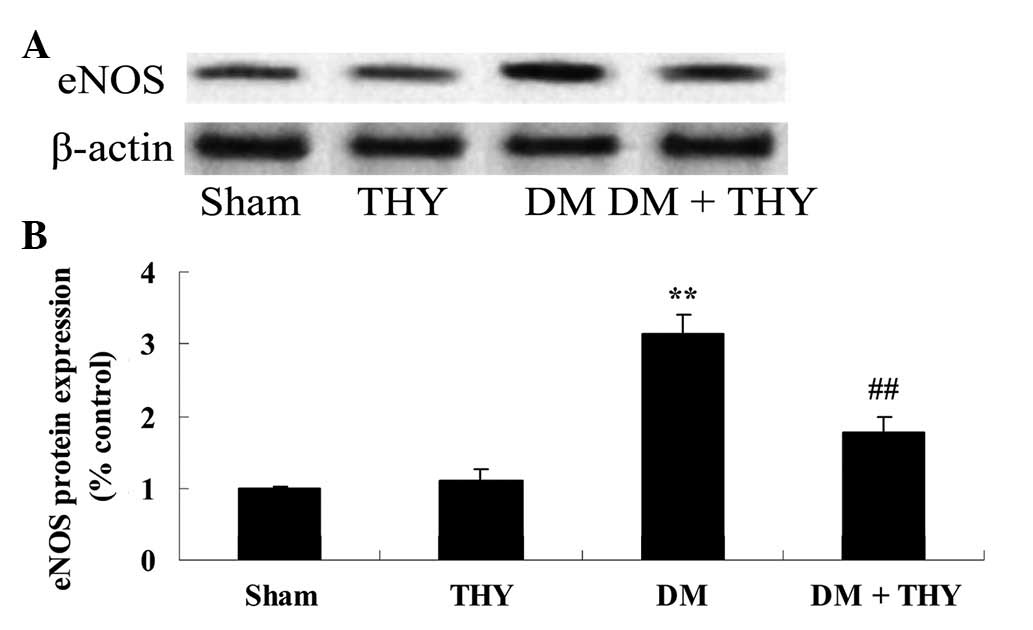
Effect of thymoquinone treatment on the
eNOS protein expression levels
As demonstrated in Fig.
8, the eNOS protein expression levels were significantly
increased in the DM group compared with the sham (P<0.01) and
thymoquinone groups. In contrast, the increase of the eNOS protein
expression levels was inhibited by treatment with thymoquinone in
the DM + THY-treated group compared with the DM group (P<0.01;
Fig. 8).
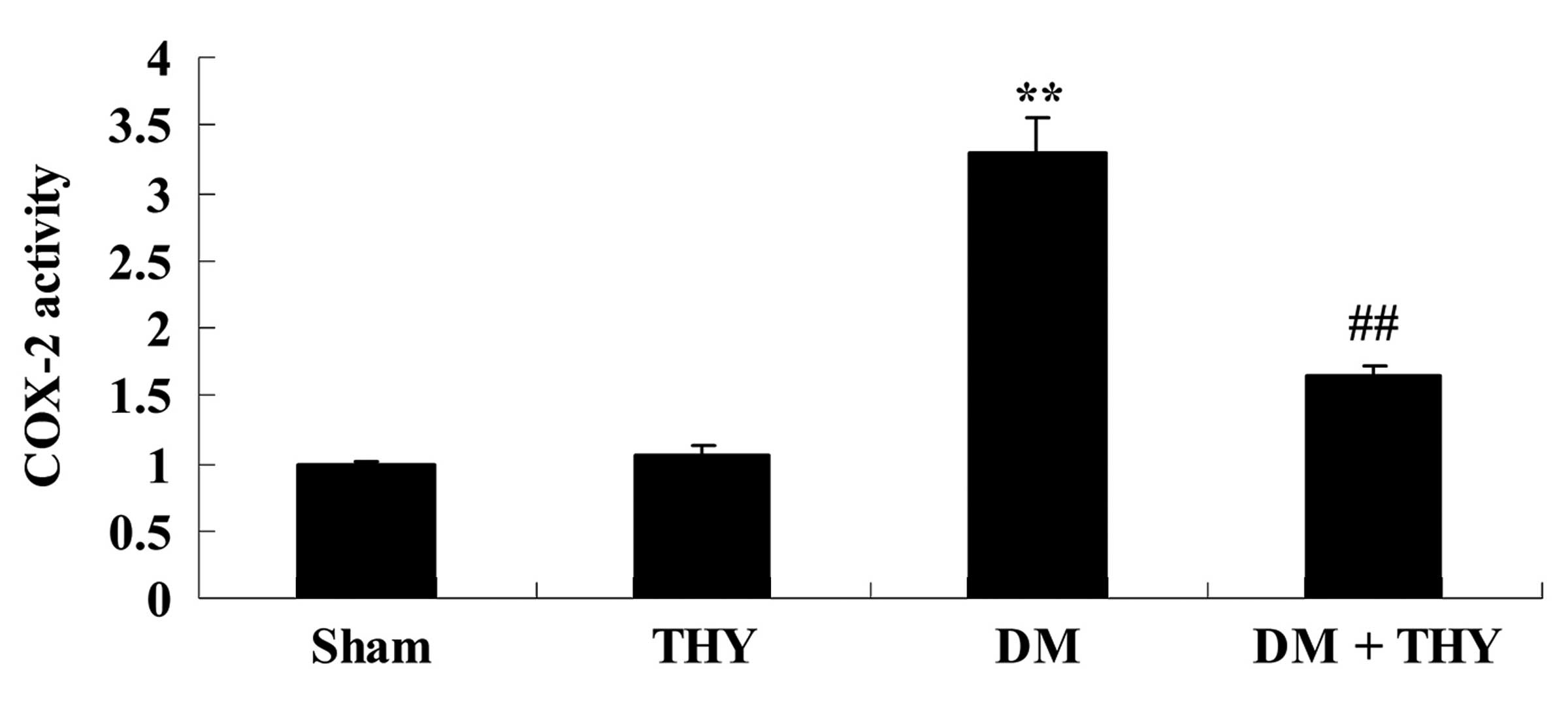
Effect of thymoquinone treatment on COX-2
activity
As demonstrated in Fig.
9, the COX-2 levels in the DM group were markedly increased
compared with the sham (P<0.01) and THY-treated groups. However,
administration of thymoquinone in the DM + THY group indicated an
inhibition of this effect compared with the DM group (P<0.01;
Fig. 9).
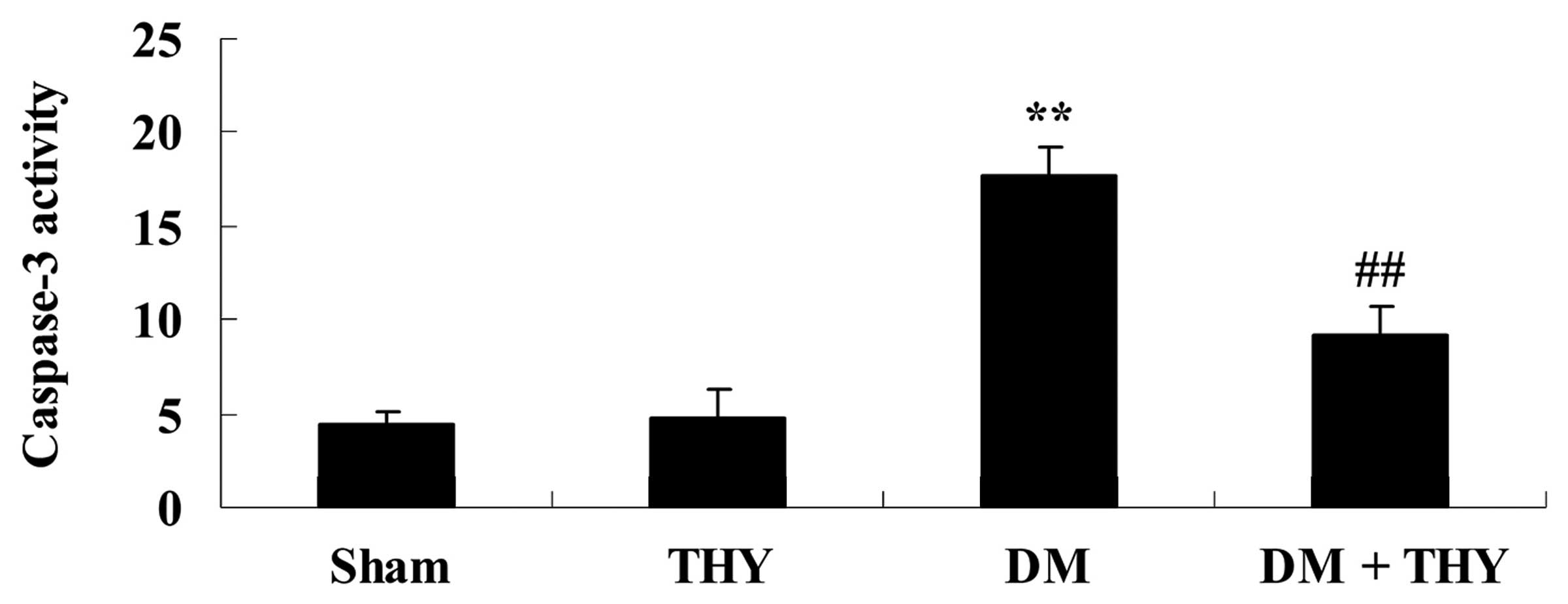
Effect of thymoquinone treatment on
caspase-3 activity
As demonstrated in Fig. 10, DM-induction increased caspase-3
activity compared with the sham (P<0.01) and THY-treated groups.
In contrast, thymoquinone treatment resulted in a significant
reduction in caspase-3 activity in the DM + THY group compared with
the DM group (P<0.01; Fig.
10).
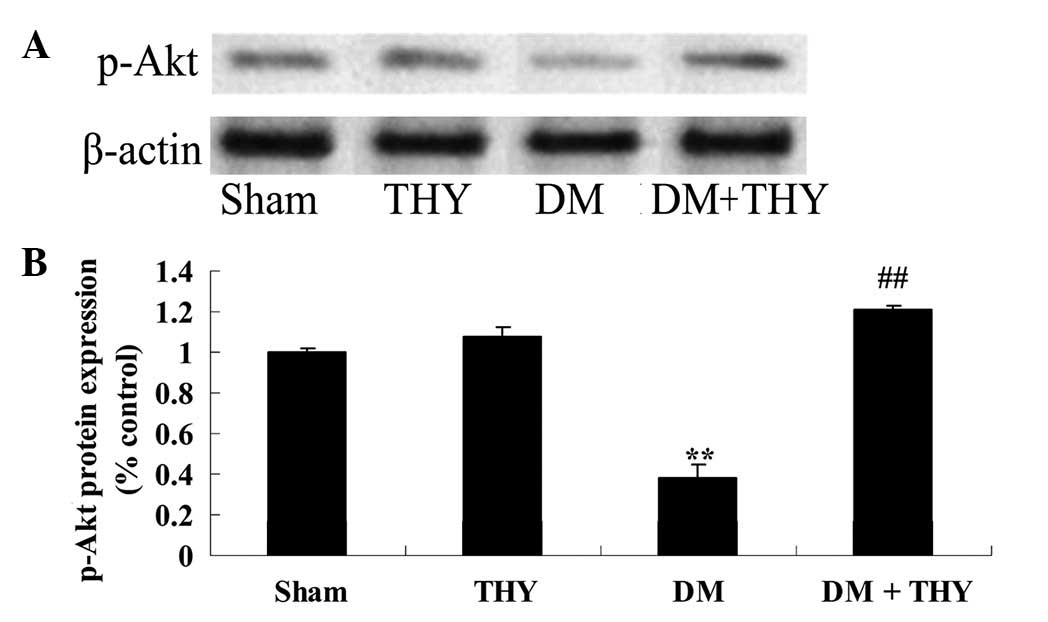
Effect of thymoquinone treatment on the
p-Akt protein expression levels
A significant reduction in the p-Akt protein
expression levels of DM rats was observed compared with the sham
(P<0.01; Fig. 11) and
THY-treated groups (Fig. 11).
Similarly, the reduction in the p-Akt levels was significantly
suppressed by administration of thymoquinone in the DM + THY group
compared with the DM group (P<0.01; Fig. 11).
Discussion
DM is a common and frequently occurring disease of
the endocrine system resulting from the combined effect of several
factors and characterized by alterations in the glucose metabolism
(12). DCD is considered the main
cause of mortality and disability in patients with DM and results
in alterations in the pathology of multiple organs, including the
kidney, eyes, heart and skin (13). The prevalence rate of DCD in
patients with DM is 40% in the USA and Europe, which is 17 times
higher than in patients without DM (14). The prevalence rate of DCD in China
increases in line with age (15),
and the prevalence is >50% in individuals over 60 years old
(16). The present study
demonstrated that thymoquinone treatment significantly increased
the insulin levels and body weight, and reduced the blood glucose
and heart rate levels in DM rats. El-Mahmoudy et al
(17) indicated that thymoquinone
protects against the STZ-induced DM via inhibition of nitric oxide.
Furthermore, Pari and Sankaranarayanan (18) demonstrated that thymoquinone
protects against hepatic dysfunction in STZ-nicotinamide-induced DM
rats. The results of the present study were consistent with those
of the previous studies, thus, thymoquinone may be an improved
agent for DCD therapy.
Oxidative stress refers to the tissue damage
resulting from the disequilibrium of the overproduction of reactive
oxygen species (ROS) and the inhibition of the antioxidant defense
mechanisms (19). A previous study
suggested that the circulating levels of markers may increase due
to ROS damage and the inability to prevent this effect (19). Patients with DM suffer from lipid
disorders that lead to the production of ROS and oxidative stress
(19,20). The activation of glucose-induced
eNOS may lead to endothelial dysfunction due to the production of
oxidative stress (21). In the
current study, pretreatment with thymoquinone significantly
inhibited the MDA levels and increased SOD activity in DM rats.
Farag et al (22)
demonstrated that thymoquinone improves the disease pathology in
injured kidney and liver tissue by resetting the
oxidant/antioxidant balance of the affected organs in rats with
acute renal ischemia/reperfusion. Mabrouk and Ben Cheikh (23) suggested that thymoquinone
supplementation reversed lead-induced oxidative stress in adult rat
testes. These results indicate that the effect of thymoquinone on
DCD may be associated with the anti-oxidative effect observed in
the DM rats of the current study.
IR and insulin secretory defects further contribute
to the pathogenesis of DM, however the mechanisms underlying this
remain to be fully understood (24). A previous study demonstrated that
various inflammatory factors are associated with DM, such as
C-reactive protein (CRP), thus, inflammation may be the key
initiator of IR (25). Cytokines
are regulators of the adipose tissue metabolism and stimulating
factors, such as overnutrition, may lead to an increase in the
secretion of IL-6 and TNF-α by the adipocytes. This may further
lead to an increase in the hepatic synthesis and secretion of CRP
(26). Furthermore, inhibition of
insulin receptor tyrosine kinase activity and the aggravation of IR
result in the production of macrophage migration inhibitory factor
(MIF) (24). MIF can induce the
production of TNF-α, and their interaction may affect the balance
of insulin levels, and lead to IR (24). Previous studies suggested that
insulin may inhibit the synthesis of CRP, as in the case of IR, the
sensitivity of the liver to insulin is suppressed, leading to a
rapid increase in the synthesis of CRP (24,25).
At present, evidence indicates that chronic inflammation serves an
important role in the pathogenesis of IR, however the mechanism
remains to be elucidated (24).
In the current study, thymoquinone treatment
significantly suppressed the TNF-α and IL-6 levels in DM rats.
Periyanayagam et al (27)
observed that thymoquinone ameliorates NLRP3-induced inflammation
in albino Wistar rats administered with ethanol and fed a high-fat
diet. Rifaioglu et al (28)
demonstrated that thymoquinone has an antioxidative and
anti-inflammatory effect in an acute pseudomonas prostatitis rat
model. These results indicated that the effect of thymoquinone on
DCD may be associated with an anti-inflammatory effect in DM
rat.
Endothelial progenitor cells (EPCs) are a type of
precursors that differentiate into vascular endothelial cells
(29). Previous studies
demonstrated that EPCs are involved in the angiogenesis of human
embryos and the repair process in endothelial injury (29,30).
EPCs have an important role in the occurrence and development of
vascular complications in DM (29,31).
eNOS is the key enzyme that regulates the production of
endothelium-derived nitric oxide (21) and has an effect on the
phosphatidylinositol 3-kinase/Akt signaling pathway (32). The PI3K/Akt pathway regulates the
metabolism, growth, migration and proliferation of various cells
(32). The results obtained from
the current study demonstrated that treatment with thymoquinone
significantly reduced the eNOS protein expression levels, COX-2 and
caspase-3 activity levels, and increased the p-Akt protein
expression levels in the DM rats. Idris-Khodja and Schini-Kerth
(33) determined that
thymoqui-none improves the aging-associated endothelial dysfunction
through eNOS in the rat mesenteric artery. Kundu et al
(34) demonstrated that in
vivo thymoquinone treatment inhibits the phorbol ester-induced
NF-κB and COX-2 expression levels in the skin from mice. Yu and Kim
(35) suggested that thymoquinone
suppressed COX-2 and caspase-3 activity levels and activated the
PI3K/Akt pathway in rabbit articular chondrocytes. The effect of
thymoquinone on DCD is suggested to be associated with the
downregulation of eNOS, COX-2 and caspase-3 activity levels and the
upregulation of PI3K/Akt pathway.
In conclusion, the present study demonstrated that
the protective effect of thymoquinone improves the cardiovascular
function through antioxidative, anti-inflammatory and
anti-apoptotic effects in DM rats. The PI3K/Akt pathway is a
possible target in future research focusing upon treatment with
thymoquinone to protect cardiovascular function of DM rats.
References
|
1
|
Yin L, Cai WJ, Chang XY, Li J, Su XH, Zhu
LY, Wang XL and Sun K: Association between fetuin-A levels with
insulin resistance and carotid intima-media thickness in patients
with new-onset type 2 diabetes mellitus. Biomed Rep. 2:839–842.
2014.PubMed/NCBI
|
|
2
|
Cheung KK, Luk AO, So WY, Ma RC, Kong AP,
Chow FC and Chan JC: Testosterone level in men with type 2 diabetes
mellitus and related metabolic effects: A review of current
evidence. J Diabetes Investig. 6:112–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li MZ, Su L, Liang BY, Tan JJ, Chen Q,
Long JX, Xie JJ, Wu GL, Yan Y, Guo XJ and Gu L: Trends in
prevalence, awareness, treatment, and control of diabetes mellitus
in mainland china from 1979 to 2012. Int J Endocrinol.
2013:7531502013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Whittemore R and He GP: The
relationship between diabetes self-management and metabolic control
in youth with type 1 diabetes: An integrative review. J Adv Nurs.
67:2294–2310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng D, Wang J, Zhang R, Tang S, Jiang F,
Chen M, Yan J, Sun X, Wang T, Wang S, et al: C-reactive protein
genetic variant is associated with diabetic retinopathy in Chinese
patients with type 2 diabetes. BMC Endocr Disord. 15:82015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CQ, Ma GZ, Tao MD, Ma XL, Feng J and
Liu QX: The relationship between renal injury and change in vitamin
D metabolism in aged rats with insulin resistance or type 2
diabetes mellitus. J Int Med Res. 36:289–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Göbl CS, Brannath W, Bozkurt L, Handisurya
A, Anderwald C, Luger A, Krebs M, Kautzky-Willer A and Bischof MG:
Sex-specific differences in glycemic control and cardiovascular
risk factors in older patients with insulin-treated type 2 diabetes
mellitus. Gend Med. 7:593–599. 2010. View Article : Google Scholar
|
|
8
|
Rutter MK and Nesto RW: Ischemia imaging
and plaque imaging in diabetes: Complementary tools to improve
cardiovascular risk management: Response to Raggi et al. Diabetes
Care;29:1187author reply 1188. 2006.PubMed/NCBI
|
|
9
|
Aycan IO, Tokgöz O, Tüfek A, Alabalık U,
Evliyaoğlu O, Turgut H, Çelik F and Güzel A: The use of
thymoquinone in nephrotoxicity related to acetaminophen. Int J
Surg. 13:33–37. 2015. View Article : Google Scholar
|
|
10
|
Khan MA, Anwar S, Aljarbou AN, Al-Orainy
M, Aldebasi YH, Islam S and Younus H: Protective effect of
thymoquinone on glucose or methylglyoxal-induced glycation of
superoxide dismutase. Int J Biol Macromol. 65:16–20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han J, Wang LU, Bian H, Zhou X and Ruan C:
Effects of paroxetine on spatial memory function and protein kinase
C expression in a rat model of depression. Exp Ther Med.
10:1489–1492. 2015.PubMed/NCBI
|
|
12
|
Cabrera SM, Rigby MR and Mirmira RG:
Targeting regulatory T cells in the treatment of type 1 diabetes
mellitus. Curr Mol Med. 12:1261–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De la Sierra A: Angiotensin receptor
blockers in hypertension and cardiovascular diseases. Cardiovasc
Hematol Agents Med Chem. 4:67–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassan MH and Abd-Allah GM: Effects of
metformin plus gliclazide versus metformin plus glimepiride on
cardiovascular risk factors in patients with type 2 diabetes
mellitus. Pak J Pharm Sci. 28:1723–1730. 2015.
|
|
15
|
Trost S, Pratley R and Sobel B: Impaired
fibrinolysis and risk for cardiovascular disease in the metabolic
syndrome and type 2 diabetes. Curr Diab Rep. 6:47–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forst T, Anastassiadis E, Diessel S,
Löffler A and Pfützner A: Effect of linagliptin compared with
glimepiride on postprandial glucose metabolism, islet cell function
and vascular function parameters in patients with type 2 diabetes
mellitus receiving ongoing metformin treatment. Diabetes Metab Res
Rev. 30:582–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Mahmoudy A, Shimizu Y, Shiina T,
Matsuyama H, El-Sayed M and Takewaki T: Successful abrogation by
thymoquinone against induction of diabetes mellitus with
streptozotocin via nitric oxide inhibitory mechanism. Int
Immunopharmacol. 5:195–207. 2005. View Article : Google Scholar
|
|
18
|
Pari L and Sankaranarayanan C: Beneficial
effects of thymo-quinone on hepatic key enzymes in
streptozotocin-nicotinamide induced diabetic rats. Life Sci.
85:830–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Zhao H, Wang Q, Liang H and Jiang X:
Fucoidan protects ARPE-19 cells from oxidative stress via
normalization of reactive oxygen species generation through the
Ca2+-dependent ERK signaling pathway. Mol Med
Rep. 11:3746–3752. 2015.PubMed/NCBI
|
|
20
|
Matsunami T, Sato Y, Sato T, Ariga S,
Shimomura T and Yukawa M: Oxidative stress and gene expression of
antioxidant enzymes in the streptozotocin-induced diabetic rats
under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 3:177–188.
2009.
|
|
21
|
Huang A, Yang YM, Feher A, Bagi Z, Kaley G
and Sun D: Exacerbation of endothelial dysfunction during the
progression of diabetes: Role of oxidative stress. Am J Physiol
Regul Integr Comp Physiol. 302:R674–R681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farag MM, Ahmed GO, Shehata RR and Kazem
AH: Thymoquinone improves the kidney and liver changes induced by
chronic cyclosporine A treatment and acute renal
ischaemia/reperfusion in rats. J Pharm Pharmacol. 67:731–739. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mabrouk A and Ben Cheikh H: Thymoquinone
supplementation reverses lead-induced oxidative stress in adult rat
testes. Gen Physiol Biophys. 34:65–72. 2015. View Article : Google Scholar
|
|
24
|
Yin L, Cai WJ, Zhu LY, Li J, Su XH, Wang
XL, Chang XY and Sun K: Association of plasma Fetuin-A and clinical
characteristics in patients with new-onset type 2 diabetes
mellitus. Int J Clin Exp Med. 8:991–999. 2015.PubMed/NCBI
|
|
25
|
Javed F, Al-Kheraif AA, Salazar-Lazo K,
Yanez-Fontenla V, Aldosary KM, Alshehri M, Malmstrom H and Romanos
GE: Periodontal inflammatory conditions among smokers and
never-smokers with and without Type 2 diabetes mellitus. J
Periodontol. 86:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schäffler A, Zeitoun M, Wobser H, Buechler
C, Aslanidis C and Herfarth H: Frequency and significance of the
novel single nucleotide missense polymorphism Val109Asp in the
human gene encoding omentin in Caucasian patients with type 2
diabetes mellitus or chronic inflammatory bowel diseases.
Cardiovasc Diabetol. 6:32007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Periyanayagam S, Arumugam G, Ravikumar A
and Ganesan VS: Thymoquinone ameliorates NLRP3-mediated
inflammation in the pancreas of albino Wistar rats fed ethanol and
high-fat diet. J Basic Clin Physiol Pharmacol. 26:623–632. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rifaioglu MM, Nacar A, Yuksel R, Yonden Z,
Karcioglu M, Zorba OU, Davarci I and Sefil NK: Antioxidative and
anti-inflammatory effect of thymoquinone in an acute Pseudomonas
prostatitis rat model. Urol Int. 91:474–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun N, Wang H and Wang L: Vaspin
alleviates dysfunction of endo-thelial progenitor cells induced by
high glucose via PI3K/Akt/eNOS pathway. Int J Clin Exp Pathol.
8:482–489. 2015.
|
|
30
|
Sorrentino SA, Bahlmann FH, Besler C,
Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A,
Limbourg F, et al: Oxidant stress impairs in vivo
reendothelialization capacity of endothelial progenitor cells from
patients with type 2 diabetes mellitus: Restoration by the
peroxisome proliferator-activated receptor-gamma agonist
rosiglitazone. Circulation. 116:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang Y, Jacobi A, Vater C, Zou X and
Stiehler M: Salvianolic acid B protects human endothelial
progenitor cells against oxidative stress-mediated dysfunction by
modulating Akt/mTOR/4EBP1, p38 MAPK/ATF2, and ERK1/2 signaling
pathways. Biochem Pharmacol. 90:34–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang T, Mao X, Li H, Qiao S, Xu A, Wang J,
Lei S, Liu Z, Ng KF, Wong GT, et al: N-Acetylcysteine and
allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via
adiponectin and attenuated myocardial postischemic injury in
diabetes. Free Radic Biol Med. 63:291–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Idris-Khodja N and Schini-Kerth V:
Thymoquinone improves aging-related endothelial dysfunction in the
rat mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol.
385:749–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kundu JK, Liu L, Shin JW and Surh YJ:
Thymoquinone inhibits phorbol ester-induced activation of NF-κB and
expression of COX-2, and induces expression of cytoprotective
enzymes in mouse skin in vivo. Biochem Biophys Res Commun.
438:721–727. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu SM and Kim SJ: The thymoquinone-induced
production of reactive oxygen species promotes dedifferentiation
through the ERK pathway and inflammation through the p38 and PI3K
pathways in rabbit articular chondrocytes. Int J Mol Med.
35:325–332. 2015.
|