Introduction
According to the 2012 Chinese cancer registration
report and statistics of the World Health Organization, the Chinese
population presents a high incidence of gastric carcinoma (1). Gastric carcinoma presented the second
largest incidence of malignant tumors in China and the third
highest rate of mortality; cases of gastric carcinoma and
associated mortality account for half the malignant tumor cases
worldwide (2). Gastric cancer in
China presents high morbidity and mortality rates, and most cases
are diagnosed at the advanced stage. The incidence rate of gastric
carcinoma in patients under the age of 30 increased from 1.7% in
the 1970s to 3.3% at present in China (3).
Nuclear factor (NF)-κB is a transcription factor
observed in various cell types that serves a role in physiological
and pathological processes through the NF-κB-inducing kinase
(NIK)/IκB kinase (IKK)/NF-κB signaling pathway (4,5).
Previous studies have demonstrated that NF-κB is associated with
proliferation, differentiation, apoptosis, invasion and metastasis
of tumor cells (6-8). In addition, aberrant activation of
NF-κB was demonstrated in gastric cancer cells and pathological
tissues (9).
X-linked inhibitor of apoptosis protein (XIAP) is an
effective caspase inhibitor and the most investigated molecular
structure of the inhibitor of apoptosis protein (IAP) family. XIAP
selectively binds to caspases-3, -7 or -9 to inhibit their activity
and prevent cell apoptosis (10).
Embelin is a small-molecule inhibitor of XIAP that binds to the
baculoviral IAP repeat 3 structural domain of XIAP and prevents
binding to caspases-3, -7 or -9, thus inducing cell apoptosis
(11). However, the anticancer
effect of embelin in human gastric carcinoma cells and the
mechanisms underlying it are poorly understood. The present study
hypothesizes that the anticancer effect of embelin induces cell
apoptosis in human gastric carcinoma cells through the p38
mitogen-activated protein kinase (MAPK) and NF-κB signaling
pathways.
Materials and methods
Chemical reagents
Invitrogen RPMI-1640 and fetal bovine serum (FBS)
were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). MTT was purchased from Sangon Biotech Co., Ltd. (Shanghai,
China). DAPI staining assays (cat. no. C1005) and caspase-3
activation commercial kits (cat. no. C1116) were obtained from
Beyotime Institute of Biotechnology (Haimen, China). A Pierce BCA
Protein Assay kit was purchased from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). An Annexin
V-FITC/PI Apoptosis Detection kit (cat. no. KGA101) was obtained
from KeyGen Biotech Co., Ltd. (Nanjing, China). An NF-κB p65
colorimetric assay kit was obtained from Elabscience Biotechnology
Co., Ltd. (Wuhan, China; cat. no. E-EL-H1388c).
Cell culture and cell proliferation
assay
The human gastric carcinoma cell line SGC7901 was
acquired from the experimental center of the Second Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) and
maintained in RPMI 1640 supplemented with 10% FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin (Sigma-Aldrich, St. Louis,
MO, USA), in a 5% CO2 atmosphere at 37°C. SGC7901 cells
were seeded in 96-well plates and treated with embelin (5, 10 or 15
µM; purity ≥98%; Sigma-Aldrich) for 1, 3 and 5 days as
previously described (12). Cell
proliferation was determined using the MTT assay. Briefly, MTT
solution (20 µl; 5 mg/l; Sangon Biotech Co., Ltd.) was added
to each well for a 4-h incubation in a 5% CO2
atmosphere, at 37°C. Following incubation, 150 µl dimethyl
sulfoxide was added to each well and shaken for 20 min at room
temperature. The optical density was determined using a 96-well
multiscanner at 570 nm (ELx808; Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Analysis of caspase-3 activity
SGC7901 cells were seeded in 6-well plates and
treated with embelin (5, 10 or 15 µM) for 5 days. Following
treatment, caspase-3 activity was assessed using caspase-3
activation commercial kits. Briefly, cells were prepared in cell
lysis buffer for 30–60 min at 4°C and centrifuged at 12,000 × g for
10 min at 4°C. The protein concentrations in the cell lysates were
measured with the Pierce BCA Protein Assay kit according to the
manufacturer's instructions. Equal amounts of protein were mixed
with the Ac-DEVD-pNA reaction buffer and incubated at 37°C for 2 h
in the dark. Following incubation, absorbance was measured at 405
nm with the XL-818 instrument.
Analysis of cell apoptosis
SGC7901 cells were seeded in 6-well plates and
treated with embelin (5, 10 or 15 µM) for 5 days. Cells were
washed twice with ice-cold phosphate-buffered saline (PBS),
collected and resuspended in annexin V binding buffer from the kit
according to the manufacturer's instructions. Following
resuspension, 5 µl annexin V-FITC and 5 µl propidium
iodide were added to the cells, and incubated for 10 min on ice in
the dark. Cell apoptosis was immediately detected using a flow
cytometer (Epics Altra; Beckman Coulter, Inc., Brea, CA, USA) to
identify annexin V- and/or PI-positive cells.
DAPI staining assay
SGC7901 cells were seeded in 6-well plates and
cultured with embelin (5, 10 or 15 µM) for 5 days. SGC7901
cells were washed twice with ice-cold PBS and fixed using 0.5 ml
paraformaldehyde (4%; Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) for 30 min at 4°C. Cells were then washed twice
with PBS and incubated with sodium citrate (0.1%; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) containing 0.1% Triton
X-100 (Sinopharm Chemical Reagent Co., Ltd.) for 5 min, at 4°C.
Cells were incubated with the DAPI staining assay for 10–15 min at
4°C in the dark. DAPI was excited by ultraviolet light to indicate
nuclear apoptosis and images were captured with an Axio Observer A1
fluorescence microscope (Zeiss AG, Oberkochen, Germany) at the
excitation wavelength of 340 nm.
Analysis of NF-κB p65 activation
SGC7901 cells were seeded in 96-well plates and
cultured with embelin (5, 10 or 15 µM) for 5 days. NF-κB p65
activation was measured using the NF-κB p65 colorimetric assay kit,
according to the manufacturer's instructions.
Western blotting
SGC7901 cells were seeded in 6-well plates and
cultured with embelin (5, 10 or 15 µM) for 5 days. Following
treatment, cells were prepared in cell lysis buffer for 30–60 min
at 4°C and centrifuged at 12,000 × g for 10 min at 4°C. The protein
concentrations in cell lysates were measured with the Pierce BCA
Protein Assay kit according to the manufacturer's protocol. Equal
volumes of proteins were resolved in 10% SDS-PAGE and transferred
to nitrocellulose membranes. Membranes were incubated overnight at
4°C with mouse anti-phosphorylated (p)-p38 MAPK monoclonal antibody
(1:1,000; cat. no. sc-7973; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-p-IκBα monoclonal antibody (1:1,000; cat. no.
ab133462; Abcam, Cambridge, MA, USA), anti-p-IKKα/β polyclonal
antibody (1:1,000; cat. no. sc-21661; Santa Cruz Biotechnology,
Inc.) and anti-β-actin polyclonal antibody (1:500; cat. no.
D110007; Sangon Biotech Co., Ltd., Shanghai, China). Following
washing with Tris-buffered saline supplemented with Tween-20, the
membranes were incubated with a horseradish peroxidase-conjugated
anti-mouse IgG secondary antibody (1:1,000; cat. no. sc-2380; Santa
Cruz Biotechnology, Inc.). for 2 h. Proteins were visualized using
enhanced chemiluminescence (Santa Cruz Biotechnology, Inc.) and
detected using Quantity One software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments and analyzed with SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). Differences
between groups were analyzed by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of embelin on tumor growth in
gastric carcinoma cells
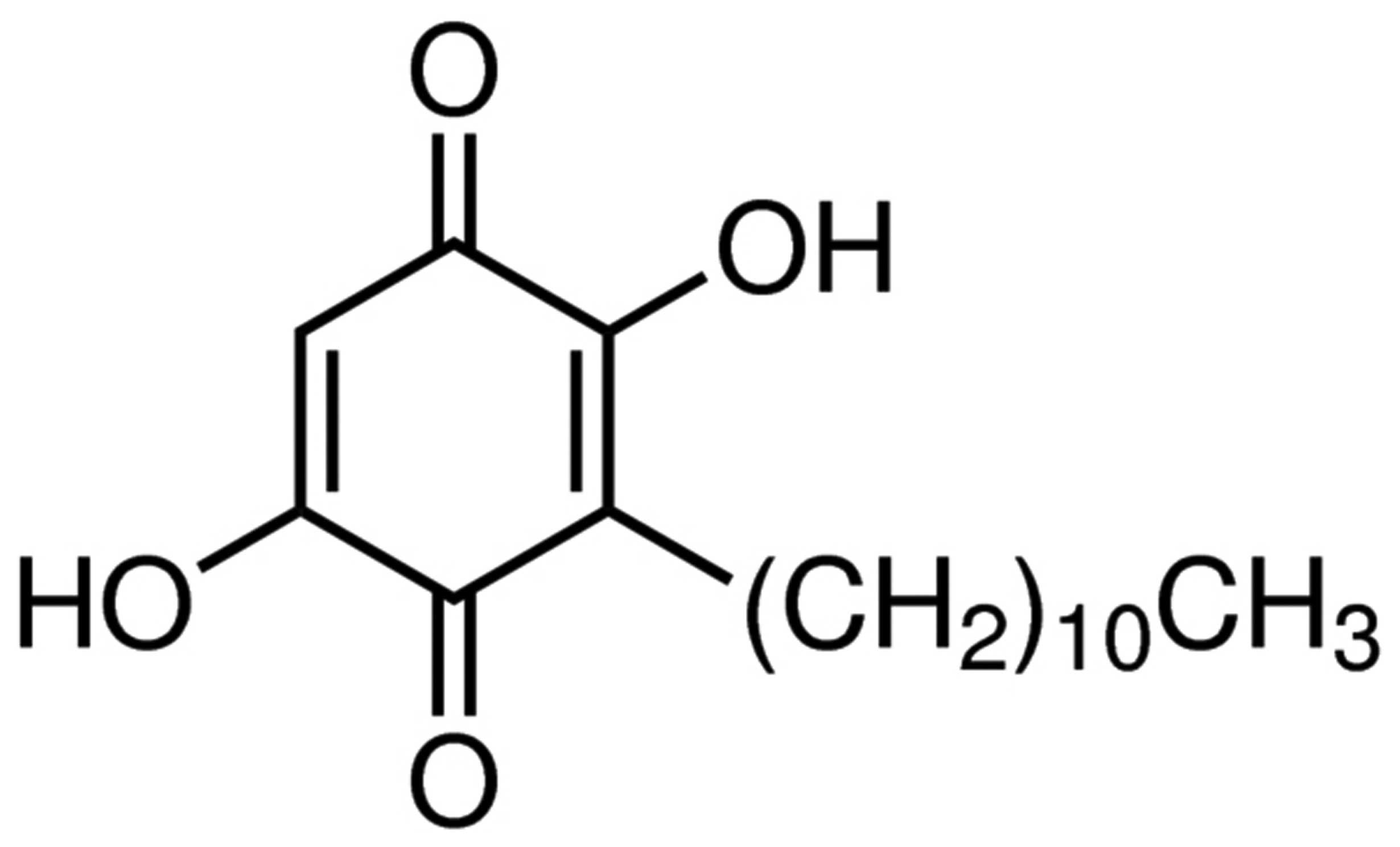
The chemical structure of embelin is presented in
Fig. 1. To investigate the
anticancer effect of embelin on tumor growth, SGC7901 cells were
treated with embelin (5, 10 or 15 µM) for 1, 3 or 5 days,
and the levels of cell proliferation were determined using the MTT
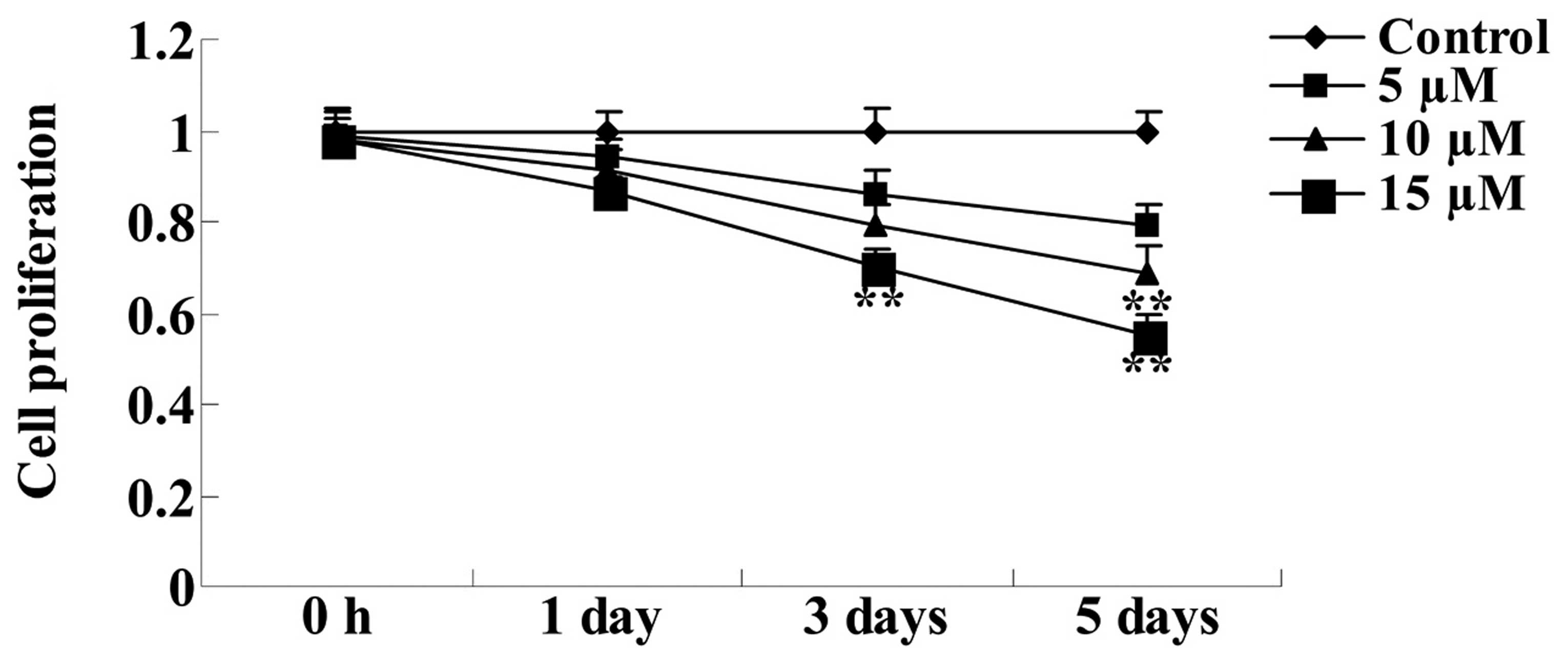
assay. As demonstrated in Fig. 2,
10 and 15 µM embelin significantly suppressed cell
proliferation following 5-days of culture, compared with the
control group (P<0.01). Therefore, further assays were performed
on SGC7901 cells treated with the various concentrations of embelin
for 5 days.
Embelin induces caspase-3 activity in
gastric carcinoma cells
To investigate the therapeutic effect of embelin on
cell apoptosis, SGC7901 cells were treated with embelin (5, 10 and
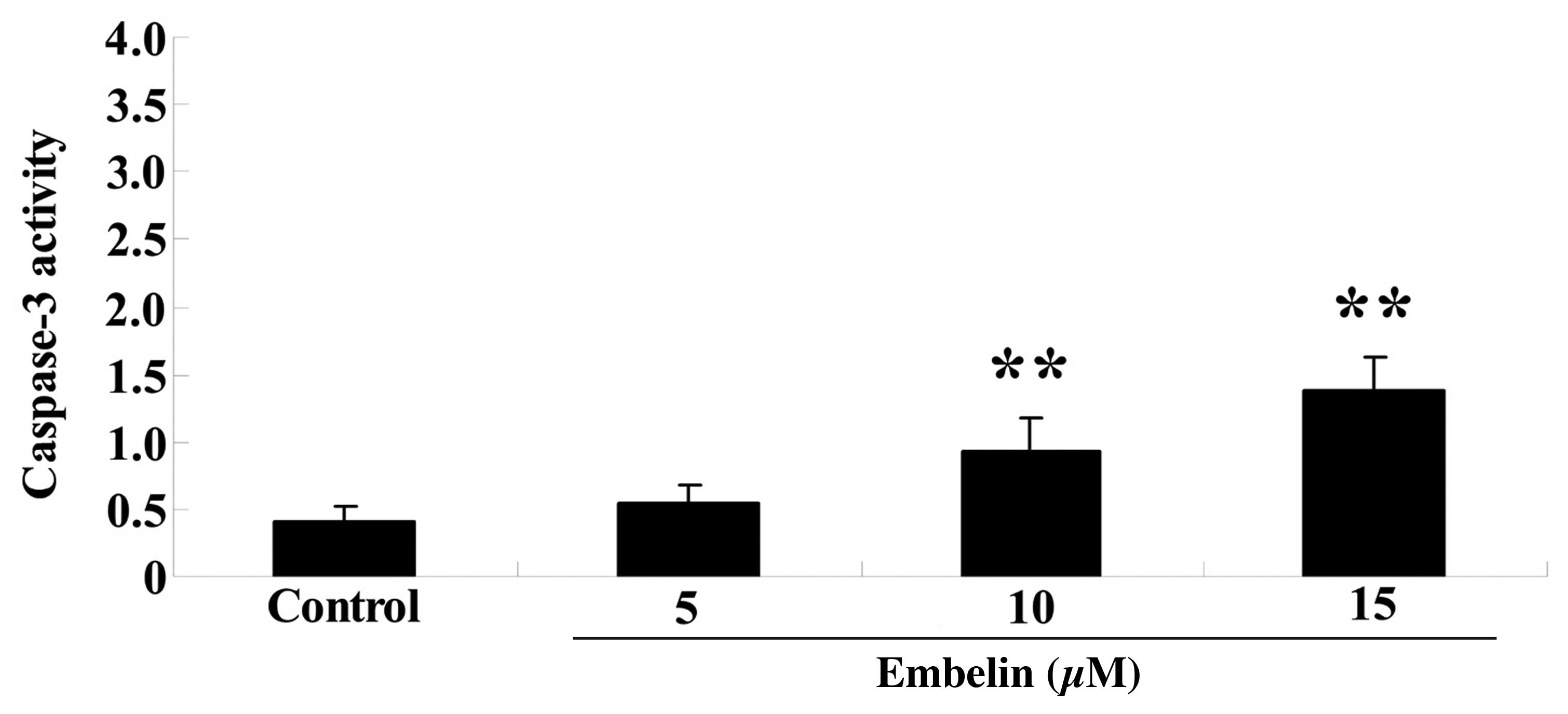
15 µM) for 4 days, and caspase-3 activity was measured. As
demonstrated in Fig. 3, 10 or 15
µM embelin significantly increased caspase-3 activity
compared with the control group (P<0.01).
Embelin induces cellular apoptosis in
gastric carcinoma cells
In order to examine the effect of embelin on cell
apoptosis, SGC7901 cells were treated with embelin (5, 10 or 15
µM) for 4 days, and the cellular apoptosis was determined.
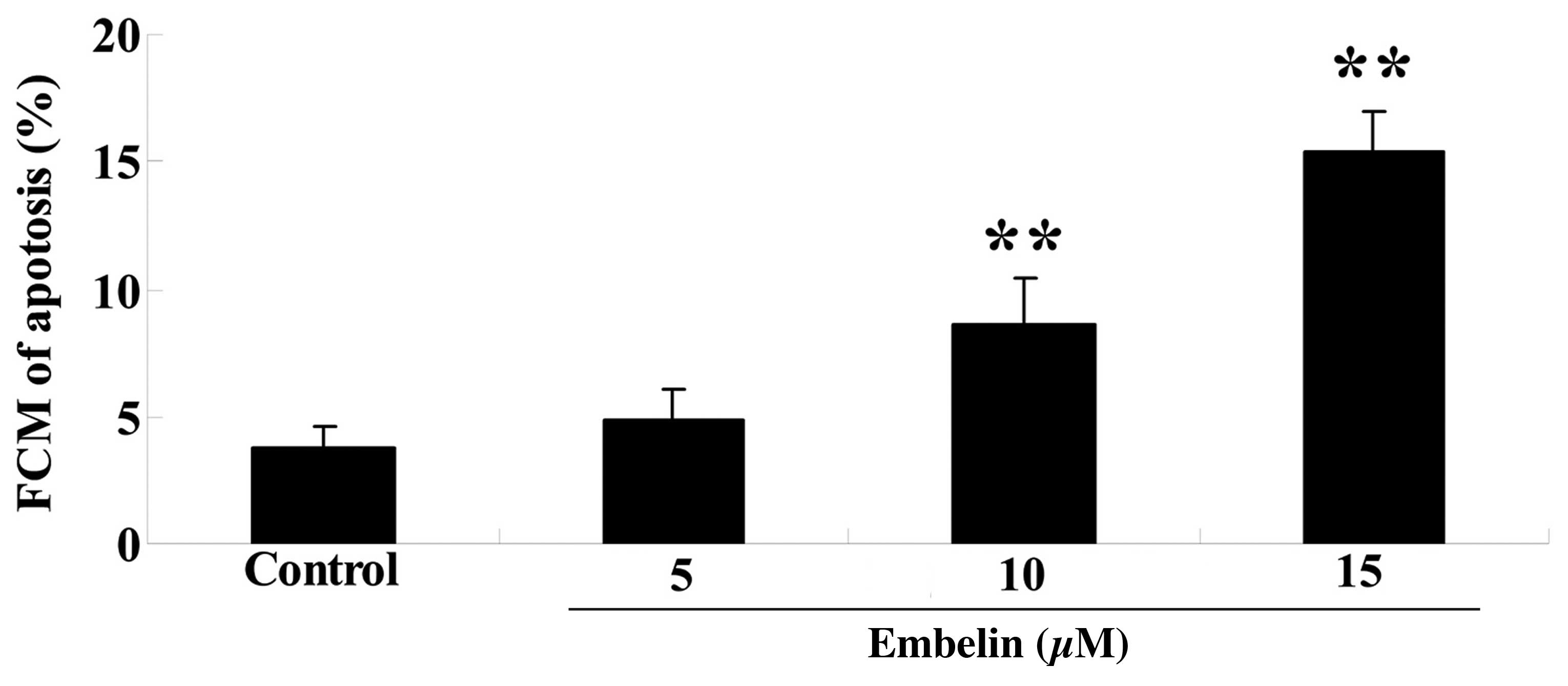
As demonstrated in Fig. 4, 10 and
15 µM embelin significantly increased the percentage of
cellular apoptosis compared with the control group (P<0.01).
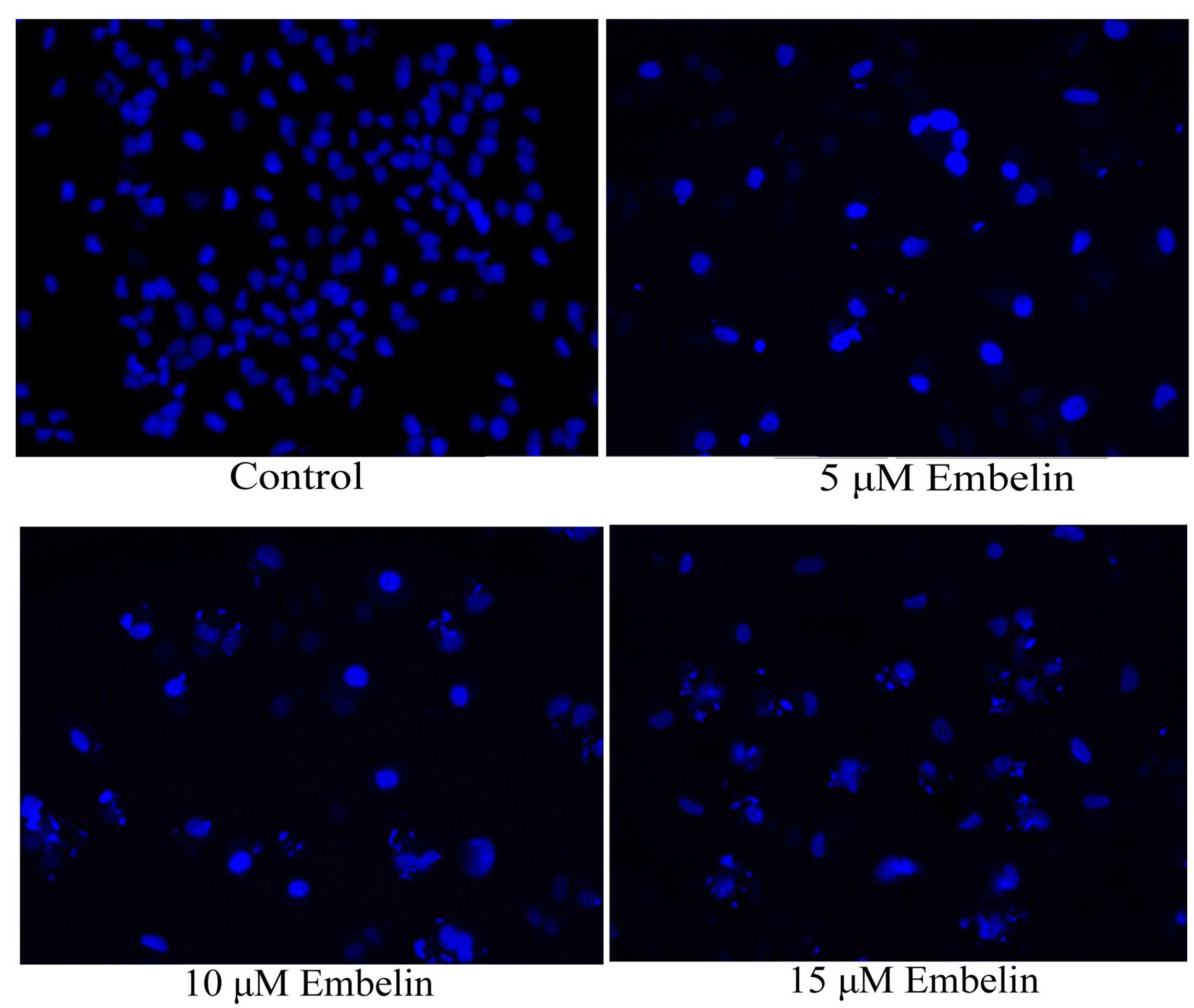
Effect of embelin induces nuclear
apoptosis in gastric carcinoma cells
In order to investigate the anticancer effect of
embelin on nuclear apoptosis, SGC7901 cells were treated with 5, 10
or 15 µM embelin for 4 days, and nuclear apoptosis was
evaluated with the DAPI staining assay. As demonstrated in Fig. 5, nuclear apoptosis was observed
following embelin treatment at all concentrations.
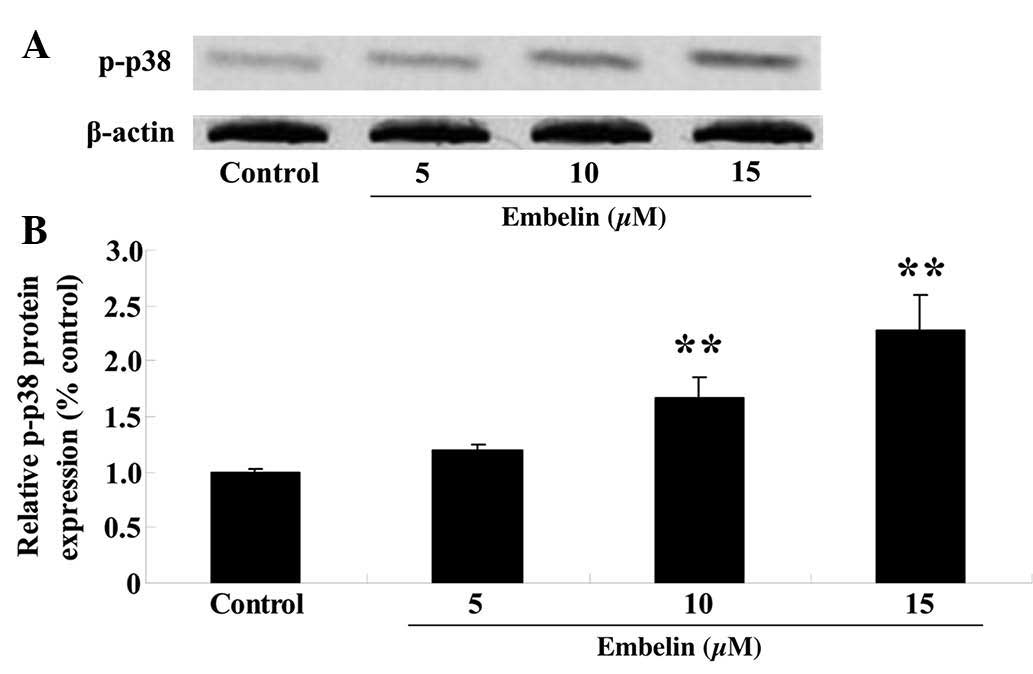
Embelin induces p-p38 MAPK protein
expression levels in gastric carcinoma cells
SGC7901 cells were treated with embelin (5, 10 or 15
µM) for 4 days, and the p-p38 MAPK protein expression levels
were measured using western blotting. The results demonstrated that
administration of embelin treatment (10 or 15 µM)
significantly upregulated the relative p-p38 MAPK protein
expression levels compared with those of the control group
(P<0.01; Fig. 6).
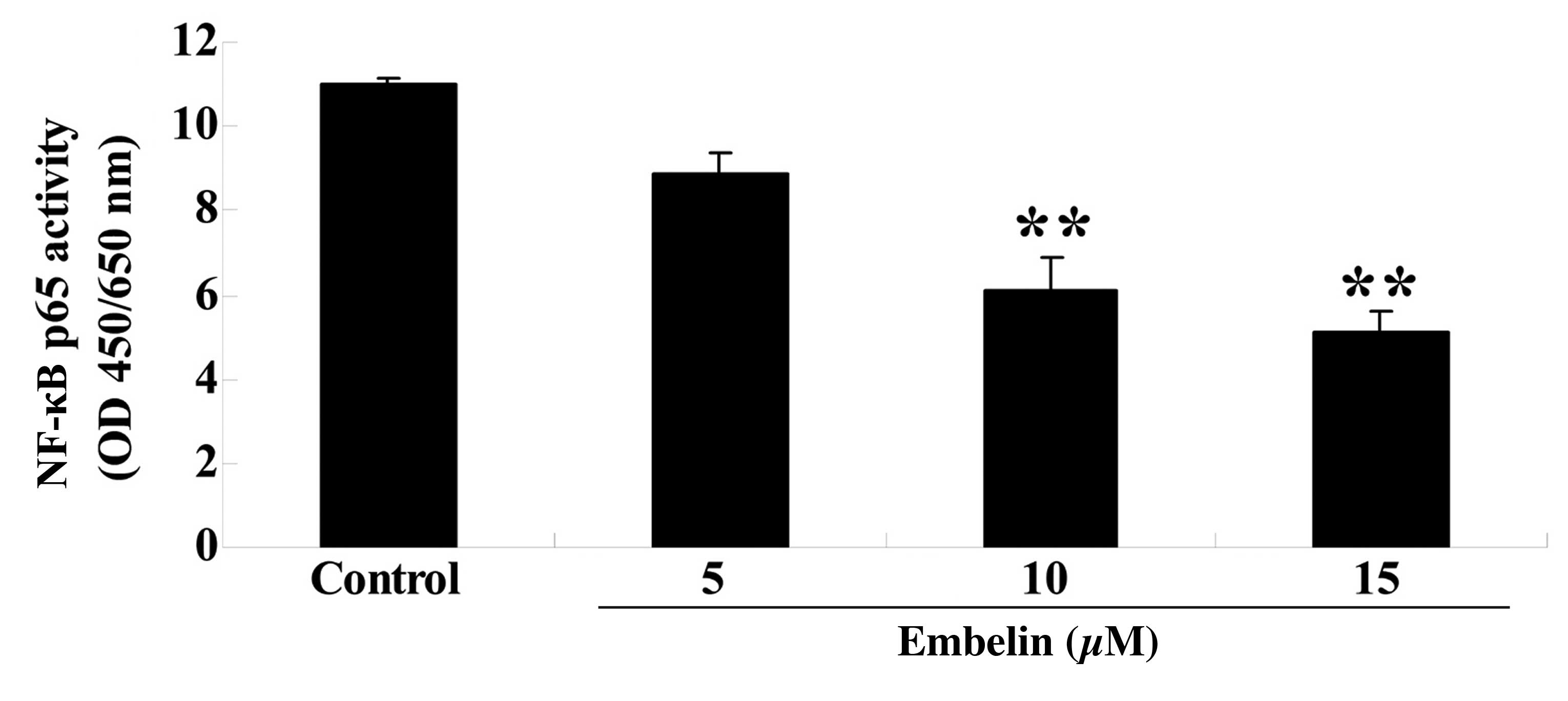
Embelin inhibits NF-κB p65 activity in
gastric carcinoma cells
SGC7901 cells were treated with embelin (5, 10 or 15
µM) for 4 days, and the NF-κB p65 activity was measured. As
demonstrated in Fig. 7, embelin
treatment (10 or 15 µM) significantly reduced NF-κB p65
activity compared with the control group (P<0.01).
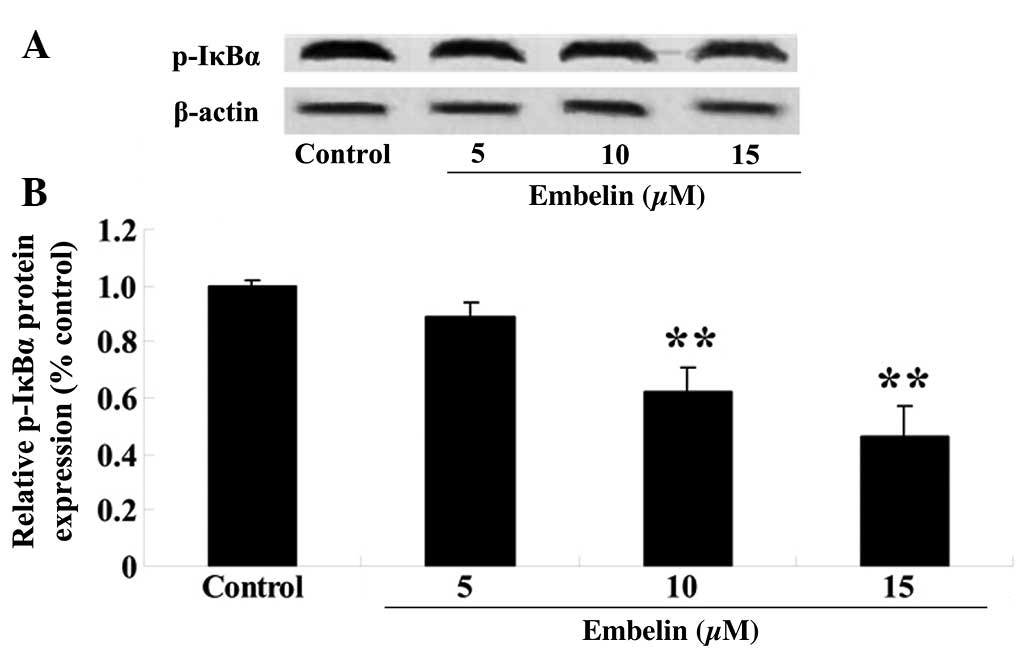
Embelin inhibits the phosphorylation of
IκBα in gastric carcinoma cells
SGC7901 cells were treated with embelin (5, 10 or 15
µM) for 4 days, and the p-IκBα expression levels relative to
β-actin were determined with western blotting. As demonstrated in
Fig. 8, embelin treatment (10 or
15 µM) significantly suppressed p-IκBα protein expression
compared with the control group (P<0.01).
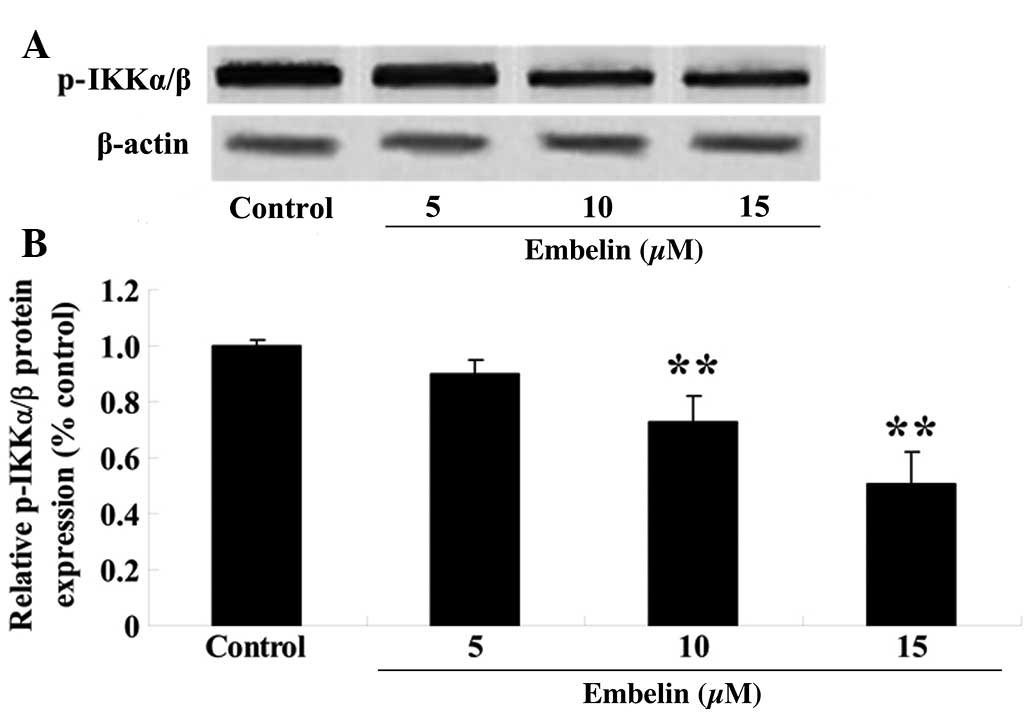
Embelin inhibits the phosphorylation of
the IKKα/β in gastric carcinoma cells
To investigate the anticancer therapeutic effect of
embelin treatment on IKKα/β protein expression, SGC7901 cells were
treated with embelin (5, 10 or 15 µM) for 4 days, and the
p-IKKα/β protein expression levels relative to β-actin were
determined with western blotting. As demonstrated in Fig. 9, embelin treatment (10 or 15
µM) significantly reduced the relative p-IKKα/β protein
expression levels compared with the control group (P<0.01).
Discussion
Malignant tumors are the second leading cause of
mortality in China, and stomach cancer presents the highest rate of
mortality associated with these tumors (13). China has the highest rate of
gastric carcinoma worldwide (14)
and the rate has risen over the past 20 years (15). In the present study, embelin
treatment significantly suppressed cell proliferation, induced
caspase-3 activation, and increased cell and nuclear apoptosis in
the SGC7901 cells. Marsh et al (16) suggested that embelin suppresses the
growth of pancreatic cancer in A549 non-small cell lung cancer
(17) and brain glioma cells
(18). Embelin is considered to be
a potential drug for the treatment of carcinoma.
MAPKs are Ser/Thr protein kinases that mediate the
cellular response and apoptosis, react to various cell growth and
mitosis promotion factors, and transduct outer signals into cells
(19). The signal transmission of
the MAPK pathway is completed by the continuous phosphorylation of
MAPKKK, MAPKK and MAPK (20).
There are five activators of the MAPK pathways; ERK, p38, JNK,
ERK3/ERK4 and ERK5 (21).
Extracellular stimuli result in different biological responses
depending on the MAPK pathway activated (22). In the present study, administration
of embelin significantly upregulated the relative p38 MAPK protein
expression in human gastric carcinoma cells. Wang et al
(23) indicated that embelin
reduced cell viability in gastric cancer cells via p38 MAPK pathway
activation. Avisetti et al (24) indicated that embelin induced
apoptosis in lung cancer cells through the activation of the
p38/JNK pathway. Activation of the p38/JNK pathway may be a
potential target for embelin treatment in human gastric carcinoma
cells.
The occurrence and development of a tumor is a
complex and multistep process that involves a series of genetic
changes, including metastasis of cells deriving from the primary
tumor into the blood and lymphatic vessels, through adhesion to
endothelial cells, resulting in the metastasis of the tumor
(25). A previous study
demonstrated that the upregulation of NF-κB leads to the occurrence
of tumors, and NF-κB mediates one of the main mechanisms by which
tumor cells resist apoptosis during tumor development (26). Upon activation, it produces
anti-apoptotic signals to aid the development of the tumor. Royuela
et al (27) demonstrated
that NF-κB induces anti-apoptosis genes, including IAG, cl-2 like
factor, TRAF1, TRAF2 and A20D. Similarly, NF-κB promotes tumor
formation via a non-apoptotic pathway, activating the
proto-oncogenes c-myc and cyclin D1 (28). Yang et al (28) demonstrated that cyclin D1 was the
target gene of NF-κB, and that NF-κB initiated the transcription of
cyclin D1, promoting the transit from phase
G1/G0 to phase S, leading to the cell
proliferation, malignant transformation and cancerization (29). In the present study, embelin
significantly reduced the NF-κB activity in human gastric carcinoma
cells. Ahn et al (30)
indicated that embelin is a potential suppressor of tumorigenesis,
as it suppresses NF-κB-regulated anti-apoptosis. Reuter et
al (12) demonstrated that
embelin suppresses osteoclastogenesis through the inhibition of the
NF-κB cell signaling pathway. Therefore, the suppression of NF-κB
may be a marker for embelin treatment in human gastric carcinoma
cells.
Pathological classification of the tumor may
indicate its invasiveness, thus, NF-κB p65 activity may have an
effect on tumor metastasis (31).
NF-κB serves a role in the activation of the immune system, cell
apoptosis and inflammatory cell chemotaxis associated with gene
transcription. As a gene encoding inflammatory molecules, NF-κB
regulates and controls the expression of numerous
inflammation-mediating genes, and NF-κB p65 activation was
previously associated with cell infiltration (32,33).
In the present study, a significant reduction in the relative
p-IκBα and p-IKKα/β protein expression levels was observed
following embelin treatment (10 or 15 µM). Park et al
(34) indicated that the
administration of embelin induced apoptosis in human glioma cells
through the degradation of p-IκBα and p-IKKα/β. This may have been
due to the decrease in p-IκBα and p-IKKα/β protein expression
induced by embelin treatment.
In conclusion, the results of the current study
suggest that embelin suppresses cell proliferation and induces
levels of apoptosis in human gastric carcinoma cells through the
inhibition of the NF-κB signaling pathway. These results indicate
that the suppression of the NF-κB signaling pathway, due to embelin
administration, is a potential therapeutic target for the treatment
of gastric carcinoma.
References
|
1
|
Lam TK, Freedman ND, Fan JH, Qiao YL,
Dawsey SM, Taylor PR and Abnet CC: Prediagnostic plasma vitamin C
and risk of gastric adenocarcinoma and esophageal squamous cell
carcinoma in a Chinese population. Am J Clin Nutr. 98:1289–1297.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Philips P, North DA, Scoggins C, Schlegel
M and Martin RC: Gastric-Esophageal Stenting for Malignant
Dysphagia: Results of Prospective Clinical Trial Evaluation of
Long-Term Gastroesophageal Reflux and Quality of Life-Related
Symptoms. J Am Coll Surg. 221:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang DS, Jin Y, Luo HY, Wang ZQ, Qui M,
Wang FH, Li Y and Xu RH: Pemetrexed for previously treated patients
with metastatic gastric cancer: A prospective phase II study. Br J
Cancer. 112:266–270. 2015. View Article : Google Scholar :
|
|
4
|
Zhang L, Ding Y, Yuan Z, Liu J, Sun J, Lei
F, Wu S, Li S and Zhang D: MicroRNA-500 sustains nuclear factor-κB
activation and induces gastric cancer cell proliferation and
resistance to apoptosis. Oncotarget. 6:2483–2495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandrasekar B, Mummidi S, Perla RP,
Bysani S, Dulin NO, Liu F and Melby PC: Fractalkine (CX3CL1)
stimulated by nuclear factor kappaB (NF-kappaB)-dependent
inflammatory signals induces aortic smooth muscle cell
proliferation through an autocrine pathway. Biochem J. 373:547–558.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu BS, Xing CG, Lin F, Fan XQ, Zhao K and
Qin ZH: Blocking NF-κB nuclear translocation leads to p53-related
autophagy activation and cell apoptosis. World J Gastroenterol.
17:478–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shostak K and Chariot A: EGFR and NF-kB:
Partners in cancer. Trends Mol Med. 21:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukherjee N, Houston TJ, Cardenas E and
Ghosh R: To be an ally or an adversary in bladder cancer: The NF-kB
story has not unfolded. Carcinogenesis. 36:299–306. 2015.
View Article : Google Scholar
|
|
9
|
Li ZM, Pu YW and Zhu BS: Blockade of NF-κB
nuclear translocation results in the inhibition of the invasiveness
of human gastric cancer cells. Oncol Lett. 6:432–436.
2013.PubMed/NCBI
|
|
10
|
Bai Y, Ahmad U, Wang Y, Li JH, Choy JC,
Kim RW, Kirkiles-Smith N, Maher SE, Karras JG, Bennett CF, et al:
Interferon-gamma induces X-linked inhibitor of apoptosis-associated
factor-1 and Noxa expression and potentiates human vascular smooth
muscle cell apoptosis by STAT3 activation. J Biol Chem.
283:6832–6842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu R, Zhu K, Li Y, Yao K, Zhang R, Wang H,
Yang W and Liu Z: Embelin induces apoptosis through down-regulation
of XIAP in human leukemia cells. Med Oncol. 28:1584–1588. 2011.
View Article : Google Scholar
|
|
12
|
Reuter S, Prasad S, Phromnoi K, Kannappan
R, Yadav VR and Aggarwal BB: Embelin suppresses osteoclastogenesis
induced by receptor activator of NF-κB ligand and tumor cells in
vitro through inhibition of the NF-κB cell signaling pathway. Mol
Cancer Res. 8:1425–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu ZJ, Zheng RS, Zhang SW, Zou XN and Chen
WQ: Nasopharyngeal carcinoma incidence and mortality in China in
2009. Chin J Cancer. 32:453–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo W, Ou G, Li X, Huang J, Liu J and Wei
H: Screening of the nutritional risk of patients with gastric
carcinoma before operation by NRS 2002 and its relationship with
postoperative results. J Gastroenterol Hepatol. 25:800–803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marsh JL, Jackman CP, Tang SN, Shankar S
and Srivastava RK: Embelin suppresses pancreatic cancer growth by
modulating tumor immune microenvironment. Front Biosci (Landmark
Ed). 19:113–125. 2014. View
Article : Google Scholar
|
|
17
|
Jiang L, Hao JL, Jin ML, Zhang YG and Wei
P: Effect of Embelin on TRAIL receptor 2 mAb-induced apoptosis of
TRAIL-resistant A549 non-small cell lung cancer cells. Asian Pac J
Cancer Prev. 14:6115–6120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang A, Zhang B, Zhang J and Wu W and Wu
W: Embelin-induced brain glioma cell apoptosis and cell cycle
arrest via the mitochondrial pathway. Oncol Rep. 29:2473–2478.
2013.PubMed/NCBI
|
|
19
|
Betti M, Ciacci C, Lorusso LC, Canonico B,
Falcioni T, Gallo G and Canesi L: Effects of tumour necrosis factor
alpha (TNFalpha) on Mytilus haemocytes: Role of stress-activated
mitogen-activated protein kinases (MAPKs). Biol Cell. 98:233–244.
2006. View Article : Google Scholar
|
|
20
|
Fiedler B, Feil R, Hofmann F, Willenbockel
C, Drexler H, Smolenski A, Lohmann SM and Wollert KC:
cGMP-dependent protein kinase type I inhibits TAB1-p38
mitogen-activated protein kinase apoptosis signaling in cardiac
myocytes. J Biol Chem. 281:32831–32840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei YY, Wang WJ, Mei JH and Wang CL:
Mitogen-activated protein kinase signal transduction in solid
tumors. Asian Pac J Cancer Prev. 15:8539–8548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shan X, Aziz F, Tian LL, Wang XQ, Yan Q
and Liu JW: Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation
inhibits melanoma cell proliferation by decreasing FUT4/LeY
expression. Int J Oncol. 46:1667–1676. 2015.PubMed/NCBI
|
|
23
|
Wang DG, Sun YB, Ye F, Li W, Kharbuja P,
Gao L, Zhang DY and Suo J: Anti-tumor activity of the X-linked
inhibitor of apoptosis (XIAP) inhibitor embelin in gastric cancer
cells. Mol Cell Biochem. 386:143–152. 2014. View Article : Google Scholar
|
|
24
|
Avisetti DR, Babu KS and Kalivendi SV:
Activation of p38/JNK pathway is responsible for embelin induced
apoptosis in lung cancer cells: Transitional role of reactive
oxygen species. PLoS One. 9:e870502014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren S, Abuel-Haija M, Khurana JS and Zhang
X: D2-40: An additional marker for myoepithelial cells of breast
and the precaution in interpreting tumor lymphovascular invasion.
Int J Clin Exp Pathol. 4:175–182. 2011.PubMed/NCBI
|
|
26
|
Wang Y, Ma W and Zheng W: Deguelin, a
novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest
and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol.
1:215–219. 2013.
|
|
27
|
Royuela M, Rodriguez-Berriguete G, Fraile
B and Paniagua R: TNF-alpha/IL-1/NF-kappaB transduction pathway in
human cancer prostate. Histol Histopathol. 23:1279–1290.
2008.PubMed/NCBI
|
|
28
|
Yang GF, Deng CS, Xiong YY, Gong LL, Wang
BC and Luo J: Expression of nuclear factor-kappa B and target genes
in gastric precancerous lesions and adenocarcinoma: Association
with Helicobactor pylori cagA (+) infection. World J Gastroenterol.
10:491–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe
EM Jr and Slominski AT: 20,23-dihydroxyvitamin D3, novel P450scc
product, stimulates differentiation and inhibits proliferation and
NF-kappaB activity in human keratinocytes. J Cell Physiol.
223:36–48. 2010.
|
|
30
|
Ahn KS, Sethi G and Aggarwal BB: Embelin,
an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein,
blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading
to suppression of NF-kappaB-regulated antiapoptotic and metastatic
gene products. Mol Pharmacol. 71:209–219. 2007. View Article : Google Scholar
|
|
31
|
Kwon HC, Kim SH, Oh SY, Lee S, Lee JH,
Jang JS, Kim MC, Kim KH, Kim SJ, Kim SG and Kim HJ:
Clinicopathologic significance of expression of nuclear factor-κB
RelA and its target gene products in gastric cancer patients. World
J Gastroenterol. 18:4744–4750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sousa LP, Carmo AF, Rezende BM, Lopes F,
Silva DM, Alessandri AL, Bonjardim CA, Rossi AG, Teixeira MM and
Pinho V: Cyclic AMP enhances resolution of allergic pleurisy by
promoting inflammatory cell apoptosis via inhibition of PI3K/Akt
and NF-kappaB. Biochem Pharmacol. 78:396–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer CD, Beatty JK, Zvaigzne CG, Morck
DW, Lucas MJ and Buret AG: Anti-Inflammatory benefits of
antibiotic-induced neutrophil apoptosis: Tulathromycin induces
caspase-3-dependent neutrophil programmed cell death and inhibits
NF-kappaB signaling and CXCL8 transcription. Antimicrob Agents
Chemother. 55:338–348. 2011. View Article : Google Scholar
|
|
34
|
Park SY, Lim SL, Jang HJ, Lee JH, Um JY,
Kim SH, Ahn KS and Lee SG: Embelin induces apoptosis in human
glioma cells through inactivating NF-κB. J Pharmacol Sci.
121:192–199. 2013. View Article : Google Scholar
|