Introduction
Mammalian oocytes are exposed to oxidative
conditions (1). The oxidative
modification of cell components caused by the increased production
of reactive oxygen species (ROS) (2) compromises membrane integrity, and
causes structural and functional changes in proteins, as well as
damage to nucleic acids (3). ROS
accelerate oocyte aging, lower oocyte quality, and induce apoptosis
in oocytes and early embryonic cells (3). Therefore, increased ROS production
negatively affects cell function and contributes significantly to
several diseases, including those which damage reproduction and
fertility (4). Accumulating
evidence has demonstrated that oocytes are major sources of ROS, as
they use oxygen to produce energy through mitochondrial oxidative
phosphorylation (5). ROS
production is higher during oocyte in vitro maturation (IVM)
than during in vivo maturation (5). The major ROS,
H2O2, is produced intracellularly during
several pathophysiologic processes, and causes oxidative damage.
Exposure to exogenous H2O2 (25–300 µM)
reportedly induces apoptosis in rat oocytes (1) and mouse zygotes (6). However, whether direct
H2O2 exposure induces apoptosis in goat
oocytes cultured in vitro remains to be elucidated.
Evidence has shown that glutathione (GSH) is
involved in maintaining cellular redox states. Thus, the GSH
content of oocytes protects the zygote and early embryos from
oxidative damage prior to genomic activation (7). The addition of cysteine to oocyte
culture media has been shown to increase intracellular levels of
GSH in buffaloes (8,9), pigs (10) and goats (11). However, the effects of
supplementation with a specific concentration of cysteine depend on
the dose, the species and the maturation medium (2). The cystine content of TCM-199 (83.2
mM), a widely used medium, is sufficient for oocyte IVM, although
the mechanism underlying its antioxidative capacity remains to be
elucidated (12). The balance
between ROS production and antioxidant defense is critical to the
function of granulosa cells and oocytes (13,14).
The oocyte environment has a marked effect on the outcomes of
assisted reproductive technologies (15). Cysteine is unstable and readily
oxidized into cystine in the medium; therefore, the addition of
cysteine and cystine to the medium decreases levels of oxidative
stress on oocytes cultured in vitro.
The present study aimed to determine the effects of
H2O2, with and without cysteine and cystine,
on the apoptosis of mature goat oocytes cultured in vitro.
The effects examined included DNA damage, caspase-3 activity,
mitochondria regulators and the gene expression of B cell
lymphoma-2-associated X protein (BAX). In addition, the
intracellular concentration of GSH in oocytes and their subsequent
parthenogenetic embryonic development capacities were
determined.
Materials and methods
Reagents
The chemicals used to culture the oocytes and
embryos in the present study were obtained from Sigma-Aldrich (St.
Louis, MO, USA), whereas media (including, TCM199, FBS,
formaldehyde and Triton X-100) were from Gibco; Thermo Fisher
Scientific, Inc., (Waltham, MA, USA). The RNeasy® Micro
kit (cat. no. 74004) was purchased from QIAGEN (Valencia, CA, USA).
SYBR Premix Ex Taq (cat. no. DRR420A) and the PrimeScript RT
reagent kit with gDNA Eraser (cat. no. DRR047S) were purchased from
Takara Biotechnology Co., Ltd. (Dalian, China). The One-Step
Terminal Deoxynucleotidyl Transferase UTP Nick End Labeling (TUNEL)
apoptosis assay kit (cat. no. C1088), the GSH and GSSG assay kits
(cat. no. S0053), and the Caspase 3 activity assay kit (cat. no.
C1116) were purchased from Beyotime Institute of Biotechnology
(Haimen, China). All other chemicals were obtained commercially and
were of reagent grade. All experiments were performed in accordance
with the Guidelines for the Care and Use of Animals of the College
of Animal Science and Technology, Nanjing Agricultural University
(Nanjing, China) (16).
Preparation of
H2O2, cystine and cysteine working
solutions
The working solutions of H2O2
(Sigma-Aldrich; cat. no. H3410), cystine (Sigma-Aldrich; cat. no.
C7602) and cysteine (Sigma-Aldrich; cat. no. C5360) were prepared
freshly prior to use. Briefly, 3.5 µl of 30%
H2O2 was diluted in 1 ml of IVM culture media
(30 mM). Subsequently, 10 and 12 µl of this solution were
further diluted in 3 ml of culture media (100 and 120 µM).
The 100 µM H2O2 solution was diluted
into further concentrations of 60 and 80 µM. The cysteine
stock solution (20 mM) and the cystine stock solution (100 mM) were
diluted in culture media to obtain final concentrations of 100, 200
and 500 µM. Thus, four IVM culture conditions were used for
the oocytes in the present study, as follows: i) control (IVM media
without chemical agent), ii) H2O2 (IVM media
with H2O2), iii) CC (IVM media with cystine
and cysteine), and iv) H2O2 and CC (IVM media
with H2O2 and CC).
Oocyte collection
Goat ovaries were collected from a slaughterhouse
(Haimen goat Research Center; Haimen, Nantong, China), delivered to
the laboratory within 3–4 h and washed with normal saline. The
connective tissues and the attached oviducts were removed. The
ovaries were transferred into Petri dishes and the 2–6 mm follicles
were sectioned. Cumulus-oocyte complexes (COCs) appropriate for IVM
were selected for IVM culture. The base medium for IVM comprised
modified TCM199 supplemented with 10 µg/ml
follicle-stimulating hormone luteinizing hormone, estrogen (all
Sigma-Aldrich) and 10% FBS. This media was further supplemented
with 100 µM cysteine and 100 µM cystine or 100
µM H2O2, according to the experimental
design. The selected COCs were cultured for 20–22 h at 38.5°C under
a saturated 5% CO2 atmosphere.
Oocyte recovery
Following IVM, the COCs were treated with 0.1%
hyaluronidase to strip their cumulus cells by repeated pipetting
through a narrow-bore pipette in culture media. Good-quality MII
oocytes with a uniform cytoplasm and a well-extruded polar body
were selected to assess for parthenogenetic activation.
In vitro culture
The parthenogenetic embryos were treated with TCM
199 containing 5 µM ionomycin for 5 min, incubated in TCM
199 containing 2 mM 6-DMAP for 4 h, and washed three times in 100
µl of M16 medium. The oocytes were then transferred into 100
µl M16 droplets under mineral oil and cultured for 6 days at
38.5°C under 5% CO2. Cleavage and development to the
blastocyst stage were observed on day 2.5 (day of parthenogenetic
activation=day 0.5) and day 6.5, respectively. Blastocysts, which
developed on day 6.5, were fixed to examine their cell numbers. The
total number of blastocysts were determined under a Nikon Eclipse
TE2000 fluorescence microscope (Nikon Corporation, Tokyo, Japan)
following Hoechst 33342 staining (Sigma-Aldrich; cat. no.
B-2261).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Mature oocytes (30 oocytes per group) were collected
to analyze the expression levels of genes associated with the
mitochondria and apoptosis in goat oocytes. Total mRNA was
extracted using the RNeasy® Micro kit, according to the
manufacturer's protocol. For the RT, total mRNA with a final volume
of 20 µl (containing 0.5 mg oligo-dT, 1X RT buffer, 10 mM
dithiothreitol and 10 mM dNTP) was reverse transcribed at 37°C for
50 min and at 70°C for 15 min, and the products were stored at 4°C
until use. The qPCR was performed using an qPCR reagent kit with
gDNA Eraser. The total reaction volume of 20 µl contained 2
µl of 5X gDNA Eraser Buffer, 1 µl of gDNA Eraser, 1
µg of total RNA, 4 µl of 5X RT Buffer, 1 µl of
qPCR enzyme mix, 1 µl of RT Primer Mix, and sufficient
nuclease-free H2O. The qPCR was performed at 42°C for 2
min, 37°C for 15 min, followed by a denaturation step at 85°C for
15 sec and cooling on ice. The primer sequences for each gene are
shown in Table I. The tests were
performed in triplicate, and the mRNA levels in each sample were
normalized to the mRNA level of GAPDH.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene and reference
sequence (GenBank accession no.) | Amplicon primer
sequence | Annealing size
(bp) | Temperature
(°C) |
|---|
| NRF-1
(AY368269) | F:
5′-AGGCTGGGGCAAAGAAAG-3′ | 303 | 58.0 |
| R:
5′-CCAACCTGGATAAGCGAGAC-3′ | | |
| PGC-1α
(AY321517) | F:
5′-CACCCACAACTCCTCCTCAT-3′ | 232 | 58.0 |
| R:
5′-GCCTTCCTTTCCTCGTGTC-3′ | | |
| BAX
(XM_002701934.1) | F:
5′-GCATCCACCAAGAAGCTGAG-3′ | 130 | 58.0 |
| R:
5′-CCGCCACTCGGAAAAAGAC-3′ | | |
| BCL2
(NM_001166486.1) | F:
5′-ATGTGTGTGGAGAGCGTCA-3′ | 182 | 58.0 |
| R:
5′-AGAGACAGCCAGGAGAAATC-3′ | | |
| GAPDH
(NM001034034) | F:
5′-CGACTTCAACAGCGACACTCAC-3′ | 118 | 58.0 |
| R:
5′-CCCTGTTGCTGTAGCCAAATTC-3′ | | |
Analysis of gene expression levels using
qPCR
All transcripts were quantified by qPCR using an ABI
7300 PRISM system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The amplification products of PGC-1α, NRF-1, BAX, Bcl-2 and
GAPDH were detected using SYBR green staining. The GenBank
accession numbers and primer sequences used to amplify the target
genes are presented in Table
I.
The qPCRs were run in triplicate in a total volume
of 20 µl, consisting of SYBR Premix Ex Taq, ROX Reference
Dye, 200 nM of each gene-specific primer and 100 ng equivalent
cDNA. The amplification conditions were as follows: DNA polymerase
activation at 95°C for 30 sec, followed by 40 amplification cycles
of 95°C for 5 sec and 58°C for 31 sec. At the end of the
amplification cycles, melting curve analysis was performed to
verify gene-specific amplification. The comparative CQ method
(17) was used to quantify the
relative expression levels of the target genes. For ease of
comparison, the average expression level of each gene from the
control group was set as one (16).
Terminal deoxynucleotidyl transferase UTP
nick end labeling (TUNEL) assay
The TUNEL method was performed using a One-Step
TUNEL Apoptosis Assay kit, as previously described. Briefly, the
control and experimental oocytes (20 oocytes per group) undergoing
shrinkage were fixed in 3.7% formaldehyde in PBS for 1 h at
18–20°C. Following fixation, the oocytes were washed in PBS and
permeabilized by incubation in 0.1% (v/v) Triton X-100 for 2 min at
4°C. The oocytes were then washed twice in PBS and incubated with
the TUNEL labeling media in the dark for 1 h at 37°C. Following
counterstaining with 10 µg/ml Hoechst 33342 for 10 min at
37°C to label all the nuclei, the oocytes were mounted with light
coverslip compression and observed under a microscope.
GSH and oxidized glutathione (GSSG)
assay
The GSH concentrations were measured in oocytes
matured in vitro under four IVM culture conditions,
according to the GSH and GSSG Assay kit. The oocytes (30 oocytes
per group) were carefully denuded by repeated pipetting in 0.1%
(w/v) hyaluronidase (Sigma-Aldrich) and washed several times in
polyvinyl alcohol-PBS to remove any trace of materials with thiol
groups. The oocytes were stored in a microtube at −20°C until they
were assayed (30 oocytes per tube). All procedures complied with
the manufacturer's protocol. Briefly, GSH was assayed using
5,5-dithio-bis (2-nitrobenzoic) acid (DTNB)-GSSG reductase
recycling. The GSSG was measured by measuring the
5-thio-2-nitrobenzoic acid (TNB) produced from the reaction of
reduced GSH with DTNB. The rate of TNB formation was measured at
412 nm using a Multiskan™ FC Microplate Photometer (Thermo Fisher
Scientific, Inc.), over 3 min. The reduced GSH concentration in the
sample was calculated by subtracting the GSSG from GSH.
Caspase-3 activity assay
Caspase-3 activity was analyzed in the control and
experimental oocytes using a Caspase-3 Activity kit, with
Ac-DEVD-pNA as the colorimetrically-specific substrate. Briefly, 30
oocytes from each group were washed twice in PBS and then lysed in
100 µl of chilled lysis buffer (Gibco) at 4°C for 10 min.
The lysate was centrifuged at 20,000 × g at 4°C for 10 min, and the
supernatant was incubated for 7 h at 37°C with 10 µl of
caspase-3 substrate. Substrate cleavage was measured using an
Hitachi Fluorescence Spectrophotometer F-7000 (Hitachi, Ltd.,
Tokyo, Japan) at 405 nm and was corrected to the protein content in
the lysate. The caspase-3 activity is presented as the fold
increase in optical density/30 oocytes.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean of triplicate samples. All percentages were subjected to
arcsine square root transformation prior to statistical analysis.
The data were analyzed using either Student's t-test or
one-way analysis of variance followed by a multiple t-test.
All statistical analysis was performed using SPSS (version 17.0;
SPSS, Inc. Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Optimal concentrations of
H2O2 and CC for oxidative stress and goat
parthenogenetic embryonic development
H2O2 induces ROS generation,
causing oxidative stress. CC is usually used to improve GSH
synthesis, as GSH protects cells against the destructive effects of
ROS. To determine the optimal concentrations of
H2O2 and CC, the present study evaluated goat
oocyte maturation rate and parthenogenesis blastocyst formation
rate. The oocytes treated with 100 µM
H2O2 exhibited significantly decreased
maturation rates and blastocyst rates, compared with the control
group (52.67±1.81, vs. 71.78±2.06 and 11.98±1.27, vs. 20.29±2.06,
respectively; P<0.05; Fig. 1).
However, oocytes treated with 120 µM
H2O2 exhibited marked decrease in oocyte
maturation rates and blastocyst formation rates (19.34±1.43 and
2.49±1.36, respectively), and the high concentration of
H2O2 resulted in a low survival rate of
oocytes and thus, the remaining surviving oocytes were
insubstantial for the completion of subsequent experiments. Thus,
100 µM H2O2 was considered the optimal
concentration for oxidative stress. In addition, when 200 µM
cysteine and 200 µM cystine were added to the IVM media, the
oocyte maturation rate and blastocyst formation rate were higher,
compared with those the control group (81.57±2.86, vs. 71.41±1.11%
and 25.04±1.34, vs. 19.80±2.66%, respectively; P<0.05).
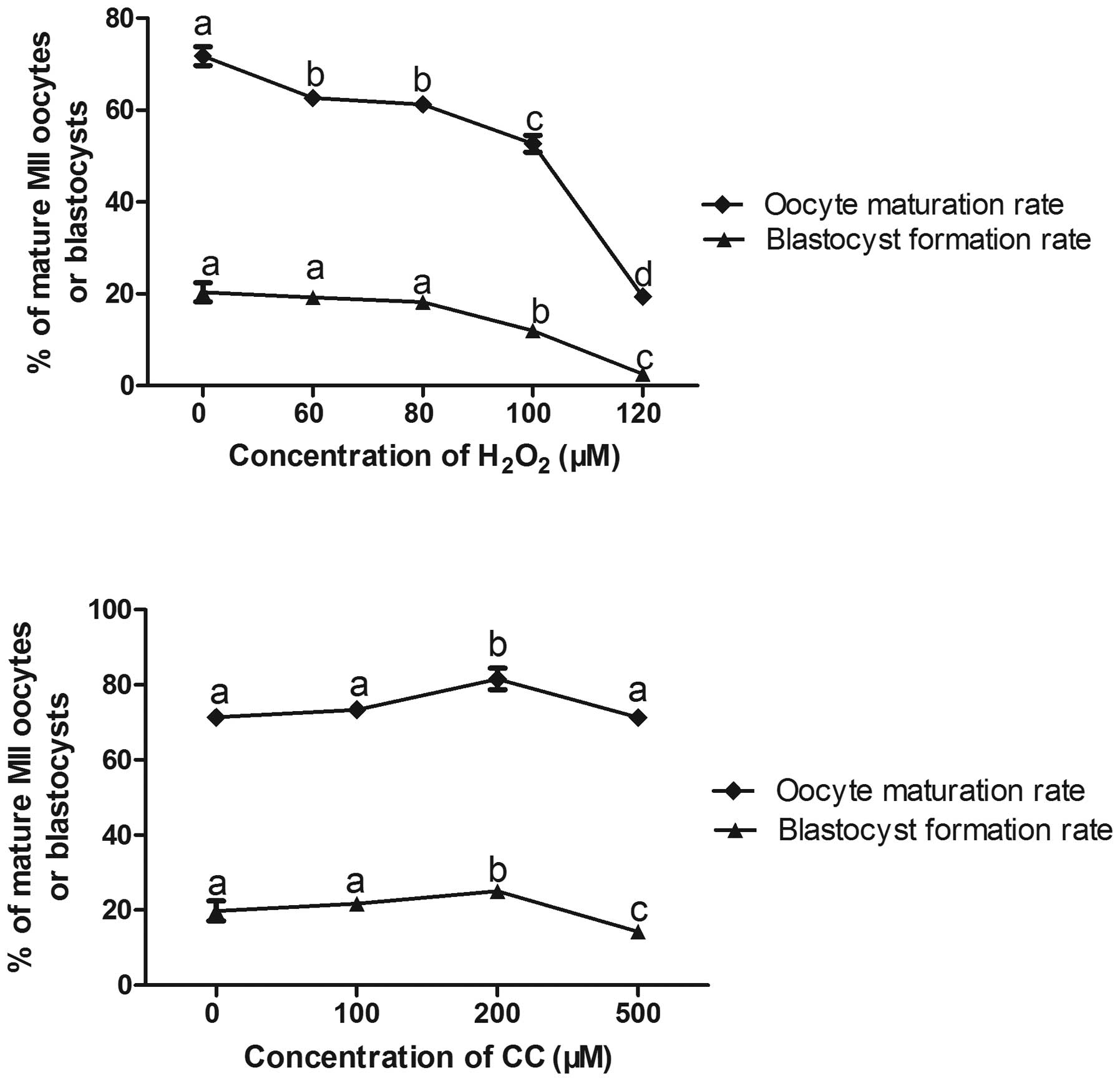 | Figure 1Optimal concentrations of
H2O2 and CC and the parthenogenetic
development of embryos. Data are expressed as the mean ± standard
error of the mean Means with different superscript letters are
statistically different (P<0.05). Oocyte maturation rate:
aP<0.05 vs. group 0; bP<0.05 vs. group
0, 100 µm and 120 µm; cP<0.05 vs. group
0, 60 µm, 80 µm and 120 µm;
dP<0.05 vs. group 0, 60 µm, 80 µm, 100
µm and 120 µm. Blastocyst formation rate:
aP<0.05 vs. group 0; bP<0.05 vs. group
0, 60 µm, 80 µm and 120 µm; c= P<0.05 vs.
group 60 µm, 80 µm and 100 µm. For Optimal
concentrations of CC: Oocyte maturation rate: aP<0.05
vs. group 0; bP<0.05 vs. group 0, 100 µm and
500 µm. Blastocyst formation rate: aP<0.05 vs.
group 0; bP<0.05 vs. group 0, 100 µm and 500
µm; cP<0.05 vs. group 0, 100 µm and 200
µm. H2O2, hydrogen peroxide; CC
cystine and cysteine. |
Effect of oxidative stress on goat oocyte
development following parthenogenesis
Culturing the goat oocytes in M199 with
H2O2 decreased the oocyte maturation rate and
blastocyst formation rate, compared with the control group
(49.47±2.65% vs. 65.71±2.74 and 13.47±1.11 vs. 20.57±0.61%
respectively; P<0.05; Table
II). The oocyte maturation and blastocyst formation rates in
the CC+H2O2 group were higher, compared with
those in the H2O2 group (69.96±3.51, vs.
49.47±2.65% and 19.19±0.53, vs. 13.47±1.11%, respectively;
P<0.05). The oocyte maturation rate and the blastocyst rate in
the CC group were also higher, compared with those in the
H2O2 group (78.36±2.83, vs. 49.47±2.65% and
23.06±0.59, vs. 13.47±1.11%, respectively; P<0.05). Furthermore,
the mean number of cells per blastocyst in the
H2O2 group was significantly lower, compared
with those in the other three groups (P<0.05), although the
embryonic cleavage rates did not differ among the four groups
(P>0.05).
 | Table IIEffect of oxidative stress on goat
oocyte development following parthenogenetic activation. |
Table II
Effect of oxidative stress on goat
oocyte development following parthenogenetic activation.
| Treatment | Oocytes examined
(n) | Maturation
rate | Cleavage rate | Blastocyst
formation rate | Cells/blastocysts
(n) |
|---|
| Control | 768 | 509
(65.71±2.74)a | 321
(62.37±1.16)a | 67
(20.57±0.61)a | 92±2.1a |
|
H2O2 | 854 | 464
(49.47±2.65)b | 255
(65.86±4.69)a | 35
(13.47±1.11)b | 75±3.6b |
| CC | 1024 | 786
(78.36±2.83)c | 500
(66.82±4.52)a | 113
(23.06±0.59)a | 99±3.4a |
|
H2O2+CC | 760 | 524
(69.96±3.51)a | 317
(62.88±1.90)a | 63
(19.19±0.53)a | 90±2.7a |
Effect of oxidative stress on apoptosis
of COCs following maturation
A TUNEL assay was used to evaluate the quality and
viability of the goat oocytes cultured in M199 with stress
inducers. The goat oocytes treated with H2O2
exhibited significantly increased DNA fragmentation, compared with
the control group (121±1.89 cells/COC, vs. 7.8±1.32 cells/COC,
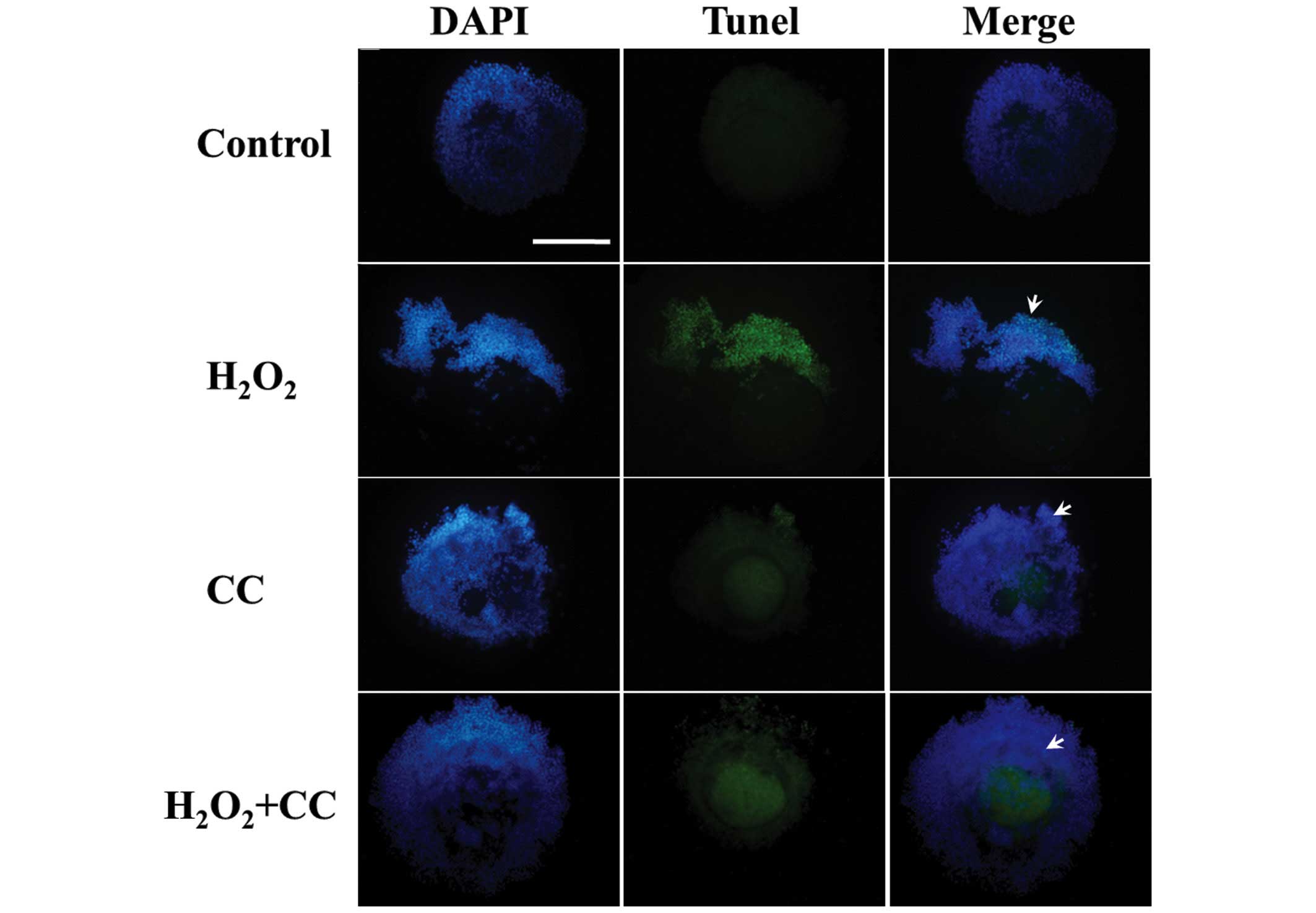
respectively; P<0.05), as shown in Figs. 2 and 3A). The H2O2 media
containing CC further reduced the number of apoptotic cells
(36±1.13 cells/COC), similar with the CC group (25±2.10 cells/COC).
These findings indicated that oxidative stress induced apoptosis
during goat oocyte IVM.
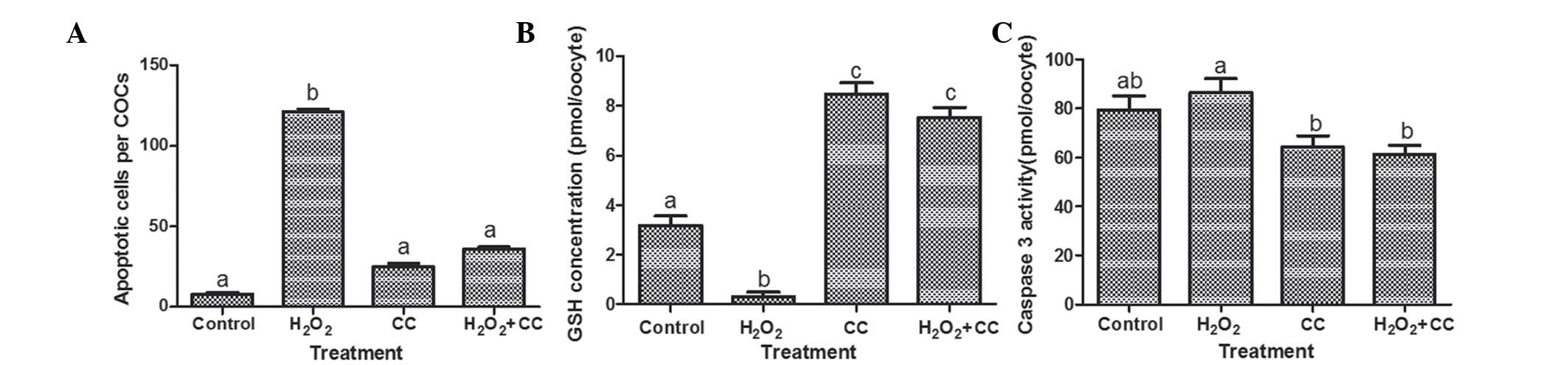 | Figure 3Effects of oxidative stress on levels
of apoptosis, intracellular GSH concentrations and caspase-3
activity in goat oocytes following maturation. (A) Number of
TUNEL-positive cells in goat cumulus-oocyte complexes, presented as
the number of TUNEL-positive nuclei. (B) Intracellular GSH
concentrations in the goat oocytes. (C) Caspase-3 activity in the
goat oocytes. Data are expressed as the mean ± standard error of
the mean. Means with different superscript letters are
statistically different (P<0.05). In (A) aP<0.05
vs. control group; b= P<0.05 vs. control group, CC group, and
H2O2+CC group. In (B) aP<0.05
vs. control group; bP<0.05 vs. control group, CC
group, and H2O2 +CC group;
cP<0.05 vs. control group and
H2O2 group. In (C) a,bP<0.05
vs. H2O2 group. GSH, glutathione; TUNEL,
terminal deoxynucleotidyl transferase UTP nick end labeling;
H2O2, hydrogen peroxide. |
GSH concentrations of goat oocytes
cultured in oxidative maturation media with and without CC
The mean intracellular GSH concentrations in the MII
oocytes treated with H2O2 were significantly
lower, compared with those in the control groups (0.31±0.18, vs.
3.18±0.38 pmol/oocyte; P<0.05; Fig.
3B). The addition of CC to the media significantly increased
the GSH concentrations, compared with the control and
H2O2 groups (7.53±0.41 vs. 0.31±0.18 and
3.18±0.38 pmol/oocyte, respectively; P<0.05). In addition, the
GSH concentration of the MII oocytes in the CC group was higher,
compared with those of the control and H2O2
groups (8.47±0.46, vs. 0.31±0.18 and 3.18±0.38 pmol/oocyte,
respectively; P<0.05).
Activity of caspase-3 activity in goat
oocytes cultured in oxidative maturation media with and without
CC
To confirm whether caspase-3 is involved in the IVM
of goat oocytes in H2O2-induced oxidative
media, caspase-3 activity was measured using its specific
substrate, Ac-DEVD-MCA. The activity of caspase-3 in the MII
oocytes with H2O2 was 86.4±5.71 pmol/oocyte,
which was significantly higher than the caspase-3 activities of the
oocytes in the CC group and the H2O2+CC group
(64.3±4.54 and 15±2.46 pmol/oocyte, respectively; P<0.05;
Fig. 3C).
Gene expression in goat oocytes cultured
in oxidative maturation media with and without CC
The expression levels of selected genes were
determined in MII oocytes during IVM, following treatment with
H2O2 and. or CC. The transcription levels
were highly variable in all treatment groups. A comparable pattern
of mRNA expression was observed for all transcripts analyzed,
although certain differences were noted (Fig. 4). The expression levels of PGC-1α
and NRF-1 were significantly higher in the
H2O2 group, compared with the control group
(P<0.05). Adding CC to the oxidative media significantly
downregulated the expression of PGC-1α and the expression of NRF-1
(P<0.05). The gene transcription levels of BAX were
significantly lower in the H2O2 group,
compared with the control group (P<0.05). The gene expression of
BCL-2 in the oocytes of the CC group was upregulated, compared with
the other three groups (P<0.05). Furthermore, the BAX:BCL-2
ratio in the H2O2 group was higher, compared
with the other three groups (P<0.05).
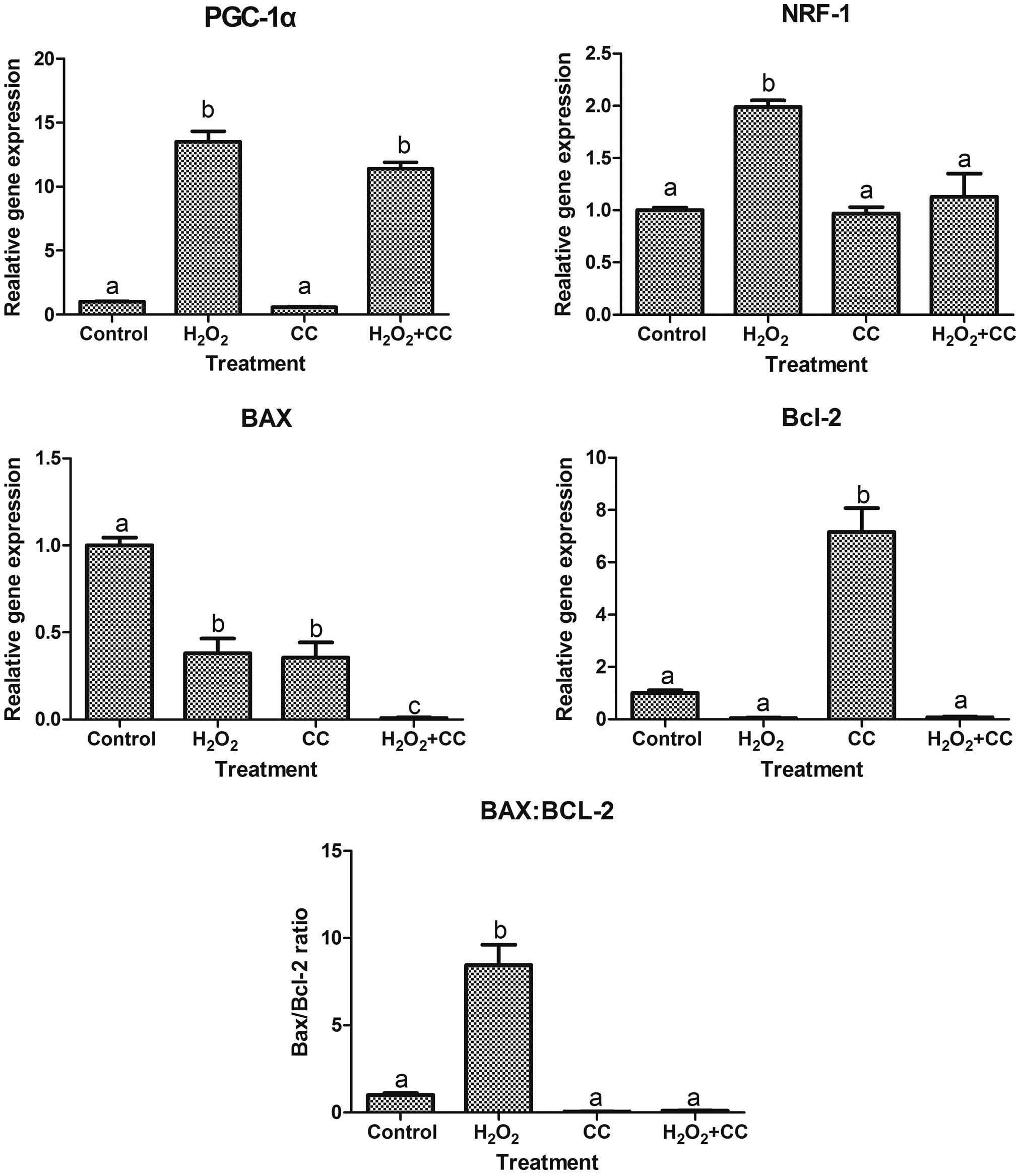 | Figure 4Gene expression levels in goat
oocytes, determined using reverese transcription-quantitative
polymerase chain reaction analysis. Data are presented as the mean
± standard error of the mean. Means with different superscript
letters are statistically different (P<0.05). For PGC-1,
aP<0.05 vs. group control; bP<0.05 vs.
group control and group CC. NRF-1, aP<0.05 vs.
control group; bP<0.05 vs. control group, CC group
and H2O2+CC group. BAX, aP<0.05
vs. control group; bP<0.05 vs. control group and
H2O +CC group, cP<0.05 vs. control group,
H2O2 group and CC group. Bcl-2,
aP<0.05 vs. control group; bP<0.05 vs.
control group, H2O2 group, and group
H2O2+CC. BAX:Bcl-2, aP<0.05 vs.
control group; bP<0.05 vs. control group, CC group
and H2O2+CC group.
H2O2, hydrogen peroxide; CC, cystine and
cysteine; PGC-1α, proliferator-activated receptor γ coactivator-1
α, NRF-1, nuclear respiratory factor-1; Bcl-2, B cell lymphoma 2;
BAX, Bcl-2 -associated X factor. |
Discussion
Mammalian embryonic development in vitro is
affected by culture conditions. In particular, ROS are
predominantly generated from the environment surrounding embryos.
Therefore, several studies have attempted to reduce levels of
oxidative stress, which causes defects in embryonic development.
The present study demonstrated that the oocytes exposed to 200
µM cysteine and 200 µM cystine exhibited improved
developed, compared with the oocytes in the other treatment groups.
By contrast, increasing the concentrations of cysteine and cystine
to 500 µM significantly decreased blastocyst formation. This
result indicated that higher concentrations of cysteine and cystine
do not improve embryonic development, possibly due to high cystine
concentrations disrupting the redox equilibrium in cells (18). However, a previous bovine study
suggested that adding 1.2 mM of cysteine to IVM media inhibits the
production of ROS in oocytes and the apoptosis of cumulus cells
(19). Different culture media,
and the combination of cysteine and cystine may affect the
concentrations used. Salmen et al confirmed that
supplementing cysteine to oocyte culture media increases the
intracellular concentration of GSH in oocytes, and promotes
pronuclear formation and embryonic development in mice (20). Yoshida et al found that
adding cysteine to the maturation medium of pig oocytes increases
GSH synthesis, producing GSH concentrations comparable to those
found in oocytes matured in vivo (21). These data suggest that culturing
early embryos in vitro with cysteine and cystine provides
more suitable conditions for embryonic growth and development. In
the present study, 100 µM H2O2 was
used to induce oocyte oxidative stress. Adding cysteine and cystine
to the oocytes under oxidative conditions improved oocyte
maturation and the parthenogenesis of embryonic development, and
also demonstrated that the addition of cysteine and cystine at
appropriate concentrations during oocyte in vitro culture
reduced oxygen tension and improved blastocyst development.
Cysteine, is arises in mammals through the
metabolism of pantetheine, a component of coenzyme A (22). Cystine is essential for GSH
synthesis, and it directly increases the GSH content of cells and
tissues. The GSH content is increased through increased synthesis
by adaptive mechanisms to oxidative stress. However, severe
oxidative stress suppresses GSH levels through deactivation of
these adaptive mechanisms (23).
GSH functions as an antioxidant through two mechanisms: i) it
functions as an ROS scavenger by reacting with certain species; and
ii) it acts as an inhibitor of transcription factor nuclear
factor-κB activation, thereby controlling the synthesis of various
enzymes (24,25). Acute increases in levels of
oxidative stress in cells significantly increases cellular
apoptosis, which can be attenuated by pretreatment with GSH-ethyl
ester (26). In the present study,
supplementation with cysteine and cystine under oxidative
conditions decreased the number of apoptotic cells and decreased
caspase-3 activity, which suggested that increasing GSH prevented
apoptosis of the goat oocytes. Kizhakkayil et al further
demonstrated that GSH depletion induces caspase-dependent and
caspase-independent apoptosis in human leukemic cells (27).
The mitochondria reportedly have anti-apoptotic and
antioxidant functions in germ cells and granulosa cells (28,29).
The mitochondrial regulators, NRF-1 and PGC-1α, interact to
regulate oxidative metabolism (30,31).
By contrast, endotoxin-induced oxidative stress causes the
mitochondrial translocation of the pro-apoptotic protein, Bax,
increases the total expression of Bax and downregulates the
expression of the antiapoptotic protein, BCL-2 (32). In the present study, PGC-1α and
NRF-1 were expressed at higher levels under oxidative conditions,
compared with the other groups, possibly due to the oocytes
requiring mitochondrial biogenesis and a high metabolism when
exposed to oxidative stress (33).
In addition, the BAX/BCL-2 ratio was highest in the oxidative
stress group, which suggested that the mitochondrial pathway may be
involved in the apoptosis mediated by
H2O2-induced oxidative stress (34).
In conclusion, the results of the present study
suggested that the addition of appropriate concentrations of
cysteine and cystine reduces the oxygen tension triggered by
H2O2 and improves parthenogenetic blastocyst
development. Apoptosis is involved in this process via the
mitochondrial pathway. These results may improve current
understanding of the apoptotic mechanisms involved in oxidative
stress, and provide novel insights into optimizing embryonic in
vitro development systems.
Acknowledgments
This study was financially supported by the National
Nature Science Foundation of China (grant no. 31272443), the
National Nature Science Foundation of Jiangsu Province, China
(grant no. BK20140541), the Ministry of Science and Technology
Support Project of China (grant no. 2011BAD19B02-7) and the Open
Research Fund of State Key Laboratory of Bioelectronics, Southeast
University, and Jiangsu University Fund (grant no. 1291270022).
References
|
1
|
Chaube SK, Prasad PV, Thakur SC and
Shrivastav TG: Hydrogen peroxide modulates meiotic cell cycle and
induces morphological features characteristic of apoptosis in rat
oocytes cultured in vitro. Apoptosis. 10:863–874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deleuze S and Goudet G: Cysteamine
supplementation of in vitro maturation media: A review. Reprod
Domest Anim. 45:e476–e482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamura H, Takasaki A, Taketani T, Tanabe
M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y and
Sugino N: The role of melatonin as an antioxidant in the follicle.
J Ovarian Res. 5:52012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agarwal A, Gupta S and Sharma RK: Role of
oxidative stress in female reproduction. Reprod Biol Endocrinol.
3:282005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goto Y, Noda Y, Mori T and Nakano M:
Increased generation of reactive oxygen species in embryos cultured
in vitro. Free Radic Biol Med. 15:69–75. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamura H, Takasaki A, Miwa I, Taniguchi K,
Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y and
Shimamura K: Oxidative stress impairs oocyte quality and melatonin
protects oocytes from free radical damage and improves
fertilization rate. J Pineal Res. 44:280–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Matos DG and Furnus CC: The importance
of having high glutathione (GSH) level after bovine in vitro
maturation on embryo development effect of beta-mercaptoethanol,
cysteine and cystine. Theriogenology. 53:761–771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gasparrini B, Boccia L, Marchandise J, Di
Palo R, George F, Donnay I and Zicarelli L: Enrichment of in vitro
maturation medium for buffalo (Bubalus bubalis) oocytes with thiol
compounds: Effects of cystine on glutathione synthesis and embryo
development. Theriogenology. 65:275–287. 2006. View Article : Google Scholar
|
|
9
|
Adona PR, de Bem TH, Mesquita LG, Rochetti
RC and Leal CL: Embryonic development and gene expression in
oocytes cultured in vitro in supplemented pre-maturation and
maturation media. Reprod Domest Anim. 46:e31–e38. 2011. View Article : Google Scholar
|
|
10
|
Kobayashi M, Asakuma S and Fukui Y:
Blastocyst production by in vitro maturation and development of
porcine oocytes in defined media following intracytoplasmic sperm
injection. Zygote. 15:93–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodríguez-González E, López·Bejar M,
Mertens MJ and Paramio MT: Effects on in vitro embryo development
and intracellular glutathione content of the presence of thiol
compounds during maturation of prepubertal goat oocytes. Mol Reprod
Dev. 65:446–453. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou P, Wu YG, Li Q, Lan GC, Wang G, Gao D
and Tan JH: The interactions between cysteamine, cystine and
cumulus cells increase the intracellular glutathione level and
developmental capacity of goat cumulus-denuded oocytes.
Reproduction. 135:605–611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tatone C, Amicarelli F, Carbone MC,
Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P and
Focarelli R: Cellular and molecular aspects of ovarian follicle
ageing. Hum Reprod Update. 14:131–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Behrman HR, Kodaman PH, Preston SL and Gao
S: Oxidative stress and the ovary. J Soc Gynecol Investig. 8(1
Suppl Proceedings): S40–S42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agarwal A and Said TM: Role of sperm
chromatin abnormalities and DNA damage in male infertility. Hum
Reprod Update. 9:331–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Z, Wan Y, Zhang Y, Wang Z, Jia R, Fan
Y, Nie H, Ying S, Huang P and Wang F: Follicular development and
expression of nuclear respiratory factor-1 and peroxisome
proliferator-activated receptor gamma coactivator-1 alpha in
ovaries of fetal and neonatal doelings. J Anim Sci. 90:3752–3761.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
18
|
Arrigo AP: Gene expression and the thiol
redox state. Free Radic Biol Med. 27:936–944. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nabenishi H, Ohta H, Nishimoto T, Morita
T, Ashizawa K and Tsuzuki Y: The effects of cysteine addition
during in vitro maturation on the developmental competence, ROS,
GSH and apoptosis level of bovine oocytes exposed to heat stress.
Zygote. 20:249–259. 2012. View Article : Google Scholar
|
|
20
|
Salmen JJ, Skufca F, Matt A, Gushansky G,
Mason A and Gardiner CS: Role of glutathione in reproductive tract
secretions on mouse preimplantation embryo development. Biol
Reprod. 73:308–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida M, Ishigaki K, Nagai T, Chikyu M
and Pursel VG: Glutathione concentration during maturation and
after fertilization in pig oocytes: Relevance to the ability of
oocytes to form male pronucleus. Biol Reprod. 49:89–94. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pitari G, Maurizi G, Flati V, Ursini CL,
Spera L, Duprè S and Cavallini D: Enzymatic synthesis of
S-aminoethyl-L-cysteine from pantetheine. Biochim Biophys Acta.
1116:27–33. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grosicka-Maciag E, Kurpios-Piec D, Grzela
T, Czeczot H, Skrzycki M, Szumiło M and Rahden-Staroń I: Protective
effect of N-acetyl-L-cysteine against disulfiram-induced oxidative
stress and apoptosis in V79 cells. Toxicol Appl Pharmacol.
248:210–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Li W and Kong AN: Anti-oxidative
stress regulator NF-E2-related factor 2 mediates the adaptive
induction of antioxidant and detoxifying enzymes by lipid
peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2:402012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aggarwal S, Dimitropoulou C, Lu Q, Black
SM and Sharma S: Glutathione supplementation attenuates
lipopolysaccharide-induced mitochondrial dysfunction and apoptosis
in a mouse model of acute lung injury. Front Physiol. 3:1612012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kizhakkayil J, Thayyullathil F, Chathoth
S, Hago A, Patel M and Galadari S: Glutathione regulates
caspase-dependent ceramide production and curcumin-induced
apoptosis in human leukemic cells. Free Radic Biol Med.
52:1854–1864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perez GI, Maravei DV, Trbovich AM,
Cidlowski JA, Tilly JL and Hughes FM Jr: Identification of
potassium-dependent and -independent components of the apoptotic
machinery in mouse ovarian germ cells and granulosa cells. Biol
Reprod. 63:1358–1369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tingaud-Sequeira A, Chauvigné F, Lozano J,
Agulleiro MJ, Asensio E and Cerdà J: New insights into molecular
pathways associated with flatfish ovarian development and atresia
revealed by transcriptional analysis. BMC Genomics. 10:4342009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patti ME, Butte AJ, Crunkhorn S, Cusi K,
Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R,
et al: Coordinated reduction of genes of oxidative metabolism in
humans with insulin resistance and diabetes: Potential role of PGC1
and NRF1. Proc Natl Acad Sci USA. 100:8466–8471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding L, Liang XG, Zhu DY and Lou YJ:
Icariin promotes expression of PGC-1alpha, PPARalpha and NRF-1
during cardiomyocyte differentiation of murine embryonic stem cells
in vitro. Acta Pharmacol Sin. 28:1541–1549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mishra DP and Dhali A: Endotoxin induces
luteal cell apoptosis through the mitochondrial pathway.
Prostaglandins Other Lipid Mediat. 83:75–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vina J, Gomez-Cabrera MC, Bor ras C, Froio
T, Sanchis-Gomar F, Martinez-Bello VE and Pallardo FV:
Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv
Rev. 61:1369–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Franco R and Cidlowski JA: Apoptosis and
glutathione: Beyond an antioxidant. Cell Death Differ.
16:1303–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|