Introduction
Acute lung injury (ALI) and its severe
manifestation, acute respiratory distress syndrome (ARDS), are
well-known life-threatening diseases with high morbidity rates
(35–50%) in critically ill patients (1). The multiple etiologies, including
severe sepsis, pneumonia, lung abscess and severe burns, cause
uncontrolled and self-amplified pulmonary inflammation, which are
at the center of the pathology of ALI/ARDS (2–5).
Studies have been confirmed that the alveolar epithelium and
pulmonary endothelium are the primary injury targets of this
disease (3–6). Although substantial progress has been
made in understanding the pathogenesis and pathophysiology of
ALI/ARDS, the treatment remains limited.
It has been confirmed that intratracheal
instillation of the endotoxin, lipopolysaccharide (LPS), can
reproducibly induce ALI (7). LPS
induces inflammatory cell infiltration into lung tissues, causing
the release of proinflammatory cytokines, reactive oxygen species
and chemotactic factors (8). These
changes can cause lung edema, alveolar hemorrhage and substantial
inflammation. In mammals, the Toll-like receptor 4 (TLR4)/myeloid
differentiation factor-2 (MD-2) complex constitutes an essential
component of the LPS receptor system (9). Common downstream signal transduction
pathways of TLR4/MD-2, which have been shown to mediate
inflammatory responses in lung injury, include nuclear factor-κB
(NF-κB) and mitogen-activated protein kinases (MAPKs) (10). Major signaling pathways regulating
cellular growth and the responses to cytokines and stress occur
through the conserved MAPK family, which consists of p42/44
extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun
N-terminal kinase (JNK) (11).
NF-κB is a critical transcription factor required for maximal
expression of several cytokines, and is involved in the
pathogenesis of acute lung injury. Reports have demonstrated that
the severity of inflammation in ALI/ARDS is markedly reduced by
inhibition of the NF-κB signaling pathway (12–15).
Therefore, drugs focusing on downregulating the TLR4/MD-2-mediated
MAPK and NF-κB signaling pathways have a potent therapeutic effect
for the treatment of ALI (16).
Radix Tetrastigmae (RT) is a commonly used Chinese
traditional medicine. The total flavonoid compounds isolated from
RT (RTFs), the root of Tetrastigma hemsleyanum Diels et
Gilg), are the predominant active ingredients and have wide
biological and pharmacological functions, including antitumor,
liver-protecting, antiviral, anti-inflammatory, immunoregulatory
effects and other pharmacological effects, with the exception that
it is basically non-toxic (17–19).
The present study investigated the effect of RTFs on mice with
LPS-induced ALI for the first time, to the best of our knowledge.
Elucidation of the mechanisms, TLR4/MD-2-mediated MAPK and NF-κB
signaling pathways and associated downstream regulators were also
investigated, with the aim of determining the therapeutic effects
of RTFs on ALI.
Materials and methods
Flavonoid isolation
The total flavonoids were extracted from RT,
according to the method previously reported (20) with mild revision. Briefly,
decoction powder of RT from Guizhou (China) was extracted twice
with 60% ethanol (v/w) in a boiling water bath for 90 min, followed
by filtering and concentration using reduced pressure distillation.
The crude extracts were dissolved in water and purified through
resin HPD826. Total flavonoid extracts were obtained with further
distillation under a vacuum with lyophilization of the eluant. The
quality of the total flavonoid extract was determined using
colorimetric HCl-Mg and aluminum chloride reactions, followed by
high-performance liquid chromatography (20,21).
Animals
Male BALB/c mice (n=60) were purchased from SLRC
Laboratory Animal, Ltd. (Shanghai, China). These mice used in the
present study were ~10-week old mice (20–25 g), matched for age and
weight. The animals were maintained in room with regulated
temperature (22–24°C), humidity (55±5%) and light (12 h light-dark
cycle), and with access to food and water ad libitum. All
procedures were performed in accordance with the Guidelines for
Animal Experimentation of Zhejiang University (Zhejiang,
China).
Mouse model of ALI
The mice were randomly divided into five groups
(n=12/group): Control [phosphate-buffered saline (PBS)]; model (2.5
mg/kg LPS) and TRF (40, 80 or 160 mg/kg+2.5 mg/kg LPS). All mice
were challenged intratracheally with either 25 µl/PBS alone
or 25 µl PBS containing 25 µg LPS (per 10 g body
weight) under anesthesia with intraperitoneal injection of chloral
hydrate (400 mg/kg). The mice were intragastrically administered
with 100 µl PBS or RTF 30 min prior to LPS challenge and for
2 days after LPS challenge. On the 4th day, mice were anesthetized
with chloral hydrate (0.4 g/kg; Sigma-Aldrich; St. Louis, MO, USA)
to collect the bronchoalveolar lavage fluid (BALF) and lung tissues
and then sacrificed by overdose of chloral hydrate. The severity of
lung injury was assessed by pathological changes in the lung
tissues and cellular profiles in the BALF.
Collection of BALF and leukocyte
counting
The BALF was collected, as previously described
(22). Briefly, under anesthesia,
the trachea was cannulated and the lungs were lavaged three times
with PBS (0.5 ml each time). The collected BALF was centrifuged at
200 × g for 5 min at 4°C, and the supernatant was frozen at −80°C
for subsequent biochemical analysis. The pelleted cells were then
re-suspended in PBS for cell counting. The numbers of leukocytes
were enumerated using a hemocytometer. For differential counts,
smears of the BALF cells (~105 cells/ml) from each mouse
were prepared by centrifugation (4°C at 200 × g for 5 min) and then
stained with Wright-Giemsa solution.
Inflammatory cytokine antibody array and
ELISA
The secretion of different cytokines from the BALF
was measured using a semi-quantitative mouse inflammation antibody
array from RayBiotech (Guangzhou, China). In brief, antibodies
against 40 different inflammatory cytokines were spotted onto the
cytokine array by the manufacturer. Initially, 100 µl
blocking buffer was added each well and incubated at room
temperature for 30 min to block the slides. Subsequently, 100
µl sample was added to each sub-array at room temperature
overnight, prior to incubation with biotin-conjugated primary
antibodies. Following washing, Cy3-conjugated streptavidin was
added for 2 h. Finally the chip was scanned using a GenePix 4000B
microarray scanner (Molecular Devices, Sunnyvale, CA, USA). The
images were analyzed using the GenePix Pro 5.0 software program
(Molecular Devices), and the levels of cytokines were quantified
according to the standard curve calibrated from the same array.
The secretion of interleukin (IL)-1β, tumor necrosis
factor (TNF)-α, IL-6 and IL-12p40 in the BALF were further measured
using an eBioscience ELISA kit (eBioscience, San Diego, CA, USA),
according to the manufacturer's protocol. In addition, activation
of the MAPK pathway in the lung tissues was also detected using
ELISA kits (Elisa Biotech, Shanghai, China), including p38,
phosphorylated (p)-p38, ERK, p-ERK, JNK and p-JNK.
Histopathologic examination of lung
tissues
To characterize the histological alterations in the
lung tissues, the lungs were excised and fixed in 10% buffered
formalin. Following dehydration with graded alcohol and embedding
in paraffin, the lung tissues were cut into 4 µm sections,
and subsequently stained with hematoxylin and eosin. The lung
injury was scored by an observer in a blinded-manner under a light
microscope, according to pathological changes, including cell
infiltration and accumulation, pulmonary edema, hemorrhage and the
thickness of the alveolar wall. Each indicator was graded according
to a five-point scale: 0=minimal damage, 1=mild damage. 2=moderate
damage, 3=severe damage and 4=maximal damage. Therefore, the
severity of lung injury was assessed according to the sum of the
individual indicator scores (23).
Western blot analysis of lung
tissues
The lung tissues were homogenized and quantified
using a BCA protein assay kit (Pierce Biotechnology, USA). Each
protein sample (20 µg) was mixed with the sample buffer to a
final concentration of 0.06 M Tris-HCl, pH 6.8, 2% SDS, 5%
β-mercaptoethanol, 10% glycerol and 0.025% bromophenol blue. Equal
quantities of protein were loaded per well, separated by SDS-PAGE
and transferred onto PVDF membranes (EMD Millipore, Billerica, CA,
USA). Subsequent to blocking with 5% nonfat milk for 1 h, the
membranes were probed with monoclonal antibodies rabbit anti-mouse
monoclonal antibodies against MD-2 (cat. no. sc-20668; Santa Cruz
Biotechnology, Inc., Danvers, MA, USA), TLR4 (cat. no. DR4841
UcallM Biotechnology Co., Ltd., Beijing, China), NF-κB p65 (cat.
no. 4764S) and p-NF-κB p65 (cat. no. 3033S) (Cell Signaling
Technology, Beverly, MA, USA) overnight at 4°C. Membranes were then
incubated with GAPDH-HRP mAb (LK9002T) and goat anti-rabbit
secondary antibody (LK2001) (Sungene Biotech, Co., Ltd., Tianjing,
China). The blots were normalized to GAPDH to correct for
differences in loading of the proteins. Densitometric values of the
immunoblot signals were obtained from three separate experiments
using Pro 3DS 6.0 image software (National Institutes of Health,
Bethesda, MA, USA).
NF-κB p65 activity assay
NF-κB activity was measured using an ELISA-based
TransAM NF-κB kit (Active Motif, Carlsbad, CA, USA) on the nuclear
protein extracts. The Nuclear Extract kit was obtained from Active
Motif for the preparation of nuclear extracts from the lung
tissues. The TransAM assay for NF-κB p65 activity was performed,
according to the manufacturer's protocol (13,14).
Briefly, oligonucleotides containing the NF-κB consensus binding
site (5′-GGGACTTCC-3′) were immobilized on a 96-well plate. The
active forms of NF-κB in the nuclear extracts were bound to the
oligonucleotides on the plate and detected colorimetrically. The
samples were measured at an absorbance of 450 nm on a
spectrophotometer, with a reference wavelength of 650 nm.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Differences between the mean values of normally
distributed data were assessed using one-way analysis of variance
(Dunnett's t-test) and two-tailed Student's t-test
using SPSS software v.19 (IBM, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
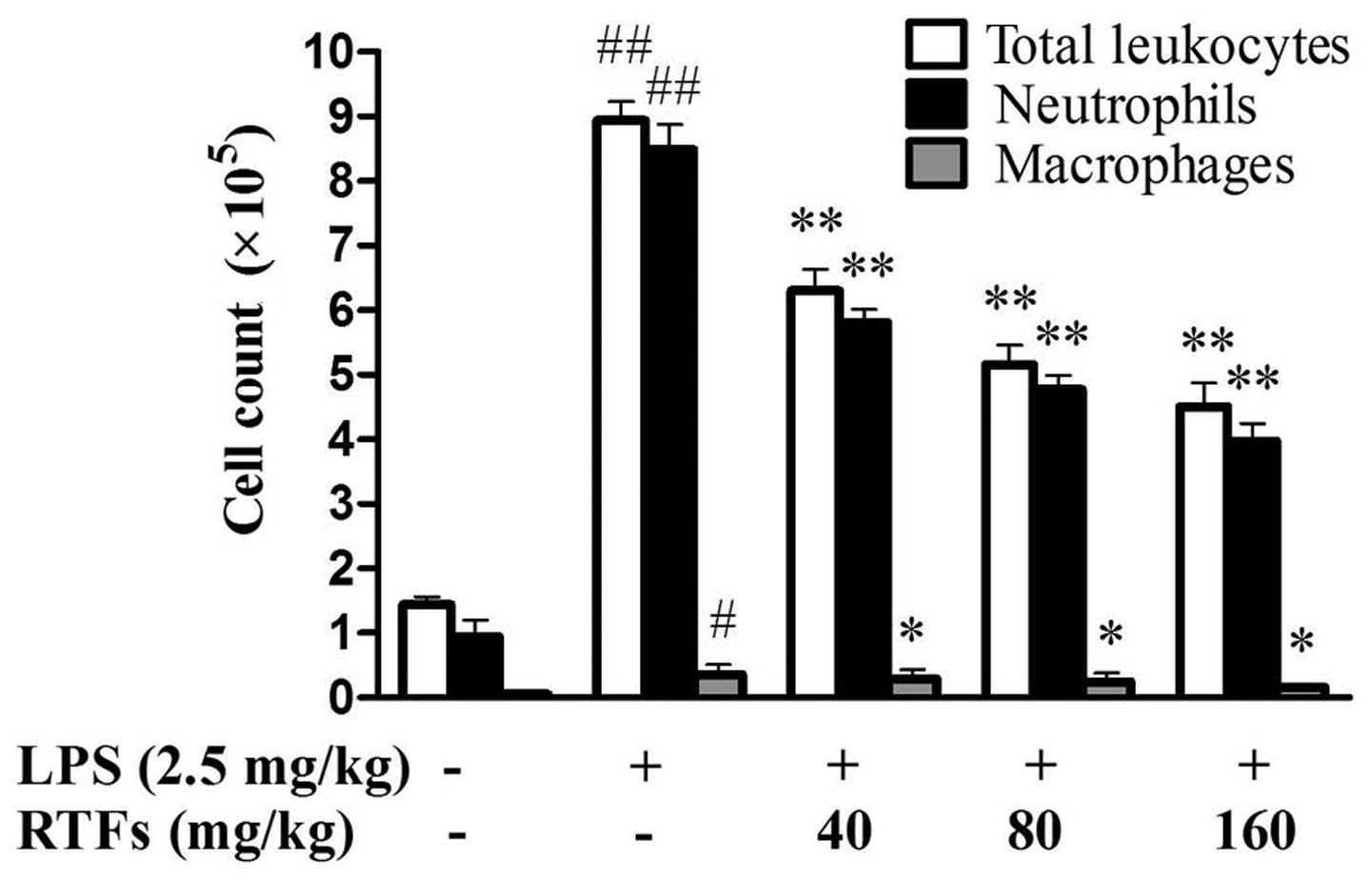
RTFs decreases LPS-induced leukocyte
infiltration in the BALF
The recruitment of inflammatory cells into pulmonary
alveoli is important in ALI. Following 3 days of LPS instillation,
the levels of total leukocytes, neutrophils and macrophages in the
BALF were significantly increased in all groups exposed to LPS,
compared with those of the control group. RTF treatment (40, 80 and
160 mg/kg) significantly reduced LPS-induced leukocyte exudation
(Fig. 1; P<0.05), particularly
the numbers of neutrophils and macrophages (P<0.01).
RTFs attenuate the secretion of
inflammatory factors in BALF
Elevation in the levels of proinflammatory cytokines
is a typical response to LPS challenge. In the present study, the
release of 40 inflammatory cytokines from the BALF of RTF-treated
mice was determined using a mouse cytokine antibody array. In the
microarray, any ≥1.5-fold increase or ≤0.65-fold decrease in signal
intensity for a single analyte between samples or groups was
considered a measurable and significant difference in expression,
provided that the sets of signals were well above background (mean
background ± two standard deviations; accuracy ~95%). Compared with
the model, the release of B lymphocyte chemoattractant (BLC),
granulocyte colony stimulating factor (GCSF), IL-1β, IL-6,
IL-12p40/p70, macrophage inflammatory protein-1α (MIP-1α), tissue
inhibitor of metalloproteinase-1 (TIMP-1) and TNF-α induced by LPS
were markedly reduced by RTF treatment (Fig. 2A). Among the eight factors, BLC,
GCSF and MIP-1α belong to the family of chemotactic factors, which
are predominantly involved in the infiltration of leukocytes. The
downregulated expression levels of these factors were consistent
with the numbers of leukocytes present in the BALF. These IL-1β,
IL-6, IL-12p40 and TNF-α proinflammatory factors may be the
functional cytokines in LPS-induced ALI.
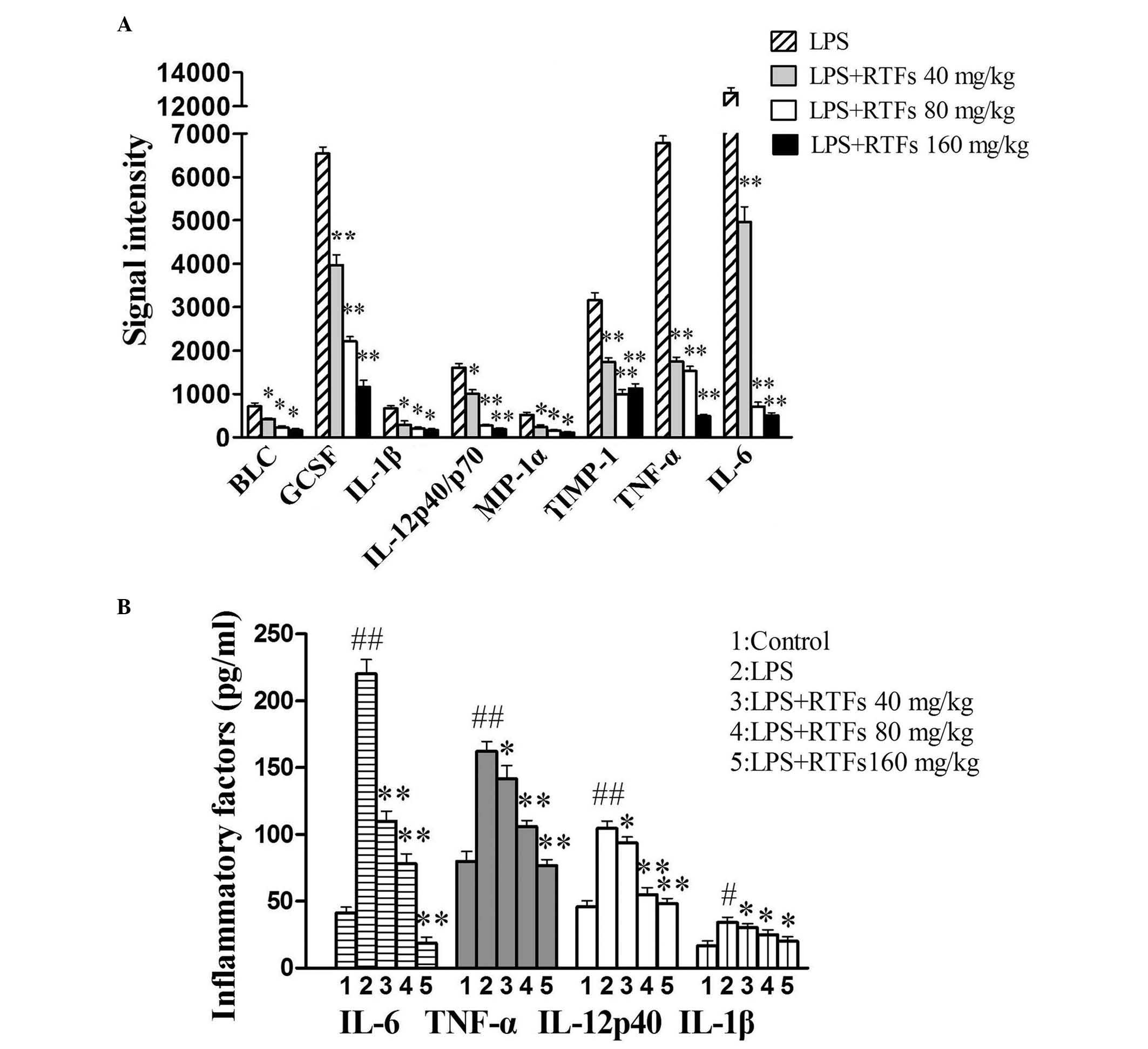 | Figure 2Effect of RTFs on the secretion of
inflammatory factors in bronchoalveolar lavage fluid from mice with
LPS-induced ALI. (A) Following treatment with LPS and/or RTFs for 3
days, several inflammatory factors were found to be significantly
altered in the inflammation antibody array. (B) Production of
IL-1β, IL-6, IL-12p40 and TNF-α was quantified using an ELISA
assay, which showed significant reductions in a dose-dependent
manner, compared with the LPS group. The data are expressed as the
mean ± standard deviation for each group. #P<0.05 and
##P<0.01, compared with the control;
*P<0.05 and **P<0.01, compared with the
LPS group. RTFs, flavonoids isolated from Radix Tetrastigmae; LPS,
lipopolysaccharide; BLC, B lymphocyte chemoattractant; GCSF,
granulocyte colony stimulating factor; IL, interleukin; MIP-1α;
macrophage inflammatory protein-1α TIMP-1; tissue inhibitor of
metalloproteinase-1; TNF-α, tumor necrosis factor-α. |
The release of IL-1β, IL-6, IL-12p40 and TNF-α was
also quantified using an ELISA assay. LPS treatment induced the
release of cytokines to a maximum. The maximal induction for IL-6
was 220±11 pg/ml, which was ~5-fold of that in the control.
Compared with the model, the production of cytokines in the
RTF-treated groups were significantly reduced in a dose-dependent
manner (Fig. 2B). By comparing the
results from the cytokine array analysis, the results from the
ELISA assay revealed a more marginal change between groups, which
may be accounted for by the sensitivity of the two different assay
systems.
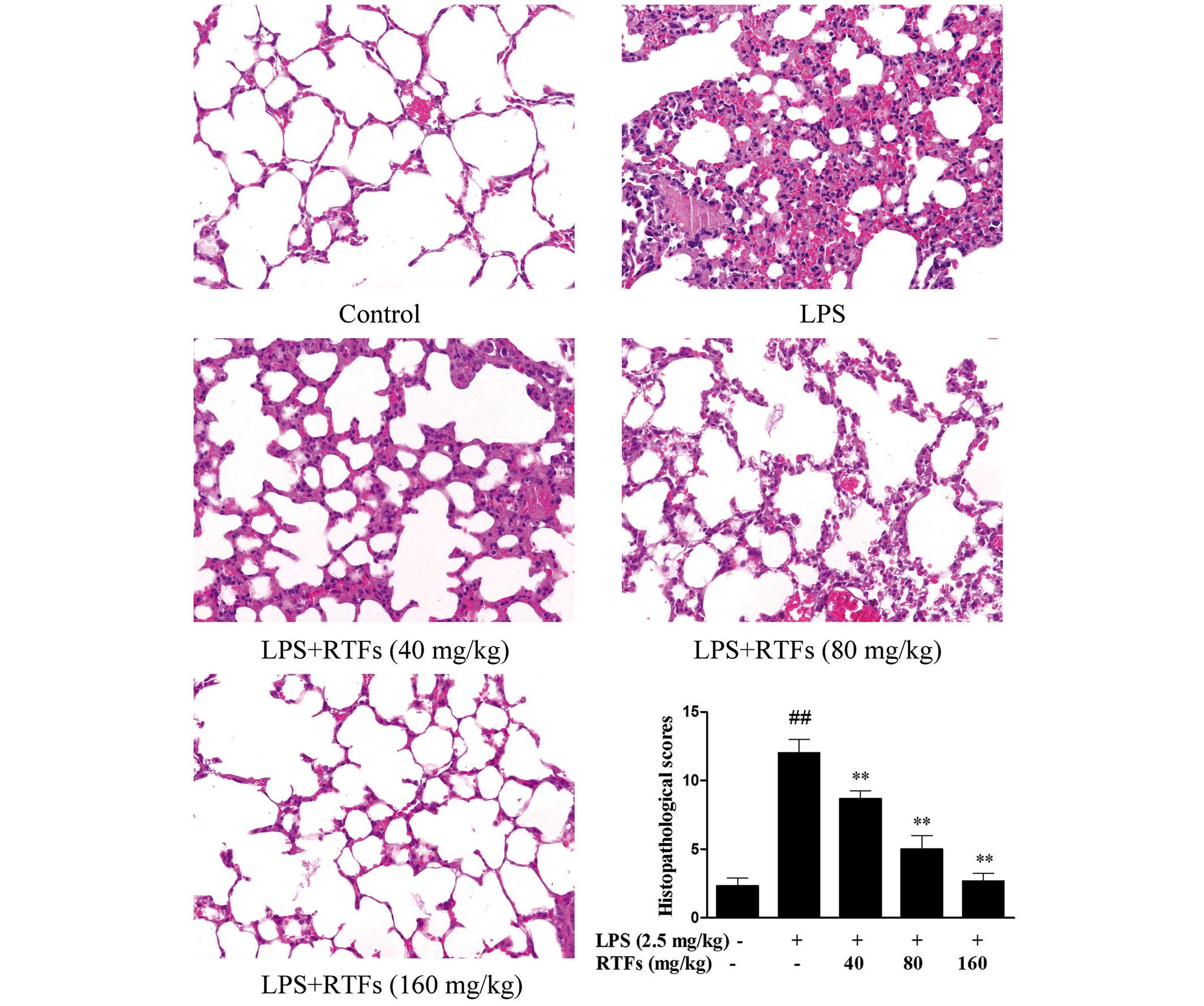
RTFs ameliorate histopathologic changes
the in lung tissues of ALI
The severity of LPS-induced ALI was further
evaluated. As shown in Fig. 3, the
control group showed normal structures, and no histopathological
changes were observed in the lung tissues. However, following
challenge with LPS, the pulmonary function of the LPS group was
markedly impaired, with various changes, including capillary
congestion, hemorrhage, the infiltration or aggregation of
neutrophils in airspace or vessel wall and thickening of the
alveolar wall. In the experimental groups, the histopathological
changes were abated by RTF treatment. In addition, similar
inhibitory effects were exhibited in the semi-quantitative assay,
in which histological changes were scored. The lung injury scores
increased in the LPS-treated groups, however, the increase was
significantly reduced by RTF treatment (Fig. 3; P<0.01). These results
demonstrated that RTF treatment attenuated the severity of lung
injury in the ALI mice induced by LPS, and improved the condition
of the lung tissues.
RTFs inhibit the expression levels of
TLR4 and MD-2 in lung tissues of ALI
To examine the mechanism underlying the
anti-inflammatory effect of RTFs, the expression levels of TLR4 and
MD-2 in the lung tissues were analyzed. LPS instillation markedly
increased the protein levels of TLR4 and MD-2 in the mouse lungs
(Fig. 4A), compared with the
control. However, RTF treatment significantly inhibited the
expression levels of TLR4 and MD-2 induced by LPS. As shown in
Fig. 4B, the inhibitory effect was
more marked on MD-2, compared with TLR4.
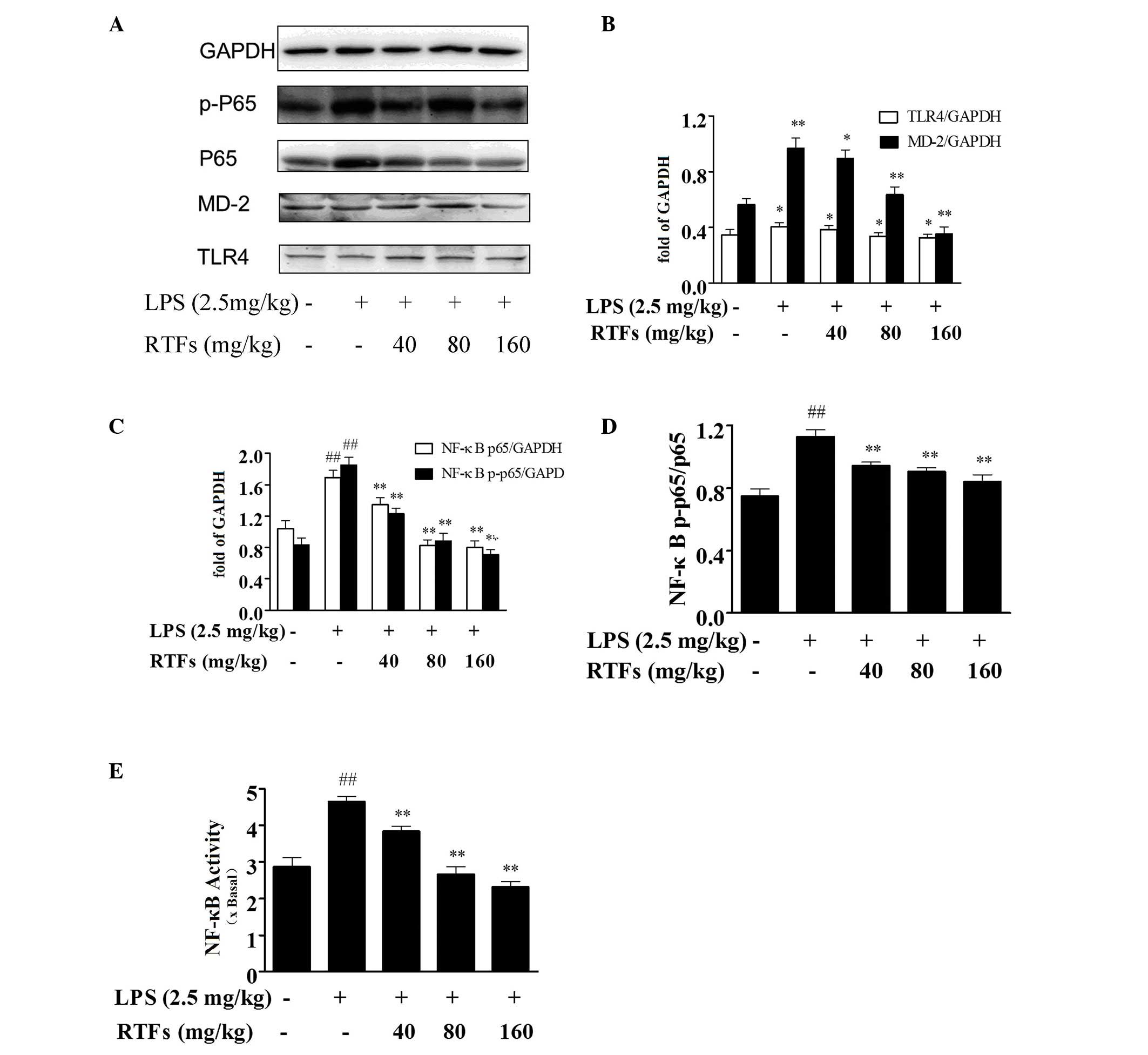 | Figure 4RTFs inhibit the TLR4/MD2-mediated
NF-κB signaling pathway in mice with LPS-induced ALI. (A) Protein
expression levels of TLR4, MD-2 and NF-κB were examined in the lung
homogenates 3 days following treatment with LPS and/or RTFs using
western blot analysis. Increased expression levels of (B) TLR4 and
MD-2, and (C) NF-κB were induced by LPS challenge. (D)
Phosphorylation of NF-κB was significantly downregulated by RTF
treatment. (E) DNA binding activity of NF-κB in nuclear extracts
from the lung tissues of the mice with LPS-induced ALI was
attenuated by RTFs, determined using a TransAM NF-κB kit. The data
are expressed as the mean ± standard deviation for each group.
##P<0.01, compared with the control;
*P<0.05 and **P<0.01, compared with the
LPS group. RTFs, flavonoids isolated from Radix Tetrastigmae; LPS,
lipopolysaccharide; ALI acute lung injury; TLR4, Toll-like receptor
4; MD-2, myeloid differentiation factor-2; NF-κB, nuclear
factor-κB; p-, phosphorylated. |
RTFs reduces NF-κB phosphorylation and
DNA binding activity in lung tissues of ALI
NF-κB is an important upstream transcription factor,
which induces the mRNA expression of various proinflammatory
cytokines following stimulation with LPS. Western blot analysis
(Fig. 4A and B) of the total
protein extracts from the lung tissues showed that LPS treatment
stimulated the expression and phosphorylation of NF-κB p65
(Fig. 4C and D), which was also
significantly downregulated by RTFs (P<0.05). Furthermore, the
effect of RTF on the transactivation of NF-κB was also detected. As
shown in Fig. 4E, RTFs
significantly reduced the DNA binding activity of NF-κB in the
nuclear extracts from lung tissues of the LPS-induced ALI mice,
which occurred in a dose-dependent manner, as determined using the
TransAM NF-κB kit (P<0.05).
RTFs alleviate MAPK activation in the
lung tissues of ALI
The MAPK signaling pathway is another important
pathway involved in inflammatory responses. Following 3 days of LPS
instillation, the MAPKs in the model group, including p38, JNK and
ERK, showed markedly upregulated levels of expression and
phosphorylation in the lungs, compared with the control group
(P<0.01). When the LPS-induced ALI mice were treated with RTFs,
the expression and phosphorylation levels of p38 and JNK were
significantly reduced. ERK and p-ERK exhibited marginal differences
between the groups, however, these differences were not
statistically significant (Fig.
5).
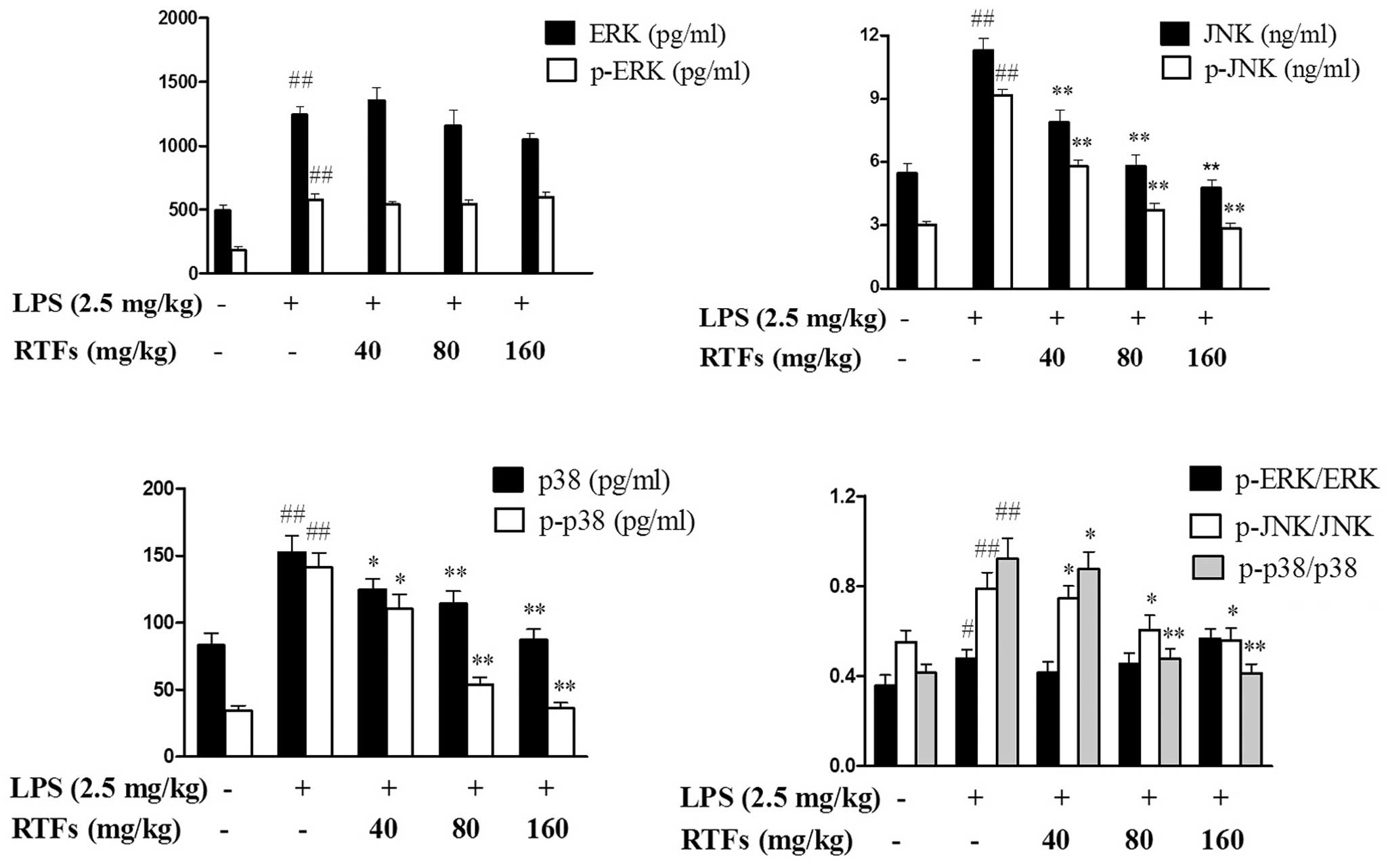 | Figure 5RTFs inhibit the mitogen-activated
protein kinase signaling pathway in lung tissues of mice with
LPS-induced acute lung injury. Following treatment with LPS and/or
RTFs for 3 days, the protein expression levels of JNK, p38, ERK and
their phosphorylated forms were examinedin the lung homogenates
using ELISA. JNK, p38 and their phosphorylated forms showed
significantly downregulated levels of expression and
phosphorylation in the RTF-treated groups. However, ERK and p-ERK
exhibited only marginal differences. The data are expressed as the
mean ± standard deviation of 10–12 mice/group.,
#P<0.05 and ##P<0.01, compared with the
control; *P<0.05 and **P<0.01, compared
with the LPS group. RTFs, flavonoids isolated from Radix
Tetrastigmae; LPS, lipopolysaccharide; JNK, c-Jun N-terminal
kinase; ERK, extracellular signal-regulated kinase; p-,
phosphorylated. |
Discussion
According to the theory of traditional Chinese
medicine, RT tuberous roots can promote the production of body
fluid and blood circulation, which results in improved lung
ventilation. RT roots have anti-inflammatory and immunomodulatory
activities, which can be used to treat bronchitis, pneumonia,
pharyngolaryngitis and viral meningitis. RT significantly inhibits
HAc-induced peritoneal capillary permeability, improves
xylene-induced ear edema in mice and improves albumin-induced paw
edema in rats (24). Feng et
al (18) initially discussed
the anti-inflammatory and antitoxic mechanisms from an
immunological viewpoint; it was found that RT may be pivotal in the
activation, proliferation and differentiation processes of T
lymphocytes. Flavonoids are one of the predominant components in RT
extract, therefore the present study aimed to elucidate the
anti-inflammatory properties and mechanisms of RTF.
The recruitment of leukocytes is critical in the
pathogenesis of ALI. It has been reported that neutrophil
accumulation in the lung parenchyma is a histological hallmark of
ALI (25). Leukocyte activation
leads to the production of reactive oxygen species and granular
enzymes, resulting in lung tissue injury by evoking an inflammatory
cascade. In the present study, it was found that RTF significantly
reduced the number of leukocytes in the BALF, including neutrophils
and macrophages, following LPS challenge. In addition, RTFs
significantly reduced macrophage infiltration in the BALF, which is
consistent with the findings in our in vitro investigation,
in which RTF treatment inhibited LPS-stimulated RAW264.7 monocyte
activation (26). These results
showed that RTFs effectively attenuated pulmonary inflammation in
LPS-induced ALI.
It is widely accepted that inflammatory cytokines
are crucial in the pathogenesis of LPS-induced ALI. Several
cytokines, particularly IL-1β, TNF-α and IL-6, have been identified
as key factors in leukocyte infiltration in the lung tissues of ALI
(27). In the present study, an
inflammation microarray was used, which detected marked increases
in the levels of a series of inflammatory mediators in the lung
tissues following LPS instillation, including the chemo-tatic
factors, GCSF, MIP-1α and BLC, and inflammatory cytokines, IL-1β,
TNF-α, IL-6 and IL-12p40. However, the secretion of these mediators
were all significantly reduced with RTF treatment. The change in
chemotactic factors is consistent with the leukocyte infiltration
in the BALF of the ALI mice. The production of inflammatory
cytokines was further confirmed using an ELISA assay. The findings
confirmed that the protective effect of RTFs on LPS-induced ALI may
be attributed to the inhibition of pulmonary inflammatory
responses.
Previous studies have indicated that TLR4 is the
major signaling receptor in LPS-induced inflammatory responses
(28) by regulating the activities
of downstream mediators, including MAPKs and NF-κB (29,30).
MD-2, which has been shown to be essential for the correct
intracellular distribution of TLR4 and efficient LPS recognition,
mediates LPS-induced responses in vitro and in vivo
(31–33). Accumulating evidence has shown that
MD-2 is the critical receptor protein for LPS and is necessary for
the ligand-dependent dimerization of the TLR4/MD-2 complex
(31–33). The binding of the TLR4/MD-2 complex
receptor by microbial products triggers a downstream signaling
cascade, culminating in the activation of NF-κB, and promoter
recognition sites direct the production of a number of
pro-inflammatory cytokines, including IL-1β, TNF-α and IL-6
(34). In the present study, RTFs
inhibited the expression of MD-2 and TLR4. Although the effect was
more marked on MD-2, compared with TLR4, they synergistically led
to a reduction in TLR4/MD-2 complex formation. These data suggested
that RTFs may attenuate the severity of LPS-induced local
inflammation, activated through the TLR4/MD-2-mediated signaling
pathway.
Activation of the TLR4/MD-2 pathway initiates a
series of MAPK and NF-κB cascades, and results in the
overproduction of cytokines, including TNF-α and IL-6 (35). NF-κB is an important transcription
factor in the LPS-TLR4 signaling pathway, regulating immune and
inflammatory processes (36,37).
The activation of MAPKs is critical in mediating a broad array of
cellular responses, including cell proliferation and
differentiation, transcription factor activation, and cytokine gene
expression and production (38–40).
Thus, inhibitors of NF-κB and MAPKs have been used as therapeutic
drugs in clinical applications for inflammation-associated human
diseases (41,42). In the present study, it was shown
that RTFs potently suppressed LPS-induced NF-κB p65 activation. In
addition to decreases in the expression and phosphorylation of
NF-κB p65 in the total protein extracted from lung tissues, a
reduction in DNA binding activity of intranuclear NF-κB p65 was
also observed following RTFs treatment, suggesting that RTFs
inhibited the mobilization of p65 into the nucleus and attenuated
NF-κB-mediated signaling transduction. In addition, RTFs inhibited
LPS-induced JNK and p38MAPK phosphorylation. Inhibition of the
activation of JNK, p38MAPK and NF-κB result in decreases in the
expression levels of cytokines, including IL-1β, IL-6, TNF-α and
IL-12 (43). Thus, the inhibitory
property of RTFs on LPS-induced generation of proinflammatory
cytokines may be associated with the attenuated activation of JNK,
p38MAPK and NF-κB.
The present study was the first, to the best of our
knowledge, to demonstrate that total flavonoids isolated from RT
significantly decreased the production of proinflammatory factors
in an LPS-induced ALI mouse model. It was found that RTFs may
interact with the expression and formation of the TLR4/MD-2 complex
to inhibit the action of LPS. RTF treatment protected the lung
tissues of the ALI mice from excessive phosphorylation of JNK,
p38MAPK and NF-κB, and down-regulated the expression and
DNA-binding activity of NF-κB through the TLR4/MD-2 pathway.
Collectively, the results of the present study provide novel
insights into the mechanisms of RTFs as anti-inflammatory agents
for LPS-mediated infections, which may be a potential therapeutic
agent for septic shock.
Acknowledgments
The authors would like to thank Dr Weihong Ge for
their expert guidance and support, and would like to thank the
members of the pharmacological team and research department of
Zhejiang Medical College for their assistance. This study was
supported by the grant of Zhejiang Modernization of Traditional
Chinese Medicine (grant no. 2010350).
Abbreviations:
|
RTFs
|
flavonoids isolated from Radix
Tetrastigmae
|
|
ALI
|
acute lung injury
|
|
JNK
|
c-Jun N-terminal kinase
|
|
TLR4
|
Toll-like receptor 4
|
|
MD-2
|
myeloid differentiation factor-2
|
|
NF-κB
|
nuclear transcription factor-κB
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Gotts JE and Matthay MA: Treating ARDS:
New hope for a tough problem. Lancet Respir Med. 2:84–85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandez-Bustamante A and Repine JE:
Chronic inflammatory diseases and the acute respiratory distress
syndrome (ARDS). Curr Pharm Des. 20:1400–1408. 2014. View Article : Google Scholar
|
|
3
|
Ware LB: Autopsy in ARDS: Insights into
natural history. Lancet Respir Med. 1:352–354. 2013. View Article : Google Scholar
|
|
4
|
Thompson BT and Matthay MA: The Berlin
definition of ARDS versus pathological evidence of diffuse alveolar
damage. Am J Respir Crit Care Med. 187:675–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Luca D, Piastra M, Tosi F, Pulitanò S,
Mancino A, Genovese O, Pietrini D and Conti G: Pharmacological
therapies for pediatric and neonatal ALI/ARDS: An evidence-based
review. Curr Drug Targets. 13:906–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu T, Wang DX, Zhang W, Liao XQ, Guan X,
Bo H, Sun JY, Huang NW, He J, Zhang YK, et al: Andrographolide
protects against LPS-induced acute lung injury by inactivation of
NF-κB. PLoS One. 8:e564072013. View Article : Google Scholar
|
|
7
|
Matute-Bello G, Frevert CW and Martin TR:
Animal models of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L379–L399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beduschi MG, Guimarães CL, Buss ZS and
Dalmarco EM: Mycophenolate mofetil has potent anti-inflammatory
actions in a mouse model of acute lung injury. Inflammation.
36:729–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song DH and Lee JO: Sensing of microbial
molecular patterns by Toll-like receptors. Immunol Rev.
250:216–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ, Lee HS, Chong YH and Kang JL: p38
mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB
activation in the development of lung injury and RAW 264.7
macrophages. Toxicology. 225:36–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arndt PG, Young SK, Lieber JG, Fessler MB,
Nick JA and Worthen GS: Inhibition of c-Jun N-terminal kinase
limits lipopolysaccharide-induced pulmonary neutrophil influx. Am J
Respir Crit Care Med. 171:978–986. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao M, Zhu T, Wang T and Wen FQ:
Hydrogen-rich saline reduces airway remodeling via inactivation of
NF-κB in a murine model of asthma. Eur Rev Med Pharmacol Sci.
17:1033–1043. 2013.PubMed/NCBI
|
|
13
|
Zhu T, Zhang W, Xiao M, Chen H and Jin H:
Protective role of andrographolide in bleomycin-induced pulmonary
fibrosis in mice. Int J Mol Sci. 14:23581–23596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-κB signalling pathway in LPS-induced acute lung
injury in a murine model. Mol Med Rep. 10:447–452. 2014.PubMed/NCBI
|
|
15
|
Jin LY, Li CF, Zhu GF, Wu CT, Wang J and
Yan SF: Effect of siRNA against NF-κB on sepsis-induced acute lung
injury in a mouse model. Mol Med Rep. 10:631–637. 2014.PubMed/NCBI
|
|
16
|
Smyth K, Garcia K, Sun Z, Tuo W and Xiao
Z: TLR agonists are highly effective at eliciting functional memory
CTLs of effector memory phenotype in peptide immunization. Int
Immunopharmacol. 15:67–72. 2013. View Article : Google Scholar :
|
|
17
|
Chen LY and Guo SH: Progress in studies of
chemical composition and pharmacological effects of Tetrastigmatis
Hems Leyani. Zhejiang Zhong Yi Xue Yuan Xue Bao. 12:1368–1370.
2012.
|
|
18
|
Feng Z, Hao W, Lin X, Fan D and Zhou J:
Antitumor activity of total flavonoids from Tetrastigma hemsleyanum
Diels et Gilg is associated with the inhibition of regulatory T
cells in mice. Onco Targets Ther. 7:947–956. 2014.PubMed/NCBI
|
|
19
|
Ma D, Li W, Ma Z, et al: Anti-liver damage
activity analysis of polysaccharide in Radix Tetrastigmatis
Hemsleyani. J Med Res. 41:33–36. 2012.
|
|
20
|
Liu B, Yang J, Ma Y, Yuan E and Chen C:
Antioxidant and angiotensin converting enzyme (ACE) inhibitory
activities of ethanol extract and pure flavonoids from Adinandra
nitida leaves. Pharm Biol. 48:1432–1438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Ding L, Yu A, Yang R, Wang X, Li
J, Jin H and Zhang H: Continuous determination of total flavonoids
in Platycladus orientalis (L.) Franco by dynamic microwave-assisted
extraction coupled with on-line derivatization and
ultraviolet-visible detection. Anal Chim Acta. 596:164–170. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo L, Li WJ, Xu MJ and Wang X: A mouse
model of acute lung inflammation induced by lipopolysaccharide
inhalation. Beijing Da Xue Xue Bao. 41:226–229. 2009.In Chinese.
PubMed/NCBI
|
|
23
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Z, Mao Q and Wei J: Evaluation of
anti-inflammatory, analgesic and antipyretic actions for the
extracts from Radix Tetrastigmae. Chinese Journal of New Drugs.
14:861–864. 2005.
|
|
25
|
Zhou X, Dai Q and Huang X: Neutrophils in
acute lung injury. Front Biosci (Landmark Ed). 17:2278–2283. 2012.
View Article : Google Scholar
|
|
26
|
Liu D, Cao G, Han L, Ye Y, SiMa Y and Ge
W: Flavonoids from Radix Tetrastigmae inhibit TLR4/MD-2 mediated
JNK and NF-κB pathway with anti-inflammatory properties. Cytokine.
84:29–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Neuhöfer P, Song L, Rabe B,
Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius
H, et al: IL-6 trans-signaling promotes pancreatitis-associated
lung injury and lethality. J Clin Invest. 123:1019–1031. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gioannini TL, Teghanemt A, Zhang D,
Coussens NP, Dockstader W, Ramaswamy S and Weiss JP: Isolation of
an endotoxin-MD-2 complex that produces Toll-like receptor
4-dependent cell activation at picomolar concentrations. Proc Natl
Acad Sci USA. 101:4186–4191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Theoharides TC, Kempuraj D, Tagen M, Conti
P and Kalogeromitros D: Differential release of mast cell mediators
and the pathogenesis of inflammation. Immunol Rev. 217:65–78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wolfs TG, Dunn-Siegrist I, van't Veer C,
Hodin CM, Germeraad WT, van Zoelen MA, van Suylen RJ,
Peutz-Kootstra CJ, Elson G, Pugin J and Buurman WA: Increased
release of sMD-2 during human endotoxemia and sepsis: A role for
endothelial cells. Mol Immunol. 45:3268–3277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schnabl B, Brandl K, Fink M, Gross P,
Taura K, Gäbele E, Hellerbrand C and Falk W: A TLR4/MD2 fusion
protein inhibits LPS-induced pro-inflammatory signaling in hepatic
stellate cells. Biochem Biophys Res Commun. 375:210–214. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Kumar A, Wheater M and Yu FS:
Lack of MD-2 expression in human corneal epithelial cells is an
underlying mechanism of lipopolysaccharide (LPS) unresponsiveness.
Immunol Cell Biol. 87:141–148. 2009. View Article : Google Scholar
|
|
34
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie G, Chen N, Soromou LW, Liu F, Xiong Y,
Wu Q, Li H, Feng H and Liu G: p-Cymene protects mice against
lipopolysaccharide-induced acute lung injury by inhibiting
inflammatory cell activation. Molecules. 17:8159–8173. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling M, Li Y, Xu Y, Pang Y, Shen L, Jiang
R, Zhao Y, Yang X, Zhang J, Zhou J, et al: Regulation of miRNA-21
by reactive oxygen species-activated ERK/NF-κB in arsenite-induced
cell transformation. Free Radic Biol Med. 52:1508–1518. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han DW, Lee MH, Kim HH, Hyon SH and Park
JC: Epigallocatechin-3-gallate regulates cell growth, cell cycle
and phosphorylated nuclear factor-κB in human dermal fibroblasts.
Acta Pharmacol Sin. 32:637–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai JN, Zong Y, Zhong LM, Li YM, Zhang W,
Bian LG, Ai QL, Liu YD, Sun J and Lu D: Gastrodin inhibits
expression of inducible NO synthase, cyclooxygenase-2 and
proinflammatory cytokines in cultured LPS-stimulated microglia via
MAPK pathways. PloS One. 6:e218912011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu HT, Huang P, Ma P, Liu QS, Yu C and Du
YG: Chitosan oligosaccharides suppress LPS-induced IL-8 expression
in human umbilical vein endothelial cells through blockade of p38
and Akt protein kinases. Acta Pharmacol Sin. 32:478–486. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu C, Zhang X, Zhou JX, Wei W, Liu DH, Ke
P, Zhang GF, Cai GJ and Su DF: The protective action of ketanserin
against lipopolysaccharide-induced shock in mice is mediated by
inhibiting inducible NO synthase expression via the MEK/ERK
pathway. Free Radic Biol Med. 65:658–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi Y, Lee MK, Lim SY, Sung SH and Kim
YC: Inhibition of inducible NO synthase, cyclooxygenase-2 and
interleukin-1beta by torilin is mediated by mitogen-activated
protein kinases in microglial BV2 cells. Br J Pharmacol.
156:933–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu X, Yin P, Wan C, Chong X, Liu M, Cheng
P, Chen J, Liu F and Xu J: Punicalagin inhibits inflammation in
LPS-induced RAW264.7 macrophages via the suppression of
TLR4-mediated MAPKs and NF-κB activation. Inflammation. 37:956–965.
2014. View Article : Google Scholar : PubMed/NCBI
|