Introduction
Cervical cancer is the most common malignant tumor
in the female reproductive tract. Previously, increased incidence
and mortality of cervical cancer, and a younger age at diagnosis
were reported, requiring further research and more effective
treatment of this deadly disease (1).
Currently, the combination of radiotherapy with
platinum-based chemotherapy is the gold-standard treatment for
advanced cervical cancer (2).
Although the addition of platinum-based chemotherapy to
radiotherapy has increased the 5-year survival of advanced-stage
cervical cancer patients (3),
systemic toxicity limits the use of high-dose chemotherapeutic
drugs.
Therefore, alternative therapies for cervical cancer
are urgently required. Complementary and alternative medicines can
perhaps benefit patients with cervical cancer as an adjunctive
therapy. Among them, Chinese traditional medicine has become
increasingly prominent and popular in cancer patients due to its
efficacy and low toxicity (4).
Glaucocalyxin (Gln), an ent-kaurane diterpenoid
isolated from the Chinese traditional medicine, Rabdosia
japonica, has been used in traditional medicine as an
antibacterial, anti-inflammatory and anticancer agent (5). Gln has three major forms, GlnA, GlnB
and GlnC. Previously, GlnA has been intensively studied for its
potent anticancer effects and its diverse molecular targets
involved in tumorigenesis (6–8).
By contrast, few studies have investigated the
functions of GlnB. GlnB has only one structural difference from
GlnA, an acetylated hydroxyl group at C14. This acetyl group
results in high liposolubility and may enhance the antitumor
activity of ent-kaurane diterpenoid. The role of GlnB in cancer
remains largely unknown. The present study reported that GlnB
exerts its anticancer effects by inducing apoptosis and autophagy,
and inhibiting proliferation in cervical cancer cells.
Materials and methods
Cell culture
Human HeLa and SiHa cervical cancer cells were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). The cell lines were
maintained in modified Eagle's media, containing 10% fetal bovine
serum (FBS) at 37°C in a 5% CO2 atmosphere.
Antibodies and reagents
The following antibodies were used in the present
study: Rabbit anti-light chain (LC)3 (cat. no. 14600-1-AP), rabbit
anti-poly (ADP-ribose) polymerase 1 (PARP1; cat. no. 13371-1-AP)
and anti-glyceraldehyde 3-phosphate dehydrogenase (cat. no.
10494-1-AP; ProteinTech, Rosemont, IL, USA); rabbit
anti-phosphorylated protein kinase B (p-)AKTS473 (cat.
no. 9271) and rabbit AKT (cat. no. 9272) (Cell Signaling
Technology, Inc., Beverly, MA, USA) (Cell Signaling Technology,
Inc., Beverly, MA, USA); and rabbit anti-phosphatase and tensin
homolog (PTEN; cat. no. ab31392 Abcam, Cambridge, MA, USA). The
annexin V-fluorescent isothiocyanate Apoptosis Detection kit was
purchased from Beyotime Institute of Biotechnology (Jiangsu,
China).
Chemicals
GlnB, kindly provided by Dr Suping Bai (School of
Pharmacy, Xinxiang Medical University, Xinxiang, China) was
dissolved in dimethylsulfoxide (DMSO) to make 64 mmol/l stock
solutions that was used for all experiments in the present study.
Both 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide
(MTT) and DMSO were purchased from MP Biomedical, LLC (Santa Ana,
CA, USA).
MTT assay
The cells were incubated overnight at 37°C in 5%
CO2 in media containing 10% FBS at a concentration of
1,000 cells/well of a 96-well plate. On the next day, the cells
were treated with either vehicle control (DMSO) or varying
concentrations of GlnB, and were allowed to grow for an additional
72 h. Cell proliferation was assessed using the MTT assay.
Following the addition of MTT (20 µl of 5 mg/ml MTT/well),
the plate was incubated at 37°C for 4–5 h. The media was
subsequently removed and 150 µl DMSO was added. The plate
was then incubated in the same conditions for 5 min. Proliferation
was quantified using a plate reader at an optical density (OD) of
570 nm. The cell growth inhibition was calculated as
(ODtest − OD0 h) / (ODcontrol − OD0
h) × 10 0%. A cell growth inhibition curve was generated by
plotting cell growth inhibition against drug concentration, and the
half-maximal inhibitory concentration (IC50) was
determined using GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA).
Apoptosis assay
Apoptosis was analyzed using annexin V/propidium
iodide (PI) staining, according to the manufacturer's protocol.
Briefly, the cells were seeded into 60-mm dishes. On the next day,
the cells were treated with either DMSO or varying concentrations
of GlnB. After 24 h, the cells were collected by trypsinization,
washed twice with cold phosphate-buffered saline and were
subsequently resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. A total of 100 µl of the solution
was transferred to a 5 ml culture tube, followed by the addition of
400 µl 1X binding buffer, and stained with annexin V and PI.
The stained cells were immediately analyzed on a FACScan flow
cytometer. The stained cells were immediately collected using a BD
FACScan flow cytometer (BD, Franklin Lakes, NJ, USA), and data were
analyzed using the Winlist program 8.0 (Verity House, Topsham, ME,
USA).
Western blotting
Cells grown in the presence or absence of GlnB were
lysed in radioimmunoprecipitation buffer. A total of 40 µg
total proteins per sample was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The proteins were
subsequently blotted onto polyvinylidene difluoride membranes and
were blocked for 1 h at room temperature with 5% bovine serum
albumen (BSA) in 1X Tris-buffered saline containing 0.1% Tween-20
(TBST). Following blocking, the membranes were probed with
anti-PARP1 (1:1,000), anti-LC3 (1:1,000), anti-PTEN (1:500),
anti-p-AKTS473 (1:1,000), anti-AKT (1:1,000) or
anti-GAPDH (1:1,000) primary antibodies diluted in 5% BSA solution
and incubated at 4°C overnight. The membranes were then washed in
TBST and incubated with horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG secondary antibody (1:1,000; cat. no. 1706515;
Bio-Rad, Hercules, CA, USA) for 1 h at room temperature. After
washing again in TBST, the membranes were developed using
Lumi-Light chemiluminescence substrate (Roche, Indianapolis, IN,
USA). Digital imaging and signal quantification were performed on a
Chemi-Genius2 Bio-Imager (Syngene, Frederick, MD, USA) using
GeneTools software Gene tools 4.01(c) (Syngene).
Statistical analysis
Statistical significances between groups were
determined by two-tailed Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results and Discussion
For >40 years, natural products have served us
well in combating cancer. Of the antitumor compounds used in
medicine, three quarters are natural products or their derivatives,
including paclitaxel (9).
Ent-kaurane diterpenoids, including GlnA, are known
to exhibit strong antitumor activities (5). Compared with GlnA, the only
structural difference of GlnB is an acetylated hydroxyl group at
C14, and this highly liposoluble acetyl group of GlnB makes the
compound easier to penetrate into the cells, thereby exhibiting
stronger anti-inflammatory properties (10). The antitumor activity of
ent-kaurane diterpenoids is through a similar structure-activity
association (5), suggesting that
GlnB may have stronger antitumor activity compared with GlnA.
Although GlnB has been reported to possess anti-inflammatory
activity (10), the role of GlnB
in cancer cells remains unclear.
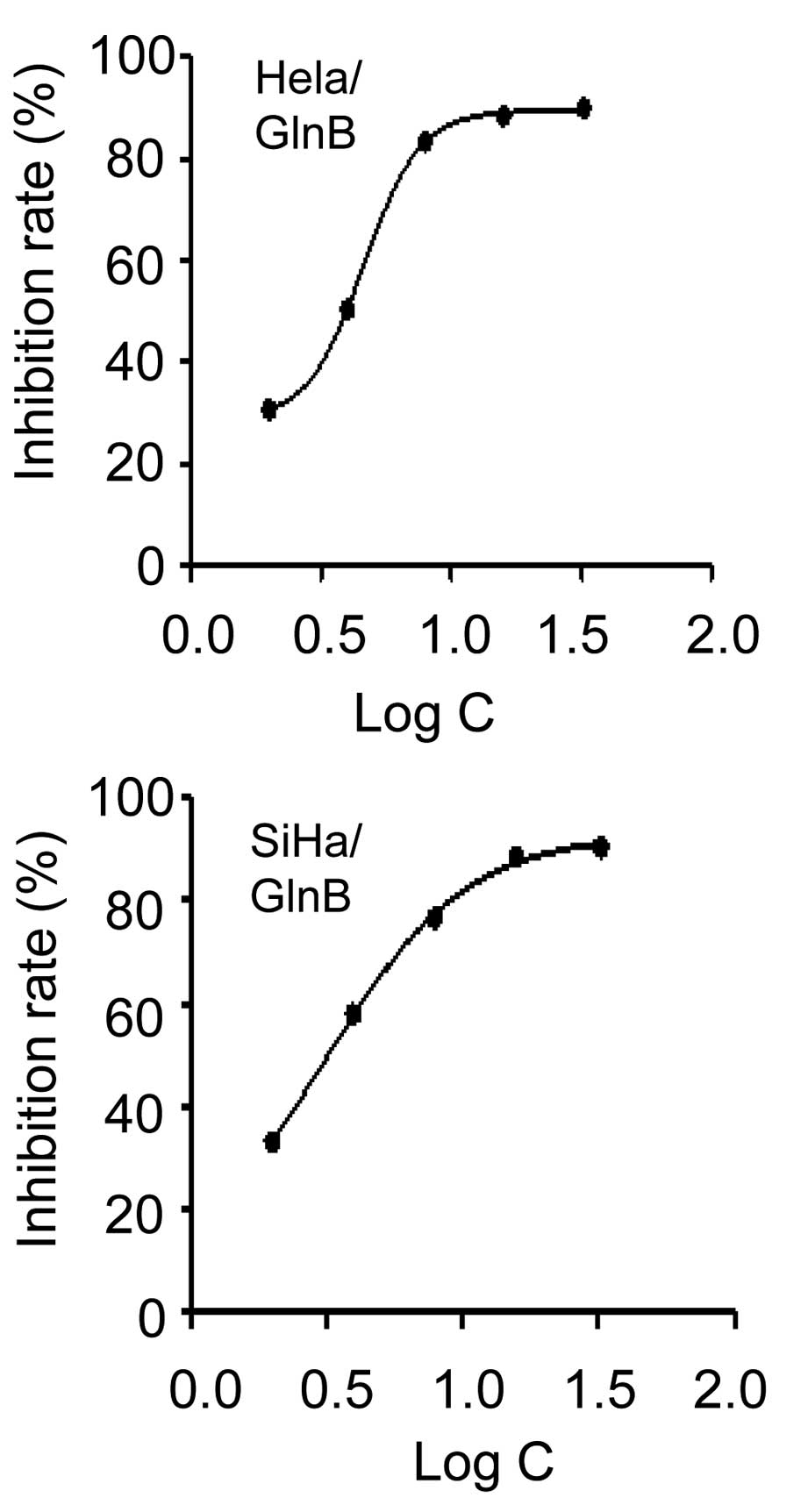
GlnB inhibits cell proliferation in
cervical cancer cells in vitro
To examine whether GlnB affects cervical cancer
proliferation, the present study first tested the effect of GlnB on
the growth of human HeLa and SiHa cervical cancer cell lines.
Following exposure to various concentrations of GlnB for 72 h, cell
growth and viability were measured using an MTT assay. GlnB
treatment inhibited the growth of HeLa and SiHa cells in a
dose-dependent manner (Fig. 1).
The IC50 of GlnB for HeLa and SiHa cells was 4.61 and
3.11 µmol/l, respectively (Fig.
1). These data suggested that GlnB treatment inhibited the
proliferation of HeLa and SiHa cells. Additionally, the
IC50 determined from in vitro proliferation
studies will assist with establishing the doses required for in
vivo studies, which will also require the pharmacokinetics data
of GlnB and the desired inhibition in vivo.
GlnB induces apoptosis and autophagy in
HeLa and SiHa cells
Yang et al (11) reported that GlnB induces apoptosis
in human HL-60 leukemia cells. Apoptosis is a major route to
eradicate cancer. Therefore, the present study investigated whether
GlnB regulated apoptotic cell death in HeLa and SiHa cells. The
cells were treated with varying doses of GlnB for 24 h and
apoptosis were assessed using annexin V staining. GlnB treatment
increased the population of annexin V/PI-positive cells in a
dose-dependent manner in each cell line (Fig. 2A). The expression of the apoptosis
marker PARP1 was examined by western blot analysis. As shown in
Fig. 2B, the level of activated
and cleaved PARP1 was upregulated in HeLa cells treated with 8
µM GlnB compared with DMSO-treated control cells. Similar
results were observed in the SiHa cells treated with 6 µM
GlnB (Fig. 2B). Autophagy is
another important mechanism for cancer cell death. Therefore, the
effect of GlnB treatment on autophagy was assessed by determining
the expression of LC3 II/I by western blot analysis. LC3 I is
cleaved to from LC3 II under such conditons. GlnB treatment
increased LC3 II/I protein cleavage in both cell lines compared
with the DMSO-treated controls (Fig.
2B). These results indicated that GlnB induces autophagy in
cervical cells.
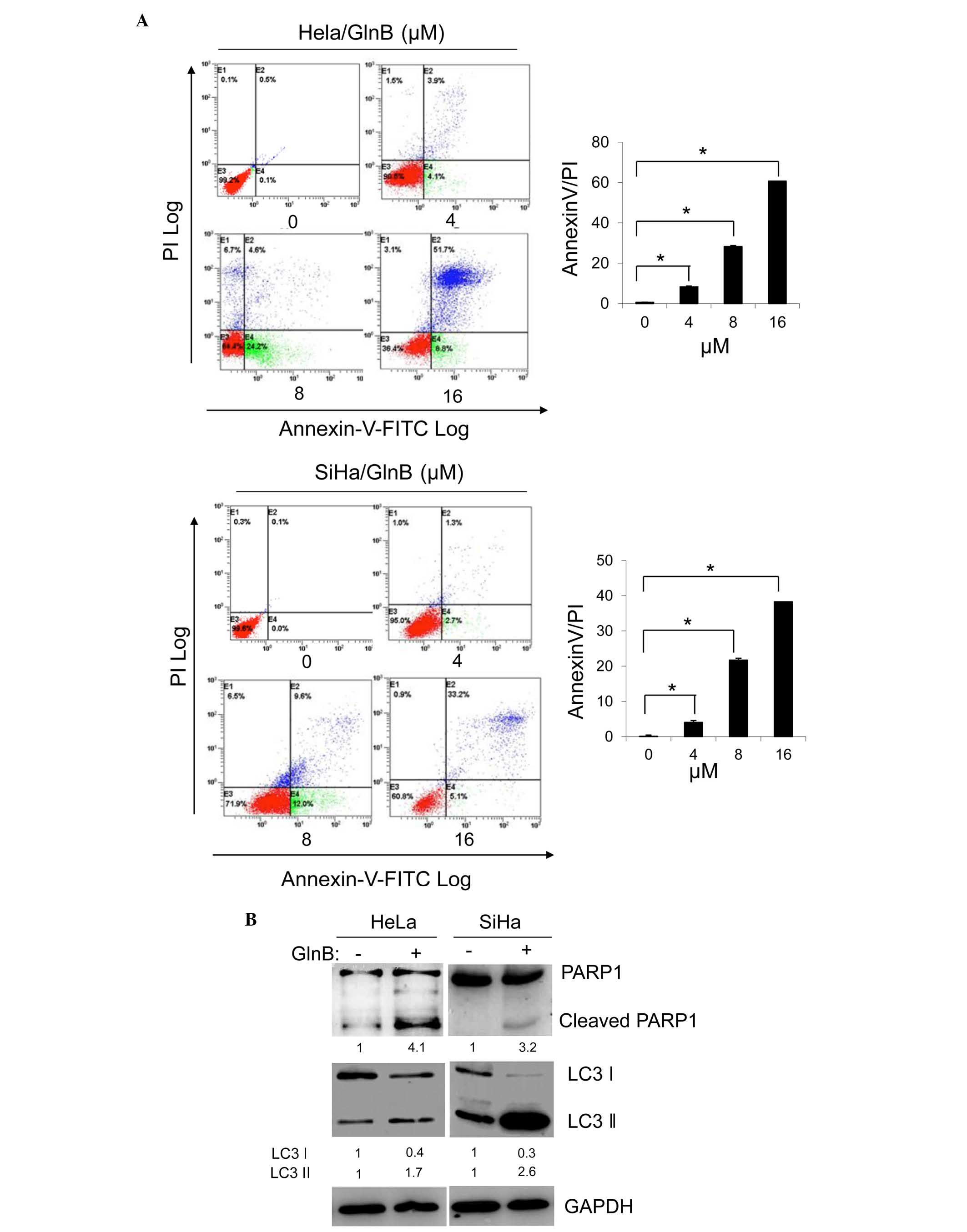 | Figure 2GlnB induces apoptosis and autophagy
in HeLa and SiHa cells. HeLa and SiHa cells were treated with
various concentrations of GlnB (0, 4, 8, and 16 µM) for 24
h. (A) Apoptosis was assessed by flow cytometric analysis of
annexin V-FITC/PI staining of HeLa and SiHa cell lines. The data
shown are representative of two independent experiments
(*P<0.05 compared with the 0 µM treatment
group). (B) HeLa cells were treated with 8 µM GlnB or DMSO
for 24 h, and the SiHa cells were treated with 6 µM GlnB or
DMSO for 24 h. The expression levels of PARP1 and LC3II/I were
examined by western blot analysis, and quantified by digitization.
GAPDH was the internal control. The data shown are representative
of two independent experiments. GlnB, glaucocalyxin B; FITC,
fluorescein isothiocyanate; PI, propidium iodide; DMSO, dimethyl
sulfoxide; PARP, poly (ADP-ribose) polymerase; LC, light chain;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/protein kinase B (Akt) is the central pathway in determining
the onset and progression of apoptosis and autophagy (12,13).
GlnA has been shown to inhibit Akt phos-phorylation, suppress
proliferation and promote apoptosis in human malignant glioma U87MG
cells (7). Therefore, the present
study next examined whether PI3K/Akt signaling is involved in
GlnB-induced apoptosis and autophagy. The expression of key
signaling proteins involved in this pathway, PTEN, Akt and
p-AktS473, were assessed by western blot analysis. The
results showed that GlnB increased the expression of PTEN and
decreased the expression of p-AktS473 (Fig. 3). The total Akt remained unchanged
by GlnB treatment (Fig. 3). These
findings indicated that the antitumor activity of GlnB is
associated with the PI3K/Akt signaling pathway.
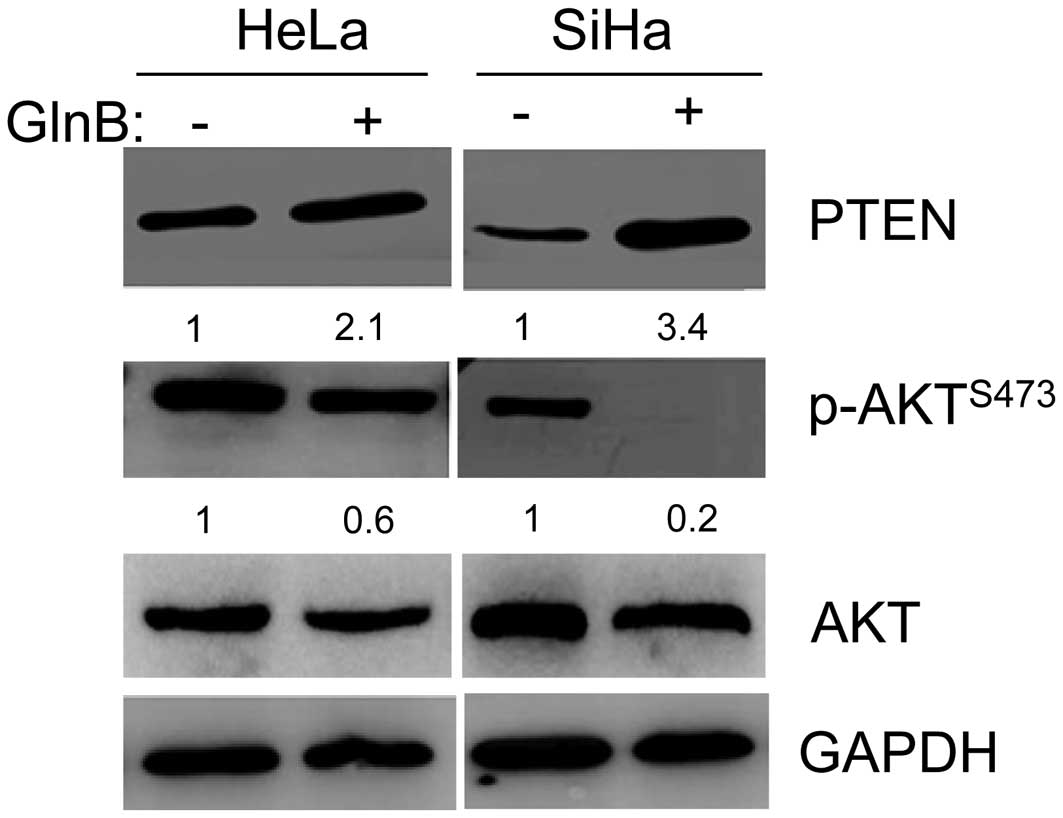 | Figure 3GlnB antitumor activity is involved
the PI3K/Akt signaling pathway. HeLa cells were treated with 8
µM GlnB or DMSO for 48 h, and SiHa cells were treated with 6
µM GlnB or DMSO for 24 h. Western blot analysis was
performed to determine the expression levels of PTEN, Akt and p-Akt
(S473) in HeLa and SiHa cells treated with GlnB. The expression
levels were quantified by digitization. GAPDH was used as an
internal control. The data shown are representative of two
independent experiments. GlnB, glaucocalyxin B; PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase; Akt, protein kinase
B; DMSO, dimethyl sulfoxide; PTEN, phosphatase and tensin homolog;
p-, phosphorylated; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
In conclusion, the present results demonstrated that
GlnB inhibited the proliferation of human cervical cancer cells
in vitro via the induction of apoptosis and autophagy, which
may be mediated by the PI3K/Akt signaling pathway. Future studies
are required to identify the direct molecular target(s) of GlnB and
to evaluate its antitumor effects in vivo using animal
models. Collectively, the present study demonstrated that GlnB
possesses antitumor properties in cervical cancer and may have a
therapeutic potential against this deadly disease.
Acknowledgments
The present study was supported by the Research
Grants of Henan Science and Technology Project (no.
142102310301).
References
|
1
|
Kim WK, Bang MH, Kim ES, Kang NE, Jung KC,
Cho HJ and Park JH: Quercetin decreases the expression of ErbB2 and
ErbB3 proteins in HT-29 human colon cancer cells. J Nutr Biochem.
16:155–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wieringa HW, van der Zee AG, de Vries EG
and van Vugt MA: Breaking the DNA damage response to improve
cervical cancer treatment. Cancer Treat Rev. 42:30–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y: The role of chinese medicine in
clinical oncology. Chin J Integr Med. 20:3–10. 2014. View Article : Google Scholar
|
|
5
|
Sun HD, Huang SX and Han QB: Diterpenoids
from isodon species and their biological activities. Nat Prod Rep.
23:673–698. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao LW, Zhang J, Yang WH, Wang B and Wang
JW: Glaucocalyxin A induces apoptosis in human leukemia HL-60 cells
through mitochondria-mediated death pathway. Toxicol In Vitro.
25:51–63. 2011. View Article : Google Scholar
|
|
7
|
Xiao X, Cao W, Jiang X, Zhang W, Zhang Y,
Liu B, Cheng J, Huang H, Huo J and Zhang X: Glaucocalyxin A, a
negative Akt regulator, specifically induces apoptosis in human
brain glioblastoma U87MG cells. Acta Biochim Biophys Sin
(Shanghai). 45:946–952. 2013. View Article : Google Scholar
|
|
8
|
Han M, Li Z, Guo Y, Zhang J and Wang X: A
nanoparticulate drug-delivery system for Glaucocalyxin A:
Formulation, characterization, increased in vitro and vivo
antitumor activity. Drug Deliv. 1–7. 2015.
|
|
9
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gan P, Zhang L, Chen Y, Zhang Y, Zhang F,
Zhou X, Zhang X, Gao B, Zhen X, Zhang J and Zheng LT:
Anti-inflammatory effects of glaucocalyxin B in microglia cells. J
Pharmacol Sci. 128:35–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang WH, Zhang Z, Sima YH, Zhang J and
Wang JW: Glaucocalyxin A and B-induced cell death is related to GSH
perturbation in human leukemia HL-60 cells. Anticancer Agents Med
Chem. 13:1280–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapoor V, Zaharieva MM, Das SN and Berger
MR: Erufosine simultaneously induces apoptosis and autophagy by
modulating the Akt-mTOR signaling pathway in oral squamous cell
carcinoma. Cancer letters. 319:39–48. 2012. View Article : Google Scholar
|
|
13
|
Le XF, Mao W, Lu Z, Carter BZ and Bast RC
Jr: Dasatinib induces autophagic cell death in human ovarian
cancer. Cancer. 116:4980–4990. 2010. View Article : Google Scholar : PubMed/NCBI
|