Introduction
Currently, ~2% of pregnant women require
non-obstetric surgery (1). In the
USA, ~75,000 pregnant women undergo non-obstetric surgery annually
(2). A number of these patients
require multiple or complicated surgeries and thus, exposure to
large amounts of inhaled anesthesia. Certain studies have suggested
that the inhalation anesthetics may be harmful. Isoflurane has been
reported to repress neuron stem cell self-renewal and influence the
differentiation and growth of neural progenitor cells (3,4).
Sevoflurane, the most widely used inhaled anesthetic for general
anesthesia, is lipophilic and thus readily crosses the placental
barrier (5). Sevoflurane was shown
to decrease the self-renewal of neuronal stem cells at clinically
relevant concentrations in vitro (6). Therefore, sevoflurane-induced
embryotoxicity has become a major health issue of interest.
However, the mechanism underlying the effects of embryotoxicity on
fetus development induced by sevoflurane remains largely
unknown.
Embryonic stem (ES) cells are derived from the inner
cell mass (ICM) of blastocysts, which is the early-stage
preimplantation embryo (7,8). Due to the unlimited self-renewal
ability and pluripotency, ES cells are widely used for research
into development. ES cell self-renewal properties mean they
proliferate rapidly under continued maintenance of pluripotency
(9). Certain cell signaling
pathways, such as leukemia inhibitory factor (Lif)/Signal
transducer and activator of transcription 3 (stat3) and
extracellular signal-regulated kinase (ERK), have been reported to
regulate the self-renewal and pluripotency of ES cells (8,10–13).
These signaling pathways act synergically to regulate self-renewal.
Activation of ERK signaling was shown to induce the differentiation
of mES cells (14). Lif/stat3
signaling, which maintains the self-renewal ability of mES cells,
significantly inhibited the activation of ERK signaling (15). Moreover, inhibition of ERK
signaling impairs mouse ES cell self-renewal (16). ERK signaling is important in
regulating mES cell self-renewal and pluripotency by acting as a
switch. Additionally, γ-aminobutyric acid (GABA) signaling, which
is important in neural regulation, has also been reported to be a
critical regulator of mES cell self-renewal (17,18).
However, the interaction between these signaling pathways that
modulate mES cell self-renewal remains largely unknown.
GABA was commonly hypothesized to be the inhibitory
neurotransmitter in the central nervous system (CNS); however,
studies have demonstrated that GABA signaling regulates
physiological and metabolic processes in numerous cell types. GABA
signaling can regulate the development of the central nervous
system (19,20). GABA signaling can also stimulate
hepatocellular carcinoma growth via the GABAA receptor
(GABAAR) (21). Studies
have demonstrated that midazolam, a GABAAR agonist, can
inhibit the proliferation of neural stem cells in vitro
(22). Midazolam can also induce
cellular apoptosis in human cancer cells (23). Sevoflurane is another
GABAAR agonist and it has been reported to induce
apoptosis in neural stem cells (24). However, whether sevoflurane can
influence the self-renewal of mES cells by GABA signaling remains
largely unknown. In the present study, mES cells were treated with
4.1% sevoflurane to detect the potential toxicity of sevoflurane to
mES cells and it was determined that sevoflurane could inhibit the
self-renewal of mES cells. Knockdown of the GABAAR
rescued the effect of sevoflurane on self-renewal. Furthermore, it
was demonstrated that sevoflurane upregulated the level of p-ERK by
GABAAR. Inhibition of ERK signaling also rescued the
sevoflurane-induced inhibition of mES cell self renewal. These
results suggested that sevoflurane inhibits the self-renewal of mES
cells by regulating the GABAAR-ERK signaling pathway.
Thus, the GABAAR-ERK signaling pathway may be a target
for preventing the toxicity of inhalation anesthetics to developing
fetal brain in the clinic, in pregnant women undergoing
non-obstetric surgery under inhalation general anesthesia.
Materials and methods
Cell culture
E14 mES cells, purchased from the Institute of
Biochemistry and Cell Biology (SCSP-204; Shanghai, China), were
seeded in plates pre-coated with 0.1% gelatin in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 15% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), glutamine (1:100; Invitrogen),
NEAA (1:100; Invitrogen; Thermo Fisher Scientific, Inc.), B-ME
(1:550; Invitrogen; Thermo Fisher Scientific, Inc.) and Lif
(1:10,000; Invitrogen; Thermo Fisher Scientific, Inc.) at a density
of 6×104 cells per well in six-well plates, and cultured
at 37°C in a humidified atmosphere containing 5%
CO2.
Cell treatment
The sevoflurane group was exposed to 4.1%
sevoflurane (Hengrui Pharmaceutical Co., Ltd., Shanghai, China), 5%
CO2 and 21% O2, and the control group was
exposed to included 5% CO2 and 21% O2 for 6
h, as previously described (25).
This concentration of sevoflurane was selected as it is clinically
relevant, and has been shown to induce apoptotic cell death and
neuroinflammation in H4 human neuroglioma cells and neurons
(26,27). A DragerVamos gas analyzer
(Drägerwerk AG, Lübeck, Germany) was used to monitor the
concentration of CO2, O2 and sevoflurane. In
the rescue experiments, cells were treated with 4.1% sevoflurane
for 6 h, prior to culture for a further 6 h in normal culture
medium. Transfection with small interfering RNA (siRNA) or addition
of compounds was then conducted. GABAAR agonist muscimol
(50 µM), GABAAR antagonist bicuculline (100
µM) and ERK signaling inhibitor PD0325901 (0.5 µM;
all Sigma-Aldrich, St. Louis, MO, USA), were added to mES cells 2 h
prior to sevoflurane exposure, respectively.
MTS assay for cell proliferation
analysis
According to a previous study, Cell Titer
96® AQueous One Solution Cell Proliferation Assay kit
(Promega Corporation, Madison, WI, USA) was used to perform an MTS
assay and absorption was detected at 490 nm using a microplate
reader as previously described (12).
Transfection of siRNA
siRNA was used for the downregulation of
GABAAR-β3. The sequence of siRNA was the same as that in
a previous study (18). siRNA
(synthesized by Biotend, Shanghai, China) was transfected into
cells using the Fugene HD transfection reagent according to the
manufacturer's instructions (Roche, Basel, Switzerland).
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted using TRIzol reagent
(Sigma-Aldrich), and 300 ng was reverse-transcribed using M-MLV
Reverse Transcriptase (Promega Corporation). The primers used were
described in a previous study (8)
and were as follows: Forward: 5′-GGATGCTGTGAGCCAAGG-3′ and reverse:
5′-GAACAAAATGATGAGTGACAGACAG-3′ for octamer-binding transcription
factor 4 (Oct4); forward: 5′-CAGGTGTTTGAGGGTAGCTC-3′ and reverse:
5′-CGGTTCATCATGGTACAGTC-3′ for Nanog; forward:
5′-GATCAGCATGTACCTCCCC-3′ and reverse: 5′-CCCTCCCAATTCCCTTGTATC-3′
for SRY (sex determining region Y)-box 2 (Sox2); forward:
5′-GGCATCTGTAAGTGGTTCAACG-3′ and reverse 5′-CCCTCCTTGAGGCTTCGGA-3′
for Lin28; forward: 5′-AATCAAAATCCCTGATCTAACCGA-3′ and reverse:
5′-AAGAGAGAAAAGGTGAATGGAAACA-3′ for GABAAR-β3; and
forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′ for GAPDH. RT-qPCR was conducted using
an Mx3000P system (Stratagene, San Diego, CA, USA). The RT-qPCR
included 40-cycles of amplification. Expression of target genes was
normalized against endogenous glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) using the 2−ΔΔCq method (28).
Western blot analysis
Cells were lysed in sodium dodecyl sulfate (SDS) and
protein concentration was measured with a BCA Protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Cell lysates
(50 µg protein) were re-suspended using 5X loading lysis
buffer (250 mM Tris-HCl, pH 6.8; 5% DTT, 10% SDS, 0.5% of 0.025 g
bromophenol blue and 50% glycerine) and separated with 10% SDS-PAGE
gel electrophoresis and transferred to polyvinyldene difluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membrane was blocked in Tris-buffered saline with Tween 20 (TBST)
containing 3% bovine serum albumin (Amresco, LLC, Solon, OH, USA)
for 1 h and then incubated with primary antibodies against p-ERK
(cat no. ab115617, Abcam, Cambridge, MA, USA), ERK (cat. no.
ab17942, Abcam), GAPDH (cat. no. ab181602, Abcam), Sox2 (cat. no.
ab79351, Abcam), Oct (cat. no. ab18976, Abcam), Nanog (cat. no.
ab80892, Abcam) and Lin28 (cat. no. ab63740, Abcam) at 4°C
overnight. Subsequently, the membranes were washed with TBST and
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (ab7090) for 1 h at room temperature. Signals
were visualized by enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.).
5′-bromo-deoxyuridine (BrdU)
incorporation analysis
Cells were incubated with 10 µM BrdU (Roche)
for 1.5 h, the labeling solution was removed and the cells were
washed twice with phosphate-buffered saline (PBS). The cells were
fixed in 4% paraformaldehyde (Sigma-Aldrich) for 20 min prior to
washing with PBS. The cells were then incubated with HCl for 30 min
and this was neutralized with 0.1 M sodium borate buffer for 30 min
at room temperature. The cells were washed with PBS and then
blocked in PBS containing 10% FBS for 1 h. Anti-BrdU (1:100; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was incubated with the
cells overnight at 4°C, subsequently, the cells were washed three
times in PBs and incubated with a secondary fluorescent antibody
(1:1,000; Abcam; ab6785) for 1 h. The cell nuclei were stained with
DAPI. The number of BrdU-positive cells were counted in at least 5
microscopic fields (Eclipse Ti-S; Nikon Corporation, Tokyo,
Japan).
Trypan blue staining
A single cell suspension was prepared in
phosphate-buffered saline, and then 0.4% trypan blue solution
(Sigma-Aldrich) was added. After 3 min, the total number of viable
cells (unstained) and total cells (stained and unstained) was
counted under a microscope to determine the cell viability
according to the following equation: Cell viability (%) = (viable
cells/total cells) × 100.
Cell apoptosis analysis
A total of 1×105 cells were suspended in
500 µl binding buffer. Annexin V-fluorescein isothiocyanate
(FITC; 5 µl) was added followed by 5 µl propidium
iodide. The mixture was incubated at room temperature for 10 min in
the dark. Flow cytometry was conducted to count the percentage of
cell in early apoptosis and late stage. Annexin V-FITC Cell
Apoptosis Detection kit (Keygentec, Nanjing, China) was used to
perform the cell apoptosis analysis.
Statistical analysis
Student's t-test was used for comparing two data
sets, and multiple comparisons were compared using two-way analysis
of variance using GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference. Values are presented as the
mean ± standard deviation.
Results
Sevoflurane represses the self-renewal of
mES cells
In this study, mES cells were used as a model to
investigate the effect of sevoflurane on embryonic development. The
mES cells were treated with 4.1% sevoflurane for 6 h as in a
previous study (25). The cells
were then cultured for another 24 h and it was demonstrated that
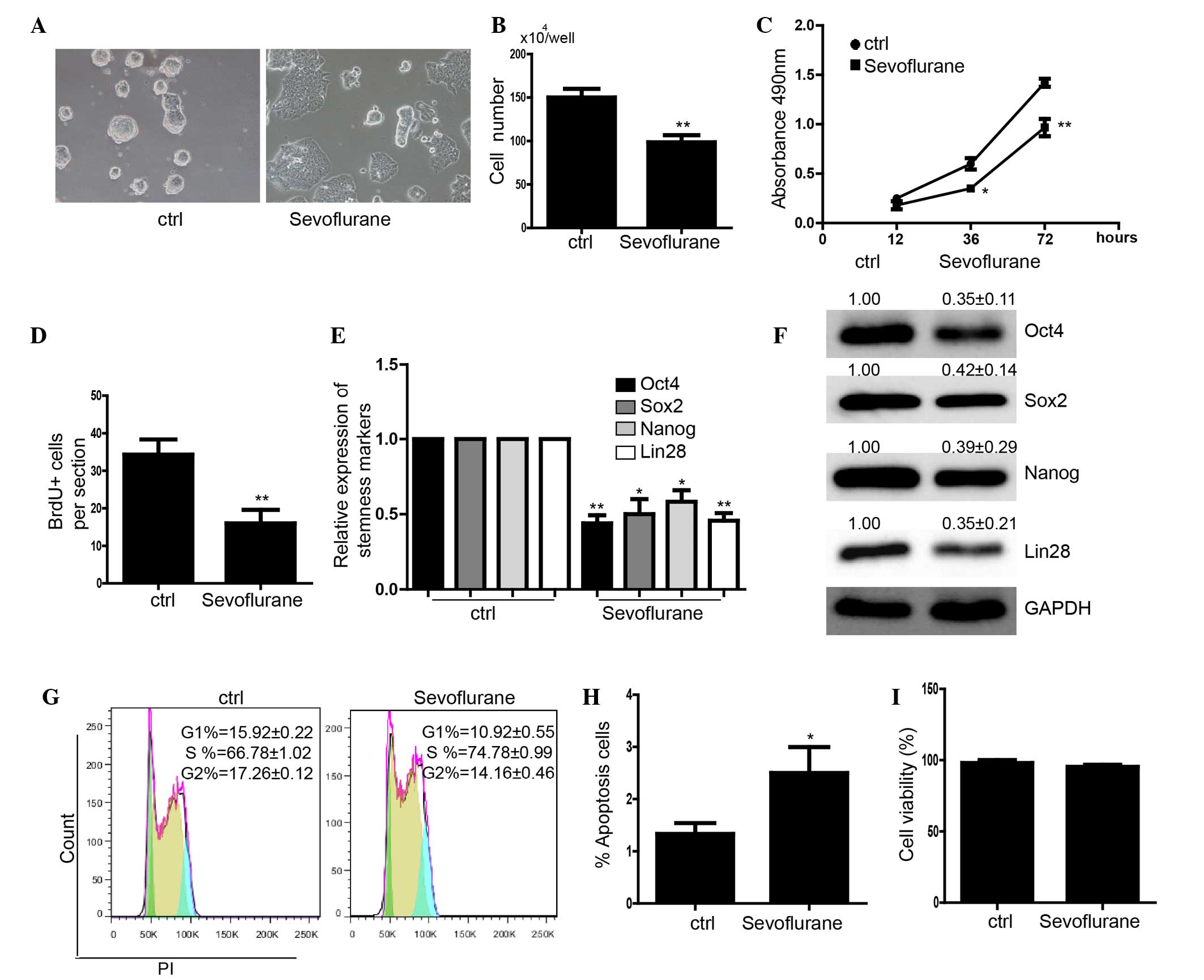
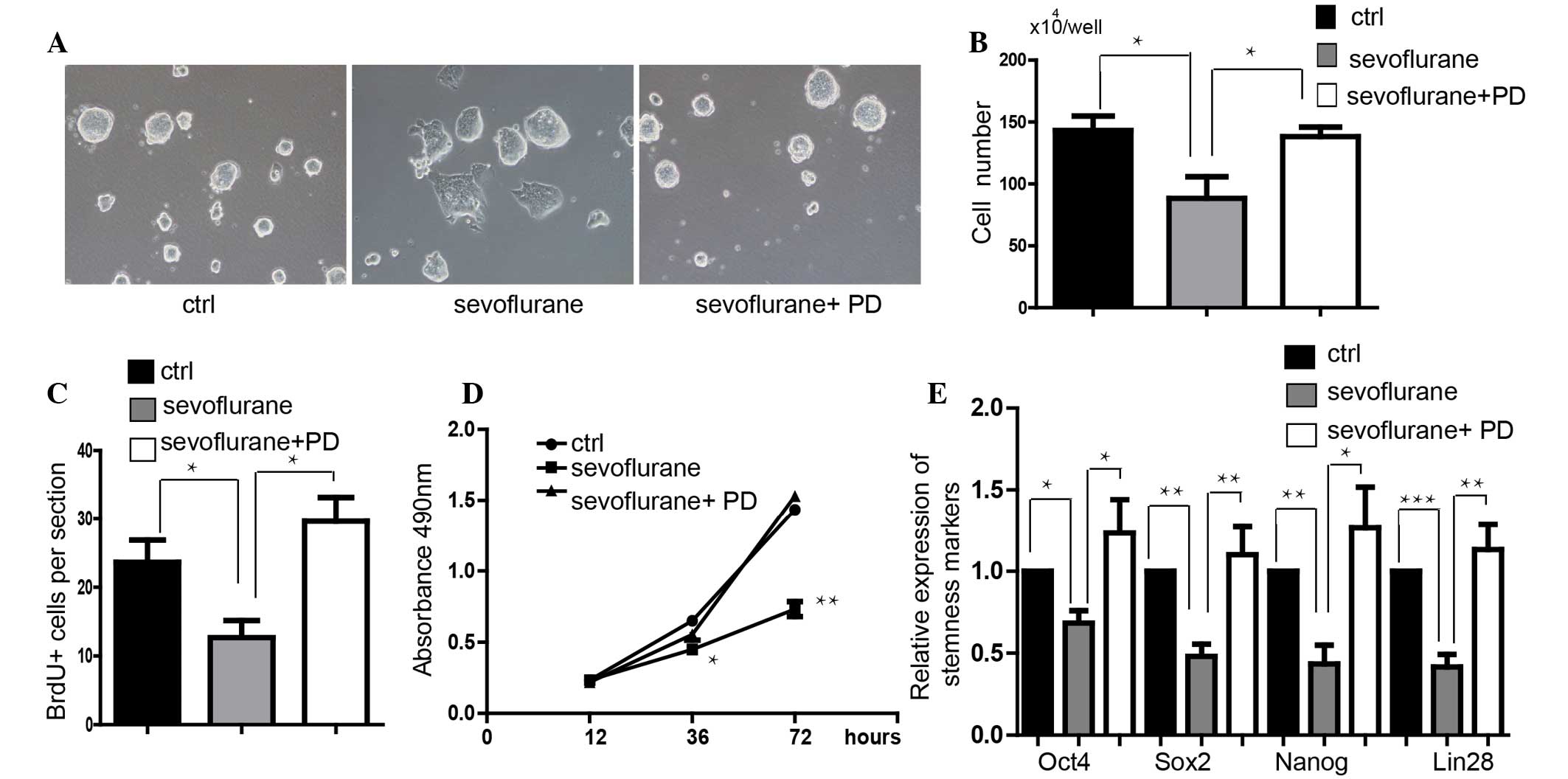
the morphology of the clones could not be maintained (Fig. 1A). The cell number in the
sevoflurane-treated mES group was significantly less than that in
the control group (Fig. 1B). The
MTS assay demonstrated that the proliferation of mES cells was
significantly inhibited by sevoflurane treatment compared with that
in control (Fig. 1C). The
BrdU+ assay also showed that the mES cells treated with
sevoflurane had a lower capacity for proliferation (Fig. 1D). The expression levels of
stemness markers Oct4, Sox2, Nanog and Lin28 were also
downregulated in the sevoflurane-treated mES cells at the mRNA and
protein level (Fig. 1E and F).
Cell cycle analysis showed that the cell cycle was arrested at S
phase following treatment with sevoflurane (Fig. 1G). Furthermore, an apoptosis assay
was conducted using fluorescence-activated cell sorting, which
determined that treatment with 4.1% sevoflurane for 6 h induced
alight apoptosis over 24 h (Fig.
1H). Trypan blue staining indicated there was no significant
cell death (Fig. 1I).
Sevoflurane upregulates the level of
p-ERK by GABAAR signalling
In order to identify the mechanism underlying the
effect of sevoflurane on mES cells, the mES cells were treated with
4.1% sevoflurane for 6 h. It was determined that sevoflurane
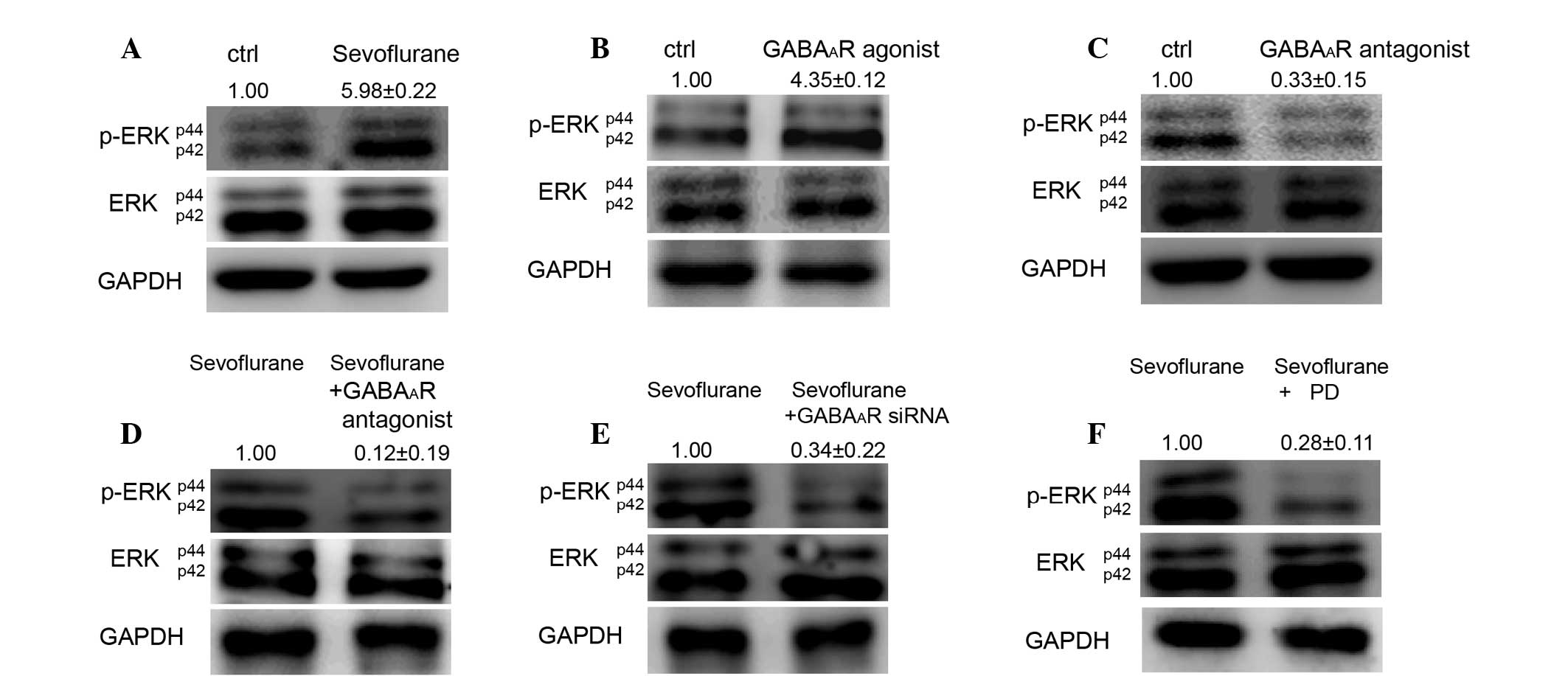
increases the level of p-ERK but not the total expression of ERK
(Fig. 2A). The GABAAR
agonist muscimol (50 µM) also upregulated the level of p-ERK
(Fig. 2B). Conversely,
GABAAR antagonist bicuculline (100 µM)
downregulated the level of p-ERK in mES cells (Fig. 2C). Notably, the GABAAR
antagonist and GABAAR siRNA attenuated the
sevoflurane-induced activation of ERK (Fig. 2D and E). PD0325901 (0.5 µM),
an inhibitor of ERK signaling, also attenuated the
sevoflurane-induced activation of ERK (Fig. 2F).
Knockdown of GABAAR-β3 rescues
the sevoflurane-induced inhibition of self-renewal in mES
cells
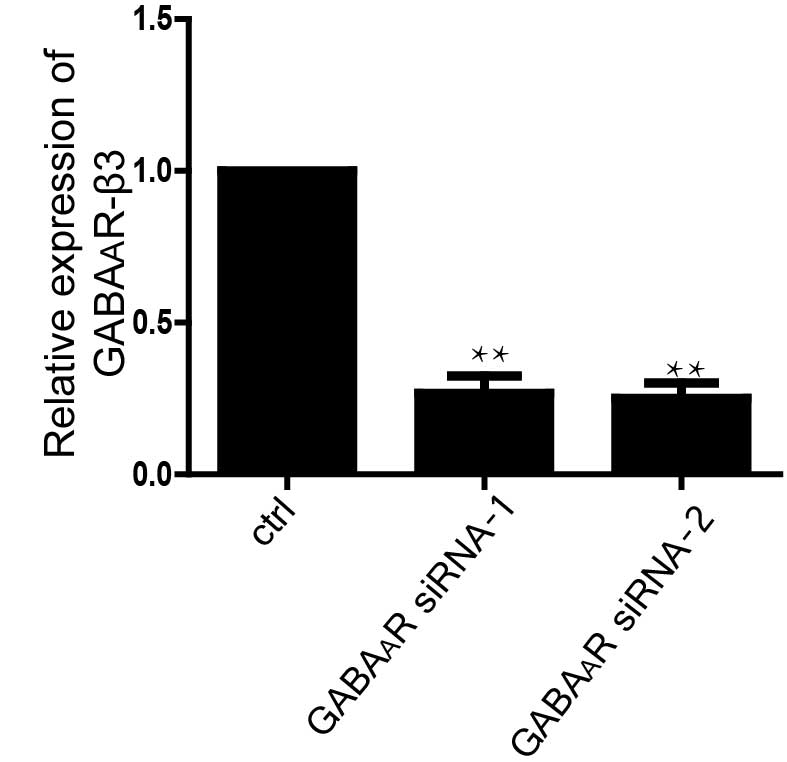
The expression level of GABAAR-β3 was
downregulated following treatment with GABAAR siRNA
(Fig. 3). The number of mES cells
with downregulated GABAAR-β3 was shown to be greater
than that in control cells (Fig. 4A
and B). Knockdown of GABAAR-β3 promoted the
proliferation of mES cells (Fig. 4C
and D). Downregulation of GABAAR-β3 increased the
expression levels of stemness markers, Oct4, Sox2 and Nanog
(Fig. 4E). Transfection of
GABAAR-β3 siRNA into mES cells rescued the
sevoflurane-induced inhibition of self-renewal in mES cells,
detected via morphological observation (Fig. 3F). Knockdown of
GABAAR-β3, rescued the decrease in cell number caused by
sevoflurane (Fig. 4G). Cell
proliferation, which can be inhibited by sevoflurane was also
rescued by downregulation of GABAAR-β3 (Fig. 4H and I). Knockdown of
GABAAR-β3 also rescued the sevoflurane-induced decrease
in expression of stemness genes in mES cells (Fig. 4J).
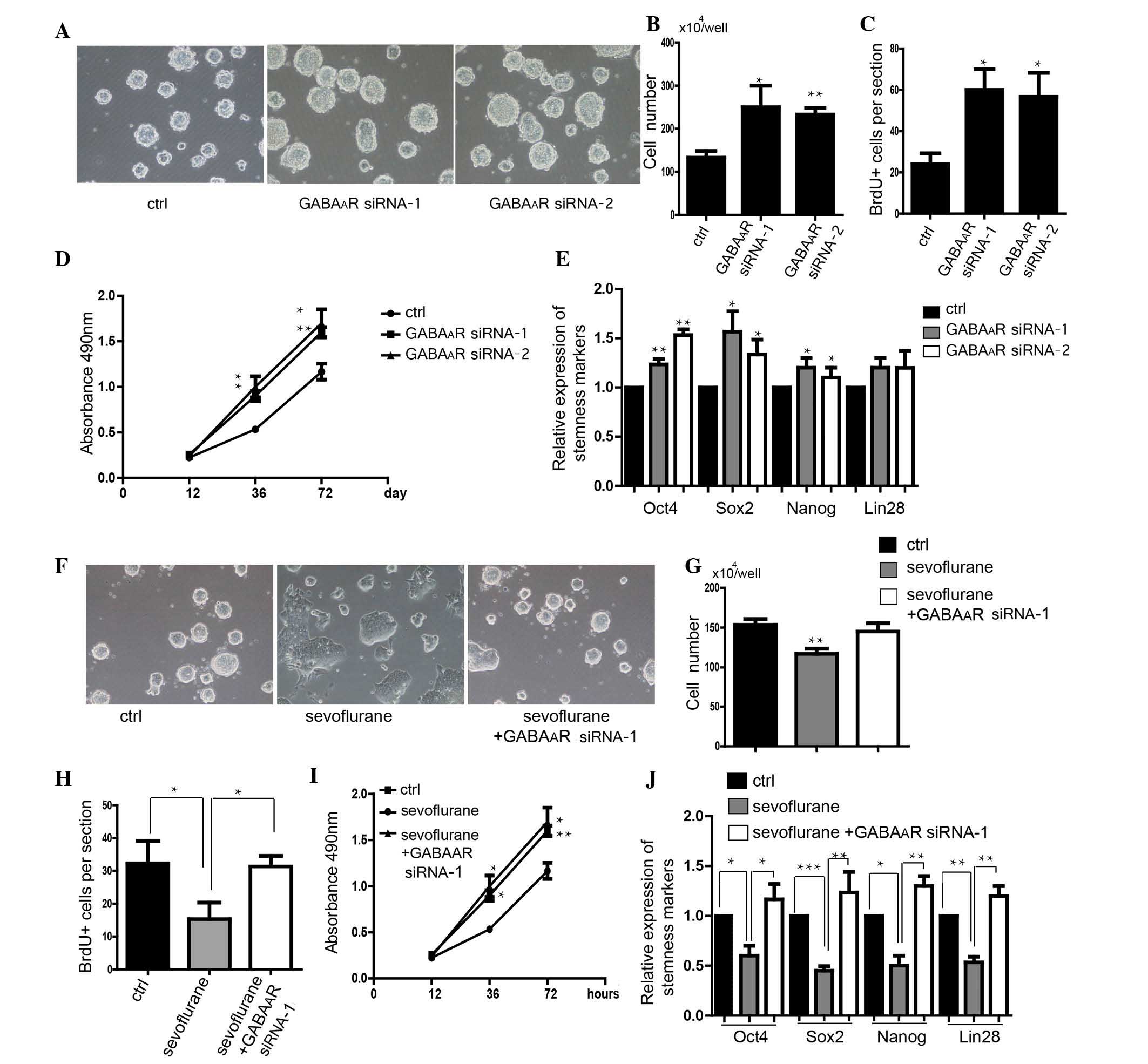 | Figure 4Knockdown of the GABAAR
rescues the effect of sevoflurane on self-renewal. (A) Morphology
of mES cells transfected with GABAAR-β3 siRNA. Scale
bar, 100 µm. (B) Number of mES cells (n=5). (C)
Quantification of the BrdU+ cells (n=50 clones). (D)
Analysis of the proliferation of mES cells using an MTS assay
(n=5). (E) Detection of stemness markers of mES cells (n=6). (F)
Transfection of GABAAR siRNA rescued the effect of
sevoflurane on mES cell clone morphology. Scale bar, 100 µm.
(G) Quantification of the cell number (n=5). (H) Analysis of the
proliferation of mES cells using a BrdU assay (n=6). (I) Analysis
of the proliferation of mES cells using an MTS assay (n=6). (J)
Detection of stemness markers of mES cells in the rescue
experiments (n=6). Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01,
***P<0.001 compared with control or sevoflurane
groups (in part I). mES cells, mouse embryonic stem cells; BrdU,
5′-bromo-deoxyuridine; PI, propidium iodide; Sox2, SRY (sex
determining region Y)-box 2 (Sox2); Oct4, octamer-binding
transcription factor 4; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; GABAAR, γ-aminobutyric acid A receptor;
siRNA, small interfering RNA. |
Inhibition of ERK attenuates the
sevoflurane-induced inhibition of self-renewal in mES cells
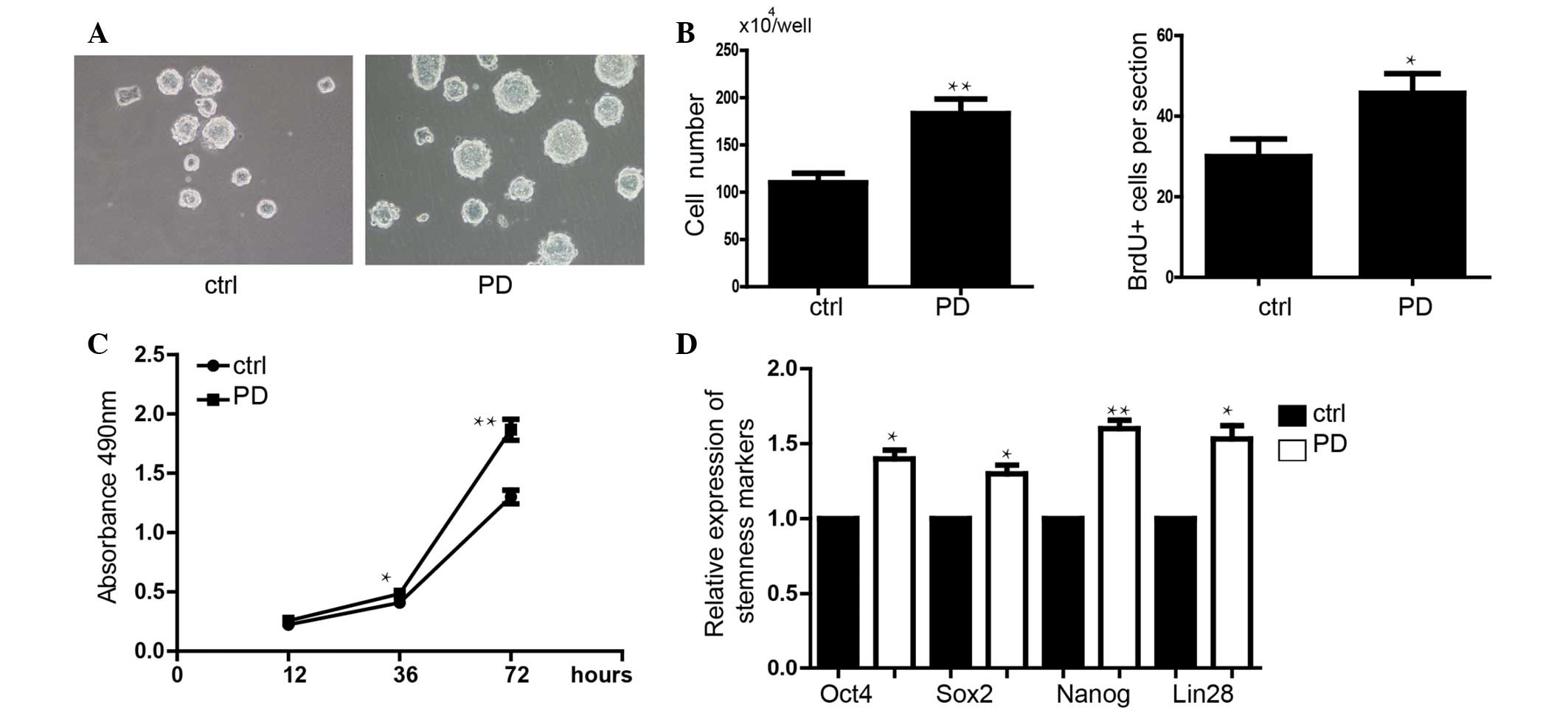
PD0325901 (0.5 µM), an inhibitor of ERK
signaling, was shown to promote the proliferation of mES cells
(Fig. 5A–D). Additionally,
PD0325901 significantly upregulated the expression level of
stemness markers Oct4, Sox2, Nanog and Lin28 (Fig. 5E). In order to detect whether the
activity of ERK signalinh mediated the function of sevoflurane on
suppressing self-renewal, a rescue experiment was conducted. It was
demonstrated that addition of PD0325901 blocked sevoflurane-induced
inhibition of self-renewal in mES cells via observation of clone
morphology (Fig. 6A). Inhibition
of ERK signaling also rescued the sevoflurane-induced decrease in
cell proliferation (Fig. 6B–D).
Furthermore, inhibition of ERK signaling also rescued stemness
marker expression, which was significantly down-regulated by
sevoflurane (Fig. 6E).
Discussion
Numerous pregnant women are exposed to inhalation
anesthetics for non-obstetric surgery (1). Inhalation anesthetics readily cross
the placental barrier and thus embryotoxicity is a major concern.
Previous studies have demonstrated that inhalation anesthetics
repressed the self-renewal of cultured rat neural stem cells and
human neural progenitor cells (3,6).
However, the mechanism underlying these effects is largely unknown.
ES cells derived from the ICM of the blastocyst proliferate rapidly
and maintain stemness, which are two critical characteristics of
self-renewal. The compromising pluripotency was assured by the
infinite and rapid self-renewal (29). In the present study, mES cells were
used as an early development model. Treatment with 4.1% sevoflurane
(the clinical concentration) for 6 h inhibited the self-renewal of
mES cells following further culture. It was shown that sevoflurane
repressed the self-renewal of mES cells, which showed destroyed
clone morphology with lower expression levels of stemness genes
(Nanog, Oct4, Sox2 and Lin28), and slower proliferation at a
clinically relevant concentration. These results suggested that
sevoflurane is a potential threat to early fetal development due to
its toxicity.
A number of general anesthetic agents, including
sevoflurane, are GABAAR modulators (30). GABA signaling occurs in numerous
cell types and regulates cell proliferation, differentiation,
immunomodulation and other physiological processes (31–33).
Previous studies showed that GABA is released by mES cells and acts
as a trophic factor regulating key developmental processes,
including the proliferation of mES cells (7,34).
Therefore, excessive or prolonged GABAergic stimulation, such as
with ethanol or valproic acid, which like sevoflurane are GABA
agonists/modulators, decreases the proliferation of mES cells. As
such, it was hypothesized that excessive GABA receptor-mediated
excitation produced in mES cells during exposure to sevoflurane may
cause decreased proliferation of these cells. Thus, the present
study treated the mES cells with 4.1% sevoflurane for 6 h, as
described previously (25), and
demonstrated that sevoflurane represses the self-renewal of mES
cells. The expression level of stemness markers Oct4, Sox2, Nanog
and Lin28 was also downregulated. In addition, the activation of
GABAAR significantly inhibited stemness maintenance and
proliferation, in order to suppress the self-renewal of mES cells
on stemness maintenance and proliferation. Inhibition of
GABAAR signaling by downregulating the GABAA
R rescued the effects of sevoflurane on self-renewal. These results
suggested that sevoflurane may act via GABAAR. As
GABAAR is a receptor, there must be downstream factors
that have direct role in the regulation of self-renewal.
The activation of ERK inhibited the self-renewal of
mES cells and promoted differentiation (35). The present study demonstrated that
sevoflurane upregulated the level of p-ERK but not the total
expression of ERK. Knockdown of the GABAAR rescued the
sevoflurane-induced activation of ERK and inhibition of
self-renewal in mES cells. Additionally, inhibition of ERK
signaling could also rescue the effects of sevoflurane. Therefore,
the present data suggest that sevoflurane may repress the
self-renewal of mES cells via a GABAAR/ERK signaling
dependent mechanism.
In conclusion, sevoflurane inhibited the
self-renewal of mES cells. In addition, GABAAR/ERK
signaling may be a potential therapeutic target for the prevention
of the embryotoxicity of sevoflurane.
References
|
1
|
Ní Mhuireachtaigh R and O'Gorman DA:
Anesthesia in pregnant patients for nonobstetric surgery. J Clin
Anesth. 18:60–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reitman E and Flood P: Anaesthetic
considerations for non-obstetric surgery during pregnancy. Br J
Anaesth. 107(suppl 1): i72–i78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Culley DJ, Boyd JD, Palanisamy A, Xie Z,
Kojima K, Vacanti CA, Tanzi RE and Crosby G: Isoflurane decreases
self-renewal capacity of rat cultured neural stem cells.
Anesthesiology. 115:754–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao X, Yang Z, Liang G, Wu Z, Peng Y,
Joseph DJ, Inan S and Wei H: Dual effects of isoflurane on
proliferation, differentiation and survival in human
neuroprogenitor cells. Anesthesiology. 118:537–549. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y and Xie
Z: Anesthetic sevoflurane causes neurotoxicity differently in
neonatal naïve and Alzheimer disease transgenic mice.
Anesthesiology. 112:1404–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Dong Y, Zheng H, Shie V, Wang H,
Busscher JJ, Yue Y, Xu Z and Xie Z: Sevoflurane inhibits
neurogenesis and the Wnt-catenin signaling pathway in mouse neural
progenitor cells. Curr Mol Med. 13:1446–1454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM: Embryonic stem
cell lines derived from human blastocysts. Science. 282:1145–1147.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niwa H, Burdon T, Chambers I and Smith A:
Self-renewal of pluripotent embryonic stem cells is mediated via
activation of STAT3. Genes Dev. 12:2048–2060. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagaoka MKU, Yuasa S, Hattori F, Chen H,
Tanaka T, Okabe M, Fukuda K and Akaike T: E-cadherin-coated plates
maintain pluripotent ES cells without colony formation. PLoS One.
1:e152006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuda T, Nakamura T, Nakao K, Arai T,
Katsuki M, Heike T and Yokota T: STAT3 activation is sufficient to
maintain an undifferentiated state of mouse embryonic stem cells.
EMBO J. 18:4261–4269. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa K, Saito A, Matsui1 H, Suzuki H,
Ohtsuka S, Shimosato D, Morishita Y, Watabe T, Niwa H and Miyazono
K: Activin-nodal signaling is involved in propagation of mouse
embryonic stem cells. J Cell Sci. 120:55–65. 2007. View Article : Google Scholar
|
|
12
|
Liu Q, Wang G, Chen Y, Li G, Yang D and
Kang J: A miR-590/ACVR2a/Rad51B axis regulates DNA damage repair
during mES cell proliferation. Stem Cell Reports. 3:1103–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunath T, Saba-El-Leil MK, Almousailleakh
M, Wray J, Meloche S and Smith A: FGF stimulation of the Erk1/2
signalling cascade triggers transition of pluripotent embryonic
stem cells from self-renewal to lineage commitment. Development.
134:2895–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Sun L, Gao F, Jiang J, Yang Y, Li C,
Gu J, Wei Z, Yang A, Lu R, et al: Stk40 links the pluripotency
factor Oct4 to the Erk/MAPK pathway and controls extraembryonic
endoderm differentiation. Proc Natl Acad Sci USA. 107:1402–1407.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JW, Hsu YC, Kao CY, Su HL and Chiu IM:
Leukemia inhibitory factor-induced Stat3 signaling suppresses
fibroblast growth factor 1-induced Erk1/2 activation to inhibit the
downstream differentiation in mouse embryonic stem cells. Stem
Cells Dev. 22:1190–1197. 2013. View Article : Google Scholar
|
|
16
|
Chen H, Guo R, Zhang Q, Guo H, Yang M, Wu
Z, Gao S, Liu L and Chen L: Erk signaling is indispensable for
genomic stability and self-renewal of mouse embryonic stem cells.
Proc Natl Acad Sci USA. 112:E5936–E5943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hjerling-Leffler J, Marmigère F, Heglind
M, Cederberg A, Koltzenburg M, Enerbäck S and Ernfors P: The
boundary cap: A source of neural crest stem cells that generate
multiple sensory neuron subtypes. Development. 132:2623–2632. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andäng M, Hjerling-Leffler J, Moliner A,
Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez
S, Nishimaru H, et al: Histone H2AX-dependent GABA (A) receptor
regulation of stem cell proliferation. Nature. 451:460–464. 2008.
View Article : Google Scholar
|
|
19
|
Behar TN, Schaffner AE, Scott CA, Greene
CL and Barker JL: GABA receptor antagonists modulate postmitotic
cell migration in slice cultures of embryonic rat cortex. Cereb
Cortex. 10:899–909. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meier J, Akyeli J, Kirischuk S and Grantyn
R: GABA (A) receptor activity and PKC control inhibitory
synaptogenesis in CNS tissue slices. Mol Cell Neurosci. 23:600–613.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YH, Liu Y, Li YD, Liu YH, Li F, Ju Q,
Xie PL and Li GC: GABA stimulates human hepatocellular carcinoma
growth through overexpressed GABAA receptor theta subunit. World J
Gastroenterol. 18:2704–2711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao S, Zhu Y, Xue R, Li Y, Lu H and Mi W:
Effect of midazolam on the proliferation of neural stem cells
isolated from rat hippocampus. Neural Regen Res. 7:1475–1482.
2012.PubMed/NCBI
|
|
23
|
Mishra SK, Kang JH, Lee CW, Oh SH, Ryu JS,
Bae YS and Kim HM: Midazolam induces cellular apoptosis in human
cancer cells and inhibits tumor growth in xenograft mice. Mol
Cells. 36:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu J, Shi P, Mao W, Zhao Y, Liu W and
Wang Y: Effect of apoptosis in neural stem cells treated with
sevoflurane. BMC Anesthesiol. 7:252015. View Article : Google Scholar
|
|
25
|
Zhang L, Zhang J, Yang L, Dong Y, Zhang Y
and Xie Z: Isoflurane and sevoflurane increase interleukin-6 levels
through the nuclear factor-kappa B pathway in neuroglioma cells. Br
J Anaesth. 110(Suppl 1): i82–i91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen X, Dong Y, Xu Z, Wang H, Miao C,
Soriano SG, Sun D, Baxter MG, Zhang Y and Xie Z: Selective
anesthesia-induced neuroinflammation in developing mouse brain and
cognitive impairment. Anesthesiology. 118:502–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng H, Dong Y, Xu Z, Crosby G, Culley
DJ, Zhang Y and Xie Z: Sevoflurane anesthesia in pregnant mice
induces neurotoxicity in fetal and offspring mice. Anesthesiology.
118:516–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Wang Y, Baskerville S, Shenoy A, Babiarz
JE, Baehner L and Blelloch R: Embryonic stem cell-specific
microRNAs regulate the G1-S transition and promote rapid
proliferation. Nat Genet. 40:1478–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maher BJ and LoTurco JJ: Stop and go GABA.
Nat Neurosci. 12:817–818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gladkevich A, Korf J, Hakobyan VP and
Melkonyan KV: The peripheral GABAergic system as a target in
endocrine disorders. Auton Neurosci. 124:1–8. 2006. View Article : Google Scholar
|
|
32
|
Jin Z, Mendu SK and Birnir B: GABA is an
effective immunomodulatory molecule. Amino Acids. 45:87–94. 2013.
View Article : Google Scholar :
|
|
33
|
Tian J, Dang H, Chen Z, Guan A, Jin Y,
Atkinson MA and Kaufman DL: γ-Aminobutyric acid regulates both the
survival and replication of human β-cells. Diabetes. 62:3760–3765.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
LoTurco JJ, Owens DF, Heath MJ, Davis MB
and Kriegstein AR: GABA and glutamate depolarize cortical
progenitor cells and inhibit DNA synthesis. Neuron. 15:1287–1298.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Liu Q, Jia W, Chen J, Wang J, Ye D,
Guo X, Chen W, Li G, Wang G, et al: MicroRNA-200a regulates Grb2
and suppresses differentiation of mouse embryonic stem cells into
endoderm and mesoderm. PLoS One. 8:e689902013. View Article : Google Scholar : PubMed/NCBI
|