Introduction
Intervertebral disc (IVD) degeneration (IDD) is the
primary cause of low back pain, which is becoming an important
socioeconomic burden. The IVD is composed of the nucleus pulposus
(NP), annulus fibrosus and cartilage endplate, and contain
extracellular matrix (ECM), which includes collagens (predominantly
type-II collagen in NP) and proteoglycans (predominantly aggrecan)
(1). Through the stimulation of
various mechanical and biochemical signals, these ECM components
may regulate cell morphology, phenotype, differentiation and ECM
production of NP cells (2). The
degradation of ECM in IVDs, particularly in the NP, is an important
cause of IDD (1).
In the body, NP cells are in a complex mechanical
environment, and their functions are affected by mechanical factors
(3–5). Under physiological conditions, the
stress in the human intervertebral space varies with postures
between 0.1–1.1 MPa (6).
Mechanical stress is important in the homeostasis of ECM in IVD
cells. Periodic mechanical stress with low frequency and amplitude
promotes the synthesis of ECM of NP cells and inhibits its
degradation (7); whereas severe
stress directly induces the dysfunction of energy metabolism and
apoptosis of NP cells (8),
possibly causing spinal diseases, including IDD. However, the
mechanisms underlying the effects of mechanical stress on the
behaviors of NP cells remains to be fully elucidated.
Integrins are a family of adhesion proteins on the
cell surface, which are important for cell adhesion, proliferation,
apoptosis and migration (9,10).
Integrins transfer extracellular mechanical signals into
intracellular chemical signals, regulating cellular metabolism via
the downstream signaling pathways (11). Structurally, integrins are
heterodimers containing α and β units, which jointly interact with
various ligands. There are 18 types of α subunits and eight types
of β subunits, constituting 24 types of integrins (12). Previous studies have shown the
presence of various integrin subunits in NP regions, including α1,
α2, α3, α5, α6, αV, β1 and β4 subunits (13–17).
However, until now, which and how these integrins mediate the
regulatory role of periodic mechanical stress in the synthesis and
migration of ECM in NPs remain to be fully elucidated.
The phosphorylation of phospholipase C-γ1 (PLCγ1)
protein, a serine theronine kinase belonging to the phospholipase C
family, is ubiquitous in various cells to regulate processes,
including cell adhesion, migration and ECM synthesis (18–20).
In our previous study, it was demonstrated that, in chondrocytes,
periodic mechanical stress activated PLCγ1 by Src through
phosphorylation at the site of Tyr783
(PLCγ1-Tyr783) to promote chondrocyte area expansion and
migration, partially via the mitogen-activated protein kinase
kinase 1/2-extracellular signal-regulated kinase 1/2 pathway
(21,22). It was also reported that periodic
mechanical stress induced the expression of ECM collagen II (Col-2)
and proteoglycan, and induced the phosphorylation of PLCγ1 protein
in NPs, whereas treatment with U73122, an inhibitor of PLCγ1,
significantly suppressed the cyclic stress-induced expression of
ECM (23). These results indicated
that PLC-γ1 may mediate the regulatory role of periodic mechanical
stress in the expression of ECM in NPs. However, how PLCγ1 is
involved in this process remains to be fully elucidated.
The current study aimed to investigate whether
integrins and PLCγ1 have regulatory roles in periodic mechanical
stress in NP cells. The present study indicated that the periodic
mechanical stress-induced expression of ECM and migration of NP
cells was mediated by the expression of integrin α1 and
phosphorylation of downstream PLCγ1. These findings provide novel
clues for investigating the mechanisms underlying the effects of
periodic mechanical stress on regulation of the behaviors of NP
cells, and to understand the pathogenesis and development of
IDD.
Materials and methods
Isolation and culturing of NP cells
NP cells were isolated and cultured, as described
previously (24). A total of 60
male Sprague-Dawley rats (4-week-old) were obtained from the Animal
Center of Nanjing Medical University (Nanjing, China), they were
maintained in standard conditions of 24±1°C, with a relative
humidity of 50%. They had access to food and water ad
libitum and were kept under a 12-h light/dark cycle. The rats
were sacrificed by cervical dislocation, following which the
thoracic and lumbar spines were collected under sterile conditions.
Following removal of the surrounded ligament and soft tissues, the
IVDs were rapidly cut open from the ventral side and digested in
1.5% type II collagenase (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C for 2 h, followed by filtration through a
200 mesh strainer. The resultant cells were cultured in Dulbecco's
modified Eagle's medium-F12 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; GE
Healthcare Life Sciences Hyclone Laboratories, Logan, UT, USA) in a
BB5060 incubator (Heraeus, Hanau, German) at 37°C and 5%
CO2. The cells were subcultured at a confluence of 80%,
and cells in the second passage were used for the following
experiments. The surgery on the animals was conducted by Hangzhou
Hibio Technology Co., Ltd. (Hangzhou, China) and approved by their
Institutional Animal Care and Use Committee.
Cell treatment
A periodic mechanical stress system was used, as
previously described (21). The
periodic mechanical stress culturing system (Taixing Experimental
Instrument Factory, Jiangsu, China), comprised a reciprocating
boost pump and a culture chamber, which provided a periodic
mechanical stress with a pressure of 0–0.3 MPa and frequency of 0–1
Hz. The cells (1×105 cells/ml) were plated on slides
(25×25 mm), and then underwent periodic mechanical stress treatment
of 0–0.2 MPa and 0.1 Hz for 6 h (stress group) or were not exposed
to stress (control group). The cells were then collected for
detection of the expression levels of integrin α1, α5, αV, collagen
2A1 (Col2A1) and aggrecan, the phosphorylation of PLCγ1 at
Tyr783 (PLCγ1-Tyr783) and cell migration of
the NPs.
In certain experiments, NPs were transfected with
either integrin α1 small interfering (si)RNA (siRNA group)
or negative control siRNA (NC group), or remained untransfected
(control group) prior to the administration of periodic mechanical
stress (0–0.2 MPa; 0.1 Hz; 6 h). NPs were also pretreated with
U73122 (Gibco; Thermo Fisher Scientific, Inc.), an inhibitor of
PLCγ1, in DMSO at a concentration of 10 µM (U73122 group) or
with DMSO alone (control group) prior to the administration of
periodic mechanical stress. After 6 h of stress, the cells were
collected for various assays.
Cell transfection
The siRNA for integrin α1 was as follows:
Sense 5′-GGUCGGGAUUGUACAGUAUGGTT-3′ and anti-sense
5′-CCAUACUGUACAAUCCCGACCTT-3′; The NC siRNA was as follows: Sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. The siRNA and the negative control
were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China).
For transfection, 75 pM of siRNA or NC, and 7.5
µl lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) were resolved in 50 µl opti-MEM medium
(Gibco; Thermo Fisher Scientific, Inc.), respectively, mixed for 5
min and added to the cells on slides (100 µl for each slide;
1×105 cells/ml). After 6 hours at 37°C, the medium was
replaced. The cells were collected for western blot analysis to
confirm successful transfection, and then underwent periodic
mechanical stress treatment.
Western blot analysis
The cells were collected and washed with
phosphate-buffered saline (PBS), and added to RIPA lysis buffer
(Beyotime Institute of Biotechnology, Nantong, China) on ice for 5
min. The cell lysate was centrifuged at 14,000 g for 5 min at 4°C,
and the supernatant was collected. The concentration of the
resultant total protein was determined using a bicinchoninic acid
assay (Beyotime Institute of Biotechnology), and the protein was
denatured and samples (50 µg) were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis with an equal
quantity of total protein for each sample. The proteins in the gel
were transferred onto polyvinylidene fluoride membranes, blocked
and incubated with primary antibodies as follows: Goat polyclonal
anti-integrin α1 (1:1,000; cat. no. sc-6584, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mouse polyclonal anti-PLCγ1
(1:1,000; cat. no. ab16955; Abcam, Cambridge, MA, USA), rabbit
polyclonal anti-PLCγ1-Tyr783 (1:1,000; cat. no. 2821;
Cell Signaling Technology, Inc., Danvers, MA, USA) and rabbit
polyclonal anti-GAPDH (1:5,000; cat. no. AP0063; Bioworld
Technology, Inc., Louis park, MN, USA) at 4°C overnight. Following
washing of the membrane PBS with Tween 20, the goat anti-rabbit
IgG-horseradish peroxidase (HRP; cat. no. BS13278; Bioworld
Technology, Inc.), goat anti-mouse IgG-HRP (cat. no. BS12478;
Bioworld Technology, Inc.) or rabbit anti-goat IgG-HRP (cat. no.
sc-2768; Santa Cruz Biotechnology, Inc.) secondary antibodies were
then added at room temperature for 1 h. Following washing, the
membrane was visualized using Immobilon™ Western Chemiluminescent
HRP substrate reagent (EMD Millipore, Billerica, MA, USA). The
blots were scanned using a Bio-Rad Gel Doc Imaging System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the band densities were
quantified and compared using QuantityOne software (Bio-Rad
Laboratories, Inc.). Expression of the target protein was
calculated as the band density of the target protein normalized to
that of GAPDH.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was extracted from the cells using
TRIzol regent (Invitrogen; Thermo Fisher Scientific, Inc.), and
transcribed into cDNA using a PrimeScript RT Master Mix kit (Takara
Bio, Inc., Shiga, Japan). The primers for integrin α1,
integrin α5, integrin αV, aggrecan and
Col2a1 were as follows: sense 5′-GGGCTACTGCTGCTAATGCT-3′ and
antisense 5′-GGCCTTTTGAAGAATCCAATC-3′ for integrin α1; sense
5′-AGCTGCATTTCCGAGTCTG-3′ and antisense 5′-CTCACACTGAAGGCTGAACG-3′
for integrin α5; sense 5′-GGTGTGGATCGAGCTGTCTT-3′ and
antisense CAAGGCCAGCATTTACAGTG-3′ for integrin αV; sense
5′-CCCTACCCTTGCTTCTCCA-3′ and antisense
5′-CTTGAGAGGCACTCATCAATGT-3′ for aggrecan; sense
5′-GACCCCCAGGTTCTAATGG-3′ and antisense 5′-GCACCTTTGGGACCATCTT-3′
for Col2A1; and sense 5′-GCAGAAGGAGATTACTGCCCT-3′ and
antisense 5′-GCTGATCCACATCTGCTGGAA-3′ for β-actin. The
primers were synthesized by Shanghai GenePharma Co., Ltd. RT-qPCR
analysis was performed using a SYBR Premix Ex Taq II kit (Takara
Bio, Inc.) and a StepOnePlus Real-Time PCR system (Thermo Fisher
Scientific, Inc.). The reaction system (20 µl) contained 10
µl 2X SYBR Premix Ex Taq II, 0.4 µl forward primer
(10 µM), 0.4 µl reverse primer (10 µM), 0.4
µl 50X ROX Reference Dye, 2 µl DNA sample, and 6.8
µl distilled water. Thermocycling conditions were as
follows: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for
30 sec. The results were quantified using the 2−ΔΔCq
method (19).
Cell migration assay
A scratch test is an effective means for the
assessment of the migration capacities of NP cells (25). Following treatment, scratches were
introduced vertically on the bottom of the slides, with the fine
end of 200 µl tips. The medium was then removed, and the
slides were washed with PBS three times to remove the detached
cells. Serum-free medium was then added for culturing in an
incubator at 37°C, 5% CO2 for 4 h. The slides were
observed under a CKX31 microscope (Olympus, Tokyo, Japan), and
images were captured under three optimal fields at 0 and 4 h
following introduction of the scratch with the tips. Cell migration
was analyzed using ImageJ 1.43 software (imagej.nih.gov). The cell migration distance
(µm) was calculated as the scratch width (shown as the
distance between the two dotted lines in Fig. 1) at 0 h minus the scratch width 4 h
following the introduction of scratch injury.
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL,
USA). The data between groups were analyzed using an unpaired
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
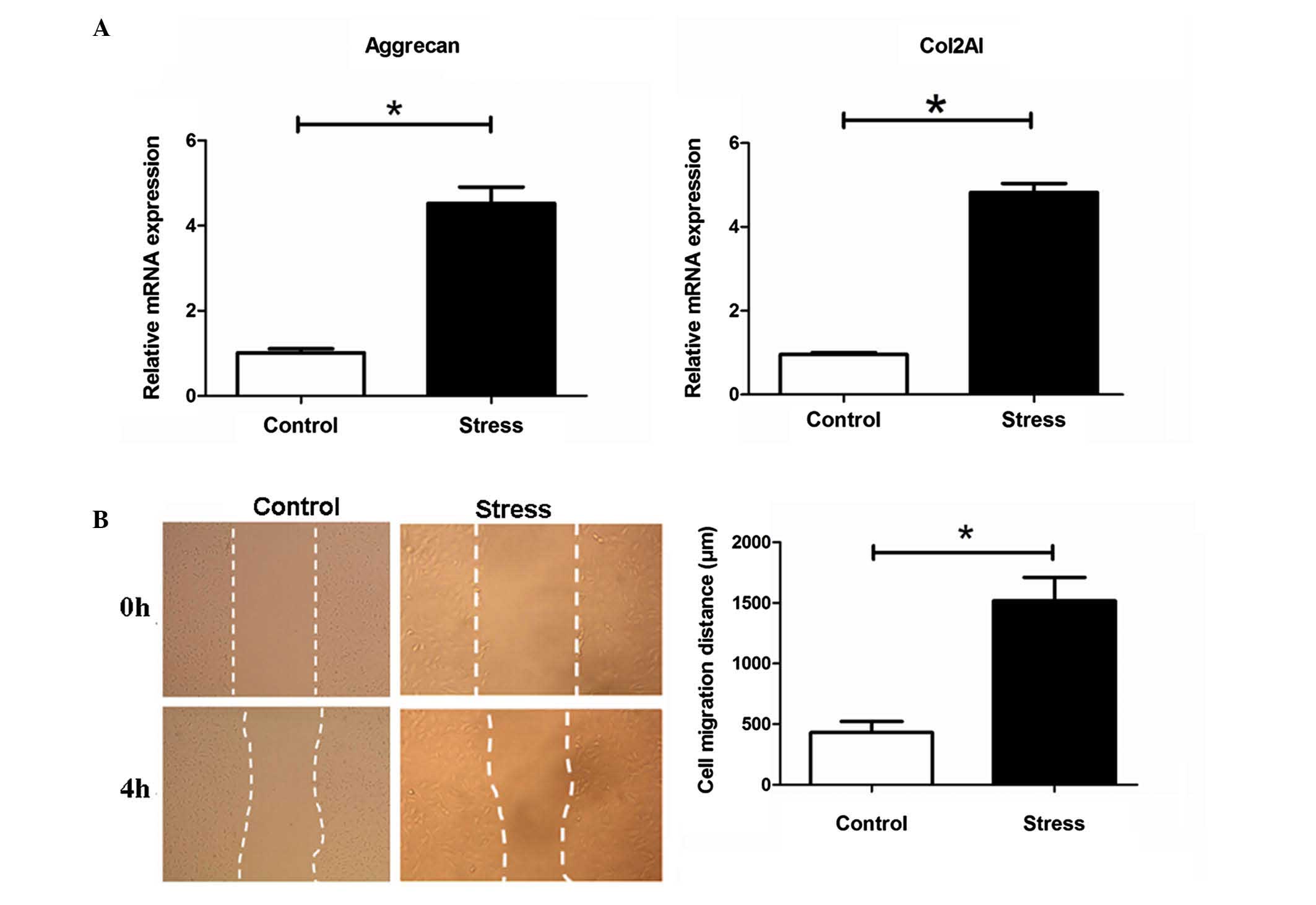
Periodic mechanical stress significantly
induces the mRNA expression of ECM Col-2A1 and aggrecan, and
promotes the migration of NP cells
Compared with the control, which was not exposed to
stress treatment, periodic mechanical stress (0.2 MPa; 0.1 Hz; 6 h)
significantly induced the mRNA expression of ECM Col2A1 and
aggrecan, as determined using RT-qPCR analysis (P<0.05, Fig. 1A), and promoted the migration of NP
cells, determined in scratch experiments (P<0.05, Fig. 1B).
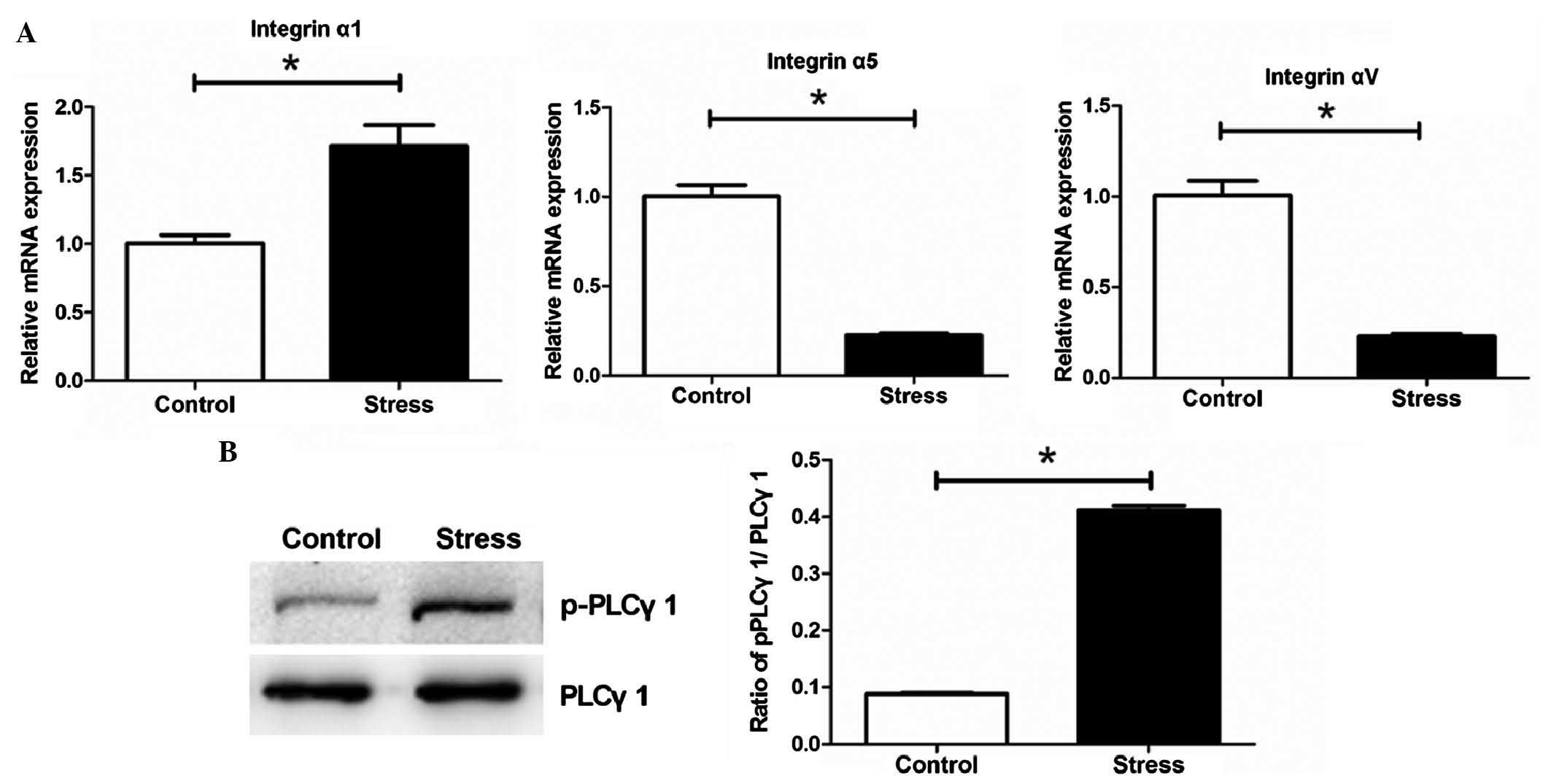
Periodic mechanical stress significantly
upregulates the mRNA expression of integrin α1 and induces the
phosphorylation of PLCγ1 in NP cells
The RT-qPCR analysis showed that periodic mechanical
stress significantly induced the mRNA expression of integrin α1,
but inhibited the expression levels of intergrin α5 and αV,
compared with the control (P<0.05; Fig. 2A). Periodic mechanical stress also
significantly induced the phosphorylation of PLC-γ1 (P<0.05;
Fig. 2B).
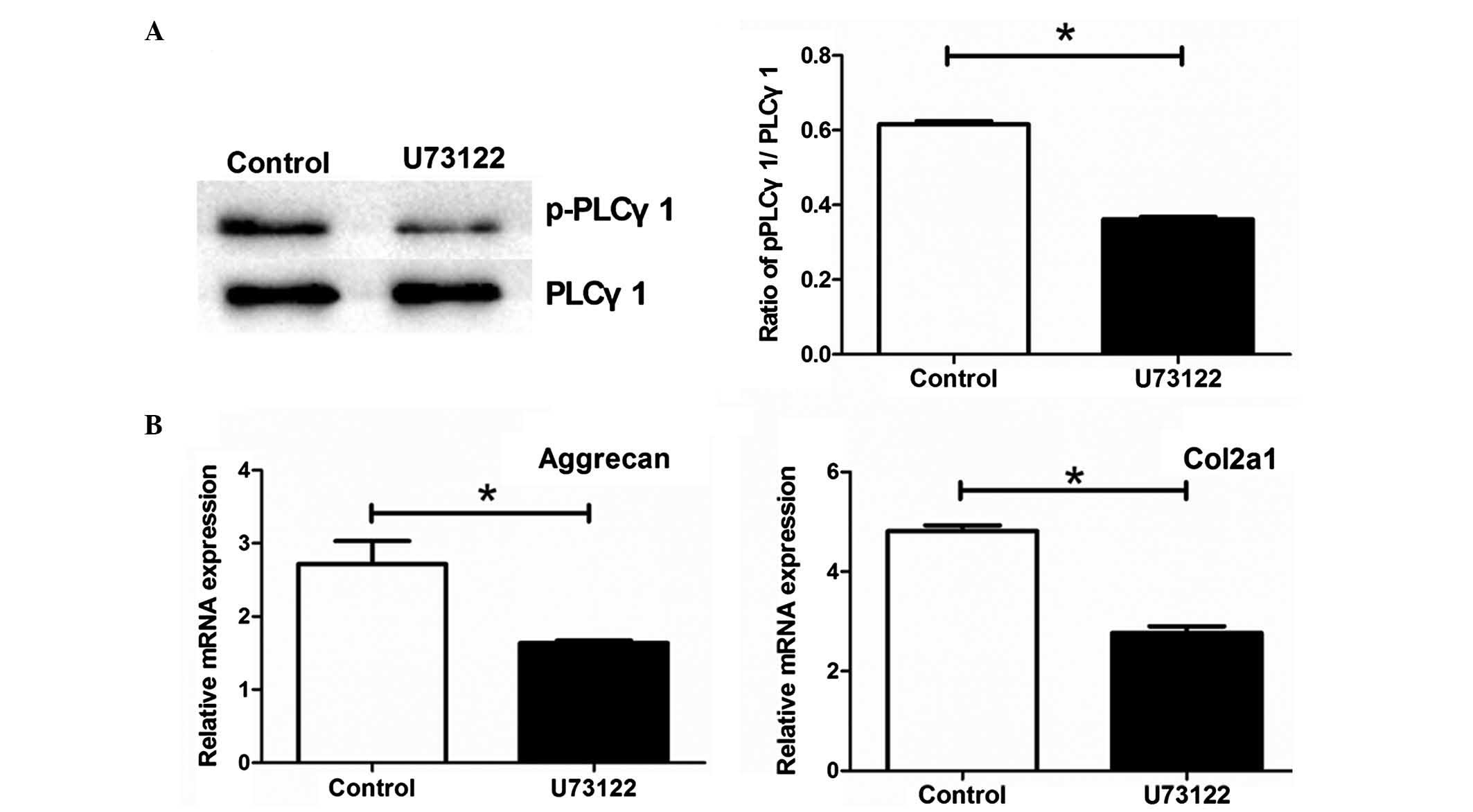
Phosphorylation of PLCγ1 is required for
the periodic mechanical stress-induced expression of ECM
To examine whether the phosphorylation of PLCγ1 is
involved in the periodic mechanical stress-induced expression of
ECM, the NP cells were pretreated with U73122 prior to the
administration of periodic mechanical stress. Pretreatment with
U73122 significantly inhibited the phosphorylation of PLCγ1 in the
NP cells (P<0.05; Fig. 3A), and
suppressed the mRNA expression levels of Col2A1 and aggrecan in the
NP cells under periodic mechanical stress (P<0.05; Fig. 3B).
siRNA-based inhibition of integrin α1
suppresses the mRNA expression of Col2A1 and aggrecan, the
migration of NPs and the phosphorylation of PLCγ1 under periodic
mechanical stress
As the results of the present study showed that
periodic mechanical stress induced the mRNA expression of integrin
α1 in NP cells under periodic mechanical stress, to determine
whether integrin α1 is involved in the regulatory effect of
periodic mechanical stress on NP cells, integrin α1 siRNA
was transfected into the NP cells. As shown in Fig. 4A, transfection with integrin
α1 siRNA resulted in a significant decrease in the protein
expression of integrin α1, as detected using western blot analysis
(P<0.05), confirming the inhibition of integrin α1 in the NP
cells of the siRNA group. Compared with the untransfected NP cells
or the cells transfected with NC siRNA, transfection with
integrin α1 siRNA suppressed the mRNA expression levels of
Col2A1 and aggrecan (Fig. 4B), and
the migration (Fig. 4C) of NP
cells under periodic mechanical stress (P<0.05).
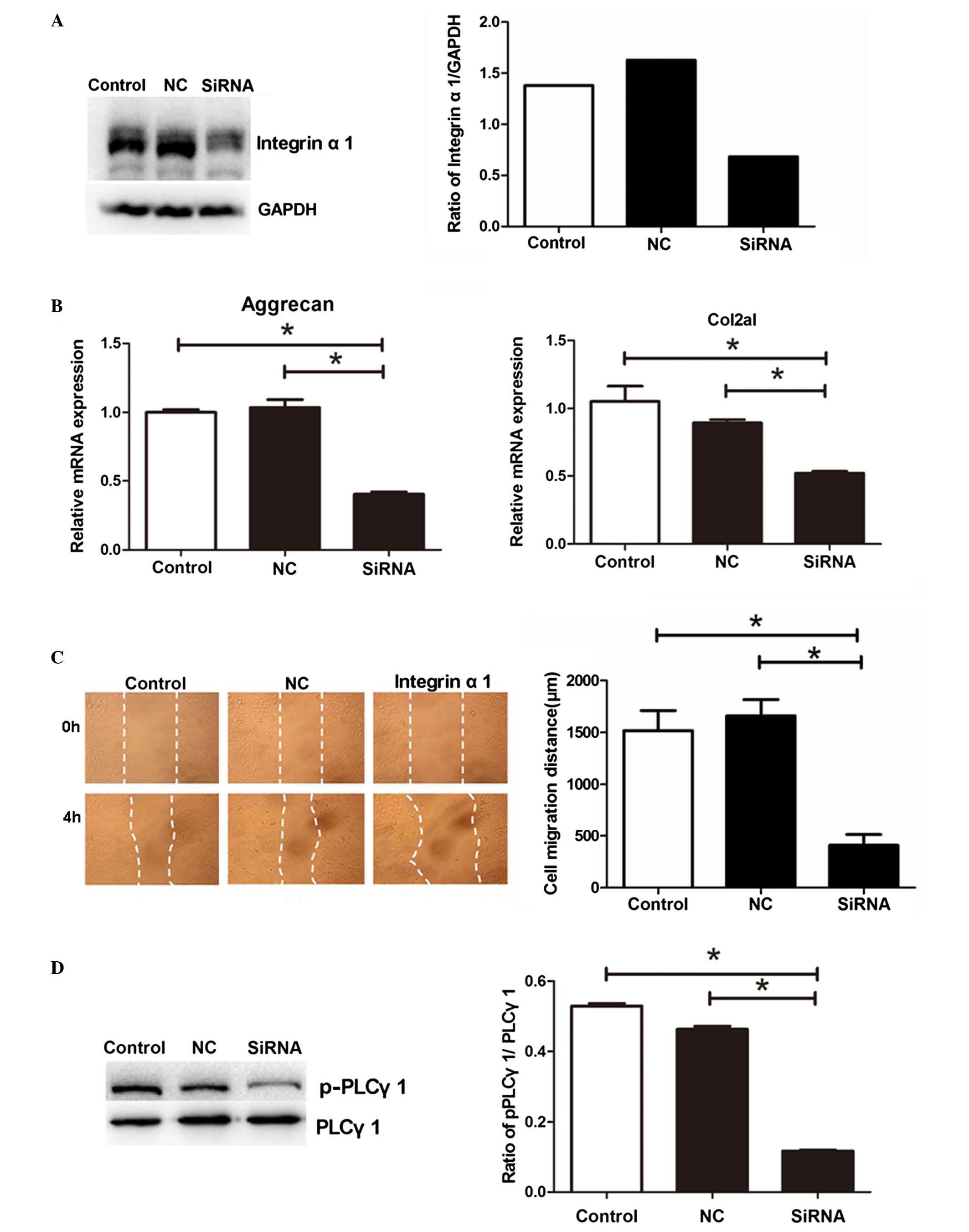 | Figure 4siRNA-based inhibition of the
expression of integrin α1 suppresses the mRNA expression levels of
Col2A1 and aggrecan, migration of NPs and phosphorylation of PLC-γ1
under periodic mechanical stress. NP cells transfected with
integrin α1 siRNA (siRNA), NC siRNA or untransfected cells
(control) were collected. (A) Protein expression of integrin α1 was
analyzed using western blot analysis. The NP cells from the siRNA,
NC and control groups were collected to measure the (B) mRNA
expression levels of Col2A1 and aggrecan, (C) migration (dotted
lines indicate scratch widths; magnification, ×200) and (D)
phosphorylation of PLC-γ1 under periodic mechanical stress. Data
are expressed as the mean ± standard deviation.
*P<0.05, compared with the control or NC group (n=3).
siRNA, small interfering RNA; Col2A1, collagen 2A1; PLCγ1,
phospholipase C-γ1; p-PLCγ1, phosphorylated PLCγ1; NP, nucleus
pulposus; NC, negative control. |
As shown in Fig.
4D, compared with the untransfected cells or the cells
transfected with NC, transfection with integrin α1, siRNA
led to a significant decrease in the phosphorylation of PLCγ1 in
the NPs under periodic mechanical stress (P<0.05).
Discussion
In NP cells, the ECM comprises predominantly Col-2
and proteoglycan, and the content gradually decreases with IDD
(26). Cell migration is important
for tissue reconstruction and repair (27). Anabolic ECM, predominantly Col2A1
and aggrecan, and the migration of NP cells are required for the
elasticity and functions of IVD; therefore, the present study
combined assessment of the expression levels of Col2A1 and
proteoglycan with cell migration to investigate the regulatory
effect of periodic mechanical stress on the functions of NP cells.
The present study aimed to investigate the mechanisms underlying
how periodic mechanical stress regulates the biological effects of
NP cells.
Depending on the magnitude, frequency and duration
of stress, NP cells exhibit diverse biological responses. Matsumoto
et al (28) found that
mechanical periodic stretch stress increased collagenous protein
synthesis in rabbit NP cells. Neidlinger-Wilke et al
(29) reported that low
hydrostatic pressure (0.25 MPa; 0.1 Hz; 30 min) promoted and high
pressure (2.5 MPa; 0.1 Hz; 30 min) decreased the expression levels
of Col-2 and aggrecan in NP cells (29). Similarly, Hee et al
(30) showed that the collagen and
glycosaminoglycan contents were significantly higher in inner NP
cells cultured under 0.2 MPa of compressive stress, compared with
untreated control cells, but were significantly lower under 0.4 MPa
of compressive stress. In our previous study, it was found that
periodic mechanical stress of 0–0.2 MPa and 0.1 Hz not only
significantly promoted the migration of chondrocytes (21), but also promoted the expression of
ECM in the NP cells (23).
Therefore, this condition was selected for investigation in the
present study. The results demonstrated that soft periodic
mechanical stress (0–0.2 Mpa; 0.1 Hz; 6 h) significantly induced
the synthesis of ECM and the migration of NP cells, which were
consistent with previous results (23,28–30).
Therefore, the results of the present and previous studies
indicated that soft stress can improve the biological functions of
NPs.
Integrins mediate interactions with the ECM of NPs,
which can promote cell attachment, survival and the biosynthesis of
NPs. Inhibiting the β1 subunit inhibits NP cell attachment to all
substrates, whereas inhibiting subunits α1, α2, α3 and α5
simultaneously inhibit NP cell attachment to laminins (14). Fibronectin fragments or dynamic
load (1.3 MPa; 1.0 Hz) induce the degeneration of NP cells and
expression of integrin α5β1, which is
reversed by silencing the expression of integrin
α5β1 or by inhibiting its activity using the
Arg-Gly-Asp peptide, indicating that integrin
α5β1 mediates the fibronectin fragment- or
severe dynamic load-induced degeneration of NP cells and catabolic
gene expression (17,31). However, until now, the effects of
integrin α1 on the behaviors of NP cells under periodic mechanical
stress, and the associated mechanisms, have not been previously
reported.
The present study examined the variation in the
expression of α1, α5 and αv, the most important α subunits in NP
cells (13), following periodic
mechanical stress treatment. It was found that soft periodic
mechanical stress increased the expression of integrin α1,
decreased the expression of integrins α5 and αv, induced the
expression of ECM Col2A1 and aggrecan, and promoted the migration
of NP cells. The siRNA-based inhibition of the expression of
integrin α1 or treatment with the PLCγ1 inhibitor, U73122,
suppressed the ECM expression and the cell migration of the NPs
under periodic mechanical stress, indicating that integrin α1 and
PLCγ1 are necessary for the effect of periodic mechanical stress on
the NP cells. In addition, transfection of the NP cells with
integrin α1 decreased the phosphorylation of PLCγ1 at
Tyr783, indicating that PLCγ1 functions downstream of
integrin α1. These results suggested that the integrin α1-mediated
phosphorylation of PLC-γ1 protein is involved in inducing the
expression of ECM and migration of NP cells under soft cyclic
stress. In addition, the decrease in the expression of α5 and αv in
NP cells in response to the periodic mechanical stress suggested
that the α5 and αv subunits function negatively in the behavior of
the NP cells under periodic mechanical stress, consistent with
previous reports (17,31).
Various studies have shown that integrins regulate
the functions of PLCγ1. The activation of PLCγ1 occurs following
α5β1 integrin activation in fibroblasts, which is required for
integrin-dependent adhesion (18).
The inhibition of PLCγ reduces α1β1
integrin-mediated cell adhesion, indicating that PLCγ is required
for α1β1-dependent signaling (32). It has also been shown that PLCγ1
modulated β1 integrin-dependent T lymphocyte migration on
fibronectin (19). However, the
interaction between integrin α1 and PLCγ1 in NP cells
has not been reported. On the basis of our previous investigation,
which revealed that periodic mechanical stress induces the
phosphorylation of PLCγ1 in NP cells and the expression of ECM
(23), the present study showed
that PLCγ1 phosphorylation (at Tyr783) was essential for
the periodic mechanical stress-induced expression of ECM and the
migration of NP cells, functioning downstream of integrin α1. How
they interact to induce the expression of ECM and migration of NP
cells under periodic mechanical stress requires further
investigation.
Calcium is an important secondary messenger for
cellular signal transduction in various physiological processes. It
has been reported that stress induces the concentration of cellular
calcium (33). Of note, PLCγ1 is
also involved in the regulation of cellular calcium concentrations
(34,35). Therefore, the regulation of
cellular calcium concentrations may involve a mechanism by which
PLCγ1 mediates the periodic mechanical stress-induced expression of
ECM and migration of NP cells. Further investigations are required
to clarify this.
In conclusion, the present study demonstrated that
periodic mechanical stress increased the expression of integrin α1
on the cell surface to induce the ECM expression and migration of
NP cells, which was mediated by the expression of integrin α1 and
the phosphorylation of downstream PLCγ1. These results provide
novel clues for further investigating the mechanisms underlying the
effects of periodic mechanical stress on the behavior of NP cells,
and understanding the pathogenesis and development of IDD.
Abbreviations:
|
IDD
|
intervertebral disk degeneration
|
|
ECM
|
extracellular matrix
|
|
NP
|
nucleus pulposus
|
|
Col2A1
|
collagen 2A1
|
|
PLCγ1
|
phospholipase C-γ1
|
|
IVD
|
intervertebral disc
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81201417).
References
|
1
|
Urban JP and Roberts S: Degeneration of
the intervertebral disc. Arthritis Res Ther. 5:120–130. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang PY, Chen J, Jing L, Hoffman BD and
Setton LA: The role of extracellular matrix elasticity and
composition in regulating the nucleus pulposus cell phenotype in
the intervertebral disc: A narrative review. J Biomech Eng.
136:0210102014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Gonzales S, Levene H, Gu W and
Huang CY: Energy metabolism of intervertebral disc under mechanical
loading. J Orthop Res. 31:1733–1738. 2013.PubMed/NCBI
|
|
4
|
Fernando HN, Czamanski J, Yuan TY, Gu W,
Salahadin A and Huang CY: Mechanical loading affects the energy
metabolism of intervertebral disc cells. J Orthop Res.
29:1634–1641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan SC, Walser J, Käppeli P, Shamsollahi
MJ, Ferguson SJ and Gantenbein-Ritter B: Region specific response
of intervertebral disc cells to complex dynamic loading: An organ
culture study using a dynamic torsion-compression bioreactor. PLoS
One. 8:e724892013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilke HJ, Neef P, Caimi M, Hoogland T and
Claes LE: New in vivo measurements of pressures in the
intervertebral disc in daily life. Spine (Phila Pa 1976).
24:755–762. 1999. View Article : Google Scholar
|
|
7
|
Kasra M, Goel V, Martin J, Wang ST, Choi W
and Buckwalter J: Effect of dynamic hydrostatic pressure on rabbit
intervertebral disc cells. J Orthop Res. 21:597–603. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo YJ, Wu LC, Sun JS, Chen MH, Sun MG and
Tsuang YH: Mechanical stress-induced apoptosis of nucleus pulposus
cells: An in vitro and in vivo rat model. J Orthop Sci. 19:313–322.
2014. View Article : Google Scholar
|
|
9
|
Ernst N, Yay A, Bíró T, Tiede S, Humphries
M, Paus R and Kloepper JE: β1 integrin signaling maintains human
epithelial progenitor cell survival in situ and controls
proliferation, apoptosis and migration of their progeny. PLoS One.
8:e843562013. View Article : Google Scholar
|
|
10
|
Wang YC, Juan HC, Wong YH, Kuo WC, Lu YL,
Lin SF, Lu CJ and Fann MJ: Protogenin prevents premature apoptosis
of rostral cephalic neural crest cells by activating the
α5β1-integrin. Cell Death Dis. 4:e6512013. View Article : Google Scholar
|
|
11
|
Huang H, Kamm RD and Lee RT: Cell
mechanics and mechanotransduction: Pathways, probes, and
physiology. Am J Physiol Cell Physiol. 287:C1–C11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nettles DL, Richardson WJ and Setton LA:
Integrin expression in cells of the intervertebral disc. J Anat.
204:515–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bridgen DT, Gilchrist CL, Richardson WJ,
Isaacs RE, Brown CR, Yang KL, Chen J and Setton LA:
Integrin-mediated interactions with extracellular matrix proteins
for nucleus pulposus cells of the human intervertebral disc. J
Orthop Res. 31:1661–1667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tran CM, Schoepflin ZR, Markova DZ, Kepler
CK, Anderson DG, Shapiro IM and Risbud MV: CCN2 suppresses
catabolic effects of interleukin-1β through α5β1 and αVβ3 integrins
in nucleus pulposus cells: Implications in intervertebral disc
degeneration. J Biol Chem. 289:7374–7387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang SD, Ma L, Gu TX, Ding WY, Zhang F,
Shen Y, Zhang YZ, Yang DL, Zhang D, Sun YP and Song YL:
17β-Estradiol protects against apoptosis induced by levofloxacin in
rat nucleus pulposus cells by upregulating integrin α2β1.
Apoptosis. 19:789–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurakawa T, Kakutani K, Morita Y, Kato Y,
Yurube T, Hirata H, Miyazaki S, Terashima Y, Maeno K, Takada T, et
al: Functional impact of integrin α5β1 on the homeostasis of
intervertebral discs: A study of mechanotransduction pathways using
a novel dynamic loading organ culture system. Spine J. 15:417–426.
2015. View Article : Google Scholar
|
|
18
|
Tvorogov D, Wang XJ, Zent R and Carpenter
G: Integrin-dependent PLC-gamma1 phosphorylation mediates
fibronectin-dependent adhesion. J Cell Sci. 118:601–610. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shannon LA, Calloway PA, Welch TP and
Vines CM: CCR7/CCL21 migration on fibronectin is mediated by
phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes. J Biol
Chem. 285:38781–38787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng G, Cui X, Liu Z, Zhao H, Zheng X,
Zhang B and Xia C: Disruption of phosphoinositide-specific
phospholipases Cγ1 contributes to extracellular matrix synthesis of
human osteoarthritis chondrocytes. Int J Mol Sci. 15:13236–13246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nong L, Yin G, Ren K, Tang J and Fan W:
Periodic mechanical stress enhances rat chondrocyte area expansion
and migration through Src-PLCgamma1-ERK1/2 signaling. Eur J Cell
Biol. 89:705–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren K, Ma Y, Huang Y, Liang W, Liu F, Wang
Q, Cui W, Liu Z, Yin G and Fan W: Periodic mechanical stress
activates MEK1/2-ERK1/2 mitogenic signals in rat chondrocytes
through Src and PLCγ1. Braz J Med Biol Res. 44:1231–1242. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie H, Nong L, Gao G, Zhou D, He J and
Chen X: Effect of the phospholipase C-γ1 protein and its inhibitor
on rat nucleus pulposus cells proliferation under periodic
mechanical stress. Chin J Exp Sur. 30:2361–2363. 2013.
|
|
24
|
Risbud MV, Guttapalli A, Stokes DG,
Hawkins D, Danielson KG, Schaer TP, Albert TJ and Shapiro IM:
Nucleus pulposus cells express HIF-1 alpha under normoxic culture
conditions: A metabolic adaptation to the intervertebral disc
microenvironment. J Cell Biochem. 98:152–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bron JL, Mulder HW, Vonk LA, Doulabi BZ,
Oudhoff MJ and Smit TH: Migration of intervertebral disc cells into
dense collagen scaffolds intended for functional replacement. J
Mater Sci Mater Med. 23:813–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roughley PJ: Biology of intervertebral
disc aging and degeneration: Involvement of the extracellular
matrix. Spine (Phila Pa 1976). 29:2691–2699. 2004. View Article : Google Scholar
|
|
27
|
Barreto Henriksson H, Lindahl A,
Skioldebrand E, Junevik K, Tängemo C, Mattsson J and Brisby H:
Similar cellular migration patterns from niches in intervertebral
disc and in knee-joint regions detected by in situ labeling: An
experimental study in the New Zealand white rabbit. Stem Cell Res
Ther. 4:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsumoto T, Kawakami M, Kuribayashi K,
Takenaka T and Tamaki T: Cyclic mechanical stretch stress increases
the growth rate and collagen synthesis of nucleus pulposus cells in
vitro. Spine (Phila Pa 1976). 24:315–319. 1999. View Article : Google Scholar
|
|
29
|
Neidlinger-Wilke C, Würtz K, Urban JP,
Börm W, Arand M, Ignatius A, Wilke HJ and Claes LE: Regulation of
gene expression in intervertebral disc cells by low and high
hydrostatic pressure. Eur Spine J. 15(Suppl 3): S372–S378. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hee HT, Zhang J and Wong HK: An in vitro
study of dynamic cyclic compressive stress on human inner annulus
fibrosus and nucleus pulposus cells. Spine J. 10:795–801. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia M and Zhu Y: Fibronectin fragment
activation of ERK increasing integrin α5 and
β1 subunit expression to degenerate nucleus pulposus
cells. J Orthop Res. 29:556–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vossmeyer D, Hofmann W, Löster K, Reutter
W and Danker K: Phospholipase Cgamma binds alpha1beta1 integrin and
modulates alpha1beta1 integrin-specific adhesion. J Biol Chem.
277:4636–4643. 2002. View Article : Google Scholar
|
|
33
|
Browning JA, Saunders K, Urban JP and
Wilkins RJ: The influence and interactions of hydrostatic and
osmotic pressures on the intracellular milieu of chondrocytes.
Biorheology. 41:299–308. 2004.PubMed/NCBI
|
|
34
|
Berridge MJ and Irvine RF: Inositol
trisphosphate, a novel second messenger in cellular signal
transduction. Nature. 312:315–321. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji QS, Winnier GE, Niswender KD, Horstman
D, Wisdom R, Magnuson MA and Carpenter G: Essential role of the
tyrosine kinase substrate phospholipase C-gamma1 in mammalian
growth and development. Proc Natl Acad Sci USA. 94:2999–3003. 1997.
View Article : Google Scholar : PubMed/NCBI
|